Summary
Background
Endoscopy surveillance is recommended for mild-moderate dysplasia and negative endoscopy findings every 3 years and 5 years, respectively, but evidence is limited. This study aimed to assess long-term esophageal cancer (EC) incidence and mortality after a single endoscopy screening.
Methods
We included individuals at high risk of EC aged 40–69 years who underwent endoscopy screening in 2007–2012 at six centres in rural China and had a baseline diagnosis of negative endoscopy findings, mild dysplasia, or moderate dysplasia. Participants were followed up for EC incidence and mortality. Cumulative incidence and mortality rates of EC were estimated by Kaplan–Meier analyses. Cox regression models were used to calculate adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between baseline endoscopy diagnosis and the risk of EC incidence and mortality. EC incidence and mortality after a single endoscopy screening were compared with those of the population in rural China by the standardized incidence ratio (SIR) and standardized mortality ratio (SMR).
Findings
A total of 42,827 participants (40,977 with negative endoscopy findings, 1562 with mild dysplasia, and 288 with moderate dysplasia) were included; 268 EC cases and 128 EC deaths were identified during a median follow-up of 10.62 years. The cumulative EC incidence at 10 years was 0.45% (0.38–0.52) in the group with negative endoscopy findings, 2.39% (1.62–3.16) in the mild dysplasia group, and 8.90% (5.57–12.24) in the moderate dysplasia group, and the cumulative EC mortality at 10 years was 0.23% (0.18–0.27), 0.96% (0.46–1.46), and 2.50% (0.67–4.33), respectively. Compared with individuals with negative endoscopy findings, the HRs for EC incidence and mortality in the mild dysplasia group were 3.52 (2.49–4.97) and 2.43 (1.41–4.19), and those in the moderate dysplasia group were 13.18 (8.78–19.76) and 6.46 (3.13–13.29), respectively. The SIR was 0.53 (0.40–0.70) for the group with negative endoscopy findings, 1.95 (1.69–2.24) for the mild dysplasia group, and 6.75 (6.25–7.28) for the moderate dysplasia group, with the SMRs of 0.43 (0.31–0.58), 1.07 (0.88–1.29) and 2.67 (2.36–3.01), respectively.
Interpretation
Individuals with negative endoscopy findings after a single endoscopy screening had a lower EC risk than the general population for up to 10.62 years, while those with mild-moderate dysplasia had an elevated risk. Our results support endoscopy surveillance for mild-moderate dysplasia every 3 years and suggest extending the interval to 10 years after a negative endoscopy finding.
Funding
National Key R&D Programme of China, Special Project of Beijing-Tianjin-Hebei Basic Research Cooperation, and Sanming Project of Medicine in Shenzhen.
Keywords: Esophageal cancer, Endoscopic screening, Endoscopy surveillance, Screening interval, Population-based
Research in context.
Evidence before this study
We searched PubMed with no language or date restrictions for studies on progression risk and surveillance intervals for individuals with negative endoscopy findings and mild and moderate dysplasia after a single esophageal cancer (EC) screening with endoscopy before June 8, 2023. We used the following search terms in either the title or the abstract: ((risk) OR (interval)) AND (((esophageal) AND (cancer)) AND (screening)). Only 4 studies reported the progression risk of EC incidence and mortality among participants with negative endoscopy findings, mild or moderate dysplasia after baseline endoscopy screening. Relative risks of EC incidence and (or) mortality among previous studies in individuals with mild or moderate dysplasia were estimated compared with those among individuals with normal squamous epithelium, without direct evidence to support the current recommended 3-year surveillance intervals. In addition, individuals with negative endoscopy findings accounted for 90% of the endoscopy screening population, however, evidence is scarce regarding the degree of EC risk and the EC risk duration from population-based prospective studies, which largely limited its recommendation quality (5-year surveillance interval) in Chinese EC screening guidelines.
Added value of this study
In this multicentre, population-based cohort from a large EC screening programme in China, we assessed long-term EC incidence and mortality by baseline endoscopy findings and estimated potential intervals for endoscopy surveillance during a median follow-up of 10.6 years. Our results showed that the cumulative EC incidence at 10 years was 0.45% for baseline negative endoscopy findings, 2.39% for mild dysplasia and 8.90% for moderate dysplasia, and the cumulative EC mortality rates were 0.23%, 0.96% and 2.50%, respectively. Individuals with mild and moderate dysplasia had a significantly higher risk of EC incidence and mortality than both individuals with baseline negative endoscopy findings and the general population, especially within the first 3 years. Individuals with negative endoscopy findings after a single endoscopy screening had a lower risk than the general population within 10 years, with an EC standardized incidence ratio of 0.53 and a standardized mortality ratio of 0.43.
Implications of all the available evidence
The disparities in the subsequent EC incidence and mortality among individuals with negative endoscopy findings and mild-moderate dysplasia indicate an urgent need to develop risk-stratified endoscopy surveillance management by baseline endoscopy findings. The findings in this study suggest that individuals with mild and moderate dysplasia should undergo endoscopy surveillance within 3 years, while individuals with negative endoscopy findings should undergo endoscopy surveillance within 10 years. Our study provides timely evidence and management strategies for population-based EC screening programmes in real practice, which would inform policy-makers to better implement EC screening programmes in China and to promote further EC screening in other countries facing the threat of a high EC burden.
Introduction
Esophageal cancer (EC) is a major public health concern worldwide and is characterized by poor survival.1, 2, 3 Endoscopic screening has been associated with reduced EC mortality,4,5 and China has the longest EC screening history and richest experience in EC prevention worldwide.6,7 In population-based EC screening, individuals can be categorized into three groups based on endoscopy diagnosis: a positive case group with a diagnosis of severe dysplasia or worse, a low-grade intraepithelial neoplasia (LGIN) group with a diagnosis of mild dysplasia or moderate dysplasia, and a group with negative endoscopy findings for individuals without any dysplasia.8 Patients in the positive case group can directly benefit from endoscopy screening through early treatment; however, the detection rate is less than 1%.9,10 In fact, many individuals are in the negative endoscopy group (more than 90%) and the LGIN group (nearly 4%) after a single endoscopy screening9 and need subsequent endoscopy surveillance to prevent EC.
Management with endoscopy surveillance, i.e., rescreening for individuals with LGIN or those with negative endoscopy findings, is an essential but overwhelming issue in the field of EC screening.8 Understanding the duration and degree of EC risk within different follow-up periods would provide crucial scientific evidence for decisions regarding endoscopy surveillance intervals. Currently, surveillance intervals are recommended for individuals with LGIN (1–3 years) and those with negative endoscopy findings (5 years) in the Chinese guidelines for EC screening; however, evidence from population-based cohorts supporting these recommendations is limited.8 There have been only two cohort studies conducted in areas with extremely high incidence rates of EC in China that estimated the progression risk of EC among individuals who underwent endoscopy screening and showed that individuals with LGIN had a higher EC risk than those with a normal squamous epithelium.11,12 No prospective study has assessed EC risk by follow-up period and provided direct evidence to support the current recommended surveillance intervals for LGIN. In addition, individuals with negative endoscopy findings have been recognized as having a low risk of EC; however, the degree of EC risk and the EC risk duration remain unclear due to a lack of evidence from cohort studies. The recommended surveillance intervals for individuals with negative endoscopy findings in the Chinese guidelines have primarily been developed on the basis of evidence from limited modelling studies on screening intervals,13, 14, 15 and the final determination is mainly based on clinical experience and screening practice. To adequately formulate and continually update these recommendations, there is an urgent need for high-quality evidence derived from robust multicentre population-based cohorts.
In light of these knowledge gaps, the specific aims of our multicentre, population-based EC screening cohort study in China were to assess long-term EC incidence and mortality after a single endoscopy and to estimate potential intervals for endoscopy surveillance. Specifically, we first assessed EC incidence and mortality among individuals with negative endoscopy findings, mild dysplasia, and moderate dysplasia during a median follow-up period of 10.6 years. Second, we examined the relationship between baseline endoscopy diagnosis and EC incidence and mortality with different follow-up periods to estimate the potential surveillance intervals for mild and moderate dysplasia; the potential surveillance intervals for individuals with negative endoscopy findings were estimated by the relative risk of EC incidence and mortality by matching sex, age, and calendar year of follow-up to those of the general population.
Methods
Study design and population
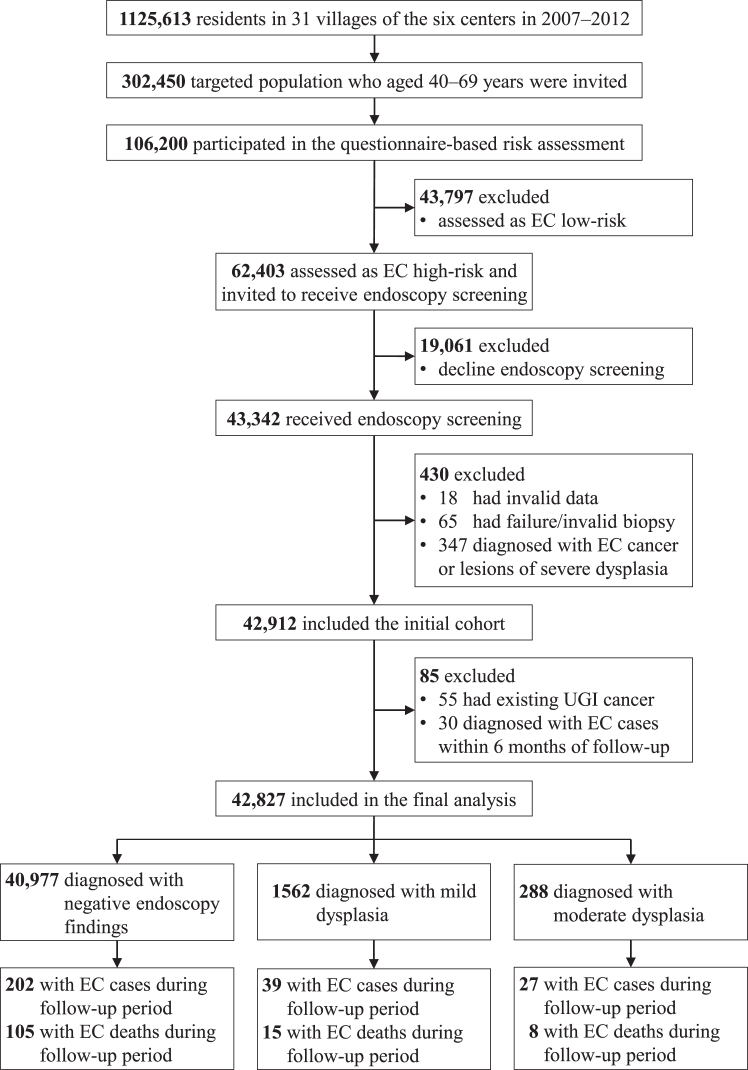
We conducted this multicentre, population-based, prospective study based on an EC screening programme in China, which was launched in 2007, and the study protocol has been described elsewhere.16,17 Six centres (counties) enrolled in this programme in 2007–2012 (Supplementary Table S1 and S2) were included in this study. A flowchart illustrating the inclusion and exclusion of the study participants is shown in Fig. 1. We included individuals at high risk of EC who underwent endoscopy screening and had a baseline diagnosis of negative endoscopy findings (with a diagnosis of normal squamous epithelium, esophagitis, or basal cell hyperplasia [BCH]), mild dysplasia, or moderate dysplasia during 2007–2012. We excluded participants with a diagnosis of upper gastrointestinal (UGI) cancer before enrolment or with a diagnosis of EC during the first 6 months of follow-up.
Fig. 1.
Flowchart of the study participant selection process. EC=Esophageal cancer. UGI=Upper gastrointestinal.
Ethics statement
All the participants provided written informed consent, and the study obtained approval from the ethics committees of China National Cancer Centre/Cancer Hospital, Chinese Academy of Medical Sciences (CICAMS) with the approval number of NCC1788. The study was conducted and reported in compliance with the STROBE guidelines for cohort studies.
Baseline procedures and data collection
The standard procedures of the entire screening process are shown in Supplementary File 1, and details have been described previously.16,17 In brief, all women and men aged 40–69 years from the 31 villages of the six included study centres (n = 302,450) were approached by trained local medical staff. After staff explained this study and obtained written informed consent, 106,200 participants (response rate of 35.11%) were administered a baseline questionnaire, which included questions on demographics (age, sex), education, cigarette smoking, alcohol consumption, disease history of the digestive system (peptic ulcer, esophagitis, and gastroenteritis), family history of any type of cancer, etc.16 Height and weight were measured for each participant, and body mass index (BMI) was calculated. The EC risk of all participants was assessed based on a scoring system developed by the National Cancer Centre (NCC) of China (Supplementary File 2). This tool includes eight variables (Supplementary Table S3), and the estimated effectiveness has been previously described in detail.16
Participants who were assessed as being at high risk for EC on the basis of the scoring system were recommended to undergo a free endoscopic examination at local hospitals designated by the programme. All UGI endoscopic examinations and biopsies with iodine staining were conducted by experts according to the protocols for endoscopic examination and pathological diagnosis in our programme, which were formulated based on the official endoscopy protocol for screening and early detection and treatment.18 Briefly, after the entire esophagus was visually examined, biopsy samples were collected from suspicious lesions, fixed in 10–13% formaldehyde, embedded in paraffin, and stained with haematoxylin and eosin. Two experienced pathologists independently reviewed the biopsy samples, and diagnostic discrepancies were adjudicated by consultation or resolved through discussion with a third pathologist. The quality control evaluation in this programme covered the development of a uniform study protocol, personnel training for all involved staff, and data collection and management to guarantee high quality; the details are summarized in Supplementary File 3.
Management of study participants, follow-up and outcomes
In our screening programme, all participants who underwent endoscopic examination were informed of their biopsy diagnosis by the doctors. Individuals with negative endoscopy findings received no surveillance endoscopy in the screening programme. Triennial and annual endoscopy re-examinations were recommended for individuals diagnosed with mild dysplasia and moderate dysplasia, respectively, at baseline endoscopy. A free endoscopy re-examination by our programme has been provided since 2012.18 In addition, patients with positive findings who were diagnosed with severe dysplasia, carcinoma in situ, intramucosal carcinoma, submucosal carcinoma, or invasive carcinoma were recommended to receive appropriate treatment according to the severity of the lesions (Supplementary Fig. S1).
In the current cohort study, all participants were followed up until December 31, 2021. We first conducted a passive follow-up by linking the cancer registry system and disease surveillance point system from the Chinese Centre for Disease Control and Prevention in each study centre. All study centres in the current cohort continuously submitted the CI5 series or China Cancer Registry Annual Report, with high-quality data from a population-based cancer registry (Supplementary Table S2). In addition, the study centres have abundant experience in follow-up through a comprehensive data collection system that includes hospital-based diagnosis and therapy information, health insurance information, and death surveillance data. Second, we conducted an active follow-up via door-to-door visits by village physicians, local program coordinators, and staff from CICAMS, aiming to further complete outcomes of cancer cases and deaths. In this step, information on cancer incidence (cancer type, date of incidence, and histology) and cancer deaths (cause of death, date of death) were collected based on medical records provided by patients or their relatives.
Newly diagnosed cancers were classified by site according to the International Classification of Diseases, version 10 (ICD-10), and by histology based on the International Classification of Disease for Oncology, version 3 (ICD-O-3). For the present study, newly diagnosed EC cases and EC deaths (ICD-10 C15) were the main outcomes of interest.
Statistical analysis
Continuous variables are presented as the means ± standard deviations (SDs) or medians and interquartile ranges (IQRs), and categorical variables are reported as counts and percentages. In the estimation of EC incidence, the person-years were calculated from the cohort entry date until the earliest occurrence of EC or administrative censoring (December 31, 2021). In the estimation of EC mortality, the person-years were calculated from the cohort entry date until EC death or administrative censoring (December 31, 2021), whichever came first. Multivariable Cox proportional hazards models were used to calculate the adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between baseline endoscopy diagnosis and the risk of EC incidence and mortality. The proportional hazards assumption was estimated by a global test based on Schoenfeld residuals at the level of P = 0.05. The adjusted factors included sex, age, cigarette smoking, alcohol consumption, and family history of EC, which met the proportional hazards assumption. Stratification analyses were conducted by 4 follow-up periods (3 years, 5 years, 7 years, and 10 years), sex (male and female), and age group (40–49 years, 50–59 years, and 60–69 years). The EC incidence and mortality in the negative endoscopy findings, mild dysplasia and moderate dysplasia groups were compared with the EC incidence and mortality rates in rural China in 2010 by matching sex, age, and calendar year of follow-up.19 The results are expressed as standardized incidence ratios (SIRs) and standardized mortality ratios (SMRs), and the 95% CIs assumed an exact Poisson distribution.20 The population-based EC incidence and mortality in China have presented a downward trend in recent decades.21 Therefore, the SIR or SIR would be overestimated if the compared rates were selected at the end of the study follow-up period (2021). In addition, all study participants were enrolled in rural China, which had a higher disease burden of EC than urban China. Based on these considerations, we selected the EC incidence and mortality rates in rural China in 2010 (the median year of study participants enrolled) to estimate SIR and SMR. The compared rates of EC incidence and mortality in rural China were reported from the NCC of China, which is the official department that collects, estimates and reports nationwide statistics for cancer incidence and mortality using population-based cancer registry data in China.22 To assess the current recommended 5-year endoscopy surveillance interval for individuals with negative endoscopy findings, SIRs and SMRs for this subgroup were calculated for the total follow-up period and for 2 other periods: 5 years or less and 5.1 to 10.0 years since the endoscopy examination. For the analysis of the period of 5.1 to 10.0 years, we excluded all individuals with a follow-up shorter than 10 years.
Five sensitivity analyses were conducted to reassess the associations between baseline endoscopy diagnosis and the risk of EC incidence and mortality with the index of univariate and multivariate HRs (95% CI). First, individuals with normal squamous epithelium were used as a reference group. Second, sub-distribution hazard models were used while taking the competing event of all-cause mortality into account. Third, the study centres increased from 2007 (n = 2) to 2012 (n = 6), and Wenshang County and Xiping County had continued data from 2007 to 2012 (Supplementary Table S1). Therefore, we included participants only from these two study centres (n = 23,277). Fourth, we excluded participants with mild and moderate dysplasia who underwent endoscopic surveillance by our program (n = 488), accounting for 26.38% of all included participants diagnosed with mild or moderate dysplasia at baseline. Fifth, we excluded participants lost to follow-up during the study period (n = 464), which accounted for 1.08% of the entire study population.
Data analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). Figures were produced using R software (R version 4.2.2). All tests were two sided, and P values of 0.05 or less were considered statistically significant.
Role of the funding source
The funder of this study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to the dataset and had final responsibility for the decision to submit for publication.
Results
Baseline characteristics
Of 42,912 eligible participants aged 40–69 years who underwent endoscopy examination in the EC screening programme between 2007 and 2012, 85 individuals were excluded based on the exclusion criteria. The remaining 42,827 participants were included in the final analysis, among whom 40,977 individuals had negative endoscopy findings (34,279 with normal squamous epithelium, 6107 with esophagitis, and 591 with BCH), 1562 individuals were diagnosed with mild dysplasia, and 288 individuals were diagnosed with moderate dysplasia (Fig. 1). The baseline demographic characteristics and other potential risk factors are summarized for all study participants and subgroups of endoscopy findings, as shown in Table 1. Among all included participants, 17,962 were males (41.94%), and the mean age was 53.29 years (SD: 7.92). Compared with individuals with negative endoscopy findings, individuals with mild or moderate dysplasia were more likely to be male, to be older, to have a lower education level, to be cigarette smokers and to have a family history of EC (Table 1).
Table 1.
Study population demographics and characteristics at baseline endoscopy screening.
| Characteristics | Baseline endoscopy diagnosis |
Total population (N = 42,827), n (%) | ||
|---|---|---|---|---|
| Negative endoscopy findings (n = 40,977), n (%) | Mild dysplasia (n = 1562), n (%) | Moderate dysplasia (n = 288), n (%) | ||
| Sex | ||||
| Male | 17,007 (41.50) | 811 (51.92) | 144 (50.00) | 17,962 (41.94) |
| Female | 23,970 (58.50) | 751 (48.08) | 144 (50.00) | 24,865 (58.06) |
| Age, yr | ||||
| Mean (SD) | 53.11 (7.90) | 57.21 (7.26) | 58.21 (6.81) | 53.29 (7.92) |
| 40–49 | 15,552 (37.95) | 262 (16.77) | 33 (11.46) | 15,847 (37.00) |
| 50–59 | 15,576 (38.01) | 657 (42.06) | 121 (42.01) | 16,354 (38.19) |
| 60–69 | 9849 (24.04) | 643 (41.17) | 134 (46.53) | 10,626 (24.81) |
| Education level | ||||
| Primary school and below | 23,629 (57.69) | 1016 (65.09) | 190 (65.97) | 24,835 (58.01) |
| Secondary school and above | 17,332 (42.31) | 545 (34.91) | 98 (34.03) | 17,975 (41.99) |
| Missing | 16 | 1 | 0 | 17 |
| BMI, kg/m2 | ||||
| <18.5 | 467 (1.14) | 27 (1.73) | 7 (2.43) | 501 (1.17) |
| 18.5 to <24 | 23,684 (57.83) | 918 (58.77) | 180 (62.50) | 24,782 (57.90) |
| 24 to <28 | 14,778 (36.09) | 534 (34.19) | 86 (29.86) | 15,398 (35.97) |
| ≥28 | 2024 (4.94) | 83 (5.31) | 15 (5.21) | 2122 (4.96) |
| Missing | 24 | 0 | 0 | 24 |
| Cigarette smoking | ||||
| No | 29,601 (72.25) | 996 (63.81) | 196 (68.06) | 30,793 (71.91) |
| Yes | 11,369 (27.75) | 565 (36.19) | 92 (31.94) | 12,026 (28.09) |
| Missing | 7 | 1 | 0 | 8 |
| Alcohol consumption | ||||
| No | 31,422 (76.71) | 1108 (70.98) | 219 (76.04) | 32,749 (76.49) |
| Yes | 9541 (23.29) | 453 (29.02) | 69 (23.96) | 10,063 (23.51) |
| Missing | 14 | 1 | 0 | 15 |
| Disease history of digestive system | ||||
| No | 35,985 (87.82) | 1364 (87.32) | 245 (85.07) | 37,594 (87.78) |
| Yes | 4992 (12.18) | 198 (12.68) | 43 (14.93) | 5233 (12.22) |
| Family history of EC | ||||
| No | 35,423 (86.67) | 1290 (82.90) | 236 (82.23) | 36,949 (86.50) |
| Yes | 5450 (13.33) | 266 (17.10) | 51 (17.77) | 5767 (13.50) |
| Missing | 104 | 6 | 1 | 111 |
BMI = body mass index; EC = esophageal cancer; SD = standard deviation; yr = year.
EC incidence and mortality rates
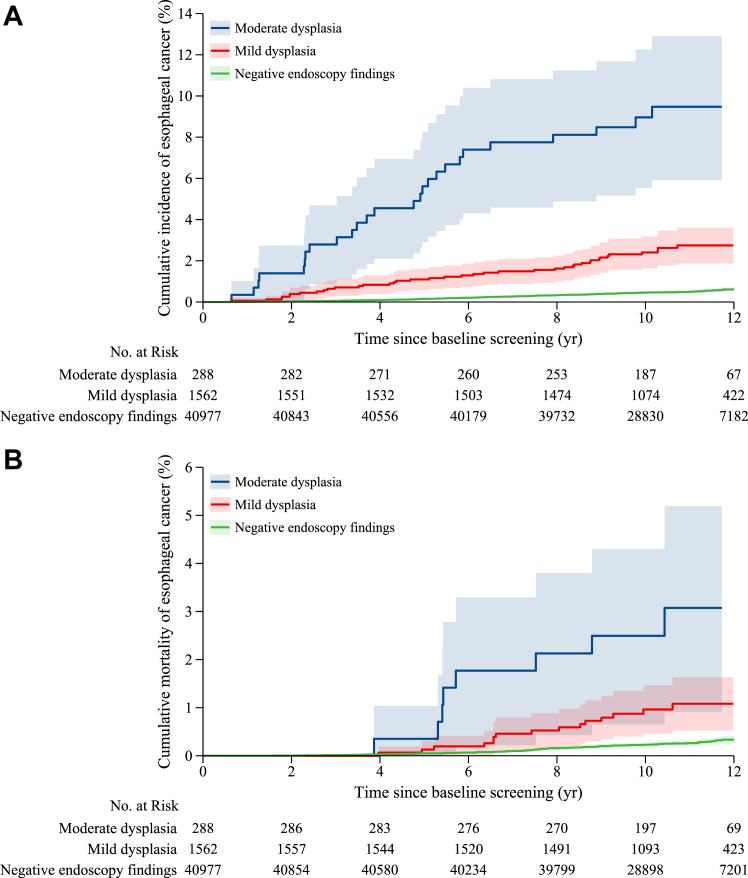
The median follow-up time was 10.62 years (IQR, 9.36–11.62 years) in the study cohort, which accounted for a total of 453555.75 person-years. Table 2 and Fig. 2 show the EC incidence and mortality in the study cohort. During the follow-up period, 268 EC cases were identified, and the main characteristics are shown in Supplementary Table S4. Among these participants with EC, 202 were in the group with negative endoscopy findings, 39 EC were in the mild dysplasia group, and 27 EC were in the moderate dysplasia group (Table 2). The cumulative EC incidence at 10 years was 0.45% (0.38–0.52) in the group with negative endoscopy findings, 2.39% (1.62–3.16) in the mild dysplasia group, and 8.90% (5.57–12.24) in the moderate dysplasia group (Fig. 2A; Supplementary Table S5), with significant differences in the rates (log-rank test: P < 0.05). The multivariate analysis showed that patients with mild dysplasia (HR = 3.52, 2.49–4.97) or moderate dysplasia (HR = 13.18, 8.78–19.76) had a higher risk of developing EC than those with negative endoscopy findings (Table 2). During the study period, 26.38% (288/1850) of individuals with a baseline diagnosis of mild or moderate dysplasia underwent subsequent endoscopic surveillance in our programme. The median time of endoscopic surveillance was 4.18 years (3.18–4.87). Among them, a total of 31 positive cases were identified (27 cases of severe dysplasia or carcinoma in situ and 4 EC cases), with a total treatment rate of 74.19%.
Table 2.
Esophageal cancer incidence and mortality, by follow-up periods after baseline endoscopy screening.
| Baseline endoscopy diagnosis | No. of participants | No. of cases | Rate per 100,000 pyrs (95% CI) | Crude HR (95% CI) | Adjusted HR (95% CI)a |
|---|---|---|---|---|---|
| Incidence | |||||
| Entire follow-up periodb | |||||
| Negative endoscopy findings | 40,977 | 202 | 46.54 (34.14–61.97) | Ref. | Ref. |
| Mild dysplasia | 1562 | 39 | 234.69 (205.62–266.72) | 5.05 (3.59–7.12) | 3.52 (2.49–4.97) |
| Moderate dysplasia | 288 | 27 | 919.50 (861.02–980.91) | 19.94 (13.34–29.79) | 13.18 (8.78–19.76) |
| Within 3 years | |||||
| Negative endoscopy findings | 40,977 | 22 | 17.94 (10.62–28.37) | Ref. | Ref. |
| Mild dysplasia | 1562 | 11 | 235.94 (206.79–268.05) | 13.16 (6.38–27.14) | 9.60 (4.62–19.98) |
| Moderate dysplasia | 288 | 8 | 942.15 (882.94–1004.28) | 52.69 (23.46–118.34) | 38.56 (17.00–87.48) |
| Within 5 years | |||||
| Negative endoscopy findings | 40,977 | 62 | 30.43 (20.59–43.33) | Ref. | Ref. |
| Mild dysplasia | 1562 | 17 | 220.10 (191.98–251.18) | 7.25 (4.24–12.39) | 5.09 (2.96–8.75) |
| Moderate dysplasia | 288 | 16 | 1149.26 (1083.77–1217.68) | 38.05 (21.96–65.93) | 26.33 (15.08–45.97) |
| Within 7 years | |||||
| Negative endoscopy findings | 40,977 | 109 | 38.37 (27.2–52.59) | Ref. | Ref. |
| Mild dysplasia | 1562 | 23 | 214.39 (186.65–245.09) | 5.60 (3.57–8.78) | 3.93 (2.50–6.18) |
| Moderate dysplasia | 288 | 22 | 1149.35 (1083.86–1217.77) | 30.25 (19.13–47.83) | 20.83 (13.10–33.10) |
| Within 10 years | |||||
| Negative endoscopy findings | 30,106 | 148 | 49.96 (37.08–65.87) | Ref. | Ref. |
| Mild dysplasia | 1170 | 30 | 265.40 (234.43–299.33) | 5.33 (3.60–7.90) | 3.66 (2.46–5.43) |
| Moderate dysplasia | 215 | 17 | 838.38 (782.58–897.11) | 16.97 (10.28–28.04) | 11.12 (6.70–18.45) |
| Mortality | |||||
| Entire follow-up periodb | |||||
| Negative endoscopy findings | 40,977 | 105 | 24.17 (15.51–35.91) | Ref. | Ref. |
| Mild dysplasia | 1562 | 15 | 89.51 (71.93–110.09) | 3.74 (2.17–6.42) | 2.43 (1.41–4.19) |
| Moderate dysplasia | 288 | 8 | 261.52 (230.78–295.21) | 10.90 (5.31–22.37) | 6.46 (3.13–13.29) |
| Within 3 years | |||||
| Negative endoscopy findings | 40,977 | 6 | 4.89 (1.562–11.513) | Ref. | Ref. |
| Mild dysplasia | 1562 | 0 | 0 | NA | NA |
| Moderate dysplasia | 288 | 0 | 0 | NA | NA |
| Within 5 years | |||||
| Negative endoscopy findings | 40,977 | 20 | 9.81 (4.67–18.14) | Ref. | Ref. |
| Mild dysplasia | 1562 | 2 | 25.77 (16.80–37.82) | 2.63 (0.61–11.24) | 1.78 (0.41–7.63) |
| Moderate dysplasia | 288 | 1 | 70.16 (54.71–88.62) | 7.17 (0.96–53.40) | 4.70 (0.63–35.31) |
| Within 7 years | |||||
| Negative endoscopy findings | 40,977 | 40 | 14.07 (7.71–23.58) | Ref. | Ref. |
| Mild dysplasia | 1562 | 7 | 64.83 (50.02–82.66) | 4.62 (2.07–10.32) | 3.15 (1.40–7.07) |
| Moderate dysplasia | 288 | 5 | 252.63 (222.44–285.78) | 18.06 (7.13–45.76) | 11.61 (4.55–29.64) |
| Within 10 years | |||||
| Negative endoscopy findings | 30,106 | 76 | 25.63 (16.69–37.66) | Ref. | Ref. |
| Mild dysplasia | 1170 | 11 | 96.37 (78.09–117.64) | 3.78 (2.01–7.11) | 2.46 (1.30–4.64) |
| Moderate dysplasia | 215 | 7 | 335.36 (300.42–373.24) | 13.21 (6.09–28.65) | 7.98 (3.66–17.39) |
CI = confidence interval; HR = hazard ratio; NA = not available; pyrs = person-years; Ref = reference.
Adjusted for sex, age, cigarette smoking, alcohol consumption, and family history of esophageal cancer.
The median follow-up time was 10.62 years.
Fig. 2.
Cumulative incidence (A) and mortality (B) of esophageal cancer by baseline endoscopy findings.
There were 128 EC-related deaths during the follow-up period, and mortality rates increased from the group with negative endoscopy findings to the mild dysplasia group and to the moderate dysplasia group (Table 2). The cumulative EC mortality at 10 years was 0.23% (0.18–0.27) in the group with negative endoscopy findings, 0.96% (0.46–1.46) in the mild dysplasia group, and 2.50% (0.67–4.33) in the moderate dysplasia group (Fig. 2B; Supplementary Table S6), with significant group differences (log-rank test: P < 0.05). The multivariate HRs were 2.43 (1.41–4.19) in the mild dysplasia group and 6.46 (3.13–13.29) in the moderate dysplasia group compared with the group with negative endoscopy findings.
The cumulative incidence and mortality rates of EC at different follow-up times are summarized in Supplementary Tables S5 and S6, respectively. Compared with those in the group with negative endoscopy findings, a significantly higher risk of developing EC in the mild dysplasia (HR = 9.60, 4.62–19.98) and moderate dysplasia groups (HR = 38.56, 17.00–87.48) was observed within three years (Table 2). Regarding the risk of death from incident EC, there were no EC deaths within three years in the mild and moderate dysplasia groups, and there were only 6 deaths with cumulative mortality of 0.02% in the group of negative endoscopy findings; a significant difference was observed only when the follow-up time reached 7 years or more (Table 2). The positive associations between endoscopy findings and EC incidence and mortality were stable in the subgroups by sex and age (Supplementary Tables S7 and S8). The association of baseline endoscopy findings with EC incidence and mortality seemed robust in the sensitivity analyses restricted to individuals with normal squamous epithelium as a reference (Supplementary Tables S9 and S10), considering competing events (Supplementary Tables S11 and S12), conducted in two study centres with continuously enrolled participants (Supplementary Table S13), excluding individuals with mild and moderate dysplasia who underwent subsequent endoscopic surveillance during the study period (Supplementary Table S14), or excluding participants lost to follow-up (Supplementary Table S15). In addition, individuals with mild and moderate dysplasia had a significantly increased risk of EC incidence and mortality compared with the general population. The SIR of EC incidence was 1.95 (1.69–2.24) in the mild dysplasia group and 6.75 (6.25–7.28) in the moderate dysplasia group, and the SMRs of EC mortality were 1.07 (0.88–1.29) and 2.67 (2.36–3.01), respectively (Supplementary Tables S16 and S17).
SIR and SMR after negative endoscopy findings
Table 3 shows the SIRs and SMRs of EC in the group with negative endoscopy findings. During a median follow-up of 10.62 years, individuals with negative endoscopy findings after a single endoscopy screening had lower EC incidence and mortality than the general population in rural China, with an SIR of 0.53 (0.40–0.70) and an SMR of 0.43 (0.31–0.58). When stratified by follow-up periods of 0–5 and 5.1–10 years, a reduced EC incidence and mortality was still observed; the SIRs at 0–5 years and 5.1–10 years were 0.44 (0.32–0.59) and 0.74 (0.59–0.93), and the SMRs at 0–5 years and 5.1–10 years were 0.24 (0.15–0.35) and 0.69 (0.53–0.86), respectively.
Table 3.
Esophageal cancer standardized incidence ratios (SIRs) and standardized mortality ratios (SMRs) after a negative endoscopy finding.
| Time after baseline endoscopy screening with negative endoscopy findings |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–5 years |
5.1–10 years |
Entire follow-up perioda |
|||||||
| No. of observed cases | No. of expected cases | SIR or SMRb (95% CI) | No. of observed cases | No. of expected cases | SIR or SMRb (95% CI) | No. of observed cases | No. of expected cases | SIR or SMRb (95% CI) | |
| Incidence | |||||||||
| All | 62 | 140 | 0.44 (0.32–0.59) | 102 | 137 | 0.74 (0.59–0.93) | 202 | 378 | 0.53 (0.40–0.70) |
| Sex | |||||||||
| Male | 46 | 93 | 0.49 (0.37–0.65) | 71 | 86 | 0.83 (0.66–1.02) | 139 | 243 | 0.57 (0.43–0.74) |
| Female | 16 | 47 | 0.34 (0.24–0.47) | 31 | 51 | 0.61 (0.47–0.78) | 63 | 135 | 0.47 (0.34–0.62) |
| Age, yr | |||||||||
| 40–49 | 4 | 12 | 0.33 (0.23–0.47) | 7 | 20 | 0.35 (0.24–0.49) | 13 | 50 | 0.26 (0.17–0.38) |
| 50–59 | 28 | 57 | 0.49 (0.36–0.65) | 38 | 63 | 0.60 (0.46–0.78) | 85 | 165 | 0.52 (0.38–0.68) |
| 60–69 | 30 | 70 | 0.43 (0.31–0.58) | 57 | 55 | 1.04 (0.85–1.26) | 104 | 162 | 0.64 (0.49–0.82) |
| Family history of EC | |||||||||
| No | 52 | 121 | 0.43 (0.31–0.58) | 83 | 117 | 0.71 (0.55–0.89) | 169 | 327 | 0.52 (0.39–0.68) |
| Yes | 10 | 18 | 0.56 (0.42–0.72) | 19 | 20 | 0.95 (0.77–1.16) | 33 | 50 | 0.66 (0.51–0.84) |
| Mortality | |||||||||
| All | 20 | 84 | 0.24 (0.15–0.35) | 61 | 89 | 0.69 (0.53–0.86) | 105 | 243 | 0.43 (0.31–0.58) |
| Sex | |||||||||
| Male | 16 | 58 | 0.28 (0.18–0.40) | 45 | 58 | 0.78 (0.61–0.96) | 78 | 162 | 0.48 (0.36–0.64) |
| Female | 4 | 26 | 0.15 (0.09–0.25) | 16 | 31 | 0.52 (0.38–0.67) | 27 | 82 | 0.33 (0.23–0.46) |
| Age, yr | |||||||||
| 40–49 | 0 | 7 | 0 | 1 | 11 | 0.09 (0.04–0.18) | 3 | 27 | 0.11 (0.06–0.20) |
| 50–59 | 11 | 32 | 0.34 (0.24–0.48) | 21 | 37 | 0.57 (0.43–0.74) | 39 | 97 | 0.40 (0.29–0.55) |
| 60–69 | 9 | 45 | 0.20 (0.12–0.31) | 39 | 42 | 0.93 (0.75–1.14) | 63 | 119 | 0.53 (0.40–0.69) |
| Family history of EC | |||||||||
| No | 18 | 72 | 0.25 (0.16–0.37) | 51 | 76 | 0.67 (0.52–0.85) | 90 | 210 | 0.43 (0.31–0.58) |
| Yes | 2 | 11 | 0.18 (0.11–0.29) | 10 | 13 | 0.77 (0.61–0.97) | 15 | 32 | 0.47 (0.34–0.62) |
CI = confidence interval; EC = esophageal cancer; SIR = standardized incidence ratio; SMR = standardized mortality ratio; yr = year.
The median follow-up time was 10.62 years.
SIR was estimated for incidence and SMR was estimated for mortality.
When stratified by sex, age, and family history of EC, the reduced EC incidence and mortality after endoscopy remained stable in all the subgroups (Table 3). In the sensitivity analyses, we further separated participants into normal squamous epithelium and esophagitis/BCH subgroups. The results showed little impact on the risk of EC incidence and mortality among individuals with esophagitis/BCH according to follow-up duration (Supplementary Tables S18 and S19); these individuals had a slightly increased risk of EC incidence and mortality in the 5.1–10-year follow-up period, with an SIR of 1.27 (1.06–1.51) and SMR of 1.30 (1.09–1.54).
Discussion
The findings of this population-based, multicentre, prospective study in the Chinese population with a median follow-up time of 10.62 years demonstrated that participants with mild or moderate dysplasia had a significantly increased risk of subsequent EC incidence and mortality compared with those with negative endoscopy findings or the general population. When assessed by follow-up period, a significantly higher risk of developing EC in the mild dysplasia and moderate dysplasia groups was observed within 3 years. In addition, individuals with negative endoscopy findings after a single endoscopy screening had a continually lower EC incidence and mortality than the general population for up to 10.6 years.
The findings in the current study that the baseline endoscopy findings were associated with subsequent risk of EC incidence and mortality are in line with those of two prospective studies.11,12 In 1985–1991, Wang et al. conducted the first cohort study among 682 individuals who underwent endoscopy screening in a high-risk area of Linzhou in China. These participants all had a previous cytological diagnosis in dysplasia, and the study found that compared with individuals with normal squamous epithelium, individuals with mild dysplasia (relative risk, RR = 2.9, 1.6–5.2) and moderate dysplasia (RR = 9.8, 5.3–18.3) had a higher risk of developing EC, with a follow-up time of 13.5 years.11 This finding was replicated in a population-based EC screening cohort with a follow-up of 8.5 years, which was conducted among 21,111 individuals from the general population from three high-risk areas in China (Linzhou, Cixian, and Feicheng) in 2005–2009.12 To our knowledge, our study provides the first evidence from a population outside high-risk areas for EC (such as Linzhou and Cixian) in China, with the largest sample size to date. These findings suggest that more frequent endoscopy surveillance is warranted for individuals with mild or moderate dysplasia.
The Chinese guidelines for EC screening recommend a repeat endoscopy examination in 1–3 years for individuals with mild or moderate dysplasia.8 The previous cohort studies in different populations in China have provided supportive evidence for this recommendation.18,23 In our previous cohort analysis of surveillance endoscopy data from this EC screening programme, we demonstrated that the median time to develop severe dysplasia or esophageal squamous cell carcinoma (ESCC) was approximately 2.39 years after the detection of mild-moderate dysplasia during a median follow-up time of 6.95 years.18 Findings from Feicheng in China also supported a screening interval of 2–3 years for patients with mild and moderate dysplasia.23 The findings of this current cohort study showed that the cumulative EC incidence within the first three years rapidly increased in those with mild or moderate dysplasia but slowly increased in those with negative endoscopy findings. The relative risk of EC incidence within the first 3 years was approximately 9–38 times higher among patients with mild and moderate dysplasia. These findings further add new evidence to support the recommendation of a three-year surveillance interval for patients with mild and moderate dysplasia.
The findings in the current study provide new insights into the management of individuals with negative endoscopy findings. Our findings showed that during the current recommended 5-year surveillance interval,8 the cumulative incidence and mortality of EC were quite low (nearly 0.1%) in the negative endoscopy findings group. In addition, compared with the general population, a lower risk of EC incidence and mortality after a negative endoscopy for up to 5 years was observed in all subgroup populations, with SIRs of 0.33–0.56 and SMRs of 0.15–0.34. These findings indicated that extending the surveillance interval beyond the recommended 5 years should be considered. Furthermore, our data supported extending the rescreening interval to 10 years for individuals with negative endoscopy findings. First, the 10-year cumulative incidence and mortality of EC in the group with negative endoscopy findings were approximately 0.45% and 0.23%, respectively, which were lower than the 3-year cumulative rates in the mild or moderate dysplasia groups. Second, the SIRs and SMRs were continually lower than those of the general population over 10 years, and the reduced EC incidence and mortality after negative endoscopy findings remained stable in all subgroups.
Our study had several limitations. First, 26.38% of individuals with mild or moderate dysplasia underwent subsequent endoscopic surveillance by our programme, which led to a slight overestimation of the HR of developing EC. This might be interpreted as EC cases being diagnosed in advance through endoscopy surveillance. In fact, this bias would not be avoided in EC screening practice because endoscopy surveillance for individuals with mild and moderate dysplasia has been recommended in the guidelines for EC screening and implemented in EC screening practice in China.6 Second, the lack of centralized histological confirmation of the endoscopic findings was another limitation, although this program has conducted strict quality control to minimize the heterogeneity of endoscopy findings from multiple centres. Third, the SIR and SMR of EC were calculated using the rate from the general population in rural China; however, participants who underwent endoscopy examinations were individuals at high risk for EC. Therefore, the SIR and SMR may have been overestimated for individuals with negative endoscopy findings. Under these circumstances, the SIRs and SMRs were well below 1, indicating that our suggestion of extending the rescreening interval to 10 years for these individuals was conservative. Finally, follow-up intervals for individuals with different endoscopy findings were determined superficially based on our cohort with a median follow-up of 10.6 years; longer-term follow-up is needed to substantiate our findings. In the future, we will further estimate the lifetime risk of EC incidence and mortality and cost-effectiveness based on our established EC Markov model,24 controlling for the potential bias in endoscopy screening practice and making up for the short follow-up period of current EC screening cohorts. These attempts would further provide evidence of endoscopy surveillance intervals for individuals with mild dysplasia, moderate dysplasia, and negative endoscopy findings after a single endoscopy.
In conclusion, our study assessed progression risk and surveillance intervals for individuals with mild and moderate dysplasia and provided the first population-based evidence of surveillance intervals for individuals with negative endoscopy findings. Individuals with negative endoscopy findings after a single endoscopy screening had a lower EC risk than the general population for up to 10.62 years, while those with mild-moderate dysplasia had an elevated risk. Our results support endoscopy surveillance for mild-moderate dysplasia every 3 years and suggest extending the interval to 10 years after a negative endoscopy finding. Further high-quality prospective cohort studies, randomized controlled trials, and cost-effectiveness studies will be valuable to gain more evidence on the optimal endoscopy surveillance intervals.
Contributors
HL, CX, and WC conceived the study. JZ, SZ, and HM contributed to the data collection, data transmission and data correction after quality control. HL and CX contributed to data management and quality control. HL drafted and finalized the paper with input from WC and CX. All authors contributed to data interpretation and manuscript revision. WC had access to all raw data and WC obtained the funding disclosed in the manuscript.
Data sharing statement
The data and code used to generate the reported estimates will be made available upon request to the corresponding author.
Declaration of interests
All authors declare no conflicts of interest.
Acknowledgements
This study was funded by the Ministry of Finance and National Health Commission of the People's Republic of China, the National Key R&D Programme of China [no. 2018YFC1313100], the Special Project of Bejing-Tianjin-Hebei Basic Research Cooperation (no. J200017), and the Sanming Project of Medicine in Shenzhen (no. SZSM201911015). We gratefully acknowledge all participants in our programme and all staff who made great contributions to the data collection, auditing, database management and verification. We thank all the individuals who contributed to this study for their important and greatly appreciated contributions to the preparation of this report.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102201.
Appendix ASupplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Chen S., Cao Z., Prettner K., et al. Estimates and projections of the global economic cost of 29 cancers in 204 countries and territories from 2020 to 2050. JAMA Oncol. 2023;9(4):465–472. doi: 10.1001/jamaoncol.2022.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allemani C., Matsuda T., Di Carlo V., et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei W.Q., Chen Z.F., He Y.T., et al. Long-term follow-up of a community assignment, one-time endoscopic screening study of esophageal cancer in China. J Clin Oncol. 2015;33(17):1951–1957. doi: 10.1200/JCO.2014.58.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen R., Liu Y., Song G., et al. Effectiveness of one-time endoscopic screening programme in prevention of upper gastrointestinal cancer in China: a multicentre population-based cohort study. Gut. 2021;70(2):251–260. doi: 10.1136/gutjnl-2019-320200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao M., Li H., Sun D., et al. Cancer screening in China: the current status, challenges, and suggestions. Cancer Lett. 2021;506:120–127. doi: 10.1016/j.canlet.2021.02.017. [DOI] [PubMed] [Google Scholar]
- 7.He Z., Ke Y. Precision screening for esophageal squamous cell carcinoma in China. Chin J Cancer Res. 2020;32(6):673–682. doi: 10.21147/j.issn.1000-9604.2020.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He J., Chen W.Q., Li Z.S., et al. China guideline for the screening, early detection and early treatment of esophageal cancer (2022, Beijing) Zhonghua Zhong Liu Za Zhi. 2022;44(6):491–522. doi: 10.3760/cma.j.cn112152-20220517-00348. [DOI] [PubMed] [Google Scholar]
- 9.Li H., Teng Y., Yan X., et al. Profiles and findings of population-based esophageal cancer screening with endoscopy in China: systematic review and meta-analysis. JMIR Public Health Surveill. 2023;9 doi: 10.2196/45360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M., Hao C., Xie S., et al. Efficacy of endoscopic treatment on patients with severe dysplasia/carcinoma in situ of esophageal squamous cell carcinoma: a prospective cohort study. Chin J Cancer Res. 2019;31(2):357–365. doi: 10.21147/j.issn.1000-9604.2019.02.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang G.Q., Abnet C.C., Shen Q., et al. Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54(2):187–192. doi: 10.1136/gut.2004.046631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei W.Q., Hao C.Q., Guan C.T., et al. Esophageal histological precursor lesions and subsequent 8.5-year cancer risk in a population-based prospective study in China. Am J Gastroenterol. 2020;115(7):1036–1044. doi: 10.14309/ajg.0000000000000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia R., Zeng H., Liu W., et al. Estimated cost-effectiveness of endoscopic screening for upper gastrointestinal tract cancer in high-risk areas in China. JAMA Netw Open. 2021;4(8) doi: 10.1001/jamanetworkopen.2021.21403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J., Wei W.Q., Niu J., Liu Z.C., Yang C.X., Qiao Y.L. Cost-benefit analysis of esophageal cancer endoscopic screening in high-risk areas of China. World J Gastroenterol. 2012;18(20):2493–2501. doi: 10.3748/wjg.v18.i20.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu B., Wang Z., Zhang Q. Age at initiation and frequency of screening to prevent esophageal squamous cell carcinoma in high-risk regions: an economic evaluation. Cancer Prev Res (Phila) 2020;13(6):543–550. doi: 10.1158/1940-6207.CAPR-19-0477. [DOI] [PubMed] [Google Scholar]
- 16.Chen W., Li H., Zheng R., et al. An initial screening strategy based on epidemiologic information in esophageal cancer screening: a prospective evaluation in a population-based cancer screening cohort in rural China. Gastrointest Endosc. 2021;93(1):110–118.e2. doi: 10.1016/j.gie.2020.05.052. [DOI] [PubMed] [Google Scholar]
- 17.Li J., Li H., Zeng H., et al. A study protocol of population-based cancer screening cohort study on esophageal, stomach and liver cancer in rural China. Chin J Cancer Res. 2020;32(4):540–546. doi: 10.21147/j.issn.1000-9604.2020.04.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H., Zhang S., Zhou J., et al. Endoscopic surveillance for premalignant esophageal lesions: a community-based multicenter, prospective cohort study. Clin Gastroenterol Hepatol. 2023;21(3):653–662.e8. doi: 10.1016/j.cgh.2022.04.039. [DOI] [PubMed] [Google Scholar]
- 19.Chen W., Zheng R., Zhang S., et al. Esophageal cancer incidence and mortality in China, 2010. Thorac Cancer. 2014;5(4):343–348. doi: 10.1111/1759-7714.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breslow N.E., Day N.E. Statistical methods in cancer research. Volume II--The design and analysis of cohort studies. IARC Sci Publ. 1987;(82):1–406. [PubMed] [Google Scholar]
- 21.Chen R., Zheng R., Zhang S., et al. Patterns and trends in esophageal cancer incidence and mortality in China: an analysis based on cancer registry data. J Natl Cancer Center. 2023;3(1):21–27. doi: 10.1016/j.jncc.2023.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei W., Zeng H., Zheng R., et al. Cancer registration in China and its role in cancer prevention and control. Lancet Oncol. 2020;21(7):e342–e349. doi: 10.1016/S1470-2045(20)30073-5. [DOI] [PubMed] [Google Scholar]
- 23.Gao D., Lu P., Zhang N., et al. Progression of precancerous lesions of esophageal squamous cell carcinomas in a high-risk, rural Chinese population. Cancer Med. 2023;12(2):1791–1800. doi: 10.1002/cam4.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia R., Li H., Shi J., et al. Cost-effectiveness of risk-stratified endoscopic screening for esophageal cancer in high-risk areas of China: a modeling study. Gastrointest Endosc. 2022;95(2):225–235.e20. doi: 10.1016/j.gie.2021.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.