Summary
Background
WHO introduced the STEPwise approach to surveillance (STEPS) to monitor trends in non-communicable diseases. For arterial hypertension, the STEPS protocol takes the average of the last two out of three standard blood pressure measurements (SBPM). This study assesses the diagnostic accuracy of SBPM, same-day and next-day unattended automated measurement (uABP), with 24 h ambulatory measurement (24 h-ABPM) as reference.
Methods
This diagnostic accuracy study was done within a population-based household survey on cardiovascular risk factors in two districts in Northern Lesotho. Adults (aged ≥ 18 years) with elevated SBPM (defined as ≥140/90 mmHg), and 2:1 age- and sex-matched participants with normal SBPM during the survey were recruited. Following SBPM, first uABP readings were obtained on survey day. Afterwards, participants received a 24 h-ABPM device. Second uABP readings were taken 24 h later, after retrieval of the 24 h-ABPM. The main outcome was overall diagnostic accuracy of all screening measurements (SBPM, first uABP, and second uABP), determined using area under the receiver operating characteristic curve (AUROC), with 24 h-ABPM as a reference.
Findings
Between November 2, 2021 and August 31, 2022, 275 participants (mean age 58 years (SD: 16 years), 163 (59%) female) were enrolled, 183 of whom had elevated and 92 had normal SBPM. Mean difference between systolic daytime 24 h-ABPM and screening measurements was highest for SBPM (mean difference: −13 mmHg; 95% CI: −14 to −11). Mean difference between diastolic daytime 24 h-ABPM and diastolic SBPM was −2 mmHg (95% CI: −4 to −1), whereas no difference was found for mean diastolic first uABP (mean difference: −1 mmHg; 95% CI: −2.0 to 0.3); and mean diastolic second uABP (mean difference: 1.0 mmHg; 95% CI: −0.4 to 2.3). White coat hypertension was highest with SBPM (55 [20%]), followed by first uABP (27 [9.8%]), and second uABP (18 [6.5%]). Using systolic daytime 24 h-ABPM as a reference, the uABPs had higher AUROC (first uABP: 87% [95% CI: 83–91]; second uABP: 88% [95% CI: 84–92]); SBPM: (79% [95% CI: 74–85]). This difference was significant between first uABP and SBPM (P = 0.0024), and between second uABP and SBPM (P = 0.0017).
Interpretation
uABP had better diagnostic performance than SBPM. Integration of uABP into STEPS protocol should be considered.
Funding
Swiss Agency for Development and Cooperation under the ComBaCaL project, and the World Diabetes Foundation.
Keywords: Survey screen blood pressure, Unattended automated office blood pressure, Ambulatory blood pressure monitor, STEPS, Sub-Saharan Africa, Lesotho
Research in context.
Evidence before this study
We reviewed a recent meta-analysis of 31 articles comparing unattended blood pressure measurement (uABP) with other methods of blood pressure measurement for identifying possible hypertension. This meta-analysis of studies which were all conducted in clinic/hospital settings and none in Africa, concluded that uABP was more accurate, and should be preferred to standard blood pressure measurements. Afterwards, we searched PubMed and Embase on 13th April 2021 for key words relating to (‘Unattended blood pressure’ OR ‘Unattended automated blood pressure’ OR ‘Automated blood pressure’ OR ‘unattended automated office blood pressure’ OR ‘automated office blood pressure’ OR ‘Out-of-office blood pressure’) AND (‘Office blood pressure’ OR ‘Standard blood pressure’) AND (‘Ambulatory blood pressure monitoring’ OR ‘Blood pressure monitoring, ambulatory’ OR ‘Ambulatory blood pressure’) AND (‘Survey’ OR ‘WHO STEPwise approach to surveillance’). Additionally, we searched for keywords relating to ‘unattended blood pressure’ AND ‘sub-Saharan Africa’ (see supplementary material, Supplementary Table S10). Of the 61 and 20 studies retrieved from PubMed and Embase respectively, none investigated uABP during a population-based survey. The only study on uABP in Africa identified participants in the community, but performed the remaining processes in a clinic setting, and found inconclusive results.
Added value of this study
This is the first study investigating the performance of standard blood pressure measurement and unattended measurements conducted real-time within a population-based survey. After four measurements (survey standard blood pressure measurement, immediate uABP, delayed uABP, and the gold standard 24-h ambulatory blood pressure monitoring) during a population-based household survey and among the same study participants, we found that unattended blood pressure measurements had better correlation with 24 h ambulatory blood pressure monitoring, higher discrimination, and lower proportions of white coat hypertension within survey conditions compared to standard blood pressure measurement recommended during World Health Organization’s STEPwise approach to surveillance (STEPS) hypertension prevalence surveys.
Implications of all the available evidence
Under survey conditions, unattended blood pressure measurements had better diagnostic performance, with lower proportions of misclassified participants compared with the widely used standard blood pressure measurement approach during population-based surveys. Furthermore, uABP was implementable during the survey as there were no additional requirements in terms of equipment, personnel, and time. Thus, integration of uABP into STEPS protocol should be considered to avoid over-reporting of prevalence figures and, likely unnecessary initiation of antihypertensive therapy.
Introduction
Elevated blood pressure is the most important cardiovascular risk factor globally, causing 7.5 million deaths annually (12.8% of all deaths).1 It accounts for 57 million disability adjusted life years (DALYS) or 3.7% of total DALYS.2 In sub-Saharan Africa, the burden is rising3 with a high proportion of individuals being unaware of their condition.4,5 To monitor national trends on elevated blood pressure and other non-communicable chronic diseases, the World Health Organization (WHO) developed and promotes the WHO STEPwise approach to surveillance (STEPS). For blood pressure measurement, STEPS recommends single-day three measurements, 3 min apart, with the mean of the second and third measurements used as final blood pressure.6 Most data on elevated blood pressure prevalence in Africa derive from population-based surveys following this protocol.
Several studies have shown that single-day blood pressure measurements have limited accuracy, misclassifying up to 30% diagnosed individuals.7, 8, 9 Therefore, for reliable estimation of blood pressure, most guidelines recommend two or more measurements on separate days.2,10, 11, 12 For population surveys, however, revisiting participants on a different day is highly resource-intensive or impossible, especially in remote rural areas or in mobile populations. Thus, for reliable blood pressure data to guide epidemiological and clinical decisions in remote or hard-to-reach settings, accurate, pragmatic, and feasible blood pressure measurement protocols, preferably implementable in one visit, are needed.13, 14, 15
Unattended automated blood pressure (uABP) protocol entails measurements where the healthcare worker leaves the room, and the participant is alone during the entire measurement period. This protocol has been shown in clinic settings to better correlate with 24-h ambulatory blood pressure monitoring (24 h-ABPM) taken as gold standard.16 uABP is reported to reduce overdiagnosis,17,18 to be easily used in different locations,19 and to have a similar profile of target organ damage predictability as 24 h-ABPM.20 Despite these advantages of uABP in clinic settings, there’s a lack of studies examining its usefulness in population-based surveys. A targeted literature search revealed no study assessing uABP for household-based screening during prevalence surveys in Africa.
In this study, we investigated within a population-based survey the diagnostic accuracy of standard survey blood pressure measurement (SBPM) protocol used in STEPS, an immediate unattended ABP (1st uABP) measurement taken on survey day, and a delayed unattended ABP (2nd uABP) measurement taken after 24 h, using the 24 h-ABPM as gold standard.
Methods
Study design
This study is part of the community-based chronic care Lesotho project (ComBaCaL; www.combacal.org). It is a diagnostic accuracy study conducted within a population-based household survey on cardiovascular risk factors implemented from 2nd November 2021 to 31st August 2022 in two districts in Northern Lesotho.21
Lesotho’s National Health Research Ethics Committee (NH-REC) approved the study protocol (NH-REC ID 139-2021; see Supplementary materials, panel S1), and participants provided written informed consent. Reporting follows the Standards for Reporting of Diagnostic Accuracy Studies (STARD) reporting guidelines.22
Sample size and participant selection
Sample size was calculated using the methods of Buderer et al.23,24 Assuming a sensitivity of 84%, and a specificity of 79% with unassisted measurement methods,25 and a prevalence of elevated blood pressure of 30%, we estimated that a minimum of 172 people with elevated blood pressure and 91 participants without elevated blood pressure would be required to estimate sensitivity and specificity with a 10% precision of a 2-sided 95% confidence interval. To account for non-response, we oversampled by 20% (see Supplementary materials, panel S1, section 5.3.2).
Participants were enrolled from the population-based survey if they were 18 years and older, gave written informed consent, had SBPM blood pressure ≥140 mmHg systolic and/or ≥90 mmHg diastolic, and lived in easy-to-reach areas of the community since it was important for survey staff to revisit for retrieval of the 24 h-ABPM device. We further enrolled a randomly selected 2:1 age- and sex-matched subsample of participants with SBPM blood pressure values <140 and 90 mmHg at survey screen. The overall prevalence survey ended on 31st August 2022. However, as by then the sample-size of this sub-study was not met, an additional 159 participants (95 with elevated blood pressure, 64 without) were recruited between 1st September and 30th November 2022. This number includes already surveyed participants revisited for inclusion in this study, and new participants surveyed and included.
Standard blood pressure measurement (SBPM)
During the household survey, blood pressure was measured using the validated WatchBP® Office ABI automatic blood pressure measurement device manufactured by Microlife®.26,27 Three measurements were taken with 2-min intervals between measurements. Measurement procedures followed the STEPS protocol. An appropriate-sized cuff was snugly applied and securely fastened to the left arm after any clothes had been rolled up, with the cuff mark aligned with the brachial artery and the lower edge of the cuff about 2.5 cm above the inner side of the elbow joint. Participants had to sit quietly for at least 15 min, with their back supported, feet flat on the floor, and arm supported with the cuff at heart level. Additionally, participants were instructed to have an empty bladder, not to drink coffee before or during measurements, and not to talk during the measurements.6 The study protocol included detailed standard operating procedures on SBPM measurement. Based on these, the survey staff was trained, regularly retrained, and supervised.
For allocation of participants to the normal vs elevated SBPM group, elevated SBPM was defined as recorded systolic blood pressure ≥140 and/or diastolic ≥90 mm Hg, calculated using the average of the final two readings.28
Unattended automated blood pressure measurement (uABP)
For the uABP, we used the same WatchBP® Office ABI as for the SBPM. The device was set to obtain three measurements with a minute interval in-between the measurements. After a minimum of 5 min of rest, the device was attached. Participants were shown how to operate the device and asked to turn it on after research assistants had left the room. Some participants were not able to follow this instruction and the device was started by the research assistants who then immediately left the room. Research assistants stayed out of the room for the duration of measurement which was about 5 min. The blood pressure measurement device stored values and labeled them accordingly as first, second or third measurements. The research assistant retrieved these values upon return to the room. The average of the second and third readings were used for analysis. The 1st uABP was taken the same-day as the SBPM, whereas the 2nd uABP was taken the next day when the research team returned to the participant’s home to take off the 24 h-ABPM device.
24-h ambulatory blood pressure measurement (24 h-ABPM)
We used the IEM 24 h Mobil-O-Graph® for 24 h ambulatory blood pressure measurement (24 h-ABPM).29 Following measurement of 1st uABP, participants were fitted with the right-sized 24 h-ABPM device. Each participant was asked for their sleep and awake times. The device was then programmed accordingly to take three measurements hourly during the day (awake time) and two measurements hourly at night (sleep time).
24 h-ABPM observations were included in the final analysis if there was 1) ≥70% of expected measurements; 2) a minimum of 20 valid daytime measurements and a minimum of 7 valid nighttime measurements; and 3) ≥2 valid daytime and ≥1 valid nighttime measurements per hour.30
Statistical analysis
Statistical analyses were conducted in Stata (16.1, StataCorp LLC, College Station, TX) and R (version 4.3.0).31 Stata was used to calculate descriptive statistics, and for determination of sensitivity, specificity, predictive values, and likelihood ratios. R was used to determine discrimination, correlation coefficients and their comparison, Bland–Altman analysis, and for categorization of participants into white coat hypertension, masked hypertension, sustained hypertension and sustained normotension.
Descriptive statistics such as mean and standard deviation; median and interquartile range were used for continuous variables, while frequency and percentage were used for categorical variables. We did not statistically compare baseline characteristics between the group with elevated and the group with normal SBPM (Table 1) as differences in cardiovascular risk profile between these groups are expected.
Table 1.
Characteristics of study participants.
| Variable | Elevated SBPM, N = 183 | Normal SBPM, N = 92 | Total, N = 275 |
|---|---|---|---|
| Female, n (%) | 110 (60.1) | 53 (57.6) | 163 (59.3) |
| Male, n (%) | 73 (39.9) | 39 (42.4) | 112 (40.7) |
| Mean age in years (SD) | 59 (16) | 56 (16) | 58 (16) |
| Diabetes mellitus, n (%) | 31 (16.9) | 2 (2.2) | 33 (12) |
| Median BMI in kg/m2 (IQR) | 27.5 (23.5–32.9) | 25.0 (21.3–28.4) | 26.6 (22.7–32.1) |
| Height in cm, median (IQR) | 159 (153–167) | 161 (154–168) | 160 (154–167) |
| Weight in kg, median (IQR) | 71 (60–85) | 66 (55–74) | 69 (59–82) |
| Abd.circ. in cm, median (IQR) | |||
|
95 (87–104) | 91 (79–100) | 94 (86–103) |
|
89 (76–102) | 79 (72–87) | 82 (75–96) |
| Mean (SD) | Mean (SD) | Mean (SD) | |
| SBPM, mmHg | |||
| Systolic | 156 (20) | 119 (12) | 144 (25) |
| Diastolic | 93 (13) | 75 (8) | 87 (14) |
| First uABP, mmHg | |||
| Systolic | 149 (24) | 121 (18) | 140 (26) |
| Diastolic | 91 (15) | 75 (9) | 86 (16) |
| Second uABP, mmHg | |||
| Systolic | 143 (24) | 119 (17) | 135 (25) |
| Diastolic | 88 (15) | 76 (13) | 84 (16) |
| 24 h-ABPM, mmHg | |||
| Average systolic | 135 (19) | 115 (13) | 128 (20) |
| Average diastolic | 85 (13) | 73 (9) | 81 (13) |
| Daytime systolic | 138 (20) | 118 (14) | 131 (20) |
| Daytime diastolic | 89 (13) | 77 (11) | 85 (13) |
| Night time systolic | 129 (22) | 111 (14) | 123 (21) |
| Night time diastolic | 80 (14) | 69 (10) | 76 (14) |
BP: blood pressure; uABP: unattended blood pressure; 24 h-ABPM: 24 h ambulatory blood pressure monitor; SD: standard deviation; IQR: interquartile range; Abd.circ.: abdominal circumference.
The reference measurement was the 24 h-ABPM. The European Society of Hypertension defines arterial hypertension as 24-h average ≥130/80 mmHg. It further defines awake hypertension as daytime measurements average ≥135/85 mmHg and nocturnal hypertension as nighttime measurements average ≥120/70 mmHg.32,33 Each screening measurement (SBPM, 1st and 2nd uABP) was compared to each of these three reference standards (24-h average, awake hypertension, nocturnal hypertension).
There is currently no consensus on the ideal threshold for diagnosis of arterial hypertension using uABP.28,33, 34, 35 To reflect different thresholds proposed for SBPM in different international guidelines,16,28,33,35, 36, 37 we calculated sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios for each screening method (1st, 2nd uABP, SBPM) under three threshold scenarios: ≥130/80, ≥135/85, and ≥140/90 mmHg.
Discrimination was determined using area under the receiver operating characteristic (AUROC) curve, as an indication of overall diagnostic accuracy of all three screening measurements. The R package pROC (version 1.18.2)38 was used to create receiver operating characteristics (ROC) curves for the screening measurements using the above-mentioned reference cut-offs for average, daytime and nighttime 24 h-ABPM. Empirical optimal cut points for SBPM, 1st uABP and 2nd uABP were estimated using Liu’s method for empirical estimation of cut points for a diagnostic test.39
Additionally, all three screening measurements (SBPM, 1st uABP, and 2nd uABP) were compared against the reference measurement using paired t-tests. Intra-class correlation coefficients were calculated for the relationship between 24 h-ABPM and each of the three screening measurements. The differences in the strengths of the correlation coefficients were calculated for SBPM against each uABP, using the R package cocor (version 1.1-4)40 for comparison of two overlapping correlations based on dependent groups. Bland–Altman analysis was used to compare agreement between the screening measurements and 24 h-ABPM. Agreement was evaluated using average 24 h-ABPM (systolic and diastolic) as well as daytime 24 h-ABPM (systolic and diastolic).
Using average 24 h-ABPM and daytime systolic and diastolic cutoffs for confirmed hypertension, participants were categorised into white coat hypertension, masked hypertension, sustained hypertension and sustained normotension. These categories were once again calculated for systolic/diastolic measurement thresholds of ≥130/80, ≥135/85, and ≥140/90 for the different screening measurements. White coat hypertension was normal blood pressure on 24 h-ABPM and blood pressure at or above threshold for screening measurements; masked hypertension was blood pressure below threshold on screening measurement but at or above thresholds in the 24 h-ABPM; sustained hypertension was blood pressure at or above threshold for screening measurements and 24 h-ABPM; sustained normotension was normal blood pressure on 24 h-ABPM and screening measurements.41,42
Participants where technical errors/non-functioning of the equipment led to missing or invalid blood pressure measurement values were excluded from the analysis.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, and data interpretation, or writing of the report. EF, TL and NDL had access to the dataset. EF and NDL had final responsibility for the decision to submit for publication.
Results
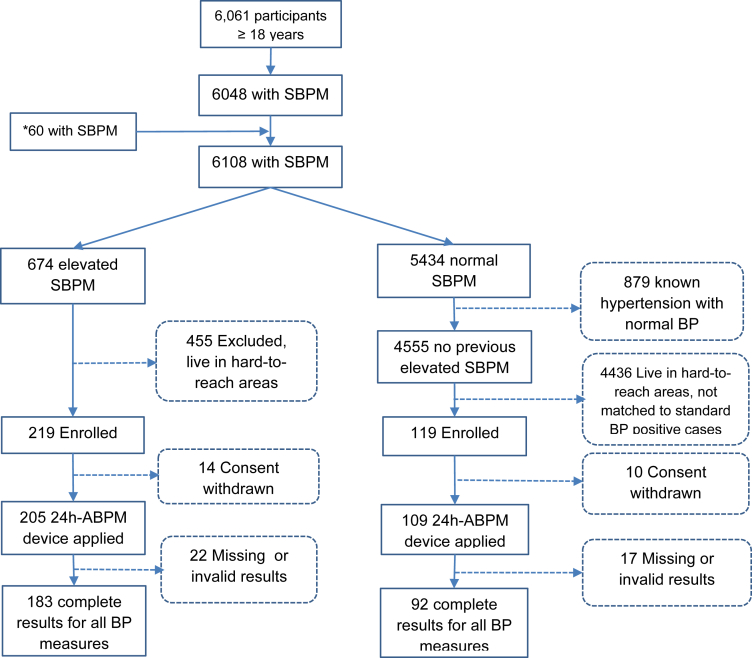
Fig. 1 shows the study flow. The survey enrolled 6061 participants with 6048 having documented SBPM values. After closure of survey on August 31st, 2022, we enrolled an additional 60 new participants, leading to a total of 6108 participants with SBPM results, out of which 674 had elevated SBPM (≥140/90 mmHg). Out of these, 219 (32.5%) were enrolled for this sub-study. Among the 4555 with SBPM < 140/90 and no prior diagnosis of elevated BP, an additional 119 age- and sex-matched individuals were enrolled. Due to invalid data or technical errors, 22 individuals with elevated and 17 with normal SBPM were retrospectively excluded, resulting in 183 individuals with elevated and 92 with normal SBPM included in the analysis. Since missingness was mainly due to technical reasons, we assumed that data were missing at random. As a result, multiple imputation was not done. Table 1 displays characteristics of participants in the elevated and normal SBPM group. Of the 183 with elevated blood pressure, 77 (42.1%) were taking antihypertensive treatment.
Fig. 1.
Study flow. SBPM = standard blood pressure measurement; 24 h-ABPM = 24 h ambulatory blood pressure monitor; BP = blood pressure; ∗Additional data between 01/09/2022 to 30/11/2022.
Correlation and level of agreement of test measurements and 24 h-ABPM
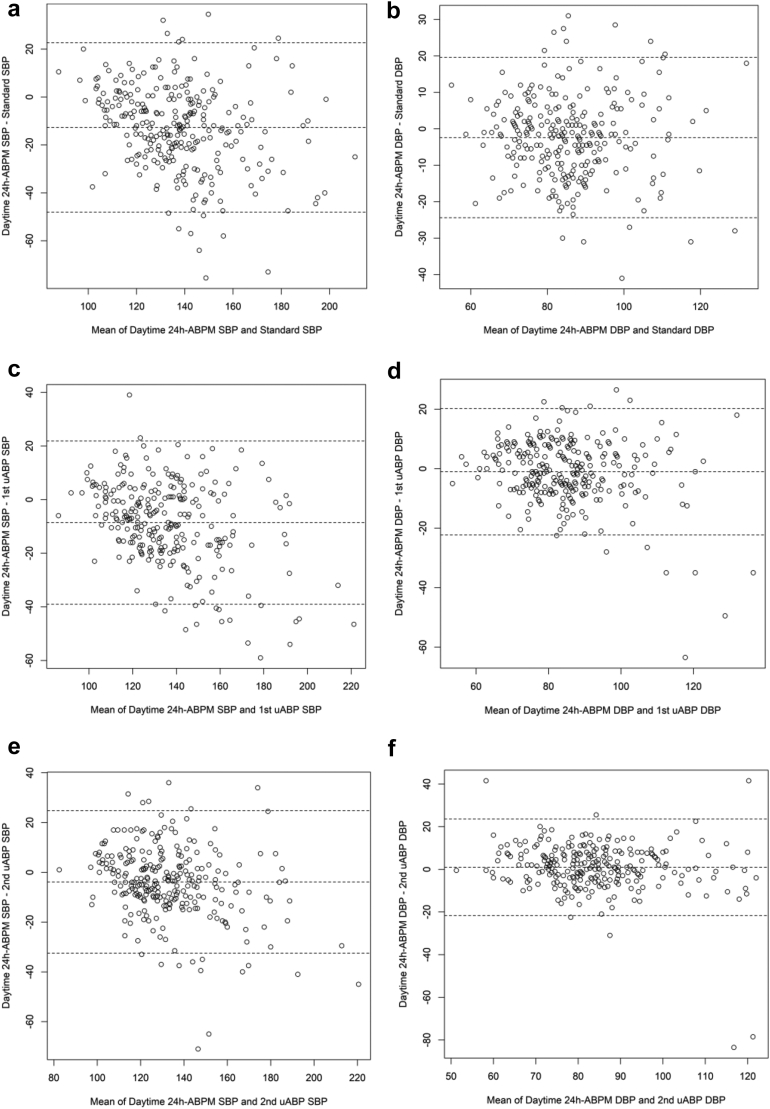
For all three screening measurements, mean systolic and diastolic blood pressure values were consistently higher than 24 h-ABPM values (average, daytime, and nighttime). Mean differences were higher for SBPM than for both uABP. There was no significant difference between diastolic values of both unattended measurements and diastolic daytime 24 h-ABPM: −1.0 mmHg (CI: −2.0 to 0.3), and 1.0 mmHg (CI: −0.4 to 2.3) for 1st uABP and 2nd uABP, respectively. Correlation coefficients of all index test measurements ranged from 0.61 to 0.81 for systolic values, and 0.57 to 0.73 for diastolic values. SBPM had lowest correlation coefficients (Table 2), which were significantly different from those of the uABPs, especially for systolic blood pressure values (see Supplementary Table S1). Agreements between the screening measurements and 24 h-ABPM are shown on Fig. 2 and Supplementary Figs. S1 and S2.
Table 2.
Mean difference and correlation between screening measurements and 24 h-ABPM values (reference).
| BP | Mean (SD) | Mean difference (95% CI) | P | Correlation coefficient (CI) | P |
|---|---|---|---|---|---|
| Standard BP (systolic) | 144 (25) | ||||
| Average | 128 (20) | −16 (−17 to −14) | <0.0001 | 0.73 (0.67–0.78) | <0.0001 |
| Daytime | 131 (20) | −13 (−15 to −11) | <0.0001 | 0.70 (0.64–0.76) | <0.0001 |
| Nighttime | 123 (21) | −21 (−24 to −19) | <0.0001 | 0.61 (0.53–0.68) | <0.0001 |
| Standard BP (diastolic) | 87 (14) | ||||
| Average | 81 (13) | −6 (−7 to −4) | <0.0001 | 0.67 (0.60–0.73) | <0.0001 |
| Daytime | 85 (13) | −2 (−4 to −1) | 0.00052 | 0.66 (0.59–0.73) | <0.0001 |
| Nighttime | 76 (14) | −11 (−12 to −9) | <0.0001 | 0.57 (0.48–0.64) | <0.0001 |
| 1st uABP (systolic) | 140 (26) | ||||
| Average | 128 (20) | −12 (−14 to −10) | <0.0001 | 0.81 (0.76–0.85) | <0.0001 |
| Daytime | 131 (20) | −9 (−10 to −7) | <0.0001 | 0.81 (0.76–0.84) | <0.0001 |
| Nighttime | 123 (21) | −17 (−20 to −15) | <0.0001 | 0.68 (0.61–0.74) | <0.0001 |
| 1st uABP (diastolic) | 86 (16) | ||||
| Average | 81 (13) | −4 (−6 to −3) | <0.0001 | 0.72 (0.66–0.77) | <0.0001 |
| Daytime | 85 (13) | −1 (−2 to 0.3) | 0.12 | 0.73 (0.67–0.78) | <0.0001 |
| Nighttime | 76 (14) | −10 (−11 to −8) | <0.0001 | 0.62 (0.54–0.68) | <0.0001 |
| 2nd uABP (systolic) | 135 (25) | ||||
| Average | 128 (20) | −7 (−9 to −5) | <0.0001 | 0.80 (0.75–0.84) | <0.0001 |
| Daytime | 131 (20) | −4 (−6 to −2) | <0.0001 | 0.80 (0.76–0.84) | <0.0001 |
| Nighttime | 123 (21) | −12 (−15 to −10) | <0.0001 | 0.69 (0.63–0.75) | <0.0001 |
| 2nd uABP (diastolic) | 84 (16) | ||||
| Average | 81 (13) | −2 (−4 to −1) | 0.00041 | 0.69 (0.63–0.75) | <0.0001 |
| Daytime | 85 (13) | 1 (−0.4 to 2.3) | 0.17 | 0.69 (0.63–0.75) | <0.0001 |
| Nighttime | 76 (14) | −8 (−9 to −6) | <0.0001 | 0.59 (0.50–0.66) | <0.0001 |
BP: blood pressure; uABP: unattended blood pressure; 24 h-ABPM: 24 h ambulatory blood pressure monitor; CI: confidence interval.
Fig. 2.
Bland–Altman plots showing levels of agreement between the three screening measurements and daytime ambulatory blood pressure monitor (24 h-ABPM). (2a, 2c, 2e) Show daytime 24 h-ABPM systolic blood pressure (SBP) vs SBP of SBPM, 1st uABP and 2nd uABP, respectively. (2b, 2d, 2f) Show daytime 24 h-ABPM diastolic blood pressure (DBP) vs DBP of SBPM, 1st uABP and 2nd uABP, respectively. All values in mmHg. SBPM = standard blood pressure measurement; uABP = unattended blood pressure.
Diagnostic categories and performance of test measurements on 24 h-ABPM
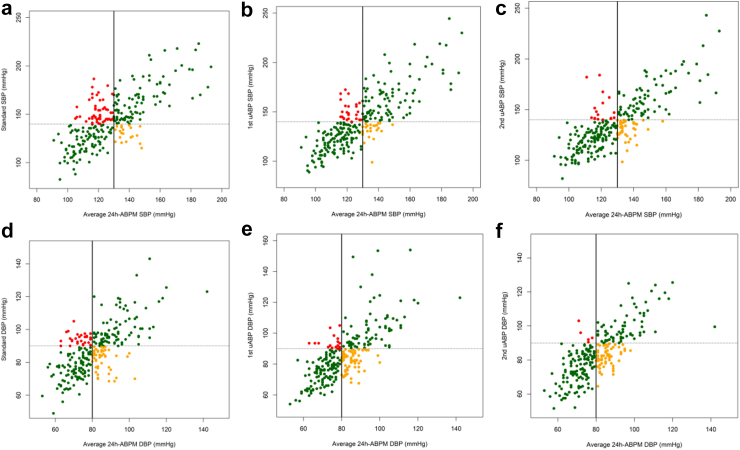
Fig. 3 shows diagnostic categories of participants using ≥140 mmHg systolic and ≥90 mmHg diastolic blood pressure as cut offs for screening measurements, against average 24 h-ABPM. For systolic blood pressure values, the category of participants with white coat hypertension was 20.0% and 9.8% with SBPM and 1st uAPB, respectively (see Supplementary Table S2 in supplementary material). The category of participants with masked hypertension was similar for both SBPM and 1st uABP: 21 (7.6%) and 25 (9.1%) respectively. There were similar results for diastolic blood pressure values. See Supplementary Figs. S3–S7 and Supplementary Table S2–S7 in supplementary material for diagnostic categories at different blood pressure thresholds.
Fig. 3.
Scatter plots of average 24-ABPM vs SBPM, 1st uABP and 2nd uABP. (a–c) Show average 24 h-ABPM SBP vs SBP of SBPM, 1st uABP and 2nd uABP respectively, using the cut off ≥140 mmHg. (d–f) Show average 24 h-ABPM DBP vs DBP of SBPM, 1st uABP and 2nd uABP respectively, using the cut off ≥90 mmHg. 24 h-ABPM = 24-h ambulatory blood pressure monitor; SBPM = standard blood pressure measurement; uABP = unattended blood pressure; SBP = systolic blood pressure; DBP = diastolic blood pressure. Black solid line is BP threshold for average 24 h-ABPM (130 mmHg systolic, 80 mmHg diastolic); dashed line is BP threshold for screening measurements (140 mmHg systolic, 90 mmHg diastolic). Green colour- BP measurements match gold standard. Orange colour-masked hypertension. Red colour-white coat hypertension.
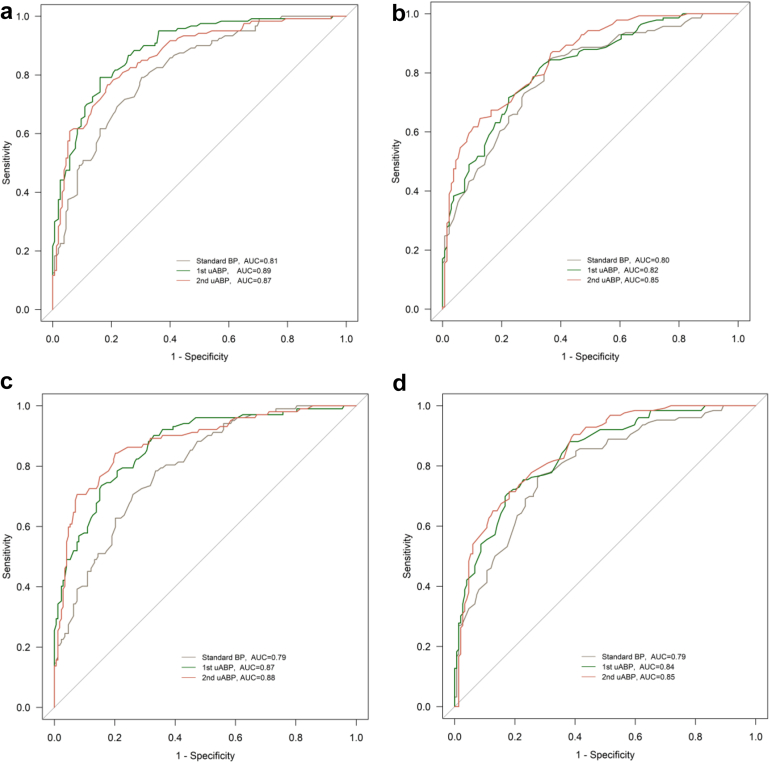
Across all three 24 h-ABPM reference thresholds, overall AUROC was lower for SBPM compared to the unattended measures. For systolic blood pressure values, using 24 h-ABPM references for average, daytime, and nighttime values, AUROCs ranged from 76% to 81% for SBPM; 79%–89% for 1st uABP; and 79%–88% for 2nd uABP measurements. AUROC differences between SBPM and 1st uABP; and between SBPM and 2nd uABP were statistically significant (see Fig. 4 and Supplementary Figs. S8 and S9). The difference in AUROC between 1st and 2nd uABP was not significant.
Fig. 4.
ROC curves using: (a) Systolic average 24 h-ABPM (≥130 mmHg) as reference vs systolic values of standard BP, 1st uABP, and 2nd uABP. P = 0.0011 for AUC difference between 1st uABP and standard BP; P = 0.047 for AUC difference between 2nd uABP and standard BP; P = 0.24 for AUC difference between 1st uABP and 2nd uABP; (b) Diastolic average 24 h-ABPM (≥80 mmHg) as reference vs diastolic values of standard BP, 1st uABP, and 2nd uABP. P = 0.45 for AUC difference between 1st uABP and standard BP; P = 0.066 for AUC difference between 2nd uABP and standard BP; P = 0.16 for AUC difference between 1st uABP and 2nd uABP; (c) systolic daytime 24 h-ABPM (≥135 mmHg) as reference vs systolic values of standard BP, 1st uABP, and 2nd uABP. P = 0.0024 for AUC difference between 1st uABP and standard BP; P = 0.0017 for AUC difference between 2nd uABP and standard BP; P = 0.56 for AUC difference between 1st uABP and 2nd uABP; (d) Diastolic daytime 24 h-ABPM (≥85 mmHg) as reference vs diastolic values of standard BP, 1st uABP, and 2nd uABP. P = 0.058 for AUC difference between 1st uABP and standard BP; P = 0.029 for AUC difference between 2nd uABP and standard BP; P = 0.49 for AUC difference between 1st uABP and 2nd uABP. ROC: receiver operating characteristics; AUC: area under the curve; uABP: unattended blood pressure; 24 h-ABPM: 24 h ambulatory blood pressure monitoring.
Across different blood pressure cut offs using average 24 h-ABPM and daytime 24 h-ABPM references, sensitivity ranged from 85% (95% CI: 74–91) to 94% (89–97) for SBPM; 72% (65–79) to 93% (87–96) for 1st uABP; and 60% (52–68) to 91% (84–95) for 2nd uABP. Specificity ranged from 38% (29–47) to 66% (56–75) for SBPM; 52% (43–61) to 82% (73–89) for 1st uABP; and 53% (44–62) to 94% (88–98) for 2nd uABP (Table 3, and Supplementary Table S8). Using average 24 h-ABPM as reference, the optimal cut point on the respective AUROCs closest to sensitivity = 100% and specificity = 100% was 135 mmHg systolic and 81 mmHg diastolic for 1st uABP. Using daytime 24 h-ABPM as reference, the optimal cut point for 1st uABP was 136 mmHg systolic and 87 mmHg diastolic. See Supplementary Table S9.
Table 3.
Accuracy of hypertension diagnosis at different blood pressure cut offs using daytime 24 h-ABPM as reference.
| BP cut off | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | LR (+) (95% CI) | LR (−) (95% CI) | AUROC (95% CI) |
|---|---|---|---|---|---|---|---|
| Standard BP measurement | |||||||
| ≥140/90 | 85 (78–91) | 57 (48–66) | 69 (61–76) | 78 (68–86) | 2.0 (1.6–2.5) | 0.3 (0.2–0.4) | 0.71 (0.66–0.77) |
| ≥135/85 | 89 (83–94) | 49 (40–58) | 66 (59–73) | 80 (70–89) | 1.8 (1.5–2.1) | 0.2 (0.1–0.4) | 0.69 (0.64–0.74) |
| ≥130/80 | 93 (88–97) | 38 (29–47) | 62 (55–69) | 84 (72–92) | 1.5 (1.3–1.7) | 0.2 (0.1–0.3) | 0.66 (0.61–0.70) |
| 1st uABP | |||||||
| ≥140/90 | 74 (66–81) | 75 (66–82) | 77 (68–84) | 72 (64–80) | 3.0 (2.1–4.1) | 0.4 (0.3–0.5) | 0.74 (0.69–0.80) |
| ≥135/85 | 85 (78–90) | 63 (54–71) | 72 (64–78) | 79 (69–86) | 2.3 (1.8–2.9) | 0.2 (0.2–0.4) | 0.74 (0.69–0.79) |
| ≥130/80 | 93 (87–96) | 52 (43–61) | 68 (61–75) | 87 (77–93) | 1.9 (1.6–2.3) | 0.1 (0.1–0.3) | 0.72 (0.67–0.77) |
| 2nd uABP | |||||||
| ≥140/90 | 64 (56–72) | 90 (84–95) | 88 (80–94) | 70 (62–77) | 6.6 (3.8–11.5) | 0.4 (0.3–0.5) | 0.77 (0.72–0.82) |
| ≥135/85 | 77 (69–84) | 70 (61–78) | 74 (66–81) | 74 (65–81) | 2.6 (2.0–3.5) | 0.3 (0.2–0.5) | 0.74 (0.68–0.79) |
| ≥130/80 | 91 (84–95) | 53 (44–62) | 68 (61–75) | 84 (74–91) | 1.9 (1.6–2.4) | 0.2 (0.1–0.3) | 0.72 (0.67–0.77) |
BP: blood pressure; uABP: unattended blood pressure; 24 h-ABPM: 24 h ambulatory blood pressure monitor; CI: confidence interval; PPV: positive predictive value; NPV: negative predictive value; LR: likelihood ratio.
Discussion
This study compared the diagnostic accuracy of SBPM, immediate (or 1st) uABP and delayed (or 2nd) uABP during a population-based survey on arterial hypertension, using 24 h-ABPM as reference standard. Unattended measurements showed significantly better correlation with 24 h-ABPM and significantly better discrimination than SBPM. Furthermore, uABP measurements had fewer participants with white coat hypertension than SBPM, while proportions of masked hypertension were similar with both procedures. The immediate uABP which is a more practical approach during surveys, had similar findings as delayed uABP. These results indicate that unattended blood pressure measurement approaches may be a feasible approach to improve accuracy of blood pressure measurement during population-based surveys, and diagnosis of arterial hypertension in community settings, where follow-up measurements are not feasible. If confirmed in other studies, unattended measurement approaches could replace the currently used blood pressure measurement protocol in the WHO STEPwise approach to surveillance (STEPS).
To our knowledge, this is the first study to directly compare diagnostic accuracy of SBPM and uABP during a community-based screening in sub-Sahara Africa. Strengths of the study are its prospective design, integration into a large survey that reflects real-life conditions of broad community-based screening, and inclusion of matched participants without elevated blood-pressure at SBPM. The main limitation of this study is that due to operational considerations, the sequence of blood pressure measurements using different approaches was not randomised, possibly introducing an order effect. However, several studies conducted in different settings did not find an important order effect.16 Secondly, there were 22 missing results in the elevated SBPM group, and 17 missing results in the normal SBPM group. Given that reasons for missing data were mainly technical, we do not expect that the exclusion of these participants results in a selection bias. Further, at increasing blood pressure values, the Bland–Altman plots show several extreme points (Fig. 2). These extreme points likely reflect the magnitude of white coat or masked hypertension effects in some participants, as well as the dependence of the white coat effect on blood pressure level.43,44 The extreme points, however, imply that at very high blood pressure values, agreement of screening measurements with ambulatory blood pressure monitoring may be low.33,44 Finally, future studies may consider hierarchical linear approaches to develop an overall model that accounts for different diagnostic test accuracy. In the current study, we focus on AUROC, sensitivity and specificity traditionally used in primary diagnostic accuracy studies.45
The burden of arterial hypertension and other cardio-vascular risk factors is increasing in sub-Sahara Africa.46 Regular surveillance of trends in prevalence is important for African countries to design health programs and plan resource-allocations. With the STEPS instrument, the WHO has created a standardized and feasible approach that allows low- and middle-income countries to estimate their burden of non-communicable diseases.47 Our study found that unattended measurements had better correlation with 24 h-ABPM and a higher area under the receiver operating characteristic curve compared to the SBPM used in STEPS. This is in line with other studies reporting better diagnostic performance of uABP measurements.16,48 One study conducted in Kenya,33 the only study assessing accuracy of uABP in sub-Sahara Africa, found modest discriminatory accuracy of uABP and concluded that uABP misclassified significant proportions of individuals undergoing hypertension screening. There, participants were identified in their homes and required to travel to clinics where study procedures were conducted. Our study, however, was carried out during a population-based survey, and all processes initiated and concluded at participants’ homesteads. Additionally, we compared the diagnostic accuracy of immediate and delayed uABP with SBPM.
Our study shows that the number of individuals with so-called white coat hypertension was twice as high for SBPM than for the unattended measurements. Previous studies suggest that up to 30% of individuals diagnosed with elevated blood pressure, commonly done with SBPM, may actually have normal blood pressure.7 By 2016, 122 countries had conducted at least one STEPS or STEPS-aligned survey, 42 of which were African countries.49 An analysis of STEPS from 17 countries in sub-Sahara Africa from 2010 to 201750 estimated that 13%–30% of 85,371 respondents had hypertension (blood pressure ≥140/90 mmHg), with markedly low access to treatment and advocating for increase in care for those diagnosed. However, considering the results of our study, a substantial proportion may have been wrongly diagnosed. Such overestimation may lead to over-reporting of prevalence figures and, possibly unnecessary initiation of antihypertensive therapy.48,51,52
In our study, for the traditional threshold of 140/90 mmHg, all screening tests had a discrimination between hypertension vs no hypertension that was in the acceptable range only. For 1st uABP using daytime 24 h-ABPM as reference, the optimal threshold was 136/87 mmHg. However, due to a lack of data, the ideal threshold for uABP is still subject to debate.28 Current evidence indicates that uABP correlates best with daytime 24 h-ABPM, for which reason 135/85 mmHg is usually taken as threshold.16,35,53 The Canadian 2020 guidelines recommend 135/85 mmHg as uABP threshold.37 This threshold is, however, based on studies that were mainly conducted among non-African participants. Population-specific studies that involve cardiovascular outcomes will be needed to identify the ideal cut-off for uABP in sub-Sahara Africa.33
Practicality and feasibility of uABP has been challenged by some experts, citing need for extra space, equipment and personnel as potential barriers to its implementation.28 In our study, we did not identify any need for additional equipment or personnel since the same care providers used the same devices for SBPM and uABP. During household-based screening, it was not a problem to find space in participants’ homes. uABP may thus be an affordable and practical alternative to labor- and logistic intensive 24 h-ABPM in resource-limited settings where remoteness of target population, availability of equipment and technical expertise for interpretation limit practicality of 24 h-ABPM.
In conclusion, during a household-based survey, uABP had better diagnostic accuracy and misclassified fewer individuals as having hypertension than the commonly used SBPM. Differences between uABP taken on day one and uABP taken on the next day were not significant, indicating that for survey settings, uABP on the survey day may be sufficient. Given the better diagnostic performance of uABP compared to SBPM, survey protocols, such as the WHO STEPwise approach to surveillance, may consider including uABP as an affordable and feasible alternative to improve accuracy of hypertension prevalence estimates obtained from population-level assessments, and as a surrogate for daytime 24 h-ABPM in population surveys.
Contributors
EF, AA, TB and NDL conceptualised the study and design. EF, LG, MM, MPS, MB, MK, IL, LR, SM and RG collected the data. EF curated, analysed and interpreted the data with support from FC, TL, TB and NDL. EF drafted the first version of the manuscript. NDL, TB, FC, LG, AA, MW, and BL reviewed the manuscript draft. All authors reviewed the results and approved the final version of the manuscript. AA and NDL are the co-principal investigators of the ComBaCaL project (www.combacal.org).
EF, TL and NDL verified the underlying data.
Data sharing statement
A dataset with the key variables of this manuscript is available at http://zenodo.org (doi:https://doi.org/10.1016/j.eclinm.2023.102197).
Declaration of interests
NDL reports grants from the Swiss National Science Foundation (Eccellenza Professorship and project grant), Botnar Foundation, Botnar Center for Child Health, Swiss Agency for Development and Cooperation, and Moritz Straus Stiftung; consulting fees from ViiV Healthcare, the Research Council of Norway, and the Swiss National Science Foundation (all paid to his division); honoraria for lectures from ETHZ and Swiss TPH (paid to his division); travel grants from Gilead Sciences and ViiV Healthcare; participation on a Data Safety Monitoring Board for Pharming (payments made to his division).
EF received travel support from the European Union’s Horizon 2020 research and innovation programme under Marie Skłodowska-Curie grant agreement (No 801076), through SSPH+ Global PhD Fellowship Programme in Public Health Sciences (GlobalP3HS).
All other authors declare no competing interests.
Acknowledgements
This study was funded by the TRANSFORM grant of the Swiss Agency for Development and Cooperation (SDC) under the ComBaCaL project (Project no. 7F-10345.01.01, www.combacal.org), obtained by AA and NDL. The study received further support from the World Diabetes Foundation, awarded to SolidarMed. EF receives his salary from the European Union’s Horizon 2020 research and innovation programme under Marie Skłodowska-Curie grant agreement (No 801076), through SSPH + Global PhD Fellowship Programme in Public Health Sciences (GlobalP3HS). NDL receives his salary from the Swiss National Science Foundation (SNSF Eccellenza PCEFP3_181355). The authors would like to thank all study participants, the ComBaCaL baseline survey team and the Ministry of Health of Lesotho.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102197.
Contributor Information
Emmanuel Firima, Email: Emmanuel.firima@usb.ch.
Niklaus Daniel Labhardt, Email: Niklaus.labhardt@usb.ch.
Appendix ASupplementary data
References
- 1.Singh S., Shankar R., Singh G.P. Prevalence and associated risk factors of hypertension: a cross-sectional study in Urban Varanasi. Int J Hypertens. 2017;2017 doi: 10.1155/2017/5491838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organisation Blood pressure/hypertension. 2023. https://www.who.int/data/gho/indicator-metadata-registry/imr-details/3155
- 3.Zhou B., Perel P., Mensah G.A., Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. 2021;18(11):785–802. doi: 10.1038/s41569-021-00559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaney T., Schutte A.E., Stergiou G.S., et al. May measurement month 2019: the global blood pressure screening campaign of the international society of hypertension. Hypertension. 2020;76(2):333–341. doi: 10.1161/HYPERTENSIONAHA.120.14874. [DOI] [PubMed] [Google Scholar]
- 5.Woodiwiss A.J., Gafane-Matemane L.F., Norton G.R., et al. May measurement Month 2019: an analysis of blood pressure screening results from South Africa. Eur Heart J Suppl. 2021;23(Supplement_B):B134–B137. [Google Scholar]
- 6.World Health Organization . World Health Organization; 2005. WHO STEPS surveillance manual: the WHO STEPwise approach to chronic disease risk factor surveillance. [Google Scholar]
- 7.Siu A.L. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(10):778–786. doi: 10.7326/M15-2223. [DOI] [PubMed] [Google Scholar]
- 8.Nsanya M.K., Ayieko P., Hashim R., et al. Sustained high blood pressure and 24-h ambulatory blood pressure monitoring in Tanzanian adolescents. Sci Rep. 2021;11(1):8397. doi: 10.1038/s41598-021-87996-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olivier S., Murray T., Matthews P., et al. Pitfalls of single measurement screening for diabetes and hypertension in community-based settings. Glob Heart. 2021;16(1):79. doi: 10.5334/gh.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vischer A.S., Burkard T. Principles of blood pressure measurement - current techniques, office vs ambulatory blood pressure measurement. Adv Exp Med Biol. 2017;956:85–96. doi: 10.1007/5584_2016_49. [DOI] [PubMed] [Google Scholar]
- 11.Groenland E.H., Bots M.L., Visseren F.L.J., McManus R.J., Spiering W. Number of measurement days needed for obtaining a reliable estimate of home blood pressure and hypertension status. Blood Press. 2022;31(1):100–108. doi: 10.1080/08037051.2022.2071674. [DOI] [PubMed] [Google Scholar]
- 12.Vischer A.S., Burkard T. How should we measure and deal with office blood pressure in 2021? Diagnostics (Basel) 2021;11(2) doi: 10.3390/diagnostics11020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills K.T., Stefanescu A., He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223–237. doi: 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maher D., Smeeth L., Sekajugo J. Health transition in Africa: practical policy proposals for primary care. Bull World Health Organ. 2010;88(12):943–948. doi: 10.2471/BLT.10.077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathers C.D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roerecke M., Kaczorowski J., Myers M.G. Comparing automated office blood pressure readings with other methods of blood pressure measurement for identifying patients with possible hypertension: a systematic review and meta-analysis. JAMA Intern Med. 2019;179(3):351–362. doi: 10.1001/jamainternmed.2018.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parati G., Stergiou G., O’Brien E. Cardiovascular variability, European society of hypertension working group on blood pressure monitoring. European society of hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31(9):1731–1768. doi: 10.1097/HJH.0b013e328363e964. [DOI] [PubMed] [Google Scholar]
- 18.Myers M.G., Asmar R., Staessen J.A. Office blood pressure measurement in the 21st century. J Clin Hypertens (Greenwich) 2018;20(7):1104–1107. doi: 10.1111/jch.13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers M.G. Automated office blood pressure measurement. Korean Circ J. 2018;48(4):241–250. doi: 10.4070/kcj.2018.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andreadis E.A., Agaliotis G.D., Angelopoulos E.T., Tsakanikas A.P., Chaveles I.A., Mousoulis G.P. Automated office blood pressure and 24-h ambulatory measurements are equally associated with left ventricular mass index. Am J Hypertens. 2011;24(6):661–666. doi: 10.1038/ajh.2011.38. [DOI] [PubMed] [Google Scholar]
- 21.González Fernández L.F., Firima E., Gupta R., et al. Prevalence and determinants of cardiovascular risk factors in Lesotho: a population-based survey. Int Health. 2023;058 doi: 10.1093/inthealth/ihad058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen J.F., Korevaar D.A., Altman D.G., et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6(11) doi: 10.1136/bmjopen-2016-012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Negida A., Fahim N.K., Negida Y. Sample size calculation guide - Part 4: how to calculate the sample size for a diagnostic test accuracy study based on sensitivity, specificity, and the area under the ROC curve. Adv J Emerg Med. 2019;3(3):e33. doi: 10.22114/ajem.v0i0.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buderer N.M. Statistical methodology: I. Incorporating the prevalence of disease into the sample size calculation for sensitivity and specificity. Acad Emerg Med. 1996;3(9):895–900. doi: 10.1111/j.1553-2712.1996.tb03538.x. [DOI] [PubMed] [Google Scholar]
- 25.Nascimento L.R., Coelli A.P., Cade N.V., Mill J.G., Molina Mdel C. Sensitivity and specificity in the diagnosis of hypertension with different methods. Rev Saude Publica. 2011;45(5):837–844. doi: 10.1590/s0034-89102011005000063. [DOI] [PubMed] [Google Scholar]
- 26.Stergiou G.S., Tzamouranis D., Protogerou A., Nasothimiou E., Kapralos C. Validation of the microlife watch BP Office professional device for office blood pressure measurement according to the international protocol. Blood Press Monit. 2008;13(5):299–303. doi: 10.1097/MBP.0b013e3283057af6. [DOI] [PubMed] [Google Scholar]
- 27.Kollias A., Ntineri A., Kyriakoulis K.G., et al. Validation of the professional device for blood pressure measurement microlife WatchBP office in adults and children according to the American National Standards Institute/Association for the Advancement of Medical Instrumentation/International Organization for standardization standard. Blood Press Monit. 2018;23(2):112–114. doi: 10.1097/MBP.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 28.Mancia Chairperson G., Kreutz Co-Chair R., Brunström M., et al. 2023 ESH guidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the European society of hypertension endorsed by the European renal Association (ERA) and the international society of hypertension (ISH) J Hypertens. 2023 doi: 10.1097/HJH.0000000000003480. [DOI] [PubMed] [Google Scholar]
- 29.Wei W., Tölle M., Zidek W., van der Giet M. Validation of the mobil-O-Graph: 24 h-blood pressure measurement device. Blood Press Monit. 2010;15(4):225–228. doi: 10.1097/MBP.0b013e328338892f. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien E., Parati G., Stergiou G. Ambulatory blood pressure measurement: what is the international consensus? Hypertension. 2013;62(6):988–994. doi: 10.1161/HYPERTENSIONAHA.113.02148. [DOI] [PubMed] [Google Scholar]
- 31.Team R.C. R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A language and environment for statistical computing. [Google Scholar]
- 32.Williams B., Mancia G., Spiering W., et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 33.Etyang A.O., Sigilai A., Odipo E., et al. Diagnostic accuracy of unattended automated office blood pressure measurement in screening for hypertension in Kenya. Hypertension. 2019;74(6):1490–1498. doi: 10.1161/HYPERTENSIONAHA.119.13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wohlfahrt P., Cífková R., Movsisyan N., et al. Threshold for diagnosing hypertension by automated office blood pressure using random sample population data. J Hypertens. 2016;34(11):2180–2186. doi: 10.1097/HJH.0000000000001076. [DOI] [PubMed] [Google Scholar]
- 35.Pappaccogli M., Di Monaco S., Perlo E., et al. Comparison of automated office blood pressure with office and out-off-office measurement techniques: a systematic review and meta-analysis. Hypertension. 2019;73(2):481–490. doi: 10.1161/HYPERTENSIONAHA.118.12079. [DOI] [PubMed] [Google Scholar]
- 36.Whelton P.K., Carey R.M., Mancia G., Kreutz R., Bundy J.D., Williams B. Harmonization of the American College of Cardiology/American Heart Association and European Society of Cardiology/European Society of Hypertension Blood Pressure/Hypertension Guidelines: comparisons, reflections, and recommendations. Circulation. 2022;146(11):868–877. doi: 10.1161/CIRCULATIONAHA.121.054602. [DOI] [PubMed] [Google Scholar]
- 37.Rabi D.M., McBrien K.A., Sapir-Pichhadze R., et al. Hypertension Canada's 2020 comprehensive guidelines for the prevention, diagnosis, risk assessment, and treatment of hypertension in adults and children. Can J Cardiol. 2020;36(5):596–624. doi: 10.1016/j.cjca.2020.02.086. [DOI] [PubMed] [Google Scholar]
- 38.Robin X., Turck N., Hainard A., et al. pROC: an open-source package for R and S+ to analyse and compare ROC curves. BMC Bioinform. 2011;12(1):1–8. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X. Classification accuracy and cut point selection. Stat Med. 2012;31(23):2676–2686. doi: 10.1002/sim.4509. [DOI] [PubMed] [Google Scholar]
- 40.Diedenhofen B., Musch J. cocor: a comprehensive solution for the statistical comparison of correlations. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0121945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papadopoulos D.P., Makris T.K. Masked hypertension definition, impact, outcomes: a critical review. J Clin Hypertens (Greenwich) 2007;9(12):956–963. doi: 10.1111/j.1524-6175.2007.07418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Celis H., Fagard R.H. White-coat hypertension: a clinical review. Eur J Intern Med. 2004;15(6):348–357. doi: 10.1016/j.ejim.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Pioli M.R., Ritter A.M., de Faria A.P., Modolo R. White coat syndrome and its variations: differences and clinical impact. Integr Blood Press Control. 2018;11:73–79. doi: 10.2147/IBPC.S152761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manios E.D., Koroboki E.A., Tsivgoulis G.K., et al. Factors influencing white-coat effect. Am J Hypertens. 2008;21(2):153–158. doi: 10.1038/ajh.2007.43. [DOI] [PubMed] [Google Scholar]
- 45.Pambabay-Calero J., Bauz-Olvera S., Nieto-Librero A., Sánchez-García A., Galindo-Villardón P. Hierarchical modeling for diagnostic test accuracy using multivariate probability distribution functions. Mathematics. 2021;9(11):1310. [Google Scholar]
- 46.Parati G., Lackland D.T., Campbell N.R.C., et al. How to improve awareness, treatment, and control of hypertension in Africa, and how to reduce its consequences: a call to action from the World hypertension league. Hypertension. 2022;79(9):1949–1961. doi: 10.1161/HYPERTENSIONAHA.121.18884. [DOI] [PubMed] [Google Scholar]
- 47.World Health Organisation Noncommunicable disease surveillance, monitoring and reporting. https://www.who.int/teams/noncommunicable-diseases/surveillance/data
- 48.Piper M.A., Evans C.V., Burda B.U., Margolis K.L., O'Connor E., Whitlock E.P. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162(3):192–204. doi: 10.7326/M14-1539. [DOI] [PubMed] [Google Scholar]
- 49.Riley L., Guthold R., Cowan M., et al. The World health Organization STEPwise approach to noncommunicable disease risk-factor surveillance: methods, challenges, and opportunities. Am J Public Health. 2016;106(1):74–78. doi: 10.2105/AJPH.2015.302962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shakil S.S., Ojji D., Longenecker C.T., Roth G.A. Early stage and established hypertension in sub-saharan Africa: results from population health surveys in 17 countries, 2010-2017. Circ Cardiovasc Qual Outcomes. 2022;15(12) doi: 10.1161/CIRCOUTCOMES.122.009046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ofori-Asenso R., Brhlikova P., Pollock A.M. Prescribing indicators at primary health care centers within the WHO African region: a systematic analysis (1995–2015) BMC Public Health. 2016;16(1):724. doi: 10.1186/s12889-016-3428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Etyang A.O., Warne B., Kapesa S., et al. Clinical and epidemiological implications of 24-hour ambulatory blood pressure monitoring for the diagnosis of hypertension in Kenyan adults: a population-based study. J Am Heart Assoc. 2016;5(12) doi: 10.1161/JAHA.116.004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Myers M.G., Godwin M. Automated office blood pressure. Can J Cardiol. 2012;28(3):341–346. doi: 10.1016/j.cjca.2011.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.