Summary
Background
Atezolizumab-bevacizumab (atezo-bev) is recommended as first-line therapy for patients with unresectable hepatocellular carcinoma (uHCC). However, its effectiveness and safety in other populations, including those with Child-Turcotte-Pugh (CTP) class B cirrhosis, is unclear.
Methods
For this systematic review and meta-analysis, electronic databases, including PubMed, Embase, and Scopus, were searched from 1st May, 2020 till 5th October, 2022; the last date of access was January 31, 2023. Pooled progression-free survival (PFS), overall survival (OS), and radiological response rate among patients receiving atezo-bev were compared between patients with CTP-A and CTP-B cirrhosis, with tyrosine kinase inhibitors (TKIs) and among those receiving the drug as first-line and later line therapy. The protocol was registered in Prospero (CRD42022364430).
Findings
Among 47 studies (n = 5400 patients), pooled PFS and OS were 6.86 (95% CI, 6.31–7.41) and 13.8 months (95% CI, 11.81–15.8), respectively. Objective response rate (ORR) and disease control rate were 26.7% (24.6–29.1) and 75.3% (73.1–77.4) using RECIST criteria, and 34% (30.3–37.8) and 73.6% (68.8–78) using mRECIST criteria, respectively. Among those receiving atezo-bev, patients with CTP-B cirrhosis had similar ORRs by RECIST (odds ratio [OR], 1.42 [0.77–2.6]; P = 0.25) and mRECIST criteria (OR, 1.33 [0.52–3.39]; P = 0.53) but shorter PFS (mean difference [MD]:3.83 months [1.81–5.84]) than those with CTP-A cirrhosis. Compared to patients receiving TKIs, those receiving atezo-bev had longer PFS (MD: 2.27 months [0.94–3.5]) and higher ORR (RECIST: OR, 1.44 [1.01–2.04] and mRECIST: OR, 1.33 [1.01–1.75]). Compared to first-line therapy, later-line therapy had lower ORR (RECIST: OR, 1.82 [1.3–2.53]; P < 0.001 and mRECIST: OR, 2.02 [1.34–3.05]) but comparable PFS (MD: 0.58 months [−0.18 to 1.35]) among nine studies. The incidence of grade ≥3 adverse events among patients with CTP-A and CTP-B cirrhosis was comparable (OR, 0.89 [0.45–1.74]) as it was for patients receiving atezo-bev and TKIs (OR, 0.86 [0.61–1.2]).
Interpretation
Our findings suggest that atezo-bev is safe and effective as first-line systemic therapy for patients with uHCC and CTP-A or CTP-B cirrhosis.
Funding
An unsolicited grant from ROCHE Products India Pvt Ltd. was received for publication.
Keywords: Immunotherapy, Liver cancer, Radiological response, Adverse events
Research in context.
Evidence before this study
Atezolizumab-bevacizumab (atezo-bev) is the preferred therapy for patients with unresectable HCC (uHCC) and Child-Turcotte-Pugh (CTP) class A cirrhosis based on level I data showing increased efficacy versus sorafenib. There are limited data evaluating the effectiveness of atezo-bev in extended patient populations, including those with CTP class B or in the second-line setting. Therefore, we performed a meta-analysis of patients with uHCC treated with atezo-bev. We used the following search terms: MeSH terms of “carcinoma, hepatocellular,” “drug therapy, combination,” and “antibodies, monoclonal” for PubMed; keywords of “monoclonal antibody” and “liver cell carcinoma” for Embase; and “atezolizumab and bevacizumab” and “hepatocellular and carcinoma” for Scopus to identify relevant articles published between 1 May, 2020 and 5 October, 2022. Only a few systematic reviews were found, including 400–500 patients treated with atezo-bev.
Added value of this study
Our systematic review identified 47 articles, including 5400 patients with uHCC treated with atezo-bev. Cumulative progression-free survival (PFS) and overall survival were 6.86 months (95% confidence interval [CI], 6.3–17.41) and 13.8 months (95% CI, 11.81–15.8), respectively. Although PFS with atezo-bev was better in CTP-A than in CTP-B, objective response rate (ORR) and incidence of adverse events were comparable. Atezo-bev yielded better ORRs in the first-line setting than in the second-line setting among nine studies. Our findings suggested that patients treated with atezo-bev in clinical practice had higher ORR and lower mortality than those treated with TKIs, although this difference was mitigated when compared to lenvatinib.
Implications of all the available evidence
Available data suggest that atezo-bev is a safe and effective therapy among patients with CTP-A or B cirrhosis.
Introduction
The incidence and mortality of hepatocellular carcinoma (HCC) has significantly increased worldwide in recent years.1,2 Over 90% of HCCs occur in patients with underlying chronic liver disease, and HCC is a major cause of morbidity and mortality in patients with cirrhosis.3 The poor prognosis of HCC has historically been related to a combination of late-stage presentation and ineffective therapies in those beyond an early stage. HCC treatment has evolved due to improved therapies, a better understanding of tumour biology, and increasing use of multidisciplinary management.4,5
Systemic therapy remains the treatment of choice for patients with unresectable HCC (uHCC). The tyrosine kinase inhibitors (TKIs), sorafenib and lenvatinib, provide a comparable overall survival (OS) of 10–13.5 months for patients with uHCC, although lenvatinib can achieve higher objective response rate (ORR) and progression-free survival (PFS) than sorafenib.6, 7, 8, 9, 10 However, neither of these drugs is effective in achieving a complete response (CR) on radiological evaluation. In 2020, the IMBrave150 trial demonstrated significantly improved PFS and overall survival (OS) with atezolizumab-bevacizumab (atezo-bev) compared to sorafenib.11 With median OS reaching 19.2 months, atezo-bev is now considered a preferred first-line therapy for patients with uHCC.12 However, the IMBrave 150 trial evaluated the efficacy of atezo-bev compared to sorafenib and its effectiveness compared to TKIs, including lenvatinib, in clinical practice is important to understand. Further, though approved as a front-line agent, the effectiveness of atezo-bev in the second-line immuno-oncological naïve setting after the failure of front-line TKIs is unknown. Like all other phase 3 studies, the IMbrave 150 trial only included patients with Child-Turcotte-Pugh (CTP) class A (CTP-A), whereas many patients in clinical practice have worse liver dysfunction at the time of HCC presentation. Understanding the safety of atezo-bev in this patient population is particularly important, given potential concerns about variceal bleeding.
Several real-world studies have examined outcomes in patients with uHCC receiving atezo-bev and provided insights into its effectiveness and safety in these extended populations. To date, only a few meta-analyses have reported the safety and effectiveness of atezo-bev, based on one to two studies.13,14 An updated meta-analysis is merited due to the rapid increase in the use of atezo-bev in real-world settings. Therefore, our systematic review and meta-analysis aimed to evaluate: a) ORR, cumulative PFS, and OS in patients treated with atezo-bev for uHCC; b) effectiveness and safety in patients with CTP-B cirrhosis and later line of therapy; c) effectiveness and safety of atezo-bev compared to TKIs in clinical practice.
Methods
Search strategy and selection criteria
We followed the latest Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines for data extraction and reporting.15 We conducted a computer-assisted search of PubMed, Embase, Google Scholar, and Scopus to identify relevant articles published between 1 May, 2020 and 5 October, 2022; the last date of access was January 31, 2023. We used the following search terms: MeSH terms of “carcinoma, hepatocellular” “drug therapy, combination,” and “antibodies, monoclonal” for PubMed; keywords of “monoclonal antibody” and “liver cell carcinoma” for Embase; and “atezolizumab and bevacizumab” and “hepatocellular and carcinoma” for Scopus (Appendix I). Abstracts from the annual meetings of the American Association for the Study of Liver Disease, International Liver Cancer Association, European Association for the Study of Liver (EASL), Asian Pacific Association for the Study of Liver, and European Society for Medical Oncology (ESMO) were also searched for relevant data.
Studies were included if they reported clinical outcomes (safety or effectiveness) among adult patients with uHCC who received atezo-bev therapy, regardless of the CTP class and line of treatment. When comparing first-line vs. second-line, we included studies that reported the line of therapy in detail. If this was not reported and authors could not provide those details when contacted, studies were excluded from first-vs. second-line subgroup analysis. We included randomised controlled trials (RCTs) and prospective and retrospective cohort studies (observational studies). Exclusion criteria included studies that reported the use of other immuno-oncological agents and/or reported only treatment with TKIs. Case reports, case series, mechanistic and experimental studies, editorials, guidelines, correspondences, and book chapters were excluded. We excluded review articles that did not include original data. We also excluded studies with incomplete data, and those reporting the same database or secondary analyses of the same data. Titles containing retracted/erratum were excluded. For publications or updated analyses from the same authors, the corresponding author was contacted for the best study to be included in the final analysis. If the author did not respond, the latest peer-reviewed published data was included in the final analysis.
All the citations were first imported into Endnote ver. 20.4. One investigator (AVK) reviewed citations from the search strategy to generate a list of potentially relevant articles. If the applicability of a study could not be determined by title or abstract alone, the full text was reviewed. Ethical approval and consent forms were waived as this was a meta-analysis.
Data collection
For each study, we recorded the number of patients, duration of follow-up, line of systemic therapy (first-line or later-line), age, sex, aetiology of HCC, liver disease severity including CTP class, Barcelona Clinic Liver cancer stage (BCLC), albumin-bilirubin (ALBI) grade, and Eastern Cooperative Oncology Group (ECOG) performance status. We also retrieved data on the proportion of patients with extrahepatic spread (EHS), macrovascular invasion (MVI), and alpha-feto protein (AFP) levels ≥400 ng/mL.
We captured the best radiological response based on both the Response Evaluation Criteria In Solid Tumours (RECIST) and modified RECIST (mRECIST) criteria.16 This radiological response assessment depends on the radiologist and can be variable and may introduce heterogeneity. Both ORR and disease control rate (DCR) was recorded, and the numbers of patients with CR, partial response (PR), stable disease (SD), and progressive disease (PD) were also calculated. Data were extracted by two separate individuals (HVT and KK), which were cross-checked by a third investigator (AVK), and any discrepancy was corrected after discussion.
Definitions
The baseline characteristics at the time of initiation of therapy were included for analysis to assess the patient demographics. OS was defined as the time from administration of the first atezo-bev dose to death. PFS was the time from atezo-bev initiation to death or disease progression.
Our primary outcomes were cumulative PFS, OS, ORR, and adverse events (AEs) in patients with uHCC treated with atezo-bev. Subgroups of interest included CTP-A vs. CTP-B, first-line vs. second-line therapy, and viral and non-viral aetiology. A secondary outcome was the effectiveness (ORR, PFS, and OS) and safety of atezo-bev compared to TKIs. The radiological response was evaluated using the RECIST and mRECIST criteria, when possible, and the cumulative responses were compared.
Quality assessment
The New Castle Ottawa scale (NOS) and the Cochrane tool were used to assess the quality of observational (cohort) studies and RCTs.17,18 Cohort studies were evaluated based on three criteria: selection (maximum 4 stars), comparability (maximum 2 stars), and outcome (maximum 3 stars). Selection included representatives of the exposed (1 star), selection of nonexposed cohort (1 star), determination of exposure (1 star), and demonstration that the outcome of interest was not present at the beginning of the study (1 star). The evaluation of the outcome of interest (1 star) with sufficient duration (1 star) and adequate follow-up (1 star) was part of the outcome assessment according to NOS. Based on NOS, the studies were classified as high (8–9 stars), moderate (6–7 stars), and low quality (<6 stars). RCTs were classified as having a high, low, or unclear risk of bias based on selection bias (random sequence generation, allocation concealment), selective reporting, blinding (performance and detection bias), attrition bias (method to address incomplete data), and other biases based on the Cochrane risk of the bias assessment tool.18 The quality of the study was assessed for each outcome by two individuals separately (HVT and KK). In case of discrepancy, a final score was assigned after a discussion with a mediator (AVK).
Statistical analysis
The baseline data were assessed using SPSS v. 29 (IBM Corp, New York, USA). Meta-analysis was performed using comprehensive meta-analysis software (CMA ver 4.0, 2022, USA). For continuous data, the median OS and PFS were entered along with the sample size, and the cumulative survival was reported as a mean with a 95% confidence interval (CI). The median was considered as the mean given an assumption of normal distribution, and the standard deviation was calculated as per the formula: standard deviation = for sample size >100 and the denominator was changed to 4.128 for sample size <60. Otherwise, the denominator was calculated in excel using = tinv (1–0.95, n-1) and used for the SD calculation. This method was used for calculating the mean difference in PFS among CTP-A and B, and ALBI grades. If the authors reported only the mean (or median), then the mean and sample size, along with the individual P-values reported by the primary studies, were input to compare different groups. This method (mean difference [MD]) was used for comparing PFS between atezo-bev and TKIs, first- and second-line therapy, and OS among different CTP classes and ALBI grades. ORR and DCR derived from each study were entered as a percentage (event rate) along with the sample size, and the cumulative ORRs and DCRs are presented with 95% CI. To assess the cumulative radiological response (CR, PR, SD, and PD), the number of events in each group was entered along with the sample size assessed and reported as cumulative rate with 95% CI.
Meta-analysis was performed using random effects models, and the prediction interval (for certainty assessment) was reported using Forest plots. Heterogeneity was assessed using measures I2 and Q. P-values <0.1 were considered statistically significant. Given anticipated significant heterogeneity due to the inclusion of studies with different lines of therapy (i.e., first vs. second line), patient characteristics (e.g., Child-Pugh class), and clinical settings (e.g., geographic location), we performed several sub-group analyses where feasible. Finally, publication bias was quantitatively evaluated using Egger's regression intercept test and qualitatively by visual examination of the symmetry of the funnel graph.
Registration and protocol
The protocol was registered in Prospero (CRD42022364430), and the data were abstracted according to a priori protocol.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had access to the data and accept the responsibility to submit for publication after approving the final draft.
Results
Study and patient characteristics
Three of the 181 studies sought for retrieval were excluded as they could not be retrieved.19, 20, 21 Of the remaining 178 articles, 43 were found to be eligible and were included in the meta-analysis. Four abstracts (through manual search) were also included in the analysis. In total, 47 studies, including 5400 patients with HCC treated with atezo-bev, were included in the analysis11,22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67 (Fig. 1:Flow chart) (Table 1 and Supplementary Table S1). The median duration of follow-up was 8.2 months.
Fig. 1.
PRISMA chart.
Table 1.
Characteristics of the included studies.
| No. | Name. Country. Year(ref.) | Total N | Age (years) | Males/Females (n) | Etiology (n) | Child class (n) | BCLC (n) | Albi (n) | MVI (n) | EHM (n) | AFP > 400 ng/mL (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ando et al. Japan. 2021(21) | 40 | 69 (47–90) | 30/10 | Viral-26 others-14 | A-40 | B-18 C-22 |
1/2a-23 2b-17 3-0 |
12 | 16 | |
| 2 | Awiwi et al. USA. 2022(22) | 55 | 66 (36–80) | 45/10 | A-30 B-25 |
5 | 18 | 27 | |||
| 3 | Casadei-Gardini et al. Italy, Germany, Portugal, Japan and the Republic of Korea. 2022(23) | 864 | 72 (65–79) | 682/182 | HCV-299 HBV-184 NASH-126 |
A-778 B-86 |
188 | 314 | |||
| 4 | Charonpongsuntorn et al. Thailand. 2022(24) | 30 | – | ||||||||
| 5 | Chen et al. Taiwan. 2022(25) | 41 | 65 (25–84) | 38/3 | HBV-30 HCV-8 Others-4 |
A-28 B-6 C-7 |
– | 1-7 2a-11 2b-19 3-4 |
30 | 30 | 18 |
| 6 | Cheng et al. China. 2022(26) | 13 | A-8 B-5 |
BCLC C-13 | |||||||
| 7 | Cheon et al. Korea, 2021(27) | 121 | 61 (36–83) | 101/20 | HBV-93 HCV-6 Nonviral-22 |
A-121 | B-25 C-96 |
1-65 2-56 | 45 | 85 | 46 |
| 8 | Chon et al. Korea. 2021(28) | 121 | 63 (57–71) | 100/21 | HBV-83 HCV-7 Nonviral-31 |
A-121 | B-20 C-101 |
1-67 2-54 3-0 |
50 | 68 | |
| 9 | Chuma et al. Japan. 2021(29) | 94 | 73 (37–87) | 73/21 | HBV-18 HCV-31 Others-45 |
A-82 B-13 |
A-1 B-45 C-48 |
1-31 2a-27 2b-33 3-3 |
22 | 32 | |
| 10 | Cosgrove et al. USA. 2022m(30) | 166 | 69 (32–88) | 126/40 | 2-92 3-15 |
49 | |||||
| 11 | D-Alessio et al. Asia/Europe/USA. 2022(31) | 202 | 69 (23–90) | 173/29 | HBV-35 HCV-72 Others-92 |
A-154 B-48 |
A-3 B-55 C-144 |
1-71 2-118 3-13 |
80 | 77 | 65 |
| 12 | De Castro et al. Germany/Austria. 2022(32) | 147 | 68.7 (30–96) | 125/22 | Alcohol-39 HBV-12 HCV-38 NASH-34 Others-24 |
A-106 B-35 C-6 |
A-1 B-23 C-116 D-7 |
1-51 2-83 3-13 |
48 | 65 | 52 |
| 13 | Eso et al. Japan. 2021(33) | 40 | 70.5 (53–82) | 35/5 | HBV-6 HCV-13 Others-21 |
A-38 B-2 |
B-21 C-19 |
1-16 2a-12 2b-12 |
|||
| 14 | Finn et al., Global. 2021(11) | 336 | 64 (56–71) | 277/59 | HBV-164 HCV-72 Others-100 |
A-335 B-1 |
A:8 B:52 C:276 |
129 | 212 | 126 | |
| 15 | Fulgenzi et al. Europe, Asia and USA. 2022(34) | 296 | 245/51 | HBV-120 HCV-75 Others-101 |
A-296 | B-92 C-204 |
1-161 2-133 3-2 |
104 | 169 | 102 | |
| 16 | Ha et al. Korea. 2022(35) | 125 | 63 | 103/22 | HBV-87 | A-113 B-12 |
|||||
| 17 | Ha and Kim et al. Korea. 2022(36) | 194 | 62.1 | 166/28 | HBV-128 Others-66 |
A-165 B-27 C-2 |
1-104 2-86 3-4 |
28 | 119 | ||
| 18 | Hayakawa et al. Japan. 2021(37) | 52 | 73 (24–89) | 42/10 | HBV-10 HCV-20 |
A-48 B-4 |
B-29 C-23 |
1-12 2a-13 2b-26 3-1 |
6 | 17 | 21 |
| 19 | Himmelsbach et al. Germany, Austria. 2022(38) | 66 | 65 (30–88) | 54/12 | HBV-9 HCV-14 Alcohol-25 NASH-18 |
A-35 B-23 C-5 |
A-1 B-22 C-35 D-8 |
1-14 2-46 3-6 |
29 | 18 | 19 |
| 20 | Iwamoto et al. Japan. 2021(39) | 51 | 71 (37–85) | 45/6 | HBV-7 HCV-19 others-25 |
A-47 B-4 |
B-24 C-27 |
1-11 2-39 3-1 |
– | – | |
| 21 | Kim et al. Korea. 2022(40) | 86 | 62 (56–71) | 70/16 | HBV-62 HCV-3 Alcohol-11 Others-10 |
A-82 B-4 |
B-18 C-68 |
– | 43 | 37 | |
| 22 | Komatsu et al. Japan. 2022(41) | 34 | 73 (45–82) | 25/9 | HBV-10 HCV-9 Others-15 |
A-32 B-2 |
B-15 C-19 |
Albi 1-11 Albi 2-23 |
3 | 13 | |
| 23 | Kulkarni et al. India. 2022(42) | 67 | 61 | 58/9 | HBV-13 HCV-11 NASH-37 Alcohol-5 Cyrptogenic-1 |
A-24 B-36 C-7 |
BCLC-B-6 C-50 D-11 |
ALBI 1-31 2-24 3-12 |
14 | 12 | 37 |
| 24 | Kuwano et al. Japan. 2022(43) | 24 | 72 (63.8–80.8) | 20/4 | A-18 B-6 |
A-1 B-11 C-12 |
6 | 5 | |||
| 25 | Kuzuya et al. Japan. 2021(44) | 50 | 73 (38–85) | 44/6 | HBV-11 HCV-9 Others-30 |
A-50 | B-19 C-31 |
1-16 2a-17 2b-17 |
7 | 24 | |
| 26a | Lee et al. Multicentre. 2020(45) | 104 | 62 (23–82) | 84/20 | HBV-51 HCV-31 Nonvira-22 |
A-98 B-6 |
B-10 C-94 |
55 | 74 | 37 | |
| 26b | Lee et al. Multicentre. 2020(45) | 60 | 60 (22–82) | 54/6 | HBV-34 HCV-11 Nonviral-15 |
A-60 | B-6 C-54 |
20 | 40 | 18 | |
| 27 | Maesaka et al. Japan. 2021(46) | 66 | 76 (49–93) | 50/16 | Viral-36 Nonviral-30 |
A-64 B-2 |
A/B-31 C-35 |
1/2a-35 2 b or 3-31 |
12 | 27 | |
| 28 | Matsumae et al. Japan. 2022(47) | 85 | 74 (65–80) | 66/19 | HBV-22 HCV-29 HBV + HCV-2 Alcohol-15 Others-17 |
A-81 B-4 |
A-6 B-31 C-48 |
15 | 38 | 43 | |
| 29 | Matsumoto et al. Japan. 2022(48) | 32 | 77 (54–91) | 19/13 | HBV-4 HCV-15 Alcohol-5 NAFLD-8 |
A-30 B-2 |
B-24 C-8 |
1-11 2a-7 2b-14 |
4 | 4 | |
| 30 | Niizeki et al. Japan. 2022(49) | 152 | 73 | 118/34 | HBV-22 HCV-61 Nonviral-69 |
A-4 B-81 C-67 |
1-58 2a-41 2b-53 |
31 | 43 | ||
| 31 | Ogawa et al. Japan. 2022(50) | 33 | 72 (48–89) | 26/7 | HBV-3 HCV-8 Alcohol-3 Others-19 |
A-33 | B-8 C-25 |
14 | 18 | ||
| 32 | Oura K et al. Japan. 2022(51) | 41 | |||||||||
| 33 | Shimose et al. Japan. 2022(52) | 130 | 72.5 (37–93) | 102/28 | HBV-19 HCV-60 Alcohol-30 NASH-21 |
B-69 C-61 |
1-40 2a-45 2b-45 |
20 | 46 | ||
| 34 | Sho et al. Japan. 2022(53) | 115 | 72 (31–89) | 95/20 | HBV-35 HCV-21 Non-viral-59 |
A-106 B-9 |
B-35 C-80 |
1-38 2-77 |
23 | 46 | 40 |
| 35 | Sinner et al. Germany and Austria. 2022(54) | 50 | 65 (50–80) | 41/9 | HCV-11 HBV-6 Alcohol-13 NAFLD-12/others-10 |
A-30 B-9 C-2 |
B-7 C-23 D-11 |
1-19 2-28 3-3 |
17 | 27 | 32 |
| 36 | Su et al. Taiwan. 2022(55) | 46 | 61.2 | 38/8 | Virus-41 Nonviral-5 |
A-40 B-6 |
B-14 C-32 |
1-20 2-23 |
24 | 15 | 28 |
| 37 | Takada et al. Japan. 2022 (56) | 27 | 72 (44–88) | B-14 C-13 |
|||||||
| 38 | Tamaki et al. Japan. 2022(57) | 91 | 74 (68–79) | 65/26 | HBV-12 HCV-36 Alcohol-16 NAFLD-8 Others-19 |
A-77 B-13 C-1 |
A-1 B-40 C-50 |
41 | |||
| 39 | Tanaka et al. Japan.2022(58) | 457 | 74 (68–79) | 368/89 | HCV-156 HBV-79 HCV and HBV-1 Alcohol-82 Other-139 |
A-427 B-30 |
0–7 A-21 B-172 C-257 |
1-162 2-292 3-3 |
85 | 154 | 125 |
| 40 | Teng et al., Taiwan2022(59) | 89 | 61.3 (56.4–67.8) | 75/14 | HBV-69 HCV-10 Non-viral-10 |
A-76 B-13 |
B-23 C-66 |
1-34 2-50 3-1 |
45 | 35 | 43 |
| 41 | Tokunaga et al. Japan. 2022(60) | 95 | A-80 B-15 |
A-3 B-43 C-49 |
1-29 2a-26 2b-39 3-1 |
12 | 38 | 27 | |||
| 42 | Tomonari et al. Japan. 2022(61) | 71 | 71 [66–79] | 58/13 | HBV-8 HCV-30 Non0viral-33 |
A-65 B-6 |
A-4 B-24 C-43 |
1-18 2a-24 2b-27 3–2 |
15 | 24 | |
| 43 | Uchikawa et al. Japan. 2022(62) | 30 | 69 (66–73) | 25/5 | HBV-4 HCV-11 Nonviral-15 |
A-23 B-7 |
1–16 2a-4 2b-8 3-2 |
11 | 12 | ||
| 44 | Vithayathil et al. Austria, Germany, Japan, Taiwan, UK, Italy, USA. 2022(63) | 191 | 68.4 (61.8–75.2) | 161/30 | NAFLD-25 Alcohol-73 HBV-37 HCV-72 Other-12 |
A-147 B-44 |
A-7 B-68 C-113 |
1-67 2-106 3-18 |
78 | 72 | 65 |
| 45 | Wang et al. Taiwan. 2022(64) | 48 | 62 (31–80) | 38/10 | HBV-28 HCV-13 Others-7 |
A-42 B-6 |
C-48 | 1-23 2-25 |
26 | 26 | |
| 46 | Xin et al. China. 2022(65) | 52 | 55.9 | 46/6 | HBV-47 Others-5 |
A-52 | C-52 | 1-43 2-9 |
37 | 26 | 30 |
| 47 | Yamada et al. Japan. 2022(66) | 20 | 76 (n = 8) 75 (n = 12) |
17/3 | HBV-8 HCV-7 Non-viral-5 |
A-15 B-5 |
B-10 C-10 |
3 | 7 | 8 |
BCLC, Barcelona Clinic Liver Cancer; ALBi, Albumin-Bilirubin; MVI, Macrovascular invasion; EHM, Extrahepatic metastasis; AFP, Alpha fetoprotein; HBV- HCV-Hepatitis C virus; Hepatitis B Virus; NASH, Non Alcoholic Steatohepatitis; NAFLD-Non-Alcoholic Fatty liver disease.
Of the 5400 included patients, 4473 patients received atezo-bev as first-line systemic therapy, whereas 900 received atezo-bev as second-line (or later-line) therapy, while the line of therapy was unclear among 27 patients. Twelve articles compared the outcomes of first vs. later-line therapies among 604 patients and 521 patients, respectively. Of them five studies provided the details of prior therapies: 124 had received lenvatinib, 60 had received sorafenib, 29 had received regorafenib, 10 had received ramucirumab, and 13 patients had received more than one prior therapy.22,38,54,60,62 Dosing of the drug was uniform in all studies: atezolizumab 1200 mg and bevacizumab 15 mg/kg, except in the four studies (three from Taiwan and one from Japan). Kuwano et al., Teng et al., and Wang et al., reported the administration of bevacizumab at a dose of 5–7.5 mg/kg, whereas Su et al. reported a fixed dose of 500 mg of bevacizumab administration for all patients.44,56,60,65 Approximately 63% (1212/1921) of patients among 17 studies reported some form of prior locoregional therapy (LRT), and 26% (423/1639) of patients among 14 studies reported surgical intervention before systemic therapy. Two studies reported prior LRT and surgery (combined) in 78.3% (137/175) of the patients.36,55 All these studies reported the use of systemic therapy following locoregional therapy or surgery except for the study by Cheng et al., Su et al., and Xin et al., which reported the use of atezo-bev concurrently with locoregional therapy (n = 79).27,56,66
Among the 42 studies that reported the age and sex distribution, the median age was 69 years, and men comprised 81.5% (4233/5194). Of 39 studies (n = 4949) that reported HCC aetiology, a viral cause was reported among 60% (2966/4949) of patients, whereas 40% (2013/4949) patients had a non-viral aetiology (of which 30 had dual aetiology).55,64 Among the reported viral causes, hepatitis B virus (HBV) infection accounted for 56% (1638/2925), hepatitis C virus infection (HCV) for 44% (1287/2925), and HBV + HCV coinfection 0.2% (7/2925). The distribution of the CTP class among 4854 patients and 41 studies were CTP-A: 88.5% (4295/4854), CTP-B: 10.7% (516/4854), and CTP-C: 0.6% (30/4854). Himmelsbach et al. could not assess four patients, and nine noncirrhotic patients in the study by Sinner et al. were excluded from the CTP classification.39,55
ECOG performance status was reported in 27 studies, including 3991 patients. A majority of treated patients had good performance status (63.2% [2525/3991] ECOG 0 and 27.31% [1090/3991] ECOG 1). Only 9.4% were ECOG 2 (375/3991) and <0.5% ECOG 3 (1/3991).
BCLC staging was reported in 38 studies, including 3854 patients. Most patients were in BCLC-C stage (65.7%; 2532/3854), with 31.3% (1205/3854) of patients having BCLC-B stage, 2% (61/3854) in BCLC-A stage, and 1% (37/3854) BCLC-D stage at the time of atezo-bev initiation. Only seven patients with BCLC-0 received atezo-bev therapy in the study by Tanaka et al.59 In the study by Sinner et al., nine patients without cirrhosis and three patients in the Vithayathil et al. study did not have complete data to report the BCLC class.55,64
In the 38 studies that reported MVI for 4816 patients, 29% (1396/4816) had MVI. In the 39 studies that reported EHS for 4907 patients, 44% (2144/4907) had EHS. Twenty-two studies reported that 36% (1048/2913) of patients had AFP levels ≥400 ng/mL.
Six studies compared the effectiveness of atezo-bev with TKIs (n = 1918; sorafenib-165; lenvatinib-1753).11,24,41,47,50,56 (Supplementary Table S2) The median age in the TKI group was 70.8 years, and 79.8% (1532/1918) were men. Among four studies that reported prior LRT, 75% (n = 1276/1700) had received some form of LRT, and among two studies, 29% (424/1467) had undergone surgical intervention before receiving TKIs. The reported aetiology was viral in 59.4% (1139/1918) of patients. Of the four studies that reported viral aetiology details among 1064 patients, HBV and HCV infections accounted for 45% (479/1064) and 55% (585/1064), respectively.11,24,41,50 Except for the study by Niizeki et al., the remaining studies reported CTP class and ECOG performance status among 1766 patients. Approximately 91% (1606/1766) were patients with CTP-A cirrhosis, and only 9% (160/1766) had CTP-B cirrhosis. The majority of patients had good performance status (71.2% [1345/1766] ECOG 0 and 23.8% [421/1766] ECOG 1–2). The study by Casadei-Gardini et al. did not report BCLC staging. Most patients were in BCLC-C stage (67.7%; 389/575), whereas 30% were in BCLC-B stage (174/575) and 2% (12/575) BCLC-A stage. Among the six studies, 26% (500/1918) patients had MVI, and 39.6% (759/1918) had EHS. Two studies reported that 34.2% (72/211) of patients had AFP levels ≥400 ng/mL.
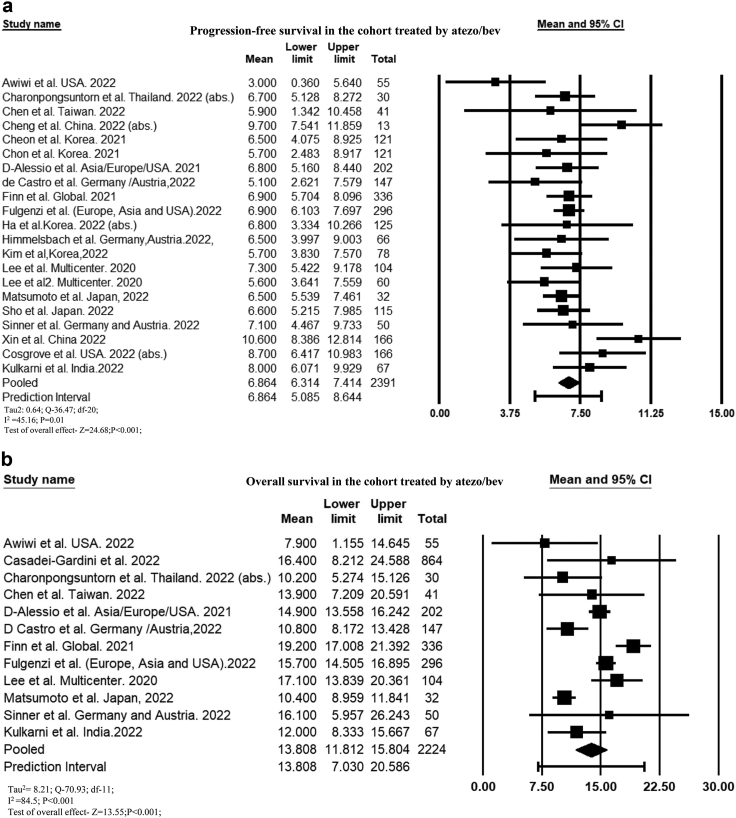
Progression-free survival
The cumulative PFS among the 21 studies that reported PFS among 2391 patients was 6.86 months (95% CI, 6.31–7.41; I2 = 45.2) (Fig. 2a). PFS was 7.1 months (95% CI, 6.52–7.64; I2 = 37.8) among 2024 patients (17 studies) who received atezo-bev as first-line therapy. The mean PFS was significantly longer in patients with CTP-A cirrhosis than in those with CTP-B cirrhosis (MD: 3.83 months [95% CI, 1.81–5.84]; P < 0.001; I2 = 0.0)32,43,55,59 (Supplementary Figure S1a). Similarly, pooled PFS was longer in patients with ALBI grade 1 than ALBI grade 2 (MD: 3.07 months [95% CI, 0.44–5.7]; P = 0.02; I2 = 76.1) and 3 (MD: 4.13 months [95% CI, 0.16–8.1]; P = 0.04; I2 = 0.0).23,35,43,54,55,59,62 (Supplementary Figure S1b). PFS was longer in patients receiving atezo-bev than those receiving TKIs (MD: 2.27 months [95% CI, 0.94–3.5]; P = 0.001; I2 = 72.1). When stratified by type of TKIs, PFS was significantly longer compared to sorafenib (MD: 2.6 months [95% CI, 1.06–4.13]; P = 0.001) but did not significantly differ compared to lenvatinib (MD: 1.4 months [95% CI, −0.9 to 3.7]; P = 0.23) (Supplementary Figure S1c). PFS was comparable among those who received atezo-bev as first-vs. second-line therapy (MD: 0.58 months [95% CI, −0.18 to 1.35]; P = 0.13; I2 = 21.7) (Supplementary Figure S1d).
Fig. 2.
Forest plot of (a) cumulative progression-free survival and (b) cumulative overall survival of patients treated with atezo-bev.
Six studies reported PFS estimates stratified by liver disease aetiology.33,39,46,54,62,65 D'Castro et al. reported PFS of 5.1 months among patients with viral aetiology, compared to 7 and 6.2 months for those with alcohol-related liver disease and NASH, respectively. Himmelschbach et al. reported PFS of 17.3 months and 6.1 months among viral and non-viral aetiology, while Lee et al. reported a PFS estimates of 4.5, 6.6, and 6.3 months for HBV, HCV and non-viral aetiologies, respectively. Sho et al. reported PFS of 6.2 months and 6.9 months among patients with viral and non-viral aetiology. While Tomonari et al. reported PFS of 9.8 and 3 months among viral and non-viral aetiology patients. Lastly, Wang et al. reported PFS of 5 months in HBV and 5.1 in HCV patients, while the PFS was 3.3 months and 5 months among non-HBV and non-HCV patients. Cumulatively, the pooled PFS in the viral group (n = 265) was 7.44 months (95% CI, 3.83–11.1) and 5.5 (95% CI, 4.2–6.8) in the non-viral group (n = 277) (P = 0.24).
Overall survival
Among 12 studies (n = 2224 patients) that reported the OS, the pooled mean OS was 13.8 months (95% CI, 11.81–15.8; I2 = 84.5) (Fig. 2b). The mean OS increased to 14.5 months (95% CI, 12.3–16.7; I2 = 86.7) among 1972 patients who received atezo-bev as first-line therapy (nine studies). The mortality rate was 32.1% (95% CI, 27–37.7; 910/2684; I2 = 86) among 17 reported studies (Supplementary Figure S2a). Survival in patients with CTP-A cirrhosis was longer than that in patients with CTP-B (MD: 7.19 [95% CI, 4.85–9.53]; P < 0.001; I2 = 0.0) and CTP-C cirrhosis (MD: 10.1 months [95% CI, 6.32–13.85]; P < 0.001; I2 = 0.0).26,32,33,43,55,59 (Supplementary Figure S2b). Pooled mean OS was 9.1 months (95% CI, 5.97–13.82; P < 0.001; I2 = 27.9) longer in ALBI grade 1 than ALBI grade 2 and 13.5 months (95% CI, 7.68–19.32); P < 0.001; I2 = 0) longer in ALBI grade 1 than ALBI grade 3.23,26,33,43 (Supplementary Figure S2c). Mortality was lower in patients treated with atezo-bev than with TKIs (OR, 0.67 [95% CI, 0.58–0.78]; P < 0.001; I2 = 0)11,24,41,47,56 (Supplementary Figure S2d). On stratifying based on the type of TKIs, mortality was significantly lower in atezo-bev group than lenvatinib (OR, 0.66 [95% CI, 0.56–0.77]; P < 0.001) but comparable with sorafenib (OR, 0.75 [95% CI, 0.51–1.1]; P = 0.14). The mean OS was similar among patients who received atezo-bev as first- or second-line therapy (MD: 3.52 months [95% CI, −1.42 to 8.46]; P = 0.16; I2 = 44.2).23,33
Two studies reported OS, stratified by viral vs. non-viral liver disease aetiology.33,39 D'Castro, reported higher OS in patients with non-viral (n = 97) liver disease (11.5 months vs. 8.6 months among 50 viral liver disease patients), whereas Himmelsbach et al. reported that median OS was not achieved among patients with viral aetiology (n = 23) compared to 11.8 months among those with non-viral liver disease (n = 43).
Radiological response
All studies reported performing computed tomography or magnetic resonance imaging at 6–12 weekly intervals. The cumulative ORR and DCR as per RECIST criteria were 26.7% (95% CI, 24.6–29.1; I2 = 42.67) and 75.3% (95% CI, 73.1–77.4; I2 = 41.41) among 33 studies (n = 3134 patients). Cumulative ORR and DCR as per mRECIST criteria were 34% (95% CI, 30.3–37.8; I2 = 60.3) and 73.6% (95% CI, 68.8–78; I2 = 75.7) in 25 studies (n = 1774 patients). ORR and DCR as per RECIST criteria were 29% (95% CI, 26.3–32; I2 = 27.7) and 77% (95% CI, 63.6–86.4; I2 = 0.0) among 1600 patients (14 studies) who received atezo-bev as first-line therapy only. ORR and DCR as per mRECIST criteria were 40.1% (95% CI, 35.5–46.3; I2 = 61.1) and 73.4% (95% CI, 64.3–81; I2 = 85.6) among 971 patients (10 studies) who received atezo-bev as first-line therapy. Cumulative CR, PR, SD, and PD in 3121 patients from 32 studies assessed according to the RECIST criteria was 2.8% (95% CI, 1.9–4; I2 = 54.4), 24.3% (95% CI, 22.2–26.6; I2 = 42.7), 49.3% (95% CI, 46.4–52.2; I2 = 56.2), and 24.8% (95% CI, 22.6–27; I2 = 42.7), respectively. Cumulative CR, PR, SD, and PD rates for 1774 patients from 25 studies were 8% (95% CI, 6.1–10.2; I2 = 42.1), 27.4% (95% CI, 24.4–30.6; I2 = 47.3), 41% (95% CI, 36.4–45.5; I2 = 70.0), and 25.2% (95% CI, 22–29; I2 = 60.6), respectively, using mRECIST criteria (Table 2).
Table 2.
Cumulative radiological response among patients treated with atezolizumab-bevacizumab.
| Response | RECIST | mRECIST | ||
|---|---|---|---|---|
| ORR | 844/3134 | 26.7% (24.6–29.1; I2 = 42.67) | 616/1774 | 34% (30.3–37.8; I2 = 60.25) |
| DCR | 2382/3134 | 75.3% (73.1–77.4; I2 = 41.41) | 1330/1774 | 73.6% (68.8–78; I2 = 75.66) |
| CR | 86/3121 | 2.8% (1.9–4; I2 = 54.38) | 135/1774 | 8% (6.1–10.2; I2 = 42.08) |
| PR | 755/3121 | 24.3% (22.2–26.6; I2 = 42.71) | 480/1774 | 27.4% (24.4–30.6; I2 = 47.25) |
| SD | 1538/3121 | 49.3% (46.4–52.2; I2 = 56.14) | 733/1774 | 41% (36.4–45.5; I2 = 70) |
| PD | 750/3121 | 24.8% (22.6–27; I2 = 42.73) | 426/1774 | 25.2% (22–29; I2 = 60.64) |
RECIST, Response Criteria evaluation in solid tumour; mRECIST, modified RECIST; ORR-Objective response rate; DCR, Disease Control Rate; CR, Complete Response; PR, Partial response; SD, Stable Disease; PD, Progressive Disease.
The proportion of patients who achieved ORR was lower with the RECIST than with the mRECIST criteria (OR, 0.68 [95% CI, 0.57–0.81]; P < 0.001) (Fig. 3a), whereas the proportion of patients who achieved DCR was comparable between patients evaluated by the RECIST and mRECIST criteria among the 15 studies reporting ORR/DCR including 1275 and 1263 patients (OR, 0.96 [95% CI, 0.8–1.16]; P = 0.72), respectively (Fig. 3b). The proportion of patients achieving CR and PR was lower, and SD was higher with the RECIST criteria than with the mRECIST criteria (Supplementary Figure S3a–c), although, both criteria labelled a similar proportion of patients receiving atezo-bev as having PD (Supplementary Figure S3d).
Fig. 3.
Forest plot comparing the (a) objective response rate and (b) disease control rate by RECIST and mRECIST criteria for patients treated with atezo-bev.
Radiological response based on the CTP score
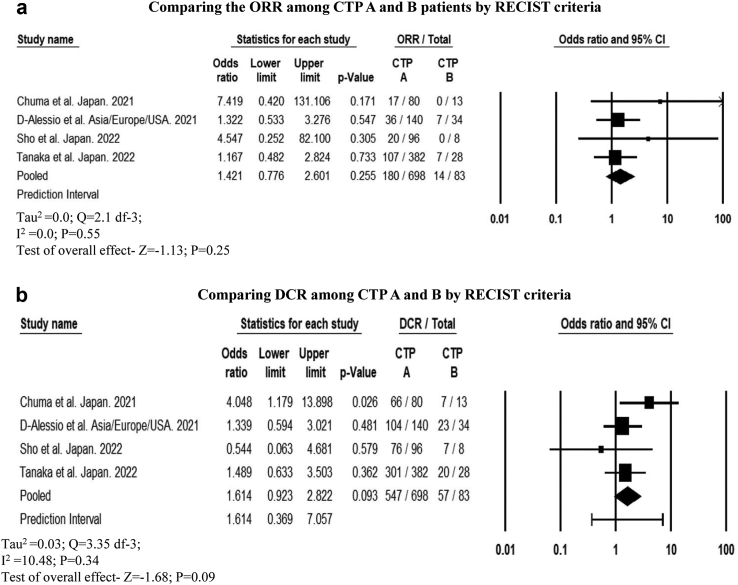
Among four studies that reported ORR and DCR among patients with CTP-A (n = 698) and CTP-B cirrhosis (n = 83), the cumulative response rate was comparable (ORR: OR, 1.42 [95% CI, 0.77–2.6]; P = 0.25 and DCR: OR, 1.61 [95% CI, 0.92–2.82]; P = 0.09) based on RECIST criteria.30,32,54,59 Similarly, ORR did not differ between patients with CTP-A (n = 247) and CTP-B cirrhosis (n = 57) as per mRECIST criteria (OR, 1.33 [95% CI, 0.52–3.39]; P = 0.53).30,40,43,54 However, DCR was significantly higher in patients with CTP-A than those with CTP-B cirrhosis (OR, 2.23 [95% CI, 1.06–4.67]; P = 0.03) (Fig. 4a–d). A higher number of patients with CTP-B cirrhosis had PD than those with CTP-A cirrhosis, based on both RECIST and mRECIST criteria (Table 3). ORR and DCR among patients with CTP-C cirrhosis was 7.1% (95% CI, 0.004–57.7; 0/6) as reported in one study.43
Fig. 4.
Forest plot comparing the (a) objective response rate and (b) disease control rate by RECIST criteria (c) objective response rate, and (d) disease control rate by mRECIST criteria among CTP A and B patients treated with atezo-bev.
Table 3.
Cumulative radiological response among patients treated with atezolizumab-bevacizumab among CTP A and B.
| RECIST |
P | mRECIST |
P | |||
|---|---|---|---|---|---|---|
| CTP A | CTP B | CTP A | CTP B | |||
| ORR | 25.9% (22.7–29.4; 180/698) | 16.8% (12–31.6; 14/83), | 0.07 | 33.6% (28–40.1; 83/247) | 30% (15.4–54.5; 17/57) | 0.58 |
| DCR | 78.4% (75–81.1; 547/698) | 68% (57–77.3; 57/83) | 0.04 | 81% (75.6–85.5; 201/247) | 67% (53.5–78; 38/57) | 0.01 |
| CR | 3.2% (17/522) | 0% (0/62) | 0.14 | 9.8% (7/71) | 13.9% (5/36) | 0.53 |
| PR | 24.1% (126/522) | 22.6% (14/62) | 0.78 | 28.2% (20/71) | 27.8% (10/36) | 0.96 |
| SD | 51.7% (311/602) | 46.7% (35/75) | 0.41 | 48.4% (73/151) | 34.7% (17/49) | 0.09 |
| PD | 21.4% (129/602) | 33.4% (25/75) | 0.02 | 15.2% (23/151) | 34.7% (17/49) | 0.003 |
RECIST, Response Criteria evaluation in solid tumour; mRECIST, modified RECIST; ORR-Objective response rate; DCR, Disease Control Rate; CR, Complete Response; PR, Partial response; SD, Stable Disease; PD, Progressive Disease.
Radiological response among patients receiving atezo-bev and TKIs
ORR was higher with atezo-bev than with TKIs according to the RECIST criteria (OR, 1.44 [95% CI, 1.01–2.04]; P = 0.04) and mRECIST criteria (OR, 1.33 [95% CI, 1.01–1.75]; P = 0.03)11,41,47,50,56 (Supplementary Figure S4a and b). However, ORR did not significantly differ between the atezo-bev and lenvatinib-treated patients (RECIST: OR, 1.02 [95% CI, 0.64–1.61]; P = 0.91 and mRECIST: OR, 1.006 [95% CI, 0.82–4.81]; P = 0.12).
DCR was similar among atezo-bev- and TKI-treated patients using RECIST evaluation (OR, 1.18 [95% CI, 0.83–1.66]; P = 0.35)11,41,47 (Supplementary Figure S4c). However, DCR using mRECIST evaluation was higher with atezo-bev than with TKIs (OR, 12 [95% CI, 7.54–19.11]; P < 0.001)11,41,47,50,56 (Supplementary Figure S4d).
A higher proportion of patients achieved CR, and a lower proportion had PD with atezo-bev therapy than with TKIs (sorafenib but not lenvatinib) using the mRECIST criteria (CR: OR, 2.08 [95% CI, 1.2–3.6]; P = 0.008; PD: 0.69 [95% CI, 0.49–0.99]; P = 0.04) but not using the RECIST criteria (CR: OR, 3.55 [95% CI, 0.97–12.91]; P = 0.05; PD: 0.84 [95% CI, 0.59–1.2]; P = 0.35). The proportion of patients who achieved PR and SD was similar between atezo-bev- and TKI-treated patients using both the RECIST and mRECIST criteria (Supplementary Figures S5a-d, S6a-d).
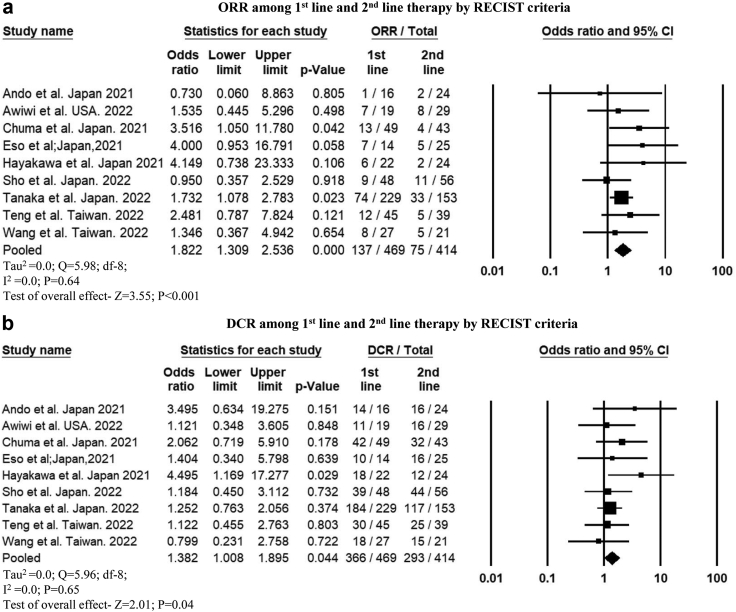
Radiological response among those who received atezo-bev as first-line vs. second-line therapy
In nine studies that reported, ORR (OR, 1.82 [95% CI 1.3–2.53]; P < 0.001) and DCR (OR, 1.38 [95% CI 1.008–1.89]; P = 0.04) were higher when atezo-bev was used as first-line therapy than as second-line therapy according to the RECIST criteria (Fig. 5a and b). Using the mRECIST criteria, ORR was higher when used as first-line therapy (OR, 2.02 [95% CI 1.34–3.05]; P = 0.001) (Supplementary Figure S7a). However, the DCR did not differ based on the line of therapy (OR, 1.5 [95% CI, 0.89–2.5]; P = 0.12) (Supplementary Figure S7b). A higher proportion of patients who received atezo-bev as first-line therapy achieved a CR and PR, whereas those who received it as second-line therapy had PD according to both the RECIST and the mRECIST criteria (Supplementary Table S3).
Fig. 5.
Forest plot comparing the (a) objective response rate and (b) disease control rate by RECIST criteria among patients who received atezo-bev as 1st line vs. 2nd line therapy.
Radiological response based on liver disease aetiology
Among four studies that report ORR using RECIST criteria stratified by liver disease aetiology, the response was comparable (OR, 1.01 [95% CI, 0.67–1.52]; P = 0.93).34,35,41,54 (Supplementary Figure S8) Two studies reported similar DCR using RECIST criteria among viral (n = 54/70) and non-viral (n = 55/73) aetiology (OR, 1.1 [95% CI, 0.5–2.4]; P = 0.8) and another reported similar CR, PR, SD and PD by aetiology.34,54 One study reported comparable ORR (23.5% vs. 321%; P = 0.38) and DCR (72.6% vs. 81.1%: P = 0.35) using mRECIST criteria among viral and non-viral aetiology.54
Adverse events
The cumulative incidence of AEs (any grade) was 82.7% (95% CI, 77.8–86.7; 2619/3332; I2 = 89.3) among 23 reported studies (Supplementary Figure S9a). The cumulative incidence of grade ≥3 AEs among 29 studies (n = 3855 patients) was 31.8% (95% CI, 25.6–38.7; I2 = 93.6) (Supplementary Figure S9b). The proportion of patients who discontinued atezo-bev due to adverse events in 25 studies (n = 2651 patients) was 13.8% (10–18.7; I2 = 88.7) (Supplementary Figure S10). Common AEs included hypertension, proteinuria, and fatigue (Table 4).
Table 4.
Cumulative adverse events among patients treated with atezolizumab-bevacizumab.
| Adverse events | n/N | Incidence |
|---|---|---|
| Hypertension | ||
| Cumulative | 1082/4073 | 27% (23.3–31.2); I2 = 84.42 |
| Grade ≥3 | 244/3804 | 6% (4.7–7.8); I2 = 64.87 |
| Fatigue | ||
| Cumulative | 987/4060 | 25.1% (22.1–28.4); I2 = 76.71 |
| Grade ≥3 | 58/3753 | 2% (1.6–2.6); I2 = 0.0 |
| Anorexia | ||
| Cumulative | 623/3240 | 18.8% (15.8–22.2); I2 = 76.36 |
| Grade ≥3 | 46/2978 | 2% (1.4–2.4); I2 = 0 |
| Fever | ||
| Cumulative | 235/1434 | 16.3% (12.4–21); I2 = 75.22 |
| Grade ≥3 | 19/1344 | 1.8% (1.2–2.7); I2 = 0 |
| Proteinuria | ||
| Cumulative | 985/3822 | 25.2% (22.2–28.6); I2 = 75.55 |
| Grade ≥3 | 207/3445 | 6.3% (5.1–7.8); I2 = 42.84 |
| Diarrhea | ||
| Cumulative | 281/2791 | 9.3% (7.3–11.8); I2 = 69.57 |
| Grade ≥3 | 26/2759 | 1.3% (0.1–1.9); I2 = 0.0 |
| Gastrointestinal bleeding | 128/2255 | 5.6% (4–8); I2 = 70.87 |
| Variceal bleeding | 103/2062 | 4.7% (3.3–6.7); I2 = 63.68 |
| Hypothyroidism | ||
| Cumulative | 178/2591 | 7% (5.2–9.4); I2 = 64.1 |
| Grade ≥3 | 4/2284 | 0.5 (0.3–1); I2 = 0.0 |
| AST elevation | ||
| Cumulative | 407/1547 | 25% (18.1–33.2); I2 = 90 |
| Grade ≥3 | 83/1497 | 6.4% (5.1–8); I2 = 9.25 |
| Others (cumulative) | ||
| Thrombocytopenia | 228/1145 | 15.3% (8.4–26); I2 = 93.3 |
| Infusion reaction | 47/762 | 3.4% (1.4–7.7); I2 = 69.95 |
| HFSR | 37/1545 | 3.1% (1.5–6); I2 = 67.21 |
| iRAE | 181/1537 | 10.2% (6.9–14.8); I2 = 72.53 |
AST, aspartate transaminase; HFSR, Hand foot Skin Reaction; iRAE-immune related adverse events.
The cumulative incidence of variceal bleeding was 4.7% in 16 studies. However, only 10 studies reported details on screening for varices.11,28,29,32,33,37,39,41,43,60 Approximately 79% (1130/1429) of patients underwent a screening endoscopy prior to atezo-bev therapy. Of these, only 46% (521/1130) of the patients had varices. Furthermore, 54% (250/465) of patients had received treatment for varices either in the form of ligation and/or nonselective beta-blocker (NSBB) (Teng et al. and Cheon et al. did not report any details on treatment). The incidence of variceal bleeding was similar for patients with CTP-A (8.55%; 29/342) and CTP-B cirrhosis (9%; 10/111) (OR, 0.83 [0.2–3.41]; P = 0.8; I2 = 57.0).32,37,43 The incidence of variceal bleeding was significantly higher in patients with main portal vein thrombosis (PVT) (11.6%; 24/208) than in those without PVT (6.2%; 21/376) (OR, 2.05 [1.1–3.82]; P = 0.02; I2 = 0.0).28,32,37,43 The incidence of variceal bleeding was similar between those who had received prior prophylactic therapy (ligation and/or NSBB) (8.2%; 7/85) and those without prior therapy (4.1%; 10/241) (OR, 2.12 [0.63–7.17]; P = 0.22; I2 = 0.0).37,39,43
The cumulative incidence of immune-related AEs (iRAE) was 10.2%. Five studies reported types of iRAE.32,33,39,51,66 The most common iRAE was hepatitis in 10 patients, followed by hypothyroidism in nine, rheumatological and musculoskeletal injury in five, skin toxicity and diarrhoea in four each, hypopituitarism, cholangitis, and rush in two each. The remaining iRAEs (pulmonary and neurologic toxicity, colitis, nephritis, mucositis, hyperparathyroidism, and type 1 diabetes mellitus) were reported in one patient each.
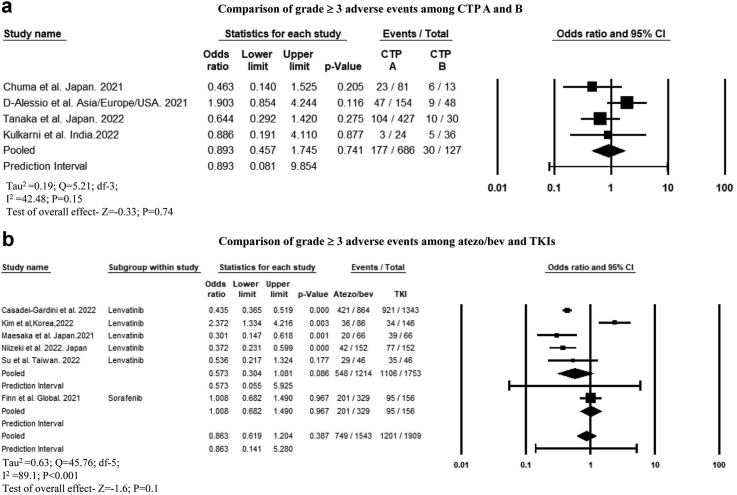
The incidence of grade ≥3 AEs among patients with CTP-A and CTP-B cirrhosis was comparable (OR, 0.89 [95% CI, 0.45–1.74]; P = 0.74; I2 = 42.5)30,32,59 (Fig. 6a). Patients treated with atezo-bev in the first-vs. second-line did not significantly differ regarding the cumulative incidence of AEs (76.2% vs. 41%; P = 0.46) and discontinuation rates (7.7% vs. 7.8; P = 0.98).38,40,54 One study reported that the incidence of grade 3 AEs was similar between those treated with atezo-bev as first-line vs. second-line therapy.38 Atezo-bev and TKIs had a similar cumulative incidence of any AEs (OR, 0.69 [95% CI, 0.14–3.5]; P = 0.53; I2 = 86.5) and grade ≥3 AEs (OR, 0.86 [95% CI, 0.61–1.2]; P = 0.38; I2 = 89.1)11,24,41,47,50,56 (Fig. 6b). The incidence of grade 3 hypertension (OR, 1.01 [95% CI, 0.64–1.6]; P = 0.95; I2 = 69.7) and gastrointestinal bleeding (OR, 1.55 [95% CI, 0.64–3.75]; P = 0.32; I2 = 22.5) were similar between patients treated with atezo-bev and TKIs (Supplementary Figure S11a and b), whereas the incidence of anorexia, fatigue, diarrhoea, proteinuria, and hand-foot skin reaction were significantly higher among patients treated with TKIs (Supplementary Figure S12a-e).
Fig. 6.
Forest plot comparing the grade ≥3 adverse events among (a) CTP A and B patients treated with atezo-bev. (b) Among atezo-bev and tyrosine kinase inhibitors.
Study quality and publication bias
Overall, there were 30 multicentre and 17 single-centre studies. Forty were retrospective, whereas six were prospective studies. The study by Finn et al. was the only RCT and had a low risk of bias, except for blinding. Six studies scored 8–9 (high quality), 25 studies scored 6–7 (moderate quality), and 15 studies scored <6 (low quality) on NOS grading (Supplementary Table S4).
We included studies from several countries with different patient characteristics that were not uniformly distributed. Therefore, the heterogeneity was high for studies reporting OS but not for PFS. However, to overcome the heterogeneity, prediction interval has been provided in the Forest plot to ascertain the measured effect. There was no heterogeneity in the studies reporting OS, PFS, ORR, DCR, or incidence of grade 3 AEs in studies comparing CTP-A and CTP-B cirrhosis. Similarly, there was no heterogeneity in studies that compared PFS and radiological response for patients receiving first- and second-line atezo-bev treatment. However, there was significant heterogeneity in studies comparing PFS, ORR, and DCR, among patients receiving TKIs and atezo-bev but not in comparing mortality rate or individual grade 3 AEs. Sensitivity analysis excluding studies that reported different dosing of atezo-bev and concomitant combination of locoregional therapy and atezo-bev did not alter the cumulative results (data not shown). Similarly, when we included only high-quality studies, the results were unaffected. Funnel plot symmetry and Egger's test assessment revealed no publication bias in most outcomes (Supplementary Figures S13–S19).
Discussion
In this meta-analysis, including 5400 patients, we found that atezo-bev yielded an ORR of 26–34%, pooled PFS of 6.86 months, and OS of 13.8 months in patients with uHCC. Notably, patients with CTP-A cirrhosis had comparable ORR but significantly longer PFS and OS than patients with CTP-B cirrhosis. We also found significantly higher ORR but similar PFS and OS among those receiving atezo-bev in the first-line setting versus second-line setting (Table 5).
Table 5.
Major results of the meta-analysis.
| Outcome | Cumulative | CTP A vs. B | vs. TKIs | 1st line vs. 2nd line |
|---|---|---|---|---|
| PFS | 6.86 m (6.31–7.41) | MD: 3.83 m (1.81–5.84)∗∗ | MD: 2.27 m (0.94–3.5)∗∗ | MD: 0.58 m (−0.18 to 1.35) |
| OS | 13.8 months (11.81–15.8) | MD: 7.19 (4.85–9.53)∗∗ | OR, 0.67 (0.58–0.78)∗∗ | MD:3.52 m (−1.42 to 8.46) |
| ORR | ||||
| RECIST | 26.7% (24.6–29.1) | OR, 1.42 (0.77–2.6) | OR, 1.44 (1.01–2.04)∗ | OR, 1.82 (1.3–2.53)∗∗ |
| mRECIST | 34% (30.3–37.8) | OR, 1.33 (0.52–3.39) | OR, 1.33 (1.01–1.75)∗ | OR, 2.02 (1.34–3.05)∗∗ |
| DCR | ||||
| RECIST | 75.3% (73.1–77.4) | OR, 1.61 (0.92–2.82) | OR, 1.18 [0.83–1.66] | OR,1.38 (1.008–1.89)∗ |
| mRECIST | 73.6% (68.8–78) | OR, 2.23 (1.06–4.67)∗ | OR, 12 (7.54–19.11)∗∗ | OR, 1.5 (0.89–2.5) |
| Grade 3 adverse events | 31.8% (25.6–38.7) | OR,0.89 (0.45–1.74) | OR, 0.86 (0.61–1.2) | – |
PFS, progression-free survival; OS, overall survival; m, months; MD, mean difference; OR, odds ratio; RECIST, Response Evaluation Criteria in Solid Tumours; mRECIST, modified RECIS; TKIs, Tyrosine Kinase inhibitors.
∗P < 0.05; ∗∗P < 0.001.
Most patients in the real world are not diagnosed in the early stage of HCC, and the safety and efficacy in advanced liver disease should be ascertained. The cumulative OS reported in our meta-analysis is much lower than the updated IMBrave analysis. These lower estimates is related to use in extended populations such as those with CTP-B cirrhosis, worse performance status, or in the second-line setting. Survival was better in patients with CTP-A than in those with CTP-B cirrhosis among those treated with atezo-bev. However, the radiological response and AE rates were similar. Therefore, atezo-bev can still be considered a therapeutic option for patients with CTP-B in real-world settings. A recent meta-analysis by Hajra et al. reported limited evidence to support the use of immunotherapy in patients with significant liver dysfunction.68 However, the review included various immune-oncological agents, and only three studies reported the use of atezo-bev in patients with advanced liver disease. Further studies, including more granular data on CTP-B (Child score 6/7 vs. 8 vs. 9) are required as this class includes a heterogeneous population.
One major obstacle to the use of TKIs is the lack of CR achievable by these oral drugs and there limited effects on OS. According to the current data, atezo-bev performs significantly better in achieving ORR without any added disadvantage of AEs. The incidence of hypertension and gastrointestinal bleeding is similar among patients treated with atezo-bev and those treated with TKIs. PFS and OS are similar among those who received atezo-bev as first-line and second-line therapy. However, ORR is significantly better in patients who receive atezo-bev as first-line therapy than as second-line therapy. The incidence of AEs was similar whether the drug was administered early (first-line) or late (second-line). Therefore, atezo-bev should be used as a first-line therapy for uHCC, although its use as a second-line therapy should not be discouraged.55,61,69
Bevacizumab, which is a vascular endothelial growth factor (VEGF) inhibitor, is associated with bleeding events including variceal bleeding. The feared complication of variceal bleeding was observed in approximately 3–5% of patients, which was much lower than expected and comparable with the corresponding proportion observed among patients treated with TKIs. The incidence of variceal bleeding was similar between patients with CTP-A and CTP-B cirrhosis and was independent of prophylactic treatment. However, patients with PVT had a higher risk of variceal bleeding. Therefore, patients with PVT should be carefully monitored for variceal bleeding as they are prone to more post-banding ulcer-related bleeding and may not benefit from prophylactic variceal ligation.
The EASL and ESMO guidelines recommend the mRECIST criteria for response evaluation for systemic therapy.70,71 We found that a lower proportion of patients were labelled as achieving ORR and CR with the RECIST than mRECIST criteria, which is consistent with prior studies, whereas both could identify PD in a similar proportion of patients. This is expected given that the former includes total tumour burden, whereas the latter simply includes the enhancing, viable component. Due to ease of assessment, the mRECIST criteria is a better tool than the RECIST criteria in clinical practice. Further studies should explore the use of iRECIST criteria for patients receiving atezo-bev.72
Further real-world data are needed to compare atezo-bev with lenvatinib, given higher ORR and PFS compared to sorafenib.73 This is particularly noteworthy in light of prolonged survival observed with lenvatinib seen in LEAP-002. Several biomarkers have been used to predict the response to immuno-oncological agents.74,75 To date, only the baseline neutrophil-to-lymphocyte ratio, low C-reactive protein, and AFP in Immunotherapy (CRAFITY score: AFP <100 ng/mL or C-reactive protein <10 mg/L) score at baseline and decline in AFP levels at 3–6 weeks, and low serum VEGF at 3 weeks post-therapy, have been reported to predict the response to atezo-bev.28, 29, 30,34,36,58,60,65 Programmed cell death-ligand 1 (PD-L1) expression on tumours has also been variably reported to predict the response.76,77 Molecular correlates such as intratumoral high expression of CD274 and CD8+ T cell density is associated with improved outcomes while high regulatory T cell to effector T cell ratio and expression of oncofetal genes was associated with poorer response to atezo-bev.78 Future studies should aim to elucidate and validate simple biomarkers (at baseline) to predict the response to atezo-bev for better prognostication.
Initial studies suggested poorer response to immune-oncological agents in patients with NASH due to aberrant T-cell activation and impaired immune surveillance.11,79 In the current study, the radiological response rate and PFS were similar among viral and non-viral HCC, though this is not a purely NASH population. Results were discordant between the two studies, with data for OS stratified by liver disease aetiology. Overall, current results suggest the effectiveness of atezo-bev does not significantly differ by liver disease aetiology. A recent post-hoc analysis of IMBrave150 similarly suggested no difference in efficacy by liver disease etiology.80 These results may be related to the complementary enhancement of the immune microenvironment by bevacizumab, which may overcome impaired immune surveillance reported in patients with NASH.79,81
The meta-analysis had some limitations, with heterogeneity being the most important limitation. However, as we included several studies from real-world data from different countries, heterogeneity in terms of tumour characteristics, aetiology, liver cirrhosis severity, and assessment of tumour response was expected and may limit the generalisability of the findings. Secondly, 60% of the patients in our meta-analysis had viral aetiology, which may be argued as one of the reasons for the better response to atezo-bev than TKIs.79 The keywords differed for each search engine, which may introduce bias in study selection. Although the studies comparing atezo-bev vs. TKI were based on five retrospective studies with good NOS scoring and one RCT, further large prospective studies and RCTs are required to confirm these findings. Few of the sub-group analyses were based on <4 studies, and data on AEs were derived from retrospective studies, which affects the cumulative results reported here. Lastly, the efficacy of only atezo-bev therapy without any LRT could not be assessed as several studies included a combination of LRT with atezo-bev, which is the current practice to improve the survival of patients with advanced HCC.
In conclusion, atezo-bev is a highly effective and safe therapy for patients with uHCC. Atezo-bev may be more beneficial when used as first-line therapy than as second-line therapy for patients with uHCC and in patients with CTP-A and CTP-B cirrhosis; however, there does not appear to be a significant difference in effectiveness by liver disease aetiology. Continued research is needed to identify treatment response biomarkers, beyond these clinical features, to select patients who benefit most from atezo-bev.
Contributors
Conceptualization and designing of the study by PNR, AVK, and DNR; AVK, HVT, and KK retrieved the data; AVK performed the meta-analysis; Data was accessed and verified by AVK, HVT, and KK; initial drafting by AVK, HVT, KK, and MP; MM, AH, TH, TT, TK, SK, NRP, AV, AP, AS, and RF critically assessed and edited the manuscript. All members had access to the data in the study and accept responsibility to submit it for publication after approving the final draft.
Data sharing statement
Reasonable request to the corresponding author with scientific rationale and sound methodology.
Declaration of interests
Dr. Atsushi Hiraoka received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Chugai, Lilly, and AstraZeneca. Dr. Takeshi Hatanaka has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Eisai. Dr. Toshifumi Tada has received payment or honoraria for lectures, from Abbvie, Eisai and Chugai. Dr. Ardnt Vogel (AV) has served as a consultant for Roche, Bayer, BMS, EISAI, AstraZeneca, Ipsen, MSD, Sirtex, BTG, Servier, Terumo, Imaging Equipment Ltd (AAA), Böhringer Ingelheim. AV has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Roche, Bayer, BMS, EISAI, AstraZeneca, Ipsen, MSD, Sirtex, BTG, Servier, Terumo, Imaging Equipment Ltd (AAA), and Böhringer Ingelheim. AV received support for attending meetings and/or travel from Roche, Bayer, and BMS and participated in the Advisory board for Roche, Bayer, BMS, Lilly, EISAI, AstraZeneca, Ipsen, MSD, Sirtex, BTG, Servier, Terumo, Imaging Equipment Ltd (AAA). Dr. Richard S. Finn has received grants from Roche Genentech, Bayer, BMS, Eisai, Merck, Pfizer, Eli Lilly to institute. RSF is also a consultant for Roche Genentech, AstraZeneca, Bayer, BMS, Eisai, Exelixis CStone Eli Lilly, Pfizer, and Merck and has received support for attending meetings and/or travel from Roche Genentech, Merck, Pfizer, Bayer. RSF has participated for Data Safety Monitoring Board/Advisory Board for AstraZeneca. Dr. Anjana Pillai has served as a consultant for Eisai Inc, Exelixis, Genentech/Roche, AstraZeneca and Replimune. Dr. Amit Singal has served as a consultant for Genentech, AstraZeneca, Eisai, Bayer, Exelixis, Boston Scientific, FujiFilm Medical Sciences, Exact Sciences, Roche, Glycotest, Universal Dx, Freenome, and GRAIL. Other authors have nothing to declare.
Acknowledgements
An unsolicited grant from ROCHE Products India Pvt Ltd. was received for publication. We thank the authors of the primary studies included in the analysis for replying to our queries.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102179.
Appendix A. Supplementary data
References
- 1.Llovet J.M., Kelley R.K., Villanueva A., et al. Hepatocellular carcinoma. Nat Rev Dis Prim. 2021;7(1):6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 2.McGlynn K.A., Petrick J.L., El-Serag H.B. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73 Suppl 1(Suppl 1):4–13. doi: 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar A., Acharya S.K., Singh S.P., et al. 2019 update of Indian National Association for Study of the liver consensus on prevention, diagnosis, and management of hepatocellular carcinoma in India: the puri II recommendations. J Clin Exp Hepatol. 2020;10(1):43–80. doi: 10.1016/j.jceh.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal P.D., Phillips P., Hillman L., et al. Multidisciplinary management of hepatocellular carcinoma improves access to therapy and patient survival. J Clin Gastroenterol. 2017;51(9):845–849. doi: 10.1097/MCG.0000000000000825. [DOI] [PubMed] [Google Scholar]
- 5.Yopp A.C., Mansour J.C., Beg M.S., et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol. 2014;21(4):1287–1295. doi: 10.1245/s10434-013-3413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kudo M., Finn R.S., Qin S., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 7.Llovet J.M., Ricci S., Mazzaferro V., et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 8.Kulkarni A.V., Fatima S., Sharma M., et al. Lenvatinib for unresectable hepatocellular carcinoma: the first Indian experience. GastroHep. 2021;3(6):407–408. [Google Scholar]
- 9.Zhao Y., Zhang Y.N., Wang K.T., Chen L. Lenvatinib for hepatocellular carcinoma: from preclinical mechanisms to anti-cancer therapy. Biochim Biophys Acta Rev Cancer. 2020;1874(1) doi: 10.1016/j.bbcan.2020.188391. [DOI] [PubMed] [Google Scholar]
- 10.Kuo Y.H., Lu S.N., Chen Y.Y., et al. Real-world lenvatinib versus sorafenib in patients with advanced hepatocellular carcinoma: a propensity score matching analysis. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.737767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finn R.S., Qin S., Ikeda M., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 12.Reig M., Forner A., Rimola J., et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fulgenzi C.A.M., D'Alessio A., Airoldi C., et al. Comparative efficacy of novel combination strategies for unresectable hepatocellular carcinoma: a network metanalysis of phase III trials. Eur J Cancer. 2022;174:57–67. doi: 10.1016/j.ejca.2022.06.058. [DOI] [PubMed] [Google Scholar]
- 14.Vogel A., Rimassa L., Sun H.C., et al. Comparative efficacy of atezolizumab plus bevacizumab and other treatment options for patients with unresectable hepatocellular carcinoma: a network meta-analysis. Liver Cancer. 2021;10(3):240–248. doi: 10.1159/000515302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lencioni R., Llovet J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells G.A., Shea B., O'Connell D., et al. 2000. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford. [Google Scholar]
- 18.Higgins J.P., Altman D.G., Gotzsche P.C., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Min L., Shasha F., Wei P., Yichao C., Yao Z., Huaxin D. Clinical efficacy of atezolizumab plus bevacizumab for the treatment of advanced hepato-cellular carcinoma. Chin J Dig Surg. 2021;20:29–31. [Google Scholar]
- 20.Xiaoyun H., Mengya Z., Qi L., Guosheng Y., Rong L., Jinzhang C. Clinical efficacy of atezolizumab plus bevacizumab for first-line treatment of unresectable hepatocellular carcinoma. Chin J Dig Surg. 2021;20:20–24. [Google Scholar]
- 21.Yi L., Junwei L., Guoliang S., et al. Clinical efficacy of atezolizumab plus bevacizumab in the first-line treatment of advanced hepatocellular carcinoma. Chin J Dig Surg. 2021;20:25–28. [Google Scholar]
- 22.Ando Y., Kawaoka T., Kosaka M., et al. Early tumor response and safety of atezolizumab plus bevacizumab for patients with unresectable hepatocellular carcinoma in real-world practice. Cancers (Basel) 2021;13(16):3958. doi: 10.3390/cancers13163958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Awiwi M.O., Elsayes K.M., Mohamed Y.I., et al. The prognostic value of baseline clinical and radiologic imaging features in patients with unresectable hepatocellular carcinoma treated with atezolizumab plus bevacizumab. J Hepatocell Carcinoma. 2022;9:913–927. doi: 10.2147/JHC.S379428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casadei-Gardini A., Rimini M., Tada T., et al. Atezolizumab plus bevacizumab versus lenvatinib for unresectable hepatocellular carcinoma: a large real-life worldwide population. Eur J Cancer. 2023;180:9–20. doi: 10.1016/j.ejca.2022.11.017. [DOI] [PubMed] [Google Scholar]
- 25.Charonpongsuntorn C., Tanasanvimon S., Korphaisarn K., et al. MO5-3 Efficacy and patient-report outcomes of atezolizumab/bevacizumab for unresectable hepatocellular carcinoma in Thailand. Ann Oncol. 2022;33:S484. doi: 10.1200/GO.22.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen C.T., Feng Y.H., Yen C.J., et al. Prognosis and treatment pattern of advanced hepatocellular carcinoma after failure of first-line atezolizumab and bevacizumab treatment. Hepatol Int. 2022;16(5):1199–1207. doi: 10.1007/s12072-022-10392-x. [DOI] [PubMed] [Google Scholar]
- 27.Cheng J., Chen B., Jing Y., Lu Y. Efficacy and safety in advanced hepatocellular carcinoma using atezolizumab plus bevacizumab combined with interventional therapy: a retrospective analysis of real-world evidence. J Clin Oncol. 2022;40(16_suppl):e16112–e. [Google Scholar]
- 28.Cheon J., Yoo C., Hong J.Y., et al. Efficacy and safety of atezolizumab plus bevacizumab in Korean patients with advanced hepatocellular carcinoma. Liver Int. 2022;42(3):674–681. doi: 10.1111/liv.15102. [DOI] [PubMed] [Google Scholar]
- 29.Chon Y.E., Cheon J., Kim H., et al. Predictive biomarkers of survival in patients with advanced hepatocellular carcinoma receiving atezolizumab plus bevacizumab treatment. Cancer Med. 2023;12(3):2731–2738. doi: 10.1002/cam4.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chuma M., Uojima H., Hattori N., et al. Safety and efficacy of atezolizumab plus bevacizumab in patients with unresectable hepatocellular carcinoma in early clinical practice: a multicenter analysis. Hepatol Res. 2022;52(3):269–280. doi: 10.1111/hepr.13732. [DOI] [PubMed] [Google Scholar]
- 31.Cosgrove D., Tan A., Hernandez S., et al. Hepatology; 2022. Wiley 111 River ST; Hoboken 07030-5774, NJ USA: 2022. Atezolizumab plus bevacizumab in patients with hepatocellular carcinoma (Hcc): real-world experience from A us community Oncology network; pp. S1308–S1309. [Google Scholar]
- 32.D'Alessio A., Fulgenzi C.A.M., Nishida N., et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: a real-world study. Hepatology. 2022;76(4):1000–1012. doi: 10.1002/hep.32468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Castro T., Jochheim L.S., Bathon M., et al. Atezolizumab and bevacizumab in patients with advanced hepatocellular carcinoma with impaired liver function and prior systemic therapy: a real-world experience. Ther Adv Med Oncol. 2022;14 doi: 10.1177/17588359221080298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eso Y., Takeda H., Taura K., Takai A., Takahashi K., Seno H. Pretreatment neutrophil-to-lymphocyte ratio as a predictive marker of response to atezolizumab plus bevacizumab for hepatocellular carcinoma. Curr Oncol. 2021;28(5):4157–4166. doi: 10.3390/curroncol28050352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fulgenzi C.A.M., Cheon J., D'Alessio A., et al. Reproducible safety and efficacy of atezolizumab plus bevacizumab for HCC in clinical practice: results of the AB-real study. Eur J Cancer. 2022;175:204–213. doi: 10.1016/j.ejca.2022.08.024. [DOI] [PubMed] [Google Scholar]
- 36.Ha Y., Yang H., Cheon J., et al. Abstract. Hepatol Int. 2022;16(S1):1–503. [Google Scholar]
- 37.Ha Y., Kim J.H., Cheon J., Jeon G.S., Kim C., Chon H.J. Risk of variceal bleeding in patients with advanced hepatocellular carcinoma receiving atezolizumab/bevacizumab. Clin Gastroenterol Hepatol. 2022;21:2421. doi: 10.1016/j.cgh.2022.07.035. [DOI] [PubMed] [Google Scholar]
- 38.Hayakawa Y., Tsuchiya K., Kurosaki M., et al. Early experience of atezolizumab plus bevacizumab therapy in Japanese patients with unresectable hepatocellular carcinoma in real-world practice. Invest New Drugs. 2022;40(2):392–402. doi: 10.1007/s10637-021-01185-4. [DOI] [PubMed] [Google Scholar]
- 39.Himmelsbach V., Pinter M., Scheiner B., et al. Efficacy and safety of atezolizumab and bevacizumab in the real-world treatment of advanced hepatocellular carcinoma: experience from four tertiary centers. Cancers (Basel) 2022;14(7):1722. doi: 10.3390/cancers14071722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwamoto H., Shimose S., Noda Y., et al. Initial experience of atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma in real-world clinical practice. Cancers (Basel) 2021;13(11):2786. doi: 10.3390/cancers13112786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim B.K., Cheon J., Kim H., et al. Atezolizumab/bevacizumab vs. lenvatinib as first-line therapy for unresectable hepatocellular carcinoma: a real-world, multi-center study. Cancers (Basel) 2022;14(7):1747. doi: 10.3390/cancers14071747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Komatsu S., Yano Y., Fujishima Y., et al. Current role of atezolizumab plus bevacizumab therapy in the sequential treatment of unresectable hepatocellular carcinoma. Anticancer Res. 2022;42(3):1403–1412. doi: 10.21873/anticanres.15610. [DOI] [PubMed] [Google Scholar]
- 43.Kulkarni A.V., Krishna V., Kumar K., et al. Safety and efficacy of atezolizumab-bevacizumab in real world: the first Indian experience. J Clin Exp Hepatol. 2023;13(4):618–623. doi: 10.1016/j.jceh.2023.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuwano A., Yada M., Narutomi F., et al. Therapeutic efficacy of atezolizumab plus bevacizumab for hepatocellular carcinoma with WNT/beta-catenin signal activation. Oncol Lett. 2022;24(1):216. doi: 10.3892/ol.2022.13337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuzuya T., Kawabe N., Hashimoto S., et al. Early changes in alpha-fetoprotein are a useful predictor of efficacy of atezolizumab plus bevacizumab treatment in patients with advanced hepatocellular carcinoma. Oncology. 2022;100(1):12–21. doi: 10.1159/000519448. [DOI] [PubMed] [Google Scholar]
- 46.Lee M.S., Ryoo B.Y., Hsu C.H., et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21(6):808–820. doi: 10.1016/S1470-2045(20)30156-X. [DOI] [PubMed] [Google Scholar]
- 47.Maesaka K., Sakamori R., Yamada R., et al. Comparison of atezolizumab plus bevacizumab and lenvatinib in terms of efficacy and safety as primary systemic chemotherapy for hepatocellular carcinoma. Hepatol Res. 2022;52(7):630–640. doi: 10.1111/hepr.13771. [DOI] [PubMed] [Google Scholar]
- 48.Matsumae T., Kodama T., Myojin Y., et al. Circulating cell-free DNA profiling predicts the therapeutic outcome in advanced hepatocellular carcinoma patients treated with combination immunotherapy. Cancers (Basel) 2022;14(14):3367. doi: 10.3390/cancers14143367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsumoto H., Tsuchiya K., Nakanishi H., et al. Clinical usefulness of monitoring muscle volume during atezolizumab plus bevacizumab therapy in patients with unresectable hepatocellular carcinoma. Cancers (Basel) 2022;14(14):3551. doi: 10.3390/cancers14143551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niizeki T., Tokunaga T., Takami Y., et al. Comparison of efficacy and safety of atezolizumab plus bevacizumab and lenvatinib as first-line therapy for unresectable hepatocellular carcinoma: a propensity score matching analysis. Target Oncol. 2022;17(6):643–653. doi: 10.1007/s11523-022-00921-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ogawa K., Kanzaki H., Chiba T., et al. Effect of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma harboring CTNNB1 mutation in early clinical experience. J Cancer. 2022;13(8):2656–2661. doi: 10.7150/jca.71494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oura K., Morishita A., Kei T., et al. Abstract. Hepatol Int. 2022;16(S1):1–503. [Google Scholar]
- 53.Shimose S., Iwamoto H., Tanaka M., et al. Association between adverse events and prognosis in patients with hepatocellular carcinoma treated with atezolizumab plus bevacizumab: a multicenter retrospective study. Cancers (Basel) 2022;14(17):4284. doi: 10.3390/cancers14174284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sho T., Suda G., Yamamoto Y., et al. Efficacy and effect on liver functional reserve of atezolizumab and bevacizumab for unresectable hepatocellular carcinoma in patients who do not meet eligibility criteria of IMbrave150. Cancers (Basel) 2022;14(16):3938. doi: 10.3390/cancers14163938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinner F., Pinter M., Scheiner B., et al. Atezolizumab plus bevacizumab in patients with advanced and progressing hepatocellular carcinoma: retrospective multicenter experience. Cancers (Basel) 2022;14(23):5966. doi: 10.3390/cancers14235966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su C.W., Teng W., Lin P.T., et al. Similar efficacy and safety between lenvatinib versus atezolizumab plus bevacizumab as the first-line treatment for unresectable hepatocellular carcinoma. Cancer Med. 2023;12(6):7077–7089. doi: 10.1002/cam4.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takada H., Maekawa S., Enomoto N. Hepatology; 2022. Wiley 111 River ST; Hoboken 07030-5774, NJ USA: 2022. Significance of plasma IP-10/CXCL10 levels in patients treated with atezolizumab and bevacizumab for unresectable hepatocellular carcinoma; p. S1364. [Google Scholar]
- 58.Tamaki N., Tada T., Kurosaki M., et al. Optimal threshold of alpha-fetoprotein response in patients with unresectable hepatocellular carcinoma treated with atezolizumab and bevacizumab. Invest New Drugs. 2022;40(6):1290–1297. doi: 10.1007/s10637-022-01303-w. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka T., Hiraoka A., Tada T., et al. Therapeutic efficacy of atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma in patients with Child-Pugh class A or B liver function in real-world clinical practice. Hepatol Res. 2022;52(9):773–783. doi: 10.1111/hepr.13797. [DOI] [PubMed] [Google Scholar]
- 60.Teng W., Lin C.C., Su C.W., et al. Combination of CRAFITY score with Alpha-fetoprotein response predicts a favorable outcome of atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma. Am J Cancer Res. 2022;12(4):1899–1911. [PMC free article] [PubMed] [Google Scholar]
- 61.Tokunaga T., Tateyama M., Kondo Y., et al. Hepatology; 2022. Wiley 111 River ST; Hoboken 07030-5774, NJ USA: 2022. Optimal management of atezolizumab plus bevacizumab therapy for patients with unresectable hepatocellular carcinoma focusing on therapeutic modifications; pp. S1351–S1352. [Google Scholar]
- 62.Tomonari T., Tani J., Sato Y., et al. Initial therapeutic results of atezolizumab plus bevacizumab for unresectable advanced hepatocellular carcinoma and the importance of hepatic functional reserve. Cancer Med. 2023;12(3):2646–2657. doi: 10.1002/cam4.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uchikawa S., Kawaoka T., Fujino H., et al. Evaluation of atezolizumab plus bevacizumab combination therapy for hepatocellular carcinoma using contrast-enhanced ultrasonography. J Med Ultrason. 2023;50(1):57–62. doi: 10.1007/s10396-022-01260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vithayathil M., D'Alessio A., Fulgenzi C.A.M., et al. Impact of older age in patients receiving atezolizumab and bevacizumab for hepatocellular carcinoma. Liver Int. 2022;42(11):2538–2547. doi: 10.1111/liv.15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J.H., Chen Y.Y., Kee K.M., et al. The prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with hepatocellular carcinoma receiving atezolizumab plus bevacizumab. Cancers (Basel) 2022;14(2) doi: 10.3390/cancers14020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xin Y., Cao F., Yang H., et al. Efficacy and safety of atezolizumab plus bevacizumab combined with hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.929141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamada T., Minami T., Tateishi R., Koike K. Limited efficacy of atezolizumab and bevacizumab for hepatocellular carcinoma previously treated with tyrosine kinase inhibitor. Liver Int. 2021;41(9):2233–2234. doi: 10.1111/liv.15010. [DOI] [PubMed] [Google Scholar]
- 68.El Hajra I., Sanduzzi-Zamparelli M., Sapena V., et al. Outcome of patients with HCC and liver dysfunction under immunotherapy: a systematic review and meta-analysis. Hepatology. 2023;77(4):1139–1149. doi: 10.1097/HEP.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 69.Singal A.G., Llovet J.M., Yarchoan M., et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023 doi: 10.1097/HEP.0000000000000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vogel A., Cervantes A., Chau I., et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv238–i255. doi: 10.1093/annonc/mdy308. [DOI] [PubMed] [Google Scholar]
- 71.European Association for the Study of the Liver. Electronic address eee. European association for the study of the L EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 72.Seymour L., Bogaerts J., Perrone A., et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Finn R., Kudo M., Merle P., et al. LBA34 primary results from the phase III LEAP-002 study: lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC) Ann Oncol. 2022;33:S1401. [Google Scholar]
- 74.Rizzo A., Cusmai A., Gadaleta-Caldarola G., Palmiotti G. Which role for predictors of response to immune checkpoint inhibitors in hepatocellular carcinoma? Expert Rev Gastroenterol Hepatol. 2022;16(4):333–339. doi: 10.1080/17474124.2022.2064273. [DOI] [PubMed] [Google Scholar]
- 75.Singal A.G., Hoshida Y., Pinato D.J., et al. International Liver Cancer Association (ILCA) white paper on biomarker development for hepatocellular carcinoma. Gastroenterology. 2021;160(7):2572–2584. doi: 10.1053/j.gastro.2021.01.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rizzo A., Ricci A.D., Di Federico A., et al. Predictive biomarkers for checkpoint inhibitor-based immunotherapy in hepatocellular carcinoma: where do we stand? Front Oncol. 2021;11 doi: 10.3389/fonc.2021.803133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Viscardi G., Tralongo A.C., Massari F., et al. Comparative assessment of early mortality risk upon immune checkpoint inhibitors alone or in combination with other agents across solid malignancies: a systematic review and meta-analysis. Eur J Cancer. 2022;177:175–185. doi: 10.1016/j.ejca.2022.09.031. [DOI] [PubMed] [Google Scholar]
- 78.Zhu A.X., Abbas A.R., de Galarreta M.R., et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat Med. 2022;28(8):1599–1611. doi: 10.1038/s41591-022-01868-2. [DOI] [PubMed] [Google Scholar]
- 79.Pfister D., Nunez N.G., Pinyol R., et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592(7854):450–456. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Espinoza M., Muquith M., Lim M., Zhu H., Singal A.G., Hsiehchen D. Disease etiology and outcomes after atezolizumab plus bevacizumab in hepatocellular carcinoma: post-hoc analysis of IMbrave150. Gastroenterology. 2023;165(1):286–288 e4. doi: 10.1053/j.gastro.2023.02.042. [DOI] [PubMed] [Google Scholar]
- 81.Hatanaka T., Kakizaki S., Hiraoka A., et al. Comparative efficacy and safety of atezolizumab and bevacizumab between hepatocellular carcinoma patients with viral and non-viral infection: a Japanese multicenter observational study. Cancer Med. 2023;12(5):5293–5303. doi: 10.1002/cam4.5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.