Abstract
To ensure the stability of biologicals over their entire shelf-life, non-ionic surface-active compounds (surfactants) are added to protect biologics from denaturation and particle formation. In this context, polysorbate 20 and 80 are the most used detergents. Despite their benefits of low toxicity and high biocompatibility, specific factors are influencing the intrinsic stability of polysorbates, leading to degradation, loss in efficacy, or even particle formation. Polysorbate degradation can be categorized into chemical or enzymatic hydrolysis and oxidation. Under pharmaceutical relevant conditions, hydrolysis is commonly originated from host cell proteins, whereas oxidative degradation may be caused by multiple factors such as light, presence of residual metal traces, peroxides, or temperature, which can be introduced upon manufacturing or could be already present in the raw materials. In this review, we provide an overview of the current knowledge on polysorbates with a focus on oxidative degradation. Subsequently, degradation products and key characteristics of oxidative-mediated polysorbate degradation in respect of different types and grades are summarized, followed by an extensive comparison between polysorbate 20 and 80. A better understanding of the radical-induced oxidative PS degradation pathway could support specific mitigation strategies. Finally, buffer conditions, various stressors, as well as appropriate mitigation strategies, reagents, and alternative stabilizers are discussed. Prior manufacturing, careful consideration and a meticulous risk-benefit analysis are highly recommended in terms of polysorbate qualities, buffers, storage conditions, as well as mitigation strategies.
Keywords: Biotherapeutic formulations, Biologicals, Surfactants, Polysorbates, Tweens®, Oxidative degradation, Polysorbate stability, Oxidation
Graphical abstract
1. Polysorbates in biotherapeutic formulations
Recombinant proteins and antibodies are an emerging class of therapeutics in biopharmaceutical industry. In 2019, seven of the ten highest-selling drugs were biologics (five antibodies and two fusion proteins) (https://pharmaintelligence.informa.com, 2022; Strickley and Lambert, 2021), and in 2021 six of ten were biologics (https://www.drugdiscoverytrends.com/50-of-2021s-best-selling-pharmaceuticals/, 2022). Those biopharmaceuticals are delivered in a wide range of concentrations up to 200 mg·mL-1 (Garidel et al., 2017). Here, tight protein packing (less than a few nanometers) is accompanied by the disadvantages of molecular crowding, protein–protein interactions, and denaturation (Blaffert et al., 2018; Philo, 2009; Mahler et al., 2009). This phenomenon is even enhanced by the protein´s surface activity leading to partial unfolding, followed by e.g. particle formation (Patten and Schellekens, 2003; Van Beers et al., 2012). However, drug products with lower concentrations are also subjected to denaturation. Especially, the contact with different interfaces (e.g., plastic polymers, glass, liquid-air, or stainless steel) upon manufacturing and drug product filling may promote protein degradation (Basu et al., 2013; Britt et al., 2012; Brett Ludwig et al., 2010; Gerhardt et al., 2014). Other stress conditions include shaking, transport, pumping or freeze–thaw processes (Khan et al., 2015; Lougheed et al., 1983). To prevent these undesired effects, protein formulations are stabilized by non-ionic surfactants to ensure quality, safety, and efficacy of the final product (Brovč et al., 2020a; Rayaprolu et al., 2018). Polysorbates (PS) are the most widely used detergents in biopharmaceutical industry due to their low toxicity, high biocompatibility, and excellent stabilizing properties (Khan et al., 2015; Lougheed et al., 1983; Jones et al., 2018; Patapoff and Esue, 2009). They are by far the most used surfactants in biopharmaceutical products (Kishore et al., 2011a; Crommelin et al., 2019; Daugherty and Mrsny, 2006). Generally, two different PS – namely polysorbate 20 (PS20; Tween®20) and polysorbate 80 (PS80; Tween®80) – are used as an essential part of registered drug products for intravenous (i.v.), subcutaneous (s.c.), and intramuscular (i.m.) applications. Both surfactants are characterized by high hydrophilic–lipophilic balance (HLB) values in the range of 15 to 17 and low critical micelle concentration ranges (CMRs) (Knoch et al., 2021), allowing them to adsorb on surfaces at low concentrations. They are commonly used in ranges between 0.01 and 1 mg·mL-1 in drug products (Martos et al., 2017). These concentrations were determined by stress tests defining the lowest effective surfactant concentration preventing protein denaturation (Kishore, 2018). Alternative surfactants like poloxamer 188 (Kolliphor®P188), lecithin, sodium dodecyl sulfate, polyoxyl 35 castor oil (Cremophor®EL or Kolliphor®EL), and alkyl saccharides are also used in parental applications (Martos et al., 2017; Bollenbach et al., 2022; Ruiz et al., 2022).
Three main mechanisms are discussed for the beneficial impact of surfactants on protein stability: (i) surfactants compete with proteins at hydrophobic interfaces and provide protection against interfacial stress and protein aggregation as well as increasing formulation stability and ensuring biotherapeutic activity (Singh et al., 2017; Gerhardt et al., 2015; Mahler et al., 2005). (ii) As second mechanism, direct interactions with hydrophobic patches are reported (Khan et al., 2015; Mahler et al., 2005), however, direct binding of PS20 and PS80 to several mAbs was shown to be minimal to negligible, depending on the investigated mAb drugs (Khan et al., 2015; Singh et al., 2017; Garidel et al., 2009). (iii) The encapsulation in the micelle interior. However, due to the discrepancy in size and number of micelles in comparison to mAbs, this protection mechanism is relatively unlikely and not possible for PS (Garidel et al., 2021). The exact stabilization mechanism of non-ionic surfactants is still under investigation (Dwivedi et al., 2020). Recently PS20 was fractionated and the protein stabilization effect of specific fractions was investigated (Diederichs et al., 2023; Tomlinson et al., 2020).
Despite their benefits, many factors are influencing the intrinsic stability of PS, leading to various degradation reactions (Kerwin, 2008). Generally, two main degradation mechanisms are discussed: (i) chemical or enzymatic hydrolysis, and (ii) oxidation (Kerwin, 2008; Donbrow et al., 1978a). It is essential to elucidate the obstacles of PS degradation to ensure quality, efficacy, and safety of the drug product over its entire shelf-life (Kishore, 2018). However, the inherent complexity of PS as well as the varying impact between biopharmaceuticals on the extent of PS degradation cause major analytical challenges.
This review provides a general overview on PS20 and PS80 followed by a detailed description of the current scientific knowledge of polysorbate oxidation, degradation products, oxidation markers, key characteristics, effect of PS quality/grade on oxidation, formulation conditions, and the different stressors used in the literature. Additionally, mitigation strategies and future perspectives are discussed.
2. Structure and heterogeneity of polysorbate 20 and 80
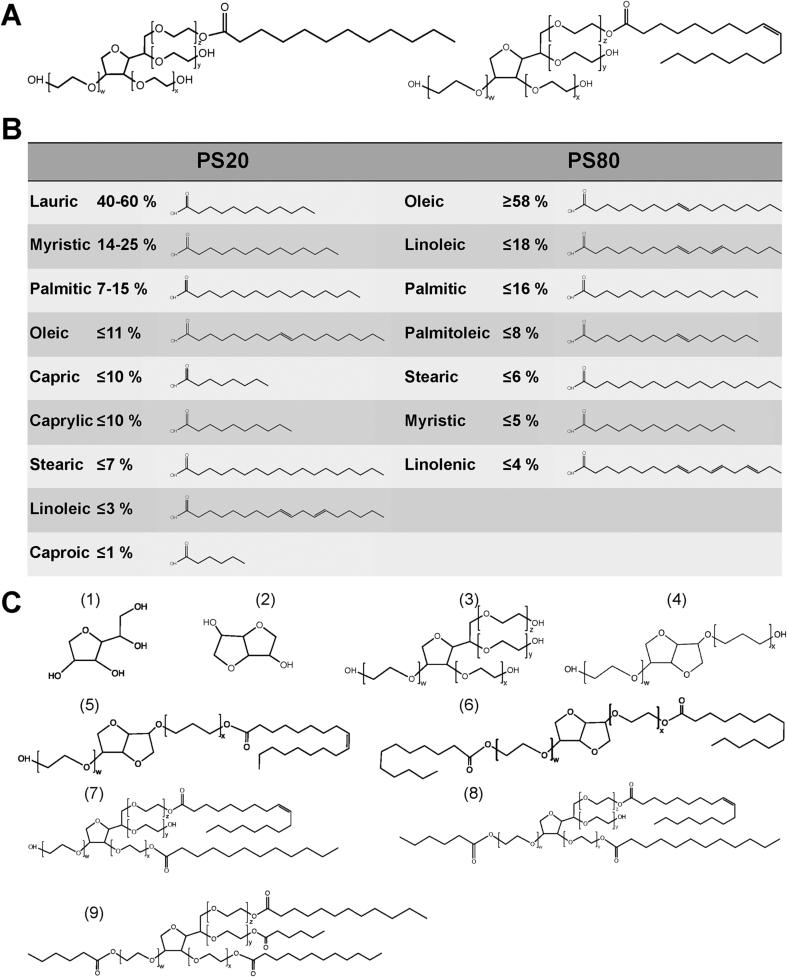
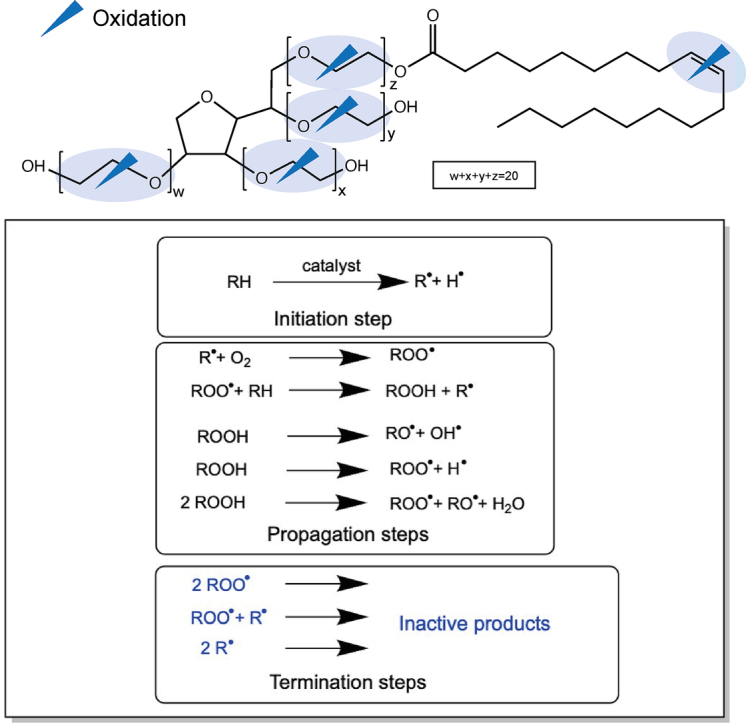
The heterogeneity of polysorbates can be derived from their synthesis and the used raw materials, starting with the dehydration of sorbitol to sorbitans and isosorbides, followed by esterification of fatty acids (FAs) and ethoxylation with ethylene oxide (Brovč et al., 2020b). The exact order of esterification and ethoxylation is not specified by the manufacturers, but usually requires high temperatures and anhydrous basic conditions (Kishore, 2018; Smidrkal et al., 2004). According to the pharmacopoeia, polysorbates are composed of a hydrophilic headgroup (sorbitan, isosorbide) esterified via up to four chains of approximately 20 polyoxyethylene (POE) moieties in total with up to four lipophilic fatty acids. The latter is adding complexity as a fatty acid composition ranging from caproic (C6:0) to linolenic acid (C18:3), depending on the polysorbate species, is esterified to the POE moieties. The actual PS is named after the most abundant FA, which is predominately monolaurate (C12:0) for PS20 (polyoxyethylene(20) sorbitan monolaurate) and mainly monooleate (C18:1) for PS80 (polyoxyethylene (20) sorbitan monooleate) (Kerwin, 2008). The expected structures of PS20 and PS80 are shown in Fig. 1A. Both structures only account for approximately 20 % (w/w) of the total material (Hewitt et al., 2011). The structures of the different fatty acids present in PS20 and PS80 are summarized in Fig. 1B. Acceptable ratios of the different fatty acids as well as other parameters such as impurities and peroxide values are defined by the pharmacopoeias (European pharmacopoeia (Ph. Eur.), United State pharmacopoeia (USP), Japanese pharmacopoeia (JP), British pharmacopoeia (BP), or Chinese pharmacopoeia (ChP)) (Evers et al., 2021). The amounts of peroxides of commercially available polysorbates for parenteral application are far below the limits of the pharmacopoeias. Nevertheless, Doshi and colleagues (2020) provided certificates of analysis for two different PS20 qualities, observing small differences between PS20 and PS80 as well as in different qualities and lots (Doshi et al., 2020a). Additionally, the raw material contains a certain amount of unesterified fatty acids (free fatty acids) and POE sorbitans and it was reported that up to approximately 28 % of PS20 corresponds to unesterified sorbitan-POE, depending on batch, quality, and vendor (Hewitt et al., 2008). Evers and colleagues (2021) presented values of unesterified fatty acids mostly below 5 % of hydrolysis, however, for C16 or C18 higher values (up to 20 %) were detected (Evers et al., 2021). The latter are ranging between 11 to 28 % (w/w) depending on the polysorbate species (Hewitt et al., 2011). The most abundant impurities as well as the di-, tri-, and tetraesters are illustrated in Fig. 1C.
Fig. 1.
Structural heterogeneity of polysorbate. (A) Idealized structures of PS20 (left) and PS80 (right), with approximately w + x + y + z = 20 (26 experimentally determined, but dependent on quality and batch (Evers et al., 2020)), referring to the number of ethylene oxide moieties defined by the pharmacopeias. (B) Structures and content of various fatty acids in PS20 and PS80 arranged according to the relative occurrence as specified in the pharmacopoeia. (C) Compounds present in PS20 and PS80 including (1) sorbitan, (2) isosorbide, (3) POE sorbitan, (4) POE isosorbide, (5) POE isosorbide monoester, (6) POE isosorbide diester (7) POE sorbitan diester, (8) POE sorbitan triester, and (9) POE sorbitan tetraester. Fatty acids with double bonds are illustrated in the trans configuration to save space.
All in all, the complexity of polysorbate can be differentiated in: (i) diversity of POE, (ii) esterification degree, (iii) head group variation, and (iv) composition of fatty acids (Kishore, 2018). Additionally, impurities can vary, depending on the used raw materials. The rational in defining all components of polysorbate can be discussed, however, Evers and coworkers (2020) revealed in total a remarkable number of more than 600 compounds which could be assigned in PS20 determined via mass spectrometry (Evers et al., 2020).
3. Pharmacopoeia’s requirements and grades
The specifications for polysorbate qualities are described by the international pharmacopoeias – Ph. Eur., USP, JP, BP, and ChP – normally agreeing on the majority of requirements like FA composition (Table 1) (Martos et al., 2017; Kishore, 2018). Impurities and specific tests for the different pharmacopoeias are given in Table 2. Currently, different grades are available: (i) multicompendial (MC) grade and (ii) ultrapure grade. The MC grades can be subdivided into high purity (HP) and super refined (SR) qualities, and the latter is claimed by the manufacturers to possess lower peroxide, impurity, and endotoxin levels. For PS20, the MC grade defines a ratio of 40–60 % of lauric acid, whereas the oleic acid content of PS80 must be ≥58 %. For a more detailed description see Martos et al. (2017) (Martos et al., 2017). Additionally, ultra-pure grades with reduced heterogeneity in the fatty acid composition were developed, denoted as all-oleate (AO), with a content of ≥98 % oleic acid for PS80 and <0.5 % for all other fatty acids as well as with a content of ≥98 % lauric acid for PS20 denoted as all-laurate (AL) (Brovč et al., 2020b). Additionally, the endotoxin level is restricted to 0.012 EU (Martos et al., 2017). To the best of our knowledge, the AL grade has only been used for research purposes (Brovč et al., 2020b), as only a few companies offer these quality standards AL for PS20 and AO (comparable to ChP grade) for PS80. The storage and handling of polysorbate upon manufacturing and filling is crucial as well (Martos et al., 2017). The actual advantages and disadvantages of the different grades in respect to oxidative degradation will be summarized and scientifically evaluated in the following chapters.
Table 1.
Fatty acid composition as listed in the different pharmacopoeias (ChP: Chinese pharmacopoeia; JP: Japanese pharmacopoeia; Ph. Eur.: European pharmacopoeia; USP: United State pharmacopoeia) for PS20 and PS80. JP has no monograph specific for PS20. For USP only the differences to the other pharmacopeias are reported.
| Pharmacopeia | Polysorbate | Fatty acid | Percentage / % | USP / % | |
|---|---|---|---|---|---|
| Ph. Eur. / ChP | PS20 | Lauric acid | C(12:0) | 40–60 | |
| Myristic acid | C(14:0) | 14–25 | |||
| Palmitic acid | C(16:0) | 7–15 | |||
| Oleic acid | C(18:1) | ≤11 | |||
| Capric acid | C(10:0) | ≤10 | |||
| Caprylic acid | C(8:0) | ≤10 | |||
| Stearic acid | C(18:0) | ≤7 | ≤11 | ||
| Linoleic acid | C(18:2) | ≤3 | |||
| Caproic acid | C(6:0) | ≤1 | |||
| Pharmacopoeia | Polysorbate | Fatty acid | Percentage / % | ChP / % | |
|---|---|---|---|---|---|
| Ph. Eur. / JP / USP | PS80 | Oleic acid | (18:1) | ≥58 | ≥98 |
| Linoleic acid | C(18:2) | ≤18 | ≤0.5 | ||
| Palmitic acid | C(16:0) | ≤16 | ≤0.5 | ||
| Palmitoleic acid | C(16:1) | ≤8 | ≤0.5 | ||
| Stearic acid | C(18:0) | ≤6 | ≤0.5 | ||
| Myristic acid | C(14:0) | ≤5 | ≤0.5 | ||
| Linolenic acid | C(18:3) | ≤4 | ≤0.5 |
Table 2.
Impurities and specific tests for PS20 and PS80.
| Pharmacopoeia | Polysorbate | Ph. Eur. | USP | JP | ChP |
|---|---|---|---|---|---|
| Peroxide value / mEq·kg-1 | PS20 | ≤10 | ≤5 | - | ≤10 |
| PS80 | ≤10 | ≤10 | ≤10 | ≤3 | |
| Heavy metal / ppm | PS20 | ≤10a | ≤10 | - | ≤10 |
| PS80 | ≤10a | ≤10a | ≤20 | ≤10 | |
| Endotoxins / EU | PS20 | - | - | - | - |
| PS80 | - | - | - | 0.012 | |
| Acid value / mgKOH·g-1 | PS20 | ≤2 | ≤2 | ≤4 | ≤2 |
| PS80 | ≤2 | ≤2 | ≤2 | ≤1 | |
| Hydroxyl value / mgKOH·g-1acetylated | PS20 | 96–108 | 96–108 | - | 96–108 |
| PS80 | 65–80 | 65–80 | 65–80 | 65–80 | |
| Saponification value / mgKOH·g-1 | PS20 | 40–50 | 40–50 | 43–55 | 40–50 |
| PS80 | 45–55 | 45–55 | 45–55 | 45–55 | |
| Ethylene oxide / ppm | PS20 | ≤1 | ≤1 | - | ≤1 |
| PS80 | ≤1 | ≤1 | ≤1 | ≤1 |
Limits deleted since Ph. Eur. 9.0 and since 01.01.2018 (USP)
4. Degradation of polysorbates and their impact on biologicals
The degradation of PS can have a significant impact on the quality of the drug product by decreasing the efficient surfactant concentration or by the formation of degradants affecting protein or product stability (Kishore et al., 2011a). The formation of protein particles bears the risk of immunogenicity (Rosenberg, 2006; Carpenter et al., 2009; Moussa et al., 2016), whereas PS-related fatty acid particles or particles in general can affect the requirements of the pharmacopoeias (European Pharmacopeia. 10.5, 2022; Das, 2012). Two different degradation pathways can be distinguished: (i) chemical/enzyme-mediated hydrolysis and (ii) oxidation (Kerwin, 2008; Donbrow et al., 1978a; Brovč et al., 2020b; Smidrkal et al., 2004). The root causes promoting each mechanism are diverse, as well as the impacts on product quality. It is crucial to shed light on the different degradation pathways starting from early stages of formulation development to the determination of the product shelf-life (Martos et al., 2017). Therefore, a huge number of different techniques and methods were developed in the last decade to analyze and investigate the composition and the properties of polysorbate, ranging from fluorescence detection to reversed-phase high performance liquid chromatography (RP-HPLC) coupled to evaporative light scattering detection (ELSD), charged aerosol detection (CAD), and/or mass spectrometry (MS), to name a few (Garidel et al., 2009; Doshi et al., 2020a; Hewitt et al., 2008; Hewitt et al., 2008; Moussa et al., 2016; Borisov et al., 2015; Dixit et al., 2016; McShan et al., 2016; Tomlinson et al., 2015; Kovner et al., 2023; Wuchner et al., 2022a; Lippold et al., 2017; Khossravi et al., 2002; Dwivedi et al., 2018; Liu et al., 2022a; Saggu et al., 2015; Siska et al., 2015). Besides liquid chromatography with CAD/ELSD coupling, which is the most used analytical method as described by the industry perspective on the use and characterization of PS, the fluorescence micelle assay (FMA) is often used for quantification (Wuchner et al., 2022a). Here, the dye N-phenyl-1-naphthylamine (NPN) can partition in hydrophobic environments like micelles followed by a detectable increase in the fluorescence quantum yield, allowing to quantify micelles. Differences between quantification by FMA and HPLC-CAD/ELSD should be considered, as FMA is highly dependent on higher-order ester species with high hydrophobicity’s and less dependent on monoesters with less hydrophobicity’s. Thus, an increased or faster degradation of hydrophobic species (higher-order esters) may lead to an overrepresentation in PS loss during FMA, which is not detected in HPLC measurements (Lippold et al., 2017). Colorimetric techniques are also used, however, they may not always be able to indicate whether polysorbate has been degraded and thus should only be performed using careful consideration (Khossravi et al., 2002). For a more detailed description of the different approaches, see Dwivedi et al. (2018) and Liu et al. (2022) (Dwivedi et al., 2018; Liu et al., 2022a).
-
a.
Chemical and enzymatic-mediated hydrolysis
Ester bond hydrolysis can occur by chemical- or enzymatic-mediated hydrolysis, both releasing free fatty acids (FFA) (Tomlinson et al., 2015; Saggu et al., 2015; Siska et al., 2015). The subsequent formation of fatty acid particles in relation to the PS concentration is discussed as a major concern in the biopharmaceutical community (Glücklich et al., 2020; Cao et al., 2015). Chemical hydrolysis of PS can be promoted by acidic and alkaline conditions (Dwivedi et al., 2020; Bates et al., 1973; Roberts and Urey, 1939; Stefanidis and Jencks, 1993). Here, pioneering work was performed by Bates and coworkers (1973) studying polysorbate degradation at different pH values and temperatures, revealing an acid- and base-catalyzed reaction of PS80 below pH 3 and above pH 7.6, respectively (Bates et al., 1973). The hydrolysis rate was inversely proportional to the surfactant concentration, discussed as being attributed to micelle formation and inaccessibility of the ester group (Bates et al., 1973). Additionally, no significant effects upon different hydrocarbon chain lengths were observed for polysorbate 40 (PS40, polyoxyethylene(20) sorbitan monopalmitate), polysorbate 60 (PS60, polyoxyethylene(20) sorbitan monostearate), and PS80 (Bates et al., 1973). More recently, Dwivedi and colleagues (2020) investigated the chemical hydrolysis of PS20 and PS80 at acidic, neutral, and alkaline conditions at different temperatures via FMA and RP-HPLC-CAD Dwivedi et al. (2020). They demonstrated that both surfactants are more prone to degradation at alkaline conditions (pH >12), whereas the hydrolysis was neglectable at pH 7 and 40 °C for 48 h (Dwivedi et al., 2020). Additionally, PS80 was found to be less sensitive to chemical hydrolysis in comparison to PS20 (Dwivedi et al., 2020).
pH-induced ester cleavage is unlikely in comparison to enzyme-mediated hydrolysis, as biopharmaceuticals are typically formulated at pH values between 5 and 7 and at storage temperature of 2–8 °C (Dwivedi et al., 2018). In particular, the presence of host cell proteins (HCPs) as impurities in the final product is described as the main root cause for enzymatic PS degradation (Kishore et al., 2011b; Borisov et al., 2015; McShan et al., 2016; Siska et al., 2015; Roberts and Urey, 1939; Stefanidis and Jencks, 1993; Glücklich et al., 2021; Hall et al., 2016; Zhang et al., 2021; Rupp et al., 2010; Li et al., 2022; Zhang et al., 2020; Chen et al., 2020; Kovner et al., 2023; Roy et al., 2021; Graf et al., 2021). These PS-degrading enzymes are classified as esterases or lipases and multiple PS-degrading enzymes were studied so far to gain fingerprints or characteristics of various lipases, as demonstrated exemplarily by McShan and coworkers (2016) (McShan et al., 2016). The analytical toolbox for identifying and quantifying the PS-degrading enzymes is essential to comprehend and mitigate the enzymatic hydrolysis of PS (Li et al., 2022). A wide range of analytical methods was developed, ranging from PS purity and content analysis over enzymatic activity assays to mass detection (Li et al., 2022). However, each HCP possesses its own substrate specificity, enzymatic optimum, and impact on PS degradation, prohibiting common guidelines and strategies. For instance, certain lipases degrade primarily higher-order esters implying a higher risk for particle formation as more individual FAs are released (Doshi et al., 2020b). Furthermore, insolubility limits of released fatty acids from PS are dependent on temperature, pH, PS concentration, and PS quality, which vary between final drug products (Glücklich et al., 2020; Doshi et al., 2015).
Different analytical methods are used to detect the hydrolytic degradation of PS. In addition to chromatographic methods, the titration of the formed fatty acid can be used, however, the apparent pKa of long chain fatty acid depends strongly on the local environment and can range from “4.2 to 10.2” (Heider et al., 2016). The degree of protonation/deprotonation has a strong impact on the hydrophilicity and solubility of the fatty acids (Heider et al., 2016). Oleic acid phase separates from water in the protonated, uncharged form, but it becomes water soluble and micelle-forming in its deprotonated anionic form (Heider et al., 2016).
Different mitigation strategies were applied to prevent enzymatic PS degradation, such as specific inhibitors (Zhang et al., 2020), cell line development via knockouts (Chiu et al., 2017; Chen et al., 2020; Kol et al., 2020,Dovgan et al., 2021), additional upstream development (harvest time, cell density, media optimization) (Li et al., 2022), adjustment/optimization of purification steps (resin, elution, buffers, and general optimizing purification steps), or improved formulation (PS type and grade, buffer system, and excipients) (Roy et al., 2021). For a more detailed description of enzymatic PS degradation, see Li et al. (2022) (Li et al., 2022).
-
b.
Oxidative degradation
Oxidative degradation of PS occurs at typical pharmaceutical conditions like pH values ranging from 5 to 7 and at temperatures between 5 and 25 °C (Donbrow et al., 1978a; Donbrow et al., 1978b; Hamburger et al., 1975). Donbrow and colleagues (1978) are pioneers in the field of polysorbate oxidation, which is portrayed as a three-phase process (Donbrow et al., 1978a). Starting with the initiation phase, oxidation can be accelerated by e.g., light exposure or transition metals in the presence of oxygen, which are common factors during pharmaceutical production processes, resulting in peroxide formation, or can be triggered by residual peroxides itself (Kerwin, 2008; Donbrow et al., 1978a; Singh et al., 2012; Larson et al., 2020). It has been demonstrated that catalysts such as copper sulfate can shorten the oxidative initiation time and increase the peroxide formation rate (Donbrow et al., 1978a). In this context, it should be mentioned that copper has presumably minor importance for the pharmaceutical industry compared to, for instance, iron, which is present nearly ubiquitously. Origin of residual H2O2 or organic peroxides may be the raw materials or the manufacturing/filling process, as H2O2 is a commonly used disinfectant agent in manufacturing environment (Sterchi, 2001; Corveleyn et al., 1997; Krishna et al., 2000). Therefore, stress tests conducted using H2O2 are certainly of practical relevance. As described above, the required peroxide limit stated in the certificate of analysis (<1 mEqO2·kg-1) for PS80 is 10-fold lower than the limit defined in the pharmacopeias (<10 mEqO2·kg-1) (Mittag et al., 2022). H2O2 can form hydroxyl radicals (OH•) for instance via the Fenton (Eq. 1) or Haber–Weiss reaction (Eq. 2), so that in the presence of redox-active metals, tiny impurities in the ppb range (as they occur during pharmaceutical production processes) are sufficient to cause significant effects (Phaniendra et al., 2015). Mittag and colleagues (2022) reported the presence of OH•, R•, RO•, and ROO• in bulk and 10 % (w/v) PS80 solutions measured via EPR spectroscopy and spin trapping. In bulk materials primarily ROO• and RO• were detected, whereas in 10 % (w/v) aqueous PS80 solutions mainly OH• was found, independent of PS80 quality and grade (Mittag et al., 2022).
| (1) |
| (2) |
Elevated temperatures accelerate oxidation of PS, and they are for instance used as accelerated stress conditions in pharmaceutical industry (Donbrow et al., 1978a).
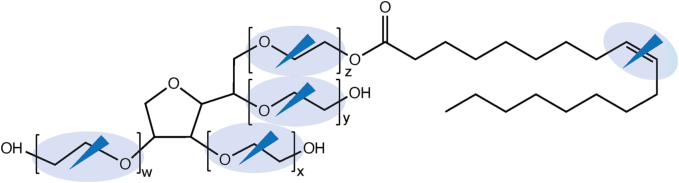
Polysorbates provide multiple potential reaction sites for oxidation such as (i) the POE moieties, also resulting in free fatty acid to a low extent, and (ii) the unsaturation site (double bond) of the fatty acid as illustrated in Fig. 2 (Donbrow et al., 1978a; Zhang et al., 2017; Yao et al., 2009).
Fig. 2.
Exemplary scheme for PS80 with its potential oxidation reaction sites (blue arrow and blue shadow).
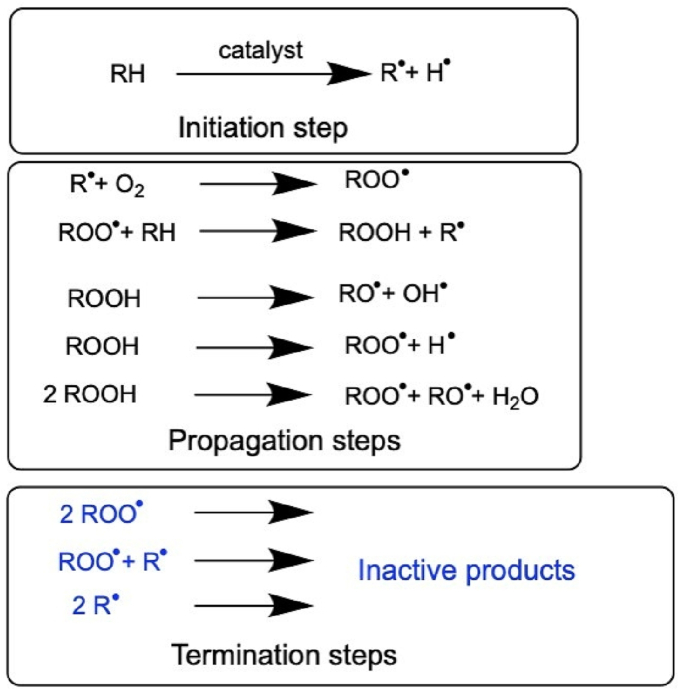
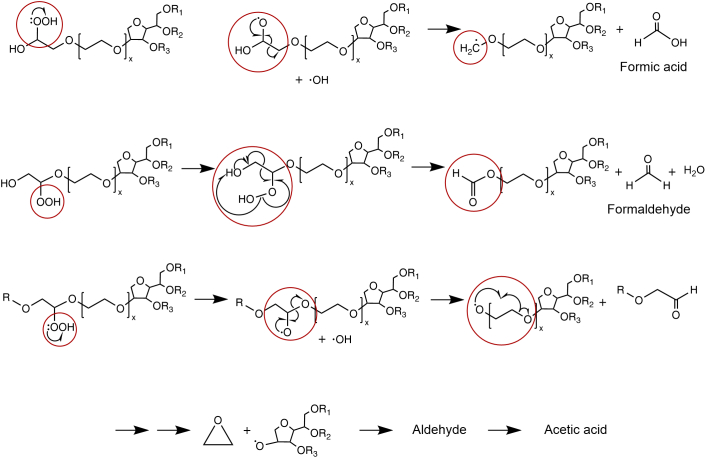
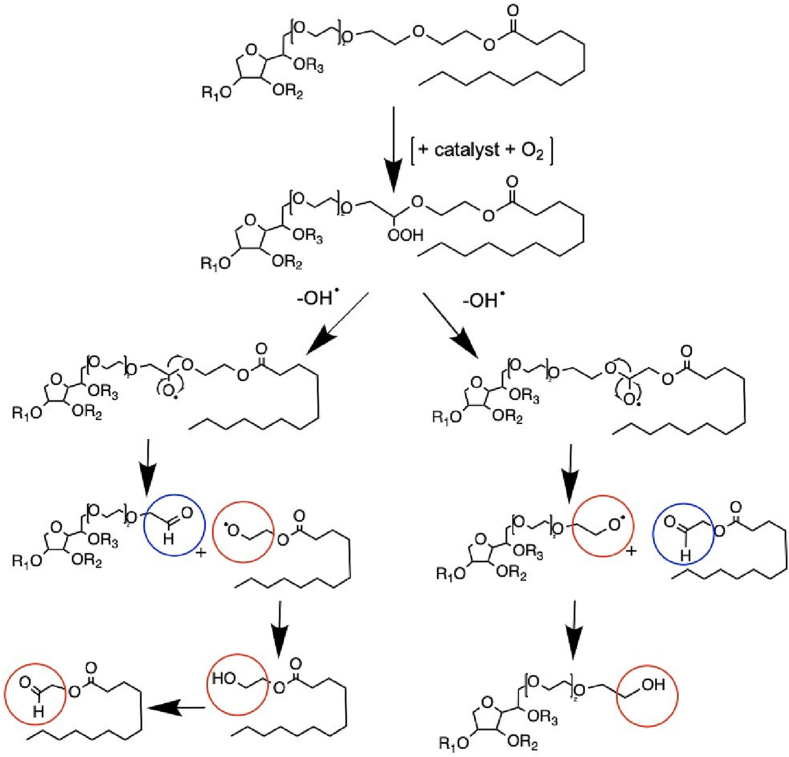
As shown in Fig. 3, oxidation is described by a radical initiation, propagation, and termination reaction (Kerwin, 2008; Kishore et al., 2011b; Dulog and Storck, 1966), similar to most polyoxyethylene systems (Donbrow et al., 1978a). Initially, an alkyl radical can be formed, promoted by e.g., light, temperature, metal traces, or residual peroxides followed by the propagation reaction with molecular oxygen. Here, Ha et al. (2002) detected an increased peroxide formation with increased temperature and light for 20 % (w/v) PS80 solutions (Ha et al., 2002). The resulting hydroperoxide is driving the reaction forward. Nevertheless, such high polysorbate concentrations are not commonly applied in pharmaceutical industry. Other free radicals are generated by homolytic or heterolytic peroxide cleavage before individual radicals are quenching each other leading to the radical termination reaction (Donbrow et al., 1978a). Another possibilities for termination are the lack of substrate or the presence of antioxidants. Molecular oxygen is essential for the oxidation, and thus, oxygen removal results in a lower initial increase in peroxides or even in prevention of peroxide formation and oxidation processes (Ha et al., 2002). The radical scheme is depicted in Fig. 3, and a more detailed mechanistic description of the POE radical-mediated oxidation is shown in Fig. 4. Different scenarios can be distinguished depending on the “location of the radical” within the POE group or within the different POE monomers in the POE chain (dependent on the hydrogen abstraction of another radical). Formic acid is formed by starting the radical-induced degradation from an alkoxyl radical (RO•) at the end of a POE chain (compare Fig. 4A), whereas initiation from a hydroperoxide (ROOH) at the terminus of the POE chain can result in the formation of an ester, formaldehyde, and water due to a six-membered intramolecular decomposition (compare Fig. 4B) (Decker and Marchal, 1974). The remaining ester will be further hydrolyzed to formic acid. There are multiple reactions possible (compare Fig. 3), as the hydroperoxide can also be homo- or heterolytically cleaved, resulting in an alkoxy radical or a peroxyl radical. In contrast, a radical reaction within the POE chain creates acetaldehyde and finally acetic acid via an epoxide intermediate (Fig. 4C) (Dulog and Storck, 1966).
Fig. 3.
Radical-induced oxidative degradation of general polyoxyethylene units with an alkyl residue R. The radical reaction scheme can be divided into initiation, propagation, and termination steps. This figure is adapted from Donbrow et al. (1978) (Donbrow et al., 1978a).
Fig. 4.
Radical-induced oxidative degradation of polyoxyethylene (POE) moieties in polysorbates. The variety of products is dependent on different factors such as the position of the attacked POE chain or C-atom within the POE chain, leading to the formation of different degradation products such as (A) formic acid, (B) formaldehyde, (C) acetic acid. This figure is adapted from Kishore et al. (2011) and Dwivedi et al. (2018) (Kishore et al., 2011a; Dwivedi et al., 2018). The reaction sites are marked by brown circles.
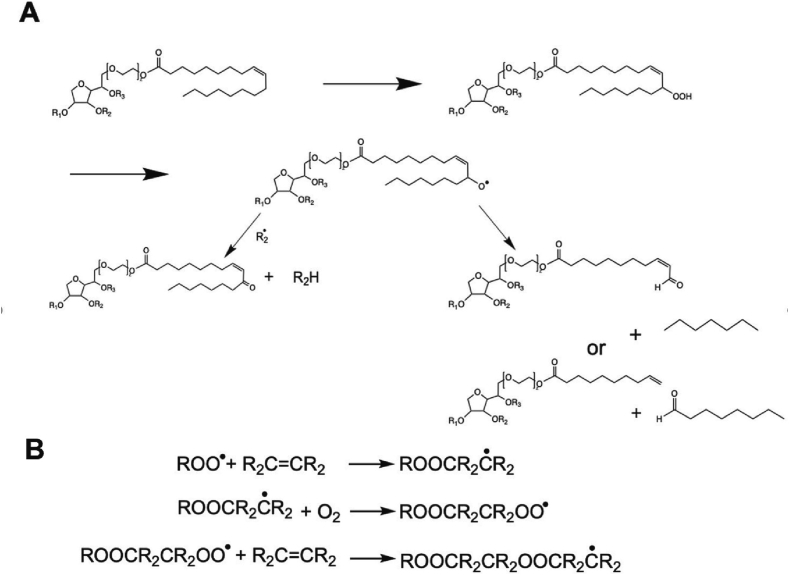
Under the consideration of an actual polysorbate monoester, radical-induced oxidation of the POE chain will result in short-chain POE esters of fatty acids and POE sorbitans/isosorbides. Potential mechanistic illustrations are given in Fig. 5 (adapted from Dahotre et al. (2018)) (Dahotre et al., 2018). Donbrow and colleagues (1978) claimed that it is challenging to break the terminal hydroxyl groups, requiring strongly acidic conditions with harsh oxidizing agents (Donbrow et al., 1978a). However, such instability may occur by reaction with a hydroperoxide radical in the α- or ß-position to the terminal hydroxyl group leading to C-C or C-O cleavage, forming the corresponding short-chain acids as degradation products (Donbrow et al., 1978a).
Fig. 5.
Two potential mechanisms for radical-induced oxidative degradation of the polyoxyethylene (POE) moieties in polysorbate leading to the formation of aldehydes (left) and alcohols (right). This figure is adapted from Dahotre et al. (2018) (Dahotre et al., 2018).
Finally, oxidative degradation can occur on the hydrocarbon site of unsaturation (Yao et al., 2009), as shown in Fig. 6. This pathway is more relevant for PS80 as it contains at least 58 % unsaturated fatty acids (only considering oleic acid), whereas PS20 comprises at least 86 % saturated fatty acids (European Pharmacopeia. 10.5, 2022). Nevertheless, PS20 can include up to 11 and 3 % oleic acid and linoleic acids, respecitvely (European Pharmacopeia. 10.5, 2022). The basis of the susceptibility of PS80 for oxidative degradation is based on electron transfer followed by hydrogen atom abstraction. The enthalpy for H-atom extraction by a hydroperoxyl radical strongly depends on the mesomeric stabilization of the radical intermediate. Unsaturated and especially multi-unsaturated fatty acids have a higher degree of delocalization regarding the mesomeric stabilization of the radical intermediate and therefore, are more prone to oxidation (Pratt et al., 2011; Ding et al., 2022). In addition, the formation of carbon centered radicals – oxygen complexes have been described as important intermediate species (Pratt et al., 2011). New insights on the oxidation of oleic acid have been published already several years ago and also very recently (Ding et al., 2022; Porter, 1986). According to the experimental results of Ding et al. (2022), isomerization of allyl, analogous to β-fragmentation, is one of the pathways to form multiple hydroperoxides (Ding et al., 2022). Nevertheless, different oxidation stages strongly affect both reactions. While hydroperoxides accumulate, β-fragmentation is taking a leading part (Ding et al., 2022). In contrast, allyl isomerization primarily appears when hydroperoxide is decomposed in large quantities (Ding et al., 2022). In total, the H-atom abstraction energy is estimated to be more favorable in the vicinity of a double bond or between double bonds than near an ethylene oxide group (Yao et al., 2009). In oils a corelation between degree of unsaturation and oxidative susceptibility could be established (Musakhanian et al., 2022). The alkyl radical in the vicinity of the double bond is reacting with molecular oxygen and H-atom abstraction (see Fig. 6A). Subsequent, homolytic cleavage of the hydroperoxide results in the formation of an alkyl radical, which can react with another radical in a termination reaction or in the formation of alkenes, aldehydes, or ketones via scission of the C-C bond (Kishore, 2018). Additionally, radicals can react with double bonds by direct reaction with similar rates as the hydrogen abstraction (see Fig. 6B) (Yao et al., 2009; Denisov, 2005).
Fig. 6.
Radical-induced oxidative degradation of polysorbate via the unsaturation site of the fatty acid. (A) Reaction with molecular oxygen in the vicinity of the double bond and (B) reaction of peroxyl radicals with double bonds. This figure is adapted from Yao et al. (2009) and Kishore (2018) (Kishore, 2018; Yao et al., 2009).
Thereby, various degradation products are formed ranging from alkenes, aldehydes, and ketones to short carboxylic acids, fatty acids, and POE esters of fatty acids (Hvattum et al., 2012). Especially fatty acid esters with short POE chains are of major concern for fatty acid particle formation, as they possess similar solubility characteristics as fatty acids, due to their short hydrophilic headgroup (Kishore et al., 2011a). Kishore and coworkers (2011) determined the logP value of various components of oxidative degraded PS20 and PS80 revealing more sparsely soluble degradants for PS20 (Kishore et al., 2011a). This change in hydrophilic-to-hydrophobic ratio results in change of the critical micelle concentration (CMC) and change of the cloud point (Donbrow et al., 1978a). Additionally, the resulting aldehydes may be harmful in respect to protein stability and may also affect the human body in a toxic manner (Lopachin and Gavin, 2014).
But not only the degradation products, also the generation of reactive oxygen species (ROS) are critical, as they are oxidizing the protein product as well as other formulation components (Yao et al., 2009; Hipper et al., 2021; Ehrenshaft et al., 2015; Michaeli and Feitelson, 1994). ROS are defined as reactive chemicals formed from O2 including free radicals (HO•, O2•−, HOO•), but also H2O2, hydroperoxide (ROOH) or singlet oxygen (1O2). The reactivity of different ROS is quite different. OH-radicals are highly reactive and only the diffusion limits its reactivity (k >109 M–1⋅s–1) (Finkelstein et al., 1980; Goldstern et al., 2004; Hawkins and Davies, 2014). In contrast, peroxyl-radicals and superoxide radicals are less reactive with reaction constants 6 to 9 orders of magnitude lower and might therefore undergo further reactions at later time points and different locations (Mittag et al., 2022; Finkelstein et al., 1980; Goldstern et al., 2004; Hawkins and Davies, 2014; Haywood, 2013; Buettner and Mason, 1990). Nevertheless, the exact mechanism of oxidation is not fully understood so far (Borisov et al., 2015). To determine the types of radicals involved in oxidative PS degradation, NMR, or EPR measurements were conducted (Mittag et al., 2022; Doyle et al., 2019).
5. Oxidation of polysorbate
Recently, an industry perspective of 16 globally acting companies was published, highlighting that PS degradation was observed through both hydrolysis (69 %) and oxidation (63 %) in at least one of their biopharmaceutical products (Wuchner et al., 2022a; Wuchner et al., 2022b). To elucidate the challenges of PS degradation, it is essential to differentiate between hydrolytic and oxidative degradation to guide appropriate mitigation strategies. Many methods like RP-HPLC coupled with CAD (Fekete et al., 2010; Christiansen et al., 2011), ELSD (Hewitt et al., 2008; Nayak et al., 2012), or FMA (Brito Rui and Vaz, 1986) were established to determine the PS content in formulations, however, they fail to clearly distinguish the main root cause of PS degradation. In the following chapter, degradation products and oxidation markers determined by MS or 1H-NMR are summarized to evaluate the root cause of PS degradation. Other indicators for oxidative degradation are the presence of peroxides (relative stable intermediate of oxidation), which were detected upon oxidative processes (Donbrow et al., 1978a; Ha et al., 2002). Additionally, shifts to more acidic pH values in weakly-buffered systems can be observed, due to the short-chain acid degradation products like for instance formic acid and acetic acid (Donbrow et al., 1978a). ROS are intermediates in the oxidation process and two assays are commonly used to monitor ROS and correlate them with PS oxidation: (i) the ROS content (every ROS oxidizing Fe2+ to Fe3+ is determined) is investigated using a dye complex consisting of Fe3+ and xylenol orange under acidic conditions (FOX assay), and (ii), the content of H2O2 is examined with the aid of horseradish peroxidase (AmplexTM Red assay) (Doshi et al., 2020a; Ha et al., 2002; Kranz et al., 2019). Another possibility to assess the root cause of degradation are specific reaction conditions like the addition of stressors, placebo formulations, or other conditions allowing only oxidation to occur (Bollenbach et al., 2022; Singh et al., 2017; Roy et al., 2021). To tackle oxidation-mediated degradation, formulations can be exposed to stress factors, which were developed to investigate the decomposition process as well as to identify degradation products (ICH Guideline Stability Testing, 1996). These stress conditions include exposure of polysorbates to evaluate the impact of e.g. temperature, iron or transition metals (Brovč et al., 2020a; Liu et al., 2022a; Hvattum et al., 2012; Kranz et al., 2019; Gopalrathnam et al., 2018; Schröter et al., 2021; Bensaid et al., 2022; Doyle et al., 2019; Kranz et al., 2020; Klair et al., 2021; Prajapati et al., 2022), H2O2 (Kishore et al., 2011a; Kranz et al., 2019; Peters et al., 2022), light exposure (Ha et al., 2002; Doyle et al., 2019; Doshi et al., 2021a; Singh et al., 2012; Agarkhed et al., 2013; Prajapati et al., 2020, Prajapati et al., 2022, Larson et al., 2020; Lei et al., 2021), or 2,2-azobis(2-amidinopropane) dihydrochloride (AAPH) (Borisov et al., 2015; Larson et al., 2020; Zhang et al., 2017; Schröter et al., 2021; Zhang et al., 2018), all promoting the formation of radicals resulting in oxidative degradation. As oxidation of polysorbates can occur at: (i) the POE moieties, which can also result in free fatty acids and (ii) the existing double bonds (Donbrow et al., 1978a; Zhang et al., 2017; Yao et al., 2009), it is discussed whether PS80 or PS20 or different subspecies are most susceptible to oxidative degradation (compare Fig. 5 and Fig. 6). Subsequently, we will summarize oxidative degradation products, elaborate key characteristics of oxidative-mediated PS degradation in respect to different PS types and grades, followed by an extensive comparison between PS20 and PS80 and their grades. Finally, we will summarize current available stress studies of polysorbate to provide a common picture of the different stress conditions as well as suitable mitigation strategies and reagents.
-
a.Degradation products and oxidative markers
-
I.Degradation products
-
I.
Technologies detecting the degradation products, such as liquid chromatography coupled to mass spectrometry (LC-MS), or 1H-NMR are suitable to track oxidation (Kishore et al., 2011b; Khossravi et al., 2002; Hvattum et al., 2012; Ilko et al., 2015; Zhang et al., 2015). These methods focus on detecting established oxidation markers and/or relevant degradation products such as aldehydes or ketones, short-chain acids, shortened POE chains, or short-chain POE fatty acid esters to verify oxidative behavior (Kishore et al., 2011a; Evers et al., 2020; Zhang et al., 2017; Dahotre et al., 2018; Hvattum et al., 2012; Kranz et al., 2020). Formaldehydes as oxidative degradation products, may be formed through C-C bond cleavage by hydroperoxides or free radicals (compare Fig. 5) (Kerwin, 2008). A broad overview of degradation products was provided by Kishore et al. (2011), analyzing PS20 and PS80 by stir-bar-assisted sorption extraction gas chromatography-mass spectrometry (SBSE-GC-MS) (Kishore et al., 2011a). In general, degradation products were divided into five groups: (i) ketones (C5-C15), (ii) aldehydes (C5-C15), (iii) furanones (C5-C9), (iv) fatty acids (C10-C14), and (v) fatty acid esters (C12-C16). Formic acid, acetic acid, acetaldehyde, and formaldehyde were found originating to arise from β-scission of alkoxy radicals, by a six-membered intramolecular decomposition, or might be generated via an epoxy intermediate (compare Fig. 4) (Kishore et al., 2011a). Many different aldehydes like decanal, nonanal, octanal, and hexanal (to name a few) were generated as secondary products from hydroperoxides by oxidation of the double bond of unsaturated oleic, linoleic, or linolenic acids. By analyzing protein formulations stored for 20 months at 25 °C, they detected primarily C10-C16 fatty acids and fatty acid esters for PS20, indicating oxidative scission of the POE chain (Kishore et al., 2011a). In contrast, C8-C9 fatty acids and the lack of oleic acid were found in PS80 samples, suggesting cleavage at the double bond (Kishore et al., 2011a). Some of those degradation products like short-chain POE monoesters have also been found by Hvattum and colleagues (2012), by using LC-MS with argon as collision gas for collision-induced dissociation (CID) and 1H-NMR spectroscopy (Hvattum et al., 2012). Using CID-MS they could compare PS80 species esterified with different fatty acids before and after incubation at 40 °C for 8 weeks. C18:1 and C18:2 species were investigated more closely, showing that C18:2 species were nearly-completely degraded after 8 weeks at 40 °C to circa 30 % and 8 % for POE sorbitan and POE isosorbide/POE esters, whereas C18:1 species degraded to approximately 86 % and 80 % for POE sorbitan and POE isosorbide/POE esters for the same conditions. Additionally, they observed oxidation products of oleic acid, such as sorbitan/isosorbide/POE esters of hydroxyl-C18:1 (C18:1-OH), epoxy-C18:0, or keto-C18:1, as well as short-chain POE esters of C18:1 (Hvattum et al., 2012). Borisov and coworkers (2015) revealed degradation products of PS20 and PS80 after stressing with AAPH at 40 °C (Borisov et al., 2015). For PS20 and PS80 5 mM and 1.5 mM AAPH for 40 h and 12.5 h were used, respectively. Comparable to Hvattum et al. (2012), they used LC-MS with CID to investigate fragments of the different fatty acids in PS20 and PS80. For PS80, 9-oxononanoic acid (oxo-C9:0), keto-C18:1, hydroxyl-C18:1, epoxy-C18:0, hydroperoxyl-C18:1, and shorter POE versions (result of ether bond scission) of them were found as degradation products, whereas for PS20 mainly shorter POE chain derivatives were observed (Borisov et al., 2015). Additionally, free fatty acids can be generated by oxidation, however, their concentration is notably lower in comparison to hydrolytic degradation (Zhang et al., 2017). Here, Zhang and coworker (2017) developed an UPLC-MS method to differentiate between hydrolysis and oxidation via 18O-labeling technique, where they were able to detect free lauric acid and POE-laurate after oxidative degradation with 1.5 mM AAPH at 40 °C (Zhang et al., 2017). Two mechanisms were proposed, (i) starting with H-abstraction from the C-H bond on the POE chain immediately adjacent to the ester bond (α-C-H bond) and (ii) H-abstraction from the ß-C-H group. Hydrogen abstraction from the ß-C-H group is proposed to be the more favored mechanism based on the higher bond dissociation energy for the C-H bond immediately adjacent to the ester bond, and as there has to be a ratio of 16O:18O of free lauric acid of 3:1, in case of α-C-H abstraction, which could not be detected experimentally (Zhang et al., 2017).
-
II.
Oxidation markers
With the improved knowledge of oxidative degradation, oxidation markers were detected and used. Generally, it can be distinguished between ‘universal markers’ which can be applied for both polysorbate species or markers which are specific for PS20 or PS80 (Table 3). Here, Dahotre and colleagues (2018) identified an aldehyde derivate of fatty acid esters as result of the POE chain scission, which can be derivatized to analytically monitor PS20 oxidation (Dahotre et al., 2018). Theoretically, this approach should be applicable for PS80 as well, as it detects the ether bond scission of the POE chain, although it was not verified for PS80 in their study. Some of the already mentioned degradation products were summarized by Kranz et al. (2020), however, also additional oxidation markers were identified via LC-MS by stressing PS20 and PS80 in the presence and absence of protein with 10 ppb Fe2+ at 40 °C (Kranz et al., 2020). As universal markers they revealed peroxyl derivatives and glycolic acid esters of POE with varying chain length (6-10 POE units), which can be found in PS20 (MC/AL) and PS80 (MC/AO) (Kranz et al., 2020). Furthermore, specific markers for PS20 (MC/AL) like POE-laurates with a terminal esterified glycolic acid moiety with POE chain length of 1-6 (or more) units and oxo-lauric acid derivates were observed (Kranz et al., 2020). However, the exact position of the keto group was not defined, allowing different isomers to be present (Kranz et al., 2020). The novel degradation markers as well as the already published degradation products for PS80 summarized by Kranz and colleagues (2020) are listed in Table 3. Acids such as the aforementioned formic acid (Donbrow et al., 1978a; Donbrow et al., 1978b; Brovč et al., 2020a), glycolic acid, and acetic acid were for instance found by Brovč et al. (2020) (Brovč et al., 2020a). They used UPLC coupled to UV detection after derivatization with 2-nitrophenylhydrazine to identify formic acid, acetic acid, glycolic acid, and propionic acid as well as formaldehyde, acetaldehyde, propanal, and acetone after stressing polysorbates at 50 °C for 2 months in histidine buffer. Elevated levels of acetaldehyde, acetone, and propanal could be detected, whereas acetaldehyde is most likely formed by oxidative scission of POE units, while acetone and propanal are more likely generated by the oxidation of oleic acid (Brovč et al., 2020a). Additionally, they found 1,9-nonanedioic acid as degradation product of PS80 (Brovč et al., 2020a). Schröter et al. (2021) used 2,4-dinitrophenyl hydrazine (DNPH) for derivatization of 4-hydroxynonenal, being a result of poly-unsaturated fatty acid degradation (Schröter et al., 2021). Their derivation assay of carbonyls with DNPH leads to hydrazones that can be detected by LC-MS. By applying different stress conditions for 5 weeks they obtained ppb concentrations of 4-hydroxynonenal in 10 % (w/v) PS80 solutions of different grades, varying in the butylhydroxytoluene (BHT) concentration (Schröter et al., 2021).
Table 3.
Oxidation markers or specific degradation products for polysorbate 20 and 80. Published oxidation markers or specific degradation products of PS20 and PS80 are summarized below. Not all identified degradation products are summarized. Only derivates, which are reported as oxidation markers or specific degradation products are listed.
| Oxidation marker | Marker type | Applied stress | Molecular weighta / g·mol-1 | Putative structure | Referencesb |
|---|---|---|---|---|---|
| PEGx10 peroxide | Universal | 10 ppb Fe2+ & 40 °C | 474.2676 | Kranz et al. (2020) (Kranz et al., 2020) | |
| PEGx9 glycolic acid ester | Universal | 10 ppb Fe2+ & 40 °C | 472.2520 | 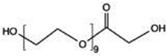 |
Kranz et al. (2020) (Kranz et al., 2020) |
| Glycolic acid ester-PEGx2-laurate | PS20 | 10 ppb Fe2+ & 40 °C | 346.2355 | Kranz et al. (2020) (Kranz et al., 2020) | |
| 12-oxo-lauric acid | PS20 | 10 ppb Fe2+ & 40 °C | 214.1569 | Kranz et al. (2020) (Kranz et al., 2020) | |
| 9-oxo-C9:0-ester† | PS80 | 1.5 mM AAPH | 1418.8232† | Borisov et al. (2015) (Borisov et al., 2015) | |
| Hydroxy-C18:1-ester† | PS80 | 40 °C | 1544.9641† | 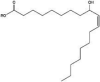 |
Hvattum et al. (2012) (Hvattum et al., 2012) |
| Hydroperoxyl-C18:1-ester† | PS80 | 1.5 mM AAPH | 1560.959† | 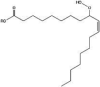 |
Borisov et al. (2015) (Borisov et al., 2015) |
| Keto-C18:1-ester† | PS80 | 40 °C | 1542.9484† | 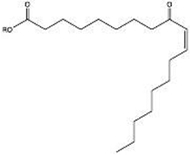 |
Hvattum et al. (2012) (Hvattum et al., 2012) |
| Epoxy-C18:0-ester | PS80 | 40 °C | - | 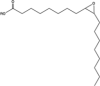 |
Hvattum et al. (2012) (Hvattum et al., 2012) |
| 1,9-nonanedioic acid‡, ⨁ | PS80 | 50 °C | - | Brovč et al. (2020) (Brovč et al., 2020a) | |
| 2-decenedioic acid-ester‡ | PS80 | Stored under air | - | 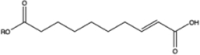 |
Liu et al. (2022) (Liu et al., 2022a) |
Molecular weight is given for the original structure and not for the measured adduct.
Reference, which first reported and verified the degradation product.
Kranz et al. (2020) provided already published oxidation markers from Borisov et al. (2015) and Hvattum et al. (2012) including their molecular weights: esters of 9-oxo-C9:0, hydroxy-C18:1, hydroperoxyl-C18:1, and keto-C18.
Specific degradation products not reported as oxidation markers.
1,9-nonanedioic acid is esterified to sorbitan-POE.
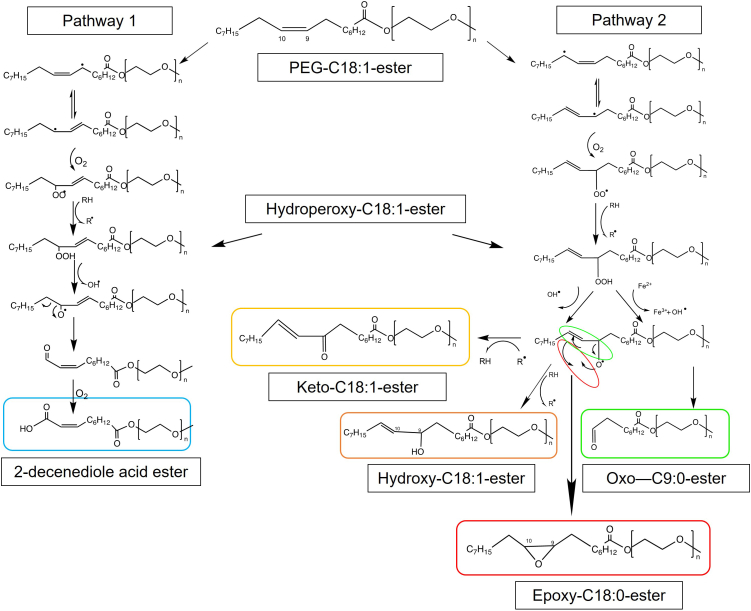
Li et al. (2022) postulated degradation pathways for PS80 and its major degradants (see Fig. 7). Many oxidation markers such as esters of oxo-C9:0, hydroxy-C18:1, epoxy-C18:0, and keto-C18:1 are presented by Li et al. (2022). They stressed polysorbates with Fe2+ at elevated temperatures in the absence of nitrogen followed by analysis via LC-MS (see Fig. 7) (Liu et al., 2022a). These markers were also described previously (compare Table 3) (Borisov et al., 2015; Hvattum et al., 2012), however, 2-decenedioic acid esters were found as novel degradation products of PS80 (Liu et al., 2022a). Additionally, two degradation pathways for oxidative degradation of PS80 including the found degradation products were postulated, starting with a hydrogen abstraction of oleic acid and distinguishing in the position of the radical in the vicinity of the double bond. In pathway 1 (see Fig. 7), the radical is formed on C8 of the oleic acid, followed by isomerization, O2 addition at C10, and formation of esters of hydroperoxyl-C18:1 (C10 position). Subsequently, the peroxide is cleaved resulting in an alkoxy radical (C10) and finally in esters of 2-decenedioic acid. Pathway 2 starts with hydrogen abstraction and radical formation on C11 of the oleic acid, followed by isomerization, O2 addition at C9, formation of hydroperoxyl-C18:1 (C9), and resulting in an alkoxy radical of oleic acid at C9. Here, different reactions are distinguished resulting in esters of either oxo-C9:0, hydroxy-C18:1, keto-C18:1, and epoxy-C18:0 (compare Fig. 7).
-
b.
Key characteristics for the oxidation of polysorbates
Fig. 7.
Two possible oxidative degradation pathways of PS80 postulated and adapted based on identified degradation products from Liu et al. (2022) (Liu et al., 2022a).
The most common oxidation mechanism involves radical-induced cleavage of the ether bonds within the POE sidechains, which is characteristic for polysorbate oxidation, but could also occur within other surfactants having POE sidechains (Kishore et al., 2011a; Donbrow et al., 1978a; Borisov et al., 2015; Larson et al., 2020). The free radical reactions naturally shorten the POE chain, impacting surface activity, CMC, and cloud points of PS20 (quality not stated) by changing the ratio between hydrophilic and hydrophobic parts as has been shown by induced oxidation by incubating polysorbate at 25, 40, 60, and 70 °C (Donbrow et al., 1978a). Nevertheless, Larson et al. (2020) observed a neglectable change in the CMC measured via pyrene fluorescence after PS80 (PS quality not stated in the paper) oxidation with AAPH, whereas higher values for the surface pressure isotherms in histidine buffer of the oxidized PS80 (approximately 18 mN·m-1 after 50 min) were detected in comparison to untreated PS80 (approximately 15 mN·m-1 after 50 min) by Langmuir trough (Larson et al., 2020).
To determine whether oxidation has occurred in a particular pharmaceutical formulation, guidance can be obtained using the key characteristics listed in this section (see also Table 4).
-
I.
pH shifts
Table 4.
Summarized key characteristics of oxidative PS degradation. Different characteristics are evaluated between PS20 and PS80 with positive (+) for true, negative (-) for false, (+/-) for controversial (not distinct), or with (n.a.) for not applicable.
| Key characteristic | PS20 | PS80 | References |
|---|---|---|---|
| pH shifts (weakly-buffered systems) | + | + | Donbrow et al. (1978) (Donbrow et al., 1978a) |
| Preferred degradation of higher-order esters | + | +/- | Kranz et al. (2019) (Kranz et al., 2019), Kranz et al. (2020) (Kranz et al., 2020), Zhang et al., (2017) (Zhang et al., 2017), Borisov et al. (2015) (Borisov et al., 2015), Liu et al. (2022) (Liu et al., 2022a), Zhang et al. (2018) (Zhang et al., 2018), Bensaid et al. (2022) (Bensaid et al., 2022), Lippold et al. (2017) (Lippold et al., 2017), Brovč et al. (2020) (Brovč et al., 2020a) |
| Species of lower hydrophobicity appearing in the chromatogram | - | + | Kranz et al. (2020) (Kranz et al., 2020), Kranz et al. (2019) (Kranz et al., 2019), Borisov et al. (2015) (Borisov et al., 2015), Lippold et al. (2017) (Lippold et al., 2017), Brovč et al. (2020) (Brovč et al., 2020a), Liu et al. (2022) (Liu et al., 2022a) |
| Dominant degradation of longer chain fatty acid esters | + | n.a. | Borisov et al. (2015) (Borisov et al., 2015), Zhang et al. (2018) (Zhang et al., 2018) |
| Competition of protein and PS oxidation | + | + | Brovč et al. (2020) (Brovč et al., 2020a), Bensaid et al. (2022) (Bensaid et al., 2022), Gopalathram et al. (2018) (Gopalrathnam et al., 2018) |
pH shifts in unbuffered polysorbate solutions as key characteristic of polysorbate oxidation was first shown nearly half a century ago (Donbrow et al., 1978a). Here, Donbrow and coworkers (1978) evaluated 3 % (w/v) PS20 (quality not stated in the paper) oxidation reactions by determining cloud points, pH values, peroxide values, and surface tensions after storage at elevated temperature ranging from 25 to 70 °C depending on the experiments with or without Cu2+ (10-4 M) or after addition of NaCl (Donbrow et al., 1978a). They demonstrated three phases of oxidation and revealed that catalysts like copper sulfate or light (daylight vs. darkness) are shortening the induction phase and accelerating peroxide formation rate as well as decreasing the cloud point and pH value for unbuffered PS solutions (Donbrow et al., 1978a). Within 10 days at 25 °C and exposed to daylight, the pH value decreased by more than 1 pH unit, whereas it drops by more than 3 pH units at 60 or 70 °C (Donbrow et al., 1978a). Nevertheless, it is important to note that such high temperatures do normally not occur during production processes. For different temperatures higher peroxide numbers (mEq·kg-1 surfactant) after light exposure were detected spectrophotometrically (Donbrow et al., 1978a; Azaz et al., 1973). Peroxide numbers >5 mEq·kg-1 surfactant are reported as autoxidation, showing a characteristic kinetic pattern with a strong increase to a maximum peroxide number value and a subsequent decrease (Donbrow et al., 1978a). Hence, they revealed that low peroxide values cannot directly be interpreted as lack of oxidation, as there is a general consumption and generation of peroxides depending on the timeframe of the oxidation process (Donbrow et al., 1978a). Initially, the rate of peroxide formation was higher than the rate of peroxide decomposition, whereas at some point the process reverses (the higher the peroxide concentration, the higher the likelihood for peroxides to react with other peroxides in decomposition reactions), and peroxides may be below the detection limit, which is in the micromolar range for the used method (Donbrow et al., 1978a; Azaz et al., 1973). Additionally, the thermal stability of peroxides has to be taken into account. Here, Ha and colleagues (2002) reported a complete loss of peroxides originated from a 20 % (w/v) PS80 solution (MC) in vacuum after 6 weeks at 60 °C and a reduction to 50 % at 40 °C after 4 weeks analyzed via the FOX assay (Ha et al., 2002). Again, the high polysorbate concentration and temperature applied in the presented study are extreme stress conditions.
-
II.
Preferred degradation of higher-order esters
Another key characteristic of oxidative degradation is that PS polyesters are significantly more affected by oxidation than monoesters as reported by various stress tests with either H2O2, AAPH, temperature, or iron (Borisov et al., 2015; Lippold et al., 2017; Liu et al., 2022a; Zhang et al., 2017; Kranz et al., 2019; Bensaid et al., 2022; Zhang et al., 2018; Kranz et al., 2020; Brovč et al., 2020c). Via CAD, Kranz and colleagues (2019) showed that higher-order esters of PS20 HP completely disappear after treatment with 10 ppb Fe2+ at 40 °C after seven days. Here, free POE moieties as indicator for monoester degradation only slightly increase, whereas for PS80 HP mono- and polyesters degrade in nearly similar rates with a slightly faster kinetic for higher-order esters. These findings of PS80 degradation were accompanied by a huge increase in free POE species up to 250 % (relative to t0) after nearly two weeks at 40 °C (Kranz et al., 2019). These findings were supported as well as extended by a second study of Kranz et al. (2020), showing similar behavior of PS20 AL and PS80 AO in respect to PS20 MC and PS80 MC, respectively (Kranz et al., 2020). Other research groups confirmed higher degradation of polyesters for PS20 AL or MC by forced oxidation via AAPH at elevated temperatures (Zhang et al., 2017; Zhang et al., 2018) as well as nearly similar degradation of mono- and polyesters for PS80 (quality not defined in the paper) using iron and temperature stress (Borisov et al., 2015). For PS80 (MC and ChP), Liu and colleagues (2022) reported using LC-MS that polyesters degraded more rapidly than monoesters for Fe2+-induced degradation at 25 °C (Liu et al., 2022a). Similarly, Bensaid et al. (2022) reported a prominent decrease in polyester and a less extensive degradation of monoester in PS80 MC in the presence of mAb (20 mg·mL-1) for stressing with 20 ppb Fe2+ in 10 mM histidine buffer pH 6.0 and 10 % (v/w) sucrose (Bensaid et al., 2022). Whereas, a common opinion about higher oxidation-induced polyester degradation is reported for PS20, some controversial is found for PS80. It is important to emphasize that higher-order esters can be degraded in monoesters, increasing the monoester fraction successively, potentially blurring the small difference for PS80 (Borisov et al., 2015). As only Kranz and colleagues (2019/2020) measured free POE moieties in parallel they could verify faster degradation of polyesters in PS20 as well as only slightly higher degradation of higher-order ester for PS80 (Kranz et al., 2019, Kranz et al., 2020). The other studies only checked on mono-, di-, tri-, and tetraesters or their rates and found similar results. Nevertheless, it should be emphasized that for instance for sorbitan-POE-laurate, a cleavage in only one chain of POEs would result in a detectable change via CAD, whereas for higher-order esters like tetraesters or triesters a change in every POE chain or in 3 of 4 POE chains would result in a detectable change via CAD, as the fatty acids were cleaved.
-
III.
Emerging of species with lower hydrophobicity
Another key characteristic for oxidative PS80 degradation is the appearance of multiple new species in the chromatograms with low hydrophobicity after forced oxidation (Brovč et al., 2020a; Borisov et al., 2015; Lippold et al., 2017; Liu et al., 2022a; Kranz et al., 2019; Kranz et al., 2020). The presence of double bonds is discussed as origin resulting in oxidation degradation products of oleic acid (Borisov et al., 2015; Liu et al., 2022a; Kranz et al., 2020). Liu et al. (2022) suggested, for instance, keto-oleic acid or hydroperoxyl-oleic acid with in-source water loss after performing LC-MS with CID as more hydrophilic degradation products of oleic acid for two of the early eluting species (Liu et al., 2022a). However, also other products were discussed as described above (see Fig. 7) (Liu et al., 2022a).
-
IV.
Dominant degradation of longer chain fatty acid esters
Additionally, Borisov and coworkers (2015) revealed a stronger degradation of PS species esterified to longer FA chains in comparison to shorter ones (Borisov et al., 2015). For instance, POE sorbitan mono-caprate (C10:0) esters are more resilient to oxidative stress with AAPH after 19 h and 40 h, respectively, compared to POE sorbitan mono-stearate (18:0) esters (Borisov et al., 2015). These results were supported by Zhang et al. (2018), demonstrating reduced APPH induced degradation of laurates in comparison to stearates and revealing another key characteristic of polysorbate oxidation (Zhang et al., 2018). Exact rationales behind the preferred oxidation of longer fatty acid POE sorbitans are unknown, however, preferential ether bond scission within the esterified POE chain were argued due to different arrangements of the POE chain (Borisov et al., 2015).
-
V.
Competition of protein and PS oxidation
Another characteristic is the "competitive" oxidation between polysorbate and the protein present in formulations (Brovč et al., 2020a; Gopalrathnam et al., 2018; Bensaid et al., 2022). Brovč and colleagues (2020) investigated the degradation of PS80 AO (0.2 mg·mL-1) formulated in 20 mM histidine chloride buffer pH 6.5 in the presence of four different mAbs (10 mg·mL-1) at 50 °C for 1 month. In all cases less PS80 oxidation in comparison to the placebo formulation was observed, with even neglectable oxidation of PS for two of the four mAbs (Brovč et al., 2020a). A mAb concentration dependent effect was shown by Bensaid et al. (2022), as less PS80 MC oxidation was observed with increasing protein concentration ranging from 10 to 140 mg·mL-1, although the samples were stressed with 50 ppb iron at 40 °C (Bensaid et al., 2022). The protective effect of protein on PS80 (quality not defined) oxidation was also reported by Gopalrathnam et al. (2018), as no PS80 oxidation was observed for samples containing mAb >40 mg·mL-1 for the storage of 10 month at 25 °C (Gopalrathnam et al., 2018). As active pharmaceutical ingredients are typically formulated in high protein concentrations, there are stochastically more protein molecules in the solution compared to surfactant molecules, which would result in higher protein oxidation for diffusion driven oxidation (OH• radicals). For example, there are >6 times more mAb molecules in a formulation with 0.2 mg·mL-1 (∼0.16 mM) polysorbate 20 and 150 mg·mL-1 (∼1 mM) antibody (Mw PS20 ∼1227 g·mol-1 and Mw mAb ∼150,000 g·mol-1). Therefore, the likelihood for radicals to attack an antibody molecule will be higher as finding a surfactant molecule based on solely stochastically assumptions. Oxidation of the protein is not intended as it can influence the drug efficacy, but it has been observed multiple times (Brovč et al., 2020a; Gopalrathnam et al., 2018; Bensaid et al., 2022). However, the presence of polysorbate itself results in increased oxidation of the proteins as shown for instance by Ha et al. (2002), Lam et al. (2011), and Klair et al. (2021) to name a few (Ha et al., 2002; Klair et al., 2021; Lam et al., 2011).
-
c.
Is PS80 more prone to oxidation than PS20?
It is usually claimed that PS80 is more susceptible to oxidative degradation compared to PS20, due to the higher ratio of esterified unsaturated fatty acids (Brovč et al., 2020a; Kishore et al., 2011a; Kishore et al., 2011b; Borisov et al., 2015; Liu et al., 2022a; Larson et al., 2020; Yao et al., 2009; Hvattum et al., 2012). However, an in-depth comparison between both polysorbate types in a concentration dependent manner and under pharmaceutical relevant conditions, is currently not available in the literature. This chapter will elaborate and summarize the public available conducted studies that examined the different degradation of PS20 compared to PS80.
A study that pursued the question which surfactant might be more susceptible on oxidative processes, was conducted by Yao et al. (2009), using the difference in reaction heats for H-absorption by hydroperoxyl radicals to demonstrate that the oxidation is energetically favored in the vicinity of the unsaturated site rather than at the POE units (Yao et al., 2009). After stressing with AAPH at 40 °C, differences in oxidizability constants between PS80 and PS20 (the authors used highest Sigma Aldrich grade at that time) of 2.65 were reported (Yao et al., 2009). It should be mentioned that quite high concentrations of PS80 and PS20 were used, ranging between 0.5 and 5.0 % (w/v) as well as between 0.5 and 4.0 % (w/v), respectively (Yao et al., 2009). However, they concluded that two-thirds of the oxidation occurs in the vicinity of the unsaturated fatty acid ester groups of PS80, as otherwise the oxidizability constants should be identical for PS20 and PS80, when oxidation is only occurring at the identical amounts of POE subunits. In PS20, oxidation is mainly occurring at the POE groups, based on the small amounts of unsaturated fatty acids (Yao et al., 2009). The different heats of reaction for H-absorption by hydroperoxyl radicals illustrates that unsaturated fatty acids are more affected by oxidation compared to POE units. Likewise, it seems logical that there would be differences in the oxidizability constants, which had to be the same for PS20 and PS80 if they had identical degradation kinetics.
A different approach was pursued by Kishore and colleagues (2010) investigating oxidation of PS20 MC and PS80 MC raw materials via thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) over a temperature range from 25 to 400 °C (Kishore et al., 2011b). By combining TGA and DSC loss/gain in weight as well as the thermodynamic heat loss/gain profiles they extracted information on oxidation. Additionally, they compared Span-80 (sorbitan ester of oleic acid) and PS80 to verify the influence of the unsaturated oleic acid upon oxidation. They revealed that thermal oxidation of PS80 is dominated by the POE chain, although it is initiated at the olefinic site (Kishore et al., 2011b). PS80 raw material showed slightly higher peroxide rates and slightly more degradation than PS20 due to the degree of unsaturation, however, the overall degradation profiles were comparable for storage at 40 °C for 24 weeks (Kishore et al., 2011b). In a second study, they compared degradation products of PS20 and PS80 via MS and were able to differentiate in POE oxidation (formation of formates) as well as in scission in the vicinity of the double bond (alkyl aldehydes, alkanes, and dicarboxylic acids) (Kishore et al., 2011a; De Sainte, 2009). GC-MS analysis of PS20 and PS80 detected hexane, pentane, and heptanal as well as butanal, pentanal, hexanal, and heptanal, respectively (Kishore et al., 2011a). Thus, while these results demonstrate oxidation occurring at the unsaturated fatty acid site, they unfortunately did not show whether oxidation is a more severe problem at PS80 compared to PS20.
Borisov and coworkers (2015) demonstrated that after stressing with the artificial radical initiator AAPH, a higher oxidative susceptibility of unsaturated fatty acids is observed (Borisov et al., 2015). The degradation product oxo-nonanoic acid was detected by LC-MS, originating from oxidation of the oleic acid (Borisov et al., 2015). Additionally, pseudo first-order rates for the decomposition of POE sorbitan esters were determined after stressing with AAPH, revealing higher rates for unsaturated fatty acids (Borisov et al., 2015). They clearly showed a faster oxidation of oleic acid species (PS80), however, the impact under pharmaceutical conditions, without additional stressors was not investigated.
Comparing PS20 and PS80 of different vendors and in different buffer systems, Brovč et al. (2020) observed higher degradation in PS80 relative to PS20 at 50 °C in some conditions, suggesting unsaturated fatty acids causing the difference (Brovč et al., 2020a). The detection of the degradation products like 1,9-nonanedioic acid additionally revealed oxidation at the double bond of oleic acid (Brovč et al., 2020a). Nevertheless, this observation was less obvious for all tested conditions, as some grades of PS80 revealed slower oxidation kinetics than PS20, as after one month more PS80 content was measured in comparison to PS20.
Liu et al. (2022) also reported that the oxidation of PS80 MC or AO mainly occurs at the double bond of unsaturated fatty acids (Liu et al., 2022a). They developed an LC-MS detection method to distinguish the degradation products of different PS80 grades and vendors by distinct fingerprints due to variations in the fatty acid compositions (Liu et al., 2022a). By monitoring specific degradation products such as 9-oxononanoic acid, keto-oleic acid, and 2-decenedioic acid species, after stressing PS80 MC or AO with 50 ppb iron at 25 °C, they demonstrated that the oxidation primarily occurs at the double bond of unsaturated fatty acids, since the POE structures remained intact (Liu et al., 2022a). Saturated fatty acid derivatives, such as POE palmitic acid monoesters showed higher stability (Liu et al., 2022a). These results were supported by Hvattum and colleagues (2012) reporting higher susceptibility of PS80 after temperature stress, by observing complete degradation of C18:2 after 8 weeks at 40 °C via LC-MS. (Hvattum et al., 2012) For the oleic acid a decrease of 20 % was monitored in short-chain POE esters and C18:1 oxidation products, however, the overall degradation rate was reported to be comparable to other esterified FAs (Hvattum et al., 2012). It needs to be clarified whether the entire degradation of C18:2 is indeed responsible for the overall accelerated degradation of PS80.
Larson and coworker (2020) stressed their PS80 samples with AAPH, showing that peroxyl radicals mainly attack PS80 on the double bond and in fact showed higher degradation, if higher amounts of double bonds like linoleic or linolenic acids were involved (Larson et al., 2020). Nevertheless, degradation products derived from both, the unsaturated fatty acids and POE units (Larson et al., 2020).
Although, there are many studies reporting and demonstrating oxidation of PS80 on the unsaturation site based on degradation products of oleic or linoleic acid, less is known about a direct comparison between PS20 and PS80 under pharmaceutical relevant conditions (without additional stressors). Or to rephrase it, is the difference of PS20 and PS80 oxidation relevant for the pharmaceutical industry and are products stored at 5 °C drastically affected? Up to now, only Kishore and colleagues (2010) measured a slightly higher peroxide formation in PS80, however, the degradation comparison between PS20 and PS80 over 24 weeks at 40 °C was nearly similar, making a conclusive statement rather difficult (Kishore et al., 2011b). Furthermore, Brovč et al. (2020) compared PS20 and PS80 from different manufactures, buffers, as well as at different pH values (pH 5.5 and 6.5) and could show that after 2 months a lower PS concentration can be monitored for PS80 (mainly in histidine chloride and sodium succinate buffer) (Brovč et al., 2020a). However, recently Kranz and colleagues (2020) observed similar polyester degradation of PS20 AL and PS80 AO, despite the higher oleic acid content of the all-oleate quality (Kranz et al., 2020). The question regarding PS20 or PS80 being the better surfactant is quite complex as it depends on many factors. To sum it up, although higher oxidation of the unsaturated fatty acids was demonstrated, there are currently not enough data to clearly show that PS80 is more prone to oxidation under pharmaceutical relevant conditions like temperatures of 5 °C. For an exact comparison, type and degree of stress should be considered as well.
-
d.
Impact of polysorbate 20 and 80 qualities on oxidation
-
I.
Purer polysorbate grades – PS20 AL and PS80 AO
While in recent years much effort has been spent on developing and commercializing purer or less heterogeneous polysorbates, studies published so far have not demonstrated any relevant advantage regarding oxidative polysorbate degradation. It seems that the functionality of PS is still not understood, as even poorer performance was reported (Brovč et al., 2020a; Doshi et al., 2020a; Liu et al., 2022a; Kranz et al., 2019; Bensaid et al., 2022; Kranz et al., 2020).
Due to the current issues and the intrinsic heterogeneity of PS, purer grades were developed, like PS20 AL and PS80 AO with higher amounts of the corresponding fatty acids. To identify advantages and disadvantages of this new polysorbate grades towards oxidative degradation, studies were conducted comparing different polysorbate qualities. Several studies demonstrated a faster oxidative degradation of the PS20 AL and PS80 AO grades after exposure to H2O2 or iron (Liu et al., 2022a; Kranz et al., 2019; Bensaid et al., 2022; Kranz et al., 2020). For instance, Kranz and colleagues (2019/20) investigated the oxidation behavior of the ultrapure polysorbates PS20 AL and PS80 AO in comparison to the MC grades of PS20 and PS80, respectively (Kranz et al., 2019; Kranz et al., 2020). They induced oxidative degradation by spiking Fe2+ or H2O2 at 40 °C (Kranz et al., 2019; Kranz et al., 2020). In the presence of H2O2 a greater loss in PS20 MC and AL as well as PS80 MC and AO concentration was observed in comparison to samples formulated in the absence of H2O2 (Kranz et al., 2019). By monitoring the polyester fractions of the different polysorbates, a slightly higher susceptibility was observed for the PS20 AL variant, whereas a drastically higher degradation was exhibited for the PS80 AO grade in comparison to their MC grades (Kranz et al., 2019). A similar behavior was detected for 10 ppb Fe2+ and 40 °C, with even faster degradation in comparison to the presence of just H2O2 (Kranz et al., 2019). Supporting results were obtained in their second study (Kranz et al., 2020). By tracking oxidation markers after oxidation stress, faster degradation for the ultrapure grades was confirmed (Kranz et al., 2020). This observation is matching with results from other studies, detecting higher oxidative susceptibility of the ultrapure grades (Brovč et al., 2020a; Liu et al., 2022a; Kranz et al., 2019; Bensaid et al., 2022; Kranz et al., 2020). Liu et al. (2022) investigated PS80 MC against ChP quality (AO) after exposure to 50 ppb Fe2+ at RT and hypothesized that the faster PS80 AO degradation is based on the approximately 10 % increased unsaturated fatty acids content (Liu et al., 2022a). Faster degradation of PS80 AO was also indicated by Brovč et al. (2020), investigating different PS80 grades from different vendors in various buffers (Brovč et al., 2020a). Similar behavior was observed in the presence of proteins, as Bensaid et al. (2022) stressed PS80 AO and MC grade in the presence of 20 mg·mL-1 mAb with 5 ppb Fe2+ at elevated temperatures and analyzed the PS degradation as well as the Met255 oxidation (Bensaid et al., 2022). Samples of the ultrapure grades (PS80 AO) exhibited faster PS decomposition as well as Met255 oxidation after 3 months at 25 °C and 1 month at 40 °C (Bensaid et al., 2022). Although many studies revealed higher susceptibility for oxidative degradation of ultrapure polysorbate qualities, protein protection against mechanical stress was not affected by the different qualities (Grabarek et al., 2020). For instance, Grabarek et al. (2020) exposed PS80 MC and AO to various mechanical stress conditions with no distinguishable differences in mAb functionality (Grabarek et al., 2020).
-
II.
PS20 HP vs. PS20 SR
Up to the authors knowledge, only one study compared polysorbate of HP and SR quality (Doshi et al., 2020a). Here, Doshi and coworkers (2020) showed that PS20 SR grade is more susceptible for oxidation than the corresponding HP quality, arguing with higher values of PS20 degradation, protein oxidation, and peroxide formation (Doshi et al., 2020a). Generally, it is reported by Doshi et al. (2020) by comparing the certificate of analysis that PS20 SR possesses a reduced content of stearate (6.3 % in PS20 HP vs. 1.1 % in PS20 SR) and correspondingly more myristate (18.5 % in PS20 HP vs. 22.5 % in PS20 SR). Also the additional proprietary flash chromatography of SR shows reduced content of impurities such as peroxides or aldehydes (Doshi et al., 2020a; Smaltz, 2013). Additionally, it was demonstrated for three different lots by ICP-MS that PS20 SR contains less transition metals like iron or chromium (4-8 ppb iron, <1-1.5 ppb chromium) in comparison to PS20 HP (11-15 ppb iron, 1-3 ppb chromium) (Doshi et al., 2020a). Therefore, the authors actually expected a higher susceptibility of PS20 HP, if only these data were considered. They tested the performance of 0.03 % (w/v) PS20 SR and HP in eight mAb formulations in histidine acetate buffer at pH 5.5 and monitored PS degradation, protein oxidation, and protein aggregation for different time periods at 2-8, 25, and 40 °C (Doshi et al., 2020a). The formulations containing PS20 HP demonstrated lower M252 oxidation, lower HMWs formation, as well as lower PS20 degradation mitigated by the presence of 10 mM methionine (Doshi et al., 2020a). The observed differences were explained by the faster peroxide formation of PS20 SR at 40 °C with PS20 HP showing a lag phase of approximately one week, monitored via the fluorometric Amplex® UltraRed HRP assay (Doshi et al., 2020a). The slight variation in oleate esters between PS20 SR and HP could also be a rationale behind the differences, however, it could not be definitively proven (Doshi et al., 2020a). This comparison of PS20 SR and HP demonstrates that a purified grade not necessarily possesses improved oxidation stability performance.
Recently, temperature-induced oxidation of two different PS20 HP qualities (0.2 % (w/v)) were compared in histidine acetate buffer and sucrose at pH 5.5 (Doshi et al., 2021b). The modified PS20 HP (RO PS20 HP) contained less stearic, palmitic, and myristic acid but increased lauric, capric, and caprylic acid content. No general difference was detected between both HP quantities after 5 weeks at 40 °C, most likely due to the small variations in fatty acid composition. However, it seems that RO PS20 HP showed initially a slightly higher rate of decrease (Doshi et al., 2021b).
-
e.
Buffers affecting polysorbate oxidation
PS oxidation can lead to shifts in the pH value, due to the formation of short-chain acids such as formic and acetic acid (Kishore, 2018; Donbrow et al., 1978a; Donbrow et al., 1978b). However, this phenomenon is often compensated by the buffer system, whereas in water (unbuffered) a decreasing pH over time can be observed (Donbrow et al., 1978a). Buffering compounds are essential excipients in biopharmaceutical formulations, contributing to the overall stability of the drug product (Zbacnik et al., 2017). Common buffer systems are acetate, histidine, citrate, phosphate, and succinate or mixtures of them (Brovč et al., 2020a; Zhou et al., 2011). Subsequently, we will evaluate the impact of these buffer systems on oxidative polysorbate degradation.
Falconer, 2019 Histidine is used to adjust the pH value in formulations between 5.5 and 6.5, and is described as being a free-radical or reactive oxygen scavenger (Wade and Tucker, 1998; Imre and Floyd, 1984; Matheson and Lee, 2008). It also may provide effective protection against oxidative stress originating from AAPH (Zhang et al., 2017). Nevertheless, there are indicators for discoloration of histidine buffers most likely due to chemical oxidation and the presence of metal ions, which are speculated as root cause to induce oxidation (Brovč et al., 2020a; Gopalrathnam et al., 2018; Chen et al., 2003). The effect of histidine on oxidative polysorbate degradation is heavily discussed in the literature (Brovč et al., 2020a; Doyle et al., 2019; Kranz et al., 2019; Gopalrathnam et al., 2018; Zhang et al., 2018). For instance, Zhang et al. (2018) reported a dual role of histidine on PS20 MC stability, by forcing oxidative degradation in the presence of AAPH spiking at 40 °C as well as by accelerated stability studies at the same temperature (Zhang et al., 2018). After stressing PS20 with AAPH, they reported that PS20 degradation is delayed in the presence of histidine-acetate buffer in comparison to solely sodium-acetate buffer. This protective role is proposed originating from histidine adducts with the peroxyl radicals formed after AAPH decomposition, whereas no positive effect was observed for histidine-acetate buffer under accelerated stability conditions of 40 °C. Especially, the degradation of higher-order esters of PS20 is promoted in histidine-acetate buffer in comparison to sodium-acetate under temperature stress (Zhang et al., 2018).
Thereby, different functionalities of histidine can be discussed based on the applied stress, however, it is important to emphasize which buffer is used as comparison as well. For instance, Brovč and colleagues (2020) compared PS20 MC and PS80 MC degradation from different suppliers at elevated temperatures in different buffer systems at pH values of 5.5 and 6.5, revealing a faster degradation in histidine-chloride buffer compared to sodium-phosphate or sodium-citrate buffers (Brovč et al., 2020a). For PS20 MC (independent of the manufacturer) a reduction of more than 50 % was observed after 1 month in histidine-chloride buffer, whereas for sodium-phosphate, succinate, and citrate nearly no degradation was detected. After 2 months also sodium-succinate showed higher degradation, whereas for sodium-phosphate and sodium-citrate only small changes were detected. Similar findings were demonstrated for PS80 MC (Brovč et al., 2020a). However, a comparison in accordance with Zhang et al. (2018) with acetate buffer was not conducted (Zhang et al., 2018).
A positive effect of histidine buffer in comparison to acetate buffer in respect to polyester degradation in PS80 was observed by Kranz et al. (2019), after inducing oxidative degradation by H2O2 (Kranz et al., 2019). For acetate buffer at pH 5.5 a degradation of PS80 AO was observed, whereas polysorbate remained stable in histidine buffer at pH 6.0. The stability of PS80 AO in histidine buffer was even enhanced in the presence of 10 mM H2O2, whereas a complete reduction of the polyesters was observed after 17 days in acetate buffer with 1 mM H2O2. These protections against H2O2 stress of histidine buffer was speculated due to complex formation between histidine and H2O2, blocking either the δ-nitrogen or deactivating the impurities in the high purity grade histidine (Kranz et al., 2019). Such mitigating effects could not be shown for iron/stainless steel-forced oxidative degradation of polysorbates in histidine buffers. They also investigated different histidine grades (Ph. Eur. grade, BioUltra grade, or reagent grade) and observed different degradation rates after incubation at 40 °C of the polyester peak area of PS80 AO (Khossravi et al., 2002; Kranz et al., 2019). They argued that the oxidative degradation is most likely induced by impurities and contaminations of the excipients, as up to 10 ppm iron can be present in histidine of Ph. Eur. grade.
Gopalrathnam et al. (2018) induced oxidative PS80 (quality not defined in the paper) degradation by exposure to stainless steel and found a stronger reduction of the oleic acids content in histidine buffer in comparison to citrate buffer (Gopalrathnam et al., 2018). In a follow up study, they extended their research by comparing PS80 MC degradation forced by temperature, light, and stainless steel in histidine, citrate, and phosphate buffer (10 mM each, compare Table 6) (Doyle et al., 2019). Exposure to 50 °C for 14 days revealed a nearly complete degradation of PS80 in phosphate buffer, whereas no degradation was detected in histidine or citrate buffer. The contact with stainless steel for 30 days and exposure to 50 °C resulted in complete PS80 degradation in phosphate and histidine buffer in comparison to no content loss in citrate buffer using high buffer concentrations (Doyle et al., 2019). However, the opposite was observed by Brovč and coworkers (2020), demonstrating a reduced degradation of PS20 MC and PS80 MC/AO in phosphate buffer in comparison to histidine buffer at elevated temperatures (Brovč et al., 2020a). Light exposure (100 % ICH Q1B) and elevated temperatures (50 °C) for 30 days revealed complete stability for PS80 MC in citrate buffer, whereas PS80 degraded in histidine and phosphate buffer, with a faster rate followed by complete PS80 loss for the latter after 14 days (Doyle et al., 2019). Currently no comprehensive studies are available, comparing oxidative degradation of PS20 and PS80 in all different buffer systems, to evaluate which of all available buffer systems revealed the lowest PS degradation.
The comparison between PS80 degradation (MC and ChP) in histidine or citrate buffer after Fe2+ stress was also performed by Liu et al. (2022), demonstrating a chelating effect of citrate, whereas PS degradation was observed in the histidine system (Liu et al., 2022a). The protective effect of high concentrated citrate for oxidative degradation of PS20 MC and PS80 MC/AO are supported by findings of Brovč et al. (2020), revealing almost no PS20/80 degradation after exposure to 50 °C for sodium-citrate buffer (20 mM) of various vendors and pH values (Brovč et al., 2020a), as well as by Gopalrathnam and colleagues (2018) for PS80 (quality not defined in the paper), as mentioned above (Liu et al., 2022a; Doyle et al., 2019; Gopalrathnam et al., 2018; Bensaid et al., 2022).
Nevertheless, increased PS80 degradation (MC/AO) can occur in citrate buffers in combination with Fe3+ and UV light exposure (Prajapati et al., 2022). Fe3+ and citrate can create a water-soluble complex, which under the influence of UV light, forms radicals, Fe2+, and ROS with the potential to degrade polysorbate (Prajapati et al., 2022). Based on spin restriction, dioxygen (triplet state) cannot react directly with iron (singlet state). However, the coordination of iron to chelators is accompanied by an energy rearrangement, allowing O2 to interact through the d-orbitals, so that iron acts as bridge between the molecular oxygen and the biomolecule/chelator, rescinding the spin restriction (Welch et al., 2002; Glusker, 1980). In absence of one of those factors, no polysorbate 80 degradation was observed (Prajapati et al., 2022).
In general, many studies were performed, investigating the effect of buffers for oxidative-mediated PS degradation. Most of them in the absence of proteins, which makes observed effects mainly relevant for placebo studies. In cases protein being present, high amounts of citrate might induce the formation of protein particles, which should be strictly avoided (Barnett et al., 2015). However, it is important to emphasize that the applied stress can have huge impact on the results obtained for the individual buffer, as demonstrated for histidine. Additionally, it is important to shed light on the effects in relation to other tested buffer conditions. For the optimal selection, each buffer system must be considered carefully prior selection. A summary of publications with different buffers is given in Table 5.
-
f.
Summary of available literature on stress factors in oxidation studies
Table 5.
Overview of buffer studies. Studies investigating polysorbate oxidation using different buffer systems in the presence (+) and absence (-) of protein. Studies independently investigating one buffer system or using different buffer systems without a direct comparison are not included.
| Buffer system | Study | Polysorbate quality | Protein |
|---|---|---|---|
| Sodium-citrate | Gopalrathnam et al. (2018) (Gopalrathnam et al., 2018) | PS80 (quality not defined) | - |
| Doyle et al. (2019) (Doyle et al., 2019) | PS80 MC | - | |
| Brovč et al. (2020) (Brovč et al., 2020a) | PS20 MC and PS80 MC/AO | - | |
| Bensaid et al., (2022) (Bensaid et al., 2022) | PS80 MC/AOa | +/- | |
| Citrate | Liu et al. (2022) (Liu et al., 2022a) b | PS80 MC/ChP | - |
| Histidine-hydrochloride | Zhang et al. (2018) (Zhang et al., 2018) | PS20 MC | - |
| Gopalrathnam et al. (2018) (Gopalrathnam et al., 2018) | PS80 (quality not defined) | - | |
| Doyle et al. (2019) (Doyle et al., 2019) | PS80 MC | - | |
| Brovč et al. (2020) (Brovč et al., 2020a) | PS20 MC and PS80 MC/AO | - | |
| Liu et al. (2022) (Liu et al., 2022a) b | PS80 MC/ChP | - | |
| Bensaid et al., (2022) (Bensaid et al., 2022) | PS80 MC/AOa | +/- | |
| Peters et al., (2022) (Peters et al., 2022) | PS80 MC | - | |
| Sodium-acetate | Zhang et al. (2018) (Zhang et al., 2018) | PS20 HP | - |
| Kranz et al. (2019) (Kranz et al., 2019) | PS20 MC/AL PS80 MC/AO |
- | |
| Bensaid et al., (2022) (Bensaid et al., 2022) | PS80 MC/AOa | +/- | |
| Sodium-phosphate | Doyle et al. (2019) (Doyle et al., 2019) | PS80 MC | - |
| Brovč et al. (2020) (Brovč et al., 2020a) | PS20 MC and PS80 MC/AO | - | |
| Peters et al., (2022) (Peters et al., 2022) | PS80 MC | - | |
| Histidine-acetate | Zhang et al. (2018) (Zhang et al., 2018) | PS20 HP | - |
| Sodium-succinate | Brovč et al. (2020) (Brovč et al., 2020a) | PS20 MC and PS80 MC/AO | - |
Bensaid et al. (2022): not clearly defined which PS80 was used in the buffer comparison.
Liu et al. (2022): not clearly defined which counter ion was used
To observe and analyze the oxidative degradation of polysorbate, several studies with a broad variety of stress factors have been conducted. Subsequently, we will summarize the different stress conditions described in the literature as well as their formulation conditions.
-
I.
Temperature studies
The most prominent example of stress potentially leading to oxidation is temperature in the presence of oxygen with nearly 20 different studies published, however, often combined with other stress factors. The investigated polysorbate content range from 0.04 mg·mL-1 (Borisov et al., 2015), over 0.1 mg·mL-1 (Kishore et al., 2011a; Agarkhed et al., 2013), to a majority of studies with 0.2 – 0.4 mg·mL-1 (Kishore et al., 2011a; Doshi et al., 2020a; Kishore et al., 2011b; Dahotre et al., 2018; Doyle et al., 2019; Kranz et al., 2019; Gopalrathnam et al., 2018; Bensaid et al., 2022; Zhang et al., 2018; Kranz et al., 2020), up to 100 mg·mL-1 (Schröter et al., 2021; Schmidt et al., 2020) or 200 mg·mL-1 (Ha et al., 2002), stressed at varying temperatures. Typically, 25 °C and 40 °C were applied, although higher temperatures such as 50 °C (Doyle et al., 2019) or up to 70 °C (Donbrow et al., 1978a) were also used.
-
II.
Transition metal studies
Usually, temperature is combined with an additional stressor, like iron (Liu et al., 2022a; Doyle et al., 2019; Kranz et al., 2019; Gopalrathnam et al., 2018; Schröter et al., 2021; Bensaid et al., 2022; Kranz et al., 2020; Klair et al., 2021; Prajapati et al., 2022) or exposure to stainless steel (Doyle et al., 2019; Gopalrathnam et al., 2018) to accelerate the oxidation process, since biologicals are in contact with stainless steel upon pharmaceutical manufacturing. Therefore, several groups have stressed PS20 and PS80 in the presence of iron or stainless steel, with different concentrations being applied, ranging from 0.005 mg·L-1 via 0.01 mg·L-1 to 10 mg·L-1 (Doyle et al., 2019; Kranz et al., 2019; Bensaid et al., 2022; Kranz et al., 2020). Normally iron is spiked in the solutions and the final concentration is not verified, except for Bensaid et al. (2022) and Klair et al. (2021), both determining the iron content by inductively coupled plasma-mass spectrometry (ICP-MS). (Bensaid et al., 2022; Klair et al., 2021) Primarily, Fe2+SO42- (Doyle et al., 2019; Gopalrathnam et al., 2018; Liu et al., 2022a; Kranz et al., 2019; Schröter et al., 2021; Kranz et al., 2020) or Fe2+Cl-2 (Bensaid et al., 2022; Klair et al., 2021) were added, except for Klair et al. (2021) and Prajapati et al. (2022), which forced oxidation by Fe3+Cl-3 addition sometimes in combination with other stressors (Klair et al., 2021; Prajapati et al., 2022). The different amounts of iron range from <2 ppb to 15 ppm, depending on the study (Liu et al., 2022a; Doyle et al., 2019; Kranz et al., 2019; Gopalrathnam et al., 2018; Schröter et al., 2021; Bensaid et al., 2022; Kranz et al., 2020; Klair et al., 2021; Prajapati et al., 2022). However, the highest iron concentration was used in combination with H2O2, which is also known as the Fenton's reagent (Schröter et al., 2021). For Fe2+ and Fe3+ multiple reactions are discussed in the literature, like the Fenton reaction (Eq. 1) or other potential reactions with organic substrates (Eq. 3) (Heiba et al., 1969), peroxides (Eq. (4), (5)) (Goldstein et al., 1993), and molecular oxygen (Eq. 6) (Bensaid et al., 2022; Hovorka and Schöneich, 2001; Feig, 1994).
| (3) |
| (4) |
| (5) |
| (6) |
In general, mainly Fe2+ is used in oxidative studies, however, due to the multitude of redox reactions converting Fe2+ to Fe3+ or vice versa, both can accelerate oxidation. With a standard potential of -0.44 V for Fe/Fe2+ and a standard potential of +0.77 V for Fe2+/Fe3+, however, Fe3+ is actually the stronger oxidizing agent (Harris, 2014). Bensaid et al. (2022), stressed PS80 MC and AO quality using Fe2+ and monitored the actual iron content via ICP-MS, followed by PS80 content and Met255 mAb oxidation determination (Bensaid et al., 2022). The latter acts as indicator for oxidative-mediated mAb modifications. Two different formulations containing mAb and PS80 MC were analyzed, containing either 20 or <2 ppb iron. At 20 ppb iron, high PS80 MC degradation (from around 180 ppm PS80 initially to 0 ppm after two weeks) and Met255 oxidation (around 20 % oxidized Met255 after two weeks) were observed, while no degradation and only a small impact on protein oxidation was shown for <2 ppb iron, illustrating huge impact of iron leachables upon manufacturing. Additionally, they observed an inverse correlation between PS80 MC depletion and Met255 oxidation at 25 °C after 3 months as well as at 40 °C after 1 month (Bensaid et al., 2022). It is challenging to compare studies differing in iron amounts, iron charge, temperatures, buffers, polysorbates, as well as in other excipients. Furthermore, the stressors were often applied for various time periods, making a comparison arduous.
Striving for clarifications on how iron and stainless-steel exposure lead to polysorbate degradation, Kranz et al. (2020) proposed a working hypothese where oxidation mainly takes place within micelles (Kranz et al., 2020). They argued that micelles possess a higher oxidation potential due to following reasons: (i) higher solubility of molecular oxygen in the apolar environment demonstrated for non-ionic surfactants at pH 8.0 (Chistyakova et al., 2012), (ii) complexion of metal ions by POE moieties (Sari et al., 2006), or (iii) attractive interactions between the micelle-dissolved negatively-charged fatty acids and the positively-charged iron ions (Kranz et al., 2020). In this context, micelles are claimed to act as "tiny" reaction vessels, as no oxidation markers for PS20 and PS80 were detected after dissolving micelles by t-BuOH. However, other inhibition mechanisms of t-BuOH are possible, like radical scavenging or competing reactions. Nevertheless, the authors claimed that these mechanisms are less probable (Kranz et al., 2020). Contrary to expectations, they observed that PS20 AL and PS80 AO are degraded at similar rates, despite their difference in hydrocarbon chain unsaturation (Kranz et al., 2020). Peters and colleagues (2022) observed similar findings, by addressing the extent of extra- and intra-micellar oxidation of PS80 by a newly developed fluorescence assay using AAPH, the lipid peroxide probe BODIPY(581/591), as well as α-tocopherol. They demonstrated that AAPH-induced oxidation occurred inside as well as outside of micelles, however, with a higher percentage of approximately 70 % and 63 % oxidation in the intra-micellar space for phosphate and histidine buffer systems, respectively (Peters et al., 2022). Nevertheless, as there are many open questions, more research has to be performed to clearly demonstrate and validate the behavior of micelle-driven oxidation.
-
III.
AAPH studies
For AAPH, six studies were performed and most of them used 1.5 mM AAPH at 40 °C, only varying in formulations and time of the stress (Borisov et al., 2015; Larson et al., 2020; Zhang et al., 2017; Schröter et al., 2021; Zhang et al., 2018; Peters et al., 2022). Only, a few studies induced oxidation with up to 10 mM AAPH (Borisov et al., 2015; Larson et al., 2020). The latter is a water-soluble azo compound often used as radical starter to accelerate oxidation processes, leading to faster outcomes. These studies are more artificial mostly used in comparative stability studies, as AAPH is normally not found in biopharmaceutical products. AAPH is thermally decomposed in two carbon-centered alkyl radicals (R•) generating peroxyl radicals (ROO•) by O2 addition (Zhang et al., 2018), however, it is often used to start the oxidation process in short time periods and in a controlled way. At 37 °C a peroxyl radical formation rate of 1.36 × 10–6 M·s–1 was reported (Werber et al., 2011; Niki, 1990).
-
IV.
H2O2studies
Another stressor is H2O2, which was applied by the minority of the studies ranging in concentrations from 1 mM to 10 mM (Kishore et al., 2011a; Kranz et al., 2019; Schröter et al., 2021). Usually, H2O2 can form hydroxyl radicals (OH•) for instance via Fenton (Eq. 1) or Haber–Weiss reaction (Eq. 2) in the presence of redox-active metals and impurities, which are even sufficient to cause significant effects in the ppb range (Phaniendra et al., 2015). Therefore, it can be combined with iron acting as Fenton reagent (Schröter et al., 2021). The presence and the concentration of H2O2 may be relevant for fill and finish process in isolator due to the fact the isolator units can be sterilized using H2O2. Nevertheless, also the stability of H2O2 is of major importance as it tends to decompose with increasing temperature. The decomposition proceeds via intermediate radical steps, which may then in turn promote polysorbate oxidation (Hartmann-Schreier, 2004; Christensen et al., 1994). In general, commercially-available H2O2 is often stabilized by chelators or sequestrants to reduce its decomposition. This issue is not described in PS oxidation studies using H2O2 and should be discussed and considered more deeply in order to compare and understand the results. As already mentioned even impurities in the ppb range of heavy metals are able to catalyze this procedure (Hartmann-Schreier, 2004). Here, Ha and colleagues (2002) demonstrated a 50 % or 100 % reduction of the peroxide concentration for a 20 % (w/v) PS80 solution under vacuum after 6 weeks at 40 or 60 °C, respectively (Ha et al., 2002). Peroxides or iron are more relevant pharmaceutical stressors, as they can be found in traces within the PS raw material (ppb levels or below) or can leach into the solution during manufacturing (Bensaid et al., 2022) or packaging (Allain and Wang, 2007). For instance Bensaid et al. (2022) reported the presence of approximately 20 ppb and <2 ppb iron measured by ICP-MS for two drug substance batches with normal and improved manufacturing processes, respectively, has a huge impact on PS80 stability at 40 °C and Met255 stability at 40 °C (Bensaid et al., 2022).
-
V.
Light studies
Finally, polysorbates can be stressed by light (Larson et al., 2020; Ha et al., 2002; Doyle et al., 2019; Agarkhed et al., 2013; Prajapati et al., 2022; Prajapati et al., 2020). As complete light protection is challenging to be implemented during manufacturing (Kaiser et al., 2021), it is a more relevant stress condition than AAPH. Consequently, different authors investigated the influence of light on polysorbate. Agarkhed and colleagues (2013) illuminated protein formulations containing polysorbate under near UV light, leading to increased turbidity and protein aggregation (Agarkhed et al., 2013). They found an increased peroxide content with an increased PS80 MC concentration after light exposure with a non-linear relationship (Agarkhed et al., 2013). While there exist some studies concerning light-induced protein oxidation (Hipper et al., 2021; Hipper et al., 2023), only limited attention is paid to the degradation of polysorbates. Doyle et al. (2019) exposed PS80 MC in different buffer systems to 20 %, 50 %, and 100 % of the light dose specified in ICH Q1B. The vials were stored at 50 °C in darkness after light exposure, and PS80 oxidation was observed in histidine buffer after 14 days, which did not occur after storage at 50 °C in darkness without previous light stress (light doses of 50 and 100 % ICH Q1B). In citrate buffer, oxidation of PS80 arose after 35 days with 100 % illumination, showing the influence of light on polysorbate oxidation in different buffer systems (Doyle et al., 2019). Ha and colleagues (2002) exposed a 20 % (w/v) PS80 (MC) solution in water to light (light conditions: 460 foot candle ≈ 4,951 lx) at 40 °C and observed an 8-fold increase in the peroxide level (1,330 mEq) after 5 weeks in comparison to samples without light stress (Ha et al., 2002). Both samples were investigated in the presence of air. By applying vacuum, light had no influence on the peroxide levels showing no relevant oxidation. Hence, storage in the absence of oxygen was recommended (Ha et al., 2002). Another study demonstrated the formation of cis/trans isomerization of e.g., oleic acid and linoleic acid, whenever the formulation contained both PS80 and monoclonal antibodies. However, the influence of the formed trans fatty acids on PS80 degradation remained unclear (Prajapati et al., 2020). Recently, the interaction of Fe3+ and citrate under the influence of light was discussed. Here, 10 μM Fe3+ was added to PS80 in 10 mM citrate buffer and irradiated with UV light, in the context of PS80 degradation. In this case an additional mechanism of radical formation (carbon dioxide radical anion (•CO2–)) was made responsible for polysorbate degradation (Prajapati et al., 2022; Subelzu and Schöneich, 2020). Nevertheless, intensity, applied energy (wavelength), and exposure time are important parameters for the light studies. Using different light sources as well as different surrounding media, varying irradiation times and conditions (for example 20 % vs. 100 % ICH Q1B) complicate a direct comparison between the individual studies (Larson et al., 2020; Ha et al., 2002; Doyle et al., 2019; Agarkhed et al., 2013; Prajapati et al., 2022; Prajapati et al., 2020).
-
VI.
Stress conditions in general
The heterogeneity of used stress conditions makes a comparative conclusion difficult. All stressors have different impact on oxidation and the formulation conditions such as buffer composition or polysorbate/protein concentrations provide another level of complexity. For instance, buffers as well as their qualities have a significant influence on polysorbate oxidation, which makes it challenging to permit generalized statements (Kranz et al., 2019). A good example that the stressor affects the oxidation process of PS is described in the buffer section as a difference between AAPH and thermal stress was observed for histidine buffer (Zhang et al., 2018). In this context, it should be noted that neither highly elevated temperatures nor AAPH are relevant factors during pharmaceutical production. Also, PS20 and PS80 as well as their different qualities have different impact on oxidation. As impurities of metals or peroxide in the ppb range can cause effects, it is extremely challenging to ensure that oxidation is only caused by the expected stressor. To mimic oxidative stress occurring during manufacturing and subsequent storage of the drug product, light exposure, iron contamination, H2O2, or the combination of these factors are valuable tools. In general, the most relevant stress conditions for polysorbate oxidation from a pharmaceutical point of view are iron, light, ambient temperatures, and H2O2, as all these factors can be found during the manufacturing process of pharmaceuticals. However, the concentration of polysorbate itself as well as the quality and buffers used should also be considered. A summary of the stress studies of polysorbate for temperature, iron, light, AAPH, and H2O2 are given subsequently (Table 6A, Table 6B, Table 6C, Table 6D, Table 6E).
-
g.
Risk assessment and mitigation of oxidative PS degradation
-
I.
General
Table 6A.
Overview of published stress studies related to polysorbate degradation using increased temperatures. Temperature, pH, buffer conditions, study duration, polysorbate concentration, and polysorbate quality are summarized. Abbreviations: His: histidine, d: day, m: month, MC: multicompendial, AO: all-oleate, AL: all-laurate, AcOH: acetate, HP: high-purity, SR: super-refined, AAPH: 2,2-azobis(2-amidinopropane) dihydrochloride, BHT: butylated hydroxytoluene, BHA: butylated hydroxyanisole.
| Study | Temperature / °C | pH | Buffer | Study duration | Polysorbate / % (w/v) | Polysorbate |
|---|---|---|---|---|---|---|
| Kranz et al. (2019) (Kranz et al., 2019) | 40 | 6.0 | 10 mM His, 8 % (w/v) sucrose | 17 d | 0.04 | PS20 MC PS80 MC/AO |
| 40 | 6.0 | 10 mM His, 8 % (w/v) sucrose, 50 μM EDTA | 7 d 17 d |
0.04 | PS20 MC/AL PS80 MC/AO |
|
| 40 | 5.5 | 10 mM AcOH, 8 % (w/v) sucrose | 17 d | 0.04 | PS20 MC/AL PS80 MC/AO |
|
| Bensaid et al. (2022) (Bensaid et al., 2022) | 25 | 6.0 | 20 mM His, 20 mg·mL-1 mAb1, 10 % (w/v) sucrose | 3 m | 0.02 | PS80 MC/AO |
| 40 | 1 m | |||||
| 40 | 5.0, 6.0 | 20 mM citrate, 20 mg·mL-1 mAb, 10 % (w/v) sucrose, 30 ppb Fe(lI)Cl2 x 4 H2O | 1 m | 0.05 | PS80 MC | |
| 40 | 7.0 | 20 mM His, 20 mg·mL-1 mAb, 10 % (w/v) sucrose, 30 ppb Fe(lI)Cl2 x 4 H2O | 1 m | 0.05 | PS80 MC | |
| 40 | 5.0, 6.0 | 20 mM AcOH, 20 mg·mL-1 mAb, 10 % (w/v) sucrose, 30 ppb Fe(lI)Cl2 x 4 H2O | 1 m | 0.05 | PS80 MC | |
| Dahotre et al. (2018) (Dahotre et al., 2018) | 2-8 | 5.4 | His-AcOH | 0, 12, 18, 24 m | 0.04 | PS20 (quality not defined) |
| 2-8 | 5.5 | His-AcOH | 13, 32 m | 0.02 | PS20 (quality not defined) | |
| Kishore et al. (2011) (Kishore et al., 2011a) | 5 | 6.0 | 20 mM His, 10 mg·mL-1 mAb1, 240 mM trehalose | 12 m | 0.05 | PS20 or PS80 (quality not defined) |
| 5 | 6.0 | 20 mM His, 10 mg·mL-1 mAb2, 240 mM trehalose | 12 m | 0.01-0.04 | PS20 and PS80 (quality not defined) | |
| 5 | 6.0 | 25 mM Na-AcOH, mAb3 10 mg·mL-1, 125 mM NaCl | 12 m | 0.025 | PS20 (quality not defined) | |
| 5 | 5.5 | 20 mM His/His-Cl, mAb4 25 mg·mL-1, 240 mM trehalose | 24 m | 0.02 | PS80 (quality not defined) | |
| Doshi et al. (2020) (Doshi et al., 2020a) | 40 | 5.5 | His-AcOH, 10 mg·mL-1 IgG1 mAb, sucrose | 1 m | 0.03 | PS20 HP/SR |
| 25 | 3 m | |||||
| 2-8 | 12 m | |||||
| Gopalrathnam et al. (2018) (Gopalrathnam et al., 2018) | 5, 25 | 5.5-6.0 | 10 mM His, 150 mM NaCl, 5, 40, 70, 100, 120 mg·mL-1 LY2951742 antibody |
10 m | 0.05-0.06 | PS80 (quality not defined) |
| Borisov et al. (2015) (Borisov et al., 2015) | 40 | - | Water, 1.5-10 mM AAPH | 19 h 40 h |
0.004 | PS20 (quality not defined) |
| 40 | - | Water, 1.5-10 mM AAPH | 12.5 h 40 h |
0.004 | PS80 (quality not defined) | |
| Kranz et al. (2020) (Kranz et al., 2020) | 40 | 6.0 | His 10 mM, 8 % (w/v) sucrose, 10 ppb Fe(II)SO4 | 12 d | 0.04 | PS20 MC/AL PS80 MC/AO |
| Ha et al. (2002) (Ha et al., 2002) | 40, 50, 60 | - | Water | 8 w | Neat and 20 | PS80 NF grade Low peroxide PS80 |
| Yao et al. (2009) (Yao et al., 2009) | 40 | 7.0 | 100 mM Sodium phosphate, 0.1 mM EDTA | - | - | PS20 PS80 high grades |
| Kishore et al. (2010) (Kishore et al., 2011b) | 5, 25, 40 | 5.5 | 20 mM His, 240 mM trehalose, 10 mg·mL-1 mAb1 or placebo | 12 m | 0.05 or 0.03 | PS20 or PS80 (quality not defined) |
| Liu et al. (2021) (Liu et al., 2022a) | 25 | 6.0 | Water | 11-20 m | 0.05 | PS80 MC/ChP |
| Donbrow et al. (1978) (Donbrow et al., 1978a) | 25, 40, 60, 70 | 6.0 | Buffer, potassium iodide | 30 d | 1 | PS20 (quality not defined) |
| Doyle et al. (2019) (Doyle et al., 2019) | 50 | 5.5 | 10 mM His, citrate, phosphate | 30 d | 0.02 | PS80 MC |
| Schmidt et al. (2020) (Schmidt et al., 2020) | 40 | - | Water | 7 w | 10 | PS20 and PS80 containing either no antioxidant or BHT or BHA at a concentration of 0.02 % (v/w) |
| Zhang et al. (2018) (Zhang et al., 2018) | 40 | 5.5 | 20 mM Na-AcOH or 20 mM His, or His-AcOH | 8 w | 0.02 | PS20 HP |
| Schröter et al. (2020) (Schröter et al., 2021) | RT | Acidic | Water + trifluoroacetic acid (0.5 mM), 10 mM H2O2, 0.1 mM Fe(II)SO4 | 5 w | 10 | PS80 (no BHT, 1 mM BHT, 0.3 mM BHT) |
| 40 | Acidic | Water + trifluoroacetic acid (0.5 mM), 1.5 mM AAPH | 5 w | 10 | PS80 (no BHT, 1 mM BHT, 0.3 mM BHT) | |
| Zhang et al. (2017) (Zhang et al., 2017) | 40 | 1 7 11 |
Water, 1.5 mM AAPH | 12, 24, 36, 48 h | 0.1 | PS20 AL |
| Agarkhed et al. (2012) (Agarkhed et al., 2013) | 25 | 6.0 | 10 mM His, NaCl, glycine, 5 mg·mL-1 mAb | 6 d | 0.01 | PS80 (SR, NF, ultrapure) |
Table 6B.
Overview of published stress studies related to polysorbate degradation in the presence of iron. Iron content, temperature, pH, buffer conditions, study duration, polysorbate concentration, and polysorbate quality are summarized. Abbreviations: His: histidine, d: day, m: month, MC: multicompendial, AO: all-oleate, AL: all-laurate, ADC: antibody drug conjugate, AcOH: acetate, AAPH: 2,2-azobis(2-amidinopropane) dihydrochloride, Arg.: arginine, Met.: methionine BHT: butylated hydroxytoluene.
| Study | Iron / ppb | Temperature / °C | pH | Buffer | Study duration | Polysorbate / % (w/v) | Polysorbate |
|---|---|---|---|---|---|---|---|
| Liu et al. (2021) (Liu et al., 2022a) | Fe(II)SO4: 50 |
25 | 6.0 | 20 mM His, 150 mM Arg |
5 d | 0.05 | PS80 ChP PS80 MC |
| Fe(II)SO4: 50 |
25 | 6.0 | 20 mM citrate, 150 mM Arg |
5 d | 0.05 | ||
| Doyle et al. (2019) (Doyle et al., 2019) | 316 Stainless steel surface | 50 | 5.5 | 10 mM His, citrate, phosphate | 30 d | 0.02 | PS80 (NF) |
| Stainless steel | 50 | 5.5 | 10 mM His with 0, 0.0625, 0.5, 1, 25, and 100 mM citrate | 24 h Stainless steel, 25 d glass vials | 0.02 | PS80 (NF) | |
| Stainless steel | 50 | 5.5 | 10 mM His with 0,1,10,100,1000 μM EDTA | 24 h Stainless steel, 25 d glass vials | 0.02 | PS80 (NF) | |
| Stainless steel | 50 | 5.5 | 10 mM His with 0, 0.0625, 0.125, 0.25, 0.5, 1 mM Citrate | 24 h Stainless steel, 24 d glass vials | 0.02 | PS80 (NF) | |
| Stainless steel | 50 | 5.5 | 10 mM His with 0, 1, 10, 100, 1000 μM EDTA | 24 h Stainless steel, 25 d glass vials | 0.02 | PS80 (NF) | |
| Fe(II)SO4: 0, 100, 1000, 10000 |
50 | - | 10 mM His, 62.5 μM citrate |
24 h Stainless steel, 24 d glass vials | 0.02 | PS80 (NF) | |
| Fe(II)SO4: 0, 100, 1000, 10000 |
50 | - | 10 mM His, 62.5 μM EDTA |
24 h Stainless steel, 24 d glass vials | 0.02 | PS80 (NF) | |
| Schröter et al. (2020) (Schröter et al., 2021) | Fe(II)SO4: 15,000 |
RT | Acidic | Water + trifluoroacetic acid (0.5 mM) | 5 w | 10 | PS80 (no BHT, 1 mM BHT, 0.3 mM BHT) |
| Kranz et al., (2020) (Kranz et al., 2020) | Fe(II)SO4: 10 |
40 | 6.0 | 10 mM His 8 % (w/v) sucrose |
12 d | 0.04 | PS20 MC/AL PS80 MC/AO |
| Gopalrathnam et al. (2018) (Gopalrathnam et al., 2018) | Stainless steel surface | 25 | 5.5-6.0 | 10 mM His, 150 mM NaCl |
7 d 30 d |
0.05-0.06 | PS80 (quality not defined) |
| Stainless steel needle | 25 | 5.5-6.0 | 10 mM His, 150 mM NaCl |
28 d | 0.05-0.06 | PS80 (quality not defined) | |
| Stainless steel needle | 25 | 6.0 | 10 mM His, 150 mM NaCl, 100 ppm EDTA |
28 d | 0.05-0.06 | PS80 (quality not defined) | |
| Compare PS20/PS80 | 25 | 5.5-6.0 | 10 mM His, 150 mM NaCl |
15 d | 0.05-0.06 | PS80 (quality not defined) | |
| Compare PS20/PS80 | 25 | 5.5-6.0 | 10 mM His, 150 mM NaCl |
15 d | 0.05-0.06 | PS20 (quality not defined) | |
| Stainless steel surface | 25 | 5.5-6.0 | 50 mM His, 150 mM NaCl |
2 w | 0.06 | PS80 (quality not defined) | |
| 25 | 5.5-6.0 | 50 mM His, 150 mM NaCl |
2 w | 0.01 | PS80 (quality not defined) | ||
| 25 | 5.5-6.0 | 5 mM His, 150 mM NaCl |
2 w | 0.06 | PS80 (quality not defined) | ||
| 25 | 5.5-6.0 | 5 mM His, 150 mM NaCl |
2 w | 0.01 | PS80 (quality not defined) | ||
| Fe(II)SO4: 500 Fe2+ |
25 | 5.5-6.0 | 10 mM His, 150 mM NaCl |
4 w | 0.05-0.06 | PS80 (quality not defined) | |
| Fe(II)SO4: 5000 Fe2+ |
25 | 5.5-6.0 | 10 mM His, 150 mM NaCl |
3 m | 0.05-0.06 | PS80 (quality not defined) | |
| Fe(II)SO4: 5000 Fe2+ |
25 | 5.5-6.0 | 10 mM His, 150 mM NaCl, Na2EDTA solution |
3 m | 0.05-0.06 | PS80 (quality not defined) | |
| Fe(II)SO4: 5000 Fe2+ |
25 | 5.5-6.0 | 10 mM His, 150 mM NaCl, 1 mM Met |
4 w | 0.05-0.06 | PS80 (quality not defined) | |
| Bensaid et al. (2022) (Bensaid et al., 2022) | Fe(II)Cl2: 20 |
25, 40 |
6.0 | 20 mM His, 20 mg·mL-1 mAb, 10 % (w/v) sucrose |
45 d | 0.02 | PS80 MC |
| Fe(II)Cl2: 20 |
40/75 % RH | 6.0 | 20 mM His, 20 mg·mL-1 mAb, 10 % (w/v) sucrose |
14 d | 0.02 | PS80 MC | |
| Fe(II)Cl2: 30 |
40 | 5.0, 6.0 | 20 mM citrate, 20 mg·mL-1 mAb, 10 % (w/v) sucrose |
1 m | 0.05 | PS80 MC | |
| 7.0 | 20 mM HIs, 20 mg·mL-1 mAb, 10 % (w/v) sucrose |
||||||
| 5.0, 6.0 | 20 mM AcOH, 20 mg·mL-1 mAb, 10 % (w/v) sucrose |
||||||
| Kranz et al. (2019) (Kranz et al., 2019) | Fe(II)SO4: 10 | 40 | 6.0 | 10 mM His, 8 % (w/v) sucrose | 7 d 17 d |
0.04 | PS20 MC/AL PS80 MC/AO |
| Fe(II)SO4: 10 | 40 | 6.0 | 10 mM His, 8 % (w/v) sucrose, 50 μM EDTA |
22 d | 0.04 | PS80 AO | |
| Klair et al. (2021) (Klair et al., 2021) | Fe(II)Cl2: 28, 56, 112, 168 Fe(III)Cl3: 28, 56,112,168 |
35 (ADC) 40 (mAb) |
5.0-6.0 | Sodium succinate, sucrose, 20 mg·mL-1 protein | 14 d | - | PS20 (quality not defined) |
| Prajapati et al. (2022) (Prajapati et al., 2022) | Fe(III)Cl3: 0-8,000 | - - | 6.0 | 10 mM citrate | 0-200 min. To near UV light |
0.02 | PS80 MC/AO |
Table 6C.
Overview of published stress studies related to polysorbate degradation using light. Light conditions, temperature, pH, buffer conditions, study duration, polysorbate concentration, and polysorbate quality are summarized. Abbreviations: His: histidine, d: day, w: week, AcOH: acetate.
| Study | Light | Temperature / °C | pH | Buffer | Study /irradiation duration | Polysorbate / % (v/w) | Polysorbate |
|---|---|---|---|---|---|---|---|
| Ha et al. (2002) (Ha et al., 2002) | 4951 lux Fluorescent light box Model BL1012, visible light a |
40, 50, 60 | - | Water | 8 w irradiation | Neat 20 |
PS80 MC |
| Doyle et al. (2019) (Doyle et al., 2019) | 300-800 nm according to 20, 50, 100 % ICH Q1B SUNTEST XLS + light chamber |
50 (After irradiation) |
5.5 | 10 mM His, citrate, phosphate | 35 d (After irradiation) |
0.02 | PS80 MC |
| Agarkhed et al. (2012) (Agarkhed et al., 2013) | 1.2 × 106 lxh, 200 Wh/m2 of near UV a Caron 6500 photostability chamber |
25 | 6.0 | 10 mM His, NaCl, glycine, 5 mg·mL-1 mAb | - | 0-1.0 | PS80 (SR, NF, ultrapure) |
| Prajapati et al. (2022) (Prajapati et al., 2022) | UV light (ICH Q1B) 6.3-21 Wh/m2 λmax=350 nm 350-416 nm emission spectrum |
- | 6.0 | Water, 10 mM citrate, 1 μM Fe3+Cl3 |
0-200 min irradiation | 0.02 | PS80 MC/AO |
| Visible light 14-57 Wh/m2 λmax=419 nm 390-475 nm emission spectrum | |||||||
| Prajapati et al. (2020) (Prajapati et al., 2020) | <40 Wh/m2 for 1 h photoirr. at λ=254 nm; <25 Wh/m2 for 1 h photoirr. at λmax = 305, 350 and 419 nm |
- | 5.5 | 20 mM or 50 mM NaAcOH | 1 h irradiation | 0.01 | PS80 (quality not defined) |
| 0.2 | |||||||
| Larson et al. (2020) (Larson et al., 2020) | Rayonet system, 4 phosphor-coated low-pressure mercury lamps, λmax= 350 nm | 4 (After irradiation) |
- | Water, 10 mM AAPH |
6 h irradiation with λmax=350 nm | 1 | PS80 (quality not defined) |
| Singh et al. (2012) | Caron 6500 series photostability chamber 1.2 × 106 luxh and 200 W hour m-2 of near UV light | 25 | 6.5 | 10 mM histidine buffer + NaCl and glycine | 6 d of irradiation | 0.01 | PS80, SR, NF, NOF (ultra-pure), another NF grade |
| 5 mg·mL-1 mAb |
no specifications of the used wavelength
Table 6D.
Overview of published stress studies related to polysorbate degradation using 2,2-azobis(2-amidinopropane) dihydrochloride (AAPH). AAPH concentration, temperature, pH, buffer conditions, study duration, polysorbate concentration, and polysorbate quality are summarized. Abbreviations: His: histidine, w: weeks, m: month, h: hour, HP: high-purity, AL: all-laurate, AcOH: acetate, BHT: butylated hydroxytoluene.
| Study | AAPH / mM | Temperature / °C | pH | Buffer | Study duration | Polysorbate / % (v/w) | Polysorbate |
|---|---|---|---|---|---|---|---|
| Borisov et al. (2015) (Borisov et al., 2015) | 1.5-10 | 40 | - | Water | 19 h 40 h |
0.004 | PS20 (quality not defined) |
| 1.5-10 | 40 | - | Water | 12.5 h 40 h |
0.004 | PS80 (quality not defined) | |
| Larson et al. (2020) (Larson et al., 2020) | 10 | 4 (After irradiation) |
- | Water | 6 h irradiation with λmax= 350 nm | 1 | PS80 (quality not defined) |
| Zhang et al. (2018) (Zhang et al., 2018) | 1.5 | 40 | 5.5 | 20 mM Na-AcOH, His, or His-AcOH | 12, 24, 36, 48, 60, 72 h | 0.02 | PS20 HP |
| 1.5 | 40 | 5.5 | 20 mM Na-AcOH, His, or His-AcOH with either 10 mM L-His or imidazole or His methyl ester |
24 h | 0.02 | PS20 HP | |
| 4.5 | 40 | 5.5 | 20 mM Na-AcOH, His, or His-AcOH with either 10 mM L-His or imidazole or His methyl ester |
8 h | 0.02 | PS20 HP | |
| 9.0 | 40 | 5.5 | 20 mM Na-AcOH, His, or His-AcOH with either 10 mM L-His or imidazole or His methyl ester |
4 h | 0.02 | PS20 HP | |
| Zhang et al. (2017) (Zhang et al., 2017) | 1.5 | 40 | 1 7 11 |
Water | 12, 24, 36, 48 h | 0.1 | PS20 AL |
| Schröter et al. (2020) (Schröter et al., 2021) | 1.5 | 40 | Acidic | Water + trifluoroacetic acid (0.5 mM) | 5 w | 10 | PS80 (no BHT, 1 mM BHT, 0.3 mM BHT) |
| Peters et al. (2022) (Peters et al., 2022) | 0-1 | 5 / 25 / 40 | 5.5 6.0 |
20 mM L-His 20 mM phosphate 0-4.5 μM α-tocopherol |
1 m | 0.2 | PS80 (quality not defined) |
Table 6E.
Overview of published stress studies of polysorbate degradation in the presence of H2O2. H2O2 concentration, temperature, pH, buffer conditions, study duration, polysorbate concentration, and polysorbate quality are summarized. Abbreviations: His: histidine, d: day, w: week, MC: multicompendial, AO: all-oleate, AL: all-laurate, AcOH: acetate, BHT: butylated hydroxytoluene.
| Study | H2O2 / mM | Temperature / °C | pH | Buffer | Study duration | Polysorbate / % (v/w) | Polysorbate |
|---|---|---|---|---|---|---|---|
| Kranz et al. (2019) (Kranz et al., 2019) | 1 10 |
40 | 6.0 | 10 mM His, 8 % (w/v) sucrose |
17 d | 0.04 | PS20 MC PS80 MC/AO |
| 1 10 |
40 | 5.5 | 10 mM AcOH, 8 % (w/v) sucrose |
17 d | 0.04 | PS20 MC/AL PS80 MC/AO |
|
| Kishore et al. (2011) (Kishore et al., 2011a) | 8.8 | 80 | 6.0 | 20 mM His, 10 mg·mL-1 mAb2, 240 mM trehalose |
7 d | 0.02 | PS20 (quality not defined) PS80 (quality not defined) |
| 0.005-0.03 | |||||||
| Schröter et al. (2020) (Schröter et al., 2021) | 10 | RT | Acidic | Water + trifluoroacetic acid (0.5 mM), 0.1 mM Fe2+SO4 | 5 w | 10 | PS80 (no BHT, 1 mM BHT, 0.3 mM BHT) |
After examination of the oxidative processes in polysorbate in detail, it remains to be answered how oxidative polysorbate degradation can be reduced or prevented. Various approaches are being pursued for this, like the addition of chelators, antioxidants, or by reducing the impurities such as metals (Doshi et al., 2020a; Doyle et al., 2019; Schmidt et al., 2020; Knepp et al., 1996; Mcclements and Decker, 2000; Waraho et al., 2009; Yarbrough et al., 2019; Kranz et al., 2019; Gopalrathnam et al., 2018; Schröter et al., 2021; Bensaid et al., 2022; Doshi et al., 2021a; Kranz et al., 2020; Brovč et al., 2020c; Doshi et al., 2021b). Alternatively, production processes or environmental conditions can be adapted, as for instance exposure to stainless steel/iron has been extensively examined as critical impact for oxidative polysorbate degradation (Liu et al., 2022a; Doyle et al., 2019; Kranz et al., 2019; Gopalrathnam et al., 2018; Schröter et al., 2021; Bensaid et al., 2022; Kranz et al., 2020; Klair et al., 2021; Prajapati et al., 2022). Here, Abernethy et al. (2010) as well as other groups demonstrated that traces of metal ions in the ppb range (depending on multiple factors) can be introduced in the solution via manufacturing processes or primary packaging (Zhou et al., 2011; Abernethy et al., 2010; Zhou et al., 2010a). More recently Klair et al. (2021) revealed iron leachables (in the range of 53 to 114 ppb or 0.9 to 2.0 μM) as source for protein oxidation after storage in Hastelloy®-based metal containers (Klair et al., 2021). As normally 316L stainless steel is used, metal traces like iron, chromium, and nickel should be carefully observed. Here, Zhou and colleagues (2012) detected an accumulation in theses metals after long-time storage via ICP-MS in the presence of stainless steel coupons (Zhou et al., 2012). They reported that the actual leached amounts depend drastically on contact time, metal chelators concentration, protein concentration and temperature and are in the lower up to medium ppb levels (e.g. from 3 ppb up to 550 ppb iron for storage of 3 months at 25 °C) (Zhou et al., 2012). Apart from avoiding exposure of the formulation to metal, it is generally advisable to not neglect the primary packaging material due to potential leachables (Zhou et al., 2011). Therefore, Gopalrathnam et al. (2018) suggested the use of glass or disposable bag systems in order to reduce the risk for oxidation (Gopalrathnam et al., 2018). Nevertheless, glass can act as potential source of leachables like aluminum, affecting the quality of the product by promoting particle formation with fatty acids (Allmendinger et al., 2021; Gregoritza et al., 2021). Although the layer of single-use bioprocess containers in direct contact with the fluid is inert, leachables from other layers could potentially migrate in the drug solution upon antibody manufacturing (Jenke, 2002; Jenke, 2006; Jenke, 2005). For instance, Xiao et al. (2016) reported leachable-induced stability issues in mAb solutions originating from plastic storage bags (Xiao et al., 2016). In general, every material and every excipient used in formulations should be examined carefully before application. Another approach to diminish oxidative degradation would be the protection from air or more particular oxygen. Here, Liu et al. (2022) suggested to protect polysorbate raw material against air by storing under nitrogen gas (Liu et al., 2022a). They investigated polysorbate samples in open bottles without nitrogen overlay after 11 to 20 months via MS at RT. No obvious differences were detected after 11 months. However, after 17 and 20 months new peaks appeared in the profiles, leading to considerations that the amount of oxygen may have a decisive influence. Other factors, such as temperature or pH could also have an influence (Liu et al., 2022a).
Obviously, the general storage conditions of PS are crucial. Here, the storage in small light-protected containers with reduced amount of oxygen, coverage with inert gas, as well as low temperatures like 5 °C are recommended (Jones et al., 2018; Wuchner et al., 2022a). In the case PS handling studies were performed, showing that PS or PS dilutions thereof are stable at e.g. 25 °C, deviation of the general recommendation can be taken. Thereby, oxidation can be controlled, however, not completely mitigated, as for instance, Wuchner et al. (2022) described that still approximately two-thirds of the companies participated in the survey, observed PS degradation through both hydrolysis and oxidation in at least one of their drug products (Wuchner et al., 2022a). However, also leachables from the primary packing material must be considered. Paying close attention to these factors will at least decrease oxidative polysorbate degradation.
-
II.
Antioxidants
Knepp and colleagues (1996) used for instance methionine and cysteine as well as other antioxidants (1.5 and 15 mM) for their studies on two different recombinant human growth/neurotrophic factors (Knepp et al., 1996). The positive effect of methionine against temperature-induced oxidation of PS20 (quality not specifically stated in the study) was verified by Kranz et al. (2020) (concentration not given) in IgG formulations of acetic acid pH 5.5 (Kranz et al., 2020), as well as by Doshi and colleagues (2020) with 10 mM methionine in histidine-acetate buffer pH 5.5 for PS20 HP and SR. (Doshi et al., 2020a) For PS80 (quality not defined) in histidine buffer, iron-induced oxidation (5 ppm Fe2+) was not mitigated in the presence of methionine (1 mM) and the author argued that it is most likely based on the low antioxidant concentration (Gopalrathnam et al., 2018). L-methionine was also found to prevent protein oxidation in mAbs formulated in 20 mM histidine pH 5.5, however, failed in this study to mitigate PS80 (quality not defined) degradation, as only a small effect was observed after addition of approximately 1.3 mM methionine (approximately 1.3 mM calculated from % (v/w) with Mw of 149.21 g·mol-1) (Yarbrough et al., 2019). Generally, discrepancies can be attributed to varying concentrations of methionine, polysorbate, or protein, all affecting the mitigation potential. Drug product formulations containing 7 mM or 23 mM of methionine, respectively, are applied in marketed products in the respective indication (U.S. Food and Drug Administration, 2022; U.S. Food and Drug Administration, 2021). In studies comparing the protective effect of EDTA and methionine, the latter was demonstrated to be less efficient to prevent PS oxidation (Gopalrathnam et al., 2018; Kranz et al., 2020; Yarbrough et al., 2019). A comprehensive comparison between different antioxidants and chelators for 0.15 % (w/v) PS80 AO in histidine-acetate buffer pH 5.5 was performed by Doshi and colleagues (2021), demonstrating a slightly better protection with higher methionine concentration of 35 mM in comparison to 10 mM (Doshi et al., 2021a). For protein containing formulations they observed high oxidative protection with methionine (5 to 30 mM) for two monoclonal antibodies (Doshi et al., 2021a).
Another alternative to prevent oxidation is the use of radical scavengers as antioxidants. For instance, butylated hydroxytoluene (BHT) or butylated hydroxyanisole (BHA) (see Fig. 8) have been tested, which are currently only used as food additives (Williams et al., 1999; Glusker, 1980). Both reagents can transform peroxyl radicals into hydroperoxides by releasing a hydrogen atom and the resulting BHT/BHA radical is mainly unreactive due to steric blockage by the tert-butyl groups, especially for BHT (Yehye et al., 2015). Schmidt and coworkers (2020) verified that PS20 and PS80 oxidations were diminished or suppressed by 0.02 % (w/v) BHT or BHA addition in water containing 10 % (w/v) polysorbate (quality not specified), as volatile aldehyde degradation products are minimized or could not be detected at all after antioxidant addition. They concluded that the antioxidant prevents peroxide formation, leading to no loss in polysorbates by reactive-oxygen species. However, they reported that oxidized products formed from the antioxidants have to be evaluated as potential source for protein modifications as well (Schmidt et al., 2020). The positive effect of BHT on polysorbate oxidation was demonstrated by Schröter et al. (2021), as BHT inhibits the formation of 4-hydroxynonenal, a degradation product of PS80 (Schröter et al., 2021). Another strategy to mitigate oxidation was the addition of low concentrations of catalase (0.0002 mg·mL-1) (Yarbrough et al., 2019). The enzyme catalyzes the decomposition of H2O2 to water and oxygen, thus catalase was expected to prevent oxidation of polysorbates by reducing the H2O2 concentration (Yarbrough et al., 2019). However, no oxidation prevention for PS80 or the protein was observed in the presence of catalase at elevated temperature in histidine buffer (Yarbrough et al., 2019).
-
III.
Chelating agents
Fig. 8.
Chemical structures of the antioxidants butylated hydroxytoluene (BHT) (A) and the two isomers of butylated hydroxyanisole (BHA) (B).
As PS80 MC was found to be not stable at 50 °C after exposure to stainless steel (completely degraded after 14 days for 0.02 (w/v) PS80), Doyle and coworkers (2019) investigated whether the addition of citrate could also prevent PS80 MC oxidation in histidine buffer. The combination of citrate (62.5 μM) with 10 mM histidine could mitigate PS80 MC (0.02 % (w/v)) oxidation induced by 0.1 ppm iron for up to 7 days at 50 °C, whereas in the absence of citrate, complete PS80 degradation was observed (Doyle et al., 2019). Whether the presence of both excipients is able to stabilize PS80 for long-time storage e.g., up to 12 months is not known and cannot be derived for a short temperature study. This outcome is unclear, because it has been reported, that the presence of histidine can avoid PS degradation (Kranz et al., 2019; Zhang et al., 2018). For stronger chelating effects, EDTA can be selected, which is an FDA-approved excipient for human use (i.v., i.m., s.c.) that forms stable complexes with many divalent cations, such as transition metals (iron), by forming a six-dentate tetraacetic acid complex. Various forms of EDTA exist, differing in their counterions as well as in their toxicity (Zhou et al., 2010b). For instance, disodium EDTA or calcium disodium EDTA are commonly used in concentrations between 0.15 – 2.97 mM and 0.27 – 2.66 mM, respectively (Rowe et al., 2009). One safety aspect of disodium EDTA is the ability to complex calcium ions, which might cause hypocalcemia for long-term use or rapid i.v. infusions (Rowe et al., 2009). Additionally, EDTA containing parenterals should be treated carefully when applied to patients with impaired cardiac or renal functions (Rowe et al., 2009). A balanced risk-benefit analysis should be performed before application. The chelating effect of EDTA preventing iron-induced surfactant oxidation was investigated in several studies revealing a protective effect against polysorbate oxidation (Doyle et al., 2019; Kranz et al., 2019; Gopalrathnam et al., 2018; Bensaid et al., 2022; Kranz et al., 2020; Mcclements and Decker, 2000; Waraho et al., 2009; Yarbrough et al., 2019). Kranz et al. (2019) demonstrated that 50 μM EDTA prevented iron-induced PS80 AO oxidation in 10 mM histidine buffer pH 6.0 and 8 % (w/v) sucrose, as without EDTA a fast decrease of PS polyesters was observed (Kranz et al., 2019). The mitigating effect of EDTA was further observed for PS20 in acetate buffer at pH 5.5 in the presence of 50 mg·mL-1 antibody (Kranz et al., 2020). Protection against oxidative degradation of PS80 was demonstrated over a range of EDTA concentrations (1, 10, 20 μM) with 20 mg·mL-1 protein in 20 mM histidine buffer at pH 6 and sucrose 10 % (w/v) after iron as well as temperature-induced stress (Bensaid et al., 2022), or with slightly different conditions by Yarbrough et al. (2019) (Yarbrough et al., 2019), Gopalrathnam et al. (2018) (Gopalrathnam et al., 2018), and Doyle et al. (2019) (Doyle et al., 2019). In general, these studies show that EDTA provides protection against oxidative degradation of polysorbates in the absence or presence of proteins as well as in different buffer systems after iron or temperature stress. Nevertheless, EDTA as complexing agent showed concentration-dependent increase in the levels of metal leachables from corresponding metal containers (Zhou et al., 2011; Zhou et al., 2012). This effect can weaken the protective effect of EDTA related to PS degradation.
Another tested chelator is the eight-dentate chelator diethylenetriaminepentaacetic acid (DTPA). DTPA is a strong complexation agent for multivalent cations due to the eight possible coordination sites, so that it can complex multivalent cations more efficiently than the six-toothed EDTA (Gregoritza et al., 2021). Its complexation ability can be decreased by lowering the pH, due to the protonation of the carboxylate groups (compare Fig. 9) (Granholm et al., 2010). As described above, EDTA was reported to prevent iron-induced polysorbate oxidation (Doyle et al., 2019; Kranz et al., 2019; Gopalrathnam et al., 2018; Bensaid et al., 2022; Kranz et al., 2020; Yarbrough et al., 2019), however, it was also claimed to drive protein oxidation under certain circumstances with ascorbate and Fe3+ (Stadtman, 1990). Similar behavior was demonstrated by Brovč and colleagues (2020), stressing proteins and polysorbate with Fe3+ and Cu2+ in the presence and absence of ascorbate as well as EDTA/DTPA (Brovč et al., 2020c). A dependency of OH• formation on chelator and on metal ions could be observed. Hydroxyl radical production increased tremendously after iron stress and EDTA addition with ascorbate, whereas DTPA slowed down OH• radical production as analyzed by the ascorbate redox assay (Brovč et al., 2020c). Thus, DTPA can be used to prevent metal-induced oxidation and possesses a good safety profile (Zhou et al., 2010b). The use of EDTA and DTPA is useful if there are traces of iron or other metals driving the oxidation of polysorbate. However, as there are also drawbacks of chelators, its minimal required concentration should be carefully evaluated. There is no reason for just adding any chelator to reduce the risk of oxidative degradation. A list of different mitigation agents and their corresponding formulation conditions (buffer, PS grade/quality, and proteins) as well as stress conditions are given in Table 7. Therefore, a clear understanding of the mechanism that promotes PS oxidation drives the specific mitigation strategy to be successful.
Fig. 9.
Chemical structures of the chelating agents ethylenediaminetetraacetic acid (EDTA) (A) and diethylenetriaminepentaacetic acid (DTPA) (B).
Table 7.
Additives to mitigate oxidative polysorbate degradation. The different antioxidants/chelators, polysorbate qualities/concentrations, buffer conditions, excipients, protein, and stressors that have been tested are summarized.
| Antioxidant/ chelators | PS quality | [PS] / % (v/w) | Buffer | pH | Excipient | Protein / mg·mL-1 | Stress | Study |
|---|---|---|---|---|---|---|---|---|
| 1.5/15 mM Met/Cys | PS80 | ∼0.24 | TRIS | 7.6 | NaCl, EDTA | rhCNTF & rhNGF | H2O2/alkyl hydroperoxide | Knepp et al. (1996) (Knepp et al., 1996) |
| Meta | PS20d | 0.04 | AcOH | 5.5 | NaCl, mannitol | <50 mAb | 25/40 °C | Kranz et al. (2020) (Kranz et al., 2020) |
| 10 mM Met | PS20 HP / PS20 HP RO | 0.2 | <15 mM His-AcOH | 5.5 | Sucrose | - | 40 °C | Doshi et al. (2021)b (Doshi et al., 2021b) |
| 1 mM Met | PS80d | 0.05-0.06 | 10 mM His | Water | - | 5 ppm Fe2+ | Gopalrathnam et al. (2018) (Gopalrathnam et al., 2018) | |
| Ca. 1.3 mM Met | PS80d | 0.02 | 20 mM His | 5.5 | 8.5 % (w/v) sucrose | 25 mAb | 5/25/40 °C | Yarbrough et al. (2019) (Yarbrough et al., 2019) |
| 10, 35 mM Met | PS80 AO | 0.15 | His-AcOH | 5.5 | Water | - | 40 °C + light + stainless steel | Doshi et al. (2021)c (Doshi et al., 2021a) |
| 10 mM Met | PS20 HP & SR | 0.03 | His-AcOH | 5.5 | Sucrose | 10 mAb | 40 °C | Doshi et al. (2020) (Doshi et al., 2020a) |
| 1 mM BHT | PS20d & PS80d | 10 | - | Water | - | 40 °C | Schmidt et al. (2020) (Schmidt et al., 2020) | |
| 0.3, 1 mM BHT | PS80d | 10 | - | Water | - | 10 mM H2O2 + 0.1 mM Fe2+ + 25°C | Schröter et al. (2021) (Schröter et al., 2021) | |
| 0.3, 1 mM BHT | PS80d | 10 | - | Water | - | 1.5 mM AAPH + 40 °C | Schröter et al. (2021) (Schröter et al., 2021) | |
| 0.3, 1 mM BHT | PS80d | 10 | - | Water | - | air + 40 °C | Schröter et al. (2021) (Schröter et al., 2021) | |
| 50 μM EDTA | PS80 AO | 0.04 | 10 mM His | 6.0 | 8 % (w/v) sucrose | - | 10 ppb Fe2+ | Kranz et al. (2019) (Kranz et al., 2019) |
| EDTAa | PS20d | 0.04 | AcOH pH | 5.5 | NaCl, mannitol | <50 mAb | 25/40 °C | Kranz et al. (2020) (Kranz et al., 2020) |
| 1, 10, 20 μM EDTA | PS80 MC/AO | 0.02 | 20 mM His | 6.0 | 10 % (w/v) sucrose | 20 mAb | 5/50 ppb Fe2+ + 25/40 °C | Bensaid et al. (2022) (Bensaid et al., 2022) |
| 0.17 mM EDTA | PS80d | 0.02 | 20 mM His | 5.5 | 8.5 % (w/v) sucrose | 25 mAb | 5/25/40 °C | Yarbrough et al. (2019) (Yarbrough et al., 2019) |
| 1, 10, 100, 1000 μM EDTA | PS80 MC | 0.02 | 10 mM His | 5.5 | Water | - | Stainless steel + 50 °C | Doyle et al. (2019) (Doyle et al., 2019) |
| 342 μM EDTA | PS80d | 0.06 | 10 mM His | 5.5-6.0 | 150 mM NaCl | - | 5 ppm Fe2+ + 25 °C |
Gopalrathnam et al. (2018) (Gopalrathnam et al., 2018) |
| 0.5 mM DTPA | PS80 AO | 0.15 | His-AcOH | 5.5 | Water | - | 40 °C + light + stainless steel | Doshi et al. (2021)c (Doshi et al., 2021a) |
concentration of methionine and EDTA not given.
Doshi et al. (2021), Evaluating a modified high purity polysorbate 20 designed to reduce the risk of free fatty acid particle formation.
Doshi et al. (2021), A comprehensive assessment of all-oleate polysorbate 80: free fatty acid particle formation, interfacial protection, and oxidative degradation.
quality not specifically given.
6. Conclusion and future perspectives
As polysorbates are by far the most prevalent surfactants in biopharmaceuticals, it is critical to solve the issues regarding polysorbate degradation. Different approaches are proposed and pursued to solve or mitigate oxidative polysorbate degradation, like (i) the use of chelating reagents (EDTA (ethylenediaminetetraacetic acid), DTPA (diethylenetriaminepentaacetic acid)) (Kranz et al., 2019; Gopalrathnam et al., 2018; Bensaid et al., 2022; Kranz et al., 2020; Doyle et al., 2019; Doshi et al., 2021a; Yarbrough et al., 2019), (ii) antioxidants (methionine, BHT (butylated hydroxytoluene), BHA (butylated hydroxyanisole)) (Doshi et al., 2020a; Gopalrathnam et al., 2018; Schröter et al., 2021; Doshi et al., 2021a; Kranz et al., 2019; Kranz et al., 2020; Doshi et al., 2021b; Schmidt et al., 2020; Knepp et al., 1996; Yarbrough et al., 2019), or (iii) changes in storage and manufacturing conditions (protection from air, light, or metal traces) (Liu et al., 2022a; Gopalrathnam et al., 2018; Zhou et al., 2011; Abernethy et al., 2010; Zhou et al., 2010a), or (iv) improving the quality/purity of excipients such as polysorbate.
Due to the structural complexity of PS, a variety of analytical methods are available. Among these, LC-MS is a very powerful method, as oxidative degradation can be monitored via separation and supposed degradation products (markers) can be identified. Different marker molecules were reported (see Table 3) (Kishore et al., 2011a; Evers et al., 2020; Dahotre et al., 2018; Kranz et al., 2020), enabling to track oxidative and/or hydrolytic degradation and to differentiate between both degradation pathways. They shed light on potential issues related to different buffer systems (Brovč et al., 2020a; Liu et al., 2022a; Doyle et al., 2019; Kranz et al., 2019; Gopalrathnam et al., 2018; Bensaid et al., 2022; Zhang et al., 2018; Prajapati et al., 2022), or on promising mitigation reagents (Doyle et al., 2019; Kranz et al., 2019; Yarbrough et al., 2019; Gopalrathnam et al., 2018; Schröter et al., 2021; Bensaid et al., 2022; Doshi et al., 2021a; Kranz et al., 2020; Brovč et al., 2020c; Schmidt et al., 2020; Knepp et al., 1996).
For oxidative polysorbate degradation, the following key characteristics were identified (compare Table 4): (i) preferential degradation of higher-order esters for PS20 (Lippold et al., 2017; Liu et al., 2022a; Zhang et al., 2017; Kranz et al., 2019; Zhang et al., 2018; Kranz et al., 2020; Brovč et al., 2020c) as well as in some cases a slight preference for higher-order esters in PS80 (Bensaid et al., 2022; Borisov et al., 2015; Liu et al., 2022a; Hvattum et al., 2012), (ii) the dominant oxidative degradation of PS20 species with longer chain fatty acids (Borisov et al., 2015; Zhang et al., 2018), (iii) the pH shifts in weakly-buffered systems upon oxidative degradation of both PS due to the formation of short chain acids (Donbrow et al., 1978b), (iv) the competition in oxidation with proteins (Brovč et al., 2020a; Gopalrathnam et al., 2018; Bensaid et al., 2022), or (v) the generation of species with lower hydrophobicity in PS80 after oxidation (Brovč et al., 2020a; Borisov et al., 2015; Lippold et al., 2017; Liu et al., 2022a; Kranz et al., 2019; Kranz et al., 2020). Liu and colleagues (2022) suggested more hydrophilic species of oleic acids as origin for the emerging peaks (Liu et al., 2022a).
Multiple forced degradation studies comparing PS20 and PS80 demonstrate a higher potential for PS80 oxidation mostly due to the higher content of unsaturation. For PS80 many degradation products involving the oleic acid double bond were identified, like for instance esters of hydroxy-C18:1, hydroperoxyl-C18:1, keto-C18:1, or epoxy-C18:0 (Borisov et al., 2015; Hvattum et al., 2012), or the shorter versions like esters of 9-oxo-C9:0, 1,9-nonanedioic acid, or 2-decenedioic acid (Brovč et al., 2020a; Borisov et al., 2015; Liu et al., 2022a). Nevertheless, less is known about a direct comparison between PS20 and PS80 oxidation under pharmaceutical-relevant conditions (2-8 °C) without additional stressors. In other words, are these differences in oxidation between PS20 and PS80 relevant for the pharmaceutical community? An additional level of complexity is provided for different grades of polysorbates. For instance, Kranz and colleagues (2020) revealed no difference in oxidative degradation of polyesters between PS20 AL and PS80 AO (Kranz et al., 2020), despite the much higher content of unsaturation for PS80 AO. The rationale behind this observation is still under debate and more studies are required for clarification. Some recent studies demonstrated a higher susceptibility for the purer and less heterogenous polysorbate grades (PS20 AL and PS80 AO) in comparison to their multicompendial grades, independently of PS20 or PS80 (Brovč et al., 2020a; Doshi et al., 2020a; Liu et al., 2022a; Kranz et al., 2019; Bensaid et al., 2022; Kranz et al., 2020). Less is known about a direct comparison between HP and SR quality. Here, Doshi and coworkers (2020) showed a higher oxidative susceptibility for PS20 SR, arguing with higher values of PS20 degradation, protein oxidation, and peroxide formation (Doshi et al., 2020a). Nevertheless, the exact rationale for the faster degradation of purer grades as well as the impact on the stabilization effect is not fully understood so far and more studies are required.
Some scientists are even searching/investigating alternative surfactants for protein stabilization (Bollenbach et al., 2022; Serno et al., 2011; Dubey and Giovannini, 2021; Wu et al., 2021; Haji Abdolvahab et al., 2014; Yue et al., 2020; Liu et al., 2022b; Schiefelbein et al., 2010; Hanson et al., 2020). The probably most frequently used alternative is poloxamer 188, which is to the best of our knowledge, the “only” relevant surfactant used in parenteral pharmaceutical formulations so far (e.g., Enspryng®, Hemlibra®). Poloxamer is a non-ionic triblock-copolymer surfactant and consists of a polypropylene oxide (PPO) block, flanked by two blocks of hydrophilic POE subunits in a POEa – PPOb – POEc fashion. The indices a, b, and c vary depending on the poloxamer species and are ranging from 25-30 PPO blocks for b as well as 75 to 85 POE moieties for a and c for P188Bollenbach et al. (2022). For a more detailed description see Bollenbach et al. (2022). Nevertheless, poloxamers 188 are also able to be oxidized, which results in oxidized POE and PPO species such as formaldehyde, propionaldehyde, and acetaldehyde depending on the buffer system and the applied stress (solution stability of poloxamer) (Bollenbach et al., 2022; Wang et al., 2019; Kim et al., 2014), which is a problem in histidine buffer as well as in presence of trace metals (Wang et al., 2019; Chen et al., 2022). Another alternative is cyclodextrin as it is already used in some formulations, with hydroxypropyl-ß-cyclodextrin (HPßCD) being the most extensively studied (Jenke, 2006; Zhou et al., 2010b; Rowe et al., 2009; Wu et al., 2021). Cyclodextrins have different structures compared to polysorbate and poloxamer, belonging to the cyclic oligosaccharides. The Ph. Eur. distinguishes between α- and ß-cyclodextrins, with six or seven glucopyranosides, respectively (Davis and Brewster, 2004). For a general overview of polysorbate alternatives see Ruiz et al. (2022) (Ruiz et al., 2022). Even though some alternatives have already been investigated, there is still a lack of information to assess, whether any other surfactant is more beneficial regarding oxidative degradation than polysorbate. Even if other excipients seem promising at first glance, such as poloxamer 188 or HPßCD, each of them has its own challenges that need to be kept in mind before use.
For an oxidative susceptibility assessment in general, all pharmaceutical substances, excipients, and conditions need to be considered with respect to quality and suitability (Wuchner et al., 2022a; Wuchner et al., 2022b). This includes interactions that may occur between protein and polysorbate as well as the impact of buffers or the polysorbate qualities themselves. A precise recommendation for an oxidative stable formulation is challenging as there are many influences upon manufacturing, formulation, and storage. PS oxidation is a highly complex, multifactorial process with synergistic degradation parameters. In general the following mitigation options have been discussed: e.g., (i) chelating reagents (Doyle et al., 2019; Kranz et al., 2019; Gopalrathnam et al., 2018; Bensaid et al., 2022; Kranz et al., 2020; Doshi et al., 2021a; Yarbrough et al., 2019), (ii) antioxidants (Doshi et al., 2020a; Gopalrathnam et al., 2018; Schröter et al., 2021; Doshi et al., 2021a; Kranz et al., 2020; Doshi et al., 2021b; Schmidt et al., 2020; Knepp et al., 1996; Yarbrough et al., 2019), or (iii) storage and manufacturing conditions in respect to for instance the purity of used materials (such as iron contaminations) or in respect to light exposure (Liu et al., 2022a; Gopalrathnam et al., 2018; Zhou et al., 2011; Abernethy et al., 2010; Zhou et al., 2010a). It is worth to mention that the applied stress in many studies is often exceeding the actual pharmaceutical conditions. For instance, formulations are stressed at significant elevated temperatures (25 and 40 °C, or in some rare cases even higher), even though they are generally stored in refrigerators at 2 to 8 °C. In these pharmaceutical relevant colder temperature conditions, polysorbate degradation is strongly reduced. Additionally, forced oxidation is often investigated in placebo formulations, so the impact on specific proteins remains unclear. A protective effect of proteins for polysorbate oxidation is observed, although the opposite effect is intended by the addition of excipients (Brovč et al., 2020a; Gopalrathnam et al., 2018; Bensaid et al., 2022). The original intention to prevent protein degradation needs to be pursued more carefully. Storage in a dark and "oxygen-free" environment has been recommended and production processes could possibly be adapted accordingly (Donbrow et al., 1978a; Ha et al., 2002). As already mentioned, there are some additives that are supposed to prevent oxidative PS degradation.
By considering the increasing number of publications in respect to the oxidation of polysorbate in the last years, it could be seen that the oxidative degradation of polysorbates is not underestimated, however, enzyme-mediated hydrolysis seems to be a more critical issue (e.g., fatty acid particle formation). Similar findings were revealed by Wuchner et al. (2022), highlighting that a huge percentage of the 16 globally acting pharmaceutical companies participated in the survey, face problems with PS oxidation in their products (Wuchner et al., 2022a). Especially minimizing contact with stainless steel or light, and the limited supply of oxygen is crucial (Liu et al., 2022a; Gopalrathnam et al., 2018; Zhou et al., 2011; Abernethy et al., 2010; Zhou et al., 2010a). Many studies have been conducted, limiting and assessing the risk of oxidation as summarized above. Nevertheless, there are still many open questions in respect to oxidative PS degradation, especially due to the susceptibility of different grades, the presence of specific excipients (salt, sugar, buffer, pH), or if micelles affect oxidation. In the end it seems there is no single universal remedy to fully avoid for oxidative PS degradation and related to the protection of proteins, both surfactants PS20 and PS80 are highly valuable. Currently, no alternative stable surfactant with comparable protein-stabilization properties as PS is available.
CRediT authorship contribution statement
Johanna Weber: Investigation, Formal analysis, Conceptualization, Visualization, Writing – original draft, Writing – review & editing, Visualization. Julia Buske: Writing – review & editing, Supervision. Karsten Mäder: Conceptualization, Writing – review & editing, Supervision, Project administration. Patrick Garidel: Conceptualization, Writing – review & editing, Supervision, Project administration. Tim Diederichs: Investigation, Conceptualization, Visualization, Writing – original draft, Writing – review & editing, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interest or personal relationship that could have appeared to influence the work reported in this manuscript.
Acknowledgement
We acknowledge Stefan Carle and Tamara Phan for their comments, ideas, and for proof reading and Holger Thie for management support.
Contributor Information
Patrick Garidel, Email: patrick.garidel@boehringer-ingelheim.com.
Tim Diederichs, Email: tim.diederichs@boehringer-ingelheim.com.
Data availability
Data will be made available on request.
References
- Abernethy D.R., DeStefano A.J., Cecil T.L., Zaidi K., Williams R.L. Metal impurities in food and drugs. Pharm. Res. 2010;27(5):750–755. doi: 10.1007/s11095-010-0080-3. [DOI] [PubMed] [Google Scholar]
- Agarkhed M., O’Dell C., Hsieh M.C., Zhang J., Goldstein J., Srivastava A. Effect of polysorbate 80 concentration on thermal and photostability of a monoclonal antibody. AAPS PharmSciTech. 2013;14(1):1–9. doi: 10.1208/s12249-012-9878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain L., Wang Q. Impact of package leachables on the stability of pharmaceutical products. Am. Pharm. Rev. 2007;10(4):38–44. [Google Scholar]
- Allmendinger A., Lebouc V., Bonati L., Woehr A., Kishore R.S.K., Abstiens K. Glass leachables as a nucleation factor for free fatty acid particle formation in biopharmaceutical formulations. J. Pharm. Sci. 2021;110(2):785–795. doi: 10.1016/j.xphs.2020.09.050. [DOI] [PubMed] [Google Scholar]
- Azaz E., Donbrow M., Hamburger R. Assay of micro-scale amounts of hydroperoxide and of iodine in aqueous non-ionic surfactant solutions by a spectrophotometric method. Analyst. 1973;98:663–672. doi: 10.1039/AN9739800663. [DOI] [Google Scholar]
- Barnett G.V., Razinkov V.I., Kerwin B.A., et al. Specific-ion effects on the aggregation mechanisms and protein-protein interactions for anti-streptavidin immunoglobulin gamma-1. J. Phys. Chem. B. 2015;119(18):5793–5804. doi: 10.1021/acs.jpcb.5b01881. [DOI] [PubMed] [Google Scholar]
- Basu P., Krishnan S., Thirumangalathu R., Randolph T.W., Carpenter J.F. IgG1 aggregation and particle formation induced by silicone-water interfaces on siliconized borosilicate glass beads: A model for siliconized primary containers. J. Pharm. Sci. 2013;102(3):852–865. doi: 10.1002/jps.23434. [DOI] [PubMed] [Google Scholar]
- Bates T.R., Nightingale C.H., Dixon E. Kinetics of hydrolysis of polyoxyethylene (20) sorbitan fatty acid ester surfactants. J. Pharm. Pharmacol. 1973;25(6):470–477. doi: 10.1111/j.2042-7158.1973.tb09135.x. [DOI] [PubMed] [Google Scholar]
- Bensaid F., Dagallier C., Authelin J.R., et al. Mechanistic understanding of metal-catalyzed oxidation of polysorbate 80 and monoclonal antibody in biotherapeutic formulations. Int. J. Pharm. 2022;615 doi: 10.1016/j.ijpharm.2022.121496. [DOI] [PubMed] [Google Scholar]
- Blaffert J., Haeri H.H., Blech M., Hinderberger D., Garidel P. Spectroscopic methods for assessing the molecular origins of macroscopic solution properties of highly concentrated liquid protein solutions. Anal. Biochem. 2018;561-562:70–88. doi: 10.1016/j.ab.2018.09.013. [DOI] [PubMed] [Google Scholar]
- Bollenbach L., Buske J., Mäder K., Garidel P. Poloxamer 188 as surfactant in biological formulations – An alternative for polysorbate 20/80? Int. J. Pharm. 2022;620 doi: 10.1016/j.ijpharm.2022.121706. [DOI] [PubMed] [Google Scholar]
- Borisov O.V., Ji J.A., Wang Y.J. Oxidative degradation of polysorbate surfactants studied by liquid chromatography-mass spectrometry. J. Pharm. Sci. 2015;104(3):1005–1018. doi: 10.1002/jps.24314. [DOI] [PubMed] [Google Scholar]
- Brett Ludwig D., Carpenter John F., Hamel Jean-Bernard, Randolph Theodore W. Protein adsorption and excipient effects on kinetic stability of silicone oil emulsions. J. Pharm. Sci. 2010;99(4):1721–1733. doi: 10.1002/jps.21982. [DOI] [PubMed] [Google Scholar]
- Brito Rui M., Vaz W.L. Determination of the critical micelle concentration of surfactants using the fluorescent probe N-Phenyl-1-Naphthylamine. Anal. Biochem. 1986;152(2):250–255. doi: 10.1016/0003-2697(86)90406-9. [DOI] [PubMed] [Google Scholar]
- Britt K.A., Schwartz D.K., Wurth C., Mahler H.C., Carpenter J.F., Randolph T.W. Excipient effects on humanized monoclonal antibody interactions with silicone oil emulsions. J. Pharm. Sci. 2012;101(12):4419–4432. doi: 10.1002/jps.23318. [DOI] [PubMed] [Google Scholar]
- Brovč E.V., Mravljak J., Šink R., Pajk S. Degradation of polysorbates 20 and 80 catalysed by histidine chloride buffer. Eur. J. Pharm. Biopharm. 2020;154:236–245. doi: 10.1016/j.ejpb.2020.07.010. [DOI] [PubMed] [Google Scholar]
- Brovč E.V., Mravljak J., Šink R., Pajk S. Rational design to biologics development: The polysorbates point of view. Int. J. Pharm. 2020;581 doi: 10.1016/j.ijpharm.2020.119285. [DOI] [PubMed] [Google Scholar]
- Brovč E.V., Pajk S., Šink R., Mravljak J. Protein formulations containing polysorbates: Are metal chelators needed at all? Antioxidants. 2020;9(5):441. doi: 10.3390/antiox9050441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner G.R., Mason R.P. Spin-trapping methods for detecting superoxide and hydroxyl free radicals in vitro and in vivo. Methods Enzymol. 1990;186:127–133. doi: 10.1016/0076-6879(90)86101-Z. [DOI] [PubMed] [Google Scholar]
- Cao X., Fesinmeyer R.M., Pierini C.J., et al. Free fatty acid particles in protein formulations, Part 1: Microspectroscopic identification. J. Pharm. Sci. 2015;104(2):433–446. doi: 10.1002/jps.24126. [DOI] [PubMed] [Google Scholar]
- Carpenter J.F., Randolph T.W., Jiskoot W., et al. Overlooking subvisible particles in therapeutic protein products: Gaps that may compromise product quality. J. Pharm. Sci. 2009;98(4):1201–1205. doi: 10.1002/jps.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Bautista R., Yu K., Zapata G.A., Mulkerrin M.G., Chamow S.M. Influence of histidine on the stability and physical properties of a fully human antibody in aqueous and solid forms. Pharm. Res. 2003;20(12):1952–1960. doi: 10.1023/b:pham.0000008042.15988.c0. [DOI] [PubMed] [Google Scholar]
- Chen D., Luo W., Hoffman J., et al. Insights into virus inactivation by polysorbate 80 in the absence of solvent. Biotechnol. Prog. 2020;36(3):2953. doi: 10.1002/btpr.2953. [DOI] [PubMed] [Google Scholar]
- Chen W., Stolz S., Wegbecher V., et al. The degradation of poloxamer 188 in buffered formulation conditions. AAPS Open. 2022;8(5) doi: 10.1186/s41120-022-00055-4. [DOI] [Google Scholar]
- Chistyakova G.V., Koksharov S.A., Vladimirova T.V. Dependence of the solubility of atmospheric oxygen in weakly alkaline aqueous solutions on surfactant concentration. Russ. J. Phys. Chem. A. 2012;86(11):1753–1755. doi: 10.1134/S0036024412110088. [DOI] [Google Scholar]
- Chiu J., Valente K.N., Levy N.E., Min L., Lenhoff A.M., Lee K.H. Knockout of a difficult-to-remove CHO host cell protein, lipoprotein lipase, for improved polysorbate stability in monoclonal antibody formulations. Biotechnol. Bioeng. 2017;114(5):1006–1015. doi: 10.1002/bit.26237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H., Sehested K., Løgager T. Temperature dependence of the rate constant for reactions of hydrated electrons with H, OH and H2O2. Radiat. Phys. Chem. 1994;43(6):527–531. doi: 10.1016/0969-806X(94)90163-5. [DOI] [Google Scholar]
- Christiansen A., Backensfeld T., Kühn S., Weitschies W. Stability of the non-ionic surfactant polysorbate 80 investigated by HPLC-MS and charged aerosol detector. Pharmazie. 2011;66(9):666–671. doi: 10.1691/ph.2011.1033. [DOI] [PubMed] [Google Scholar]
- Corveleyn S., Vandenbossche G., Remon J. Near-Infrared (NIR) monitoring of H2O2 vapor concentration during vapor hydrogen peroxide (VHP) sterilisation. Pharm. Res. 1997;14(3):294–298. doi: 10.1023/a:1012085702372. [DOI] [PubMed] [Google Scholar]
- Crommelin D.J.A., Hawe A., Jiskoot W. In: Pharmaceutical Biotechnology: Fundamentals and Applications. Crommelin D., Sindelar R., Meibohm B., editors. Springer; Cham: 2019. Formulation of biologics including biopharmaceutical considerations; pp. 83–103. [DOI] [Google Scholar]
- Dahotre S., Tomlinson A., Lin B., Yadav S. Novel markers to track oxidative polysorbate degradation in pharmaceutical formulations. J. Pharm. Biomed. Anal. 2018;157:201–207. doi: 10.1016/j.jpba.2018.05.031. [DOI] [PubMed] [Google Scholar]
- Das T.K. Protein particulate detection issues in biotherapeutics development-current status. AAPS PharmSciTech. 2012;13(2):732–746. doi: 10.1208/s12249-012-9793-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A.L., Mrsny R.J. Formulation and delivery issues for monoclonal antibody therapeutics. Adv. Drug Deliv. Rev. 2006;58(5–6):686–706. doi: 10.1016/j.addr.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Davis M.E., Brewster M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004;3(12):1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- De Sainte Claire P. Degradation of PEO in the solid state: A theoretical kinetic model. Macromolecules. 2009;42(10):3469–3482. doi: 10.1021/ma802469u. [DOI] [Google Scholar]
- Decker C., Marchal J. Autoxydation radio-induite du poly(oxyéthylène) en solution aqueuse, 7. Cinétique de la consommation d’oxygène. Die Makromol. Chemie. 1974;175:3531–3540. doi: 10.1002/macp.1974.021751218. [DOI] [Google Scholar]
- Denisov E.T. Afanasev IB. Oxidation and antioxidants in organic. Chem. Biol. 2005 doi: 10.1201/97814200300853. [DOI] [Google Scholar]
- Diederichs T., Mittag J.J., Humphrey J., Voss S., Carle S., Buske J., Garidel P. Existence of a superior polysorbate fraction in respect to protein stabilization and particle formation? Int. J. Pharm. 2023;635(December 2022) doi: 10.1016/j.ijpharm.2023.122660. [DOI] [PubMed] [Google Scholar]
- Ding C., Wang L., Yao Y., Li C. Mechanism of the initial oxidation of monounsaturated fatty acids. Food Chem. 2022;392 doi: 10.1016/j.foodchem.2022.133298. [DOI] [PubMed] [Google Scholar]
- Dixit N., Salamat-Miller N., Salinas P.A., Taylor K.D., Basu S.K. Residual host cell protein promotes polysorbate 20 degradation in a sulfatase drug product leading to free fatty acid particles. J. Pharm. Sci. 2016;105(5):1657–1666. doi: 10.1016/j.xphs.2016.02.029. [DOI] [PubMed] [Google Scholar]
- Donbrow M., Azaz E., Pillersdorf A. Autoxidation of polysorbates. J. Pharm. Sci. 1978;67(12):1676–1681. doi: 10.1002/jps.2600671211. [DOI] [PubMed] [Google Scholar]
- Donbrow M., Hamburger R., Azaz E., Pillersdorf A. Development of acidity in non-ionic surfactants: Formic and acetic acid. Analyst. 1978;103:400–402. doi: 10.1039/AN9780300400. [DOI] [Google Scholar]
- Doshi N., Demeule B., Yadav S. Understanding particle formation: Solubility of free fatty acids as polysorbate 20 degradation byproducts in therapeutic monoclonal antibody formulations. Mol. Pharm. 2015;12(11):3792–3804. doi: 10.1021/acs.molpharmaceut.5b00310. [DOI] [PubMed] [Google Scholar]
- Doshi N., Fish R., Padilla K., Yadav S. Evaluation of super refinedTM polysorbate 20 with respect to polysorbate degradation, particle formation and protein stability. J. Pharm. Sci. 2020;109(10):2986–2995. doi: 10.1016/j.xphs.2020.06.030. [DOI] [PubMed] [Google Scholar]
- Doshi N., Martin J., Tomlinson A. Improving prediction of free fatty acid particle formation in biopharmaceutical drug products: Incorporating ester distribution during polysorbate 20 degradation. Mol. Pharm. 2020;17(11):4354–4363. doi: 10.1021/acs.molpharmaceut.0c00794. [DOI] [PubMed] [Google Scholar]
- Doshi N., Giddings J., Luis L., et al. A comprehensive assessment of all-oleate polysorbate 80: Free fatty acid particle formation, interfacial protection and oxidative degradation. Pharm. Res. 2021;38(3):531–548. doi: 10.1007/s11095-021-03021-z. [DOI] [PubMed] [Google Scholar]
- Doshi N., Ritchie K., Shobha T., et al. Evaluating a modified high purity polysorbate 20 designed to reduce the risk of free fatty acid particle formation. Pharm. Res. 2021;38(9):1563–1583. doi: 10.1007/s11095-021-03087-9. [DOI] [PubMed] [Google Scholar]
- Dovgan T., Golghalyani V., Zurlo F., et al. Targeted CHO cell engineering approaches can reduce HCP-related enzymatic degradation and improve mAb product quality. Biotechnol. Bioeng. 2021;118(10):3821–3831. doi: 10.1002/bit.27857. [DOI] [PubMed] [Google Scholar]
- Doyle L.M., Sharma A.N., Gopalrathnam G., Huang L., Bradley S. A mechanistic understanding of polysorbate 80 oxidation in histidine and citrate buffer systems—Part 2. PDA J. Pharm. Sci. Technol. 2019;73(4):320–330. doi: 10.5731/pdajpst.2018.009639. [DOI] [PubMed] [Google Scholar]
- Dubey S., Giovannini R. Stability of biologics and the quest for polysorbate alternatives. Trends Biotechnol. 2021;39(6):546–549. doi: 10.1016/j.tibtech.2020.10.007. [DOI] [PubMed] [Google Scholar]
- Dulog V.L., Storck G. Die Oxydation von Polyepoxiden mit molekularem Sauerstoff. Macromol. Chem. Phys. 1966;91(1):50–73. doi: 10.1002/macp.1966.020910104. [DOI] [Google Scholar]
- Dwivedi M., Blech M., Presser I., Garidel P. Polysorbate degradation in biotherapeutic formulations: Identification and discussion of current root causes. Int. J. Pharm. 2018;552(1–2):422–436. doi: 10.1016/j.ijpharm.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Dwivedi M., Buske J., Haemmerling F., Blech M., Garidel P. Acidic and alkaline hydrolysis of polysorbates under aqueous conditions: Towards understanding polysorbate degradation in biopharmaceutical formulations. Eur. J. Pharm. Sci. 2020;144 doi: 10.1016/j.ejps.2019.105211. [DOI] [PubMed] [Google Scholar]
- Ehrenshaft M., Deterding L.J., Mason R.P. Tripping up Trp: modification of protein tryptophan residues by reactive oxygen species, modes of detection, and biological consequences. Free Radic. Biol. Med. 2015;89:220–228. doi: 10.1016/j.freeradbiomed.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers D.H., Schultz-Fademrecht T., Garidel P., Buske J. Development and validation of a selective marker-based quantification of polysorbate 20 in biopharmaceutical formulations using UPLC QDa detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020;1157 doi: 10.1016/j.jchromb.2020.122287. [DOI] [PubMed] [Google Scholar]
- Evers D.H., Carle S., Lakatos D., Hämmerling F., Garidel P., Buske J. Hydrolytic polysorbate 20 degradation – Sensitive detection of free fatty acids in biopharmaceuticals via UPLC-QDa analytics with isolator column. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021;1174 doi: 10.1016/j.jchromb.2021.122717. [DOI] [PubMed] [Google Scholar]
- Falconer R.J. Advances in liquid formulations of parenteral therapeutic proteins. Biotechnol. Adv. 2019;37(7) doi: 10.1016/j.biotechadv.2019.06.011. [DOI] [PubMed] [Google Scholar]
- Feig A.L. Reactions of nonheme iron(II) centers with dioxygen in biology and chemistry. Chem. Rev. 1994;94(3):759–805. doi: 10.1021/cr00027a011. [DOI] [Google Scholar]
- Fekete S., Ganzler K., Fekete J., acute. Fast and sensitive determination of polysorbate 80 in solutions containing proteins. J. Pharm. Biomed. Anal. 2010;52(5):672–679. doi: 10.1016/j.jpba.2010.02.035. [DOI] [PubMed] [Google Scholar]
- Finkelstein E., Rosen G.M., Rauckman E.J., Spin trapping. Kinetics of the reaction of superoxide and hydroxyl radicals with nitrones. J. Am. Chem. Soc. 1980;102(15):4994–4999. doi: 10.1021/ja00535a029. [DOI] [Google Scholar]
- Garidel P., Hoffmann C., Blume A. A thermodynamic analysis of the binding interaction between polysorbate 20 and 80 with human serum albumins and immunoglobulins: A contribution to understand colloidal protein stabilisation. Biophys. Chem. 2009;143(1–2):70–78. doi: 10.1016/j.bpc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Garidel P., Kuhn A.B., Schäfer L.V., Karow-Zwick A.R., Blech M. High-concentration protein formulations: How high is high? Eur. J. Pharm. Biopharm. 2017;119:353–360. doi: 10.1016/j.ejpb.2017.06.029. [DOI] [PubMed] [Google Scholar]
- Garidel P., Blech M., Buske J., Blume A. Surface tension and self-association properties of aqueous polysorbate 20 HP and 80 HP solutions: Insights into protein stabilisation mechanisms. J. Pharm. Innov. 2021;16(4):726–734. doi: 10.1007/s12247-020-09488-4. [DOI] [Google Scholar]
- Gerhardt A., McGraw N.R., Schwartz D.K., Bee J.S., Carpenter J.F., Randolph T.W. Protein aggregation and particle formation in prefilled glass syringes. J. Pharm. Sci. 2014;103(6):1601–1612. doi: 10.1002/jps.23973. [DOI] [PubMed] [Google Scholar]
- Gerhardt A., McUmber A.C., Nguyen B.H., et al. Surfactant effects on particle generation in antibody formulations in pre-filled syringes. J. Pharm. Sci. 2015;104(12):4056–4064. doi: 10.1002/jps.24654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glücklich N., Dwivedi M., Carle S., Buske J., Mäder K., Garidel P. An in-depth examination of fatty acid solubility limits in biotherapeutic protein formulations containing polysorbate 20 and polysorbate 80. Int. J. Pharm. 2020;591 doi: 10.1016/j.ijpharm.2020.119934. [DOI] [PubMed] [Google Scholar]
- Glücklich N., Carle S., Buske J., Mäder K., Garidel P. Assessing the polysorbate degradation fingerprints and kinetics of lipases – how the activity of polysorbate degrading hydrolases is influenced by the assay and assay conditions. Eur. J. Pharm. Sci. 2021;166 doi: 10.1016/j.ejps.2021.105980. [DOI] [PubMed] [Google Scholar]
- Glusker J.P. Citrate conformation and chelation: Enzymatic implications. Acc. Chem. Res. 1980;13:345–352. doi: 10.1021/ar50154a002. [DOI] [Google Scholar]
- Goldstein S., Meyerstein D., Czapski G. The fenton reagents. Free Radic. Biol. Med. 1993;15(4):435–445. doi: 10.1016/0891-5849(93)90043-T. [DOI] [PubMed] [Google Scholar]
- Goldstern S., Rosen G.M., Russo A., Samuni A. Kinetics of spin trapping superoxide, hydroxyl, and aliphatic radicals by cyclic nitrones. J. Phys. Chem. A. 2004;108(32):6679–6685. doi: 10.1021/jp048441i. [DOI] [Google Scholar]
- Gopalrathnam G., Sharma A.N., Dodd S.W., Huang L. Impact of stainless steel exposure on the oxidation of polysorbate 80 in histidine placebo and active monoclonal antibody formulation. PDA J. Pharm. Sci. Technol. 2018;72(2):163–175. doi: 10.5731/pdajpst.2017.008284. [DOI] [PubMed] [Google Scholar]
- Grabarek A.D., Bozic U., Rousel J., et al. What makes polysorbate functional? Impact of polysorbate 80 grade and quality on IgG stability during mechanical stress. J. Pharm. Sci. 2020;109(1):871–880. doi: 10.1016/j.xphs.2019.10.015. [DOI] [PubMed] [Google Scholar]
- Graf T., Seisenberger C., Wiedmann M., Wohlrab S., Anderka O. Best practices on critical reagent characterization, qualification, and life cycle management for HCP immunoassays. Biotechnol. Bioeng. 2021;118(10):3633–3639. doi: 10.1002/bit.27881. [DOI] [PubMed] [Google Scholar]
- Granholm K., Harju L., Ivaska A. Desorption of metal ions from kraft pulps. BioResources. 2010;5:206–226. doi: 10.15376/biores.5.1.206-226. [DOI] [Google Scholar]
- Gregoritza K., Cai S.K., Siketanc M., et al. Metal-induced fatty acid particle formation resulting from hydrolytic polysorbate degradation. J. Pharm. Sci. 2021;111(3):743–751. doi: 10.1016/j.xphs.2021.09.044. [DOI] [PubMed] [Google Scholar]
- Ha E., Wang W., Wang Y.J. Peroxide formation in polysorbate 80 and protein stability. J. Pharm. Sci. 2002;91(10):2252–2264. doi: 10.1002/jps.10216. [DOI] [PubMed] [Google Scholar]
- Haji Abdolvahab M., Fazeli A., Fazeli M.R., ez Brinks V., Schellekens H. The effects of dodecyl maltoside and sodium dodecyl sulfate surfactants on the stability and aggregation of recombinant interferon Beta-1b. J. Interf. Cytokine Res. 2014;34(11):894–901. doi: 10.1089/jir.2013.0131. [DOI] [PubMed] [Google Scholar]
- Hall T., Sandefur S.L., Frye C.C., Tuley T.L., Huang L. Polysorbates 20 and 80 degradation by group XV lysosomal phospholipase A2 isomer X1 in monoclonal antibody formulations. J. Pharm. Sci. 2016;105(5):1633–1642. doi: 10.1016/j.xphs.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Hamburger R., Azaz E., Donbrow M. Autoxidation of polyoxyethylenic non-ionic surfactants and of polyethylene glycols. Pharm. Acta Helv. 1975;50(1–2):7–10. https://api.semanticscholar.org/CorpusID:5897509 [PubMed] [Google Scholar]
- Hanson M.G., Katz J.S., Ma H., et al. Effects of hydrophobic tail length variation on surfactant-mediated protein stabilization. Mol. Pharm. 2020;17(11):4302–4311. doi: 10.1021/acs.molpharmaceut.0c00737. [DOI] [PubMed] [Google Scholar]
- Harris D.C. In: Lehrbuch Der Quantitativen Analyse. 8th ed. Werner G., Werner T., editors. Springer Spektrum; Berlin, Heidelberg: 2014. [DOI] [Google Scholar]
- Hartmann-Schreier J. RÖMPP; 2004. Wasserstoffperoxid.https://roempp.thieme.de/lexicon/RD-23-00389 Online. Published. Accessed August 29, 2022. [Google Scholar]
- Hawkins C.L., Davies M.J. Detection and characterisation of radicals in biological materials using EPR methodology. Biochim. Biophys. Acta, Gen. Subj. 2014;1840(2):708–721. doi: 10.1016/j.bbagen.2013.03.034. [DOI] [PubMed] [Google Scholar]
- Haywood R. In: Encyclopedia of Biophysics. Roberts G.C.K., editor. Springer; Berlin, Heidelberg: 2013. Spin-trapping: Theory and applications. [DOI] [Google Scholar]
- Heiba E.I., Dessau R.M., Koehl W.J. Oxidation by metal salts V. Cobaltic acetate oxidation of alkylbenzenes. J. Am. Chem. Soc. 1969;91:6830–6837. doi: 10.1021/JA01052A049. [DOI] [Google Scholar]
- Heider M., Hause G., Mäder K. Does the commonly used pH-stat method with back titration really quantify the enzymatic digestibility of lipid drug delivery systems? A case study on solid lipid nanoparticles (SLN) Eur. J. Pharm. Biopharm. 2016;109:194–205. doi: 10.1016/j.ejpb.2016.10.007. [DOI] [PubMed] [Google Scholar]
- Hewitt D., Zhang T., Kao Y.H. Quantitation of polysorbate 20 in protein solutions using mixed-mode chromatography and evaporative light scattering detection. J. Chromatogr. A. 2008;1215(1–2):156–160. doi: 10.1016/j.chroma.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Hewitt D., Alvarez M., Robinson K., et al. Mixed-mode and reversed-phase liquid chromatography-tandem mass spectrometry methodologies to study composition and base hydrolysis of polysorbate 20 and 80. J. Chromatogr. A. 2011;1218(15):2138–2145. doi: 10.1016/j.chroma.2010.09.057. [DOI] [PubMed] [Google Scholar]
- Hipper E., Blech M., Hinderberger D., Garidel P., Kaiser W. Photo-oxidation of therapeutic protein formulations: from radical formation to analytical techniques. Pharmaceutics. 2021;14(1):72. doi: 10.3390/pharmaceutics14010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipper E., Lehmann F., Kaiser W., Hübner G., Buske J., Blech M., Hinderberger D., Garidel P. Protein photodegradation in the visible range? Insights into protein photooxidation with respect to protein concentration. Int. J. Pharm. X. 2023:5. doi: 10.1016/j.ijpx.2022.100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovorka S.W., Schöneich C. Oxidative degradation of pharmaceuticals: Theory, mechanisms and inhibition. J. Pharm. Sci. 2001;90(3):253–269. doi: 10.1002/1520-6017(200103)90:3<253::AID-JPS1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 2022. https://pharmaintelligence.informa.comhttps://pharmaintelligence.informa.com Accessed June 22.
- 2022. https://www.drugdiscoverytrends.com/50-of-2021s-best-selling-pharmaceuticals/https://www.drugdiscoverytrends.com/50-of-2021s-best-selling-pharmaceuticals/ Accessed June 22.
- Hvattum E., Yip W.L., Grace D., Dyrstad K. Characterization of polysorbate 80 with liquid chromatography mass spectrometry and nuclear magnetic resonance spectroscopy: Specific determination of oxidation products of thermally oxidized polysorbate 80. J. Pharm. Biomed. Anal. 2012;62:7–16. doi: 10.1016/j.jpba.2011.12.009. [DOI] [PubMed] [Google Scholar]
- ICH Guideline Stability Testing . 1996. Photostability Testing of New Drug Substances and Products Q1B. Published online November. [Google Scholar]
- Ilko D., Braun A., Germershaus O., Meinel L., Holzgrabe U. Fatty acid composition analysis in polysorbate 80 with high performance liquid chromatography coupled to charged aerosol detection. Eur. J. Pharm. Biopharm. 2015;94:569–574. doi: 10.1016/j.ejpb.2014.11.018. [DOI] [PubMed] [Google Scholar]
- Imre Z.N., Floyd R.A. Hydroxyl free radical reactions with amino acids and proteins studied by electron spin resonance spectroscopy and spin-trapping. Biochim. Biophys. Acta. 1984;790(3):238–250. doi: 10.1016/0167-4838(84)90028-1. [DOI] [PubMed] [Google Scholar]
- Jenke D. Extractable/leachable substances from plastic materials used as pharmaceutical product containers/devices. PDA J. Pharm. Sci. Technol. 2002;56(6):332–371. https://journal.pda.org/content/56/6/332 [PubMed] [Google Scholar]
- Jenke D.R. Linking extractables and leachables in container/closure applications. PDA J. Pharm. Sci. Technol. 2005;59(4):265–281. https://journal.pda.org/content/59/4/265 [PubMed] [Google Scholar]
- Jenke D. Extractable substances from plastic materials used in solution contact applications: an updated review. PDA J. Pharm. Sci. Technol. 2006;60(3):191–207. https://journal.pda.org/content/60/3/191 [PubMed] [Google Scholar]
- Jones M.T., Mahler H.C., Yadav S., et al. Considerations for the use of polysorbates in biopharmaceuticals. Pharm. Res. 2018;35(8):148. doi: 10.1007/s11095-018-2430-5. [DOI] [PubMed] [Google Scholar]
- Kaiser W., Schultz-Fademrecht T., Blech M., Buske J., Garidel P. Investigating photodegradation of antibodies governed by the light dosage. Int. J. Pharm. 2021;604 doi: 10.1016/j.ijpharm.2021.120723. [DOI] [PubMed] [Google Scholar]
- Kerwin B.A. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: Structure and degradation pathways. J. Pharm. Sci. 2008;97(8):2924–2935. doi: 10.1002/jps.21190. [DOI] [PubMed] [Google Scholar]
- Khan T.A., Mahler H.C., Kishore R.S.K. Key interactions of surfactants in therapeutic protein formulations: A review. Eur. J. Pharm. Biopharm. 2015;97:60–67. doi: 10.1016/j.ejpb.2015.09.016. [DOI] [PubMed] [Google Scholar]
- Khossravi M., Kao Y.H., Mrsny R.J., Sweeney T.D. Analysis methods of polysorbate 20: A new method to assess the stability of polysorbate 20 and established methods that may overlook degraded polysorbate 20. Pharm. Res. 2002;19(5):634–639. doi: 10.1023/a:1015306112979. [DOI] [PubMed] [Google Scholar]
- Kim H.L., McAuley A., Livesay B., Gray W.D., McGuire J. Modulation of protein adsorption by poloxamer 188 in relation to polysorbates 80 and 20 at solid surfaces. J Pharm Sci. 2014;103(4):1043–1049. doi: 10.1002/jps.23907. Epub 2014 Feb 15. PMID: 24532194. [DOI] [PubMed] [Google Scholar]
- Kishore S.K.R. In: Challenges in Protein Product Development. AAPS Advances in the Pharmaceutical Sciences. Warne N., Mahler H.C., editors. Vol. 38. Springer; Cham: 2018. Polysorbate degradation and quality; pp. 25–62. [DOI] [Google Scholar]
- Kishore R.S.K., Kiese S., Fischer S., Pappenberger A., Grauschopf U., Mahler H.C. The degradation of polysorbates 20 and 80 and its potential impact on the stability of biotherapeutics. Pharm. Res. 2011;28(5):1194–1210. doi: 10.1007/s11095-011-0385-x. [DOI] [PubMed] [Google Scholar]
- Kishore R.S.K., Pappenberger A., Dauphin I.B., et al. Degradation of polysorbates 20 and 80: Studies on thermal autoxidation and hydrolysis. J. Pharm. Sci. 2011;100(2):721–731. doi: 10.1002/jps.22290. [DOI] [PubMed] [Google Scholar]
- Klair N., Kim M.T., Lee A., Xiao N.J., Patel A.R. Stress temperature studies in small scale Hastelloy® drug substance containers lead to increased extent of and increased variability in antibody-drug conjugate and monoclonal antibody aggregation: Evidence for novel oxidation-induced crosslinking in monoc. J. Pharm. Sci. 2021;110(4):1615–1624. doi: 10.1016/j.xphs.2020.09.052. [DOI] [PubMed] [Google Scholar]
- Knepp V.M., Whatley J.L., Muchnik A., Calderwood T.S. Identification of antioxidants for prevention of peroxide-mediated oxidation of recombinant human ciliary neurotrophic factor and recombinant human nerve growth factor. PDA J. Pharm. Sci. Technol. 1996;50(3):163–171. https://journal.pda.org/content/50/3/163 [PubMed] [Google Scholar]
- Knoch H., Ulbrich M.H., Mittag J.J., Buske J., Garidel P., Heerklotz H. Complex micellization behavior of the polysorbates Tween 20 and Tween 80. Mol. Pharm. 2021;18(8):3147–3157. doi: 10.1021/acs.molpharmaceut.1c00406. [DOI] [PubMed] [Google Scholar]
- Kol S., Ley D., Wulff T., et al. Multiplex secretome engineering enhances recombinant protein production and purity. Nat. Commun. 2020;11(1):1908. doi: 10.1038/s41467-020-15866-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovner D., Yuk I.H., Shen A., et al. Characterization of recombinantly-expressed hydrolytic enzymes from chinese hamster ovary cells: Identification of host cell proteins that degrade polysorbate. J. Pharm. Sci. 2023:(23). doi: 10.1016/j.xphs.2023.01.003. [DOI] [PubMed] [Google Scholar]
- Kranz W., Wuchner K., Corradini E., Berger M., Hawe A. Factors influencing polysorbate’s sensitivity against enzymatic hydrolysis and oxidative degradation. J. Pharm. Sci. 2019;108(6):2022–2032. doi: 10.1016/j.xphs.2019.01.006. [DOI] [PubMed] [Google Scholar]
- Kranz W., Wuchner K., Corradini E., Menzen T., Hawe A. Micelle driven oxidation mechansim and novel oxidation markers for different grades of polysorbate 20 and 80. J. Pharm. Sci. 2020;109(10):3064–3077. doi: 10.1016/j.xphs.2020.06.028. [DOI] [PubMed] [Google Scholar]
- Krishna A.K., Lodhi S.A., Harris M.R. Isolation technology for research and development applications: From concept to production. Pharm. Dev. Technol. 2000;5(4):507–520. doi: 10.1081/PDT-100102034. [DOI] [PubMed] [Google Scholar]
- Lam X.M., Lai W.G., Chan E.K., Ling V., Hsu C.C. Site-specific tryptophan oxidation induced by autocatalytic reaction of polysorbate 20 in protein formulation. Pharm. Res. 2011;28(10):2543–2555. doi: 10.1007/s11095-011-0482-x. [DOI] [PubMed] [Google Scholar]
- Larson N.R., Wei Y., Prajapati I., et al. Comparison of polysorbate 80 hydrolysis and oxidation on the aggregation of a monoclonal antibody. J. Pharm. Sci. 2020;109(1):633–639. doi: 10.1016/j.xphs.2019.10.069. [DOI] [PubMed] [Google Scholar]
- Lei M., Quan C., Wang J.Y., Kao Y.H., Schöneich C. Light-induced histidine adducts to an IgG1 molecule via oxidized histidine residue and the potential impact of polysorbate-20 concentration. Pharm. Res. 2021;38(3):491–501. doi: 10.1007/s11095-021-03010-2. [DOI] [PubMed] [Google Scholar]
- Li X., Wang F., Li H., Richardson D.D., Roush D.J. The measurement and control of high-risk host cell proteins for polysorbate degradation in biologics formulation. Antib Ther. 2022;5(1):42–54. doi: 10.1093/abt/tbac002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippold S., Koshari S.H.S., Kopf R., et al. Impact of mono- and poly-ester fractions on polysorbate quantitation using mixed-mode HPLC-CAD/ELSD and the fluorescence micelle assay. J. Pharm. Biomed. Anal. 2017;132:24–34. doi: 10.1016/j.jpba.2016.09.033. [DOI] [PubMed] [Google Scholar]
- Liu H., Jin Y., Menon R., et al. Characterization of polysorbate 80 by liquid chromatography-mass spectrometry to understand its susceptibility to degradation and its oxidative degradation pathway. J. Pharm. Sci. 2022;111(2):323–334. doi: 10.1016/j.xphs.2021.08.017. [DOI] [PubMed] [Google Scholar]
- Liu Y., Li H., Yan Z., Zhang L., Sun P. Discovery and reduction of tryptophan oxidation-induced IgG1 fragmentation in a polysorbate 80-dependent manner. Eur. J. Pharm. Biopharm. 2022;173:45–53. doi: 10.1016/j.ejpb.2022.02.015. [DOI] [PubMed] [Google Scholar]
- Lopachin R.M., Gavin T. Molecular mechanisms of aldehyde toxicity: a chemical perspective. Chem. Res. Toxicol. 2014;27(7):1081–1091. doi: 10.1021/tx5001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lougheed W.D., Albisser A.M., Martindale H.M., Chow J.C., Clement J.R. Physical stability of insulin formulations. Diabetes. 1983;32(5):424–432. doi: 10.2337/diab.32.5.424. [DOI] [PubMed] [Google Scholar]
- Mahler H.C., Müller R., Frieß W., Delille A., Matheus S. Induction and analysis of aggregates in a liquid IgG1-antibody formulation. Eur. J. Pharm. Biopharm. 2005;59(3):407–417. doi: 10.1016/j.ejpb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Mahler H.C., Senner F., Maeder K., Mueller R. Surface activity of a monoclonal antibody. J. Pharm. Sci. 2009;98(12):4525–4533. doi: 10.1002/jps.21776. [DOI] [PubMed] [Google Scholar]
- Martos A., Koch W., Jiskoot W., et al. Trends on analytical characterization of polysorbates and their degradation products in biopharmaceutical formulations. J. Pharm. Sci. 2017;106(7):1722–1735. doi: 10.1016/j.xphs.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Matheson I., Lee J. Chemical reaction rates of amino acids with singlet oxygen. Photochem. Photobiol. 2008;29:879–881. doi: 10.1111/j.1751-1097.1979.tb07786.x. [DOI] [Google Scholar]
- Mcclements D.J., Decker E.A. Lipid oxidation in oil-in-water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000;65(8):1270–1282. doi: 10.1111/j.1365-2621.2000.tb10596.x. [DOI] [Google Scholar]
- McShan A.C., Kei P., Ji J.A., Kim D.C., Wang Y.J. Hydrolysis of polysorbate 20 and 80 by a range of carboxylester hydrolases. PDA J. Pharm. Sci. Technol. 2016;70(4):332–345. doi: 10.5731/pdajpst.2015.005942. [DOI] [PubMed] [Google Scholar]
- Michaeli A., Feitelson J. Reactivity of singlet oxygen toward amino acids and peptides. Photochem. Photobiol. 1994;59(3):284–289. doi: 10.1111/j.1751-1097.1994.tb05035.x. [DOI] [PubMed] [Google Scholar]
- Mittag J., Trutschel M.L., Kruschwitz H., Mäder K., Buske J., Garidel P. Characterization of radicals in polysorbate 80 using electron paramagnetic resonance (EPR) spectroscopy and spin trapping. Int. J. Pharm. 2022;4:100–123. doi: 10.1016/j.ijpx.2022.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussa E.M., Panchal J.P., Moorthy B.S., et al. Immunogenicity of therapeutic protein aggregates. J. Pharm. Sci. 2016;105(2):417–430. doi: 10.1016/j.xphs.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Musakhanian J., Rodier J.D., Dave M. Oxidative Stability in Lipid Formulations: a Review of the Mechanisms, Drivers, and Inhibitors of Oxidation. AAPS Pharm Sci Tech. 2022;23(5):151. doi: 10.1208/s12249-022-02282-0. Erratum in: AAPS PharmSciTech. 2022 Jun 14;23(5):165. PMID: 35596043. [DOI] [PubMed] [Google Scholar]
- Nayak V.S., Tan Z., Ihnat P.M., Russell R.J., Grace M.J. Evaporative light scattering detection based HPLC method for the determination of polysorbate 80 in therapeutic protein formulations. J. Chromatogr. Sci. 2012;50(1):21–25. doi: 10.1093/chromsci/bmr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki E. Free radical initiators as source of water- or lipid-soluble peroxyl radicals. Methods Enzymol. 1990;186:100–108. doi: 10.1016/0076-6879(90)86095-d. [DOI] [PubMed] [Google Scholar]
- Patapoff T.W., Esue O. Polysorbate 20 prevents the precipitation of a monoclonal antibody during shear viscosity of monoclonal antibodies under shear. Pharm. Dev. Technol. 2009;14(6):659–664. doi: 10.3109/10837450902911929. [DOI] [PubMed] [Google Scholar]
- Patten P.A., Schellekens H. The immunogenicity of biopharmaceuticals. Lessons learned and consequences for protein drug development. Dev. Biol. 2003;112:81–97. http://europepmc.org/abstract/MED/12762507 [PubMed] [Google Scholar]
- Peters B.H., Wei Y., Middaugh C.R., Schöneich C. Intra-micellar and extra-micellar oxidation in phosphate and histidine buffers containing polysorbate 80. J. Pharm. Sci. 2022;111(9):2435–2444. doi: 10.1016/j.xphs.2022.06.011. [DOI] [PubMed] [Google Scholar]
- Phaniendra A., Jestadi D.B., Periyasamy L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015;30(1):11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Pharmacopeia 10.5. 2022. [Google Scholar]
- Philo J.S. A critical review of methods for size characterization of non-particulate protein aggregates. Curr. Pharm. Biotechnol. 2009;10(4):359–372. doi: 10.2174/138920109788488815. [DOI] [PubMed] [Google Scholar]
- Porter N.A. Mechanisms for the Autoxidation of Polyunsaturated Lipids. Acc. Chem. Res. 1986;19(9):262–268. doi: 10.1021/ar00129a001. [DOI] [Google Scholar]
- Prajapati I., Peters B.H., Larson N.R., et al. Cis/trans isomerization of unsaturated fatty acids in polysorbate 80 during light exposure of a monoclonal antibody–containing formulation. J. Pharm. Sci. 2020;109(1):603–613. doi: 10.1016/j.xphs.2019.10.068. [DOI] [PubMed] [Google Scholar]
- Prajapati I., Subelzu N., Zhang Y., Wu Y., Schöneich C. Near UV and visible light photo-degradation mechanisms in citrate buffer: One-electron reduction of peptide and protein disulfides promotes oxidation and cis/trans isomerization of unsaturated fatty acids of polysorbate 80. J. Pharm. Sci. 2022;111(4):991–1003. doi: 10.1016/j.xphs.2022.01.026. [DOI] [PubMed] [Google Scholar]
- Pratt D.A., Tallman K.A., Porter N.A. Free radical oxidation of polyunsaturated lipids: New mechanistic insights and the development of peroxyl radical clocks. Acc. Chem. Res. 2011;44(6):458–467. doi: 10.1021/ar200024c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayaprolu B.M., Strawser J.J., Anyarambhatla G. Excipients in parenteral formulations: selection considerations and effective utilization with small molecules and biologics. Drug Dev. Ind. Pharm. 2018;44(10):1565–1571. doi: 10.1080/03639045.2018.1483392. [DOI] [PubMed] [Google Scholar]
- Roberts I., Urey H. The mechanisms of acid catalyzed ester hydrolysis, esterification and oxygen exchange of carboxylic acids. J. Am. Chem. Soc. 1939;61:2584–2587. doi: 10.1021/ja01265a003. [DOI] [Google Scholar]
- Rosenberg A.S. Effects of protein aggregates: An Immunologic perspective. AAPS J. 2006;8(3):501–507. doi: 10.1208/aapsj080359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe R.C., Sheskey P.J., Quinn M.E. In: Handbook of Pharmaceutical Excipients. 6th ed. Rowe R.C., Sheskey P.J., Quinn M.E., editors. Pharmaceutical Press and the American Pharmacists Association; 2009. Handbook of pharmaceutical excipients; pp. 242–244. [Google Scholar]
- Roy I., Patel A., Kumar V., et al. Polysorbate degradation and particle formation in a high concentration mAb: Formulation strategies to minimize effect of enzymatic polysorbate degradation. J. Pharm. Sci. 2021;110(9):3313–3323. doi: 10.1016/j.xphs.2021.05.012. [DOI] [PubMed] [Google Scholar]
- Ruiz A.J.C., Boushehri M.A.S., Phan T., Carle S., Garidel P., Buske J., Lamprecht A. Alternative excipients for protein stabilization in protein therapeutics: Overcoming the limitations of polysorbates. Pharmaceutics. 2022;14(12):2575. doi: 10.3390/pharmaceutics14122575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp C., Steckel H., Müller B.W. Mixed micelle formation with phosphatidylcholines: The influence of surfactants with different molecule structures. Int. J. Pharm. 2010;387(1–2):120–128. doi: 10.1016/j.ijpharm.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Saggu M., Liu J., Patel A. Identification of subvisible particles in biopharmaceutical formulations using raman spectroscopy provides insight into polysorbate 20 degradation pathway. Pharm. Res. 2015;32(9):2877–2888. doi: 10.1007/s11095-015-1670-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari N., Kahraman E., Sari B., Özgün A. Synthesis of some polymer-metal complexes and elucidation of their structures. J. Macromol. Sci. Part A Pure Appl. Chem. 2006;43(8):1227–1235. doi: 10.1080/10601320600737484. [DOI] [Google Scholar]
- Schiefelbein L., Keller M., Weissmann F., Luber M., Bracher F., Frieß W. Synthesis, characterization and assessment of suitability of trehalose fatty acid esters as alternatives for polysorbates in protein formulation. Eur. J. Pharm. Biopharm. 2010;76(3):342–350. doi: 10.1016/j.ejpb.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Koulov A., Huwyler J., Mahler H.C., Jahn M. Stabilizing polysorbate 20 and 80 against oxidative degradation. J. Pharm. Sci. 2020;109(6):1924–1932. doi: 10.1016/j.xphs.2020.03.003. [DOI] [PubMed] [Google Scholar]
- Schröter A., Koulov A.V., Huwyler J., Mahler H.C., Jahn M. 4-Hydroxynonenal is an oxidative degradation product of polysorbate 80. J. Pharm. Sci. 2021;110(6):2524–2530. doi: 10.1016/j.xphs.2021.01.027. [DOI] [PubMed] [Google Scholar]
- Serno T., Geidobler R., Winter G. Protein stabilization by cyclodextrins in the liquid and dried state. Adv. Drug Deliv. Rev. 2011;63(13):1086–1106. doi: 10.1016/j.addr.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Singh S.R., Zhang J., O’Dell C., et al. Effect of polysorbate 80 quality on photostability of a monoclonal antibody. AAPS PharmSciTech. 2012;13(2):422–430. doi: 10.1208/s12249-012-9759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.M., Bandi S., Jones D.N.M., Mallela K.M.G. Effect of polysorbate 20 and polysorbate 80 on the higher-order structure of a monoclonal antibody and its Fab and Fc fragments probed using 2D nuclear magnetic resonance spectroscopy. J. Pharm. Sci. 2017;106(12):3486–3498. doi: 10.1016/j.xphs.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siska C.C., Pierini C.J., Lau H.R., Latypov R.F., Fesinmeyer R.M., Litowski J.R. Free fatty acid particles in protein formulations, Part 2: Contribution of polysorbate raw material. J. Pharm. Sci. 2015;104(2):447–456. doi: 10.1002/jps.24144. [DOI] [PubMed] [Google Scholar]
- Smaltz P.A. Focus on Eliminating Formulation Impurities Drives of New Polysorbate Products. 2013. www.avantormaterials.com/askavantor
- Smidrkal J., Cervenkova R., Filip V. Two-stage synthesis of sorbitan esters, and physical properties of the products. Eur. J. Lipid Sci. Technol. 2004;106(12):851–855. doi: 10.1002/ejlt.200401003. [DOI] [Google Scholar]
- Stadtman E.R. Metal ion-catalyzed oxidation of proteins: biochemical mechanism and biological consequences. Free Radic. Biol. Med. 1990;9(4):315–325. doi: 10.1016/0891-5849(90)90006-5. [DOI] [PubMed] [Google Scholar]
- Stefanidis D., Jencks W.P. General base catalysis of ester hydrolysis. J. Am. Chem. Soc. 1993;115(14):6045–6050. doi: 10.1021/ja00067a020. [DOI] [Google Scholar]
- Sterchi A.C. Swiss Federal Institute of Technology in Zürich (ETH Zürich); 2001. Hydrogen Peroxide in Pharma-Isolators, Investigations about Behavior, Measurement and Effects.https://api.semanticscholar.org/CorpusID:94691418 [Google Scholar]
- Strickley R.G., Lambert W.J. A review of formulations of commercially available antibodies. J. Pharm. Sci. 2021;110(7):2590–2608. doi: 10.1016/j.xphs.2021.03.017. [DOI] [PubMed] [Google Scholar]
- Subelzu N., Schöneich C. Near UV and visible light induce iron-dependent photodegradation reactions in pharmaceutical buffers: mechanistic and product studies. Mol. Pharm. 2020;17(11):4163–4179. doi: 10.1021/acs.molpharmaceut.0c00639. [DOI] [PubMed] [Google Scholar]
- Tomlinson A., Demeule B., Lin B., Yadav S. Polysorbate 20 degradation in biopharmaceutical formulations: Quantification of free fatty acids, characterization of particulates, and insights into the degradation mechanism. Mol. Pharm. 2015;12(11):3805–3815. doi: 10.1021/acs.molpharmaceut.5b00311. [DOI] [PubMed] [Google Scholar]
- Tomlinson A., Zarraga I.E., Demeule B. Characterization of polysorbate ester fractions and implications in protein drug product stability. Mol. Pharm. 2020;17(7):2345–2353. doi: 10.1021/acs.molpharmaceut.0c00093. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration Full Prescribing Information: Clinimix E. 2021. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8469d6fb-d6ef-476f-8cc3-0905192de0a8
- U.S. Food and Drug Administration Full Prescribing Information: Premasol - Sulfite-Free. 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9afdcc3e-0d06-47f4-86ca-40da48b2b02b
- Van Beers M.M.C., Gilli F., Schellekens H., Randolph T.W., Jiskoot W. Immunogenicity of recombinant human interferon beta interacting with particles of glass, metal, and polystyrene. J. Pharm. Sci. 2012;101(1):187–199. doi: 10.1002/jps.22744. [DOI] [PubMed] [Google Scholar]
- Wade A.M., Tucker H.N. Antioxidant characteristics of L-histidine. J. Nutr. Biochem. 1998;9(6):308–315. doi: 10.1016/S0955-2863(98)00022-9. [DOI] [Google Scholar]
- Wang T., Markham A., Thomas S.J., Wang N., Huang L., Clemens M., Rajagopalan N. Solution Stability of Poloxamer 188 Under Stress Conditions. J. Pharm. Sci. 2019;108(3):1264–1271. doi: 10.1016/j.xphs.2018.10.057. Epub 2018 Nov 9. PMID: 30419275. [DOI] [PubMed] [Google Scholar]
- Waraho T., Cardenia V., Rodriguez-Estrada M.T., Julian Mcclements D., Decker E.A. Prooxidant mechanisms of free fatty acids in stripped soybean oil-in-water emulsions. J. Agric. Food Chem. 2009;57(15):7112–7117. doi: 10.1021/jf901270m. [DOI] [PubMed] [Google Scholar]
- Welch K.D., Davis T.Z., Aust S.D. Iron autoxidation and free radical generation: Effects of buffers, ligands, and chelators. Arch. Biochem. Biophys. 2002;397(2):360–369. doi: 10.1006/abbi.2001.2694. [DOI] [PubMed] [Google Scholar]
- Werber J., Wang Y., Milligan M., Li X., Ji J. Analysis of 2,2 ’-Azobis (2-Amidinopropane) dihydrochloride degradation and hydrolysis in aqueous solutions. J. Pharm. Sci. 2011;100(8):3307–3315. doi: 10.1002/jps.22578. [DOI] [PubMed] [Google Scholar]
- Williams G.M., Iatropoulos M.J., Whysner J. Safety assessment of butylated hydroxyanisole and butylated hydroxytoluene as antioxidant food additives. Food Chem. Toxicol. 1999;37(9–10):1027–1038. doi: 10.1016/s0278-6915(99)00085-x. [DOI] [PubMed] [Google Scholar]
- Wu H.H., Garidel P., Blech M. HP-β-CD for the formulation of IgG and Ig-based biotherapeutics. Int. J. Pharm. 2021;601 doi: 10.1016/j.ijpharm.2021.120531. [DOI] [PubMed] [Google Scholar]
- Wuchner K., Yi L., Chery C., Nikels F., Junge F., Crotts G., Rinaldi G., Starkey J.A., Bechtold-Peters K., Shuman M., Leiss M., Jahn M., Garidel P., de Ruiter R., Richer S.M., Cao S., Sylvain Huille S., Wang T., Le Brun V. Industry perspective on the use and characterization of polysorbates for biopharmaceutical products part 1: Survey report on current state and common practices for handling and control of polysorbates. J. Pharm. Sci. 2022;111(5):1280–1291. doi: 10.1016/j.xphs.2022.02.009. [DOI] [PubMed] [Google Scholar]
- Wuchner K., Yi L., Chery C., Nikels F., Junge F., Crotts G., Rinaldi G., Starkey J.A., Bechtold-Peters K., Shuman M., Leiss M., Jahn M., Garidel P., de Ruiter R., Richer S.M., Cao S., Sylvain Huille S., Wang T., Le Brun V. Industry perspective on the use and characterization of polysorbates for biopharmaceutical products part 2: Survey report on control strategy preparing for the future. J. Pharm. Sci. 2022;111(11):2955–2967. doi: 10.1016/j.xphs.2022.08.021. [DOI] [PubMed] [Google Scholar]
- Xiao N., Medley C.D., Shieh I., et al. Assessing the risk of leachables from single-use bioprocess containers through protein quality characterization. PDA J. Pharm. Sci. Technol. 2016 doi: 10.5731/pdajpst.2015.006338. Published online January 1. [DOI] [PubMed] [Google Scholar]
- Yao J., Dokuru D.K., Noestheden M., et al. A quantitative kinetic study of polysorbate autoxidation: The role of unsaturated fatty acid ester substituents. Pharm. Res. 2009;26(10):2303–2313. doi: 10.1007/s11095-009-9946-7. [DOI] [PubMed] [Google Scholar]
- Yarbrough M., Hodge T., Menard D., et al. Edetate disodium as a polysorbated degradation and monoclonal antibody oxidation stabilizer. J. Pharm. Sci. 2019;108(4):1631–1635. doi: 10.1016/j.xphs.2018.11.031. [DOI] [PubMed] [Google Scholar]
- Yehye W.A., Rahman N.A., Ariffin A., et al. Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): A review. Eur. J. Med. Chem. 2015;101:295–312. doi: 10.1016/j.ejmech.2015.06.026. [DOI] [PubMed] [Google Scholar]
- Yue L., Yan Z., Li H., Liu X., Sun P. Brij-58, a potential injectable protein-stabilizer used in therapeutic protein formulation. Eur. J. Pharm. Biopharm. 2020;146:73–83. doi: 10.1016/j.ejpb.2019.12.001. [DOI] [PubMed] [Google Scholar]
- Zbacnik T.J., Holcomb R.E., Katayama D.S., et al. Role of buffers in protein formulations. J. Pharm. Sci. 2017;106(3):713–733. doi: 10.1016/j.xphs.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Wang A., Meng Y., et al. NMR method for accurate quantification of polysorbate 80 copolymer composition. Anal. Chem. 2015;87(19):9810–9816. doi: 10.1021/acs.analchem.5b02096. [DOI] [PubMed] [Google Scholar]
- Zhang L., Yadav S., Demeule B., Wang Y.J., Mozziconacci O., Schӧneich C. Degradation mechanisms of polysorbate 20 differentiated by 18O-labeling and mass spectrometry. Pharm. Res. 2017;34(1):84–100. doi: 10.1007/s11095-016-2041-y. [DOI] [PubMed] [Google Scholar]
- Zhang L., Yadav S., John Wang Y., Mozziconacci O., Schӧneich C. Dual effect of histidine on polysorbate 20 stability: Mechanistic studies. Pharm. Res. 2018;35(2):33. doi: 10.1007/s11095-017-2321-1. [DOI] [PubMed] [Google Scholar]
- Zhang S., Xiao H., Molden R., Qiu H., Li N. Rapid polysorbate 80 degradation by liver carboxylesterase in a monoclonal antibody formulated drug substance at early stage development. J. Pharm. Sci. 2020;109(11):3300–3307. doi: 10.1016/j.xphs.2020.07.018. [DOI] [PubMed] [Google Scholar]
- Zhang S., Xiao H., Li N. Degradation of Polysorbate 20 by Sialate O-Acetylesterase in Monoclonal Antibody Formulations. J. Pharm. Sci. 2021;110(12):3866–3873. doi: 10.1016/j.xphs.2021.09.001. [DOI] [PubMed] [Google Scholar]
- Zhou S., Lewis L., Singh S.K. Metal leachables in therapeutic biologic products: Origin, impact and detection. Am. Pharm. Rev. 2010;13:76–80. [Google Scholar]
- Zhou S., Zhang B., Sturm E., et al. Comparative evaluation of disodium edetate and diethylenetriaminepentaacetic acid as iron chelators to prevent metal-catalyzed destabilization of a therapeutic monoclonal antibody. J. Pharm. Sci. 2010;99(10):4239–4250. doi: 10.1002/jps.22141. [DOI] [PubMed] [Google Scholar]
- Zhou S., Schöneich C., Singh S.K. Biologics formulation factors affecting metal leachables from stainless steel. AAPS PharmSciTech. 2011;12(1):411–421. doi: 10.1208/s12249-011-9592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Evans B., Schöneich C., Singh S.K. Biotherapeutic formulation factors affecting metal leachables from stainless steel studied by design of experiments. AAPS PharmSciTech. 2012;13(1):284–294. doi: 10.1208/s12249-011-9747-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.