Abstract
The primary aim of this review was to systematically evaluate the literature regarding the effect of pre-, pro-, or synbiotic supplementation in infant formula on the gastrointestinal microbiota. The Cochrane methodology for systematic reviews of randomized controlled trials (RCTs) was employed. Five databases were searched and 32 RCTs (2010–2021) were identified for inclusion: 20 prebiotic, 6 probiotic, and 6 synbiotic. The methods utilized to evaluate gastrointestinal microbiota varied across studies and included colony plating, fluorescence in situ hybridization, quantitative real-time polymerase chain reaction, or tagged sequencing of the 16S rRNA gene. Fecal Bifidobacterium levels increased with supplementation of prebiotics and synbiotics but not with probiotics alone. Probiotic and synbiotic supplementation generally increased fecal levels of the bacterial strain supplemented in the formula. Across all pre-, pro-, and synbiotic-supplemented formulas, results were inconsistent regarding fecal Clostridium levels. Fecal pH was lower with some prebiotic and synbiotic supplementation; however, no difference was seen with probiotics. Softer stools were often reported in infants supplemented with pre- and synbiotics, yet results were inconsistent for probiotic-supplemented formula. Limited evidence demonstrates that pre- and synbiotic supplementation increases fecal Bifidobacterium levels. Future studies utilizing comprehensive methodologies and additional studies in probiotics and synbiotics are warranted.
Keywords: gastrointestinal microbiota, infant, prebiotic, probiotic, synbiotic, systematic review
Introduction
Human milk (HM) is the preferred source of nutrition in early life, and exclusive HM feeding is recommended, when possible, for infants until six months of age (American Academy of Pediatrics 2019; World Health Organization 2021). HM provides numerous benefits to infants, including lowering risks of infections (e.g., otitis media, gastrointestinal tract infections) and allergic diseases (Jeurink et al. 2013; World Health Organization 2021). HM contains immunoglobulins, growth peptides, and over 200 types of human milk oligosaccharides (HMOs). HMOs act as prebiotics and promote the growth of beneficial bacteria while blocking pathogens from binding to epithelial cells (Cheng and Yeung 2021; Wiciński et al. 2020). This translates into prevention of gastrointestinal and respiratory tract infections (Andreas, Kampmann, and Mehring Le-Doare 2015). The microorganisms transmitted from mother to infant through HM, which act as probiotics (e.g., Bifidobacterium, Lactobacillus), as well as HMOs, facilitate growth and colonization of the gastrointestinal microbiota (Andreas, Kampmann, and Mehring Le-Doare 2015; Bergmann et al. 2014; Hunt et al. 2011; Lyons et al. 2020; Zimmermann and Curtis 2020).
When HM feeding is not feasible, the next suitable alternative is infant formula (IF) (World Health Organization 2021; American Academy of Pediatrics 2012). The various types of commercially available IF products, while isocaloric, typically differ in the sources and proportion of macro-/micronutrients (Green Corkins and Shurley 2016). For example, sources of carbohydrates in IF products include lactose, rice starch, corn syrup, maltodextrin, sucrose, and galactooligosaccharides, among others (Green Corkins and Shurley 2016). Fat sources include palm olein, soy, coconut, soybean oil, and safflower oil (Green Corkins and Shurley 2016). Protein sources are skim milk; free amino acids; casein; whey; enzymatically hydrolyzed whey protein isolates; concentrates of whey protein, sodium caseinate, and hydrolyzed or nonhydrolyzed soy protein isolate; or combinations thereof (Green Corkins and Shurley 2016). While IF products are designed to mimic the macronutrient composition of HM, most do not contain significant quantities of pre- and/or probiotics (Salminen et al. 2020).
Various prebiotics, probiotics, and/or combinations of the two (synbiotics) have been added to IF products in an attempt to recapitulate the benefits of HM (Green Corkins and Shurley 2016; Reverri et al. 2018). Prebiotics are defined as “substrates that are selectively utilized by host microorganisms conferring a health benefit,” probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host,” and synbiotics are defined as “a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host” (Gibson et al. 2017; Hill et al. 2014; Swanson et al. 2020). Intervention studies of formulas supplemented with pre-, pro-, or synbiotics typically select only a few bacteria (probiotics), and/or oligosaccharides (prebiotics, e.g., galactooligosaccharides (GOS), fructooligosaccharides (FOS)), and/or HMOs, whereas HM contains a diverse microbial community and an array of HMOs. Further, there is little synthesis of the totality of literature related to the impacts of pre-/pro-/synbiotics on the gastrointestinal microbiota and/or associated health benefits (Andreas, Kampmann, and Mehring Le-Doare 2015). To that end, the primary objective of this systematic review was to comprehensively evaluate scientific evidence regarding the effects of pre-, pro-, and/or synbiotics added to IF on the gastrointestinal microbiota in exclusively formula-fed (FF) infants. The secondary objectives were to evaluate the impacts of pre-, pro-, and/or synbiotics added to IF on gastrointestinal metabolites, stool characteristics, disposition, and gastrointestinal, dermatological, and immunologic markers in exclusively FF infants.
Materials and Methods
The systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) 2020 statement (Page et al. 2021) and registered in the PROSPERO database (CRD42021271028).
Outcome measures
The primary outcome of interest was infant gastrointestinal microbiota: total bacterial counts, abundances, and/or colony-forming units (CFU) of genera or species of gastrointestinal bacteria. Secondary outcomes of interest included gastrointestinal metabolites, infant disposition (e.g., fussiness, crying), gastrointestinal markers (e.g., flatulence, stool consistency, or stool pH), dermatological markers (e.g., eczema, atopic dermatitis), and infection and immunologic markers (e.g., vaccine response).
Search strategy
An initial literature search was conducted on February 12, 2021 (LEF, SK, JT). In accordance with the systematic review process, prior to manuscript submission this literature search was rerun (November 4, 2021) to identify any new potential articles to be included. Studies published from 2010 and later were considered for inclusion in this systematic review. The following databases were utilized to search literature: Cochrane Central Register of Controlled Trials (also known as CENTRAL), PubMed, Web of Science, Cumulative Index of Nursing and Allied Health Literature (also known as CINAHL), and Scopus. Also, hand searching (LEF, LNC) was conducted to find additional eligible studies. Details of the number of articles identified, reviewed, included, and excluded (with reasons for exclusion) in our systematic review are provided as a PRISMA flowchart in Figure 1.
Figure 1.
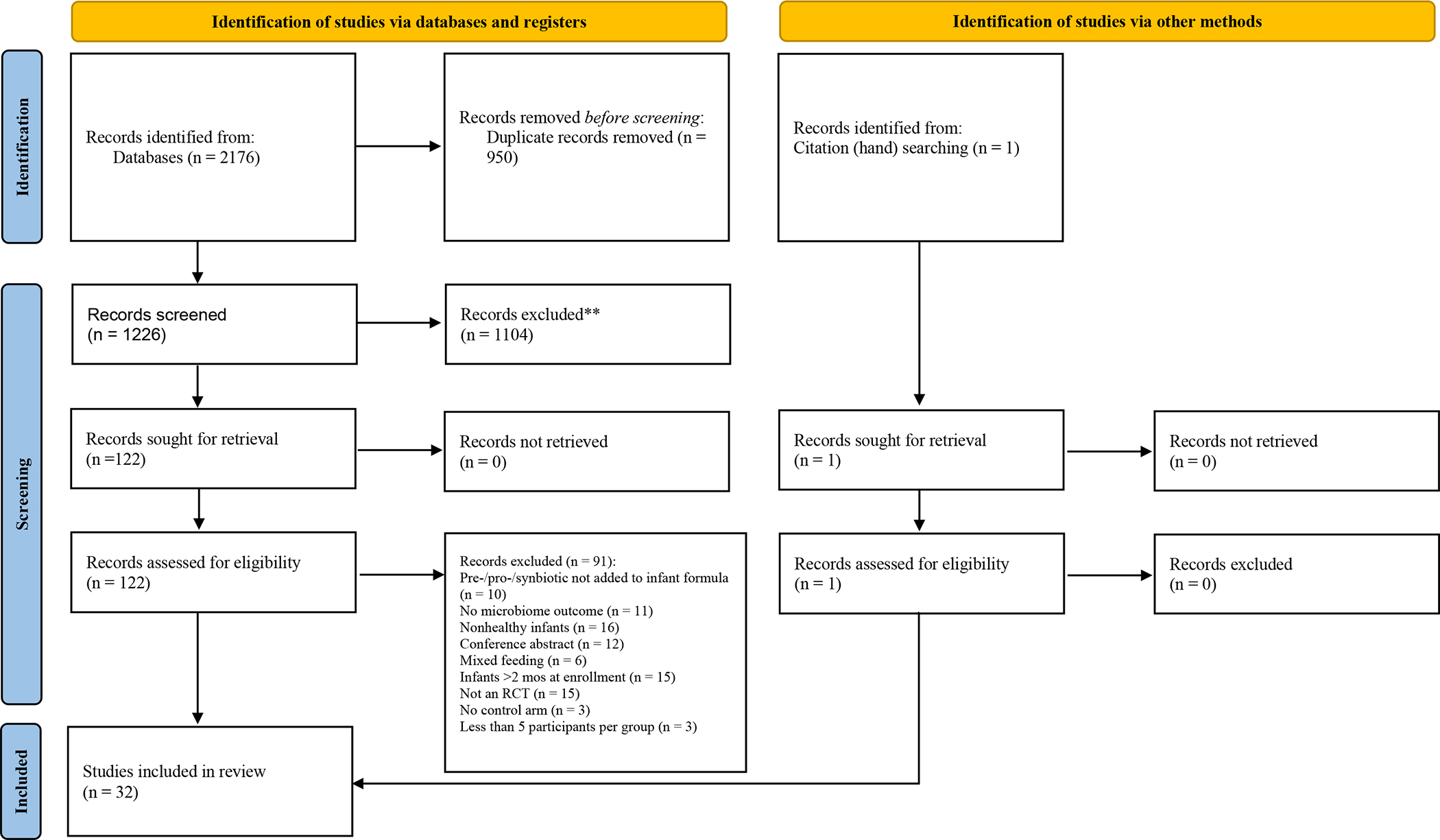
PRISMA 2020 diagram. An outline of studies identified, screened, and included in this systematic review.
Excerpt from a sample search:
((“Synbiotics”[Mesh] OR “Prebiotics”[Mesh] OR “Inulin”[Mesh] OR “Probiotics”[Mesh] OR Synbiotics[title/abstract] OR Synbiotic[title/abstract] OR Prebiotics[title/abstract] OR Prebiotic[title/abstract] OR Inulin[title/abstract] OR GOS[title/abstract] OR scGOS[title/abstract] OR Galactooligosaccharide[title/abstract] OR Galactooligosaccharides[title/abstract] OR FOS[title/abstract] OR scFOS[title/abstract] OR lcFOS[title/abstract] OR Fructooligosaccharide[title/abstract] OR Fructooligosaccharides[title/abstract] OR 2’FL[title/abstract] OR 2’Fucosyllactose[title/abstract] OR Oligofructose[title/abstract]))
Criteria for considering studies for this review
Inclusion criteria were randomized controlled trials of healthy, term (≥37 weeks’ gestation), exclusively FF infants randomized to an experimental IF containing prebiotics, probiotics, or synbiotics or to a control formula without supplementation. Infants must be enrolled within the first two months of life and followed longitudinally, and all studies must have at least five subjects per group and measure gastrointestinal microbiota outcomes (i.e., the primary outcome). Exclusion criteria were any studies that did not assess gastrointestinal microbiota or that included one or more of the following: nonhealthy infants, preterm infants, mixed-fed infants (i.e., feeding IF and HM), or enrollment of infants older than two months.
Screening of studies
First, two reviewers (LEF, LNC) independently reviewed study titles and abstracts. Next, these reviewers screened eligible studies by full text review to determine inclusion into the systematic review. Any discrepancies between the reviewers were discussed with the entire workgroup (LEF, LNC, JKK, JED, ESF, JT, KB, KR) until consensus was reached. Title and abstract screening as well as full text review were documented using Covidence software. (“Covidence systematic review software” 2021) Full details of inclusion and exclusion criteria, search methods, and study selection can be found in Supplemental Table S1 (online).
Data extraction
Following the screening, results of all included studies were extracted. Initial data extraction was conducted independently by the same two reviewers (LEF, LNC) using a standard data extraction form. The data extraction table was then reviewed by each workgroup member independently. Again, any discrepancies between reviewers were discussed with the entire workgroup (LEF, LNC, JKK, JED, ESF, JT, KB, KR) until consensus was reached.
Data synthesis
Included studies were stratified by pre-, pro-, or synbiotics. When outlining results, our focus was twofold: (1) microbiota outcomes during the study intervention (beginning, middle, end) and (2) time points prior to the introduction of solid foods, when possible, to investigate the effect of formula only.
Assessment of risk of bias
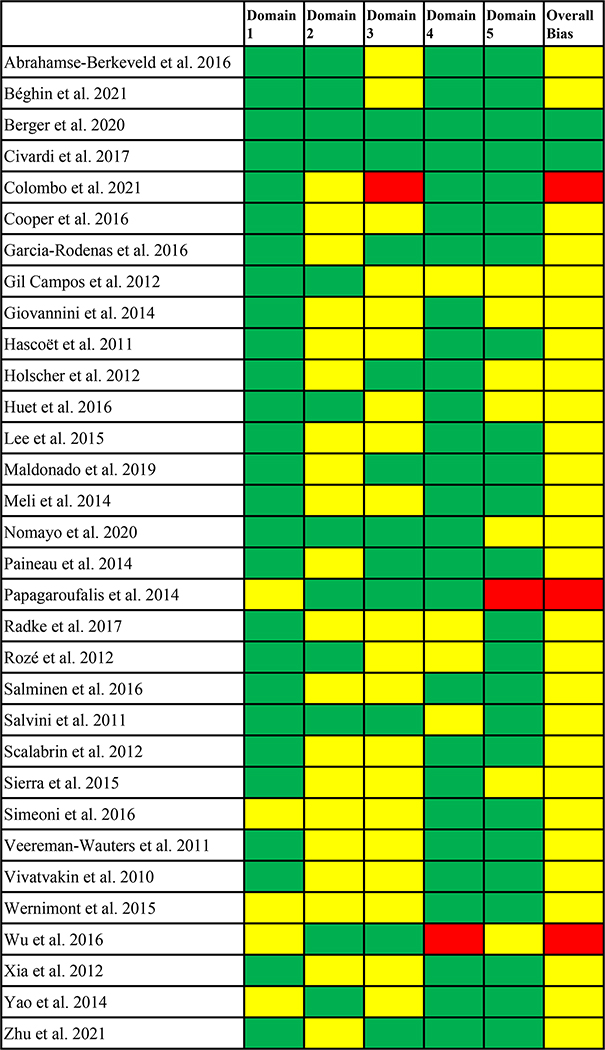
Workgroup members (LEF, LNC, JKK, JED, ESF, JT, KB, KR) independently assessed each included study for five domains of bias using criteria of the Cochrane Handbook for Systematic Reviews of Interventions and the Cochrane Risk of Bias Tool (Higgins et al. 2020). Following these criteria, each article was assessed for five domains: (1) bias arising from the randomization process, (2) bias due to deviations from intended interventions, (3) bias due to missing outcome data, (4) bias in measurement of the outcome, and (5) bias in selection of the reported result (see Supplemental Table S1). The workgroup members reviewed bias ratings for each domain and an overall bias rating of each study until consensus was reached based on the following guidance from the Cochrane Risk of Bias Tool (Higgins et al. 2020): studies in which all five domains were low concern were given a low overall assessment of bias; studies in which one or more domains were high concern or there was some concern for multiple domains that lowered the confidence in study results were given a high overall assessment of bias; studies in which at least one domain raised some concern but no high concern was given in any domains were given a moderate overall assessment of bias.
Results
A total of 2176 records were identified from the five databases searched. After removing duplicates, a total of 1226 studies were to be screened. From here, 1104 records were excluded at the title and abstract review stage. The remaining 122 records were reviewed in full text review and 91 were excluded, leaving 31 studies that matched the inclusion criteria for this systematic review. Hand searching identified one study that matched inclusion criteria; therefore, a total of 32 studies were included in this systematic review.
When assessing the quality of each study based on the Cochrane Risk of Bias Tool (Higgins et al. 2020), three studies attained an overall low bias rating (Béghin et al. 2021; Berger et al. 2020; Civardi et al. 2017), three attained an overall high bias rating (Colombo et al. 2021; Papagaroufalis et al. 2014; Wu et al. 2016), and all other 26 studies attained an overall moderate bias rating (see Figure 2).
Figure 2.
Overall bias ratings for each study: domain 1, bias arising from the randomization process; domain 2, bias due to deviations from intended interventions; domain 3, bias due to missing outcome data; domain 4, bias in measurement of the outcome; domain 5, bias in selection of the reported result. Green indicates low concern for risk of bias, yellow indicates some concern for risk of bias, and red indicates high concern for risk of bias within each domain. For the overall risk of bias, green indicates low risk, yellow indicates moderate risk, and red indicates high risk.
Prebiotics
Twenty articles examined the effect of prebiotics on gastrointestinal microbiota outcomes (Béghin et al. 2021; Berger et al. 2020; Civardi et al. 2017; Colombo et al. 2021; Giovannini et al. 2014; Holscher et al. 2012; Huet et al. 2016; Lee et al. 2015; Nomayo et al. 2020; Paineau et al. 2014; Salminen et al. 2016; Salvini et al. 2011; Scalabrin et al. 2012; Sierra et al. 2015; Veereman-Wauters et al. 2011; Vivatvakin et al. 2010; Wernimont et al. 2015; Xia et al. 2012; Yao et al. 2014; Zhu et al. 2021) (Table 1). Four studies supplemented formulas with GOS (Civardi et al. 2017; Giovannini et al. 2014; Nomayo et al. 2020; Sierra et al. 2015); one study supplemented with FOS (Xia et al. 2012); one study supplemented with short-chain FOS (scFOS) (Paineau et al. 2014); one supplemented with a GOS/scFOS mixture (Holscher et al. 2012); three supplemented with short-chain GOS (scGOS)/long-chain FOS (lcFOS) mixtures (Béghin et al. 2021; Huet et al. 2016; Salvini et al. 2011); one study supplemented with GOS, FOS, and oligofructose (OF) (Veereman-Wauters et al. 2011); three studies supplemented with unspecified types of GOS/FOS mixtures (Lee et al. 2015; Vivatvakin et al. 2010; Zhu et al. 2021); two studies supplemented with OF (Wernimont et al. 2015; Yao et al. 2014); one study supplemented with a 2’-fucosyllactose (2’FL)/lacto-N-neotetraose (LNnT) mixture, both of which are HMOs (Berger et al. 2020); and three studies supplemented with polydextrose (PDX)/GOS mixtures (Colombo et al. 2021; Salminen et al. 2016; Scalabrin et al. 2012). Four studies also supplemented with a high SN-2 palmitate oil (also known as high oleic-palmitic-oleic (OPO) oil) (Civardi et al. 2017; Nomayo et al. 2020; Yao et al. 2014; Zhu et al. 2021). Prebiotic concentrations in each intervention group in each study are summarized in Figure 3 (A).
Table 1.
Summary of the 20 included prebiotic studies
| Reference, study design, study location, overall biasa | Study enrollment and intervention duration | Interventions used in study groupsb | Fecal sample collection, tests, compositionc | Fecal microbial endpoints | Health outcomes |
|---|---|---|---|---|---|
|
Béghin et al. 2021 Double blind, multicenter France, Germany, Italy MODERATEa |
Infants enrolled by day 7 of life, on study formula for 6 mos (complementary foods allowed after 4 mos) | 1) CTRL: Standard cow-milk-based formula, n=70 (ITT) and n=47 (PP) 2) FERM: CTRL formula + bioactive compounds only (B. breve C50 and S. thermophilus O65), n=70 (ITT) and n=43 (PP) 3) PRE: CTRL formula + scGOS/lcFOS (9:1) mixture only (0.52 g/100mL GOS), n=70 (ITT) and n=35 (PP) 4) FERM/PRE: FERM formula + scGOS/lcFOS (9:1 ratio) prebiotic mixture, n=70 (ITT) and n=46 (PP) 5) BF reference, n=70 (ITT) and n=38 (PP) Total: N=280 (ITT) and N=209 (PP) |
n=148 samples collected at baseline, 2, 4, and 6 mos (microbiome not analyzed at 6 mos) Fecal SCFA: gas chromatography Fecal pH: pH meter Composition: FISH using 16S-rRNA targeted oligonucleotide probes, specifically targeting Atopbium cluster, Bacteroides distasonis group, Bacteroides fragilis group, Bifidobacterium spp., Blautia coccoides group, Clostridium histolyticum group, Clostridium lituseburense group, subset Enterobacteriaceae, Eubacterium rectale and B. coccoides group, Lactobacillus-Enterococcus group, Roseburia and E. rectale group |
Bifidobacterium (relative abundance, %) higher in PRE v. CTRL at 4 mos (P=0.002) Bacteroides distasnois (relative abundance, %) significantly lower in PRE v. CTRL at 4 mos Clostridium histolyticum and Clostridium lituseburense (relative abundance, %) significantly lower in PRE v. CTRL and in FERM/PRE v. CTRL at 4 mos Blautia coccoides (relative abundance, %) significantly lower in PRE v. CTRL at 4 mos Median pH values lower in PRE v. CTRL at both 2 and 4 mos D-lactate (P=0.001) and L-lactate (P=0.004) detected in a higher proportion (presence/absence) in PRE v. CTRL at 4 mos Isovaleric acid detected in a lower proportion (presence/absence) in PRE v. CTRL at 4 mos (P<0.001) |
Stool frequency significantly higher in all formula groups v. CTRL in ITT population at 4 mos |
|
Berger et al. 2020 Double blind, multicenter Belgium, Italy LOWa |
Infants enrolled by day 14 of life, on study formula for 6 mos (complementary foods allowed after 4 mos) | 1) CTRL: intact protein cow-milk-based (whey-predominant) formula with long-chain PUFAs, n=87 (ITT) and n=63 (PP) 2) PRE: CTRL formula + 2 HMOs (2'FL, LNnT) at 1.0–1.2 and 0.5– 0.6 g/liter reconstituted formula, respectively, n=88 (ITT) and n=58 (PP) 3) BF reference, n=38 (ITT) and n=38 (PP) Total: N=175 (ITT) and N=121 (PP) |
n=156, 116 samples collected at 3 and 12 mos, respectively Composition: qPCR for total bacterial abundance, 16S rRNA gene sequencing to the species level |
Faith phylogenic diversity (alpha diversity index) lower in PRE v. CTRL at 3 mos (P<0.05) Global difference in microbiota composition (redundancy analysis) different in the PRE v. CTRL at 3 mos (P=0.036) Bifidobacterium (relative abundance, %) higher (P=0.0093, FDR 0.13) and Escherichia (P=0.0078, FDR 0.13), unclassified Peptostreptococcaceae (P=0.0275, FDR 0.16)), and Streptococcus (P=0.0372, FDR 0.17) lower in PRE v. CTRL at 3 mos |
Not assessed |
|
Civardi et al. 2017 Double blind, single center Italy LOWa |
Infants enrolled by 21 days of life, on study formula until 135 days of life | 1) CTRL: standard formula, n=62 (RAND) and n=59 (AN) 2) PRE: CTRL formula + GOS (7 g/L), beta-palmitate, and acidified milk, n=55 (RAND) and n=51 (AN) Total: N=117 (RAND) and N=110 (AN) |
n=105, 98, 103 samples collected at baseline, day 60, and day 135, respectively Composition: qPCR, specifically targeted Bifidobacterium and Clostridium |
Log increase of bifidobacteria (CFU) higher in PRE v. CTRL at day 135 v. baseline (P=0.028) | No significant differences between formula groups for number of stools per day, stool consistency, frequency of gas, and bowel cramps. |
|
Colombo et al. 2021 Double blind, multicenter USA HIGHa |
Infants enrolled at 14–35 days of life, on study formula until 112 days of life | 1) CTRL: standard formula marketed previously as Enfamil, n=82 (RAND) and n=66 (AN) 2) PRE: routine cow-milk-based formula with a prebiotic blend of PDX and GOS (4 g/L, 1:1 ratio), n=79 (RAND) and n=65 (AN) Total: N=161 (RAND) and N=131 (AN) |
n=11 samples collected at baseline and day 112 Composition: 16S rRNA gene sequencing |
Beta diversity shift in PRE group between baseline and day 112 (P=0.001); no significant shift observed in CTRL group between baseline and day 112 Lachnospiraceae (relative abundance, %) higher in PRE at baseline v. day 112 (P=0.036) Coriobacteriaceae (relative abundance, %) higher in CTRL v. PRE by day 112 (P=0.02) |
Medically confirmed adverse events higher in PRE v. CTRL (P=0.021) |
|
Giovannini et al. 2014 Double blind, multicenter Italy MODERATEa |
Infants enrolled at or before day 15 of life, on study formula until complementary foods introduced (≥120 days intervention) | 1) CTRL: standard formula, n=80 (ITT) 2) PRE: CTRL formula + GOS at 0.4g/100mL, n=83 (ITT) 3) BF reference, n=199 (ITT) Total: N=362 (ITT) |
n=75 samples collected in a subset of infants born vaginally at baseline, 30 and 60 days of life, and a final visit (just before solid food introduction) Composition: qPCR, specifically targeting Lactobacillus, Bifidobacterium, and coliforms, plating method used for Clostridium |
Bacterial (total count) at baseline and 30 days not different between CTRL v. PRE Clostridium total count (P<0.05) and Clostridium/(Lactobacillus + Bifidobacterium) ratio (P=0.02) lower at 60 days in PRE v. CTRL No other differences in microbiota (total count) at any other time points |
Colic and regurgitation risk lower in PRE v. CTRL (P<0.05) |
|
Holscher et al. 2012 Double blind, multicenter USA MODERATEa |
Infants enrolled at 2–8 weeks of life, on study formula for 6 weeks | 1) CTRL: partially hydrolyzed whey formula, n=46 (ITT) and n=33 (PP) 2) PRE: CTRL formula + 4 g/L GOS and scFOS (9:1 ratio), n=43 (ITT) and n=36 (PP) 3) BF reference, n=50 (ITT) and n=33 (PP) Total: N=139 (ITT) and N=102 (PP) |
n=102 samples collected at baseline, 3 and 6 weeks Fecal SCFA: gas chromatography Fecal pH: pH meter Composition: FISH using 16S-rRNA targeted oligonucleotide probes, specifically targeting: Bifidobacterium spp., Bacteroides/Prevotella, C. difficile, and Lactobacillus spp. |
Bifidobacteria (absolute number, P=0.0083; proportion, P=0.0219) higher in PRE v. CTRL for all visits combined Clostridium difficile (abundance, CFU/g wet feces) significantly less abundant in PRE group for all visits combined Fecal pH (P=0.0161), propionate and butyrate concentrations (P<0.0001), and propionate proportion (P=0.0026) lower in PRE v. CTRL for all visits combined Acetate proportion higher in PRE v. CTRL for all visits combined (P=0.0007) |
No significant differences in caregiver reports of crying, fussiness, colic, spitting up, vomiting, or flatulence |
|
Huet et al. 2016 Double blind, multicenter France, Belgium, Ireland MODERATEa |
Infants enrolled by 28 days of life, on study formula until 17 weeks | 1) CTRL (50% FERM): formula containing 50% fermented formula, n=107 (ITT) and n=65 (PP) 2) PRE + 50% FERM: formula containing 50% fermented formula CTRL formula plus prebiotics (scGOS/lcFOS; 0.8 g/100 mL, 9:1 ratio), n= 109 (ITT) and n=79 (PP) 3) PRE + 15% FERM: formula containing 15% fermented formula plus prebiotics, n=111 (ITT) and n=79 (PP) 4) PRE: nonfermented commercially available formula with prebiotics, n=104 (ITT) and n=75 (PP) Total: N=431 (ITT) and N=298 (PP) |
n=120 samples collected at baseline and 17 weeks Fecal pH: pH meter Fecal sIgA: ELISA Fecal SCFA: gas chromatography Lactate: enzymatic commercial kit Composition: qPCR, specifically targeted C. difficile |
Clostridium difficile present in lower proportions in PRE + 50% FERM v. CTRL at 17 weeks (P<0.05) Isobutyrate and isovalerate present in lower proportion in PRE v. CTRL at 17 weeks (P<0.05) Valerate present in higher proportion in PRE + 50% FERM v. CTRL at 17 weeks (P<0.05) L-lactate, D-lactate present in higher proportions in PRE + 50% FERM v. CTRL at 17 weeks (P<0.05) sIgA concentration higher in PRE + 50% FERM v. CTRL at 17 weeks (P<0.05) pH lower in PRE + 50% FERM v. CTRL at 17 weeks (P<0.05) |
Not assessed |
|
Lee et al. 2015 Double blind, single center Singapore MODERATEa |
Infants enrolled by 14 days of life, on study formula until 4 mos | 1) CTRL: standard Nestlé formula with L. reuteri at 108 CFU/g, n=68 (ITT) and n=61 (PP) 2) PRE: CTRL formula + 5.5 g/L GOS and 0.36 g/L FOS, n=72 (ITT) and n=62 (PP) Total: N=140 (ITT) and N=123 (PP) |
n= 60 samples collected at 2 mos Composition: FISH using 16S-rRNA targeted oligonucleotide probes, L. reuteri quantified by culture plating |
Total bacterial counts (median, P<0.01), bifidobacteria counts (median, P<0.001), and lactobacilli and enterobacteria ratio (P=0.07) higher in PRE v. CTRL at 2 mos | Not assessed |
|
Nomayo et al. 2020 Double blind, multicenter Germany MODERATEa |
Infants enrolled at or before day 10 of life, on study formula for at least 12 weeks, some to 5–6 mos at the onset of weaning | 1) CTRL: standard formula + <10% SN-2 (beta-palmitic acid), n=47 (ITT) and n=27 (PP) 2) PRE: CTRL formula + 20–25% SN-2 + 0.5 g/100mL GOS, n=45 (ITT) and n=30 (PP) 3) BF reference, n=34 (ITT) and n=18 (PP) Total: N=126 (ITT) and N=75 (PP) |
n=75 samples collected at 6 and 12 weeks Composition: qPCR specifically targeted at the genus Bifidobacterium and total bacteria |
Bifidobacteria proportion (P<0.025) and bifidobacteria count (total, P<0.0001) higher in PRE v. CTRL at 12 weeks | No significant difference with regards to gastrointestinal or respiratory infections during the first year of life |
|
Paineau et al. 2014 Double blind, multicenter France MODERATEa |
Infants enrolled by day 7 of life, on study formula for 4 mos | 1) CTRL: standard formula, n=27 (ITT) and n=15 (PP) 2) PRE: CTRL formula + scFOS (4 g/L), n=31 (ITT) and n=18 (PP) Total: N=58 (ITT) and N=33 (PP) |
n=33 samples collected at enrollment, 2, 3, 4 mos (microbiome not analyzed at 4 mos) Fecal sIgA: ELISA Composition: qPCR, specifically targeting total Bifidobacterium |
Bifidobacterium counts (change in CFU/g) between baseline and 2 mos (P=0.03) and baseline and 3 mos (P=0.003) higher in PRE v. CTRL Bifidobacterium counts (CFU/g) increase in PRE group over time between baseline and 3 mos (ITT) (P=0.008) |
No significant differences in abdominal pain, diarrhea, nausea |
|
Salminen et al. 2016 Double blind, multicenter USA MODERATEa |
Infants enrolled at 21–30 days of life, on study formula for 60 days | 1) CTRL: cow-milk-based formula, n=80 (RAND) and n=73 (AN) 2) PRE: CTRL formula + 4 g/L PDX/GOS (1:1), n=77 (RAND) and n=67 (AN) 3) BF reference, n=71 (RAND) and n=56 (AN) Total: N=157 (RAND) and N=196 (AN) |
n=unknown number of samples collected at baseline, 30, and 60 days Composition: qPCR specifically targeting Lactobacillus strains (L. acidophilus, L. casei, L. delbrueckii, L. fermentum, L. paracasei, L. plantarum, L. reuteri, L. rhamnosus), Lactobacillus group (Lactobacillus, Fructobacillus, Leuconostoc, Pediococcus, Weissella), Staphylococcus aureus |
Lactobacilli counts (log10 CFU/g feces) higher in PRE v. CTRL at 30 and 60 days combined (P=0.035) | Not assessed |
|
Salvini et al. 2011 Double blind, single center Italy MODERATEa |
Infants enrolled at birth, on study formula until 6 mos (no information given on complementary foods) | 1) CTRL: standard bovine milk formula + 8 g/L maltodextrin as placebo, n=10 (PP) 2) PRE: CTRL formula + 8 g/L prebiotic mixture (scGOS and lcFOS at a 9:1 ratio), n= 10 (PP) Total: N=22 (EN) and N=20 (PP) |
n=20 samples collected at birth, 3, 6, and 12 mos Fecal pH: pH meter Composition: Fecal counts of bifidobacteria and lactobacilli were measured via serial dilution followed by culturing for bifidobacteria and lactobacilli |
Bifidobacteria and lactobacilli (counts, CFU/g) increased during the first 3 mos in both groups (P<0.0001) and remained stable afterward (P>0.05) Bifidobacteria and lactobacilli (counts, CFU/g) higher in PRE v. CTRL at 3 (P=0.0014 v. P=0.0125), 6 (P=0.0014 v. P=0.0054), and 12 mos (P=0.0016 v. P=0042), respectively Fecal pH lower in PRE v. CTRL at 3 mos (P=0.0006) and 6 mos (P=0.0011) |
Not assessed |
|
Scalabrin et al. 2012 Double blind, multicenter USA MODERATEa |
Infants enrolled at 21–30 days of life, on study formula for 60 days | 1) CTRL: cow-milk-based formula, n=101 (EN) and n=81 (completed) 2) PRE: CTRL formula + 4 g/L PDX/GOS (1:1), n=100 (EN) and n=78 (completed) 3) BF reference, n=88 (EN) and n=71 (completed) Total: N=289 (EN) and N=230 (completed) |
n= 222, 226, 221 samples collected at baseline, 30, and 60 days, respectively Fecal sIgA: ELISA Composition: FISH and qPCR-FISH probes included total bacteria, genus-specific Bifi164, C. lituseburense group (Clostridium cluster IX), C. histolyticum group. qPCR primers included Bifidobacterium genus, B. adolescentis, B. bifidum, B. breve, B. catenulatum, B. infantis, B. animalis, B. longum group, Clostridium coccoides group, Clostridium difficile |
Bifidobacterium spp. (absolute counts) by qPCR higher in PRE v. CTRL at 60 days (P=0.002) B. longum higher in PRE v. CTRL at 60 days (P<0.05) B. infantis higher in PRE v. CTRL at 30 days (P=0.002) but not 60 days C. coccoides higher in PRE v. CTRL at 60 days (P=0.005) B. catenulatum and B. infantis had higher change from baseline in PRE v. CTRL at 30 days (P=0.004) and 60 days (P=0.024) and for B. longum at 60 days (P=0.035) Larger increase in the number of Bifidobacterium spp. detected (presence/absence) after 30 days (P=0.008) and 60 days of feeding (P=0.021) |
Stool consistency scores different between all groups, with BF the highest and CTRL the lowest (P<0.05) |
|
Sierra et al. 2015 Double blind, multicenter Spain MODERATEa |
Infants enrolled at or before 2 mos, on study formula until 6 mos (complementary foods allowed at 4 mos) | 1) CTRL: standard formula, n=177 (ITT) and n=132 (PP) 2) PRE: CTRL formula + 0.44 g/dl GOS, n=188 (ITT) and n=132 (PP) Total: N=365 (ITT) and N=264 (PP) |
n=81, 69 samples collected at enrollment and 4 mos, respectively SCFA: Gas chromatography Fecal pH: pH meter Fecal sIgA: ELISA Composition: qPCR specifically using primers for Bifidobacterium, Lactobacillus, Bacteroides, Bifidobacterium species, and Clostridium difficile |
Changes in Bifidobacterium spp. (log10 CFU/g) from 2 to 4 mos greater in PRE v. CTRL (P=0.01) Percentage of infants with detectable C. difficile (presence /absence) lower in PRE v. CTRL at 4 mos (P=0.037) Percentage of infants with detectable B. breve higher in PRE v. CTRL at 4 mos (P<0.05) Fecal pH lower in PRE v. CTRL at 4 mos (P=0.019) Acetic acid (%/total SCFA) higher in PRE v. CTRL at 4 mos (P=0.005) Proprionic acid (P=0.015) and butyric acid (P=0.040) (%/total SCFA) lower in PRE v. CTRL at 4 mos |
Defecation frequency higher in PRE v. CTRL at 3 mos (P<0.05) and 4 mos (P<0.05) Soft feces percentage higher in PRE v. CTRL at 3, 4, and 6 mos (P<0.05) |
|
Veereman-Wauters et al. 2011 Double blind, multicenter Belgium MODERATEa |
Infants enrolled at or before 5 days of life, on study formula for 28 days | 1) CTRL: standard formula, n=21 (EN) and n=14 (AN) 2) GOS/FOS: CTRL formula + GOS/FOS (90:10) added at 0.8 g/dL, n=19 (EN) and n=10 (AN) 3) LOW PRE: CTRL formula + OF/FOS (50:50) added at 0.4 g/dL, n=21 (EN) and n=14 (AN) 4) HIGH PRE: CTRL formula + OF/FOS (50:50) added at 0.8 g/dL, n=20 (EN) and n=12 (AN) 5) BF reference, n=29 (EN) and n=22 (AN) Total: N=110 (EN) and N=72 (AN) |
n=72 samples collected on days 3, 14, and 28 Composition: FISH, specifically targeting total bacteria, Bacteroides, Prevotella, all Parabacteroides species, Barnesiella viscericola, Odoribacter splanchnicus, Bifidobacterium species, Parascardovia denticolens, Clostridium cluster I and II, lactic acid bacteria |
Total bacteria (cells/g feces) increase in LOW PRE, HIGH PRE, and GOS/FOS, but not CTRL, on days 14 and 28 v. baseline (day 3) (P<0.05) Bifidobacterium counts (cells/g feces) increased in HIGH PRE and GOS/FOS on days 14 and 28 v. baseline (day 3) (P<0.05) Bifidobacterium counts (cells/g feces) increased in HIGH PRE and GOS/FOS groups v. CTRL on day 14 (P<0.05) but not day 28 |
Softer stools seen in all prebiotic supplemented groups v. CTRL at week 2 (P<0.05) Softer stools seen in GOS/FOS and HIGH PRE IF only at week 4 (P<0.05) |
|
Vivatvakin et al. 2010 Double blind, single center Thailand MODERATEa |
Infants enrolled at or before 30 days of life, on study formula until 4 mos | 1) CTRL: standard formula, n=73 (ITT) and n=59 (completed) 2) PRE: CTRL formula + GOS/FOS (9:1) mixture at 4 g/L, n=71 (ITT) and n=53 (completed) 3) BF reference, n=80 (ITT) and n=57 (completed) Total: N=224 (ITT) and N=169 (completed) |
n=90 samples collected at baseline and 2 mos Composition: FISH, targeting bifidobacteria, lactobacilli, Enterobacteriaceae, clostridia, Bacteroides |
No significant difference in any of the measured bacteria (CFU/g feces, mean and median) between PRE v. CTRL at baseline or 2 mos | Hard stool frequency lower in PRE v. CTRL (P<0.001, mean over study duration) Soft stool frequency higher in PRE v. CTRL (P<0.05, mean over study duration) |
|
Wernimont et al. 2015 Double blind, multicenter USA MODERATEa |
Infants enrolled at or before day 13 of life, on study formula for 8 weeks | 1) CTRL: standard formula with alpha-lactalbumin, n=48 (EN) and n=24 (PP) 2) PRE: CTRL formula + oligofructose (3 g/L), n=47 (EN) and n=19 (PP) 3) BF reference, n=50 (EN) and n=27 (PP) Total: N=145 (EN) and N=70 (PP) |
n=70 samples collected at baseline and after 1, 2, 4, and 8 week time points Composition: FISH, specifically targeting Bacteroides, bifidobacteria, clostridia, Enterobacteriaceae, Lactobacillus, Enterococcus, Lactobacillus Bacillus subbranch, Staphylococcus |
Bifidobacterium (log counts/g dry feces) increase significantly higher in PRE v. CTRL from baseline to week 8 (P=0.008) No significant difference in any of the other measured bacteria between PRE v. CTRL at baseline, 1, 2, 4, or 8 week time points. |
Stools were softer in PRE v. CTRL at week 8 (P=0.015) |
|
Xia et al. 2012 Double blind, multicenter USA MODERATEa |
Infants enrolled at or before day 6 of life, on study formula for 4 weeks | 1) CTRL: cow-milk-based formula, n=24 (ITT) 2) LOW PRE: CTRL IF + 2.0 g FOS/L, n=25 (ITT) 3) HIGH PRE: CTRL IF + 3.0 g FOS/L, n=26 (ITT) 4) BF reference, n=22 (ITT) Total: N=97 (ITT) |
n=65 samples collected at day 28 Composition: qPCR specifically targeting all bacteria, Bacteroides, Prevotella, Bifidobacterium, C. difficile, E. coli, Lactobacillus |
No significant difference in any of the measured bacteria between LOW PRE (2.0 g FOS/L) v. CTRL and HIGH PRE (3.0 g FOS/L) v. CTRL at day 28 | No significant differences found for mean stool consistency, average daily number of stools, spitting up, or vomiting |
|
Yao et al. 2014 Double blind, single center Philippines MODERATEa |
Infants enrolled at 7–14 days of life, on study formula for 8 weeks | 1) CTRL: bovine-milk-based, alpha-lactalbumin-enriched formula, n=75 (EN) and n=74 (completed) 2) SN-2: CTRL formula + high SN-2 palmitate formula, n=74 (EN) and n=72 (completed) 3) SN-2 + LOW PRE: SN-2 formula + 3 g/L OF, n=76 (EN) and n=75 (completed) 4) SN-2 + HIGH PRE: SN-2 IF + 5 g/L OF, n=75 (EN) and n=75 (completed) 5) BF reference, n=75 (EN) Total: N=375 (EN) and N=369 (completed) and n=73 (completed) |
n=170 samples collected at baseline and week 8 Composition: FISH, specifically targeting genus Bifidobacterium |
Bifidobacteria concentrations higher in SN-2 (P=0.033), SN-2 + LOW PRE (P=0.0002), and SN-2 + HIGH PRE v. CTRL (P=0.0022) at week 8 | Stool soap palmitic acid lower in SN-2, SN-2 + LOW PRE, and SN-2 + HIGH PRE v. CTRL at 8 weeks (P<0.001) Total stool soap fatty acids (mean values) lower in SN-2, SN-2 + LOW PRE, and SN-2 + HIGH PRE v. CTRL at 8 weeks (P<0.01) Mean percentage of mushy, soft stools higher in SN-2 v. CTRL at 8 weeks (P=0.026) Percentage of formed stools lower in SN-2 v. CTRL at 8 weeks (P=0.003) SN-2 + LOW PRE, and SN-2 + HIGH PRE had higher reductions in the percentage of formed stools than did SN-2 v. CTRL (P<0.001), and SN-2 + HIGH PRE showed increased percentage of runny stools |
|
Zhu et al. 2021 Double blind, multicenter China MODERATEa |
Infants enrolled at or before 1 mo, on study formula for 4 mos | 1) CTRL: standard formula, n=18 (EN), n=13 (completed) 2) PRE: CTRL IF + SN-2 (4.0 g/100g) + FOS (0.8 g/100 g) + GOS (0.6 g/100g), n=31 (EN), n=24 (completed) 3) BF reference, n=59 (EN), n=49 (completed) Total: N=108 (EN) and N=86 (completed) |
n=83 samples collected at 4 mos Composition: 16S rRNA gene sequencing |
Proteobacteria relative abundance higher in PRE v. CTRL (P=0.015) Actinobacteria relative abundance lower in PRE v. CTRL (P=0.011) Microbial diversity as assessed by Chao1 differed between PRE IF and CTRL IF (P<0.01) |
No significant differences found for crying, occurrence of spitting, and daily frequency of stool |
OVERALL BIAS: The overall bias rating based on ratings of five individual-domain-level bias ratings (see Figure 3 and Supplemental Table S1).
Groups: BF, breastfeeding reference group; CTRL, control group; PRE, prebiotic group; FERM, bioactive compounds group. Other groups are specified by study. Interventions: FOS, fructooligosaccharide; GOS, galactooligosaccharide; lcFOS, long-chain fructooligosaccharide; LNnT, lacto-N-neotetraose; OF, oligofructose; PDX, polydextrose; scFOS, short-chain fructooligosaccharide; scGOS, short-chain galactooligosacchride; SN-2 oil, high oleic-palmitic-oleic oil; 2'FL, 2’-fucosyllactose. We also specify analyzed (AN), enrolled (EN), intent-to-treat (ITT), per-protocol (PP) analyses, and randomized (RAND).
CFU, colony-forming units; ELISA, enzyme-linked immunosorbent assay; FISH, fluorescence in situ hybridization; SCFA, short-chain fatty acid; sIgA, secretory immunoglobulin A; qPCR, quantitative polymerase chain reaction.
Figure 3.
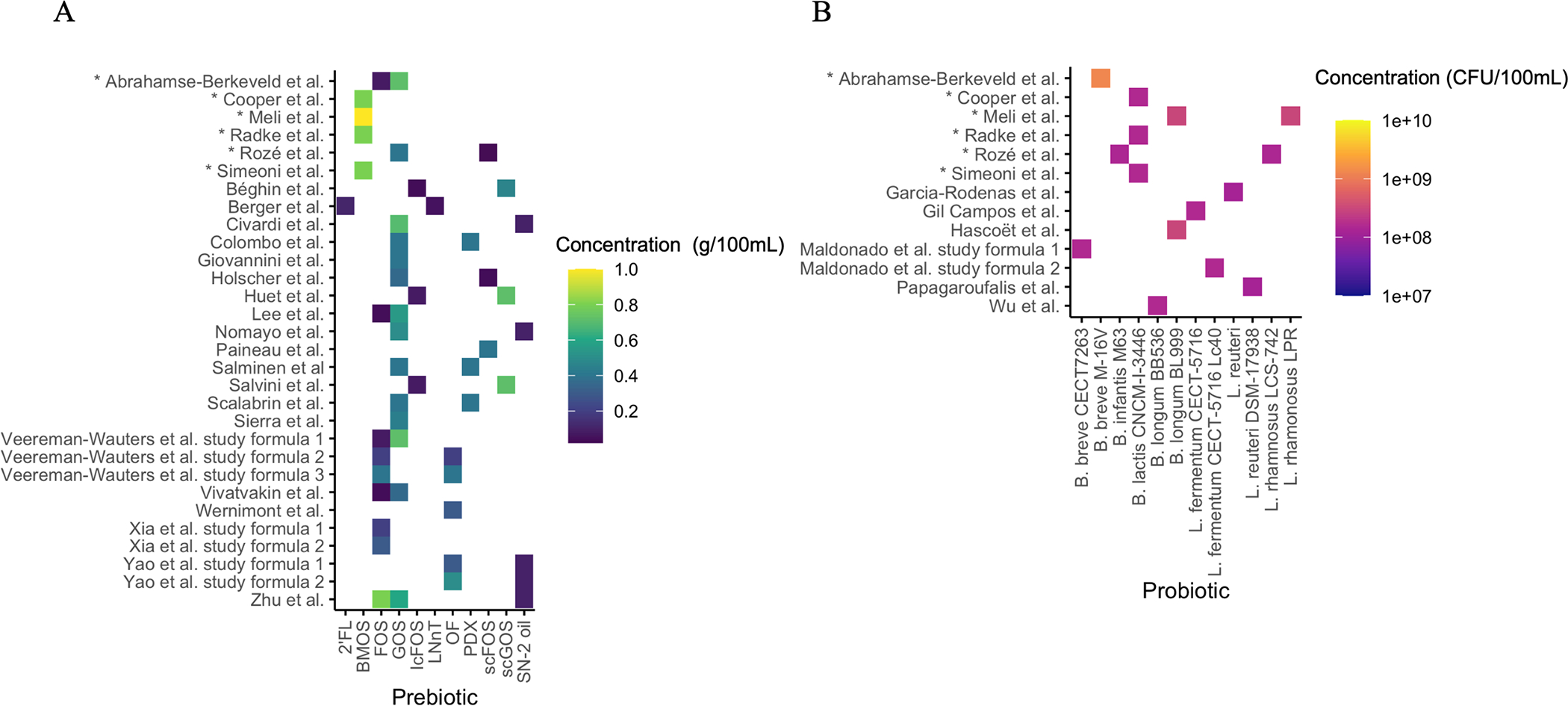
Prebiotic (A) and probiotic (B) concentrations in each formula intervention group. Pre- and probiotic concentrations reported in studies were converted to a common unit (prebiotics: g/100 mL; probiotics: CFU/100 mL). Prebiotic abbreviations: 2’FL, 2’-fucosyllactose; BMOS, bovine milk oligosaccharide; FOS, fructooligosaccharide; GOS, galactooligosaccharide; lcFOS, long-chain FOS; LNnT, lacto-N-neotetraose; OF, oligofructose; PDX, polydextrose; scFOS, short-chain FOS; scGOS, short-chain GOS; SN-2 oil, high oleic-palmitic-oleic oil.
Microbiota outcomes
All 20 articles reported gastrointestinal microbiota outcomes at the genus level (Béghin et al. 2021; Berger et al. 2020; Civardi et al. 2017; Colombo et al. 2021; Giovannini et al. 2014; Holscher et al. 2012; Huet et al. 2016; Lee et al. 2015; Nomayo et al. 2020; Paineau et al. 2014; Salminen et al. 2016; Salvini et al. 2011; Scalabrin et al. 2012; Sierra et al. 2015; Veereman-Wauters et al. 2011; Vivatvakin et al. 2010; Wernimont et al. 2015; Xia et al. 2012; Zhu et al. 2021; Yao et al. 2014). Eight also reported outcomes at the species level (Béghin et al. 2021; Berger et al. 2020; Colombo et al. 2021; Holscher et al. 2012; Huet et al. 2016; Scalabrin et al. 2012; Sierra et al. 2015; Xia et al. 2012). Intervention periods ranged from 28 days to six months. For the five studies in which the intervention extended beyond four months of age (Béghin et al. 2021; Berger et al. 2020; Huet et al. 2016; Salvini et al. 2011; Sierra et al. 2015), four studies indicated that parents were allowed to introduce solids at four months of age (Béghin et al. 2021; Berger et al. 2020; Huet et al. 2016; Sierra et al. 2015).
Overall, addition of prebiotics to IF resulted in higher fecal Bifidobacterium levels at the last time point that microbiota was measured in all but three of the 15 studies that assessed bifidobacteria (Béghin et al. 2021; Berger et al. 2020; Civardi et al. 2017; Holscher et al. 2012; Lee et al. 2015; Nomayo et al. 2020; Paineau et al. 2014; Salvini et al. 2011; Sierra et al. 2015; Veereman-Wauters et al. 2011; Wernimont et al. 2015; Yao et al. 2014). Of these 12 studies, the prebiotics supplemented were OF (Wernimont et al. 2015; Yao et al. 2014), 2’FL/LNnT (Berger et al. 2020), GOS (Civardi et al. 2017; Nomayo et al. 2020; Sierra et al. 2015), scFOS (Paineau et al. 2014), scGOS/FOS (Béghin et al. 2021), GOS/scFOS (Holscher et al. 2012), scGOS/lcFOS (Salvini et al. 2011), GOS/FOS (chain length not specified) (Lee et al. 2015), and GOS/OF/FOS (Veereman-Wauters et al. 2011). Of the three studies that reported no differences in fecal Bifidobacterium, the infants were fed formulas with GOS/FOS (chain length not specified) (Vivatvakin et al. 2010), GOS (Giovannini et al. 2014), or FOS (Xia et al. 2012) as the prebiotic.
Five studies assessed Lactobacillus (Salminen et al. 2016; Salvini et al. 2011; Vivatvakin et al. 2010; Wernimont et al. 2015; Xia et al. 2012). One study found that fecal Lactobacillus counts were higher in infants fed scGOS/lcFOS (Salvini et al. 2011) as the prebiotic supplement during the intervention (at three- and six-month time points) and afterward (at a 12-month follow-up). Another study also found higher fecal Lactobacillus (CFU/g) with a shorter intervention, noting significance at both 30- and 60-day time points with PDX/GOS (Salminen et al. 2016) as the prebiotic supplement. Three studies using GOS/FOS (Vivatvakin et al. 2010), OF (Wernimont et al. 2015), or FOS (Xia et al. 2012) found no significant differences in fecal Lactobacillus at intervention endpoints (2 months, 8 weeks, and 28 days, respectively) in the prebiotics group.
Four studies quantified Clostridium levels. Two studies supplemented with GOS: at the last time point that microbiota was measured, one study found lower levels of Clostridium in the prebiotic group (Giovannini et al. 2014), but the other study found no difference between groups (Civardi et al. 2017). The two other studies supplemented with GOS/FOS (Vivatvakin et al. 2010) or OF (Wernimont et al. 2015) and found no differences in fecal Clostridium levels between groups at the last time point that microbiota was measured.
Levels of C. difficile, a pathogenic species of the Clostridium genus, were assessed in four studies. Three of these studies found a significant difference in fecal C. difficile levels between groups: two studies, one using GOS (Sierra et al. 2015) and the other using scGOS/lcFOS (Huet et al. 2016) as the prebiotic, found lower levels of C. difficile in the prebiotic group at the last time point that microbiota was measured; the third study, which supplemented with GOS/scFOS as the prebiotic, also found lower levels of C. difficile in the probiotic group, but the analysis combined all time points (Holscher et al. 2012). The fourth study, which supplemented with FOS as the prebiotic, found no significant differences in C. difficile levels between groups (Xia et al. 2012).
Fecal Bacteroides levels were measured in three studies, which supplemented with GOS/FOS (Vivatvakin et al. 2010), OF (Wernimont et al. 2015), or FOS (Xia et al. 2012) as the prebiotic. None of these studies found significant differences.
Four of the 20 articles assessed effects of prebiotics on total bacterial count (Giovannini et al. 2014; Lee et al. 2015; Veereman-Wauters et al. 2011; Xia et al. 2012). Two studies, which supplemented with GOS/FOS (Lee et al. 2015) or with GOS, OF, or FOS (Veereman-Wauters et al. 2011), found higher amounts of total bacteria at the end of the intervention period in the prebiotic groups. The other two studies, which supplemented with GOS (Giovannini et al. 2014) or FOS (Xia et al. 2012), found no differences in total bacteria at the last time point that microbiota was measured.
Three studies used 16S rRNA gene sequencing to evaluate microbial diversity (Berger et al. 2020; Colombo et al. 2021; Zhu et al. 2021). Alpha diversity was higher in the prebiotic group in one study (Zhu et al. 2021) and lower in the second study (Berger et al. 2020). The third study found a shift in beta diversity between the prebiotic-supplemented group at baseline and the last intervention time point (Colombo et al. 2021).
Fecal metabolite outcomes
Four of the 20 prebiotic studies assessed fecal short-chain fatty acids (SCFAs); two studies supplemented with scGOS/lcFOS (Béghin et al. 2021; Huet et al. 2016), one supplemented with GOS/scFOS (Holscher et al. 2012), and one supplemented with GOS alone (Sierra et al. 2015). Of these studies, the two that supplemented with GOS (Sierra et al. 2015) or GOS/scFOS (Holscher et al. 2012) found higher acetic acid levels in the prebiotic group; one study analyzed the last time point that microbiota was measured (Sierra et al. 2015), and the other analyzed all intervention time points combined (Holscher et al. 2012). Both studies found that fecal propionate/propionic acid was lower in infants fed the prebiotic supplement.
Regarding SCFAs, three studies that supplemented with scGOS/lcFOS (Béghin et al. 2021), GOS/scFOS (Sierra et al. 2015), or GOS (Holscher et al. 2012) as prebiotics assessed butyrate/butyric acid: two of these found it to be lower in the infants fed the prebiotic-supplemented IF (Holscher et al. 2012; Sierra et al. 2015), and one study found no significant differences (Béghin et al. 2021). Two studies reported that the proportion of fecal isovalerate/isovaleric acid was lower in infants fed the prebiotic-supplemented IF at the last time point that microbiota was measured (Béghin et al. 2021; Huet et al. 2016). Two studies assessed L- and D-lactate levels; both supplemented with scGOS/lcFOS as the prebiotic, and both reported higher proportions of fecal L-lactate and D-lactate in the prebiotic-supplemented infants at the last time point that microbiota was measured (Béghin et al. 2021; Huet et al. 2016).
Fecal pH was assessed in five studies that supplemented with scGOS/lcFOS (Béghin et al. 2021; Huet et al. 2016; Salvini et al. 2011), GOS/scFOS (Holscher et al. 2012), or GOS (Sierra et al. 2015) as the prebiotic. All five studies found a lower fecal pH at the last time point that microbiota was measured in infants fed the prebiotic-supplemented IF.
Two studies measured fecal secretory immunoglobulin A (sIgA), and both supplemented with scGOS/lcFOS as the prebiotic (Béghin et al. 2021; Huet et al. 2016): one found a higher fecal sIgA concentration in the prebiotic-supplemented infants (Huet et al. 2016), and the other found no significant difference between groups (Béghin et al. 2021). Another study, which supplemented with scFOS as the prebiotic (Paineau et al. 2014), measured fecal levels of poliovirus-specific IgA and found no significant differences between formula groups.
Health outcomes
Health outcomes were measured in 15 of the 20 studies (Béghin et al. 2021; Civardi et al. 2017; Colombo et al. 2021; Giovannini et al. 2014; Holscher et al. 2012; Nomayo et al. 2020; Paineau et al. 2014; Scalabrin et al. 2012; Sierra et al. 2015; Veereman-Wauters et al. 2011; Vivatvakin et al. 2010; Wernimont et al. 2015; Xia et al. 2012; Yao et al. 2014; Zhu et al. 2021). The most frequently assessed health outcomes were stool frequency and consistency.
Ten studies assessed stool frequency (Béghin et al. 2021; Civardi et al. 2017; Colombo et al. 2021; Scalabrin et al. 2012; Sierra et al. 2015; Veereman-Wauters et al. 2011; Wernimont et al. 2015; Xia et al. 2012; Yao et al. 2014; Zhu et al. 2021). Two studies supplemented the IF with scGOS/lcFOS (Béghin et al. 2021) or GOS (Sierra et al. 2015) and found higher stool frequency in formula groups supplemented with prebiotics at the last time point that microbiota was measured. The eight other studies, which supplemented with PDX/GOS (Colombo et al. 2021; Scalabrin et al. 2012), GOS (Civardi et al. 2017), OF (Wernimont et al. 2015; Yao et al. 2014), FOS (Xia et al. 2012), GOS/OF/FOS (Veereman-Wauters et al. 2011), or 1,3-olein-2-palmitin (high SN-2 oil)/GOS/FOS (Zhu et al. 2021), found no significant difference in stool frequency between prebiotic intervention and control groups.
Nine studies assessed stool consistency (Civardi et al. 2017; Colombo et al. 2021; Scalabrin et al. 2012; Sierra et al. 2015; Veereman-Wauters et al. 2011; Vivatvakin et al. 2010; Wernimont et al. 2015; Xia et al. 2012; Yao et al. 2014). Six studies, which supplemented with OF (Wernimont et al. 2015; Yao et al. 2014), GOS (Sierra et al. 2015), PDX/GOS (Scalabrin et al. 2012), GOS/FOS (Vivatvakin et al. 2010), or GOS/OF/FOS (Veereman-Wauters et al. 2011), found softer stools in infants fed the prebiotic-supplemented IF at the last time point that microbiota was measured. The three other studies that supplemented with GOS (Civardi et al. 2017), PDX/GOS (Colombo et al. 2021), or FOS (Xia et al. 2012) found no significant differences between prebiotic intervention and control groups.
Seven studies assessed colic, fussiness, and/or crying (Colombo et al. 2021; Giovannini et al. 2014; Holscher et al. 2012; Scalabrin et al. 2012; Veereman-Wauters et al. 2011; Vivatvakin et al. 2010; Zhu et al. 2021). One study found that colic was lower in infants fed GOS-supplemented IF at the last time point that microbiota was measured (Giovannini et al. 2014). Five studies, which supplemented with PDX/GOS (Colombo et al. 2021; Scalabrin et al. 2012), GOS/scFOS (Holscher et al. 2012), GOS/FOS (Vivatvakin et al. 2010), or SN-2/GOS/FOS (Zhu et al. 2021), found no significant differences between groups. The last study, which supplemented with GOS/OF/FOS, also found no significant differences between groups but found that crying increased over time in all formula groups (Veereman-Wauters et al. 2011).
Seven studies assessed spitting up, vomiting, nausea, and/or regurgitation (Giovannini et al. 2014; Holscher et al. 2012; Paineau et al. 2014; Veereman-Wauters et al. 2011; Vivatvakin et al. 2010; Xia et al. 2012; Zhu et al. 2021). One study found that GOS-supplemented IF resulted in lower regurgitation risk (Giovannini et al. 2014). The other six studies, which supplemented with GOS/scFOS (Holscher et al. 2012), scFOS (Paineau et al. 2014), GOS/OF/FOS (Veereman-Wauters et al. 2011), GOS/FOS (Vivatvakin et al. 2010), FOS (Xia et al. 2012), or SN-2/GOS/FOS (Zhu et al. 2021), found no significant differences between prebiotic-supplemented IF and control formula at study endpoints.
Four studies assessed frequency of gas or flatulence at study endpoints using GOS (Civardi et al. 2017), PDX/GOS (Colombo et al. 2021; Scalabrin et al. 2012), or GOS/scFOS (Holscher et al. 2012) and found no significant differences between groups.
Lastly, three studies assessed viral infection (Nomayo et al. 2020; Scalabrin et al. 2012; Sierra et al. 2015). Two studies supplemented with GOS (Nomayo et al. 2020; Sierra et al. 2015), and one study supplemented with PDX/GOS (Scalabrin et al. 2012). Overall, no significant differences were found between probiotic-supplemented IF and control groups for gastrointestinal (Nomayo et al. 2020), respiratory (Nomayo et al. 2020; Sierra et al. 2015), and respiratory syncytial virus infection (Scalabrin et al. 2012).
Probiotics
Six studies explored the effect of probiotics on microbiota outcomes (Garcia Rodenas et al. 2016; Gil-Campos et al. 2012; Hascoët et al. 2011; Maldonado et al. 2019; Papagaroufalis et al. 2014; Wu et al. 2016) (Table 2). Among them, two studies used the same concentrations of Lactobacillus reuteri DSM 17938 as the probiotic (Garcia Rodenas et al. 2016; Papagaroufalis et al. 2014). Two studies supplemented IF with L. fermentum CECT-5716 (Gil-Campos et al. 2012; Maldonado et al. 2019), one of which had another study arm using Bifidobacterium breve at the same concentration (Maldonado et al. 2019). Lastly, two studies supplemented with B. longum, but one supplemented with B. longum BL 999 (Hascoët et al. 2011), whereas the other added B. longum BB 536 (Wu et al. 2016). Detailed information on probiotic concentrations for each intervention group for each study is given in Figure 3 (B).
Table 2.
Summary of the 6 included probiotic studies
| Reference, study design, study location, overall biasa | Study enrollment and intervention duration | Interventions used in study groupsb | Fecal sample collection, tests, compositionc | Fecal microbial endpoints | Health outcomes |
|---|---|---|---|---|---|
|
Garcia-Rodenas et al. 2016 Double-blind, multicenter Greece MODERATEa |
Infants enrolled by day 3 of life, on study formula for 6 mos (complementary foods allowed after 4 mos) | 1) Ct (CTRL): starter formula, n=44 (recruited) and n=31 (AN) 2) Lr (PRO): CTRL + L. reuteri 1.2×109 CFU/l, n=44 (recruited) and n=31 (AN) Total: N=88 (recruited) and N=62 (AN) |
Randomized groups further stratified based on vaginal (V) or C-section (C) delivery n=40 samples collected at 2 weeks and 4 mos: CLr, n=9; VLr, n= 11; CCt, n=10; VCt, n=10 Composition: 454 16S rRNA pyrosequencing |
Enterobacteriaceae (relative abundance, %) reduced in CLr v. CCt at 2 weeks (P=0.004) but not at 4 mos Actinobacteria (relative abundance, %) increased in CLr v. CCt at 2 weeks (P=0.015) but not at 4 mos, specifically due to Bifidobacterium Lactobacillus (relative abundance, %) higher in CLr v. CCt at 2 weeks (P=0.027) and 4 mos (P=0.051) Lactobacillus (relative abundance, %) higher in VLr v. VCt infants at 2 weeks (P=0.045) and 4 mos (P=0.012) Microbial richness and diversity as measured by Chao1 and Shannon indices did not differ among formula groups. |
Not assessed |
|
Gil-Campos et al. 2012 Double-blind, multicenter Spain MODERATEa |
Infants enrolled at 1 mo of age, on study formula for 5 mos (complementary foods introduced according to ESPGHAN guidelines)d | 1) CTRL: standard formula + GOS (0.3g/100mL), n=71 (ITT) and n=63 (PP) 2) PRO: CTRL formula + L. fermentum CECT-5716 1×107 CFU/g, n=66 (ITT) and n=63 (PP) Total: N=137 infants (ITT) |
n = unknown number of samples collected at 1 (baseline), 4, and 6 mos of age Fecal SCFA: gas chromatography Fecal IgA concentration: ELISA Composition: colony-plating and nested qPCR for Lactobacillus fermentum |
Lactobacillus, Bifidobacterium, Clostridium, Bacteroidaceae (CFU/g) did not differ between groups at each time point, but both groups showed significant increases in these bacterial groups over time (statistic not provided) L. fermentum CECT5716 detected alive in 53% of PRO group and only 2 CTRL infants (statistic not provided) |
Diarrhea incidence rate lower in PRO v. CTRL (P=0.018) |
|
Hascoët et al. 2011 Double blind, single center France MODERATEa |
Infants enrolled at or before day 7 of life, on study formula for 4 mos | 1) CTRL: standard formula, n=38 (ITT) and n=33 (PP) 2) STUDY: formula with low protein and phosphate, high lactose, predominantly whey protein, n=39 (ITT) and n=32 (PP) 3) PRO: STUDY formula + B. longum BL999 2×107 CFU/g, n=40 (ITT) and n=32 (PP) 4) BF reference, n=73 (ITT) and n=44 (PP) Total: N = 190 (ITT) and N = 140 (PP) |
n=140 samples collected at 1 and 2 mos Quantification of Bifidobacterium longum BL999 via plating followed by qPCR Fecal IgA concentration: ELISA Composition: FISH using 16S-rRNA targeted oligonucleotide probes |
B. longum BL999 detected in PRO infants at 1 mo, but not at 2 mos | Soft stool frequency higher in PRO v. STUDY (P<0.05) |
|
Maldonado et al. 2019 Double blind, multicenter Spain MODERATEa |
Infants enrolled at 1 month of age, on study formula until 12 mos of age (complementary foods introduced according to ESPGHAN guidelines)d | 1) CTRL: standard formula, n=77 (ITT) and n=61 (PP) 2) Lf (PRO): CTRL formula + L. fermentum CECT5716 Lc40, 107 CFU/g, n=83 (ITT) and n=65 (PP) 3) Bb (PRO): CTRL formula + B. breve CECT7263, 107 CFU/g, n=76 (ITT) and n=63 (PP) Total: N=236 (ITT) and N=189 (PP) |
n=236 samples collected at baseline, 4, 6, 9, and 12 mos Composition: qPCR specifically targeting, Lactobacillus spp., Bifidobacterium spp., Clostridium spp., Bacteroides spp., and Escherichia coli |
Lactobacillus higher in Bb v. CTRL at 4 mos (P<0.001) Bifidobacterium lower in Lf v. CTRL at 4 mos (P=0.038) |
Lower risk of long episodes of crying in Bb v. CTRL (P=0.001) |
|
Papagaroufalis et al. 2014 Double blind, multicenter Greece HIGHa |
Infants enrolled at 0–72 hours of life, on study formula until 28 days of age | 1) CTRL: starter infant formula, n=44 (ITT), n=35 (PP day 28), and n=31 (PP days 112, 168) 2) PRO: CTRL + L. reuteri DSM-17938 1.2×106 CFU, n=44 (ITT), n=36 (PP day 28), and n=31 (PP days 112, 168) Total: N=88 (ITT), N=71 (PP day 28), and N=62 (PP days 112, 168) |
n=71 (day 28), 62 (day 112) samples collected at 14 and 112 day visits Composition: PCR for quantification of L. reuteri and FISH, specifically bifidobacteria, lactobacilli, Enterobacteriaceae, Clostridium difficile |
Bifidobacterium, Lactobacillus, and L. reuteri detectability (presence/absence) higher in PRO v. CTRL at day 14 (P=0.005–0.032) and day 112 (P=0.006–0.024) | Lower number of spitting events (median) in PRO v. CTRL at day 28 (P=0.048) and 4 mos (P=0.047). Lower frequency of hard stools (P=0.001) and higher percentage of soft stools (P=0.018) in PRO v. CTRL at day 28 |
|
Wu et al. 2016 Double blind, single center China HIGHa |
Infants enrolled at or before day 7 of life, on study formula until 6 mos of age (no information given on complementary foods) | 1) CTRL: commercially available standard formula, n=148 (EN) and n=129 (AN) 2) PRO: CTRL formula + B. longum BB536 1×107 CFU/g, n=152 (EN) and n=135 (AN) Total: N=300 (EN) and N=264 (AN) |
n=264 samples collected at 2, 4, and 11 mos of age Composition: Plating techniques for total bacteria count, lactobacilli count, and Enterobacteriaceae count, PCR for bifidobacteria |
Bifidobacteria (log CFU/g) higher in PRO v. CTRL at 2 mos (P<0.0001) and 4 mos (P=0.0096) Bifidobacteria/Enterobacteriaceae ratio higher in PRO v. CTRL at 2 mos (P<0.0001) and 4 mos (P=0.03) |
Not assessed |
OVERALL BIAS: The overall bias rating based on ratings of five individual-domain-level bias ratings (see Figure 3 and Supplemental Table S1).
CTRL, control group; PRO, probiotic group; BF, breast-fed group. Other groups are specified by study. Interventions: GOS, galactooligosaccharide; CFU, colony-forming units. We also specify intent-to-treat (ITT), enrolled (EN), analyzed (AN), and per-protocol (PP) analyses if given in the article.
SCFA, short-chain fatty acid; FISH, fluorescence in situ hybridization; qPCR, quantitative polymerase chain reaction; ELISA, enzyme-linked immunosorbent assay.
ESPGHAN guidelines: an authoritative guidance for the incorporation of complementary foods into the infant diet.
Microbiota outcomes
All six articles reported fecal microbiota outcomes at the genus level (Garcia Rodenas et al. 2016; Gil-Campos et al. 2012; Hascoët et al. 2011; Maldonado et al. 2019; Papagaroufalis et al. 2014; Wu et al. 2016). Three studies also reported microbiota outcomes at the species level (Gil-Campos et al. 2012; Hascoët et al. 2011; Papagaroufalis et al. 2014). Across the six studies, the intervention periods ranged from 28 days to 11 months. Among the four studies with intervention extending beyond four months of age (Garcia Rodenas et al. 2016; Gil-Campos et al. 2012; Maldonado et al. 2019; Wu et al. 2016), three studies indicated that parents were allowed to introduce solids at four months of age (Garcia Rodenas et al. 2016; Gil-Campos et al. 2012; Maldonado et al. 2019).
The four studies that examined effects of probiotics on fecal Bifidobacterium levels did not find consistent effects, regardless of measurement units (log CFU/g feces, CFU/g feces, or presence/absence) (Gil-Campos et al. 2012; Maldonado et al. 2019; Papagaroufalis et al. 2014; Wu et al. 2016). Two studies found that infants fed IF with probiotics (either B. longum BB 536 (Wu et al. 2016) or L. reuteri DSM 17938 (Papagaroufalis et al. 2014)) had higher levels of Bifidobacterium at the last time point that microbiota was measured. Two studies reported that infants fed IF with L. fermentum CECT-5716 had either no significant difference (Gil-Campos et al. 2012) or lower fecal Bifidobacterium levels (Maldonado et al. 2019) at the last time point that microbiota was measured.
Fecal Lactobacillus levels were measured in four of the six studies (Garcia Rodenas et al. 2016; Gil-Campos et al. 2012; Maldonado et al. 2019; Papagaroufalis et al. 2014). Three of these found higher fecal Lactobacillus levels in infants fed the probiotic-supplemented IF (either L. reuteri DSM 17938, L. reuteri DSM 17938 (Papagaroufalis et al. 2014), or B. breve (Maldonado et al. 2019)) at the last time point that the microbiota was measured. One study found no significant differences in fecal Lactobacillus levels between groups at the last time point that microbiota was measured but found a significant increase in fecal Lactobacillus over time in both the L. fermentum CECT 5716–supplemented group and the control group (Gil-Campos et al. 2012).
With respect to microbial diversity, five of the studies did not present microbiome sequencing data, but one presented 16S rRNA gene sequencing data (Garcia Rodenas et al. 2016). Both richness and diversity, measured by Chao1 and Shannon indices, did not differ between L. reuteri DSM 17938–supplemented IF and control groups (Garcia Rodenas et al. 2016).
Fecal metabolite outcomes
Four studies measured fecal pH (Papagaroufalis et al. 2014) or fecal L-lactate and D-lactate (Papagaroufalis et al. 2014), SCFAs (Gil-Campos et al. 2012), and/or IgA concentrations (Gil-Campos et al. 2012; Hascoët et al. 2011). One study found no significant differences in fecal pH or in L-lactate or D-lactate levels between the L. reuteri DSM 17938–supplemented IF and control groups (Papagaroufalis et al. 2014). Similarly, another study found no significant differences in acetate, propionate, or butyrate SCFA levels between infants fed IF with L. fermentum CECT 5716 and the control group (Gil-Campos et al. 2012). Two studies found no significant differences in fecal IgA levels between the probiotic-supplemented IF (L. fermentum CECT 5716 (Gil-Campos et al. 2012) or B. longum BL 999 (Hascoët et al. 2011)) and the control group.
Health outcomes
Four of the six studies measured health outcomes (Gil-Campos et al. 2012; Hascoët et al. 2011; Maldonado et al. 2019; Papagaroufalis et al. 2014). Two studies that supplemented with L. reuteri DSM 17938 (Papagaroufalis et al. 2014) or B. longum BL 999 (Hascoët et al. 2011) found a higher frequency or percentage of soft stools in the infants fed the probiotics. One study reported that L. fermentum CECT 5716 supplementation resulted in a lower incidence of diarrhea (Gil-Campos et al. 2012). One study that supplemented with L. reuteri DSM 17938 found a lower number of spitting-up episodes in the probiotic-fed group versus the control group (Papagaroufalis et al. 2014). Two of the six studies, which supplemented with L. fermentum CECT 5716 (Maldonado et al. 2019) and L. reuteri DSM 17938 (Papagaroufalis et al. 2014), reported no difference in flatulence between groups. One study, which supplemented with L. fermentum CECT 5716 (Maldonado et al. 2019), reported no significant results with respect to fecal deposition number or color; gastrointestinal, respiratory, and urinary tract infections; dermatitis; or febrile episodes.
Synbiotics
A total of six studies described the effect of synbiotics (pre- and probiotics) on microbiota outcomes (Abrahamse-Berkeveld et al. 2016; Cooper et al. 2016; Meli et al. 2014; Radke et al. 2018; Rozé et al. 2012; Simeoni et al. 2016) (Table 3). Among them, three studies examined synbiotic IF that contained GOS and 3’- and 6’-sialyllactose (a bovine milk oligosaccharide) with B. lactis (Cooper et al. 2016; Radke et al. 2018; Simeoni et al. 2016). Other synbiotic combinations were GOS and FOS with B. breve (Abrahamse-Berkeveld et al. 2016), GOS and 3’- and 6’-sialyllactose with B. longum/L. rhamnosus (Meli et al. 2014), and GOS/scFOS with B. infantis/L. rhamnosus (Rozé et al. 2012).
Table 3.
Summary of the 6 included synbiotic studies
| Reference, study design, study location, overall biasa | Study enrollment and intervention duration | Interventions used in study groupsb | Fecal sample collection, tests, compositionc | Fecal microbial endpoints | Health outcomes |
|---|---|---|---|---|---|
|
Abrahamse-Berkeveld et al. 2016 Double blind, multicenter Germany MODERATEa |
Infants enrolled by day 35 of life, on study formula for 13 weeks | 1) CTRL: standardized extensively hydrolyzed whey protein-based powder without synbiotics, n=111 (ITT) and n=57 (PP) 2) SYN: CTRL formula + 0.8 g/100 mL GOS/FOS (9:1 ratio) + 1.3×109 CFU/100 mL B. breve M-16V), n=100 (ITT) and n=45 (PP) Total: N=211 infants (ITT) and N=102 infants (PP) |
n=60 (n=36 CTRL, n=24 SYN) samples collected at baseline, week 1, week 13 (subset) Fecal SCFA: gas chromatography Fecal pH: pH meter Composition: FISH using 16S rRNA-targeted oligonucleotide probes, specifically targeting bifidobacteria, lactobacilli, Bacteroides/Prevotella, Clostridium histolyticum/C. lituseburense, Enterobacteriaceae, C. coccoides/Eubacterium rectale |
Bifidobacteria (%) higher in SYN v. CTRL at week 13 (P=0.014 (ITT)) C. coccoides/E. rectale cluster (%) lower in SYN v. CTRL at week 13 (P=0.013 (PP)) Potential pathogens (%, C. histolyticum/C. lituseburense ratio) lower in SYN v. CTRL at week 1 (P=0.003) and week 13 (P=0.013) in both ITT (P=0.043) and PP (P=0.058) Fecal pH lower and D-lactate concentration higher in SYN v. CTRL at week 13 (P=0.04) |
Stool consistency score lower in SYN v. CTRL in ITT subpopulation in first 4 weeks (P=0.035), but not at 13 weeks Diaper (nappy) rash severity lower in SYN v. CTRL in ITT subpopulation in first 4 weeks (P=0.026), but not at 13 weeks |
|
Cooper et al. 2016 Double blind, multicenter South Africa MODERATEa |
Infants enrolled by day 3 of life, on study formula until 6 mos of age (complementary food allowed after 4 mos) | 1) CTRL: standard formula, n= 214 (RAND) and n= 129 (completed 4 mos) 2) SYN: CTRL formula + BMOS (whey permeate containing GOS and 3’- and 6’-sialyllactose) (8 g/L in reconstituted formula) and a probiotic (B. lactis CNCM-I-3446 at 1×107 CFU/g of powder formula), n=207 (RAND) and n=138 (completed 4 mos) Total: N=421 (RAND) and N=267 (completed 4 mos) |
n=168 samples collected at day 10; unknown fecal sample n collected at day 3, 4 weeks, and 3 mos Fecal IgA: ELISA Fecal pH: pH meter Composition: Plating methods, PCR used to determine B. lactis CNCM I-3446, Staphylococcus, enterobacteria, Escherichia coli, and Klebsiella counts FISH used to determine total bacterial counts, bifidobacteria, lactobacilli, Bacteroides, and Clostridium |
Bifidobacteria (CFU) higher in SYN v. CTRL at day 28 (P=0.001) and 3 mos (P<0.001) in C-section-born infants Bifidobacteria (CFU) greater increase in SYN v. CTRL in vaginally born infants at day 28 (P<0.001) and day 84 (P<0.001) Bifidobacteria species detected (presence/absence) in higher proportion in SYN v. CTRL at days 10 and 28 only in C-section born infants (P<0.025) B. lactis detected (presence/absence) in higher proportion of infants in SYN v. CTRL at day 10, week 4, and 3 mos (P<0.025) Lactobacillus detected (presence/absence) in higher proportion in SYN v. CTRL at 3 mos for vaginally born infants, and day 28 only for C-section-born infants (no p-value given) Clostridium eubacteria detected (presence/absence) in lower proportion in SYN v. CTRL at 3 mos (P<0.025) Enterobacteriaceae detected (presence/absence) in lower proportion in SYN v. CTRL at days 10 and 28 in vaginally born infants (P<0.025) E. coli detected (presence/absence) in lower proportion in SYN v. CTRL at days 3 and 28 in vaginally born infants (P<0.025) Klebsiella spp. detected (presence/absence) in lower proportion of infants in SYN v. CTRL at days 3 and 28 in vaginally born infants (P<0.025) Staphylococcus lower in SYN v. CTRL in vaginally born infants at days 10 and 28 only (P<0.025) Fecal pH lower in SYN v. CTRL among both vaginally and C-section born infants at day 10 and week 4, but significance only remained at month 3 for the C-section-born infants |
Harder stool consistency lower in proportion of infants in SYN v. CTRL among vaginally born (P=0.002) and C-section-born infants (P=0.001) up until 6 mos of age Formed stools greater in proportion of CTRL v. SYN infants among C-section-born (P=0.045) and vaginally born (P=0.055) infants Liquid stools (frequency) higher in SYN among C-section-born infants (P<0.001) |
|
Meli et al. 2014 Double-blind, single center Italy MODERATEa |
Infants enrolled by 14 days of life, on study formula until 4 mos of age | 1) CTRL: standard infant formula, n= 84 (RAND), n= 63 (primary analysis), and n=57 (PP) 2) PRE: CTRL formula + 10 g/L BMOS (whey permeate containing GOS and 3’- and 6’-sialyllactose) in the reconstituted formula, n=99 (RAND), n=62 (primary analysis), and n=60 (PP) 3) SYN: CTRL formula + 10 g/L BMOS (whey permeate containing GOS and 3’- and 6’-sialyllactose) + probiotics B. longum (B1999) and L. rhamnosus (LPR), each at 2×107 CFU/g, n=98 (RAND), n=64 (primary analysis), and n=56 (PP) 4) BF reference group, n=30 (RAND), n=12 (primary analysis), and n=12 (PP) Total: N=281 (RAND), N=201 (primary analysis), and N=185 infants (PP) |
n=71 samples collected in a subset of infants at 2 mos of age Composition: FISH analyzing the following bacterial species: bifidobacteria, lactobacilli, enterobacteria, clostridia, and Bacteroides; Bl999 and LPR quantified via plating technique |
Fecal Bifidobacterium and Lactobacillus counts (median) higher in SYN v. CTRL at 2 mos (P<0.05) Clostridium counts (median) lower in PRE and SYN v. CTRL at 2 mos (P<0.05) |
Daily stool frequency higher in PRE and SYN v. CTRL (P=0.0001) Lower odds of harder stools in PRE and SYN v. CTRL (P=0.0001, P=0.0003, respectively) Investigator-diagnosed colic lower in CTRL v. PRE (P=0.01) |
|
Radke et al. 2017 Double blind, multicenter France, Germany, Netherlands MODERATEa |
Infants enrolled at or before day 14 of life, on study formula from enrollment to 6 mos of age (complementary foods allowed at 4 mos) | 1) CTRL: standard formula, n=180 (ITT), n=157 (PP) 2) SYN: CTRL formula + BMOS (whey permeate containing GOS and 3’- and 6’-sialyllactose) (8 g/L) + B. lactis (CNCM I-3446, 1×107 CFU/g), n=179 (ITT) and n=150 (PP) 3) BF reference, n=59 (ITT) and n=49 (PP) Total: N=359 (ITT) and N=307 (PP) |
n=unknown samples collected in a subset of infants at 3 and 6 mos of age Fecal sIgA and alpha-1 antitrypsin: ELISA Fecal pH: pH meter Composition: FISH using 16S-rRNA targeted oligonucleotide probes (details not provided) |
Bifidobacterium and lactobacilli counts higher in SYN v. CTRL at 3 mos (P<0.01) Clostridia/eubacteria counts lower in SYN v. CTRL at 3 mos (P < 0.01) B. lactis detection (presence/absence) higher in SYN vs CTRL at 3 (P<0.001) Fecal pH (mean) lower in SYN v. CTRL at 3 mos (P<0.001, ITT) Fecal sIgA (mean) concentrations (mg/L) higher in SYN v. CTRL at 3 mos (P<0.0001, P<0.0001, respectively) (ITT) Stool alpha-1 antitrypsin higher in SYN v. CTRL at 3 mos (P=0.03) (ITT) |
Daily stool frequency higher in SYN v. CTRL in first 3 mos (P<0.005) Infants who ever had flatulence (proportion) higher in SYN v. CTRL at 3 mos (P<0.01) |
|
Rozé et al. 2012 Double blind, multicenter France MODERATEa |
Infants enrolled by day 3 of life, on study formula until 6 mos of age (no information given on complementary foods) | 1) CTRL: standard formula, n=49 (RAND) and n=38 (PP) 2) SYN: CTRL formula + L. rhamnosus LCS-742 (1.4×108 CFU) and B. infantis M63 (1.4×108 CFU) + 96% GOS (0.4 g/100mL) and 4% scFOS (0.02 g/100mL); also enriched with alpha-lactalbumin, n=48 (RAND) and n=35 (PP) Total: N=97 (RAND) and N=73 (PP) |
n=43, 34 samples collected at 1 and 6 mos, respectively Fecal sIgA: ELISA Composition: plate spreading for quantification of main genera, PCR for specific genus and species as well as 16S rDNA sequencing |
Bifidobacteria presence did not differ between SYN v. CTRL at 1 and 6 months (P=0.14 and P=0.99, respectively) and colonization (CFU/g) did not differ at 6 months (P=0.07) Lactobacilli colonization (CFU/g) higher in SYN v. CTRL at 1 month (P<0.0001), but not at 6 mos Clostridium presence did not differ between SYN v. CTRL at 1 and 6 months (P=0.97 and P=0.29, respectively) Incidence (presence/absence) rate and colonization of staphylococci (CFU/g) were higher in SYN v. CTRL at 1 and 6 mos (P=0.02, P=0.02) Fecal sIgA concentrations were similar at 1 and 6 mos in SYN, but decreased from 1 to 6 mos in CTRL (no statistics given) |
During the 3 days preceding the 1-mo clinical visit, SYN exhibited less crying or agitation, more quiet behavior v. CTRL (P=0.03 for ITT, P<0.02 for PP) SYN associated with reduced risk of atopic dermatitis during the study (P<0.05) Number of stools per day greater in SYN v. CTRL at 1 month (P=0.05) |
|
Simeoni et al. 2016 Double blind, multicenter France, Poland MODERATEa |
Infants enrolled at or before 14 days of life, on study formula until 3 mos of age | 1) CTRL: standard, starter infant formula, n=37 (EN) and n=18 (PP) 2) SYN: CTRL formula + BMOS (whey permeate containing GOS and 3’- and 6’-sialyllactose) (8 g/L + B. lactis CNCM I-3446 (1×107 CFU/g), n=39 (EN) and n=21 (PP) 3) BF reference, n=39 (EN) and n=23 (PP) Total: N=115 (EN) and N=62 infants (PP) |
n=62 samples collected at baseline, 6, and 12 weeks of age 16S rRNA gene sequencing Composition: qPCR used for total bacterial cell counts Lactobacillus and Bifidobacterium, as well as counts for individual species of bifidobacteria |
Bifidobacterium (P<0.001), total bacteria (P<0.05), and B. longum (P<0.05) counts higher in SYN v. CTRL at 6 and 12 weeks Escherichia (count) decreased over time in all groups but less so in CTRL v. SYN (P<0.01). B. animalis present in most fecal samples in SYN, but nearly absent in CTRL (P<0.001) Diversity index (P<0.01) and fecal pH (P=0.016) higher in CTRL v. SYN at 6 weeks but not at 12 weeks |
Liquid stool frequency significantly higher in SYN v. CTRL (no p-value reported) |
OVERALL BIAS: The overall bias rating based on ratings of five individual-domain-level bias ratings (see Figure 3 and Supplemental Table S1).
BF, breastfeeding reference group; CTRL, control group; PRE, prebiotic group; SYN, synbiotic group. Other groups are specified by study. Interventions: BMOS, bovine milk oligosaccharide; CFU, colony-forming units; FOS, fructooligosaccharide; GOS, galactooligosaccharide; scFOS, short-chain fructooligosaccharide;. We also specify enrolled (EN), intent-to-treat (ITT), per-protocol (PP), randomized (RAND) analyses if given in the article.
ELISA, enzyme-linked immunosorbent assay; FISH, fluorescence in situ hybridization; PCR, polymerase chain reaction; SCFA, short chain fatty acid; sIgA, secretory immunoglobulin A.
Microbiota outcomes
All six articles reported fecal microbiota outcomes at the genus level (Abrahamse-Berkeveld et al. 2016; Cooper et al. 2016; Meli et al. 2014; Radke et al. 2018; Rozé et al. 2012; Simeoni et al. 2016); four also reported outcomes at the species level (Abrahamse-Berkeveld et al. 2016; Cooper et al. 2016; Radke et al. 2018; Simeoni et al. 2016). Across the six studies, the intervention periods ranged three to six months. For the three studies in which the intervention extended beyond four months of age (Cooper et al. 2016; Radke et al. 2018; Rozé et al. 2012), two studies indicated that parents were allowed to introduce solids at four months of age (Cooper et al. 2016; Radke et al. 2018). Units of measurement varied (% abundance, CFU/g feces, median, or counts) but did not affect outcome.
Overall, one of the six studies found no significant difference in Bifidobacterium in infants supplemented with L. rhamnosus, B. infantis, GOS/scFOS, and enriched with alpha-lactalbumin (Rozé et al. 2012). Five of the six studies found that infants fed IF with a Bifidobacterium species plus a prebiotic (GOS and 3’- and 6’-sialyllactose with B. lactis (Cooper et al. 2016; Radke et al. 2018; Simeoni et al. 2016), GOS and 3’- and 6’-sialyllactose with B. longum/L. rhamnosus (Meli et al. 2014), or GOS/FOS with B. breve (Abrahamse-Berkeveld et al. 2016)) had higher levels of fecal Bifidobacterium at the last time point that microbiota was measured. Within the Bifidobacterium genus, two of the six studies supplemented GOS and 3’- and 6’-sialyllactose with B. lactis and found a higher presence of fecal B. lactis in the synbiotic group at the last time point that microbiota was measured (Cooper et al. 2016; Radke et al. 2018); one of these studies also assessed fecal B. longum and B. animalis counts, which were higher in the synbiotic IF group at the last time point that microbiota was measured (Simeoni et al. 2016).
Four of the six studies found that infants fed a synbiotic formula containing GOS and 3’- and 6’-sialyllactose with either B. lactis (Cooper et al. 2016; Radke et al. 2018, Simeoni et al., 2016) or B. longum/L. rhamnosus (Meli et al. 2014) had higher fecal Lactobacillus counts at the last time point that microbiota was measured. Another study, which supplemented with GOS/scFOS with B. infantis/L. rhamnosus, found higher fecal Lactobacillus colonization at the one-month time point, but this increase was no longer significant at the end of the intervention period (six-month time point) (Rozé et al. 2012). Two studies found no significant differences in fecal Lactobacillus counts between control and supplemented formulas (Abrahamse-Berkeveld et al. 2016; Simeoni et al. 2016).
Two of the six studies found that infants fed synbiotic formula containing GOS and 3’- and 6’-sialyllactose with either B. lactis (Cooper et al. 2016) or B. longum/L. rhamnosus (Meli et al. 2014) had lower levels of fecal Clostridium at the last time point that microbiota was measured. However, one study found no significant differences in fecal Clostridium in the supplemented group (L. rhamnosus, B. infantis, GOS/scFOS, alpha lactalbumin) (Rozé et al. 2012).
Only two of the six studies analyzed fecal Staphylococcus (Cooper et al. 2016; Rozé et al. 2012), but the findings were inconsistent. An IF containing GOS and 3’- and 6’-sialyllactose with B. lactis resulted in lower Staphylococcus at midpoints compared with the control formula group, but this difference was no longer significant at the last time point that microbiota was measured (Cooper et al. 2016). An IF containing GOS/scFOS with B. infantis/L. rhamnosus resulted in a significantly higher presence of Staphylococcus and a significantly lower relative abundance at the last time point that microbiota was measured compared with the control formula group (Rozé et al. 2012).
Similar to studies of pre- or probiotics, most synbiotics studies did not present fecal microbiome sequencing data. One study, which supplemented with GOS and 3’- and 6’-sialyllactose with B. lactis (Simeoni et al. 2016), presented 16S rRNA gene sequencing data showing that at the six-week midpoint the control formula group had a higher diversity index than the synbiotic-supplemented IF group, but this difference was not seen at the study endpoint.
Fecal metabolite outcomes
Fecal pH was assessed in four of the six studies, which fed GOS/FOS with B. breve (Abrahamse-Berkeveld et al. 2016) or GOS and 3’- and 6’-sialyllactose with B. lactis (Cooper et al. 2016; Radke et al. 2018; Simeoni et al. 2016). One study found lower fecal pH in the synbiotic group at the midpoint (Simeoni et al. 2016), and the others at the study endpoint (Abrahamse-Berkeveld et al. 2016; Cooper et al. 2016; Radke et al. 2018).
Regarding SCFAs, one study, which supplemented with GOS/FOS and B. breve, assessed acetate and butyrate levels and found no significant differences between the synbiotic and control groups (Abrahamse-Berkeveld et al. 2016).
Only two of the six studies assessed sIgA concentrations, using GOS and 3’- and 6’-sialyllactose with B. lactis (Radke et al. 2018) or GOS/scFOS with B. infantis/L. rhamnosus (Rozé et al. 2012). Their results were inconsistent. The group fed IF with GOS and 3’- and 6’-sialyllactose with B. lactis had significantly higher stool alpha-1 antitrypsin at the midpoint of the study only (Radke et al. 2018). In the same study, fecal sIgA concentrations were higher in the synbiotic group at both the midpoint and endpoint of the study (Radke et al. 2018). When IF with GOS/scFOS and B. infantis/L. rhamnosus was fed to infants, fecal sIgA concentrations remained the same at mid- and endpoints of the study, while in the control group fecal sIgA decreased between the mid- and endpoints (Rozé et al. 2012).
Health outcomes
Several health outcomes were assessed in infants fed the following combinations: GOS/FOS with B. breve (Abrahamse-Berkeveld et al. 2016), GOS and 3’- and 6’-sialyllactose with B. lactis (Cooper et al. 2016; Radke et al. 2018; Simeoni et al. 2016), GOS and 3’- and 6’-sialyllactose with B. longum/L. rhamnosus (Meli et al. 2014), or GOS/scFOS with B. infantis/L. rhamnosus (Rozé et al. 2012). Studies reported the synbiotic IF groups had higher daily stool frequency (Meli et al. 2014; Radke et al. 2018), softer stool consistency (Cooper et al. 2016; Meli et al. 2014; Simeoni et al. 2016), reduced risk of atopic dermatitis (Rozé et al. 2012), and higher proportion of infants without flatulence (Radke et al. 2018), all measured at the endpoint of each study.
Four studies examined stool consistency, and of these, three found softer stool consistency in the synbiotic formula groups (Cooper et al. 2016; Meli et al. 2014; Simeoni et al. 2016). The other one study found no significant difference at the last time point that stool was assessed but softer consistency in the synbiotic group at the four-week midpoint (Abrahamse-Berkeveld et al. 2016).
Among the five studies that assessed flatulence, only one found significance (Radke et al. 2018). The other four found no significant differences in flatulence between groups (Abrahamse-Berkeveld et al. 2016; Cooper et al. 2016; Meli et al. 2014; Simeoni et al. 2016).
With regard to crying, fussiness, and agitation, all six studies found no significant differences between formula groups at the study endpoint (Abrahamse-Berkeveld et al. 2016; Cooper et al. 2016; Meli et al. 2014; Radke et al. 2018; Rozé et al. 2012; Simeoni et al. 2016). However, one study reported that infants in the synbiotic group experienced less crying or agitation at the one-month time point (Rozé et al. 2012).
Of the five studies that assessed for spitting up and regurgitation, none found significant differences between groups (Abrahamse-Berkeveld et al. 2016; Cooper et al. 2016; Meli et al. 2014; Rozé et al. 2012; Simeoni et al. 2016). Similarly, of the four studies that assessed vomiting, no differences were seen between groups (Abrahamse-Berkeveld et al. 2016; Cooper et al. 2016; Rozé et al. 2012; Simeoni et al. 2016).
Discussion
Summary of findings
This systematic review analyzed 32 randomized controlled trials that evaluated the effect of adding pre-, pro-, or synbiotics to IF on gastrointestinal microbiota markers (primary outcomes) and other associated health outcomes (secondary outcomes). For studies included in this review, infants must have been full term, exclusively formula fed, and enrolled by (or before) two months of age, and groups must have a sample size of at least five subjects per arm.
Most prebiotic supplementation studies showed an increase in fecal Bifidobacterium levels; in the three exceptions, which supplemented with GOS (Giovannini et al. 2014), FOS (Xia et al. 2012), or GOS/FOS (Vivatvakin et al. 2010), the prebiotic supplementation resulted in no difference in Bifidobacterium between supplemented IF and respective control groups. With respect to fecal Lactobacillus, the effects of prebiotic supplementation were inconsistent, with equivocal positive and negative findings (Salminen et al. 2016; Salvini et al. 2011; Vivatvakin et al. 2010; Wernimont et al. 2015; Xia et al. 2012). Regarding fecal Clostridium, the effects of prebiotic supplementation were also inconsistent, but results tended to indicate no significant effect (Civardi et al. 2017; Giovannini et al. 2014; Vivatvakin et al. 2010; Wernimont et al. 2015). For C. difficile, all studies that supplemented GOS alone or in combination with another prebiotic found that levels of C. difficile were decreased (Holscher et al. 2012; Huet et al. 2016; Sierra et al. 2015). Among the few prebiotic studies that assessed fecal SCFAs, propionic, butyric, and isovaleric acid levels were lower while acetic acid was higher in infants fed formula supplemented with prebiotics (Béghin et al. 2021; Holscher et al. 2012; Huet et al. 2016; Sierra et al. 2015). Supplementation with GOS or GOS/FOS mixture increased D-/L-lactate (Béghin et al. 2021; Huet et al. 2016) and lowered fecal pH (Béghin et al. 2021; Holscher et al. 2012; Huet et al. 2016; Salvini et al. 2011; Sierra et al. 2015). Regarding the impact of prebiotics on health outcomes, among the limited observations on stool frequency, many found softer stools in infants supplemented with two prebiotics (e.g., GOS/FOS or GOS/PDX) (Scalabrin et al. 2012; Vivatvakin et al. 2010). When only a single prebiotic was supplemented, results were inconsistent regarding stool softness (Civardi et al. 2017; Sierra et al. 2015; Wernimont et al. 2015; Xia et al. 2012).
For the studies in which probiotics were supplemented, fecal Bifidobacterium levels were not closely correlated with Bifidobacterium supplementation in IF. However, in general, regardless of probiotic species supplemented, fecal Lactobacillus was higher in infants supplemented with a probiotic. Probiotic supplementation had no effect on fecal metabolite outcomes, such as L- and D-lactate or SCFAs. Regarding health outcomes, the impact of probiotic supplemented IF on stool frequency and consistency varied.
Among the studies in which synbiotics were supplemented, most found higher fecal Bifidobacterium levels. Inconsistent impacts of symbiotic IF were seen on fecal Lactobacillus, Clostridium, and Staphylococcus, as well as fecal metabolites. Fecal pH was generally lower in most of the synbiotic supplement studies. Regarding health outcomes, the addition of synbiotics to IF resulted in softer stools and/or higher stool frequency in most studies (Cooper et al. 2016; Meli et al. 2014; Radke et al. 2018; Rozé et al. 2012; Simeoni et al. 2016). No effect on spitting up and regurgitation was reported.
Regardless of the pre-, pro-, or synbiotic intervention, no studies reported significant adverse events in their intervention groups, and all studies reported that experimental formulas were well tolerated by the infants.
Comparisons with other systematic reviews
Previous systematic reviews have evaluated the effects of pre-, pro-, and/or synbiotics on growth and clinical outcomes (Braegger et al. 2011; Janmohammadi et al. 2021; Mugambi et al. 2012; Skórka et al. 2017, Skórka et al. 2018) or the effects of pre- and probiotics on the prevention of allergic disease and food hypersensitivity (Osborn and Sinn 2007; Osborn and Sinn 2013). To the best of our knowledge, however, this systematic review is the first with a primary outcome of infant gastrointestinal microbiota and with related clinical/health outcomes as secondary outcomes. Further, in this review, each included study was comprehensively validated using the Cochrane Risk of Bias Tool for quality assessment (Higgins et al. 2020). Therefore, this review is a timely update that provides comprehensive information on IF supplementations and their implications for infant health.
The use of the Cochrane Collaboration methodology served as a strength. Our review clearly defines a PICO formatted question—one that includes population, intervention, comparison, and outcome being assessed. The primary (gastrointestinal microbiota) and secondary (fecal metabolites and related health) outcomes were identified a priori. Methodologic steps included screening of articles, full text review, and data extraction by two independent workgroup members, and data extraction review and bias assessment independently conducted by all workgroup members. Most of the studies included had total sample sizes of 100 or more, and most studies presented gastrointestinal microbiota outcomes at the genus level.
Studies included in this systematic review had a few limitations. Regarding the IF composition, our goal was to identify studies in which the control formula was identical to that of the experimental formula except for the addition of pre-, pro-, or synbiotics. However, not all studies presented sufficient details on IF composition, preventing us from drawing firm conclusions on true similarities or differences. Relatedly, the intervention trials did not consistently provide detailed information on consumption of solid foods, which potentially introduce significant variation in gastrointestinal microbiota. Many studies followed infants to an age when complementary foods may be added to the diet, but some studies failed to mention when and if infants began consuming solid foods during the intervention. For our systematic review, we elected to synthesize results at intervention time points prior to four months of age when possible; if a study clearly designated that no complementary foods had been consumed, we also assessed later time points when available. Therefore, this limits our systematic review to only very early stages of infant life, when IF serves as the sole source of nutrition.
Implications for research and future directions
Given the period of this systematic review (2010 and later), most studies used technologies such as colony plating, fluorescence in situ hybridization, quantitative real-time polymerase chain reaction, and/or tagged 16S rRNA gene sequencing. While assessment of bias was rated according to each methodology, it made comparisons across multiple studies challenging. Future research that assesses gastrointestinal microbiota outcomes should aim to utilize similar methods across studies to quantify and analyze the microbiota. In addition, because the introduction of solid foods can dramatically influence gastrointestinal microbiota and other health outcomes assessed in this review, it is imperative for future studies to explicitly discuss the introduction of solid foods during the intervention period. Similarly, all studies should present details on product composition, either in the main text or as a supplemental table.
Summary
To summarize, the primary aim of our work was to systematically review the literature regarding the effect of pre-, pro-, or synbiotic supplementation in IF on the gastrointestinal microbiota. The 20 prebiotic, 6 probiotic, and 6 synbiotic studies analyzed here, which evaluated gastrointestinal microbiota using several methods, found that fecal Bifidobacterium levels increased with prebiotic and synbiotic supplementation but not with probiotics alone. Generally, all groups fed formulas supplemented with Lactobacillus observed increased fecal Lactobacillus levels. Results regarding fecal Clostridium were inconsistent across all pre-, pro-, and synbiotic studies. Fecal pH was lower with some prebiotic supplementations and most synbiotic supplementations but not with probiotic supplementation. Infants supplemented with pre- and synbiotics often had softer stools, but results were inconsistent for probiotics. The totality of evidence suggests that prebiotic or synbiotic supplementation in IF increases fecal Bifidobacterium levels. Future studies would benefit from using similar microbiota assessment methods across studies and should explicitly discuss introduction of solid foods during the intervention period and present details on formula composition.
Supplementary Material
Funding
This work was supported in part by the National Institutes of Health grant HD094908.
Footnotes
Disclosure statement
J.T. consults for Byheart, Inc. on infant nutrition clinical trial design. E.S.F consults for Astarte Medical and Enzymetrics Biosciences.
References
- Abrahamse-Berkeveld M, Alles M, Franke-Beckmann E, Helm K, Knecht R, Köllges R, Sandner B, Knol J, Ben Amor K, and Bufe A. 2016. “Infant formula containing galacto-and fructo-oligosaccharides and Bifidobacterium breve M-16V supports adequate growth and tolerance in healthy infants in a randomised, controlled, double-blind, prospective, multicentre study.” J Nutr Sci 5: e42. 10.1017/jns.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreas NJ, Kampmann B, and Mehring Le-Doare K. 2015. “Human breast milk: A review on its composition and bioactivity.” Early Hum Dev 91 (11): 629–35. 10.1016/j.earlhumdev.2015.08.013. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics. Breastfeeding and the Use of Human Milk. March 2012. Accessed November 3, 2021. https://pediatrics.aappublications.org/content/129/3/e827
- American Academy of Pediatrics. 2019. Pediatric Nutrition. 8th ed. Itasca, IL: American Academy of Pediatrics. [Google Scholar]
- Béghin L, Tims S, Roelofs M, Rougé C, Oozeer R, Rakza T, Chirico G, Roeselers G, Knol J, Rozé JC, and Turck D. 2021. “Fermented infant formula (with Bifidobacterium breve C50 and Streptococcus thermophilus O65) with prebiotic oligosaccharides is safe and modulates the gut microbiota towards a microbiota closer to that of breastfed infants.” Clin Nutr 40 (3): 778–787. 10.1016/j.clnu.2020.07.024. [DOI] [PubMed] [Google Scholar]
- Berger B, Porta N, Foata F, Grathwohl D, Delley M, Moine D, Charpagne A, Siegwald L, Descombes P, Alliet P, Puccio G, Steenhout P, Mercenier A, and Sprenger N. 2020. “Linking Human Milk Oligosaccharides, Infant Fecal Community Types, and Later Risk To Require Antibiotics.” mBio 11 (2). 10.1128/mBio.03196-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann H, Rodríguez JM, Salminen S, and Szajewska H. 2014. “Probiotics in human milk and probiotic supplementation in infant nutrition: a workshop report.” Br J Nutr 112 (7): 1119–28. 10.1017/s0007114514001949. [DOI] [PubMed] [Google Scholar]
- Braegger C, Chmielewska A, Decsi T, Kolacek S, Mihatsch W, Moreno L, Pieścik M, Puntis J, Shamir R, Szajewska H, Turck D, and van Goudoever J. 2011. “Supplementation of infant formula with probiotics and/or prebiotics: a systematic review and comment by the ESPGHAN committee on nutrition.” J Pediatr Gastroenterol Nutr 52 (2): 238–50. 10.1097/MPG.0b013e3181fb9e80. [DOI] [PubMed] [Google Scholar]
- Cheng YJ, and Yeung CY. 2021. “Recent advance in infant nutrition: Human milk oligosaccharides.” Pediatr Neonatol 62 (4): 347–353. 10.1016/j.pedneo.2020.12.013. [DOI] [PubMed] [Google Scholar]
- Civardi E, Garofoli F, Longo S, Mongini ME, Grenci B, Mazzucchelli I, Angelini M, Castellazzi A, Fasano F, Grinzato A, Fanos V, Budelli A, and Stronati M. 2017. “Safety, growth, and support to healthy gut microbiota by an infant formula enriched with functional compounds.” Clin Nutr 36 (1): 238–245. 10.1016/j.clnu.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Colombo J, Carlson SE, Algarín C, Reyes S, Chichlowski M, Harris CL, Wampler JL, Peirano P, and Berseth CL. 2021. “Developmental effects on sleep-wake patterns in infants receiving a cow’s milk-based infant formula with an added prebiotic blend: a Randomized Controlled Trial.” Pediatr Res 89 (5): 1222–1231. 10.1038/s41390-020-1044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper Peter, Bolton Keith D., Velaphi Sithembiso, Nanda de Groot Shahram Emady-Azar, Pecquet Sophie, and Steenhout Philippe. 2016. “Early Benefits of a Starter Formula Enriched in Prebiotics and Probiotics on the Gut Microbiota of Healthy Infants Born to HIV+ Mothers: A Randomized Double-Blind Controlled Trial.” Clinical Medicine Insights: Pediatrics (10): 119–130. 10.4137/CMPed.s40134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covidence systematic review software. Last modified 2021. Accessed November 3, 2021. https://www.covidence.org/
- Garcia Rodenas CL, Lepage M, Ngom-Bru C, Fotiou A, Papagaroufalis K, and Berger B. 2016. “Effect of Formula Containing Lactobacillus reuteri DSM 17938 on Fecal Microbiota of Infants Born by Cesarean-Section.” J Pediatr Gastroenterol Nutr 63 (6): 681–687. 10.1097/mpg.0000000000001198. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, and Reid G. 2017. “Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics.” Nat Rev Gastroenterol Hepatol 14 (8): 491–502. 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- Gil-Campos M, López MÁ, Rodriguez-Benítez MV, Romero J, Roncero I, Linares MD, Maldonado J, López-Huertas E, Berwind R, Ritzenthaler KL, Navas V, Sierra C, Sempere L, Geerlings A, Maldonado-Lobón JA, Valero AD, Lara-Villoslada F, and Olivares M. 2012. “Lactobacillus fermentum CECT 5716 is safe and well tolerated in infants of 1–6 months of age: a randomized controlled trial.” Pharmacol Res 65 (2): 231–8. 10.1016/j.phrs.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Giovannini M, Verduci E, Gregori D, Ballali S, Soldi S, Ghisleni D, and Riva E. 2014. “Prebiotic effect of an infant formula supplemented with galacto-oligosaccharides: randomized multicenter trial.” J Am Coll Nutr 33 (5): 385–93. 10.1080/07315724.2013.878232. [DOI] [PubMed] [Google Scholar]
- Green Corkins K, and Shurley T. 2016. “What’s in the Bottle? A Review of Infant Formulas.” Nutr Clin Pract 31 (6): 723–729. 10.1177/0884533616669362. [DOI] [PubMed] [Google Scholar]
- Hascoët JM, Hubert C, Rochat F, Legagneur H, Gaga S, Emady-Azar S, and Steenhout PG. 2011. “Effect of formula composition on the development of infant gut microbiota.” Journal of Pediatric Gastroenterology & Nutrition 52 (6): 756–762. http://search.ebscohost.com/login.aspx?direct=true&db=rzh&AN=108190054&site=ehost-live. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook. [Google Scholar]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, and Sanders ME. 2014. “Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic.” Nat Rev Gastroenterol Hepatol 11 (8): 506–14. 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Holscher HD, Faust KL, Czerkies LA, Litov R, Ziegler EE, Lessin H, Hatch T, Sun S, and Tappenden KA. 2012. “Effects of prebiotic-containing infant formula on gastrointestinal tolerance and fecal microbiota in a randomized controlled trial.” JPEN Journal of Parenteral & Enteral Nutrition 36 (1): 95S–105S. http://search.ebscohost.com/login.aspx?direct=true&db=rzh&AN=104629362&site=ehost-live. [DOI] [PubMed] [Google Scholar]
- Huet F, Abrahamse-Berkeveld M, Tims S, Simeoni U, Beley G, Savagner C, Vandenplas Y, and Hourihane JO. 2016. “Partly Fermented Infant Formulae With Specific Oligosaccharides Support Adequate Infant Growth and Are Well-Tolerated.” J Pediatr Gastroenterol Nutr 63 (4): e43–53. 10.1097/mpg.0000000000001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt KM, Foster JA, Forney LJ, Schütte UM, Beck DL, Abdo Z, Fox LK, Williams JE, McGuire MK, and McGuire MA. 2011. “Characterization of the diversity and temporal stability of bacterial communities in human milk.” PLoS One 6 (6): e21313. 10.1371/journal.pone.0021313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmohammadi P, Nourmohammadi Z, Fazelian S, Mirzababaei A, Alizadeh S, Zarei M, Daneshzad E, and Djafarian K. 2021. “Does infant formula containing synbiotics support adequate growth in infants? A meta-analysis and systematic review of randomized controlled trials.” Crit Rev Food Sci Nutr: 1–12. 10.1080/10408398.2021.1952548. [DOI] [PubMed] [Google Scholar]
- Jeurink PV, van Bergenhenegouwen J, Jiménez E, Knippels LM, Fernández L, Garssen J, Knol J, Rodríguez JM, and Martín R. 2013. “Human milk: a source of more life than we imagine.” Benef Microbes 4 (1): 17–30. 10.3920/bm2012.0040. [DOI] [PubMed] [Google Scholar]
- Lee Y, Bharani R, Biswas A, Lee J, Tran LA, Pecquet S, and Steenhout P. 2015. “Normal growth of infants receiving an infant formula containing Lactobacillus reuteri, galacto-oligosaccharides, and fructo-oligosaccharide: a randomized controlled trial.” Matern Health Neonatol Perinatol 1: 9. 10.1186/s40748-015-0008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons KE, Ryan CA, Dempsey EM, Ross RP, and Stanton C. 2020. “Breast Milk, a Source of Beneficial Microbes and Associated Benefits for Infant Health.” Nutrients 12 (4). 10.3390/nu12041039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado J, Gil-Campos M, Maldonado-Lobón JA, Benavides MR, Flores-Rojas K, Jaldo R, Jiménez Del Barco I, Bolívar V, Valero AD, Prados E, Peñalver I, and Olivares M. 2019. “Evaluation of the safety, tolerance and efficacy of 1-year consumption of infant formula supplemented with Lactobacillus fermentum CECT5716 Lc40 or Bifidobacterium breve CECT7263: a randomized controlled trial.” BMC Pediatr 19 (1): 361. 10.1186/s12887-019-1753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meli Ferdinando, Puccio Giuseppe, Cajozzo Cinzia, Giovanni Licata Ricottone Sophie Pecquet, Sprenger Norbert, and Steenhout Philippe. 2014. “Growth and safety evaluation of infant formulae containing oligosaccharides derived from bovine milk: a randomized, double-blind, noninferiority trial.” BMC Pediatrics 14 (1): 306–306. 10.1186/s12887-014-0306-3. http://search.ebscohost.com/login.aspx?direct=true&db=rzh&AN=109725521&site=ehost-live. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugambi Mary N., Musekiwa Alfred, Lombard Martani, Young Taryn, and Blaauw Reneé. 2012. “Synbiotics, probiotics or prebiotics in infant formula for full term infants: a systematic review.” Nutrition journal 11: 81–81. 10.1186/1475-2891-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomayo A, Schwiertz A, Rossi R, Timme K, Foster J, Zelenka R, Tvrdik J, and Jochum F. 2020. “Infant formula with cow’s milk fat and prebiotics affects intestinal flora, but not the incidence of infections during infancy in a double-blind randomized controlled trial.” Mol Cell Pediatr 7 (1): 6. 10.1186/s40348-020-00098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn DA, and Sinn JKH. 2007. “Probiotics in infants for prevention of allergic disease and food hypersensitivity.” Cochrane Database of Systematic Reviews (4). 10.1002/14651858.CD006475.pub2. [DOI] [PubMed] [Google Scholar]
- Osborn DA, and Sinn JKJ. 2013. “Prebiotics in infants for prevention of allergy.” Cochrane Database of Systematic Reviews (3). 10.1002/14651858.CD006474.pub3. [DOI] [PubMed] [Google Scholar]
- Page Matthew J., McKenzie Joanne E., Bossuyt Patrick M., Boutron Isabelle, Hoffmann Tammy C., Mulrow Cynthia D., Shamseer Larissa, Tetzlaff Jennifer M., Akl Elie A., Brennan Sue E., Chou Roger, Glanville Julie, Grimshaw Jeremy M., Asbjørn Hróbjartsson Manoj M. Lalu, Li Tianjing, Loder Elizabeth W., Evan Mayo-Wilson Steve McDonald, McGuinness Luke A., Stewart Lesley A., Thomas James, Tricco Andrea C., Welch Vivian A., Whiting Penny, and Moher David. 2021. “The PRISMA 2020 statement: an updated guideline for reporting systematic reviews.” BMJ 372: n71. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paineau Damien, Respondek Frédérique, Menet Vincent, Sauvage Roland, Bornet Francis, and Wagner Anne. 2014. “Effects of short-chain fructooligosaccharides on faecal bifidobacteria and specific immune response in formula-fed term infants: a randomized, double-blind, placebo-controlled trial.” Journal of Nutritional Science & Vitaminology 60 (3): 167–175. http://search.ebscohost.com/login.aspx?direct=true&db=rzh&AN=107798157&site=ehost-live. [DOI] [PubMed] [Google Scholar]
- Papagaroufalis K, Fotiou A, Egli D, Tran LA, and Steenhout P. 2014. “A Randomized Double Blind Controlled Safety Trial Evaluating d-Lactic Acid Production in Healthy Infants Fed a Lactobacillus reuteri-containing Formula.” Nutr Metab Insights 7: 19–27. 10.4137/nmi.S14113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke M, Picaud JC, Loui A, Cambonie G, Faas D, Lafeber HN, de Groot N, Pecquet SS, Steenhout PG, and Hascoet JM. 2018. “Starter formula enriched in prebiotics and probiotics ensures normal growth of infants and promotes gut health: a randomized clinical trial.” Pediatr Res 83 (1–1): 190. 10.1038/pr.2017.245. [DOI] [PubMed] [Google Scholar]
- Reverri Elizabeth J., Devitt Amy A., Kajzer Janice A., Baggs Geraldine E., and Borschel Marlene W.. 2018. “Review of the Clinical Experiences of Feeding Infants Formula Containing the Human Milk Oligosaccharide 2’-Fucosyllactose.” Nutrients 10 (10). 10.3390/nu10101346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozé JC, Barbarot S, Butel MJ, Kapel N, Waligora-Dupriet AJ, De Montgolfier I, Leblanc M, Godon N, Soulaines P, Darmaun D, Rivero M, and Dupont C. 2012. “An α-lactalbumin-enriched and symbiotic-supplemented v. a standard infant formula: a multicentre, double-blind, randomised trial.” Br J Nutr 107 (11): 1616–22. 10.1017/s000711451100479x. [DOI] [PubMed] [Google Scholar]
- Salminen S, Endo A, Isolauri E, and Scalabrin D. 2016. “Early Gut Colonization With Lactobacilli and Staphylococcus in Infants: The Hygiene Hypothesis Extended.” J Pediatr Gastroenterol Nutr 62 (1): 80–6. 10.1097/mpg.0000000000000925. [DOI] [PubMed] [Google Scholar]
- Salminen S, Stahl B, Vinderola G, and Szajewska H. 2020. “Infant Formula Supplemented with Biotics: Current Knowledge and Future Perspectives.” Nutrients 12 (7). 10.3390/nu12071952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvini F, Riva E, Salvatici E, Boehm G, Jelinek J, Banderali G, and Giovannini M. 2011. “A specific prebiotic mixture added to starting infant formula has long-lasting bifidogenic effects.” J Nutr 141 (7): 1335–9. 10.3945/jn.110.136747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalabrin DM, Mitmesser SH, Welling GW, Harris CL, Marunycz JD, Walker DC, Bos NA, Tölkkö S, Salminen S, and Vanderhoof JA. 2012. “New prebiotic blend of polydextrose and galacto-oligosaccharides has a bifidogenic effect in young infants.” J Pediatr Gastroenterol Nutr 54 (3): 343–52. 10.1097/MPG.0b013e318237ed95. [DOI] [PubMed] [Google Scholar]
- Sierra C, Bernal MJ, Blasco J, Martínez R, Dalmau J, Ortuño I, Espín B, Vasallo MI, Gil D, Vidal ML, Infante D, Leis R, Maldonado J, Moreno JM, and Román E. 2015. “Prebiotic effect during the first year of life in healthy infants fed formula containing GOS as the only prebiotic: a multicentre, randomised, double-blind and placebo-controlled trial.” Eur J Nutr 54 (1): 89–99. 10.1007/s00394-014-0689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeoni U, Berger B, Junick J, Blaut M, Pecquet S, Rezzonico E, Grathwohl D, Sprenger N, Brüssow H, Szajewska H, Bartoli JM, Brevaut-Malaty V, Borszewska-Kornacka M, Feleszko W, François P, Gire C, Leclaire M, Maurin JM, Schmidt S, Skórka A, Squizzaro C, and Verdot JJ. 2016. “Gut microbiota analysis reveals a marked shift to bifidobacteria by a starter infant formula containing a synbiotic of bovine milk-derived oligosaccharides and Bifidobacterium animalis subsp. lactis CNCM I-3446.” Environ Microbiol 18 (7): 2185–95. 10.1111/1462-2920.13144. [DOI] [PubMed] [Google Scholar]
- Skórka A, Pieścik-Lech M, Kołodziej M, and Szajewska H. 2017. “To add or not to add probiotics to infant formulae? An updated systematic review.” Benef Microbes 8 (5): 717–725. 10.3920/bm2016.0233. [DOI] [PubMed] [Google Scholar]
- Skórka A, Pieścik-Lech M, Kołodziej M, and Szajewska H. 2017. “Infant formulae supplemented with prebiotics: Are they better than unsupplemented formulae? An updated systematic review.” Br J Nutr 119 (7): 810–825. 10.1017/s0007114518000120. [DOI] [PubMed] [Google Scholar]
- Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, Scott KP, Holscher HD, Azad MB, Delzenne NM, and Sanders ME. 2020. “The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics.” Nat Rev Gastroenterol Hepatol 17 (11): 687–701. 10.1038/s41575-020-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veereman-Wauters G, Staelens S, Van de Broek H, Plaskie K, Wesling F, Roger LC, McCartney AL, and Assam P. 2011. “Physiological and bifidogenic effects of prebiotic supplements in infant formulae.” J Pediatr Gastroenterol Nutr 52 (6): 763–71. 10.1097/MPG.0b013e3182139f39. [DOI] [PubMed] [Google Scholar]
- Vivatvakin Boosba, Mahayosnond Atchara, Theamboonlers Apiradee, Steenhout Philippe G., and Conus Nelly J.. 2010. “Effect of a whey-predominant starter formula containing LCPUFAs and oligosaccharides (FOS/GOS) on gastrointestinal comfort in infants.” Asia Pacific Journal of Clinical Nutrition 19 (4): 473–480. http://search.ebscohost.com/login.aspx?direct=true&db=rzh&AN=104993938&site=ehost-live. [PubMed] [Google Scholar]
- Wernimont Susan, Northington Robert, Kullen Martin J., Yao Manjiang, and Bettler Jodi. 2015. “Effect of an α-Lactalbumin-Enriched Infant Formula Supplemented With Oligofructose on Fecal Microbiota, Stool Characteristics, and Hydration Status: A Randomized, Double-Blind, Controlled Trial.” Clinical Pediatrics 54 (4): 359–370. 10.1177/0009922814553433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Infant and young child feeding. Last Modified June 9, 2021. Accessed November 3, 2021. https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding
- Wiciński M, Sawicka E, Gębalski J, Kubiak K, and Malinowski B. 2020. “Human Milk Oligosaccharides: Health Benefits, Potential Applications in Infant Formulas, and Pharmacology.” Nutrients 12 (1). 10.3390/nu12010266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu BB, Yang Y, Xu X, and Wang WP. 2016. “Effects of Bifidobacterium supplementation on intestinal microbiota composition and the immune response in healthy infants.” World J Pediatr 12 (2): 177–82. 10.1007/s12519-015-0025-3. [DOI] [PubMed] [Google Scholar]
- Xia Q, Williams T, Hustead D, Price P, Morrison M, and Yu Z. 2012. “Quantitative analysis of intestinal bacterial populations from term infants fed formula supplemented with fructo-oligosaccharides.” J Pediatr Gastroenterol Nutr 55 (3): 314–20. 10.1097/MPG.0b013e3182523254. [DOI] [PubMed] [Google Scholar]
- Yao M, Lien EL, Capeding MR, Fitzgerald M, Ramanujam K, Yuhas R, Northington R, Lebumfacil J, Wang L, and DeRusso PA. 2014. “Effects of term infant formulas containing high sn-2 palmitate with and without oligofructose on stool composition, stool characteristics, and bifidogenicity.” J Pediatr Gastroenterol Nutr 59 (4): 440–8. 10.1097/mpg.0000000000000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Zheng S, Lin K, Xu X, Lv L, Zhao Z, and Shao J. 2021. “Effects of Infant Formula Supplemented With Prebiotics and OPO on Infancy Fecal Microbiota: A Pilot Randomized Clinical Trial.” Front Cell Infect Microbiol 11: 650407. 10.3389/fcimb.2021.650407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, and Curtis N. 2020. “Breast milk microbiota: A review of the factors that influence composition.” J Infect 81 (1): 17–47. 10.1016/j.jinf.2020.01.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.