Abstract
Aims
To evaluate whether melatonin (MT) supplementation during in vitro maturation (IVM) of human oocytes can reverse the age-related decline in oocyte quality.
Main methods
We enrolled 172 patients aged ≥35 years (older reproductive-aged women) and 83 patients aged <35 years (young women) who underwent in vitro fertilization between 2019 and 2022. We conducted IVM with and without 10 μM MT in immature oocytes of different ages. Oocyte fertilization and embryo development were observed using a stereomicroscope. We assessed the immunofluorescence intensity of mitochondrial function, measured the copy number of mitochondrial DNA (mtDNA), and examined the spindle and chromosome composition in in vitro mature stage II (IVM-MII) oocytes using immunofluorescence and second-generation sequencing.
Key findings
MT supplementation significantly improved the redox level in the IVM medium and IVM-MII oocytes in older reproductive-aged women. It also significantly increased the proportion of circular mtDNA and the adenosine triphosphate content in IVM-MII oocytes. In addition, the IVM-MII oocytes obtained with MT supplementation showed a significant improvement in the normal composition of the spindle and chromosomes. Thus, the aged immature oocytes also showed significantly improved maturation and blastocyst formation rates owing to the role of MT.
Significance
Supplementation with 10 μM MT in the IVM medium reverses the age-related decline in oocyte quality. Our findings provide a viable solution for enhancing fertility in older reproductive-aged women.
Keywords: Melatonin, Human oocyte, Older reproductive-aged women, In vitro maturation, Mitochondrial function
1. Introduction
Assisted reproductive technology (ART) has made significant progress in the treatment of infertility. However, dealing with the decline in the number and quality of oocytes caused by ovarian aging in older women remains a challenge for ART. Female fertility begins to decline at the age of 35 and further declines after reaching 40 years old [1,2]. The live birth rate per oocyte is 26% for women younger than 35 years, 24% for women aged 35 years, and 1% for those older than 42 years [3]. In addition, the incidence of abortion, congenital birth defects, and fetal aneuploidy is higher in women older than 35 years [4]. Embryonic aneuploidy is mainly related to impaired microtubule assembly during spindle formation, which is one of the most energy-consuming processes in meiosis [5]. Therefore, insufficient adenosine triphosphate (ATP) synthesis in mitochondria is considered a potential cause of abnormal karyotypes [6]. Reactive oxygen species (ROS) are generated during the oxidative phosphorylation process in mitochondria [7]. While ROS are essential for intracellular signal transmission, they are also highly reactive, causing damage to vital components of cells, such as DNA and proteins. The molecular damage caused by increased ROS production is known as oxidative stress [8]. The cumulative impact of oxidative stress, caused by both congenital and environmental factors, is considered the primary cause of aging [9]. Consequently, mitochondrial dysfunction and increased oxidative stress are the key factors contributing to the decline in oocyte developmental potential associated with aging [10].
Melatonin (MT) is a tryptophan derivative primarily secreted by the pineal gland at night [11,12]. In addition to regulating circadian rhythms and circulatory cycles, MT possesses potent antioxidant functions, such as directly scavenging free radicals and regulating antioxidant enzymes via receptors [13,14]. Notably, MT is present in the follicular fluid produced by ovaries and granulosa cells [15]. Across various mammalian species, MT plays crucial roles in follicular development and the regulation of ovarian function [16,17]. Extensive research has investigated the beneficial effects of MT on female fertility [18]. A recent report showed that MT concentration in follicular fluid can serve as an indicator for predicting ovarian reserve and in vitro fertilization (IVF) results [19]. Another study reported low MT concentrations in the follicular fluid of older women of reproductive age [20]. Therefore, exogenous MT supplementation in vivo or in vitro may represent an effective therapeutic approach for improving fertility. In a prospective, longitudinal cohort study involving 46 older reproductive-aged women (38–42 years), a statistically higher number of mature oocytes, fertilization rate, and both total and high-quality embryos transferred were observed compared to their previous IVF cycle. However, no difference was observed in the number of retrieved oocytes [20]. The effectiveness of oral MT for improving oocyte quality has been reported. Nevertheless, the direct impact of MT on advanced oocytes through in vitro maturation (IVM) culture requires further investigation. Recently, it has been suggested that including MT during IVM can enhance the maturation of immature human oocytes and contribute to the development of healthy offspring [21]. Based on these findings, it is worth investigating whether IVM cultures supplemented with MT can effectively counteract the decline in gamete quality observed in older women of reproductive age.
Therefore, we designed an IVM study using immature oocytes from older reproductive-aged women to examine the redox levels, mitochondrial function, euploid status, and developmental potential of in vitro-matured metaphase II (IVM-MII) oocytes. This study aimed to explore the potential benefits of MT supplementation in improving oocyte quality and subsequent developmental outcomes, specifically within this age group.
2. Materials and methods
2.1. Ethical approval and patients
This study was approved by the Ethics Committee of Anhui Medical University (No. 2015013). All participating patients signed a written informed consent for the use of normally discarded immature oocytes in this research.
This study population consisted of women who underwent intracytoplasmic single sperm injection (ICSI) at our center between 2019 and 2022. However, certain patient groups, such as those with chromosomal abnormalities, immune disorders, and pelvic tumors, were excluded from the study.
2.2. Ovarian stimulation
All patients underwent ovarian stimulation, either long-term or short-term, involving the administration of a combination of GnRH analogs and r-hFSH for superovulation. When at least 2–3 oocytes reached a diameter of 18 mm or greater, patients received an intramuscular injection of 10,000 IU hCG (Lizhu Pharmaceutical Trading Co., Ltd., Zhuhai, China). Following hCG injection, transvaginal ultrasound-guided cumulus-oocyte complexes retrieval was performed at 36 h [22].
2.3. Oocyte collection and IVM
We collected 129 immature oocytes at the germinal vesicle (GV) stage from 83 young patients (age <35 years) and 265 immature oocytes from 172 older reproductive-aged patients (age ≥35 years). After ultrasound-guided follicle extraction, the corona cumulus complexes of the oocytes were collected. Subsequently, these cumulus complexes were partially denuded using hyaluronidase (VitroLife, Gotebor, Sweden) to assess the developmental stage (MII, MI, or GV stage). All GV oocytes with sparse cumulus cells were collected and placed into IVM medium, with or without MT, for 24 h of in vitro culture at 37 °C and 6% CO2 [22]. Only the oocytes that developed to the MII stage were used for subsequent experiments.
2.4. Study design
Based on our previous study, 10 μM MT was found to be an optimal and safe concentration for the IVM culture of human oocytes [21]. In the present study, the collected GV oocytes were divided into three groups based on the patient's age and the addition of MT in the IVM medium: the Young group (age <35 years without MT), Aged group (age ≥35 years without MT), and Aged + MT group (age >35 years with MT supplementation). In the latter two groups, oocytes from patients of the same age were randomly and equally assigned.
2.5. Determination of ROS and reduced glutathione levels in oocytes
The amount of ROS generated in the oocytes was evaluated using a dichlorofluorescin diacetate (DCFH-DA) assay kit (S0033S; Beyotime Biotechnology Inc., China). Briefly, oocytes were incubated in a medium containing an oxidation-sensitive fluorescent probe (DCFH-DA) at 37 °C for 30 min in the dark and then washed three times with phosphate-buffered saline (PBS). Upon transfer to a glass medium, the oocytes were observed under a laser scanning microscope (LSM 800; Zeiss, Germany). Fluorescent photographs of the oocytes were obtained using single-layer scanning, and the image acquisition parameters were consistent for all examined oocytes. The fluorescence intensity of each oocyte was measured using the ZEN software (Carl Zeiss IMT Co., Ltd., Germany).
Glutathione (GSH) levels in oocytes were assessed using CellTracker™ Fluorescent Probes (C12881; Thermo Fisher Scientific, USA). Each group of tested oocytes was stained with 10 μM CellTracker for 30 min at 37 °C in the dark and subsequently washed with PBS to remove surface fluorescence. The fluorescence intensity was measured using a laser scanning confocal microscope. The acquired photographs were analyzed using the ZEN software to measure the staining brightness of each oocyte.
2.6. Determination of ROS and GSH levels in IVM medium
The IVM culture medium was divided into two groups: a control group that did not receive MT intervention and an MT group supplemented with melatonin. The oocytes obtained on designated days were evenly distributed between the two culture groups. For instance, if Patient A had four GV oocytes, two were placed in the IVM culture medium of the MT group, while the remaining two were assigned to the control group. After a 24-h culture period for the collected GV oocytes in IVM medium, ensuring a minimum of 5 oocytes in 500 μl of culture medium, the IVM culture medium from both the control and MT groups was collected. Subsequently, ROS and GSH levels in the collected IVM culture medium were promptly measured.
The ROS levels were measured using a serum reactive oxygen species assay kit (BB-475016; BestBio, China). For each sample, 10 μl was added to a 96-well plate, followed by 10–20 μl of the O13 probe. The wells were mixed by pipetting, and the plates were incubated at 37 °C for 30 min at room temperature in the dark. Fluorescence intensity was measured using a fluorescence photometer at an excitation wavelength of 535 nm and emission wavelength of 610 nm.
GSH levels were measured using a reduced-glutathione content kit (LCSSH-0206F; Lun Chang Shuo Biotech, China). The spectrophotometer was then preheated for 30 min. The wavelength was adjusted to 412 nm, and distilled water was used for zeroing. The samples were then added to the detection reagents and analyzed directly using a spectrophotometer.
2.7. DNA extraction and real-time quantitative PCR
Total DNA was extracted from single isolated IVM-MII oocytes using the REPLI-g Single Cell Kit (HY16119; Qiagen, Germany). Three oocytes were extracted from the Young, Aged, and Aged + MT groups. The extracted DNA was equally divided into two samples, and one DNA sample was subjected to digestion using a nuclease (Exonuclease V; New England Biolabs Inc.) to remove all linear DNA from the mitochondria [23]. The specific digestion systems are presented in Supplemental Table 1.
DNA samples before and after nuclease treatment were used as templates to determine the copy number of mitochondrial DNA (mtDNA) by real-time quantitative polymerase chain reaction (qPCR), using SYBR green DNA intercalator on the Roche LightCycler®480 II (Roche, USA), in a 20-μl reaction volume. The reactions were performed as follows: initial denaturing at 95 °C for 5 min, followed by 40 cycles at 95 °C for 30 s and 60 °C for 30 s [24]. The reference primer used was GAPDH, and 3243 primers were used for mtDNA quantification (Supplemental Table 2).
2.8. Detection of ATP content in oocytes
The ATP content was determined using an enhanced ATP assay kit (S0026; Beyotime Biotechnology Inc.) according to the manufacturer's instructions. After washing three times with PBS, 10 oocytes were placed into polymerase chain reaction (PCR) tubes containing 20 μl of ATP lysate and then stored at −80 °C for future examination. Briefly, the ATP test working solution was added to a dark 96-well plate with 100 μl per well and incubated at room temperature for 5 min. The samples were then transferred to each well, mixed, and. Examined using a luminometer. The ATP content was calculated using linear regression of the generated ATP standard curve. The experiment was independently repeated three times during this period.
2.9. Observation of spindle morphology
The spindles of IVM-MII oocytes were stained using immunofluorescence. The collected oocytes were fixed with 4% paraformaldehyde for 30 min, permeabilized with 0.2% Triton-X-100 for 25 min, blocked with 1% bovine serum albumin in PBS for 1 h, and incubated overnight at 4 °C with anti-α-tubulin-FITC (1:300) primary antibody. Subsequently, the oocytes were washed three times in PBS with 0.01% Triton-X 100 and 1% Tween 20 and counterstained with DAPI for 10 min. After staining, the oocytes were immediately observed under a laser scanning microscope.
2.10. Examination of aneuploidy
To examine aneuploidy, 18 IVM-MII oocytes were collected from the three groups (Young group [n = 6], Aged group [n = 6], and Aged + MT group [n = 6]). Subsequently, we removed the first polar body through micromanipulation. Finally, the zona pellucida was digested to examine aneuploidy using next-generation sequencing (NGS) technology.
2.11. Oocyte development
IVM-MII oocytes were collected and subjected to ICSI using donated sperms, followed by early embryonic culture. The same embryologist evaluated the developmental status of fertilized oocytes throughout the experiment.
Embryo development during the cleavage stage was examined on day 3 of in vitro culture. The number of blastomeres and the degree of fragmentation were carefully assessed. Embryos with 7–9 blastomere and less than 20% fragmentation were classified as high quality. Subsequently, the embryos were transferred to a blastocyst culture medium for further incubation lasting 2–3 days. On days 5 and 6, the resulting blastocysts were evaluated using the Gardner blastocyst grading method. High-quality blastocysts were defined as those with a score of 3BB or higher on day 5 and 4BB or higher on day 6 [25].
2.12. Statistical analysis
Categorical data regarding developmental potential are presented as counts and percentages and analyzed using the chi-square test. The remaining data are expressed as mean ± standard deviation and analyzed using a one-way analysis of variance, followed by Bonferroni tests. All data analyses were performed using GraphPad Prism 8.0 software (GraphPad Software, San Diego, CA, USA), with a significance level set at P < 0.05.
3. Results
3.1. MT intervention prevents age-related oxidative stress damage in oocytes
We aimed to investigate whether human oocytes are subjected to increased oxidative stress with age and whether this can be reversed by MT supplementation.
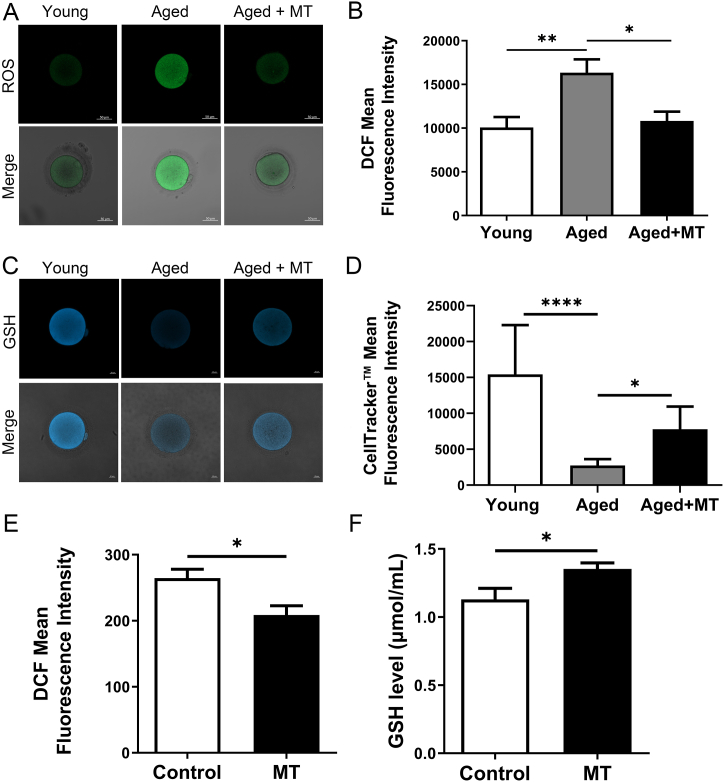
As expected, ROS levels in oocytes were significantly higher in the Aged group than in the Young group (P < 0.05), indicating that oocytes from aged women were vulnerable to oxidative stress. However, in the Aged + MT group, ROS levels were significantly lower compared to the Aged group (P < 0.05). Notably, there were no significant difference in ROS levels between the Young and Aged + MT groups (P > 0.05; Fig. 1A and B). Similarly, GSH levels in oocytes were significantly lower in the Aged group compared to the Young group (P < 0.05). However, in the Aged + MT group, GSH levels were significantly higher compared to the Aged group (P < 0.05; Fig. 1C and D).
Fig. 1.
Effects of MT intervention on ROS and GSH levels in IVM-MII oocytes and IVM culture medium. (A) Representative images of ROS levels in oocytes in the Young, Aged, and Aged + MT groups. (B) Differences in ROS levels among the three groups. Nine oocytes in each group from more than three independent experiments were tested. (C) Representative images of GSH levels in oocytes in the Young, Aged, and Aged + MT groups. (D) Differences in GSH levels among the three groups. Ten oocytes in each group from more than three independent experiments were tested. (E) Difference in ROS levels in IVM medium between the control and MT groups. Ten parts culture medium in each group from more than three independent experiments were tested. (F) Difference in GSH levels in IVM medium between the control and MT groups. Ten parts culture mediums in each group from more than three independent experiments were tested. Scale bar = 50 μm *:P < 0.05, **:P < 0.01, ***:P < 0.001, * ***:P < 0.0001.
In addition to the oocyte itself, the environment of the IVM culture medium affects intracellular oxidative stress. Therefore, we measured the ROS and GSH levels in the culture media of the control and MT groups. ROS levels in the MT group were significantly lower compared to the control group, while GSH levels were higher than those in the control group (both P < 0.05, Fig. 1E and F). These results suggest that MT supplementation improves oocyte quality in older women of reproductive age by reducing oxidative stress.
3.2. MT intervention increases ATP production by increasing the proportion of circular mtDNA in aged oocytes
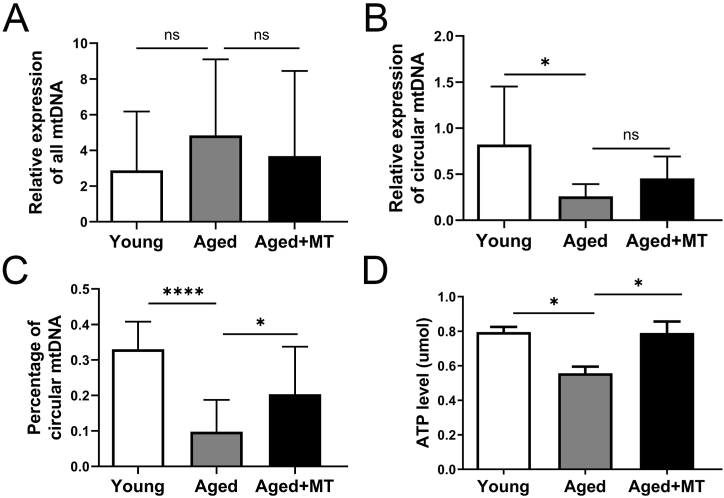
Mitochondrial function is closely related to mtDNA copy number. mtDNA exists in circular and linear forms, with linear mtDNA being an intermediate product of damaged double-stranded mtDNA degradation. The percentage of circular mtDNA reflects the mitochondrial function of cells to some extent [23,24]. Oxidative damage can impair mitochondrial function [26], and MT intervention can reduce oxidative stress in oocytes. Therefore, our objective was to determine whether MT intervention could increase the percentage of circular mtDNA in older women of reproductive age, thereby improving mitochondrial function. Analysis of a single IVM-MII oocyte using qPCR revealed no significant differences in the copy number of all mtDNA among the three groups (P > 0.05, Fig. 2A). We observed a significant decrease in the proportion of circular mtDNA in oocytes from the Aged group compared to the Young group (P < 0.05; Fig. 2B). However, following MT intervention, there was an increase in the amount of circular mtDNA (P > 0.05, Fig. 2B) and the percentage of circular mtDNA in aged oocytes (P < 0.05, Fig. 2C).
Fig. 2.
MT intervention increases the proportion of circular mtDNA and ATP levels in IVM-MII oocytes of older reproductive-aged women. (A) Relative expression of mtDNA in oocytes in the Young, Aged, and Aged + MT groups. (D) Relative expression of circular mtDNA in oocytes in the Young, Aged, and Aged + MT groups. (C) Rate of circular mtDNA in oocytes in the Young, Aged, and Aged + MT groups. Six oocytes in each group from more than three independent experiments were tested. (D) ATP levels were measured in oocytes in the Young, Aged, and Aged + MT groups. Six oocytes in each group from three independent experiments were tested.
In eukaryotic cells, ATP production primarily depends on the mitochondria [27]. To assess whether oocyte ATP production declines with age and can be restored by MT intervention, we investigated the level of ATP in the three groups. Our findings revealed that oocytes in the Aged group exhibited a lower ATP content than those in the Young group (P < 0.05, Fig. 2D). Additionally, the ATP content was significantly higher in the Aged + MT group than in the Aged group (P < 0.05). There was no significant difference in ATP content between the Young and Aged + MT groups (P > 0.05). These results suggest that MT intervention improves ATP production by increasing circular mtDNA.
3.3. MT intervention ameliorates age-related meiotic defects in oocytes by increasing ATP production
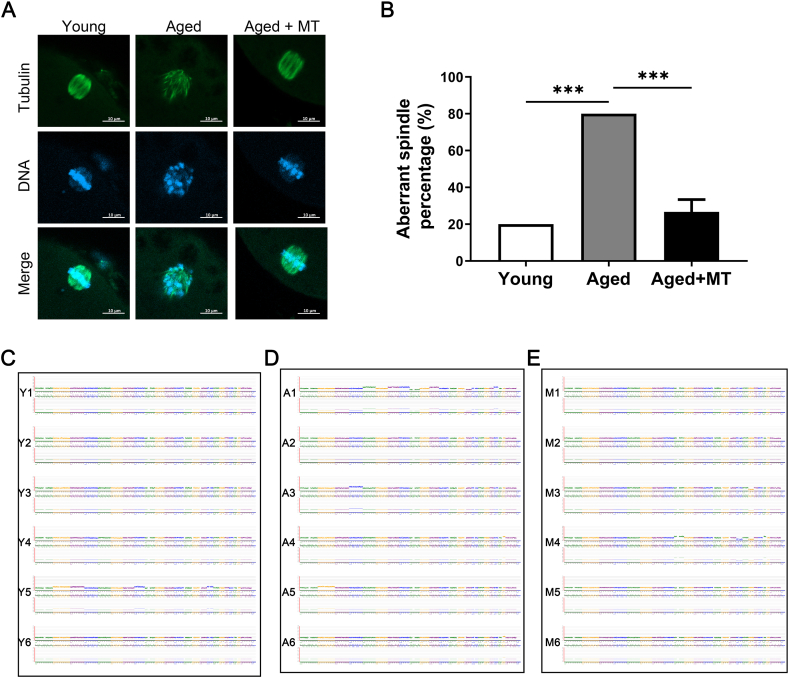
Chromosomal abnormalities are primarily associated with spindle assembly, which is one of the most energy-intensive steps in meiosis. Consequently, insufficient ATP synthesis may lead to chromosomal abnormalities [28]. The amount of ATP in oocytes gradually decreases with age, and MT intervention can rescue this trend. Therefore, we investigated whether human oocytes' spindle structure and chromosomal alignment were damaged with advancing age, leading to aneuploidy and whether MT intervention could rescue this damage. The proportion of oocytes with spindle disorders was significantly higher in the Aged group than in the Young group (P < 0.05; Fig. 3B). However, the proportion of oocytes with spindle disorders was lower in the Aged + MT group than in the Aged group (P < 0.05; Fig. 3A and B). The NGS results supported this finding (Fig. 3C–E). These results suggest that MT intervention reduces the incidence of chromosomal abnormalities in oocytes from older reproductive-aged women by increasing ATP production.
Fig. 3.
MT ameliorates the meiotic defects of IVM-MII oocytes from older reproductive-aged women. (A) Representative images of spindle morphology and chromosomal alignment in oocytes in the Young, Aged, and Aged + MT groups. Spindles were stained with an antibody against α-tubulin (green), and chromosomes were counter-stained with Hoechst (blue). Scale bar = 10 μm. (B) Rate of aberrant spindles with misaligned chromosomes in oocytes in the Young, Aged, and Aged + MT groups. Fifteen oocytes in each group from more than three independent experiments were tested. (C) Next-generation sequencing (NGS) graph shows the results of aneuploidy detection for oocytes in the Young group (01–06). 01–04, and 06: normal karyotype; 05: −2. (D) NGS graph shows the results of aneuploidy detection for oocytes in the Aged group (C1–C6). C2: normal karyotype; C1: −9; C3: +4; C4 and C6: +22; C5: +2. (E) NGS graph shows the results of aneuploidy detection for oocytes in the Aged + MT group (M1–M6). M1–M2 and M5–M6: normal karyotype; M3: −18; M4: +9. There were six IVM-MII oocytes in each group, and the oocytes corresponding to the serial numbers of the Aged and Aged + MT groups originated from the same woman. Each NGS graph indicates the copy number assignments on the y-axis and chromosome number on the x-axis. Trisomy and monosomy were denoted as +and –, respectively.
3.4. MT intervention improves the developmental potential of oocytes in older reproductive-aged women
Finally, to investigate whether MT intervention improves the developmental potential of oocytes from older reproductive-aged women, we collected 160 IVM-MII oocytes (Young group [n = 51], Aged group [n = 55], and Aged + MT group [n = 54]). The basic clinical information of each group is shown in Table 1. Developmental parameters (rates of maturation, fertilization, cleavage, high-quality embryos, blastocyst formation, and high-quality blastocysts) are shown in Table 2. We observed that the developmental potential of the Aged group was significantly lower than that of the Young group, especially regarding the rates of maturation, blastocyst formation, and high-quality blastocysts (all P < 0.05). Developmental parameters in the Aged + MT group were higher than those in the Aged group, with significant differences observed in the rates of maturation and blastocyst formation (both P < 0.05). However, there were no significant differences in any developmental parameters between the Young and Aged + MT groups (all P > 0.05). These results suggest that the developmental potential of oocytes decreases with age and that MT intervention rescues this age-related degradation of developmental potential.
Table 1.
Basic clinical information of patients.
| Index | Young group | Aged group | Aged + MT group | Adjusted P1-value | Adjusted P2-value | Adjusted P3-value |
|---|---|---|---|---|---|---|
| Retrieved oocytes (n) | 51 | 55 | 54 | – | – | – |
| Age(years) | 27.23 ± 2.74 | 37.68 ± 2.56 | 36.60 ± 3.50 | <0.0001 | <0.0001 | 0.8406 |
| Infertility duration(years) | 2.23 ± 1.01 | 5.24 ± 3.78 | 3.50 ± 2.12 | 0.0134 | >0.9999 | 0.3801 |
| BMI (kg/m2) | 22.42 ± 3.24 | 22.07 ± 2.15 | 23.33 ± 2.86 | >0.9999 | >0.9999 | 0.5078 |
| FSH (IU/L) | 7.48 ± 1.87 | 7.77 ± 2.40 | 7.97 ± 2.09 | >0.9999 | >0.9999 | >0.9999 |
| LH (IU/L) | 11.62 ± 6.85 | 4.87 ± 2.59 | 4.67 ± 3.63 | <0.0001 | 0.0004 | >0.9999 |
| E2 (pmol/L) | 59.84 ± 93.28 | 372.7 ± 1262 | 233.3 ± 300.4 | >0.9999 | >0.9999 | >0.9999 |
| P (nmol/L) | 84.98 ± 84.83 | 4.17 ± 8.74 | 2.07 ± 0.82 | <0.0001 | <0.0001 | >0.9999 |
| PRL (ng/ml) | 8.46 ± 9.34 | 12.52 ± 5.65 | 23.35 ± 46.35 | >0.9999 | 0.2214 | 0.3709 |
| T (nmol/L) | 1.42 ± 1.19 | 3.46 ± 10.40 | 1.16 ± 0.45 | >0.9999 | >0.9999 | >0.9999 |
P1: Young group & Aged group P2: Young group & Aged + MT group P3: Aged group & Aged + MT group. BMI: Body mass index; FSH: Follicle-stimulating hormone; LH: Luteinizing hormone; E2: Estradiol; P: Progesterone; PRL: Prolactin; T: Testosterone; HCG: Human chorionic gonadotropin. Measurement data are presented as means ± standard deviations (SD); comparisons between two groups were performed using the t-test.
Table 2.
Results of early embryo development in different groups.
| Index | Young group | Aged group | Aged + MT group | P1-value | P2-value | P3-value |
|---|---|---|---|---|---|---|
| Retrieved oocytes (n) | 51 | 55 | 54 | – | – | – |
| Maturation rate | 76.47(39/51) | 43.64(24/55) | 75.93(41/54) | 0.0006* | 0.9478 | 0.0006* |
| Fertility rate (%) | 74.36(29/39) | 75(18/24) | 75.61(31/41) | 0.9547 | 0.8972 | 0.9561 |
| Cleavage rate (%) | 93.1(27/29) | 83.33(15/18) | 96.77(30/31) | 0.5691 | 0.9527 | 0.2647 |
| High-quality embryo rate (%) | 37.04(10/27) | 33.33(5/15) | 50(15/30) | 0.8103 | 0.3247 | 0.2888 |
| Blastocyst formation rate (%) | 51.85(14/27) | 13.33(2/15) | 53.33(16/30) | 0.033* | 0.9109 | 0.0098* |
| High-quality blastocyst rate (%) | 25.93(7/27) | 0(0/15) | 20(6/30) | 0.0382* | 0.5944 | 0.1629 |
P1: Young group & Aged group P2: Young group & Aged + MT group P3: Aged group & Aged + MT group. High-quality embryo: 7–9 blastomeres at equal size on day 3, with no fragmentation or less than 15%. High-quality blastocyst: embryos that were ≥3BB on day 5 or ≥4BB on day 6 by the blastocyst grading system according to Gardner's criteria. Enumeration data were reported as percentages and compared by the χ2 test; *:P < 0.05, difference was statistically significant.
4. Discussion
The global changes in social, economic, and technological circumstances have resulted in a trend of delayed childbearing. One consequence of this delay is that older women of reproductive age are at an increased risk of infertility, miscarriage, fetal aneuploidy, stillbirth, and congenital disabilities [4]. With the continuous development of ART, an increasing number of families have successfully achieved healthy offspring. Older reproductive-aged women rely more on this technology than their young counterparts. However, the quality of their embryos is often lower compared to younger women, which is mainly attributed to the fact that older women have fewer oocytes and a higher incidence of aneuploidy. Therefore, optimizing the current culture system and improving the use of oocytes to enhance the cycle outcomes of older reproductive-aged women is an urgent problem that needs to be solved using ART.
In our previous studies, we found that the developmental potential of oocytes could be improved by supplementing the IVM medium with 10 μM MT [21]. Due to the scarcity of oocyte retrieval in older reproductive-aged women and the low maturation rate of immature oocytes in IVM, the number of aged oocytes in our previous experimental samples was small. Therefore, we investigated whether the addition of MT had the same effect on aged oocytes. For this purpose, we utilized 265 oocytes from older reproductive-aged women, of which 109 were analyzed to assess their developmental potential. Consistent with the results of our previous studies, the addition of MT significantly improved the rates of maturation, fertilization, cleavage, high-quality embryo formation, blastocyst formation, and high-quality blastocyst formation in aged oocytes. Therefore, MT plays a role in improving the developmental potential of oocytes in older women of reproductive age.
Furthermore, we found that MT significantly reduced the occurrence of aneuploidy in IVM cultures of aged oocytes, primarily due to the increased production of ATP and the balance of antioxidative stress. Oocytes experience oxidative stress with age, as shown by the age-dependent decrease in MT levels in the follicular fluid, where follicles grow and mature [19]. Elevated ROS levels are important indicators of oxidative stress, which is one of the mechanisms underlying aging. In our study, the elevated intracellular ROS levels in the Aged group indicated that oocytes from older reproductive-aged women were subjected to oxidative damage. MT is an antioxidant that inhibits ROS generation, thereby preventing damage to aged oocytes. An animal model of oocyte exposure to exogenous MT in aged mice in vivo and in vitro showed the scavenging of accumulated ROS [29]. During in vitro culture of aged oocytes, the oocytes produce more ROS and less GSH, and this excess ROS flows into the culture fluid, which can disrupt the redox homeostasis of the culture environment and be detrimental to oocyte development. The addition of MT neutralizes excess ROS in the culture medium, thereby maintaining the culture medium's redox homeostasis and optimizing the oocytes' survival environment. In addition, MT can directly enter the cells and neutralize excess ROS. Oxidative damage is closely associated with mitochondrial dysfunction [30]. One of the main functions of the mitochondria is to generate energy for many cellular processes. In this study, at an older age, the proportion of circular mtDNA in oocytes decreased, and ATP production was reduced. Excessive ROS may damage part of the mtDNA and adversely affect ATP by damaging the electron transport chain and tricarboxylic acid cycle [31]. MT increased the expression of SIRT1 and LC3, which are associated with cellular homeostasis and autophagy, during in vitro culture of aging mouse oocytes. In addition, MT supplementation increased ATP content, improved mitochondrial function, and enhanced autophagy process [32].
In the present study, we observed that the addition of MT positively affected oocyte health. Specifically, MT supplementation reduced intracellular ROS levels, thereby minimizing mitochondrial damage. This reduction in ROS levels contributes to an increased proportion of circular mitochondrial DNA in the oocytes, indicating improved mitochondrial function. Ultimately, this improvement in mitochondrial function increases ATP production in the oocytes. Increased ATP supply during the first meiotic division of oocytes reduces the probability of chromosomal segregation errors. This process reduces the incidence of chromosomal abnormalities in the oocytes of older reproductive-aged women, thereby laying the foundation for better embryo development and cycle outcomes. In addition, mitochondria are maternally inherited, while sperm mitochondrial proteins degrade through autophagy following their entry into the oocyte [[33], [34], [35]]. As a result, mitochondria derived from oocytes become the primary source of ATP during preimplantation embryonic development [36]. This finding helps explain why MT supplementation protects the mitochondrial function of oocytes from older reproductive-aged women and their subsequent developmental potential.
Due to the limited sample size, we could not investigate the detailed mechanisms underlying MT supplementation. Therefore, further exploration of this mechanism is necessary for future studies.
5. Conclusions
Overall, this study suggests that supplementation with 10 μM MT in an IVM medium reverses the age-related decline in oocyte quality. Our findings provide a viable solution for enhancing fertility in women of reproductive age.
Funding
Supported by National Natural Science Foundation of China (No.82071724), 145 National Key R&D Program (No. 2022YFC2703001) and Wu Jieping Medical Foundation (No. 320.6750.2022-06-45).
Author contribution statement
Dandan Yang: Yaoqin Mu: Jing Wang: Performed the experiments; Wrote the paper. Weiwei Zou: Huijuan Zou: Analyzed and interpreted the data. Han Yang: Chao Zhang: Yongqi Fan: Heng Zhang: Huan Zhang: Contributed reagents, materials, analysis tools or data. Beili Chen: Zhiguo Zhang: Conceived and designed the experiments.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful to the patients their willingness to donate their immature discarded oocytes.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19366.
Contributor Information
Beili Chen, Email: aydchenbeili@ahmu.edu.cn.
Zhiguo Zhang, Email: zhangzhiguo@ahmu.edu.cn.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Eijkemans M.J.C., Van Poppel F., Habbema D.F., Smith K.R., Leridon H., Te Velde E.R. Too old to have children? Lessons from natural fertility populations. Hum. Reprod. 2014;29:1304–1312. doi: 10.1093/humrep/deu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan T.Y., Lau M.S.K., Loh S.F., Tan H.H. Female ageing and reproductive outcome in assisted reproduction cycles. Singap. Med. J. 2014;55:305–309. doi: 10.11622/smedj.2014081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silber S.J., Kato K., Aoyama N., Yabuuchi A., Skaletsky H., Fan Y., Shinohara K., Yatabe N., Kobayashi T. Intrinsic fertility of human oocytes. Fertil. Steril. 2017;107:1232–1237. doi: 10.1016/j.fertnstert.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Magnus M.C., Wilcox A.J., Morken N.H., Weinberg C.R., Håberg S.E. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ. 2019;364:1–8. doi: 10.1136/bmj.l869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eichenlaub-Ritter U., Staubach N., Trapphoff T. Chromosomal and cytoplasmic context determines predisposition to maternal age-related aneuploidy: brief overview and update on MCAK in mammalian oocytes. Biochem. Soc. Trans. 2010;38:1681–1686. doi: 10.1042/BST0381681. [DOI] [PubMed] [Google Scholar]
- 6.Wiernsperger N., Nivoit P., Kraemer De Aguiar L.G., Bouskela E. Microcirculation and the metabolic syndrome. Microcirculation. 2007;14:403–438. doi: 10.1080/10739680701285617. [DOI] [PubMed] [Google Scholar]
- 7.Figueira T.R., Barros M.H., Camargo A.A., Castilho R.F., Ferreira J.C.B., Kowaltowski A.J., Sluse F.E., Souza-Pinto N.C., Vercesi A.E. Mitochondria as a source of reactive oxygen and nitrogen species: from molecular mechanisms to human health. Antioxidants Redox Signal. 2013;18:2029–2074. doi: 10.1089/ars.2012.4729. [DOI] [PubMed] [Google Scholar]
- 8.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 9.Perkins A.T., Das T.M., Panzera L.C., Bickel S.E. Oxidative stress in oocytes during midprophase induces premature loss of cohesion and chromosome segregation errors. Proc. Natl. Acad. Sci. U.S.A. 2016;113:E6823–E6830. doi: 10.1073/pnas.1612047113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.May-Panloup P., Boucret L., de la Barca J.M.C., Desquiret-Dumas V., Ferré-L’Hotellier1 V., Morinière C., Descamps P., Procaccio V., Reynier P. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum. Reprod. Update. 2016;22:725–743. doi: 10.1093/humupd/dmw028. [DOI] [PubMed] [Google Scholar]
- 11.Reiter R.J., Tan D.X., Galano A. Melatonin: exceeding expectations. Physiology. 2014;29:325–333. doi: 10.1152/physiol.00011.2014. [DOI] [PubMed] [Google Scholar]
- 12.Reiter R.J., Mayo J.C., Tan D.X., Sainz R.M., Alatorre-Jimenez M., Qin L. Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 2016:253–278. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- 13.Albarrán M.T., López-Burillo S., Pablos M.I., Reiter R.J., Agapito M.T. Endogenous rhythms of melatonin, total antioxidant status and superoxide dismutase activity in several tissues of chick and their inhibition by light. J. Pineal Res. 2001;30:227–233. doi: 10.1034/j.1600-079X.2001.300406.x. [DOI] [PubMed] [Google Scholar]
- 14.Okatani Y., Wakatsuki A., Kaneda C. Melatonin increases activities of glutathione peroxidase and superoxide dismutase in fetal rat brain. J. Pineal Res. 2000;28:89–96. doi: 10.1034/j.1600-079X.2001.280204.x. [DOI] [PubMed] [Google Scholar]
- 15.Reiter R.J., Tamura H., Tan D.X., Xu X.Y. Melatonin and the circadian system: contributions to successful female reproduction. Fertil. Steril. 2014;102:321–328. doi: 10.1016/j.fertnstert.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L., Zhang Z., Wang J., Lv D., Zhu T., Wang F., Tian X., Yao Y., Ji P., Liu G. Melatonin regulates the activities of ovary and delays the fertility decline in female animals via MT1/AMPK pathway. J. Pineal Res. 2019;66:1–12. doi: 10.1111/jpi.12550. [DOI] [PubMed] [Google Scholar]
- 17.Li Y., Liu H., Wu K., Liu H., Huang T., Chen Z.J., Zhao S., Ma J., Zhao H. Melatonin promotes human oocyte maturation and early embryo development by enhancing clathrin-mediated endocytosis. J. Pineal Res. 2019;67:1–12. doi: 10.1111/jpi.12601. [DOI] [PubMed] [Google Scholar]
- 18.Yong W., Ma H., Na M., Gao T., Zhang Y., Hao L., Yu H., Yang H., Deng X. Roles of melatonin in the field of reproductive medicine. Biomed. Pharmacother. 2021;144 doi: 10.1016/j.biopha.2021.112001. [DOI] [PubMed] [Google Scholar]
- 19.Jing T., Shile S., Sun Y., Li H., Li W.P., Cong Z., Chen Z.J. Melatonin levels in follicular fluid as markers for IVF outcomes and predicting ovarian reserve. Reproduction. 2017;153:443–451. doi: 10.1530/REP-16-0641. [DOI] [PubMed] [Google Scholar]
- 20.Unfer V., Raffone E., Rizzo P., Buffo S. Effect of a supplementation with myo-inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: a prospective, longitudinal, cohort study. Gynecol. Endocrinol. 2011;27:857–861. doi: 10.3109/09513590.2011.564687. [DOI] [PubMed] [Google Scholar]
- 21.Zou H., Chen B., Ding D., Gao M., Chen D., Liu Y., Hao Y., Zou W., Ji D., Zhou P., Wei Z., Cao Y., Zhang Z. Melatonin promotes the development of immature oocytes from the COH cycle into healthy offspring by protecting mitochondrial function. J. Pineal Res. 2020;68:1–13. doi: 10.1111/jpi.12621. [DOI] [PubMed] [Google Scholar]
- 22.Wang M., Yang Q., Liu J., Hu J., Li D., Ren X., Xi Q., Zhu L., Jin L. GVBD rate is an independent predictor for pregnancy in ICSI patients with surplus immature oocytes. Front. Endocrinol. 2023;13:1–10. doi: 10.3389/fendo.2022.1022044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma M.J.L., Zhang H., Jiang P., Sin S.T.K., Lam W.K.J., Cheng S.H., Lee W.S., Gai W., Olivia Tse O.Y., Peng W., Wong J., Raghupathy R., Wong R.S.M., Sahota D., Leung T.Y., Allen Chan K.C., Chiu R.W.K., Dennis Lo Y.M. Topologic analysis of plasma mitochondrial DNA reveals the coexistence of both linear and circular molecules. Clin. Chem. 2019;65:1161–1170. doi: 10.1373/clinchem.2019.308122. [DOI] [PubMed] [Google Scholar]
- 24.Peeva V., Blei D., Trombly G., Corsi S., Szukszto M.J., Rebelo-Guiomar P., Gammage P.A., Kudin A.P., Becker C., Altmüller J., Minczuk M., Zsurka G., Kunz W.S. Linear mitochondrial DNA is rapidly degraded by components of the replication machinery. Nat. Commun. 2018;9:1–11. doi: 10.1038/s41467-018-04131-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao H., Liu H., Li M., Wu K. Clinical outcomes following frozen-thawed blastocyst transfers with blastocysts derived from different cell numbers on day 3: a retrospective cohort study. J. Assist. Reprod. Genet. 2020;37:641–648. doi: 10.1007/s10815-019-01664-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slimen I.B., Najar T., Ghram A., Dabbebi H., Ben Mrad M., Abdrabbah M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review, Int. J. Hyperth. 2014;30:513–523. doi: 10.3109/02656736.2014.971446. [DOI] [PubMed] [Google Scholar]
- 27.Giacomello M., Pyakurel A., Glytsou C., Scorrano L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 2020;21:204–224. doi: 10.1038/s41580-020-0210-7. [DOI] [PubMed] [Google Scholar]
- 28.van der Reest J., Nardini Cecchino G., Haigis M.C., Kordowitzki P. Mitochondria: their relevance during oocyte ageing. Ageing Res. Rev. 2021;70:87–100. doi: 10.1016/j.arr.2021.101378. [DOI] [PubMed] [Google Scholar]
- 29.Zhang M., Lu Y., Chen Y., Zhang Y., Xiong B. Insufficiency of melatonin in follicular fluid is a reversible cause for advanced maternal age-related aneuploidy in oocytes. Redox Biol. 2020;28 doi: 10.1016/j.redox.2019.101327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kauppila T.E.S., Kauppila J.H.K., Larsson N.G. Mammalian mitochondria and aging: an update. Cell Metabol. 2017;25:57–71. doi: 10.1016/j.cmet.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 31.Brookes P.S., Yoon Y., Robotham J.L., Anders M.W., Sheu S.S. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol.: Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 32.Almohammed Z.N.H., Moghani-Ghoroghi F., Ragerdi-Kashani I., Fathi R., Tahaei L.S., Naji M., Pasbakhsh P. The effect of melatonin on mitochondrial function and autophagy in in vitro matured oocytes of aged mice. Cell J. 2020;22:9–16. doi: 10.22074/cellj.2020.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cummins J.M., Wakayama T., Yanagimachi R. Fate of microinjected spermatid mitochondria in the mouse oocyte and embryo. Zygote. 1998;6:213–222. doi: 10.1017/S0967199498000148. [DOI] [PubMed] [Google Scholar]
- 34.Hajjar C., Sampuda K.M., Boyd L. Dual roles for ubiquitination in the processing of sperm organelles after fertilization. BMC Dev. Biol. 2014;14 doi: 10.1186/1471-213X-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song W.H., Ballard J.W.O., Yi Y.J., Sutovsky P. Regulation of mitochondrial genome inheritance by autophagy and ubiquitin-proteasome system: implications for health, fitness, and fertility. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/981867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Babayev E., Seli E. Oocyte mitochondrial function and reproduction. Curr. Opin. Obstet. Gynecol. 2015;27:175–181. doi: 10.1097/GCO.0000000000000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.