Summary
Marine group II (MGII) is the most abundant planktonic heterotrophic archaea in the ocean. The evolutionary history of MGII archaea is elusive. In this study, 13 new MGII metagenome-assembled genomes were recovered from surface to the hadal zone in Challenger Deep of the Mariana Trench; four of them from the deep ocean represent a novel group. The optimal growth temperature (OGT) of the common ancestor of MGII has been estimated to be at about 60°C and OGTs of MGIIc, MGIIb, and MGIIa at 47°C–50ºC, 37°C–44ºC, and 30°C–37ºC, respectively, suggesting the adaptation of these species to different temperatures during evolution. The estimated OGT range of MGIIc was supported by experimental measurements of cloned β-galactosidase that showed optimal enzyme activity around 50°C. These results indicate that MGIIc may have originated from a common ancestor that lived in warm or even hot marine environment, such as hydrothermal vents.
Subject areas: Geomicrobiology, Microbiology, Aquatic biology
Graphical abstract

Highlights
-
•
MGIIc is a novel family based on metagenome-assembled genomes from the deep sea
-
•
Optimal growth temperature calculations suggest a thermophilic origin of MGIIc
-
•
The optimal growth temperature of the common ancestor of MGII is about 60°C
Geomicrobiology; Microbiology; Aquatic biology
Introduction
Early application of environmental 16S rRNA gene sequencing allowed the identification of marine planktonic archaea initially labeled as Marine Group I (MGI) and Marine Group II (MGII),1,2 which were later expanded by including Marine Groups III and IV.3,4 Among these groups, MGI (now called Thaumarchaeota) and MGII are much more abundant, with MGI dominating the lower photic zone and the deep ocean5 and MGII the epipelagic ocean.6,7
Still lacking a pure culture today, MGII were classified as MGIIa and MGIIb according to the phylogenetic analysis of their 16S rRNA genes.8,9,10 MGIIa is mainly distributed in epipelagic waters and MGIIb from surface to bathypelagic habitats in the global ocean.7,11 A third group, MGIIc, was tentatively identified based on only short reads of the 16S rRNA gene from the deep water (∼3000 m) of the Pacific Ocean12 and the Mariana Trench down to 8,727 m13; however, its phylogenetic position was uncertain and no genomic characterization was available.
Phylogenetic studies based on hundreds of metagenome-assembled genomes (MAGs) recovered from epi- and mesopelagic waters suggested MGII to be an order-level lineage with the proposed name Candidatus Poseidoniales, under which MGIIa is called Candidatus Poseidonaceae and MGIIb Candidatus Thalassarchaeaceae.14,15,16,17 Culture-independent metagenomic studies indicated that MGIIa and MGIIb are photoheterotrophs or chemoheterotrophs14,18,19,20 and their subclades have distinct biogeographic ranges.16,17
In the bathypelagic realm, MGII MAGs were found to be diverse in deep-sea hydrothermal vent plumes.19 For example, MGIIa, MGIIb as well as MGIIc were present in Mid-Cayman rising plumes, Guaymas Basin, and Eastern Lau Spreading Center.19 MGIIc was found in deep oceans including both hydrothermal vent plumes19 and hadal zones.13 However, due to the relatively low quality of MGIIc MAGs, little is known about their ecology and evolution.
The evolution of MGI has been discussed substantially, which was agreed that they derived from terrestrial hot springs and evolved into low temperature soil and the ocean.15,21,22,23,24,25 By contrast, the evolutionary origin of MGII, equally important in global ocean carbon cycle as marine Thaumarchaeota,6,7 is still unknown. Previous studies have demonstrated that environmental temperatures strongly influenced the GC composition of archaeal ribosomal RNA genes.26,27,28,29 The strong correlation between rRNA stem GC contents and optimal growth temperatures (OGTs) has been implemented as a molecular thermometer to infer the OGT of the archaeal ancestor.30 Therefore, it is feasible to calculate the OGTs of the uncultured MGII and their ancestors according to the GC contents of their 16S rRNA genes or stem sequences, which may provide insights into their living habitats.
Here we report 13 MGII MAGs recovered from surface to hadal waters in the Mariana Trench with three MGII MAGs from 8,000 m to 10,500 m water depths. One of them was ascribed to MGIIa and two to MGIIc based on the phylogenetic analysis of full-length 16S rRNA gene sequences as well as concatenated alignments of marker genes of MGII MAGs. These hadal species of MGII have genomic features indicative of adaptation to the deep-sea niches; in particular, MGIIc was characterized by the key enzyme 2-dehydro-3-deoxygluconokinase (kdgk) of the Entner-Doudoroff (ED) pathway missing in MGIIa and MGIIb, and by the presence of abundant genes potentially mediating osmolyte transport and production that may facilitate the organisms’ adaptation to increased environmental stress such as high hydrostatic pressure (HHP) in the deep ocean. The high GC contents of 16S rRNA genes and activity of β-galactosidase measurements suggest a thermophilic origin of MGIIc, with the common ancestor of MGII having an estimated OGT about 60°C.
Results
Phylogenetic analysis of MGII species
Thirteen MGII MAGs (completeness >50% and contamination <2%) were recovered from the Mariana Trench at the surface and at the depths of 2,000, 4,000, 8,000, and 10,500 m (Table 1). These MAGs had GC contents ranging from 42.1% to 63.8%, genome sizes 1.09–2.14 Mbp, and the number of coding sequences ranging from 931 to 1746, with lowest values all occurring in the surface water and highest values all occurring in the hadal waters (Table 1). Nine of the thirteen MAGs were classified as MGIIa and MGIIb, while the other four MAGs (F2MGII_2, P4F4MGII_2, F8MGII_2, and MT10K_MGII) did not cluster with any previously described genomes (Table S1). These four MAGs were classified as MGIIc according to the phylogenomic analysis and the 16S rRNA genes (see the following text). Presently, more than 500 MGII MAGs have been published,15,16,17 which are available from NCBI and IMG databases. We collected all these MGII MAGs and combined them with our own MAGs for annotation.
Table 1.
General features of MGII MAGs recovered from Mariana Trench water column
| MAG name | Family | Depth (m) | Completeness | Contamination | GC content | Size (Mbp) | CDS |
|---|---|---|---|---|---|---|---|
| P0F0MGII_1 | MGIIa | 0 | 90.65% | 0.00% | 58.5% | 1.89 | 1607 |
| P0F0MGII_2 | MGIIa | 0 | 71.50% | 0.47% | 42.1% | 1.64 | 1388 |
| P0F0MGII_3 | MGIIa | 0 | 64.64% | 0.93% | 44.2% | 1.37 | 1188 |
| P0F0MGII_4 | MGIIb | 0 | 84.58% | 0.00% | 47.8% | 1.30 | 1128 |
| P0F0MGII_5 | MGIIb | 0 | 57.48% | 0.00% | 50.6% | 1.09 | 931 |
| F2MGII_1 | MGIIb | 2000 | 72.59% | 0.00% | 59.6% | 1.13 | 1006 |
| F2MGII_2 | MGIIc | 2000 | 59.86% | 0.93% | 61.9% | 1.12 | 1242 |
| F2MGII_3 | MGIIb | 2000 | 73.83% | 0.93% | 61.8% | 1.25 | 1105 |
| P4F4MGII_1 | MGIIb | 4000 | 89.25% | 1.40% | 61.2% | 1.45 | 1298 |
| P4F4MGII_2 | MGIIc | 4000 | 51.11% | 0.54% | 62.3% | 1.33 | 1510 |
| F8MGII_1 | MGIIa | 8000 | 92.06% | 1.87% | 54.0% | 2.14 | 1746 |
| F8MGII_2 | MGIIc | 8000 | 92.06% | 1.87% | 62.5% | 1.99 | 1576 |
| MT10K_MGII | MGIIc | 10000 | 70.56% | 1.01% | 63.8% | 1.42 | 1302 |
The maximum-likelihood phylogenetic tree (Figure 1A) of the Mariana Trench MGII MAGs and representatives of different MGII genera in previous studies was constructed by IQ-TREE31 based on the marker genes identified using MarkerFinder.32 The three MGIIa MAGs recovered from the surface water belonged to genera M, K1, and L1, respectively, according to the classification of Rinke et al.16 An additional MGIIa MAG belonging to genus J3 was assembled from 8,000 m. Five MGIIb MAGs were recovered from surface, 2,000 m and 4,000 m, in which only one MAG from surface belonged to genus O5 and the other four MAGs belonged to genus O4 (Figure 1A).
Figure 1.
Phylogenetic analysis of MGII
(A) The phylogenetic tree of MGII MAGs. The MAGs in red were recovered from the Mariana Trench waters in this study. Numbers in the column below “%GC” indicate the GC contents of MGII MAGs.
(B) The phylogenetic tree of complete 16S rRNA genes of MGII. Sequences in red were recovered from the Mariana Trench waters in this study. Numbers in the column below “%GC” indicate the GC contents of 16S rRNA genes.
Only 24 full-length 16S rRNA genes of MGII were retrieved from all available MAGs. They were combined together with other 24 full-length 16S rRNA genes of the cultured marine Euryarchaeota and a maximum-likelihood tree was constructed (Figure 1B). Among the 24 full-length MGII 16S rRNA genes, three were from Mariana hadal waters. F8MGII_1 belonging to genera J3 of MGIIa was from an 8,000 m depth. Two clades (F8MGII_2 and MT10K_MGII) from 8,000 m and 10,500 m, respectively, were classified as MGIIc. The GC contents of MGIIc 16S rRNA genes (Figure 1B) ranged from 56.4% to 56.9% (n = 4), with the average value 56.6% being significantly higher than those of MGIIb (54.1%–55.7%; average value = 55.2%, n = 13) and MGIIa (52.7%–54.2%; average value = 53.8%, n = 9) (t test, p < 0.001 for both comparisons). Among the cultured marine Euryarchaeota, the 16S rRNA GC content of Methanohalobium evestigatum Z-7303 was closest to MGIIc with the value of 55.9%, while 16S rRNA GC contents of other cultured marine Euryarchaeota were higher, with the values ranging from 58.3% to 68.1% (Figure 1B).The GC contents of the MAGs (Figure 1A) were also significantly higher in MGIIc (39.4%–62.8%; average value = 58.6%, n = 8) than in MGIIa (34.5%–62.7%; average value = 47.5%, n = 23) or MGIIb (35.7%–61.8%; average value = 48.1%, n = 22) (t test, p < 0.01 for both comparisons).
Unique metabolic features of MGIIc
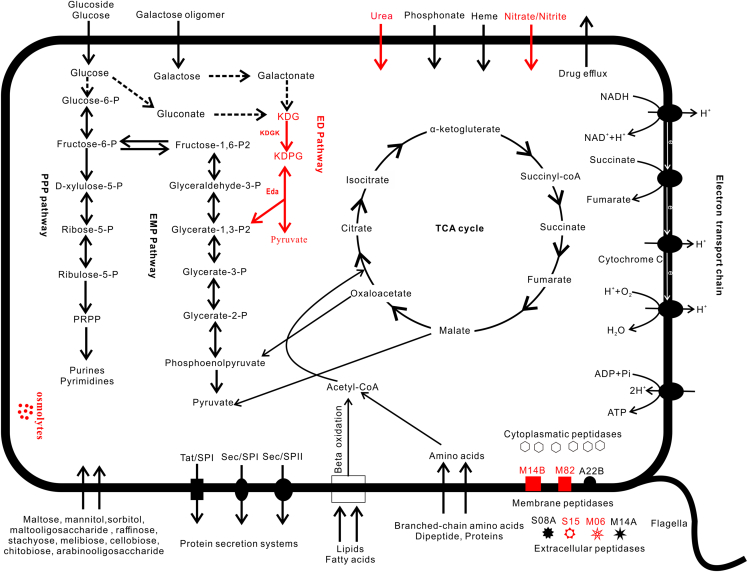
Similar to MGIIa and MGIIb in the Mariana Trench water column, MGIIc did not contain genes related to autotrophic pathways but contained genes related to the degradation of proteins, lipids, and sugars, indicating their heterotrophic nature. MGIIc also contained abundant ABC-type transporters for sugars, lipids, amino acids, as well as diverse glycoside hydrolases (GHs), glycosyl transferases, and cytoplasmatic or membrane peptidases, which were also present in Mariana Trench MGIIa and MGIIb (Figure 2).
Figure 2.
Potential metabolic pathways of MGIIc
Pathways or functions which were present in MGIIa, MGIIb, and MGIIc were colored in black, and those particularly present in MGIIc were colored in red.
MGIIc, coded for the key enzymes 2-dehydro-3-deoxygluconokinase (kdgk) and 2-dehydro-3-deoxyphosphogluconate aldolase (eda), was involved in the degradation of glucose via the ED pathway (Figure 2). The former was only detected in the basal clades (general J1-3 and genus P) in MGIIa and MGIIb, while the latter in the genus P. Our results indicate that these genes are part of the core genome of MGIIc, as opposed in the other two groups. Thus, MGIIc had two pathways for glycolysis and the formation of pyruvate. Another feature of MGIIc was that they have abundant genes potentially mediating osmolyte transport and production that may facilitate the organisms’ adaptation to increased environmental stress in the deep ocean. Osmolytes are thought to be important chemicals for life to ameliorate stresses such as changing salinity, temperature, or hydrostatic pressure.33,34
In general, MGIIc contained more genes related to HHP adaptation than MGIIa and MGIIb. For example, trehalose is an important osmolyte to respond to HHP35 and the sugC gene encoding trehalose of ATP-binding protein was present in both MGIIc and hadal MGIIa but absent in most MGIIa and MGIIb in the Mariana Trench shallower water column (Figure 3). Mannitol can also act as organic osmolyte or thermal protectant36 and the related genes encoding alpha-mannosidase (mngB) and mannitol-1-phosphate 5-dehydrogenase (mtlD) were also mainly present in MGIIc and hadal MGIIa but absent in most of other MGIIa and MGIIb MAGs (Figures 3, S1, and S2).
Figure 3.
Phylogenetic tree of Mariana Trench MGII MAGs and distributional heatmap of seven functional genes
The phylogenetic tree was conducted based on the 36 concatenated ribosomal protein alignments. The functional genes encoding the following proteins: pop, proteorhodpsin; PhrB, deoxyribodipyrimidine photolyase; pimA, phosphatidyl-myo-inositol mannosyltransferase; sugC, trehalose import ATP-binding protein; mngB, mannosylglycerate hydrolase; mtlD, mannitol-1-phosphate 5-dehydrogenase; Hsp60, chaperonin GroEL.
MGIIc also may be different from MGIIa and MGIIb in cell envelope. Phosphatidyl-myo-inositol mannosyltransferase (pimA) catalyzes the biosynthesis of phosphatidyl-myo-inositol mannosides (PIMs), which are key components of the cell envelope and commonly found in mycobacteria.37 Interestingly, pimA encoding genes were detected in MGIIc of this study as well as in MAG Cayman_69_deep recovered from the hydrothermal vent plume;19 but they were absent from MGIIa and MGIIb (Figure 3). The exact reason why MGIIc contained pimA is unknown, but it may have relevance to MGIIc’s need to cope with the extreme environment in the deep sea. PIMs could improve the stability of membrane and serve as the barrier to resist drugs.38 The presence of pimA in MGIIc possibly could help them to produce PIMs and improve their adaptation to cold and HHP environments.
Previous studies have demonstrated that gaining of proteorhodopsin (pop) from bacteria promoted the proliferation of MGII in the ocean surface.39 In the Mariana trench water samples, all five MGII MAGs from the surface contained pop genes (Figure 3). The phylogenetic analysis indicated that the pop genes of surface MGII at the Mariana Trench were possibly gained from Bacteroidetes, Proteobacteria, and Firmicutes through horizontal gene transfer (HGT) (Figure S3). All MGIIc MAGs from the Mariana Trench water column were recovered from deep waters and none of them contained pop gene. This was consistent with previous studies that only photic zone MGII contained pop gene.39 The deoxyribodipyrimidine photolyase (phrB) gene, which could repair DNA damage caused by ultraviolet rays, had a similar distribution than the pop gene in MGII MAGs at the Mariana Trench (Figure 3). From the phylogenetic analysis we found that the phrB genes of MGII were possibly gained from Proteobacteria, Bacteroidetes, Planctomycetes, or Verrucomicrobia through HGT (Figure S4).
Metagenomic recruitment of MGII MAGs and bathypelagic distribution of MGIIc
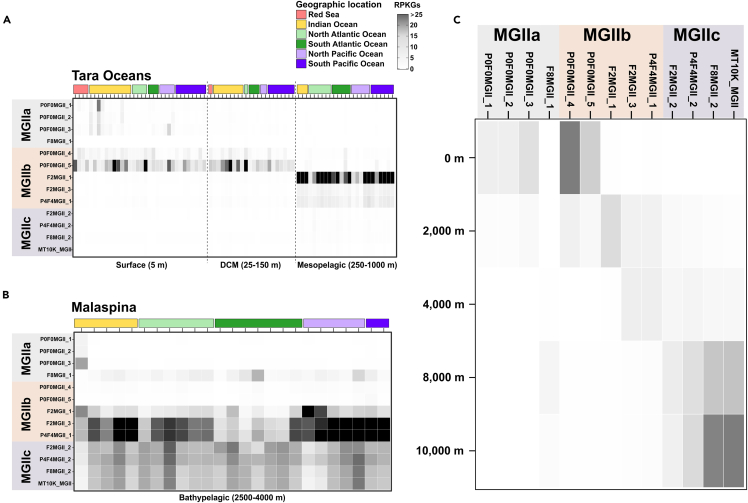
In order to attain the true bathypelagic behavior of MGIIc, we recruited its MAGs along with those of MGIIa and MGIIb against the Tara Oceans40 and Malaspina41 metagenomes (Figures 4A and 4B) and the Mariana Trench metagenomes in this study (Figure 4C). Results showed that in photic and mesopelagic waters, only genomes from MGIIa, with a patchy distribution, and MGIIb, with a more widespread distribution in the open ocean, were detected (Figure 4A), in line with previous reports.16,17 Besides, their recruitment also correlated to the depth distribution of our retrieved MAGs: MAGs from surface waters were recruited to those at 5 m and at the Deep Chlorophyll Maximum (DCM) while MAGs recovered at 2,000 and 4,000 m were recruited more abundantly at mesopelagic and bathypelagic depths. MGIIc MAGs were only recruited below 2,000 m in the Malaspina metagenomes (Figure 4B) and started to appear at bathypelagic waters, with faint recruitment values at 2,000 m and increasing toward hadal waters (>8,000 m) (Figure 4C). The synteny analysis of MGII MAGs also showed that MGIIc from hadal zones had the most synteny blocks with MGIIc from other bathypelagic habitats, such as hydrothermal vent plumes (Figure S5).
Figure 4.
The recruitment of MGII MAGs in this study
(A) against the TARA Oceans metagenomes from photic, DCM and mesopelagic waters, (B) against Malaspina metagenomes from bathypelagic waters, and (C) Mariana Trench metagenomes from surface to hadal waters.
Correlation between OGT and GC content of MGII species supported by enzymatic analysis
The correlation between OGT and GC contents of 16S rRNA genes of known marine archaea was established for evaluating the optimal growth temperatures of MGII species. Pyrococcus species had the highest OGTs and GC contents of 16S rRNA genes in marine Euryarchaeota, followed by Methanopyrus, Thermococcus, Methanocaldococcus, and Thermoplasma (Table 2; Figures 5A and 5B). Within the cultured marine Euryarchaeota, Aciduliprofundum boonei T469 is the closest relative to MGIIc (78.7% identity) according to 16S rRNA gene similarity. Its GC content of 16S rRNA gene was higher than MGIIc and its OGT is 70°C (Table 2). For comparison, the OGTs of cultured marine Thaumarchaeota were much lower, ranging between 22°C and 32°C (Table 2; Figures 5A and 5B). In order to test the effect of phylogenetic signal bias,42 we also analyzed the correlation between OGTs and GC contents using the pgls of phytools.43 The results showed that the effect of phylogenetic signal bias is negligible (Figures S6A and S6B).
Table 2.
OGTs and 16S rRNA gene GC contents of cultured marine Euryarchaeota and Thaumarchaeota
| Genome name | GC% | OGT (°C) | Isolated habitat | Reference |
|---|---|---|---|---|
| Methanothermococcus thermolithotrophicus DSM 2095 | 58.8% | 65 | geothermally heated marine sediment | (Huber et al.)47 |
| Thermococcus kodakarensis KOD1 | 66.0% | 85 | marine hydrothermal vents | (Fukui et al.)48 |
| Thermococcus gammatolerans EJ3 | 66.1% | 88 | hydrothermal vent chimneys | (Zivanovic et al.)49 |
| Thermococcus onnurineus NA1 | 65.7% | 80 | hydrothermal vent sediment | (Lee et al.)50 |
| Thermococcus guaymasensis DSM11113 | 66.0% | 88 | Guaymas Basin | (Canganella et al.)51 |
| Thermococcus paralvinellae ES1 | 65.8% | 82 | deep-sea hydrothermal vent | (Pledger and Baross)52 |
| Thermococcus cleftensis CL1 | 65.8% | 85 | hydrothermal vent | (Jung et al.)53 |
| Thermococcus eurythermalis A501 | 66.0% | 85 | Guaymas Basin | (Zhao and Xiao)54 |
| Pyrococcus furiosus DSM3638 | 66.3% | 100 | shallow marine solfatara | (Fiala and Stetter)55 |
| Pyrococcus kukulkanii NCB100 | 66.5% | 105 | deep-sea hydrothermal vent | (Callac et al.)56 |
| Pyrococcus abyssi GE5 | 66.3% | 103 | deep-sea hydrothermal vent | (Erauso et al.)57 |
| Pyrococcus horikoshii OT3 | 66.3% | 98 | deep-sea hydrothermal vent | (Gonzalez et al.)58 |
| Aciduliprofundum boonei T469 | 62.0% | 70 | marine hydrothermal sediment | (Reysenbach et al.)59 |
| Ferroglobus placidus DSM 10642 | 65.7% | 85 | hydrothermal sediment | (Hafenbradl et al.)60 |
| Methanopyrus kandleri AV19 | 68.1% | 98 | deep-sea hydrothermal vent | (Kurr et al.)61 |
| Methanohalobium evestigatum Z-7303 | 55.9% | 50 | salt lagoon mud | (Ishida et al.)62 |
| Methanocaldococcus vulcanius M7 | 64.0% | 80 | deep sea hydrothermal vent chimney | (Jeanthon et al.)63 |
| Methanofervidicoccus abyssi HHB | 62.2% | 70 | Cayman hydrothermal vent | (Sakai et al.)64 |
| Methanocaldococcus jannaschii DSM2661 | 64.4% | 85 | deep-sea hydrothermal vent | (Bult et al.)65 |
| Methanocaldococcus infernus ME | 64.8% | 85 | deep-sea hydrothermal vent | (Jeanthon et al.)66 |
| Nitrosopumilus maritimus SCM1 | 52.6% | 30 | marine aquarium | (Martens-Habbena et al.)67 |
| Nitrosopumilus cobalaminigenes strain HCA1 | 52.8% | 32 | Hood Canal station P10 | (Qin et al.)68 |
| Nitrosopumilus ureiphilus strain PS0 | 52.5% | 31 | Puget Sound main basin | (Qin et al.)68 |
| Candidatus Nitrosopelagicus brevis CN25 | 51.9% | 22 | Marine water | (Santoro et al.)69 |
| Candidatus Nitrosomarinus catalina SPOT01 | 52.2% | 23 | Marine water | (Ahlgren et al.)70 |
| Candidatus Nitrosopumilus piranensis D3C | 52.4% | 32 | coastal surface water | (Bayer et al.)71 |
| Candidatus Nitrosopumilus koreensis AR1 | 52.5% | 25 | marine sediment | (Park et al., 2012)72 |
| Candidatus Nitrosopumilus adriaticus NF5 | 52.4% | 32 | adriatic sea | (Bayer et al.)71 |
Figure 5.
The estimated OGTs of MGIIa, MGIIb, and MGIIc, as well as their ancestors
(A) The relationship between the OGTs and the GC contents of 16S rRNA gene of cultured marine archaea (Euryarchaeota and Thaumarchaeota). The OGTs of MGIIa, MGIIb, and MGIIc were estimated.
(B) The relationship between the OGTs and the GC contents of 16S rRNA stem of cultured marine archaea. The OGTs of the common ancestors of MGIIa, MGIIb, MGIIc, and all MGII were estimated.
(C) Reconstruction of the OGTs of MGII ancestors. Ancestral OGTs of MGIIa, MGIIb, MGIIc, and total MGII were shown at the corresponding nodes (top numbers) together with their confidence interval (numbers in bracket).
(D) Enzyme activities of β-galactosidases in MGIIc from Mariana Trench hadal water assayed at different temperatures. (E) Enzyme activities of β-galactosidases in MGIIc from Caribbean Sea hydrothermal vent plume assayed at different temperatures.
Based on the linear regression of 16S rRNA gene and OGT (Figure 5A), we estimated the OGTs of MGIIc to be 47°C–50°C, those of MGIIb 37°C–44°C, and those of MGIIa 30°C–37°C. To estimate the OGT of the common ancestor of all MGII and the OGTs of ancestors of MGIIa, MGIIb, and MGIIc, we performed a linear regression of OGTs of cultured marine archaea versus their 16S rRNA stem GC contents (Figure 5B, R2 > 0.84, p < 0.001) according to previous studies.26,27,28,30 The OGT of the common ancestor of MGII (Figure 5B) was then estimated to be at 60°C with a confidence internal of 52°C–65ºC; the OGTs of ancestors of MGIIa, MGIIb, and MGIIc were estimated at 46°C, 49ºC, and 57ºC, with confidence intervals of 43°C–49ºC, 45°C–52ºC, and 56°C–58ºC, respectively (Figure 5C).
Enzymatic analysis was performed to corroborate the OGT data. Genes of MGIIc β-galactosidases were synthesized, and the proteins were overexpressed at different temperatures in Escherichia coli. The activities were assayed against ortho-nitrophenyl β-D-galactopyranoside (ONPG), which is a superior β-galactosidase substrate and its product is completely soluble and easily detectable. The maximum enzymatic activity of all MGIIc β-galactosidases was at ∼50°C (Figures 5D and 5E), which provides biochemical evidence for the thermophilic properties of MGIIc enzymes.
Discussion
Identification of novel MGII archaea
Four MAGs from the Mariana Trench water column were significantly different from genera of MGIIa or MGIIb (Figure 1A). These MAGs were exclusively recovered from deep water samples (2,000–10,500 m), and the representative MAG F8MGII_2 (92.06% completeness, 1.87% contamination) was recovered from 8,000 m. We named this new cluster as genus H. Genera Q1 and Q2, which though classified as MGIIb in Rinke et al.16 were more similar with genus H than with other MGIIb genera in this phylogenetic tree. This was also supported by amino acid identity analysis (Table S2).
The 16S rRNA gene phylogenetic tree displayed a clear differentiation of MGIIc from MGIIa and MGIIb (Figure 1B). The 16S rRNA gene identity between MGIIc with MGIIa and MGIIb ranged from 86% to 89% (Table S3). The maximum likelihood tree of a ribosomal protein uL6 (182 aa length) separated MGIIc from MGIIa and MGIIb clearly (Figure S7), which was in accordance with the 16S rRNA gene phylogenetic result. Based on the taxonomic threshold of 86.5% identity for archaeal family using the 16S rRNA gene sequence identity,44 we proposed MGIIc to be a new family of MGII, including three genera: H, Q1, and Q2, which may represent the deep water MGII ecotype at depth below 2,000 m. Furthermore, given its high genome quality (92.06% completeness) we named MAG F8MGII_2 as a new species (Candidatus Qianlongarchaea bathyliensis fam. sp. nov.).
Compared with MGIIa and MGIIb, the novel group MGIIc is exclusively distributed in deep oceans, mainly below 2,000 m (Figure 4). As solar energy is not available in their habitats, no pop or phrB gene was present in MGIIc MAGs (Figure 3). There were several unique features of MGIIc, which might help them to adapt to bathypelagic environments. These included abundant genes related to osmolyte transport and production, which is essential for them to thrive in HHP stress. Because most labile organic matter produced by phytoplankton is utilized by microbes from surface and shallow oceans, it is necessary for the microbes living in deep ocean to develop preference in utilization of complex organic matter. In addition to the Embden-Meyerhof-Parnas (EMP) pathway, MGIIc also had the ED pathway, potentially improving their utilization of sugars.
Thermophilic features and evolutionary implications of MGII
The decreasing trend of OGTs from MGIIc to MGIIb and MGIIa (Figure 5A) and the OGT of their ancestors (Figure 5B) appeared to indicate that MGIIc possibly was the closest to a thermophilic ancestor while MGIIa was probably the most lately evolved in MGII. To avoid the effect of phylogenetic signal bias, the pgls analysis was conducted and the results had the same conclusion (Figure S6 and Table S4). In addition, genes responding to high temperatures (such as hsp60)35 were present in most MGIIc MAGs but only in a few MGIIa and MGIIb MAGs (Figure 3). These results indicated that MGIIc possibly had a moderately thermophilic origin and adapted to cooler environments during their evolution. This would be consistent with evolutionary processes of Thaumarchaeota and other Euryarchaeota.26,28,30 The maximum enzymatic activity of MGIIc β-galactosidases at ∼50°C provided additional evidence for the moderately thermophilic feature of MGIIc.
We also performed correlation analysis between OGTs and GC contents of the whole genomes of the isolated marine archaea, which was much less significant. The exact reason for this poor correlation is unclear. We posit that the functional genes are more easily experiencing HGT than the 16S rRNA genes during adaptation to changing nutrients (e.g., nitrogen availability),45 temperature, or pressure; thus the whole genome GC contents are collectively more variable than the 16S rRNA genes, making the former to be poorly correlated with OGT.
Conclusion
The 13 MGII MAGs from the Mariana Trench water samples in this study contain a novel MGII family MGIIc. The name Candidatus Qianlongarchaea bathyliensis fam. sp. nov. is proposed for the dominant species of MGIIc, with “Qianlong” in Chinese meaning “diving dragon” and bathyliensis meaning organisms in deep ocean water below 2,000 m. Candidatus Qianlongarchaea bathyliensis is distinguished from MGIIa and MGIIb by adaptation to deep oceans with enriched genes related to high pressure and lacks of genes related to surface oceans, such as rhodopsin genes and deoxyribodipyrimidine photolyase genes (phrB). The analyses based on 16S rRNA stem GC contents and OGTs indicate a thermophilic origin of Candidatus Qianlongarchaea bathyliensis (OGT 47°C–50°C), which is supported by β-galactosidase measurements.
Limitations of the study
Although the main findings in this study lead to the proposal that MGII originated from subsurface geothermal environment and have adapted from higher to lower temperatures, it is unknown how HGT might have played an important role for MGIIa and MGIIb to become competitive in the photic environment by gaining rhodopsin genes and photolyase genes from bacteria.46 The exact pathways leading to niche speciation of these MGII groups are elusive, particularly the adaptation of MGIIc in the deep subsurface. We expect that our hypothesis can be validated in the future when MGIIa, MGIIb, or MGIIc are brought into pure culture in the lab.
STAR★Methods
Key resources table
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Chuanlun Zhang (zhangcl@sustech.edu.cn).
Materials availability
This study did not generate new unique reagents.
Method details
Sampling, DNA extraction and sequencing
Water samples (50-100 liters) were collected at depths of 0 m, 2,000 m, 4,000 m and 8,000 m in the Challenger Deep of the Mariana Trench (11°21.847'N, 142°20.775'E) aboard R/V Dong Fang Hong 2 during March 2017 (Zhong et al.).73 The 10,500 m water sample was collected during September 2016 at the same station using the same research vessel.74 After being brought to deck by Niskin bottles of a CTD (Conductivity, Temperature and Depth) recorder equipment, seawater was filtered through 3 μm and 0.22 μm polycarbonate membranes subsequently. The filter samples were then stored in liquid nitrogen until they were transported to home laboratories.
DNA from all filter samples were extracted and then sequenced using the same methods as described in our previous work.74 Metagenomic sequencing was conducted using Illumina HiSeq X-Ten in Novogene Bioinformatics Technology Co., Ltd. (Beijing, China).
Assembly, binning, refinement, reassembly, and annotation
The read-qc module of MetaWRAP75 was used to perform the quality control of raw reads. The assembly, binning, refinement and reassembly modules of the MetaWRAP were used subsequently to do deal with the clean data. The qualities (completeness and contamination) of these reassembled bins were measured by CheckM 1.0.1177 and then the bins were classified against GTDB-Tk 0.1.6.77 MAGs of MGII (completeness > 50% and contamination < 5%) were selected and annotated using Prodigal 2.6.379, BLAST+ 2.9.080, and Prokka 1.14.681 against Uniprot database.81
Metagenomic recruitment
Metagenomes from different environmental datasets were used to study our MGII MAGs distribution. Raw reads from Tara Oceans expedition40 were downloaded from the European Nucleotide Archive (PRJEB1787). Raw reads from Malaspina41 were downloaded from JGI Integrated Microbial Genomes and Microbiomes (IMG/MER) (GOLD study ID Gs0053074). A metagenomic data set from the Mariana Trench profile73 was also downloaded from NCBI-SRA (SRR8404393 to SRR8404400). Metagenomic reads were trimmed using Trimmomatic v0.3683 and only those reads with a phred score ≥ 30, ≥ 50 bp long and with no ambiguous bases (Ns) were kept. These high-quality metagenomic reads were then aligned using BLASTN,83 using a cut-off of 98% nucleotide identity over a minimum alignment length of 50 nucleotides. We required ≥70% of each genome to be covered by reads. They were used to compute the RPKG (reads recruited per Kb of genome per Gb of metagenome) values that provide a normalized number comparable across various metagenomes. Since different data sets with different read lengths (Illumina Hiseq 2 × 100 and 2 × 150 bp) were used for the recruitment, each metagenome was also normalized, dividing the size of the database by its average read size. Genomes that recruited less than three RPKG were considered not present in the sample.
Phylogenetic analysis
All published MGII MAGs and newly assembled MGII MAGs in this study were annotated using the same method described above. The complete 16S rRNA genes were selected from available MGII MAGs. The 16S rRNA genes of cultured marine Euryarchaeota were used as the outgroup. The phylogenetic tree of full-length 16S rRNA genes were constructed by MEGA7 using the maximum likelihood method.84 The phylogenetic trees of functional genes (including kdgk, mtlD, proteorhodopsin and phrB) were constructed using the same method. Reference MAGs of different MGII genera and MGIII85 were selected according to their quality and were combined with the newly assembled MGII MAGs, with cultured Euryarchaeota (Aciduliprofundum boonei T469, Thermoplasma acidophilum DSM1728, Thermoplasma volcanium GSS1) as the outgroup. Marker genes of all MAGs and genomes were predicted by MarkerFinder.32 Then they were concatenated for constructing the phylogenetic tree using IQ-TREE31 with LG+C60+F model.
Potential metabolism of MGII
Protein sequences of MGII MAGs generated by Prokka were searched against KEGG database using GhostKOALA with KEGG GENES database ‘genus_prokaryotes + family_eukaryotes’.86,87 The ABC-type transporters, amino acids biosynthesis, metabolic pathways, electron transport chain were then predicted according to KEGG annotations. Coding sequences of MGII MAGs were analyzed for identification of carbohydrate-active enzymes against dbCAN88 using HMMER89 and DIAMOND90 with default parameters. Peptidases of MGII were predicted by blasting coding sequences against MEROPS database91 using DIAMOND. The locations (membrane, cytoplasmic or extracellular) of identified peptidases were predicted with PSORTb 3.0.393 and secretory proteins were identified with SignalP 5.0.93
OGT prediction of ancestors of all MGII as well as the three families
A dataset of 16S rRNA sequences of 28 cultured marine Euryarchaeota and Thaumarchaeota as well as their OGTs information were extracted from NCBI database. The 16S rRNA stem positions of both these cultured marine archaea and MGII were predicted by aligning against the RNA STRAND sequences, which were downloaded from the RNA STRAND database (V2.0: http://www.rnasoft.ca/strand/). The stem positions of RNA STRAND sequences were selected as the reference stem region (891 stem positions). The 16S rRNA stem GC contents of cultured marine Euryarchaeota and Thaumarchaeota were calculated and the linear regression between them and their OGTs was established. The evolutionary model of the 16S rRNA sequences along the reference tree was estimated by a nonhomogeneous model as described in Groussin and Gouy28 using bppml (BppSuite, V2.4.1).94 Then 100 replicates of ancestral sequences were reconstructed for corresponding nodes (ancestral nodes of all MGII, as well as their three families) using the program BppAncestor with the estimated parameters and HKY85 model. The 16S rRNA stem GC contents of these ancestral sequences were calculated and then the OGTs with confidence intervals of them were inferred against the linear regression.
Synteny analysis
The translated proteins of MGII MAGs were compared among themselves by BLASTP60 using a reciprocal best-hit analysis of putative homologs. Synteny blocks were detected using MCScanX95 and then the synteny blocks between the MGII MAGs from hydrothermal vent plumes and those from other marine habitats was plotted using Circos.96
Bacterial strains, plasmids, growth conditions, and chemicals
All bacterial strains and plasmids used for enzymatic activity experiments are listed in the key resources table. E. coli strains were cultivated at 37°C in lysogeny broth (LB) medium supplemented with 50 μg/ml kanamycin. Analytically pure chemical ONPG was purchased from Yeasen Biological Technology Co., Ltd. (Shanghai, China). Other Analytically pure chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Expression and purification of glycoside hydrolases (GHs)
Genes encoding GHs without signal and cytoplasmic region were synthesized in E. coli codon usage by Synbio Technologies (Suzhou, China) and subsequently cloned into the expression vector pET-28a between the BamHI and XhoI sites (the optimized DNA sequences are listed in supporting information). GH genes were overexpressed in E. coli BL21 (DE3) with 0.2 mM IPTG in a total volume of 1.0 L. Cells were harvested by centrifugation at 6 000 × g for 10 min, washed twice with washing buffer (50 mM phosphate buffer pH 7.6), then lysed by sonication in lysed buffer (50 mM phosphate buffer pH 7.6, 200 mM NaCl, 10% glycerol). The cell lysate was centrifuged at 19,000 × g for 45 min, after which the supernatant was collected and applied to a Ni-NTA column using the AKTA system. After washing with buffer (50 mM phosphate buffer pH 7.6, 200 mM NaCl, 10% glycerol, 20 mM imidazole), the fusion protein was eluted with the elution buffer (50 mM phosphate buffer pH 7.6, 200 mM NaCl, 10% glycerol, 250 mM imidazole), and then dialyzed in storage buffer (50 mM phosphate buffer pH 7.6, 200 mM NaCl, 10% glycerol).
Enzyme assays
The enzyme reaction mixture contained 150 μL Z buffer (100 mM Sodium phosphate, 10 mM KCl, 2 mM MgSO4, pH 7.0), 20 μL ONPG (5 mg/ml), and a suitable amount of purified enzyme (50-75 μg). The reactions were started by the addition of enzyme and stopped by adding 20 μL 2.5 M NaOH after 3-8 min in a water bath at the corresponding temperature ranging from 16°C to 70°C (and finally the pH reached 12.4). The mixture without enzyme was used as a control. The release of o-nitrophenol (ONP) was measured spectrophotometrically at 420 nm and the activity was calculated using a molar extinction coefficient of 3.3×103 M–1 cm–1 calculated at pH 12.4.
The specific activity assay of GHs was detected in Z buffer. The increase in absorbance at 420 nm was measured at the optimal temperature of 50°C at interval of 1min with a PerkinElmer Lambda 25 UV–VIS spectrometer in a 1.0 × 1.0-cm quartz cuvette. Finally, the release of ONP was measured spectrophotometrically at 420 nm and the activity was calculated using a molar extinction coefficient of 4.5×103 M–1 cm–1.97
Quantification and statistical analysis
Statistical analysis
The statistical difference analysis between the GC contents of MGIIc and MGIIb as well as MGIIa were used T test.
Acknowledgments
We would like to thank all the scientists and crews on the R/V Dong Fang Hong 2 of Ocean University of China for their assistance with sampling during the cruise. We thank Wei Qin and Shengwei Hou for their valuable suggestions during the revision of the manuscript as well as Xiaotong Tang for helping with the pgls analysis of phylogenetic signals. This work was financially supported by the State Key R&D project of China grant (No. 2018YFA0605802), the National Natural Science Foundation of China (91851210, 41906136, and 91751202), the Stable Support Plan Program of Shenzhen Natural Science Fund (No. 20200925173954005), the Shenzhen Key Laboratory of Marine Archaea Geo-Omics, Southern University of Science and Technology (ZDSYS201802081843490), and the Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou) (Guangzhou) (No. K19313901). Computation in this study was supported by the Center for Computational Science and Engineering at the Southern University of Science and Technology. J.H.M. and F.R.V. were supported by “VIREVO” CGL2016-76273-P [MCI/AEI/FEDER, EU], (co-founded with FEDER funds) from the Spanish Ministerio de Ciencia e Innovación. F.R.V. supervision was supported by Russian Science Foundation (RSF) Project 21-64-00018.
Author contributions
H.L., L.Q., Y.Zhu, and C.Z. proposed the main idea of this paper. H.L., Y.Zheng, and J.L. collected the water samples, extracted the community DNA, and performed genomic analyses. X.-H.Z. provided the MT10K_MGII MAG and critical ideas for its analyses. H.L., B.X., F.R.V., and J.H.M. performed the bioinformatic analysis of metagenomic data. J.H.M. and F.R.V. provided critical suggestions during the project. W.-W.L. and N.-Y.Z. designed and performed the enzyme activity experiment. J.T. and C.Z. designed the cruise and the large-volume water sampler. All authors edited and approved the final manuscript.
Declaration of interests
The authors declare no competing financial interests.
Published: August 17, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107664.
Contributor Information
Francisco Rodriguez-Valera, Email: frvalera@umh.es.
Chuanlun Zhang, Email: zhangcl@sustech.edu.cn.
Supplemental information
Data and code availability
-
•
All clean reads of metagenomic datasets used in this study have been uploaded to NCBI Sequence Read Archive in our recent work (Zhong et al.).73 The 13 assembled MGII MAGs have been deposited in NCBI. Accession numbers are listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.DeLong E.F. Archaea in coastal marine environments. Proc. Natl. Acad. Sci. USA. 1992;89:5685–5689. doi: 10.1073/pnas.89.12.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuhrman J.A., McCallum K., Davis A.A. Novel major archaebacterial group from marine plankton. Nature. 1992;356:148–149. doi: 10.1038/356148a0. [DOI] [PubMed] [Google Scholar]
- 3.Fuhrman J.A., Davis A.A. Widespread archaea and novel bacteria from the deep sea as shown by 16S rRNA gene sequences. Mar. Ecol. Prog. Ser. 1997;150:275–285. doi: 10.3354/meps150275. [DOI] [Google Scholar]
- 4.López-García P., Moreira D., López-López A., Rodríguez-Valera F. A novel haloarchaeal-related lineage is widely distributed in deep oceanic regions. Environ. Microbiol. 2001;3:72–78. doi: 10.1046/j.1462-2920.2001.00162.x. [DOI] [PubMed] [Google Scholar]
- 5.Karner M.B., DeLong E.F., Karl D.M. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature. 2001;409:507–510. doi: 10.1038/35054051. [DOI] [PubMed] [Google Scholar]
- 6.Santoro A.E., Richter R.A., Dupont C.L. Planktonic Marine Archaea. Ann. Rev. Mar. Sci. 2019;11:131–158. doi: 10.1146/annurev-marine-121916-063141. [DOI] [PubMed] [Google Scholar]
- 7.Zhang C.L., Xie W., Martin-Cuadrado A.B., Rodriguez-Valera F. Marine Group II Archaea, potentially important players in the global ocean carbon cycle. Front. Microbiol. 2015;6:1108. doi: 10.3389/fmicb.2015.01108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belmar L., Molina V., Ulloa O. Abundance and phylogenetic identity of archaeoplankton in the permanent oxygen minimum zone of the eastern tropical South Pacific. FEMS Microbiol. Ecol. 2011;78:314–326. doi: 10.1111/j.1574-6941.2011.01159.x. [DOI] [PubMed] [Google Scholar]
- 9.Galand P.E., Casamayor E.O., Kirchman D.L., Potvin M., Lovejoy C. Unique archaeal assemblages in the Arctic Ocean unveiled by massively parallel tag sequencing. ISME J. 2009;3:860–869. doi: 10.1038/ismej.2009.23. [DOI] [PubMed] [Google Scholar]
- 10.Orsi W.D., Smith J.M., Liu S., Liu Z., Sakamoto C.M., Wilken S., Poirier C., Richards T.A., Keeling P.J., Worden A.Z., Santoro A.E. Diverse, uncultivated bacteria and archaea underlying the cycling of dissolved protein in the ocean. ISME J. 2016;10:2158–2173. doi: 10.1038/ismej.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H., Zhang C.L., Yang C., Chen S., Cao Z., Zhang Z., Tian J. Marine Group II Dominates Planktonic Archaea in Water Column of the Northeastern South China Sea. Front. Microbiol. 2017;8:1098. doi: 10.3389/fmicb.2017.01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia X., Guo W., Liu H. Basin Scale Variation on the Composition and Diversity of Archaea in the Pacific Ocean. Front. Microbiol. 2017;8:2057. doi: 10.3389/fmicb.2017.02057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian J., Fan L., Liu H., Liu J., Li Y., Qin Q., Gong Z., Chen H., Sun Z., Zou L., et al. A nearly uniform distributional pattern of heterotrophic bacteria in the Mariana Trench interior. Deep-Sea Research Part I-Oceanographic Research Papers. 2018;142:116–126. doi: 10.1016/j.dsr.2018.10.002. [DOI] [Google Scholar]
- 14.Martin-Cuadrado A.B., Garcia-Heredia I., Moltó A.G., López-Úbeda R., Kimes N., López-García P., Moreira D., Rodriguez-Valera F. A new class of marine Euryarchaeota group II from the Mediterranean deep chlorophyll maximum. ISME J. 2015;9:1619–1634. doi: 10.1038/ismej.2014.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orellana L.H., Ben Francis T., Krüger K., Teeling H., Müller M.C., Fuchs B.M., Konstantinidis K.T., Amann R.I. Niche differentiation among annually recurrent coastal Marine Group II Euryarchaeota. ISME J. 2019;13:3024–3036. doi: 10.1038/s41396-019-0491-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rinke C., Rubino F., Messer L.F., Youssef N., Parks D.H., Chuvochina M., Brown M., Jeffries T., Tyson G.W., Seymour J.R., Hugenholtz P. A phylogenomic and ecological analysis of the globally abundant Marine Group II archaea (Ca. Poseidoniales ord. nov.) ISME J. 2019;13:663–675. doi: 10.1038/s41396-018-0282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tully B.J. Metabolic diversity within the globally abundant Marine Group II Euryarchaea offers insight into ecological patterns. Nat. Commun. 2019;10:271. doi: 10.1038/s41467-018-07840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iverson V., Morris R.M., Frazar C.D., Berthiaume C.T., Morales R.L., Armbrust E.V. Untangling genomes from metagenomes: revealing an uncultured class of marine Euryarchaeota. Science. 2012;335:587–590. doi: 10.1126/science.1212665. [DOI] [PubMed] [Google Scholar]
- 19.Li M., Baker B.J., Anantharaman K., Jain S., Breier J.A., Dick G.J. Genomic and transcriptomic evidence for scavenging of diverse organic compounds by widespread deep-sea archaea. Nat. Commun. 2015;6:8933. doi: 10.1038/ncomms9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie W., Luo H., Murugapiran S.K., Dodsworth J.A., Chen S., Sun Y., Hedlund B.P., Wang P., Fang H., Deng M., Zhang C.L. Localized high abundance of Marine Group II archaea in the subtropical Pearl River Estuary: implications for their niche adaptation. Environ. Microbiol. 2018;20:734–754. doi: 10.1111/1462-2920.14004. [DOI] [PubMed] [Google Scholar]
- 21.Alves R.J.E., Minh B.Q., Urich T., von Haeseler A., Schleper C. Unifying the global phylogeny and environmental distribution of ammonia-oxidising archaea based on amoA genes. Nat. Commun. 2018;9:1517. doi: 10.1038/s41467-018-03861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gubry-Rangin C., Kratsch C., Williams T.A., McHardy A.C., Embley T.M., Prosser J.I., Macqueen D.J. Coupling of diversification and pH adaptation during the evolution of terrestrial Thaumarchaeota. Proc. Natl. Acad. Sci. USA. 2015;112:9370–9375. doi: 10.1073/pnas.1419329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren M., Feng X., Huang Y., Wang H., Hu Z., Clingenpeel S., Swan B.K., Fonseca M.M., Posada D., Stepanauskas R., et al. Phylogenomics Suggests Oxygen Availability as a Driving Force in Thaumarchaeota Evolution. ISME J. 2019;13:2150–2161. doi: 10.1038/s41396-019-0418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y., Huang J.M., Cui G.J., Nunoura T., Takaki Y., Li W.L., Li J., Gao Z.M., Takai K., Zhang A.Q., Stepanauskas R. Genomics insights into ecotype formation of ammonia-oxidizing archaea in the deep ocean. Environ. Microbiol. 2019;21:716–729. doi: 10.1111/1462-2920.14518. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y., Zhang C., Lenton T.M., Yan X., Zhu M., Zhou M., Tao J., Phelps T.J., Cao Z. The Evolution Pathway of Ammonia-Oxidizing Archaea Shaped by Major Geological Events. Mol. Biol. Evol. 2021;38:3637–3648. doi: 10.1093/molbev/msab129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abby S.S., Kerou M., Schleper C. Ancestral Reconstructions Decipher Major Adaptations of Ammonia-Oxidizing Archaea upon Radiation into Moderate Terrestrial and Marine Environments. mBio. 2020;11 doi: 10.1128/mBio.02371-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boussau B., Blanquart S., Necsulea A., Lartillot N., Gouy M. Parallel adaptations to high temperatures in the Archaean eon. Nature. 2008;456:942–945. doi: 10.1038/nature07393. [DOI] [PubMed] [Google Scholar]
- 28.Groussin M., Gouy M. Adaptation to environmental temperature is a major determinant of molecular evolutionary rates in archaea. Mol. Biol. Evol. 2011;28:2661–2674. doi: 10.1093/molbev/msr098. [DOI] [PubMed] [Google Scholar]
- 29.Kimura H., Mori K., Tashiro T., Kato K., Yamanaka T., Ishibashi J.I., Hanada S. Culture-Independent Estimation of Optimal and Maximum Growth Temperatures of Archaea in Subsurface Habitats Based on the G plus C Content in 16S rRNA Gene Sequences. Geomicrobiol. J. 2010;27:114–122. doi: 10.1080/01490450903456699. [DOI] [Google Scholar]
- 30.Galtier N., Lobry J.R. Relationships between genomic G+C content, RNA secondary structures, and optimal growth temperature in prokaryotes. J. Mol. Evol. 1997;44:632–636. doi: 10.1007/pl00006186. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen L.T., Schmidt H.A., Von Haeseler A., Minh B.Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez-Gutierrez C.A., Aylward F.O. Phylogenetic Signal, Congruence, and Uncertainty across Bacteria and Archaea. Mol. Biol. Evol. 2021;38:5514–5527. doi: 10.1093/molbev/msab254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamali A., Jahmidi-Azizi N., Oliva R., Winter R. Deep sea osmolytes in action: their effect on protein-ligand binding under high pressure stress. Phys. Chem. Chem. Phys. 2022;24:17966–17978. doi: 10.1039/d2cp01769e. [DOI] [PubMed] [Google Scholar]
- 34.Qin Q.L., Wang Z.B., Su H.N., Chen X.L., Miao J., Wang X.J., Li C.Y., Zhang X.Y., Li P.Y., Wang M., et al. Oxidation of trimethylamine to trimethylamine N-oxide facilitates high hydrostatic pressure tolerance in a generalist bacterial lineage. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abf9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y., Li X., Bartlett D.H., Xiao X. Current developments in marine microbiology: high-pressure biotechnology and the genetic engineering of piezophiles. Curr. Opin. Biotechnol. 2015;33:157–164. doi: 10.1016/j.copbio.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Tonon T., Li Y., McQueen-Mason S. Mannitol biosynthesis in algae: more widespread and diverse than previously thought. New Phytol. 2017;213:1573–1579. doi: 10.1111/nph.14358. [DOI] [PubMed] [Google Scholar]
- 37.Guerin M.E., Schaeffer F., Chaffotte A., Gest P., Giganti D., Korduláková J., van der Woerd M., Jackson M., Alzari P.M. Substrate-induced conformational changes in the essential peripheral membrane-associated mannosyltransferase PimA from mycobacteria: implications for catalysis. J. Biol. Chem. 2009;284:21613–21625. doi: 10.1074/jbc.M109.003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bansal-Mutalik R., Nikaido H. Mycobacterial outer membrane is a lipid bilayer and the inner membrane is unusually rich in diacyl phosphatidylinositol dimannosides. Proc. Natl. Acad. Sci. USA. 2014;111:4958–4963. doi: 10.1073/pnas.1403078111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frigaard N.U., Martinez A., Mincer T.J., DeLong E.F. Proteorhodopsin lateral gene transfer between marine planktonic Bacteria and Archaea. Nature. 2006;439:847–850. doi: 10.1038/nature04435. [DOI] [PubMed] [Google Scholar]
- 40.Sunagawa S., Coelho L.P., Chaffron S., Kultima J.R., Labadie K., Salazar G., Djahanschiri B., Zeller G., Mende D.R., Alberti A., et al. Ocean plankton. Structure and function of the global ocean microbiome. Science. 2015;348 doi: 10.1126/science.1261359. [DOI] [PubMed] [Google Scholar]
- 41.Acinas S.G., Sánchez P., Salazar G., Cornejo-Castillo F.M., Sebastián M., Logares R., Royo-Llonch M., Paoli L., Sunagawa S., Hingamp P., et al. Deep ocean metagenomes provide insight into the metabolic architecture of bathypelagic microbial communities. Commun. Biol. 2021;4:604. doi: 10.1038/s42003-021-02112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Felsenstein J. Phylogenies and the Comparative Method. Am. Nat. 1985;125:1–15. doi: 10.1086/703055. https://www.journals.uchicago.edu/doi/abs/10.1086/284325 [DOI] [PubMed] [Google Scholar]
- 43.Revell L.J. Phytools: An R package for phylogenetic comparative biology (and other things) Methods Ecol. Evol. 2011;3:217–223. doi: 10.1111/j.2041-210X.2011.00169.x. [DOI] [Google Scholar]
- 44.Yarza P., Yilmaz P., Pruesse E., Glöckner F.O., Ludwig W., Schleifer K.H., Whitman W.B., Euzéby J., Amann R., Rosselló-Móra R. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat. Rev. Microbiol. 2014;12:635–645. doi: 10.1038/nrmicro3330. [DOI] [PubMed] [Google Scholar]
- 45.Mende D.R., Bryant J.A., Aylward F.O., Eppley J.M., Nielsen T., Karl D.M., DeLong E.F. Environmental drivers of a microbial genomic transition zone in the ocean's interior. Nat. Microbiol. 2017;2:1367–1373. doi: 10.1038/s41564-017-0008-3. [DOI] [PubMed] [Google Scholar]
- 46.Deschamps P., Zivanovic Y., Moreira D., Rodriguez-Valera F., López-García P. Pangenome evidence for extensive interdomain horizontal transfer affecting lineage core and shell genes in uncultured planktonic thaumarchaeota and euryarchaeota. Genome Biol. Evol. 2014;6:1549–1563. doi: 10.1093/gbe/evu127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huber H., Thomm M., Konig H., Thies G., Stetter K.O. Methanococcus Thermolithotrophicus, a Novel Thermophilic Lithotrophic Methanogen. Arch. Microbiol. 1982;132:47–50. doi: 10.1007/Bf00690816. [DOI] [Google Scholar]
- 48.Fukui T., Atomi H., Kanai T., Matsumi R., Fujiwara S., Imanaka T. Complete genome sequence of the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1 and comparison with Pyrococcus genomes. Genome Res. 2005;15:352–363. doi: 10.1101/gr.3003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zivanovic Y., Armengaud J., Lagorce A., Leplat C., Guérin P., Dutertre M., Anthouard V., Forterre P., Wincker P., Confalonieri F. Genome analysis and genome-wide proteomics of Thermococcus gammatolerans, the most radioresistant organism known amongst the Archaea. Genome Biol. 2009;10:R70. doi: 10.1186/gb-2009-10-6-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee H.S., Kang S.G., Bae S.S., Lim J.K., Cho Y., Kim Y.J., Jeon J.H., Cha S.S., Kwon K.K., Kim H.T., et al. The complete genome sequence of Thermococcus onnurineus NA1 reveals a mixed heterotrophic and carboxydotrophic metabolism. J. Bacteriol. 2008;190:7491–7499. doi: 10.1128/JB.00746-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canganella F., Jones W.J., Gambacorta A., Antranikian G. Thermococcus guaymasensis sp. nov. and Thermococcus aggregans sp. nov., two novel thermophilic archaea isolated from the Guaymas Basin hydrothermal vent site. Int. J. Syst. Bacteriol. 1998;48 Pt 4:1181–1185. doi: 10.1099/00207713-48-4-1181. [DOI] [PubMed] [Google Scholar]
- 52.Pledger R.J., Baross J.A. Characterization of an Extremely Thermophilic Archaebacterium Isolated from a Black Smoker Polychaete (Paralvinella Sp) at the Juan De Fuca Ridge. Syst. Appl. Microbiol. 1989;12:249–256. doi: 10.1016/S0723-2020(89)80070-0. [DOI] [Google Scholar]
- 53.Jung J.H., Holden J.F., Seo D.H., Park K.H., Shin H., Ryu S., Lee J.H., Park C.S. Complete genome sequence of the hyperthermophilic archaeon Thermococcus sp. strain CL1, isolated from a Paralvinella sp. polychaete worm collected from a hydrothermal vent. J. Bacteriol. 2012;194:4769–4770. doi: 10.1128/JB.01016-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao W., Xiao X. Complete genome sequence of Thermococcus eurythermalis A501, a conditional piezophilic hyperthermophilic archaeon with a wide temperature range, isolated from an oil-immersed deep-sea hydrothermal chimney on Guaymas Basin. J. Biotechnol. 2015;193:14–15. doi: 10.1016/j.jbiotec.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 55.Fiala G., Stetter K.O. Pyrococcus-Furiosus Sp-Nov Represents a Novel Genus of Marine Heterotrophic Archaebacteria Growing Optimally at 100-Degrees C. Arch. Microbiol. 1986;145:56–61. doi: 10.1007/Bf00413027. [DOI] [Google Scholar]
- 56.Callac N., Oger P., Lesongeur F., Rattray J.E., Vannier P., Michoud G., Beauverger M., Gayet N., Rouxel O., Jebbar M., Godfroy A. Pyrococcus kukulkanii sp. nov., a hyperthermophilic, piezophilic archaeon isolated from a deep-sea hydrothermal vent. Int. J. Syst. Evol. Microbiol. 2016;66:3142–3149. doi: 10.1099/ijsem.0.001160. [DOI] [PubMed] [Google Scholar]
- 57.Erauso G., Reysenbach A.L., Godfroy A., Meunier J.R., Crump B., Partensky F., Baross J., Marteinsson V., Barbier G., Pace N., Prieur D. Pyrococcus-Abyssi Sp-Nov, a New Hyperthermophilic Archaeon Isolated from a Deep-Sea Hydrothermal Vent. Arch. Microbiol. 1993;160:338–349. [Google Scholar]
- 58.González J.M., Masuchi Y., Robb F.T., Ammerman J.W., Maeder D.L., Yanagibayashi M., Tamaoka J., Kato C. Pyrococcus horikoshii sp. nov., a hyperthermophilic archaeon isolated from a hydrothermal vent at the Okinawa Trough. Extremophiles. 1998;2:123–130. doi: 10.1007/s007920050051. [DOI] [PubMed] [Google Scholar]
- 59.Reysenbach A.L., Liu Y., Banta A.B., Beveridge T.J., Kirshtein J.D., Schouten S., Tivey M.K., Von Damm K.L., Voytek M.A. A ubiquitous thermoacidophilic archaeon from deep-sea hydrothermal vents. Nature. 2006;442:444–447. doi: 10.1038/nature04921. [DOI] [PubMed] [Google Scholar]
- 60.Hafenbradl D., Keller M., Dirmeier R., Rachel R., Rossnagel P., Burggraf S., Huber H., Stetter K.O. Ferroglobus placidus gen nov, sp nov, a novel hyperthermophilic archaeum that oxidizes Fe2+ at neutral pH under anoxic conditions. Arch. Microbiol. 1996;166:308–314. doi: 10.1007/s002030050388. [DOI] [PubMed] [Google Scholar]
- 61.Kurr M., Huber R., Konig H., Jannasch H.W., Fricke H., Trincone A., Kristjansson J.K., Stetter K.O. Methanopyrus-Kandleri, Gen and Sp-Nov Represents a Novel Group of Hyperthermophilic Methanogens, Growing at 110-Degrees-C. Arch. Microbiol. 1991;156:239–247. doi: 10.1007/Bf00262992. [DOI] [Google Scholar]
- 62.Ishida Y., Inouye K., Ming O., Inouye M. A CUGGU/UUGGU-specific MazF homologue from Methanohalobium evestigatum. Biochem. Biophys. Res. Commun. 2019;518:533–540. doi: 10.1016/j.bbrc.2019.08.076. [DOI] [PubMed] [Google Scholar]
- 63.Jeanthon C., L'Haridon S., Reysenbach A.L., Corre E., Vernet M., Messner P., Sleytr U.B., Prieur D. Methanococcus vulcanius sp. nov., a novel hyperthermophilic methanogen isolated from East Pacific Rise, and identification of Methanococcus sp. DSM 4213T as Methanococcus fervens sp. nov. Int. J. Syst. Bacteriol. 1999;49 Pt 2:583–589. doi: 10.1099/00207713-49-2-583. [DOI] [PubMed] [Google Scholar]
- 64.Sakai S., Takaki Y., Miyazaki M., Ogawara M., Yanagawa K., Miyazaki J., Takai K. Methanofervidicoccus abyssi gen. nov., sp. nov., a hydrogenotrophic methanogen, isolated from a hydrothermal vent chimney in the Mid-Cayman Spreading Center, the Caribbean Sea. Int. J. Syst. Evol. Microbiol. 2019;69:1225–1230. doi: 10.1099/ijsem.0.003297. [DOI] [PubMed] [Google Scholar]
- 65.Bult C.J., White O., Olsen G.J., Zhou L., Fleischmann R.D., Sutton G.G., Blake J.A., FitzGerald L.M., Clayton R.A., Gocayne J.D., et al. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 66.Jeanthon C., L'Haridon S., Reysenbach A.L., Vernet M., Messner P., Sleytr U.B., Prieur D. Methanococcus infernus sp. nov., a novel hyperthermophilic lithotrophic methanogen isolated from a deep-sea hydrothermal vent. Int. J. Syst. Bacteriol. 1998;48:913–919. doi: 10.1099/00207713-48-3-913. [DOI] [PubMed] [Google Scholar]
- 67.Martens-Habbena W., Berube P.M., Urakawa H., de la Torre J.R., Stahl D.A. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature. 2009;461:976–979. doi: 10.1038/nature08465. [DOI] [PubMed] [Google Scholar]
- 68.Qin W., Heal K.R., Ramdasi R., Kobelt J.N., Martens-Habbena W., Bertagnolli A.D., Amin S.A., Walker C.B., Urakawa H., Könneke M., et al. Nitrosopumilus maritimus gen. nov., sp. nov., Nitrosopumilus cobalaminigenes sp. nov., Nitrosopumilus oxyclinae sp. nov., and Nitrosopumilus ureiphilus sp. nov., four marine ammonia-oxidizing archaea of the phylum Thaumarchaeota. Int. J. Syst. Evol. Microbiol. 2017;67:5067–5079. doi: 10.1099/ijsem.0.002416. [DOI] [PubMed] [Google Scholar]
- 69.Santoro A.E., Dupont C.L., Richter R.A., Craig M.T., Carini P., McIlvin M.R., Yang Y., Orsi W.D., Moran D.M., Saito M.A. Genomic and proteomic characterization of "Candidatus Nitrosopelagicus brevis": An ammonia-oxidizing archaeon from the open ocean. P Natl Acad Sci USA. 2015;112:1173–1178. doi: 10.1073/pnas.1416223112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahlgren N.A., Chen Y., Needham D.M., Parada A.E., Sachdeva R., Trinh V., Chen T., Fuhrman J.A. Genome and epigenome of a novel marine Thaumarchaeota strain suggest viral infection, phosphorothioation DNA modification and multiple restriction systems. Environ. Microbiol. 2017;19:2434–2452. doi: 10.1111/1462-2920.13768. [DOI] [PubMed] [Google Scholar]
- 71.Bayer B., Vojvoda J., Reinthaler T., Reyes C., Pinto M., Herndl G.J. Nitrosopumilus adriaticus sp. nov. and Nitrosopumilus piranensis sp. nov., two ammonia-oxidizing archaea from the Adriatic Sea and members of the class Nitrososphaeria. Int. J. Syst. Evol. Microbiol. 2019;69:1892–1902. doi: 10.1099/ijsem.0.003360. [DOI] [PubMed] [Google Scholar]
- 72.Park S.J., Kim J.G., Jung M.Y., Kim S.J., Cha I.T., Kwon K., Lee J.H., Rhee S.K. Draft Genome Sequence of an Ammonia-Oxidizing Archaeon, "Candidatus Nitrosopumilus koreensis" AR1, from Marine Sediment. J. Bacteriol. 2012;194:6940–6941. doi: 10.1128/Jb.01857-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhong H., Lehtovirta-Morley L., Liu J., Zheng Y., Lin H., Song D., Todd J.D., Tian J., Zhang X.H. Novel insights into the Thaumarchaeota in the deepest oceans: their metabolism and potential adaptation mechanisms. Microbiome. 2020;8:78. doi: 10.1186/s40168-020-00849-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu J., Zheng Y., Lin H., Wang X., Li M., Liu Y., Yu M., Zhao M., Pedentchouk N., Lea-Smith D.J., et al. Proliferation of hydrocarbon-degrading microbes at the bottom of the Mariana Trench. Microbiome. 2019;7:47. doi: 10.1186/s40168-019-0652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Uritskiy G.V., DiRuggiero J., Taylor J. MetaWRAP-a flexible pipeline for genome-resolved metagenomic data analysis. Microbiome. 2018;6:158. doi: 10.1186/s40168-018-0541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parks D.H., Imelfort M., Skennerton C.T., Hugenholtz P., Tyson G.W. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chaumeil P.A., Mussig A.J., Hugenholtz P., Parks D.H. GTDB-Tk: a toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics. 2019;36:1925–1927. doi: 10.1093/bioinformatics/btz848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hyatt D., Chen G.L., Locascio P.F., Land M.L., Larimer F.W., Hauser L.J. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinf. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., Bealer K., Madden T.L. BLAST+: architecture and applications. BMC Bioinf. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 81.Apweiler R., Bairoch A., Wu C.H., Barker W.C., Boeckmann B., Ferro S., Gasteiger E., Huang H., Lopez R., Magrane M., et al. UniProt: the Universal Protein knowledgebase. Nucleic Acids Res. 2004;32:D115–D119. doi: 10.1093/nar/gkh131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 84.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haro-Moreno J.M., Rodriguez-Valera F., López-García P., Moreira D., Martin-Cuadrado A.B. New insights into marine group III Euryarchaeota, from dark to light. ISME J. 2017;11:1102–1117. doi: 10.1038/ismej.2016.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kanehisa M., Sato Y., Kawashima M., Furumichi M., Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44:D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kanehisa M., Sato Y., Morishima K. BlastKOALA and GhostKOALA: KEGG Tools for Functional Characterization of Genome and Metagenome Sequences. J. Mol. Biol. 2016;428:726–731. doi: 10.1016/j.jmb.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 88.Yin Y., Mao X., Yang J., Chen X., Mao F., Xu Y. dbCAN: a web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2012;40:W445–W451. doi: 10.1093/nar/gks479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Finn R.D., Clements J., Eddy S.R. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 2011;39:W29–W37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Buchfink B., Xie C., Huson D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods. 2015;12:59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- 91.Rawlings N.D., Barrett A.J., Bateman A. Using the MEROPS Database for Proteolytic Enzymes and Their Inhibitors and Substrates. Curr. Protoc. Bioinformatics. 2014;48:1.25.1–1.25.33. doi: 10.1002/0471250953.bi0125s48. [DOI] [PubMed] [Google Scholar]
- 92.Yu N.Y., Wagner J.R., Laird M.R., Melli G., Rey S., Lo R., Dao P., Sahinalp S.C., Ester M., Foster L.J., Brinkman F.S.L. PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics. 2010;26:1608–1615. doi: 10.1093/bioinformatics/btq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Almagro Armenteros J.J., Tsirigos K.D., Sønderby C.K., Petersen T.N., Winther O., Brunak S., von Heijne G., Nielsen H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019;37:420–423. doi: 10.1038/s41587-019-0036-z. [DOI] [PubMed] [Google Scholar]
- 94.Guéguen L., Gaillard S., Boussau B., Gouy M., Groussin M., Rochette N.C., Bigot T., Fournier D., Pouyet F., Cahais V., et al. Bio++: efficient extensible libraries and tools for computational molecular evolution. Mol. Biol. Evol. 2013;30:1745–1750. doi: 10.1093/molbev/mst097. [DOI] [PubMed] [Google Scholar]
- 95.Wang Y., Tang H., Debarry J.D., Tan X., Li J., Wang X., Lee T.H., Jin H., Marler B., Guo H., et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krzywinski M., Schein J., Birol I., Connors J., Gascoyne R., Horsman D., Jones S.J., Marra M.A. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saranpuetti C., Tanaka M., Sone T., Asano K., Tomita F. Determination of enzymes from Colletotrichum sp. AHU9748 essential for lepidimoide production from okra polysaccharide. J. Biosci. Bioeng. 2006;102:452–456. doi: 10.1263/jbb.102.452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All clean reads of metagenomic datasets used in this study have been uploaded to NCBI Sequence Read Archive in our recent work (Zhong et al.).73 The 13 assembled MGII MAGs have been deposited in NCBI. Accession numbers are listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.