Abstract
Chitosan (CS) is only soluble in weak acid medium, thereby limiting its wide utilisation in the field of biomedicine, food, and agriculture. In this report, we present a method for preparing water-soluble CS oligosaccharides (COSs) at high concentration (∼10%, w/v) via the oxidative hydrolysis of CS powder with molecular weight (Mw) ∼90,000 g/mol) in 2% H2O2 solution at ambient temperature by a two-step process, namely, the heterogeneous hydrolysis step and homogeneous hydrolysis step. The resultant COSs were characterised by gel permeation chromatography (GPC), fourier transforms infrared spectroscopy (FT-IR), ultraviolet–visible spectroscopy (UV–Vis), proton nuclear magnetic resonance spectroscopy (1H NMR) and X-ray diffraction (XRD) spectroscopy. The resulting products were composed of COSs (Mw of 2000−6600 g/mol) that were completely soluble in water. The results also indicated that the structure of COSs was almost unchanged compared with the original CS unless Mw was low. Accordingly, COSs with low Mw (∼2000 g/mol) and high concentration (10%, w/v) could be effectively prepared by the oxidative hydrolysis of CS powder using hydrogen peroxide under ambient conditions.
Keywords: Chitosan oligosaccharide, Oxidative hydrolysis, Powder, Hydrogen peroxide
1. Introduction
In recent decades, many studies have shown that chitosan oligosaccharide (COS) is a biomaterial with potential application in biomedical pharmaceutical and agricultural applications. COS with low Mw can completely dissolve in water, creating remarkably high biological properties such as antibacterial, antioxidant and inflammatory functions; moreover, it can lower blood pressure, deliver drugs, prevent obesity, avoid HIV and decrease cholesterol in the blood [1]. In agriculture, low-Mw CS acts as a potent bio-stimulant, which induces anaphylactic reactions in plants and activates the production of resistance against pathogen invasion [2].
CS has high Mw and a high degree of polymerization (DP) so it has low solubility at neutral or alkaline pH; the high viscosity of CS solutions is also their limiting factor when applied to cosmetic, food, medical and agricultural industries [3]. In terms of solubility, CS with DP < 10 is readily soluble in water; however, the water solubility of CS depends on the degree of redox reduction and the pH of the solution [4]. Currently, the Mw of CS can be adjusted to that of COS to improve solubility via many methods. In the physical method, CS is hydrolyzed with the help of microwaves and added with chemical agents such as salts or acids [5] by gamma Co-60 irradiation [6] or a combination of gamma Co-60 irradiation with H2O2 treatment [7], ultrasound [8], microwave [9] and plasma solution [10,11]. Photocatalysis method is oxidation of CS solution by fluorescent light source with TiO2 catalyst [12]. In the electrochemical method, COS can be prepared by placing Ti/TiO2–RuO2 [13] or Ti/Sb–SnO2 electrodes [14] in CS solution. The disadvantage of the electrochemical method is the short life of the electrode. In the biological method, chitinase, chitosanase, glucanase, protease, and lysozyme enzymes are used to cleave the β-¼ glycoside bonds of CS; this method is safe but costly [15].
Chemical methods to adjust the Mw of CS include the use of phosphonic acid [16], hydrochloric acid [17], and nitrous acid [18]; the use of oxidants such as hydrogen peroxide, ozone, sodium perborate and potassium persulfate [19]; or the use of H2O2 with a phosphotungstic acid catalyst [20]. The method of using hydrogen peroxide to degrade CS has been reported as inexpensive and safe for the environment [21]. In this method, if using a high concentration of H2O2 or high temperature, the hydrolysis reaction occurs quickly but affects the structure of COS such as opening the glucosamine ring or creating a carboxyl functional group (–COOH) [22]. The suitable concentration of H2O2 to carry out the oxidative hydrolysis of CS has been reported to be 2% (v/v) [20,23]. Most of the previously published studies on oxidative hydrolysis of CS using H2O2 were carried out in an acidic CS solution. In this report, we use a new approach involving the oxidation of CS powder in 2% H2O2 solution to generate water-soluble COSs at pH 7−10 with high concentration (10%, w/v).
2. Materials and methods
2.1. Materials
Chemicals used in the experiment were of analytical grade, including H2O2 30%, NaOH, NH4OH and CH3COOH of Xilong, China; potassium permanganate of Duc Giang, Vietnam; CS powder with particle size ≤0.1 mm and Mw ∼90,000 g/mol (Suntze Chemical, Vietnam); and pullulan with Mw of 1300; 6000; 12000; 22000; 50000 and 110000 g/mol (Sigma Aldrich, Germany). Deionized water with pH 7 was used throughout the experiment.
2.2. Methods
2.2.1. Preparation of water-soluble COSs
About 10 g of CS powder was soaked in 100 mL of 2% H2O2 (v/v) solution in a 250 mL beaker and the 12 identical samples were prepared and placed at room temperature (25 °C −28 °C). After every 6 h, the mixture of CS/H2O2 solution was stirred for about 5 min. In the first step of the degradation process namely, namely, the heterogeneous hydrolysis step, after 48 h (2 days) of the reaction time, the mixture of CS/H2O2 was filtered to collect a solution containing water-soluble CS and unreacted H2O2. The concentration of unreacted H2O2 was determined by titration with KMnO4 [24]. Undissolved CS powder was also collected to determine the dissolved CS yield. On the basic of the results of the determined H2O2, the equally consumed amount of H2O2 was added to the remaining CS/H2O2 samples to reach 2% (v/v). In the second step of the degradation process, namely, the homogeneous hydrolysis step, when all CS powder was dissolved into solution, then H2O2 was not further added to compensate for the reacted amount of H2O2 and the soluble CS/H2O2 solution was left to proceed continuously to the hydrolysis reaction without compensation of H2O2. After every 2 days, a certain amount of the soluble CS/H2O2 solution was removed and vacuum dried to constant weight to obtain a powder sample. Notably, the beaker containing sample after being used to determine the amount of unreacted H2O2 and the characteristic properties of COS was not used further. The experiment was carried out with three replicates and the values were presented as mean ± standard error (SE).
The amount of water-soluble COS in the studied samples was determined according to the method of Herdiana et al. [25]: the mixture of CS/H2O2 solution was filtered on a quantitative filter paper with a pore size of 2 μm to collect the insoluble part of CS and dried to constant weight (m). The amount of COS dissolved in water (S) was calculated according to the following Formula (1):
| (1) |
The filtrate containing dissolved COS was placed in a 250 mL beaker and vacuum dried to constant weight, the obtained COS powder was used to determine the specific properties and solubility at pH 7−10. The solubility of COS at different pH values was conducted following the method of Vasilieva et al. [26]: 1g of COS was dissolved in 10 mL of deionized water and the pH of the solution was adjusted by NH4OH. The amount of COS dissolved was determined by filtration.
2.2.2. Structure and DD determination of CS and COS by 1H NMR spectroscopy
The 1H NMR spectra of original CS and COS were measured on a 500 Mhz Advance III HD nuclear magnetic resonance spectrometer (Bruker Biospin, Switzerland) by using a 5 mm BBO probe. The solvent used was D2O + CD3COOH. DD of CS and COS was calculated from the 1H NMR spectrum according to Formula (2) [27]:
| (2) |
2.2.3. Determination of Mw, retention time (RT) of COS by GPC
The COS powder sample was dissolved in a mixture of 0.25 M CH3COOH + 0.25 M CH3COONa. The Mw and retention time (RT) were determined by GPC LC-20AD (Shimadzu, Japan). This device uses an RID 20A detector, Shodex SB803 column HQ, using pullulan standard with Mw from 780 g/mol to 105 g/mol.
2.2.4. Determination of ptical properties of COS by UV–Vis
CS and COS samples were disolved at 0,1% (w/v) in 0.2% CH3COOH (v/v) and the UV–Vis spectra were measured on UV–Vis JASCO V630 equipment (Japan) [28].
2.2.5. Determination of crystal structures of materials by XRD
The XRD patterns were measured on XRD D8-Advance (Bruker, Germany), using Cu Kα (λ = 1.5405 Å) radiation with a constant voltage of 40 kV and diffraction angle 2θ scans from 10° to 80° [29].
2.2.6. Determination of functional groups of materials by FT-IR
The FT-IR spectra of COS samples were determined on a FT-IR 8400S (Shimadzu, Japan) with a wavenumber range of 4000−400 cm−1 [30].
3. Results
3.1. Yield of water-soluble COS
The results in Table 1 indicated that the yield of water-soluble CS increased with the increase in the hydrolysis time of CS powder by H2O2. Simultaneously, the Mw of the resultant soluble COS decreased gradually with increasing reaction time.
Table 1.
Yield of water-soluble COS, Mw, retention time (RT), polydispersity index (PI) and H2O2 reacted after every 48 h according to the reaction time.
| Steps | Samples | Mw × 10−3 (g/mol) | PI | H2O2 reacted (%) | H2O2 in solution (%) | Yield of soluble CS (%) |
|---|---|---|---|---|---|---|
| Step1: heterogeneous hydrolysisa | CS | 90.06 ± 0.17 | 1.95 ± 0.02 | 0.00 | 2,00 | ∼0.00 |
| COS2d | 9.98 ± 0.08 | 2.74 ± 0.02 | 1.01 | 2,00 | 18.96 ± 0.25 | |
| COS4d | 9.24 ± 0.07 | 2.43 ± 0.02 | 1.95 | 2.00 | 46.33 ± 0.43 | |
| COS6d | 8.89 ± 0.06 | 1.84 ± 0.02 | 2.82 | 2.00 | 76.15 ± 0.81 | |
| Step 2: homogeneous hydrolysisb | COS8d | 6.57 ± 0.05 | 1.76 ± 0.01 | 3.60 | 1.22 | ∼100 |
| COS10d | 3.97 ± 0.05 | 1.54 ± 0.02 | 4.20 | 0.62 | ∼100 | |
| COS12d | 2.68 ± 0.07 | 1.37 ± 0.02 | 4.60 | 0.22 | ∼100 | |
| COS14d | 2.22 ± 0.05 | 1.23 ± 0.02 | 4.64 | 0.18 | ∼100 | |
| COS16d | 2.09 ± 0.05 | 1.23 ± 0.02 | 4.71 | 0.11 | ∼100 | |
| COS18d | 2.07 ± 0.04 | 1.22 ± 0.02 | 4.76 | 0.06 | ∼100 | |
| COS20d | 2.07 ± 0.03 | 1.22 ± 0.02 | 4.79 | 0.03 | ∼100 | |
| COS22d | 2.06 ± 0.01 | 1.22 ± 0.01 | 4.80 | 0.02 | ∼100 | |
| COS24d | 2.04 ± 0.02 | 1.22 ± 0.01 | 4.82 | ∼0.00 | ∼100 |
Notes: PI = Mw/Mn; The number after the COS symbol was the reaction time in days.
With compensation of the reacted H2O2 after every 48 h.
Without compensation of the reacted H2O2.
In step 1, when the Mw of CS was still high and not completely dissolved in water, the amount of H2O2 was reduced after 48 h cycle by 40–50%, calculated as 2% H2O2 at the beginning of each additional cycle (sample COS2–COS6). Therefore, the first step of replenishing the amount of H2O2 lost is necessary to shorten the hydrolysis time of CS. The second step of CS hydrolysis without the compensation of H2O2, at the end of the hydrolysis process, the amount of H2O2 is completely reacted or consumed to obtain COS solution without remaining H2O2.
Interestingly, the results of PI of COS in Table 1 increased from 1.95 (CS) to 2.74 (COS2d) and then gradually decreased to 1.84 (COS6d) in step 1. As the reaction time increased, the PI of COS decreased spontaneously from 1.76 (COS8d) to 1.22 (from COS18d to COS24d) in step 2. The results in Table 1 also showed that the total amount of reacted H2O2 to obtain COS with Mw ∼2000 g/mol was 4.82% after the reaction time of 24 days. In general, a low the PI results in a narrower Mw distribution. In the heterogeneous hydrolysis of CS powder in step 1, the oxidizing agent H2O2 preferentially attacks CS molecules with high Mw only on the interface of solid and liquid phases. The highest PI value of COS was 2.74 after 2 days of reaction (COS2d). After this reaction time, the PI of COS was in a competitive distribution from the degradation of soluble COS in solution and the degradation of CS in the solid phase. Therefore, the PI of COS should be decreased to a moderate level after this period. After three H2O2 compensations, COS8d was almost soluble in the reaction medium, so the hydrolysis reaction of COS occurred in a homogeneous medium. So, the PI value decreased gradually with the increase in the reaction time. Furthermore, the obtained results of Mw, PI and yield of dissolve CS in Table 1 showed that the repeatability of the experiment was fairly good. The mode of the hydrolysis reaction of COS in step 2 was almost similar to that in the study of Hai et al. [21], who carried out the homogeneous hydrolysis of CS in acid solution by H2O2 at ambient temperature. The heterogeneous hydrolysis of CS using 2% H2O2 in step 1 with three H2O2 compensations created a COS solution with a high concentration (10%, w/v) that was difficult to prepare by dissolving CS in acid solution. This COS concentration was significantly higher than that in the research results of other authors listed in Table 2.
Table 2.
Comparison of various methods for the degradation of CS to prepare COS..
| Reagent /equipment/To |
Final CS conc. (%, w/v) | Degraded time | Mw of COS | Reference |
|---|---|---|---|---|
| H2O2/Vc, ultrasound irradiation, 25 °C | 0.2 | 1 h | ∼7.67 kDa | [8] |
| H2O2, 80 °C | ∼3 | 3 h | 1360 g/mol | [9] |
| H2O2, 80 °C, microwave irradiation | ∼3 | 25 min | 1460 | [9] |
| H2O2, solution plasma, RT°C | 0.5 | 1 h | ∼1.44−4.92 kDa | [10] |
| Solution plasma, RT°C | 0.5 | 0.5−1.5 h | ∼4.6−7.8 kDa | [11] |
| Purified cellulase, 50 °C | ∼5 | 12−24 h | ∼1.4−2.3 kDa | [31] |
| H2O2, RT°C | 3 | 360 h | ∼4.5 kDa | [21] |
| Commercial protease, 50 °C | ∼4 | 0,5−1 h | DP 3−8 | [32] |
| H2O2, 65 °C | ∼2 | 0−48 h | ∼1.2−7.7 kDa | [22] |
| Purified hemicellulase, 50 °C | ∼5 | 4 h | 1.4 kDa | [33] |
| H2O2, Phosphotungstic acid, 65 °C | ∼1 | 40 min | DP 2−9 | [20] |
| Branchzyme, 50 °C | ∼2 | 24 h | DP 2−20 | [34] |
| Chitosanase, 30 °C | ∼2 | 0.5−6 h | DP 2−7 | [35] |
| H2O2, gamma irradiation, RT°C | 5 | 4−16 h | 8.6−2.7 kDa | [36] |
| Gamma irradiation, RT°C | 5 | 24 h | ∼10 kDa | [36] |
| H2O2, food-grade cellulase, 55 °C | ∼9 | 6 h | DP 2−9 | [37] |
| H2O2, two-step, RT°C | ∼10 | 16−24 days | ∼2 kDa | This study |
In addition, COS prepared by a two-step degradation process, particularly heterogeneous and homogeneous hydrolysis steps, had a low Mw (2000 g/mol) without using organic acid for solubilizing CS. Thus, the method used in this study could be favorably applied to large-scale applications to produce water-soluble COS with low Mw and high concentration because of the relatively simple production process. Furthermore, this method was carried out at ambient temperature and the solvent of the COS product was only water, not acid solution. Although this method had a long reaction time, it did not require energy for heating and treating reaction media. Therefore, this method could be considered an energy saving method [21].
Notably, after a hydrolysis time of 18 days, the amount of H2O2 remaining in the solution was less than 0.06%. The time needed to completely decompose H2O2 in the COS18d sample to obtain COS24d at room temperature was about 6 days which could be recognized by adding 1 drop of KMnO4 solution into the COS24d sample; the purple-violet color of KMnO4 in COS24d solution did not disappeared [23].
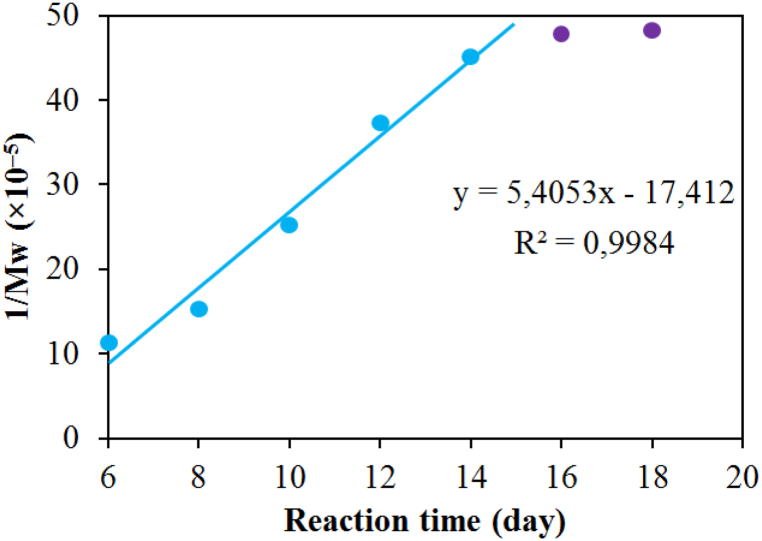
According to Hai et al. [21] and Chang et al. [38], the oxidative reaction of H2O2 for the degradation of CS belongs to a pseudo-first-order kinetic model. The relationship between the reciprocal of Mw and the reaction time in step 2 is presented in Fig. 1. The linear relationship of the oxidative hydrolysis of COS solution in the initial period was from 8 days to 14 days. The rate constant k (1/time) and k’ (mol/g/time) can be calculated according to Formula (3) as follows [27]:
| (3) |
Where the MMw is the average Mw of CS with DD of 93.47% calculated based on Formula (4) [39]:
| MMw = DD × Mwglucosamine + (1 – DD) × MwN –acetylglucosamine | (4) |
Fig. 1.
Dependence of 1/Mw on reaction time.
MMw calculated by Formula (4) was 181.92 g/mol k’ and k could be inferred to be 2.25 × 10−6 mol/g/h and 4.09 × 10−4 h−1, respectively. The rate constant (k’ and k) values obtained in this study for the oxidative hydrolysis of CS using 2% H2O2 were four times higher than that using a low H2O2 concentration of 1% in the study of Hai et al. [21]. The degraded reaction occurred at a high rate, so the reaction time could be shortened, and the obtained COS still retained the original CS structure (see sections 3.3−3.5).
The breakdown of CS to COS with a smaller Mw was due to the formation of free radicals •OH according to Equations (5), (6), which then act through scission reaction on polysaccharide (CS) to form oligosaccharide (COS) [40].
| (5) |
| (6) |
The •OH group is a strong oxidizing agent. It reacted with CS by capturing a hydrogen atom from the C–H bond on the glucose ring, followed by transposing to the 1,4-β-D-glucoside bond in the CS chain [38,40,41], according to Equations (7), (8):
| (7) |
| (8) |
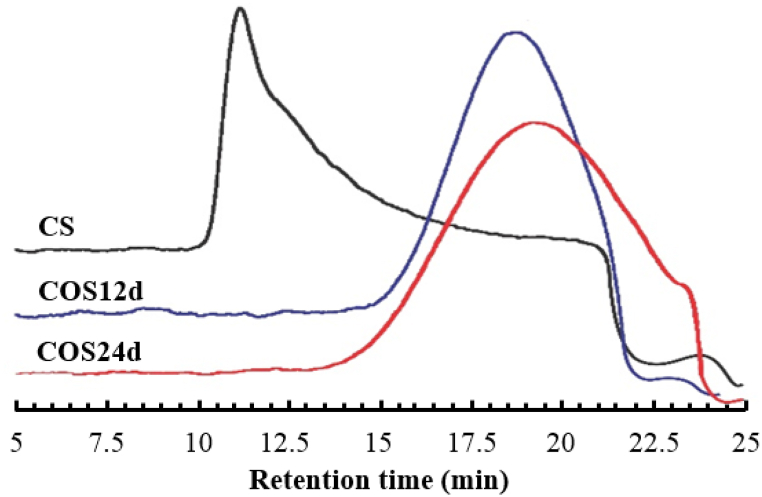
The GPC chromatograms of the original CS and COSs that were completely soluble at pH 8−10 (COS12d with Mw ∼2600 g/mol), and COS24d with Mw ∼2000 g/mol) are shown in Fig. 2.
Fig. 2.
GPC chromatogram of CS, COS12d and COS24d.
3.2. Solubility of COS in water and alkaline pH medium
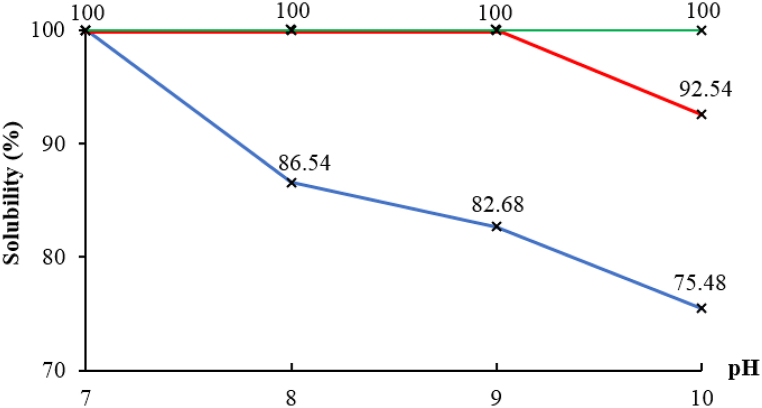
The water-soluble COS samples obtained from the oxidative hydrolysis of CS powder by H2O2 with a yield of solubility at 100% were used to investigate the solubility at pH 7−10. COS powder samples dissolved in different pH solutions with a concentration of 10% (w/v) and the results of the solubility percentage are presented in Fig. 3.
Fig. 3.
Solubility of COS samples at pH 7−10.
The results in Fig. 3 showed that COS8d with Mw of 6570 g/mol was completely soluble at pH 7 but the solubility at pH 8, 9 and 10 was 86.54, 82.68% and 75.48%, respectively. COS10d was completely soluble at pH 7−9 but had a solubility of 92.54% at pH 10. COS12d to COS24d with Mw ≤ 2680 g/mol completely dissolved at pH 7−10. Thus, the solubility of COS depended on Mw; the smaller the Mw, the higher the solubility at weak alkaline pH. According to Quin et al. [33], CS with low Mw reduces the ability of intermolecular interactions because reduced steric effects such as van der Waals forces and hydrogen bonding, so it had high solubility in aqueous solvents. This study showed that the solubility of COS at different pH depended on Mw, which was similar to the findings of Thuy et al. [7], who reported that a small Mw leads to high solubility of COS in alkaline pH. Chang et al. [42] also suggested that low Mw COS reduces intramolecular hydrogen bonding strength, thereby increasing its solubility in water due to increasing the flexibility of hydroxyl and amine groups.
3.3. 1H NMR spectra and deacetylation degree of the original CS and soluble COS
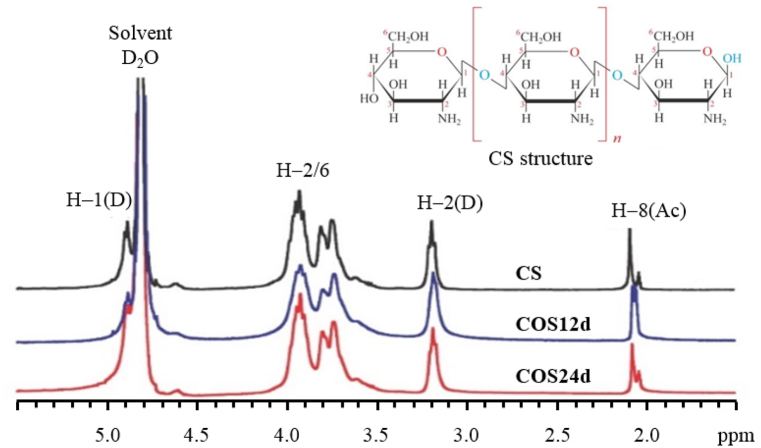
Fig. 4 presents the 1H NMR spectra of the original CS and the COSs that were completely soluble at pH 7−10 (COS12d and COS24d). The 1H NMR spectra of all samples exhibited resonance signal peaks at chemical shifts characterizing CS molecules according to Pereira et al. [43], Vidal et al. [44], Badawy et al. [45], and Jafari et al. [46].
Fig. 4.
1H NMR spectra of original CS, COS12d and COS24d.
The characteristic signals of CS in the 1H NMR spectrum include δ 2.0−2.1 ppm of 03 protons belonging to the acetyl group and δ 3.2 ppm characterised the H2 proton. Some overlapping signals were observed at positions δ 3.5−4.0 ppm characterising the H3−6 proton linked to C3 and C4 of the glucose pyranose ring. δ at position 4.9 ppm represents the H1 proton. DD data were calculated using the Equation of Lavertu et al. [27].
The 1H NMR spectra in Fig. 4 and Formula (2) for DD calculation showed that the original CS had DD of 93.47% ± 0.40%. After oxidative hydrolysis to form COS, the DD values decreased slightly to 92.50% ± 0.16% and 91.18% ± 0.23% for COS12d and COS24d, respectively. This change was not significantly different from the initial CS sample. Thus, the hydrolysis of CS powder at room temperature with 2% H2O2 solution hardly changed the DD of COS compared with the original CS. It was almost similar to the results of some previous authors using an H2O2 concentration of 1%, combined with gamma irradiation [7] or microwave irradiation [46]. The results of the 1H NMR spectroscopy and DD calculation of CS and COS above confirmed that almost no change occurred in the molecular structure during oxidative hydrolysis except low-Mw COS with C=O group at the end of the COS molecular chain (see Fig. 4, Fig. 5).
Fig. 5.
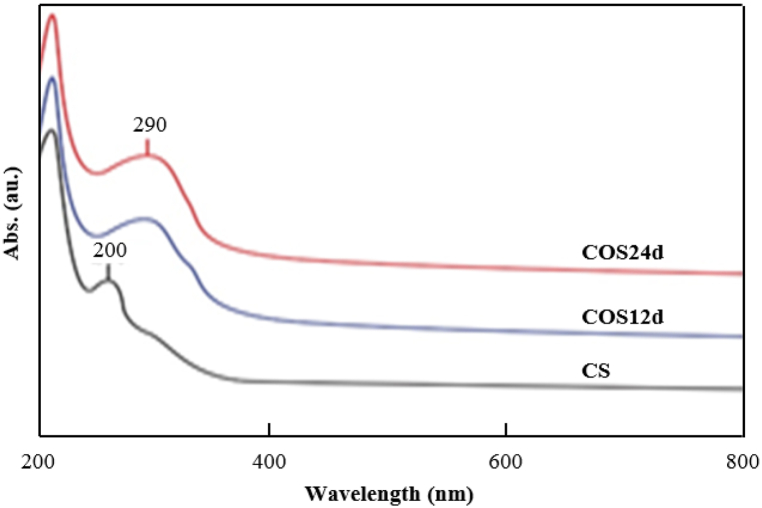
UV–Vis spectrum of original CS, COS12d and COS24d.
3.4. UV–Vis spectrum of CS and COSs
The UV–Vis spectra of the original CS, COS12d and COS24d are shown in Fig. 5. The original CS had a peak of 210 nm corresponding to the n→σ* electron shift of the amino group [23]. A peak at 256 nm could be typical for the carboxyl or carbonyl group possibly due to a previously oxidized CS material; this peak shifted to high wavelength, which might be due to the degradation of glycosidic bonds [47,48]. The peak at around 290 nm appeared for COS12d and COS24d, which was assigned to the carbonyl group (–C=O) [23,49]. The carbonyl group formed during the hydrolytic breakage of glycosidic bonds at C1 and C4 [50].
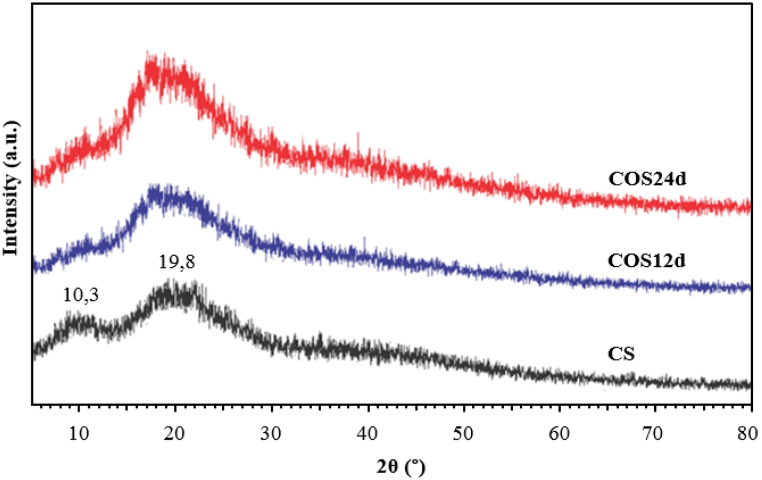
3.5. XRD patterns of CS and COSs
The XRD patterns of the studied samples CS, COS12d and COS24d are shown in Fig. 6. The XRD pattern of the original CS had signal peaks at 2θ angle located at 10.3° and 19.8° which were typically assigned for CS [[51], [52], [53]]. In the XRD patterns of the COS samples with Mw of 2040 g/mol and 2680 g/mol (COS24d and COS12d), the peak at the angle of 2θ ∼10.3° disappeared and the diffraction intensity at the angle of 2θ ∼19.8° was significantly reduced, showing that the oxidative hydrolysis of CS powder in H2O2 reduced crystallization of CS as previously reported [7,54,55].
Fig. 6.
XRD patterns of original CS, COS12d and COS24.
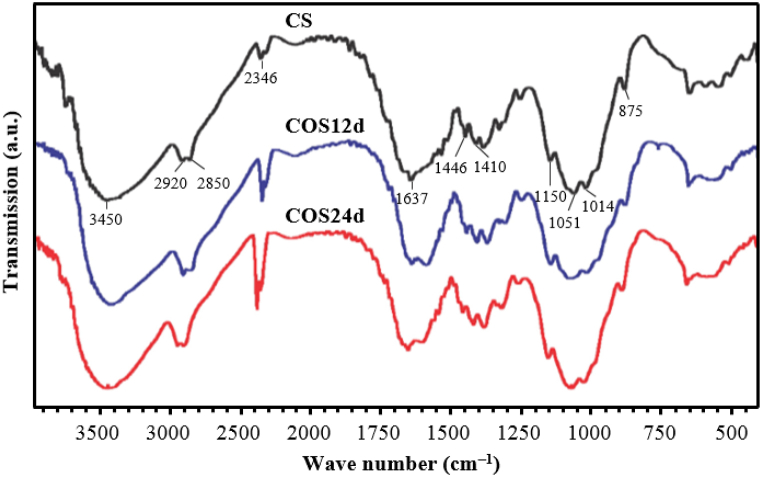
3.6. FT-IR spectra of CS and COSs
The FT-IR spectra in Fig. 7 showed that the COS samples did not appear as new peaks compared with the original CS. The signals that characterised the vibrations of the bonds in CS and COS molecules were consistent with the previous authors’ studies as follows: the O–H and N–H stretching vibration of CS at 3450 cm−1 [21,56,57] shifted to a low wavenumber for COS samples due to the reduction of intramolecular hydrogen bonding [58]. The characteristic peaks for the O–H and N–H stretching vibrations of CS in previous studies by other authors correspond to low wavenumber at 3292.31 cm−1 [59] or 3290-3270 cm−1 [60]. The above difference might be due to this characteristic vibration depending on the DD of CS, CS with low DD; the characteristic vibration was at a low wavenumber [60,61]. The peaks at 2920−2850 cm−1 were characteristic for C–H symmetric and asymmetric stretching vibrations [56,59,62,63].
Fig. 7.
FT-IR spectra of original CS, COS12d and COS24d.
The FT-IR spectra in Fig. 7 also appeared a functional group –C–OH (the secondary OH group of C3) at 2346 cm−1 [64], this vibration also appeared at 2350−2380 cm−1 in some previous studies [65,66]. The peak at 1637 cm−1 represented the C=O group bonding [62]. The covalent bond of the CH2 bending vibration was observed at the wave range of 1410−1446 cm−1 [62,67]. The peak at 1150 cm−1 represented the C–O–C stretching [61] and the peaks at 1014−1051 cm−1 represented the C–O stretching [62,63]. In the FT-IR spectrum of the original CS sample, a peak was observed at the wave number of 875 cm−1, which was typical for the C–H bending vibration of the polysaccharide [59,63].
4. Conclusion
Degradation of CS powder by oxidative hydrolysis in H2O2 solution at ambient temperature can be considered a potential method for the preparation of water-soluble COSs with low Mw (∼2000 g/mol) and high concentration (10%, w/v). The advantages of this method are environmentally safe, energy efficiency and no requirement for an organic acid solution to dissolve CS; the obtained COS solution did not contain H2O2. Furthermore, this method could be used to carry out the production of COSs on large scale because of the relatively favorable production process. The resultant COSs with low Mw that were soluble in water at neutral and slightly alkaline pH could be favorably applied in various fields.
Funding
This research was funded by the priority scientific directions of the Vietnam Academy of Science and Technology (grant No: VAST03.05/22−23) and of the Tra Vinh provincial Department of Science and Technology (grant No: CT.NN.07−2021).
Author contribution statement
Bui Duy Du: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Nguyen Quoc Hien: Conceived and designed the experiments; Wrote the paper.
Nguyen Trong Hoanh Phong, Tran Phuoc Tho: Performed the experiments.
Le Nghiem Anh Tuan: Analyzed and interpreted the data.
Data availability statement
Data included in article/supp. material/referenced in article.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
We would like to give special thanks to the Institute of Applied Materials Science-Vietnam Academy of Science and Technology for supporting our research project.
References
- 1.Naveed M., Phil L., Sohail M., Hasnat M., Baig M.M.F.A., Ihsan A.U., Shumzaid M., Kakar M.U., Khan T.M., Akabar M.D. Chitosan oligosaccharide (COS): an overview. Int. J. Biol. Macromol. 2019;129:827–843. doi: 10.1016/j.ijbiomac.2019.01.192. [DOI] [PubMed] [Google Scholar]
- 2.Orzali L., Corsi B., Forni C., Riccioni L. Chitosan in agriculture: a new challenge for managing plant disease. Biol. Act. Appl. Mar. Polysacch. 2017:17–36. [Google Scholar]
- 3.Xia W., Liu P., Zhang J., Chen J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011;25:170–179. [Google Scholar]
- 4.Liaqat F., Eltem R. Chitooligosaccharides and their biological activities: a comprehensive review. Carbohydr. Polym. 2018;184:243–259. doi: 10.1016/j.carbpol.2017.12.067. [DOI] [PubMed] [Google Scholar]
- 5.Xing R., Liu S., Yu H., Guo Z., Wang P., Li C., Li Z., Li P. Salt-assisted acid hydrolysis of chitosan to oligomers under microwave irradiation. Carbohydr. Res. 2005;340:2150–2153. doi: 10.1016/j.carres.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 6.Zainol I., Akil H.M., Mastor A. Effect of γ-irradiation on the physical and mechanical properties of chitosan powder. Mater. Sci. Eng. C. 2009;29:292–297. [Google Scholar]
- 7.Thuy N.N., Quy H.D., Duy N.N., Hien N.Q., Hai D.N. Preparation, characterization, and antioxidant activity of water-soluble oligochitosan. Green Process. Synth. 2017;6:461–468. [Google Scholar]
- 8.Wu T., Wu C., Xiang Y., Huang J., Luan L., Chen S., Hu Y. Kinetics and mechanism of degradation of chitosan by combining sonolysis with H2O2/ascorbic acid. RSC Adv. 2016;6:76280–76287. [Google Scholar]
- 9.Xing R., Liu Y., Li K., Yu H., Liu S., Yang Y., Chen X., Li P. Monomer composition of chitooligosaccharides obtained by different degradation methods and their effects on immunomodulatory activities. Carbohydr. Polym. 2017;157:1288–1297. doi: 10.1016/j.carbpol.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Chokradjaroen C., Rujiravanit R., Watthanaphanit A., Theeramunkong S., Saito N., Yamashita K., Arakawa R. Enhanced degradation of chitosan by applying plasma treatment in combination with oxidizing agents for potential use as an anticancer agent. Carbohydr. Polym. 2017;167:1–11. doi: 10.1016/j.carbpol.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Davoodbasha M., Lee S.-Y., Kim J.-W. Solution plasma mediated formation of low molecular weight chitosan and its application as a biomaterial. Int. J. Biol. Macromol. 2018;118:1511–1517. doi: 10.1016/j.ijbiomac.2018.06.168. [DOI] [PubMed] [Google Scholar]
- 12.Jawad A.H., Nawi M.A., Mohamed M.H., Wilson L.D. Oxidation of chitosan in solution by photocatalysis and product characterization. J. Polym. Environ. 2017;25:828–835. [Google Scholar]
- 13.Cai Q., Gu Z., Chen Y., Han W., Fu T., Song H., Li F. Degradation of chitosan by an electrochemical process. Carbohydr. Polym. 2010;79:783–785. [Google Scholar]
- 14.Gu Z., Cai Q., Liu Y., Li F. Electrochemical degradation of chitosan using Ti/Sb–SnO2 electrode. J. Polym. Environ. 2013;21:479–486. [Google Scholar]
- 15.Cheng C.-Y., Chang C.-H., Wu Y.-J., Li Y.-K. Exploration of glycosyl hydrolase family 75, a chitosanase from Aspergillus fumigatus. J. Biol. Chem. 2006;281:3137–3144. doi: 10.1074/jbc.M512506200. [DOI] [PubMed] [Google Scholar]
- 16.Jia Z., Shen D. Effect of reaction temperature and reaction time on the preparation of low-molecular-weight chitosan using phosphoric acid. Carbohydr. Polym. 2002;49:393–396. [Google Scholar]
- 17.Trombotto S., Ladavière C., Delolme F., Domard A. Chemical preparation and structural characterization of a homogeneous series of chitin/chitosan oligomers. Biomacromolecules. 2008;9:1731–1738. doi: 10.1021/bm800157x. [DOI] [PubMed] [Google Scholar]
- 18.Tømmeraas K., Köping-Höggård M., Vårum K.M., Christensen B.E., Artursson P., Smidsrød O. Preparation and characterisation of chitosans with oligosaccharide branches. Carbohydr. Res. 2002;337:2455–2462. doi: 10.1016/s0008-6215(02)00334-8. [DOI] [PubMed] [Google Scholar]
- 19.Tishchenko G., Šim\uunek J., Brus J., Netopilík M., Pekárek M., Walterová Z., Koppová I., Lenfeld J. Low-molecular-weight chitosans: preparation and characterization. Carbohydr. Polym. 2011;86:1077–1081. [Google Scholar]
- 20.Xia Z., Wu S., Chen J. Preparation of water soluble chitosan by hydrolysis using hydrogen peroxide. Int. J. Biol. Macromol. 2013;59:242–245. doi: 10.1016/j.ijbiomac.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 21.Hai N.T.T., Thu L.H., Nga N.T.T., Hoa T.T., Tuan L.N.A., Phu D.V., Hien N.Q. Preparation of chitooligosaccharide by hydrogen peroxide degradation of chitosan and its effect on soybean seed germination. J. Polym. Environ. 2019;27:2098–2104. [Google Scholar]
- 22.Qin C., Du Y., Xiao L., Liu Y., Yu H. Moisture retention and antibacterial activity of modified chitosan by hydrogen peroxide. J. Appl. Polym. Sci. 2002;86:1724–1730. [Google Scholar]
- 23.Wang S.-M., Huang Q.-Z., Wang Q.-S. Study on the synergetic degradation of chitosan with ultraviolet light and hydrogen peroxide. Carbohydr. Res. 2005;340:1143–1147. doi: 10.1016/j.carres.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Klassen N.V., Marchington D., McGowan H.C. H2O2 determination by the I3– method and by KMnO4 titration. Anal. Chem. 1994;66:2921–2925. [Google Scholar]
- 25.Herdiana Y., Wathoni N., Shamsuddin S., Muchtaridi M. Cytotoxicity enhancement in MCF-7 breast cancer cells with depolymerized chitosan delivery of α-mangostin. Polymers. 2022;14:3139. doi: 10.3390/polym14153139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasilieva T., Sigarev A., Kosyakov D., Ul'Yanovskii N., Anikeenko E., Chuhchin D., Ladesov A., Hein A.M., Miasnikov V. Formation of low molecular weight oligomers from chitin and chitosan stimulated by plasma-assisted processes. Carbohydr. Polym. 2017;163:54–61. doi: 10.1016/j.carbpol.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 27.Lavertu M., Xia Z., Serreqi A.N., Berrada M., Rodrigues A., Wang D., Buschmann M.D., Gupta A. A validated 1H NMR method for the determination of the degree of deacetylation of chitosan. J. Pharm. Biomed. Anal. 2003;32:1149–1158. doi: 10.1016/s0731-7085(03)00155-9. [DOI] [PubMed] [Google Scholar]
- 28.Wasikiewicz J., Yeates S. “Green” molecular weight degradation of chitosan using microwave irradiation. Polym. Degrad. Stab. 2013;98:863–867. [Google Scholar]
- 29.Dey S., Al-Amin M., Rashid T., Sultan Z., Ashaduzzaman, Sarker M., Shamsuddin S. Preparation, characterization and performance evaluation of chitosan as an adsorbent for remazol red. Int J Latest Res Eng Technol. 2016;2:52–62. [Google Scholar]
- 30.Brugnerotto J., Lizardi J., Goycoolea F.M., Argüelles-Monal W., Desbrières J., Rinaudo M. An infrared investigation in relation with chitin and chitosan characterization. Polymer. 2001;42:3569–3580. [Google Scholar]
- 31.Qin C., Zhou B., Zeng L., Zhang Z., Liu Y., Du Y., Xiao L. The physicochemical properties and antitumor activity of cellulase-treated chitosan. Food Chem. 2004;84:107–115. [Google Scholar]
- 32.Li J., Du Y., Yang J., Feng T., Li A., Chen P. Preparation and characterisation of low molecular weight chitosan and chito-oligomers by a commercial enzyme. Polym. Degrad. Stab. 2005;87:441–448. [Google Scholar]
- 33.Qin C., Du Y., Zong L., Zeng F., Liu Y., Zhou B. Effect of hemicellulase on the molecular weight and structure of chitosan. Polym. Degrad. Stab. 2003;80:435–441. [Google Scholar]
- 34.Montilla A., Ruiz-Matute A.I., Corzo N., Giacomini C., Irazoqui G. Enzymatic generation of chitooligosaccharides from chitosan using soluble and immobilized glycosyltransferase (branchzyme) J. Agric. Food Chem. 2013;61:10360–10367. doi: 10.1021/jf403321r. [DOI] [PubMed] [Google Scholar]
- 35.Qin Z., Chen Q., Lin S., Luo S., Qiu Y., Zhao L. Expression and characterization of a novel cold-adapted chitosanase suitable for chitooligosaccharides controllable preparation. Food Chem. 2018;253:139–147. doi: 10.1016/j.foodchem.2018.01.137. [DOI] [PubMed] [Google Scholar]
- 36.Hien N.Q., Phu D.V., Duy N.N., Lan N.T.K. Degradation of chitosan in solution by gamma irradiation in the presence of hydrogen peroxide. Carbohydr. Polym. 2012;87:935–938. doi: 10.1016/j.carbpol.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 37.Li M., Han J., Xue Y., Dai Y., Liu J., Gan L., Xie R., Long M. Hydrogen peroxide pretreatment efficiently assisting enzymatic hydrolysis of chitosan at high concentration for chitooligosaccharides. Polym. Degrad. Stab. 2019;164:177–186. [Google Scholar]
- 38.Chang K.L.B., Tai M.-C., Cheng F.-H. Kinetics and products of the degradation of chitosan by hydrogen peroxide. J. Agric. Food Chem. 2001;49:4845–4851. doi: 10.1021/jf001469g. [DOI] [PubMed] [Google Scholar]
- 39.Gritsch L., Lovell C., Goldmann W.H., Boccaccini A.R. Fabrication and characterization of copper(II)-chitosan complexes as antibiotic-free antibacterial biomaterial. Carbohydr. Polym. 2018;179:370–378. doi: 10.1016/j.carbpol.2017.09.095. [DOI] [PubMed] [Google Scholar]
- 40.Fang J.M., Sun R.C., Salisbury D., Fowler P., Tomkinson J. Comparative study of hemicelluloses from wheat straw by alkali and hydrogen peroxide extractions. Polym. Degrad. Stab. 1999;66:423–432. [Google Scholar]
- 41.Ulanski P., von Sonntag C. OH-Radical-induced chain scission of chitosan in the absence and presence of dioxygen. J. Chem. Soc. Perkin Trans. 2000;2:2022–2028. [Google Scholar]
- 42.Chang S.-H., Wu C.-H., Tsai G.-J. Effects of chitosan molecular weight on its antioxidant and antimutagenic properties. Carbohydr. Polym. 2018;181:1026–1032. doi: 10.1016/j.carbpol.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 43.Pereira A.G.B., Muniz E.C., Hsieh Y.-L. 1H NMR and 1H–13C HSQC surface characterization of chitosan–chitin sheath-core nanowhiskers. Carbohydr. Polym. 2015;123:46–52. doi: 10.1016/j.carbpol.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 44.Vidal R.R.L., Desbrières J., Borsali R., Guibal E. Oil removal from crude oil-in-saline water emulsions using chitosan as biosorbent. Sep. Sci. Technol. 2020;55:835–847. [Google Scholar]
- 45.Badawy M.E.I., Rabea E.I. A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant pathogens and their applications in crop protection. Int. J. Carbohydr. Chem. 2011;2011 [Google Scholar]
- 46.Jafari H., Delporte C., Bernaerts K.V., De Leener G., Luhmer M., Nie L., Shavandi A. Development of marine oligosaccharides for potential wound healing biomaterials engineering. Chem. Eng. J. Adv. 2021;7 [Google Scholar]
- 47.Czechowska-Biskup R., Rokita B., Lotfy S., Ulanski P., Rosiak J.M. Degradation of chitosan and starch by 360-kHz ultrasound. Carbohydr. Polym. 2005;60:175–184. [Google Scholar]
- 48.Islam N., Wang H., Maqbool F., Ferro V. In vitro enzymatic digestibility of glutaraldehyde-crosslinked chitosan nanoparticles in lysozyme solution and their applicability in pulmonary drug delivery. Molecules. 2019;24:1271. doi: 10.3390/molecules24071271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dung P.D., Hung L.T., Le Truc Ha T., Luan L.Q., Van Le B., Thang N.T. Study on the biological effects of oligochitosan fractions, prepared by synergistic degradation method, on capsicum. Int. J. Polym. Sci. 2018;2018 [Google Scholar]
- 50.Tahtat D., Mahlous M., Benamer S., Khodja A.N., Youcef S.L. Effect of molecular weight on radiation chemical degradation yield of chain scission of γ-irradiated chitosan in solid state and in aqueous solution. Radiat. Phys. Chem. 2012;81:659–665. [Google Scholar]
- 51.Ali M.E.A., Aboelfadl M.M.S., Selim A.M., Khalil H.F., Elkady G.M. Chitosan nanoparticles extracted from shrimp shells, application for removal of Fe(II) and Mn(II) from aqueous phases. Sep. Sci. Technol. 2018;53:2870–2881. [Google Scholar]
- 52.Kumar S., Koh J. Physiochemical, optical and biological activity of chitosan-chromone derivative for biomedical applications. Int. J. Mol. Sci. 2012;13:6102–6116. doi: 10.3390/ijms13056102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El-Sawy N.M., Abd El-Rehim H.A., Elbarbary A.M., Hegazy E.-S.A. Radiation-induced degradation of chitosan for possible use as a growth promoter in agricultural purposes. Carbohydr. Polym. 2010;79:555–562. [Google Scholar]
- 54.Chmielewski A.G., Migdal W., Swietoslawski J., Swietoslawski J., Jakubaszek U., Tarnowski T. Chemical-radiation degradation of natural oligoamino-polysaccharides for agricultural application. Radiat. Phys. Chem. 2007;76:1840–1842. [Google Scholar]
- 55.Duy N.N., Phu D.V., Anh N.T., Hien N.Q. Synergistic degradation to prepare oligochitosan by γ-irradiation of chitosan solution in the presence of hydrogen peroxide. Radiat. Phys. Chem. 2011;80:848–853. [Google Scholar]
- 56.Jawad A.H., Abdulhameed A.S., Wilson L.D., Hanafiah M., Nawawi W.I., Alothman Z.A., Rizwan Khan M. Fabrication of Schiff's base chitosan-glutaraldehyde/activated charcoal composite for cationic dye removal: optimization using response surface methodology. J. Polym. Environ. 2021;29:2855–2868. [Google Scholar]
- 57.Arafat A., Samad S.A., Masum S.Md, Moniruzzaman M. Preparation and characterization of chitosan from shrimp shell waste. IJSER. 2015;6:538–541. [Google Scholar]
- 58.Shin G.H., Kim J.T. Comparative study of chitosan and oligochitosan coatings on mucoadhesion of curcumin nanosuspensions. Pharmaceutics. 2021;13:2154. doi: 10.3390/pharmaceutics13122154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mubarak N.S.A., Chuan T.W., Khor H.P., Jawad A.H., Wilson L.D., Sabar S. Immobilized Fe-loaded chitosan film for methyl orange dye removal: competitive ions, reusability, and mechanism. J. Polym. Environ. 2021;29:1050–1062. [Google Scholar]
- 60.Cheng J., Zhu H., Huang J., Zhao J., Yan B., Ma S., Zhang H., Fan D. The physicochemical properties of chitosan prepared by microwave heating. Food Sci. Nutr. 2020;8:1987–1994. doi: 10.1002/fsn3.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mourya V.K., Inamdar N.N., Choudhari Y.M. Chitooligosaccharides: synthesis, characterization and applications. Polym. Sci. Ser. A. 2011;53:583–612. [Google Scholar]
- 62.Jawad A.H., Nawi M.A. Characterizations of the photocatalytically-oxidized cross-linked chitosan-glutaraldehyde and its application as a sub-layer in the TiO2/CS-GLA bilayer photocatalyst system. J. Polym. Environ. 2012;20:817–829. [Google Scholar]
- 63.Queiroz M.F., Teodosio Melo K.R., Sabry D.A., Sassaki G.L., Rocha H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs. 2014;13:141–158. doi: 10.3390/md13010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lusiana R.A., Sasongko N.A., Sangkota V.D.A., Prasetya N.B.A., Siahaan P., Kiswandono A.A., Othman M.H.D. In-Vitro study of polysulfone-polyethylene glycol/chitosan (PEG-PSf/CS) membranes for urea and creatinine permeation. J. Kim. Sains Dan Apl. 2020;23:283–289. [Google Scholar]
- 65.Yadav H.K.S., H.G Shivakumar. In Vitro and in vivo evaluation of pH-sensitive hydrogels of carboxymethyl chitosan for intestinal delivery of theophylline. ISRN Pharm. 2012;2012 doi: 10.5402/2012/763127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petrovici A.R., Anghel N., Dinu M.V., Spiridon I. Dextran-chitosan composites: antioxidant and anti-inflammatory properties. Polymers. 2023;15 doi: 10.3390/polym15091980. 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vino A.B., Ramasamy P., Shanmugam V., Shanmugam A. Extraction, characterization and in vitro antioxidative potential of chitosan and sulfated chitosan from Cuttlebone of Sepia aculeata Orbigny, 1848. Asian Pac. J. Trop. Biomed. 2012;2:S334–S341. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.