Abstract
Objective
To study the influence of concomitant use of hormonal contraception and non-steroidal anti-inflammatory drugs (NSAIDs) on the risk of venous thromboembolism.
Design
Nationwide cohort study.
Setting
Denmark through national registries.
Participants
All 15-49 year old women living in Denmark between 1996 and 2017 with no medical history of any venous or arterial thrombotic event, cancer, thrombophilia, hysterectomy, bilateral oophorectomy, sterilisation, or infertility treatment (n=2 029 065).
Main outcome measure
A first time discharge diagnosis of lower limb deep venous thrombosis or pulmonary embolism.
Results
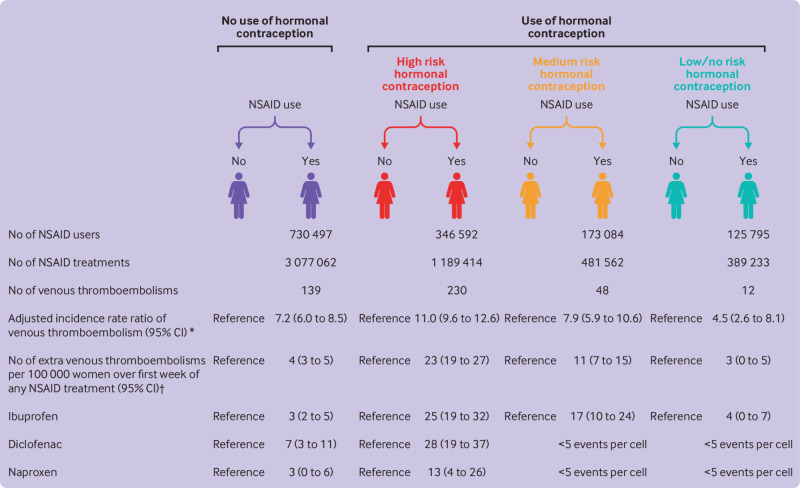
Among 2.0 million women followed for 21.0 million person years, 8710 venous thromboembolic events occurred. Compared with non-use of NSAIDs, use of NSAIDs was associated with an adjusted incidence rate ratio of venous thromboembolism of 7.2 (95% confidence interval 6.0 to 8.5) in women not using hormonal contraception, 11.0 (9.6 to 12.6) in women using high risk hormonal contraception, 7.9 (5.9 to 10.6) in those using medium risk hormonal contraception, and 4.5 (2.6 to 8.1) in users of low/no risk hormonal contraception. The corresponding numbers of extra venous thromboembolic events per 100 000 women over the first week of NSAID treatment compared with non-use of NSAIDs were 4 (3 to 5) in women not using hormonal contraception, 23 (19 to 27) in women using high risk hormonal contraception, 11 (7 to 15) in those using medium risk hormonal contraception, and 3 (0 to 5) in users of low/no risk hormonal contraception.
Conclusions
NSAID use was positively associated with the development of venous thromboembolism in women of reproductive age. The number of extra venous thromboembolic events with NSAID use compared with non-use was significantly larger with concomitant use of high/medium risk hormonal contraception compared with concomitant use of low/no risk hormonal contraception. Women needing both hormonal contraception and regular use of NSAIDs should be advised accordingly.
Introduction
Use of hormonal contraception and use of non-steroidal anti-inflammatory drugs (NSAIDs) have individually been associated with an increased risk of venous thromboembolism.1 2 3 Evidence on the magnitude of the risk of venous thromboembolism in women using hormonal contraception and NSAIDs concomitantly is lacking.
Use of combined hormonal contraception, containing oestrogen and progestin, is an acknowledged risk factor for lower limb deep venous thrombosis and pulmonary embolism.1 2 4 Studies have shown that the magnitude of the increased risk depends on the dose of oestrogen and type of progestin.1 2 4 Oestrogen is known to cause hypercoagulability by promoting transcription of genes for multiple coagulation factors.5 The influence of progestin on the coagulation system is more complex. Use of the progestin-only contraception injection, which delivers a high dose and potency of progestin, has been associated with an increased risk of venous thromboembolism6 7; however, studies have reported a detectable hypocoagulability and reduced risk of venous thromboembolism with use of the levonorgestrel releasing intrauterine device, which delivers only a small dose of progestin to the blood.2 6 7 8
Use of systemic non-aspirin NSAIDs has also been positively associated with the development of thrombogenic disease, including venous thromboembolic events.3 Both use of traditional NSAIDs, such as ibuprofen, diclofenac, and naproxen, and use of newer highly selective cyclo-oxygenase-2 inhibitors have been linked to an increased risk of both arterial and venous thromboses.3 9 Through their targeted inhibition of cyclo-oxygenase enzymes, NSAIDs promote platelet aggregation,10 which contributes to activation of the coagulation system and thereby potentially to the formation of a venous thrombosis.11 Most of the existing evidence reports on the association between NSAID use and risk of arterial thrombogenic disease. Meta-analyses based on randomised controlled trials and observational studies both support an increased risk of arterial thrombogenic disease with use of NSAIDs,9 12 leading the US Food and Drug Administration and European Medicine Agency to require that the summary of product characteristics of any systemic non-aspirin NSAID should carry a warning of an increased risk of heart attacks and strokes. Less evidence exists on the risk of venous thromboembolism with use of NSAIDs, but observational studies and meta-analyses of these report a positive association, including for traditional NSAIDs.3 13
Although both hormonal contraceptives and NSAIDs contain active ingredients that may affect blood coagulability, how concomitant use of the two drug classes affects the risk of development of venous thromboembolism in women of reproductive age remains to be explored. Considering the worldwide prevalent use of these drugs and the severity of venous thromboembolic disease, this is an important public health matter. We report a nationwide pharmacoepidemiological analysis of the incidence of venous thromboembolism in women using hormonal contraception and NSAIDs simultaneously.
Methods
Study design and population
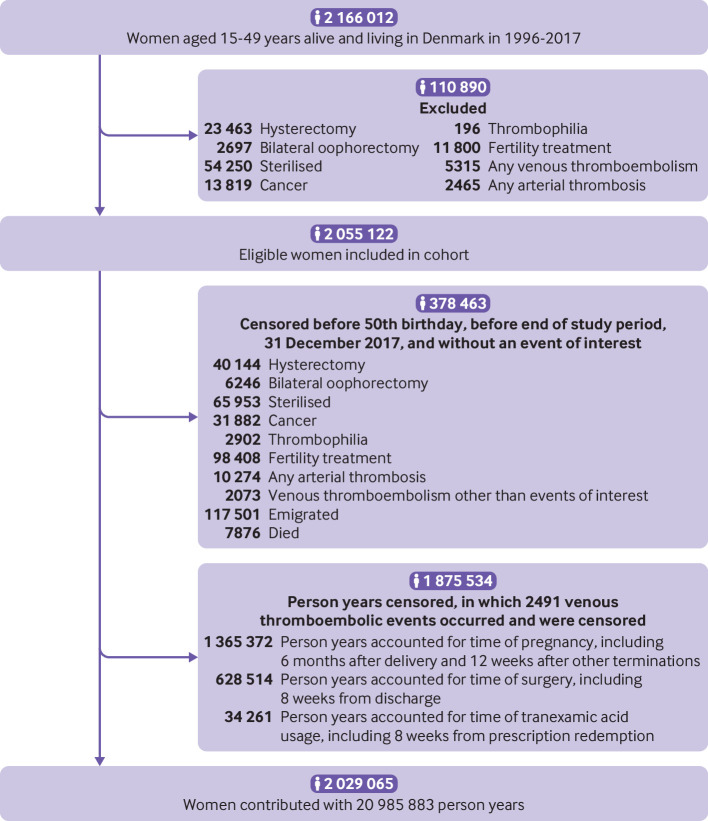
We conducted a nationwide historical cohort study, including all 15-49 year old women living in Denmark between 1996 and 2017 with no medical history of any venous or arterial thrombotic event, cancer (except non-melanoma skin cancer), thrombophilia, hysterectomy, bilateral oophorectomy, sterilisation, or infertility treatment. Women were followed from 1 January 1996 or from their 15th birthday if this occurred after the start of the study. To ensure information on eligibility, women who immigrated entered the cohort one year after the date of immigration. Women were censored at 31 December 2017, death, emigration, or the occurrence of any exclusion criterion.
We identified study participants and their follow-up times by using the Civil Registration System, the National Registry of Causes of Death, the National Patient Registry, and the National Registry of Medicinal Product Statistics.14 15 16 17 We used the personal identification number given to all Danish citizens at birth or immigration to reliably link the registries. Exact definitions of the exclusion and censoring criteria are outlined in supplementary table A.
Hormonal contraception and NSAIDs
Time updated, individual level information on purchase of hormonal contraception and systemic non-aspirin NSAIDs was provided by the National Registry of Medicinal Product Statistics, which holds information on all prescriptions filled by the Danish population since 1995.17 The registry receives its information electronically from the digital accounting systems of Danish pharmacies that primarily use the systems to secure reimbursement from the National Health Service.17 The information includes data on date of redemption of the prescription and amount and type of redeemed drug.17
We defined time exposed to hormonal contraception from the date of the filled prescription. For hormonal contraceptives administrated orally or by patch, injection, or vaginal ring, we determined duration of use by amount of defined daily doses purchased. We assumed duration of usage of intrauterine systems and implants to be one year less than the maximum approved duration of usage to account for the potential for early discontinuation. We extended all exposure periods by 28 days to account for delays in initiation of use.
On the basis of findings reported in the literature, we categorised use of hormonal contraception according to the association with venous thromboembolism into use of high risk hormonal contraceptives, medium risk hormonal contraceptives, and low/no risk hormonal contraceptives. Thus, based on findings from previous studies, high risk hormonal contraceptives included combined oestrogen and progestin patch, vaginal ring, and tablets containing 50 µg ethinyl oestradiol, or the progestins desogestrel, gestodene, drospirenone, or the anti-androgen cyproterone. Medium risk hormonal contraception included all other combined oral contraceptives as well as the medroxyprogesterone injection. We considered progestin-only tablets, implants, and hormone intrauterine devices to be low/no risk hormonal contraceptives.1 2 6 Supplementary table B provides a detailed list of all hormonal contraceptives accessible during the study period.
We assumed time exposed to systemic non-aspirin NSAIDs (Anatomical Therapeutic Chemical (ATC) code M01A, except glucosamine M01AX05) to last one week from the date of the filled prescription, as 98% of prescriptions for NSAIDs redeemed by the cohort consisted of a single package of maximum 30 tablets. As a sensitivity analysis, we expanded the exposure window for NSAIDs to 30 days from filled prescription. Supplementary table C provides a detailed list of all systemic non-aspirin NSAIDs available during the study period. When time exposed to hormonal contraception overlapped with time exposed to NSAIDs, we considered the woman to be a concomitant user.
Venous thromboembolism
We used the National Patient Registry and the National Registry of Causes of Death for the identification of all primary venous thromboembolic events, defined as a first diagnosis (classified according to the international classification of diseases, 10th revision) of any lower limb deep venous thrombosis (I801-3) or pulmonary embolism (I26).15 16 We considered diagnoses that were exclusively made in the emergency department without subsequently being repeated by a medical department to be tentative and therefore did not include them.
We did a sensitivity analysis including exclusively diagnoses either given after relevant imaging (Danish Classification of Examinations and Clinical Physiology and Nuclear Medicine, code UXUG (ultrasonography of lower extremity), UXCC/UXAC, UXCG/UXAG (computed tomography scan/angiography of thorax or lower extremity), WLHGS/WLHSS (lung scintigraphy)) or followed by redemption of a prescription for an anticoagulant (ATC B01). We chose this confirmation strategy to ensure valid confirmation of diagnoses of venous thromboembolic events throughout the entire study period, as imaging examinations were not coded at the beginning of the study period and subsequent anticoagulative treatment was not provided by prescription in the last part of the period.
Confounding variables
Time updated information on age, calendar time, educational level, pregnancy, surgery, hypertension, diabetes, polycystic ovary syndrome, endometriosis, migraine, systemic connective tissue disorders, and inflammatory polyarthropathies and use of oral tranexamic acid was available for the entire study population (supplementary table A). Women were censored temporarily during the occurrence of temporary confounding factors. A woman was censored temporarily during pregnancy and for six months after delivery and 12 weeks after other types of pregnancy terminations. The Danish Medical Birth Registry and the Registry of Legally Induced Abortions hold information on all births and induced abortions in Denmark since 1973, respectively, including date of birth/abortion and gestational age at birth/abortion, allowing calculation of the estimated date of conception and thereby the temporary censoring of women during and after pregnancy.18 19 Similarly, miscarriages, extrauterine pregnancies, and pregnancies with unknown locations diagnosed or treated in a hospital setting are registered in the National Patient Registry with date of termination and gestational age at termination.16 The National Patient Registry also holds information on all surgical procedures undergone in Danish hospitals since 1976.16 If a surgery resulted in hospital admission for at least one day, a woman would be censored temporarily for eight weeks from the day of discharge. Both hormonal contraceptives and NSAIDs may be used in the treatment of heavy menstrual bleeding. To avoid overlap with use of the potentially thrombogenic drug tranexamic acid,20 which is also used in the management of heavy menstrual bleeding, women were temporarily censored for eight weeks from the date of redemption of a prescription for tranexamic acid.
Temporarily censored time periods did not contribute with person time or venous thromboembolic events in the analyses, and women experiencing an event, death, emigration, or any exclusion criterion during a temporarily censored time period were permanently censored from the date of such incident. Data on both body mass index and smoking status just before pregnancy were available for parous women from the calendar year 2004, as it was recorded at the first visit to a midwife in the Medical Birth Registry (supplementary table A).19
Statistical analysis
Using Poisson regression, we calculated adjusted incidence rate ratios of venous thromboembolism according to user status of hormonal contraception and NSAIDs. The main analyses included an exposure variable with eight categories: no use of hormonal contraception and NSAIDs, high risk hormonal contraception use only, medium risk hormonal contraception use only, low/no risk hormonal contraception use only, use of (any) NSAIDs alone, concomitant use of high risk hormonal contraception and any NSAID, concomitant use of medium risk hormonal contraception and any NSAID, and concomitant use of low/no risk hormonal contraception and any NSAID.
In a sensitivity analysis, use of NSAIDs was further divided according to active ingredient (ibuprofen, diclofenac, naproxen, others, multiple). In another sensitivity analysis, ibuprofen use was divided according to drug unit size into use of ≥600 mg tablets and use of ≤400 mg tablets.
To compute a P value for each of the three interaction terms of interest (NSAID*high risk hormonal contraception, NSAID*medium risk hormonal contraception, and NSAID*low/no risk hormonal contraception), we did three likelihood ratio tests comparing a model including the exposure as four binary independent variables (NSAID use, yes/no; high risk hormonal contraception use, yes/no; medium risk hormonal contraception use, yes/no; and low/no risk hormonal contraception use, yes/no) with three models each also including one of the interaction terms of interest.
Using the distribution of age, calendar time, educational level, hypertension, diabetes, polycystic ovary syndrome, endometriosis, migraine, systemic connective tissue disorders, and inflammatory polyarthropathies in the entire cohort as the standard, we calculated standardised incidence rate differences of venous thromboembolism per 100 000 women over the first week of NSAID treatment compared with non-use of NSAIDs according to user status for hormonal contraception. In addition, we repeated the main statistical analyses in a subpopulation restricted to women without a history of comorbidities such as cardiovascular diseases and musculoskeletal disorders. Exclusion and censoring criteria defining this subcohort are outlined in supplementary table D. We managed data with SAS software, version 9.4, and analysed them using R software version 4.2.1.
Patient and public involvement
Except for the women of reproductive age in the research group, no patients or members of the public were involved in the design, analysis, or writing up of the study, as the research project was undertaken without funds for patient and public involvement measures. The results of the study will, however, be disseminated to the public and health professionals by press releases and presentations at scientific conferences.
Results
From 1996 through 2017, we followed 2 029 065 women aged 15 to 49 years for a median of 10.0 (interquartile range 4.3-16.5) years (fig 1). During a total of 21.0 million person years, 8710 incident venous thromboembolisms occurred. Of these, 2715 (31.2%) were coded as pulmonary embolisms and the rest were registered as isolated deep venous thromboses of the lower extremity. A total of 7043 (81%) of the 8710 outcome diagnoses were made after relevant imaging or were followed by redemption of a prescription for anticoagulation therapy. A total of 228 (2.6%) women died within 30 days of their diagnosis of venous thromboembolic. Table 1 shows the characteristics of the study population.
Fig 1.
Flow diagram of formation of study cohort
Table 1.
Characteristics of study population. Values are numbers (percentages)* unless stated otherwise
| Exposure | Person years | Age† ‡ | University education | BMI† | Smoking‡ | Comorbidities§ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypertension | Diabetes | Polycystic ovary syndrome | Endometriosis | Migraine | Systemic connective tissue disorders | Inflammatory polyarthropathies | ||||||
| Non-use of hormonal contraception and NSAIDs | 13 632 179 | 34 (24-42) | 2 554 784 (18.7) | 23 (20-26) | 500 787 (19.1) | 53 257 (0.4) | 152 011 (1.1) | 35 265 (0.3) | 78 225 (0.6) | 819 696 (6.0) | 71 747 (0.5) | 200 725 (1.5) |
| Hormonal contraception use only | ||||||||||||
| High risk hormonal contraception | 4 298 461 | 24 (20-32) | 595 293 (13.8) | 23 (21-26) | 152 054 (22.9) | 8528 (0.2) | 34 561 (0.8) | 12 332 (0.3) | 24 011 (0.6) | 287 818 (6.7) | 25 114 (0.6) | 54 191 (1.3) |
| Medium risk hormonal contraception | 1 852 352 | 25 (20-33) | 253 149 (13.7) | 23 (21-27) | 67 408 (23.0) | 4171 (0.2) | 15 845 (0.9) | 5130 (0.3) | 9240 (0.5) | 118 720 (6.4) | 11 868 (0.6) | 24 702 (1.3) |
| Low/no risk hormonal contraception | 1 116 421 | 37 (31-43) | 359 413 (32.2) | 23 (21-26) | 95 609 (15.5) | 7543 (0.8) | 20 032 (1.8) | 4627 (0.4) | 18 060 (1.6) | 164 257 (14.7) | 11 323 (1.0) | 23 269 (2.1) |
| NSAID use only | ||||||||||||
| Any | 51 864 | 39 (32-45) | 8975 (17.3) | 24 (21-29) | 3208 (27.5) | 505 (1.0) | 1509 (2.9) | 256 (0.5) | 684 (1.3) | 8928 (17.2) | 917 (1.8) | 3012 (5.8) |
| Ibuprofen | 28 586 | 39 (31-45) | 4908 (17.2) | 24 (21-29) | 2205 (28.1) | 270 (0.9) | 890 (3.1) | 168 (0.6) | 346 (1.2) | 4370 (15.3) | 457 (1.6) | 1380 (4.8) |
| Diclofenac | 11 162 | 39 (32-45) | 1984 (17.8) | 24 (21-29) | 574 (26.9) | 105 (0.9) | 292 (2.6) | 44 (0.4) | 154 (1.4) | 2056 (18.4) | 180 (1.6) | 623 (5.6) |
| Naproxen | 3426 | 36 (25-43) | 538 (15.7) | 24 (21-28) | 115 (24.7) | 30 (0.9) | 79 (2.3) | 13 (0.4) | 56 (1.6) | 467 (13.6) | 44 (1.3) | 183 (5.3) |
| Concomitant use of hormonal contraception and NSAIDs | ||||||||||||
| High risk hormonal contraception and any NSAID | 20 036 | 28 (22-36) | 2323 (11.6) | 24 (21-28) | 1261 (30.9) | 103 (0.5) | 303 (1.5) | 84 (0.4) | 278 (1.4) | 3390 (16.9) | 340 (1.7) | 795 (4.0) |
| Medium risk hormonal contraception and any NSAID | 8073 | 30 (23-38) | 935 (11.6) | 24 (21-29) | 553 (31.8) | 46 (0.6) | 136 (1.7) | 32 (0.4) | 97 (1.2) | 1317 (16.3) | 134 (1.7) | 333 (4.1) |
| Low/no risk hormonal contraception and any NSAID | 6497 | 39 (33-44) | 1500 (23.1) | 24 (21-28) | 732 (22.5) | 81 (1.3) | 221 (3.4) | 42 (0.6) | 181 (2.9) | 1757 (27.0) | 162 (2.5) | 330 (5.1) |
BMI=body mass index; NSAID=non-steroidal anti-inflammatory drug.
Descriptive percentages are percentages of person time with given characteristic.
Weighted median (interquartile range). Weight calculated as person time with given characteristic divided by total amount of person time during which specific exposure was used.
Information on smoking and BMI was available for parous women only. Information on smoking status was available from 1991 for 500 547 women for 4.2 million person years. Information on body mass index was available from 2004 for 357 503 women for 2.2 million person years.
Person years (percentage of total number of person years in exposure category).
Hormonal contraception and NSAIDs were used concomitantly by 529 704 women. Of the total amount of risk time in which concomitant use was observed, 58% was contributed by the combination of NSAIDs and high risk hormonal contraception, 23% by concomitant use with medium risk hormonal contraception, and 19% by concomitant use with low/no risk hormonal contraception.
In concomitant users, the median number of redeemed prescriptions of NSAIDs was 2 (interquartile range 1-4), and 93-95% were redeemed at least one year after initiation of hormonal contraception across the three groups of hormonal contraception. Ibuprofen was the most frequently used NSAID (60%), followed by diclofenac (20%) and naproxen (6%). Ibuprofen was primarily prescribed as tablets of 400-600 mg, diclofenac as 50 mg tablets, and naproxen as 500-550 mg tablets (supplementary table E). The mode of administration of hormonal contraception was primarily oral for high risk and medium risk hormonal contraception use (97-98%); use of low/no risk hormonal contraception was mostly accounted for by the use of the levonorgestrel releasing intrauterine system (89%) (supplementary table F). We observed similar drug use patterns in users of monotherapy (supplementary tables E and F). Of all NSAID prescriptions redeemed by the entire cohort, 98% were for a single package containing a maximum of 30 tablets.
Table 2 shows the adjusted incidence rate ratios of venous thromboembolism according to exposure status with non-use of both hormonal contraceptives and NSAIDs as the reference group. The age, calendar year, and education adjusted incidence rate ratios were 4.2 (95% confidence interval 4.0 to 4.4) for high risk hormonal contraception use only, 3.0 (2.8 to 3.2) for medium risk hormonal contraception use only, 1.1 (1.0 to 1.3) for low/no risk hormonal contraception use only, and 8.1 (6.9 to 9.6) for use of NSAIDs alone (table 2). We observed a positive association for ibuprofen, diclofenac, and naproxen, with the strongest association occurring with use of diclofenac (table 2). We found no difference in risk when comparing use of ≥600 mg tablets with ≤400 mg tablets of ibuprofen (0.9, 0.7 to 1.2).
Table 2.
Adjusted incidence rate ratios of venous thromboembolism according to hormonal contraception use only, NSAID use only, and concomitant use of hormonal contraception and NSAIDs, with non-use as reference
| Exposure | Person years | Events (PE) | Age standardised incidence rate* (95% CI) | Simple adjusted IRR† (95% CI) | Multiple adjusted IRR‡ (95% CI) |
|---|---|---|---|---|---|
| Non-use of hormonal contraception and NSAIDs | 13 632 179 | 3664 (1058) | 2.5 (2.4 to 2.6) | 1.0 (reference) | 1.0 (reference) |
| Hormonal contraception use only | |||||
| High risk hormonal contraception | 4 298 461 | 3283 (1100) | 11.2 (10.7 to 11.7) | 4.2 (4.0 to 4.4) | 4.1 (3.9 to 4.3) |
| Medium risk hormonal contraception | 1 852 352 | 977 (349) | 6.7 (6.2 to 7.2) | 3.0 (2.8 to 3.2) | 3.0 (2.8 to 3.2) |
| Low/no risk hormonal contraception | 1 116 421 | 357 (109) | 3.0 (2.6 to 3.4) | 1.1 (1.0 to 1.3) | 1.1 (1.0 to 1.2) |
| NSAID use only | |||||
| Any | 51 864 | 139 (30) | 24.8 (20.2 to 30.6) | 8.1 (6.9 to 9.6) | 7.2 (6.0 to 8.5) |
| Ibuprofen | 28 586 | 59 (8) | 18.9 (13.4 to 25.9) | 6.3 (4.9 to 8.2) | 5.7 (4.4 to 7.4) |
| Diclofenac | 11 162 | 51 (14) | 42.3 (28.9 to 59.8) | 13.6 (10.3 to 17.9) | 12.0 (9.1 to 15.8) |
| Naproxen | 3426 | 7 (3) | 16.3 (6.5 to 33.7) | 7.4 (3.5 to 15.5) | 6.6 (3.1 to 13.8) |
| Concomitant use of hormonal contraception and NSAIDs | |||||
| High risk hormonal contraception and any NSAID | 20 036 | 230 (50) | 121.7 (105.9 to 139.7) | 50.6 (44.2 to 57.8) | 44.8 (39.2 to 51.3) |
| Medium risk hormonal contraception and any NSAID | 8073 | 48 (15) | 64.1 (47.9 to 85.8) | 26.1 (19.6 to 34.7) | 23.4 (17.6 to 31.1) |
| Low/no risk hormonal contraception and any NSAID | 6497 | 12 (4) | 17.2 (8.4 to 35.2) | 5.7 (3.3 to 10.1) | 4.9 (2.8 to 8.7) |
CI=confidence interval; NSAID=non-steroidal anti-inflammatory drug; PE=pulmonary embolism.
Number of events per 10 000 person years.
Adjusted for age (1 year intervals), calendar time (1 year intervals), and educational level (elementary school only, secondary school only, skilled worker, theoretical education, theoretical education with research).
Adjusted for age, calendar time, educational level, hypertension, diabetes, polycystic ovary syndrome, endometriosis, migraine, systemic connective tissue disorders, and inflammatory polyarthropathies.
The corresponding rate ratios were 50.6 (44.2 to 57.8), 26.1 (19.6 to 34.7), and 5.7 (3.3 to 10.1) for concomitant use of NSAIDs and high risk, medium risk, and low/no risk hormonal contraception, respectively (table 2). The associations persisted after further adjustment for the occurrence of migraine, connective tissue disorder, inflammatory polyarthropathies, endometriosis, polycystic ovary syndrome, hypertension, and diabetes (table 2). The P values for interaction terms were <0.001, 0.42, and 0.01 for the interaction between NSAID use and high risk, medium risk, and low/no risk hormonal contraception, respectively.
In the subcohort restricted to the 1 709 341 healthy women without a history of these diseases, the adjusted incidence rate ratios increased to 5.7 (5.2 to 6.2) for high risk hormonal contraception use only and 4.1 (3.6 to 4.6) for medium risk hormonal contraception use only, whereas the null association persisted for low/no risk hormonal contraception use only (1.1, 0.8 to 1.5) (supplementary table G). Compared with the adjusted rate ratios estimated in the entire cohort, the rate ratios of venous thromboembolism associated with NSAID use increased around twofold in the subcohort of healthy women to 15.0 (10.8 to 20.8) for use of NSAIDs alone and to 111.7 (89.6 to 139.3), 43.2 (25.0 to 74.6), and 13.0 (3.2 to 51.9) for concomitant use of NSAIDs and high risk, medium risk, and low/no risk hormonal contraception, respectively (supplementary table G). Although non-significant, the stronger association of diclofenac with the risk of venous thromboembolism compared with ibuprofen remained, whereas no events occurred during the 1533 person years of naproxen use in the subcohort of healthy women (supplementary table G). The adjusted rate ratios of venous thromboembolism according to use of hormonal contraception and NSAIDs estimated in the entire cohort and in the healthy subcohort remained similar in sensitivity analyses exclusively including confirmed venous thromboembolic events.
Figure 2 shows the adjusted incidence rate ratios of venous thromboembolism with use of NSAIDs compared with non-use of NSAIDs according to user status of hormonal contraception. Compared with women not using hormonal contraception, the rate ratio of venous thromboembolism with NSAID use compared with non-use was higher among women using high risk hormonal contraception and lower in women using low/no risk hormonal contraception (fig 2). The increased risk of venous thromboembolism with NSAID use compared with non-use remained significantly higher in high risk hormonal contraception users compared with non-users of hormonal contraception in sensitivity analyses of only confirmed events and when expanding the exposure window of NSAIDs to 30 days from filled prescription (supplementary table H). Similarly, the ratio was lower in concomitant users of low/no risk hormonal contraception throughout sensitivity analyses (supplementary table H).
Fig 2.
Adjusted incidence rate ratios of venous thromboembolism and number of extra venous thromboembolic events in non-steroidal anti-inflammatory drug (NSAID) users versus non-users according to use of hormonal contraception. *Incidence rate ratios adjusted for age, calendar time, educational level, hypertension, diabetes, polycystic ovary syndrome, endometriosis, migraine, systemic connective tissue disorders, and inflammatory polyarthropathies. †Standardised incidence rate differences per 100 000 person weeks; standardised according to distribution of age, calendar time, educational level, hypertension, diabetes, polycystic ovary syndrome, endometriosis, migraine, systemic connective tissue disorders, and inflammatory polyarthropathies in entire cohort. CI=confidence interval
Compared with non-use of NSAIDs, the adjusted number of extra venous thromboembolic events per 100 000 women over the first week of NSAID treatment was 4 (95% confidence interval 3 to 5) in women not using hormonal contraception (fig 2). Although the number remained similar in women concomitantly using low/no risk hormonal contraception (3, 0 to 5), it increased to 23 (19 to 27) in women concomitantly using high risk hormonal contraception and 11 (7 to 15) with concomitant use of medium risk hormonal contraception (fig 2). The increased number of extra venous thromboembolisms with NSAID use versus non-use remained higher during concomitant use of high risk hormonal contraception compared with non-use of hormonal contraception for ibuprofen, diclofenac, and naproxen (fig 2).
Discussion
In a nationwide cohort of women of reproductive age, we found use of NSAIDs to be positively associated with the development of venous thromboembolism, with the magnitude of the increased risk being dependent on the user status for hormonal contraception. Most concomitant use occurred as incident NSAID use in prevalent users of hormonal contraception. Compared with non-use of hormonal contraception, the association between NSAID use and venous thromboembolism was stronger in women using high risk hormonal contraception and lower in users of low/no risk hormonal contraception.
Strengths and limitations of study
Using high quality, linkable, national registries, this nationwide study adds new knowledge on the risk of a potentially fatal event during concomitant use of two drug classes often prescribed to otherwise healthy women. First time diagnosis of deep venous thrombosis or pulmonary embolism in the Danish National Patient Registry has previously been shown to have a high positive predictive value of 80-90%.21 In this study, 81% of diagnoses of venous thromboembolism in lower extremities or lungs were made after relevant imaging or were followed by subsequent purchase of anticoagulants, and all findings persisted when we restricted the outcome to confirmed diagnoses.
The National Registry of Medicinal Product Statistics provided valid, daily updated, individual level information on purchase of hormonal contraception and NSAIDs, thereby eliminating recall bias.17 Individual level data on over-the-counter NSAID purchases were not available. However, a Danish study on the effect of misclassification of over-the-counter NSAID use as non-use concluded that such misclassification is minor and has negligible influence on estimates of associations with NSAID use, making the Danish National Registry of Medicinal Product Statistics a valid data source for assessing effects of NSAID use.22
The fixed daily dose of hormonal contraceptives and the dispensing through repetitive redemption of prescriptions allowed reliable estimation of time of exposure using information on date of dispensing and type and amount of hormonal contraception purchased. On the other hand, the NSAID dosage may vary among users, and information on dosage regimens was not available from the National Registry of Medicinal Product Statistics. Nevertheless, the assumption that NSAIDs were used within the first week from redemption of the prescription seems reasonable considering that 98% of all prescriptions for NSAIDs redeemed by the entire cohort were for a single package containing a maximum of 30 tablets, that the median number of redeemed NSAID prescriptions was two for NSAID users throughout a median follow-up time of 10 years, and the general good health of women of reproductive age, all indicative of NSAIDs being used for short term relief of an acute, temporary pain. Reassuringly, the increased risk of venous thromboembolism with NSAID use and the changes in the magnitude of the association according to hormonal contraception use persisted when we expanded the exposure window to 30 days from redemption of prescription, although the likely misclassification of time of non-use as use, occurring with such a wide exposure window, contributed to a dilution of the associations. The possibility of studying a dose effect for NSAIDs was hampered by the rather narrow range of recommended doses and predominantly isolated prescriptions.
We were able to control for a wealth of potential confounding factors by design and statistical adjustments, but exposure, being based on real world data, was not randomised, leaving the possibility of residual confounding and unmeasured confounding. Although we had information about smoking and obesity for 355 086 women, this information was missing for most of the included person time, which hampered adjustment for these factors. However, in Denmark, smoking and obesity are highly correlated with educational status, and we adjusted for education in all our analyses. Furthermore, for hormonal contraception, our incidence rate ratios were similar to those reported in studies that did adjust for smoking and obesity.1 Finally, smoking and obesity are unlikely to cause the observed differences in the magnitude of the effect of NSAID use on risk of venous thrombosis across hormonal contraception use.
Confounding by indication and protopathic bias could occur when the association between NSAID use and venous thromboembolic events is studied. We tried to eliminate confounding by indication as much as possible through control of indications for NSAIDs, such as chronic pain or inflammatory conditions, that are potentially thrombogenic themselves or increase the likelihood of immobilisation. Although residual confounding by indication may occur, the indications for the very short term NSAID use observed in this population of rather healthy young women seem unlikely to explain the sevenfold higher risk of venous thromboembolism observed during NSAID use compared with non-use. The number of person years with cardiovascular disease or chronic pain/inflammatory conditions was limited, reducing the potential impact of a collider bias. That protopathic bias would be differently distributed according to hormonal contraception use also seems unlikely, and this would need to be the case to explain the observed differences in the association between NSAID use and risk of venous thromboembolism in users of different hormonal contraceptives.
Comparison with other studies
In the largest study on the association between NSAID use and unprovoked venous thromboembolism, Schmidt et al found use of NSAIDs to be associated with a twofold higher risk of venous thromboembolism compared with non-use.13 Schmidt et al showed that the positive association persisted in long term users, reducing the likelihood of protopathic bias, and in strata according to the indication for NSAID use, minimising the effect of confounding by indication.13 Considering the general very good health status of women of reproductive age and their sparse use of NSAIDs, we did not have the data foundation to study the association in such disease strata or in long term users.
Schmidt et al assumed that use of NSAIDs occurred within 60 days after purchase, which likely caused misclassification of time of non-use as time of use and thereby a dilution of the actual association. Such dilution with the expansion of exposure window was confirmed in sensitivity analyses in their study as well as in ours.13 Furthermore, Schmidt et al’s study population included older and more morbid participants than those in our study, causing the reference group to have a much higher baseline risk of venous thromboembolism compared with the healthy, young women included in our study.13 This confounding by baseline rate was also observable in our study, as the associations doubled in the subcohort from which women with cardiovascular comorbidities were excluded.
A meta-analysis of observational studies from 2015 confirmed the findings of Schmidt et al of a twofold higher risk of venous thromboembolism among users of NSAIDs.3 Again, the broad time window of exposure and the inclusion of older participants at cardiovascular risk may explain the smaller relative association observed in this study compared with ours.3 A recent study by Kinsey et al (2020) also found a higher risk of venous thromboembolism among users of NSAIDs compared with non-users, but the association became null when the comparator was use of paracetamol.23 The authors interpret this finding as indicative of the existence of confounding by indication or baseline characteristics of analgesic users causing a higher cardiovascular risk profile compared with non-users.23 However, this study defined exposure time by extrapolating annually collected information on use of analgesics. Whether such a method would result in a valid definition of time of use of NSAIDs only and paracetamol only, securing non-overlapping use, is questionable. Furthermore, use of paracetamol cannot be considered a valid negative exposure control, as its pharmacodynamics have been shown to include inhibition of the cyclo-oxygenase enzyme (similar to NSAIDs) and it has been linked to increased risks of cardiovascular diseases.24 25 Similarly, we did not consider use of stronger analgesics, such as opioids, as negative exposure controls to be valid, as indications for these differ from common indications for use of NSAIDs and could create confounding by indication.
A meta-analysis of 280 trials of NSAIDs versus placebo (124 513 participants; 68 342 person years) and 474 trials of one NSAID versus another NSAID (229 296 participants; 165 456 person years) concluded that diclofenac in particular was associated with increased cardiovascular risk and had a similar cardiovascular risk profile to the withdrawn cyclo-oxygenase-2 inhibitor rofecoxib.9 In our study, the association with venous thromboembolism was stronger for diclofenac than for ibuprofen or naproxen. Diclofenac is a traditional NSAID like ibuprofen and naproxen but has similar affinity/selectivity to newer cyclo-oxygenase-2 inhibitors for cyclo-oxygenase-2, which is believed to promote platelet aggregation and activation of coagulation when inhibited.10
As the pharmacodynamics of both hormonal contraception and NSAIDs include an effect on the coagulation system,5 10 11 the observed superadditive joint effect between high/medium risk hormonal contraception and NSAIDs could represent a synergistic drug interaction between the drug classes. However, further research is warranted to determine the potential existence and mechanism of such drug interaction.
Conclusion and implications
In conclusion, we found the influence of NSAID use on venous thromboembolic risk to be significantly greater in women using high risk hormonal contraception compared with no use of hormonal contraception and smaller in women using low/no risk hormonal contraception. However, further research is needed to clarify whether these findings represent an actual drug interaction.
Despite the high incidence rate ratios, the absolute risk of venous thromboembolic event in the first week after NSAID purchase remained low even in users of high risk hormonal contraception (0.02%). However, considering the highly prevalent indications for use of hormonal contraception and of NSAIDs, studying this association further would be of public interest, especially in regular users of NSAIDs, who might benefit from a low/no risk hormonal contraceptive rather than a high/medium risk hormonal contraceptive.
What is already known on this topic
Use of combined hormonal contraceptives is an acknowledged risk factor for venous thromboembolism
Observational studies and meta-analyses have reported an increased risk of venous thromboembolism with use of non-steroidal anti-inflammatory drugs (NSAIDs)
Evidence is lacking on the venous thromboembolic risk with concomitant use of hormonal contraception and NSAIDs
What this study adds
In this nationwide study of women of reproductive age, use of NSAIDs was associated with an increased risk of venous thromboembolism
The magnitude of the increased risk depended on the user status of hormonal contraception
Compared with non-use of hormonal contraception, the association between NSAID use and venous thromboembolism was stronger in women using high risk hormonal contraception
Web extra.
Extra material supplied by authors
Web appendix: Supplementary tables
Contributors: CTP initiated the study. AM designed the study, with contributions from all co-authors. AM conducted the data management. AM did the analyses, with contribution from RKS and TAG. All authors, led by AM, were involved in data interpretation and the writing of the manuscript. AM is the guarantor. The corresponding author attests that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted.
Funding: The study was supported by the Danish Heart Foundation. The Danish Heart Foundation did not contribute to any part of study conduct or publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: support from the Danish Heart Foundation, which funded AM’s salary, including this study; LSM has received grants from the health insurance organisation “Denmark,” the Danish Cancer Society’s Scientific Committee, and Novo Nordisk for research unrelated to the present study; CTP has received grants from Novo Nordisk and Bayer outside the current study; no other relationships or activities that could appear to have influenced the submitted work.
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: We plan to disseminate results to relevant medical societies in the form of press releases from the authors’ departments.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
According to Danish law, studies based on the national Danish registries are not required to gain patient consent or ethical approval. This study was approved by the Danish Health Data Board (approval ID: FSEID-00005931) and the Danish Data Protection Agency (approval ID: P-2019-280).
Data availability statement
Raw data used to conduct this study are accessible only through approval from the Danish Data Protection Agency and the Danish Health Data Board. Although anonymised, the data were available at an individual level, making data sharing restricted by the General Data Protection Regulation of EU law.
References
- 1. Vinogradova Y, Coupland C, Hippisley-Cox J. Use of combined oral contraceptives and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ 2015;350:h2135. 10.1136/bmj.h2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lidegaard Ø, Nielsen LH, Skovlund CW, Skjeldestad FE, Løkkegaard E. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9. BMJ 2011;343:d6423. 10.1136/bmj.d6423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ungprasert P, Srivali N, Wijarnpreecha K, Charoenpong P, Knight EL. Non-steroidal anti-inflammatory drugs and risk of venous thromboembolism: a systematic review and meta-analysis. Rheumatology (Oxford) 2015;54:736-42. 10.1093/rheumatology/keu408 [DOI] [PubMed] [Google Scholar]
- 4. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ 2013;347:f5298. 10.1136/bmj.f5298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tchaikovski SN, Rosing J. Mechanisms of estrogen-induced venous thromboembolism. Thromb Res 2010;126:5-11. 10.1016/j.thromres.2010.01.045 [DOI] [PubMed] [Google Scholar]
- 6. van Hylckama Vlieg A, Helmerhorst FM, Rosendaal FR. The risk of deep venous thrombosis associated with injectable depot-medroxyprogesterone acetate contraceptives or a levonorgestrel intrauterine device. Arterioscler Thromb Vasc Biol 2010;30:2297-300. 10.1161/ATVBAHA.110.211482 [DOI] [PubMed] [Google Scholar]
- 7. Bergendal A, Persson I, Odeberg J, et al. Association of venous thromboembolism with hormonal contraception and thrombophilic genotypes. Obstet Gynecol 2014;124:600-9. 10.1097/AOG.0000000000000411 [DOI] [PubMed] [Google Scholar]
- 8. van Vliet HA, Tchaikovski SN, Rosendaal FR, Rosing J, Helmerhorst FM. The effect of the levonorgestrel-releasing intrauterine system on the resistance to activated protein C (APC). Thromb Haemost 2009;101:691-5. 10.1160/TH08-09-0621 [DOI] [PubMed] [Google Scholar]
- 9. Bhala N, Emberson J, Merhi A, et al. Coxib and traditional NSAID Trialists’ (CNT) Collaboration . Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013;382:769-79. 10.1016/S0140-6736(13)60900-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bacchi S, Palumbo P, Sponta A, Coppolino MF. Clinical pharmacology of non-steroidal anti-inflammatory drugs: a review. Antiinflamm Antiallergy Agents Med Chem 2012;11:52-64. 10.2174/187152312803476255 [DOI] [PubMed] [Google Scholar]
- 11. Heemskerk JW, Bevers EM, Lindhout T. Platelet activation and blood coagulation. Thromb Haemost 2002;88:186-93. 10.1055/s-0037-1613209 [DOI] [PubMed] [Google Scholar]
- 12. McGettigan P, Henry D. Cardiovascular risk with non-steroidal anti-inflammatory drugs: systematic review of population-based controlled observational studies. PLoS Med 2011;8:e1001098. 10.1371/journal.pmed.1001098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmidt M, Christiansen CF, Horváth-Puhó E, Glynn RJ, Rothman KJ, Sørensen HT. Non-steroidal anti-inflammatory drug use and risk of venous thromboembolism. J Thromb Haemost 2011;9:1326-33. 10.1111/j.1538-7836.2011.04354.x [DOI] [PubMed] [Google Scholar]
- 14. Pedersen CB. The Danish Civil Registration System. Scand J Public Health 2011;39(Suppl):22-5. 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 15. Helweg-Larsen K. The Danish Register of Causes of Death. Scand J Public Health 2011;39(Suppl):26-9. 10.1177/1403494811399958 [DOI] [PubMed] [Google Scholar]
- 16. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health 2011;39(Suppl):30-3. 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 17. Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health 2011;39(Suppl):38-41. 10.1177/1403494810394717 [DOI] [PubMed] [Google Scholar]
- 18. Blenstrup LT, Knudsen LB. Danish registers on aspects of reproduction. Scand J Public Health 2011;39(Suppl):79-82. 10.1177/1403494811399957 [DOI] [PubMed] [Google Scholar]
- 19. Knudsen LB, Olsen J. The Danish Medical Birth Registry. Dan Med Bull 1998;45:320-3. [PubMed] [Google Scholar]
- 20. Meaidi A, Mørch L, Torp-Pedersen C, Lidegaard O. Oral tranexamic acid and thrombosis risk in women. EClinicalMedicine 2021;35:100882. 10.1016/j.eclinm.2021.100882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sundbøll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open 2016;6:e012832. 10.1136/bmjopen-2016-012832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gaster N, Hallas J, Pottegård A, Friis S, Schmidt M. The Validity of Danish Prescription Data to Measure Use of Aspirin and Other Non-Steroidal Anti-Inflammatory Drugs and Quantification of Bias Due to Non-Prescription Drug Use. Clin Epidemiol 2021;13:569-79. 10.2147/CLEP.S311450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kinsey TL, Stürmer T, Funk MJ, Poole C, Simpson RJ, Glynn RJ. Incidence of venous thromboembolism following initiation of non-steroidal anti-inflammatory drugs in U.S. women. Rheumatology (Oxford) 2020;59:2502-11. 10.1093/rheumatology/kez653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hinz B, Cheremina O, Brune K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J 2008;22:383-90. 10.1096/fj.07-8506com [DOI] [PubMed] [Google Scholar]
- 25. Chan AT, Manson JE, Albert CM, et al. Nonsteroidal antiinflammatory drugs, acetaminophen, and the risk of cardiovascular events. Circulation 2006;113:1578-87. 10.1161/CIRCULATIONAHA.105.595793 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary tables
Data Availability Statement
Raw data used to conduct this study are accessible only through approval from the Danish Data Protection Agency and the Danish Health Data Board. Although anonymised, the data were available at an individual level, making data sharing restricted by the General Data Protection Regulation of EU law.