Abstract
The abundance of host-seeking Ixodes scapularis nymphs, the principal vector for the Lyme disease agent, Borrelia burgdorferi, in Old Lyme, Lyme, and East Haddam, Connecticut, was compared with the incidence of reported human Lyme disease in the 12-town area around the Connecticut River and the State of Connecticut for the period 1989 to 1996. Ticks were sampled from lawns and woodlands by dragging flannel over the vegetation and examined for the presence of B. burgdorferi by indirect fluorescent antibody staining. The infection rate of the nymphal ticks by B. burgdorferi during the 9-year period was 14.3% (of 3,866), ranging from 8.6% (1993) to 24.4% (1996). The incidence of Lyme disease was positively correlated with tick abundance in the 12 town area (r = 0.828) and the State of Connecticut (r = 0.741). An entomological risk index based upon the number of I. scapularis ticks infected by B. burgdorferi was highest in 1992, 1994, and 1996 and was highly correlated with the incidence of Lyme disease in Connecticut (r = 0.944). The number of Lyme disease cases has been influenced, in part, by annual changes in population densities of I. scapularis and, presumably, a corresponding change in the risk of contact with infected ticks. Based upon tick activity and spirochetal infection rates, epidemiologically based Lyme disease case reports on a regional scale appear to reflect real trends in disease.
Lyme disease is the most frequently reported vector-borne illness in the United States (8). This disease is a tick-borne zoonotic infection caused by the spirochete Borrelia burgdorferi sensu lato. Since surveillance for Lyme disease was begun by the Centers for Disease Control and Prevention (CDC) in 1982, the number of human cases has increased from 491 to 16,461 in 1996 (6). The majority of these cases were reported in the northeastern United States, where the principal vector is the blacklegged tick, Ixodes scapularis (also commonly known as the deer tick). The increase in reported cases can be attributed to greater recognition, improved reporting, and a true increase in incidence (4). This increase may also reflect a change in tick abundance and, for Connecticut at least, an expansion of the geographical distribution of the tick (11). The incidence of Lyme disease in several Rhode Island communities was strongly correlated with the density of spirochete-infected nymphal I. scapularis (13). There were slight decreases in reported cases nationally in 1993 and 1995, and vector surveillance data in Connecticut, New York, and Rhode Island had indicated decreased tick abundance during those years (3, 5). Rising numbers of Lyme disease cases in Connecticut and Rhode Island in 1996 were associated with increased population densities of I. scapularis (6).
Since 1991, Connecticut has reported the highest incidence of Lyme disease, with 94 cases per 100,000 people reported in 1996. In a study of the habitat distribution of I. scapularis in southeastern Connecticut from 1989 to 1991 (18), tick populations appeared to correspond with the number of human cases of Lyme disease reported in the state and the surveyed towns. The majority of Lyme disease cases was associated with nymphal I. scapularis during the summer months (10). Tick surveys were continued from 1992 through 1997 to monitor the activity of host-seeking I. scapularis nymphs and the infection rate in those ticks. The purpose of our study was to characterize the relationship between tick abundance, the prevalence of B. burgdorferi in those ticks, and the incidence of Lyme disease in Connecticut and the 12-town region around the Connecticut River.
MATERIALS AND METHODS
Tick sampling.
The relative abundance of host-seeking I. scapularis nymphs from 1989 to 1997 was determined by “dragging” a 1.2-m2 piece of flannel cloth over the vegetation at 10 residential properties in the four towns of East Haddam (three sites), Old Lyme (two sites), Lyme (four sites), and Chester (one site) (18) (Fig. 1). The flannel cloth (95 by 130 cm) was stapled along a 105-cm wooden dowel with the ends of a rope attached to each end of the dowel. The sites included woodlands and woodland edge, where I. scapularis predominates, and generally both lawns and forested areas were sampled. The total area sampled ranged from 0.15 to 0.50 ha, with woodlands comprising 0.024 to 0.25 ha at each site. The area sampled at each site was determined by measuring the site with a metric measuring wheel. From 1992, samples were taken from 10- by 25-m woodland subplots in the same locations at eight of the residences in East Haddam, Lyme, and Old Lyme. Host-seeking I. scapularis at each site was sampled once a month from April through October in 1989, twice each month from April through October from 1990 through 1993, and twice each month from May through August in 1994. Any ticks found on the drag cloth were placed in vials with a blade of grass for moisture and returned to the laboratory for identification and testing for B. burgdorferi. The presence of B. burgdorferi in host-seeking nymphs was determined by indirect fluorescent antibody staining of tick midgut tissues with murine monoclonal antibody (H5332) as previously described (12).
FIG. 1.
The 12-town region of Connecticut. The towns of Old Lyme, Lyme, and East Haddam lie east of the Connecticut River, while the remaining nine towns are west of the river.
Disease surveillance.
Connecticut has had a passive, physician-based surveillance system for Lyme disease since July 1987 (1). The CDC case definition for Lyme disease acquired in an area of endemicity was used to classify cases (7). In 1989, the Lyme disease surveillance system was expanded by the Connecticut Department of Public Health to include a supplemental Lyme disease case report form. Follow-up questionnaires were sent to physicians who reported a case of Lyme disease without supplying clinical information. Reports without clinical information were not counted as cases. In 1990, Connecticut case reports were based upon the new national surveillance case definition for Lyme disease adopted by the Council of State and Territorial Epidemiologists in 1990 (2). Therefore, the case reports for 1989 were based on the previous CDC case definition. Active surveillance for Lyme disease in the original 12-town area studied by Steere et al. (19) (i.e., Lyme, Old Lyme, East Haddam, Haddam, Chester, Deep River, Essex, Old Saybrook, Westbrook, Clinton, Killingworth, and Madison) began in 1991 (Fig. 1). Case reports for the 12-town area were used as a measure of Lyme disease incidence in the region for the period 1989 through 1996.
Methods of analysis.
The number of ticks collected from each woodland plot and lawn for each site was standardized by conversion to the number collected per hectare. Nymphal abundance for each month and year was the average for all site visits during each month and from mid-May through mid-August, respectively. Tick populations between years were compared by analysis of variance on log-transformed biweekly tick abundance and Fisher’s protected least significance difference (LSD) test (17). Patterns of tick activity within each month for each year were examined by their departure from the overall mean for each month for the entire study period (e.g., difference in mean tick abundance for May 1989 from mean tick abundance for May, 1989 to 1997). An annual entomological risk index (ERI), similar to that used by Mather et al. (13), was calculated as the product of tick abundance (average number of nymphs per hectare) and the proportion of ticks infected by B. burgdorferi. The ERI is the average number of infected ticks per hectare and reflects the risk of acquiring a bite from an infected tick. Differences between years in the rate of infection by B. burgdorferi were compared by analysis of variance on transformed (arcsin √x) site-specific infection rates. The incidences of reported human Lyme disease for the state and 12-town area were compared to annual tick abundance and the ERI by simple linear regression for the period 1989 to 1996. A projection of the number of cases in Connecticut and the 12-town area is based upon a simple linear regression of tick abundance and the number of Lyme disease cases for the 8-year period.
RESULTS
Tick abundance and prevalence of infection.
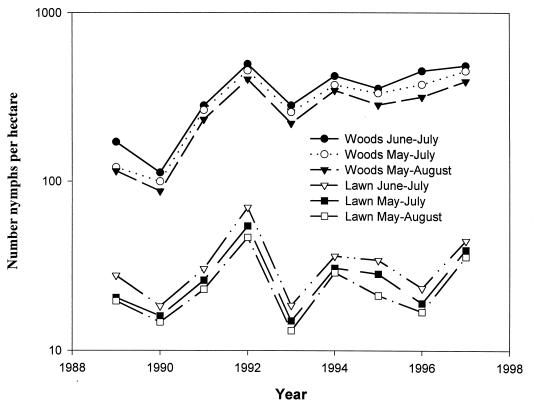
The abundance of nymphal I. scapularis in residential woodlots in southeastern Connecticut increased dramatically in 1991 over that of 1989 or 1990 (Fig. 2). Tick numbers increased again by 70% in 1992 compared to relative abundance in 1991. Tick abundance increased in 1994 by 57% over that recorded in 1993 and again by 14% in 1996 compared to 1995. Nymphal densities were lower in 1993 and 1995. In 1997, densities of I. scapularis nymphs were higher than in 1996. Similar trends were observed in tick activity on the lawn. These differences in the annual fluctuation of tick abundance were highly significant, both in the woods (F = 7.801, df = 8, 402; P ≤ 0.001) and on the lawns (F = 4.691, df = 8, 429; P ≤ 0.001). Tick abundance in 1992, 1994, and 1997 was significantly higher than in most other years on both the lawn (LSD = 0.3578; P ≤ 0.044) and woodlands (LSD = 0.7856; P ≤ 0.048), although the number of I. scapularis nymphs in 1994 was not significantly higher than that in 1991. There was little difference in the overall annual trends between the three seasonal periods used to initially tabulate tick abundance (i.e., mid-May through mid-August, mid-May through July, and June and July only) (Fig. 1). However, variation in the seasonal activity of the I. scapularis nymphs each year could influence risk of exposure to tick bites and number of human cases of Lyme disease, particularly during the beginning and end of the tick season.
FIG. 2.
Numbers of I. scapularis nymphs per hectare for three seasonal periods (mid-May through mid-August, mid-May through July, and June and July only) collected from woods or lawns during the period 1989 to 1997 in Old Lyme, Lyme, and East Haddam.
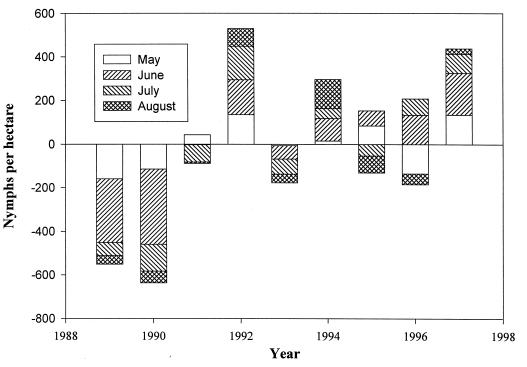
There were pronounced differences in nymphal tick activity during each month of the summer tick season over the 9-year study (Fig. 3). Not only was nymphal tick activity in the woodland plots above the 9-year baseline for each month of May, June, July, and August in 1992, 1994, and 1997, but I. scapularis nymphs were also more abundant at the beginning (May) and end (August) of the tick season. In 1995, I. scapularis was abundant in the late-May and early-June samples, with subsequent tick activity dropping rapidly through July and August, when Connecticut was experiencing a drought. Tick activity was below the long-term baseline for all 4 months in 1989, 1990, and 1993. In 1996, nymphal blacklegged ticks were abundant during the peak months of June and July, but their activity was low in May and August. The abundance of I. scapularis nymphs on the lawns shows a pattern similar to that in the woods with above-average activity in 1992, 1994, and 1997.
FIG. 3.
Departures of the mean number of I. scapularis nymphs collected during the months of May, June, July, and August from the woods in Old Lyme, Lyme, and East Haddam, for each year, 1989 to 1997, from a grand average baseline (0 on the graph) for the entire study period. Values do not represent measures of tick abundance.
The overall infection rate of the host-seeking nymphal ticks by B. burgdorferi during the 8-year period was 14.3% (of 3,866). However, the infection rate varied significantly between years (F = 2.538, df = 8,179; P = 0.012) during the study period (Table 1). The fewest ticks were found to be infected in 1993 (8.6%), and the highest infection rate was recorded in 1996 (24.4%). While infection rate estimates are highly variable among individual samples, values for most years are based on testing several hundred ticks from all the sites over the entire period of nymphal tick activity and, consequently, is probably a reasonable estimate of the general infection rate in the region for any given year. There was no significant difference between the months of May, June, July, and August in the proportion of nymphs infected with B. burgdorferi. Based upon the ERI, the years of greatest overall risk for Lyme disease were 1991, 1992, 1994, and particularly 1996 (Table 1). During 1992 and 1994, the risk of acquiring a bite from an infected nymph was high even in May and August.
TABLE 1.
Proportions of nymphal I. scapularis ticks infected with B. burgdorferi collected by sampling the vegetation at 8 to 10 residential sites in Old Lyme, Lyme, and East Haddam and ERIs, 1989 to 1996
| Yr | % infected (no. tested)a | ERIb
|
|
|---|---|---|---|
| Woods | Lawn | ||
| 1989 | 15.6 (283) | 17.9 | 3.1 |
| 1990 | 13.3 (408) | 11.6 | 2.0 |
| 1991 | 14.6 (903) | 34.5 | 3.3 |
| 1992 | 12.0 (108) | 48.3 | 5.6 |
| 1993 | 8.6 (216) | 18.9 | 1.1 |
| 1994 | 11.6 (560) | 40.0 | 3.3 |
| 1995 | 13.1 (436) | 36.3 | 2.8 |
| 1996 | 24.4 (513) | 76.9 | 4.1 |
| 1997 | 9.8 (439) | 39.9 | 3.6 |
Determined by indirect fluorescent antibody staining of tick midgut tissues for B. burgdorferi.
Based upon the mean number of nymphs per hectare and the rate of infection by B. burgdorferi.
Lyme disease incidence and tick abundance.
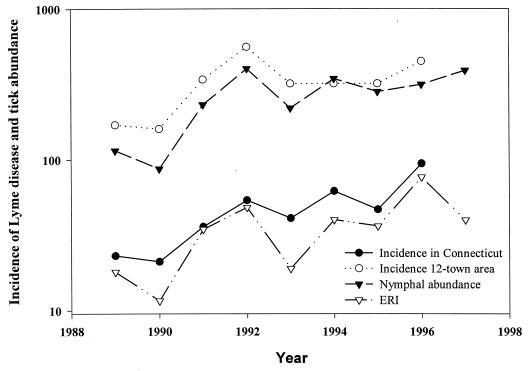
The incidence of human cases of Lyme disease in Connecticut increased dramatically in 1991, 1992, 1994, and 1996, with rates of 36, 54, 62, and 94 per 100,000 people, respectively (Fig. 4). A similar trend for the same years was observed in the 12-town region with an incidence of 340, 560, 320, and 450 per 100,000, respectively. The rate per 100,000 residents exceeded 1,000 in the town of Old Lyme in 1991, 1992, and 1994 and exceeded 1,000 for the town of Lyme in 1992 and Chester in 1996. The number of cases in the state decreased in 1993 and 1995 from the previous years. These periods of higher and lower incidence of Lyme disease coincide with the activity of I. scapularis nymphs. In 1991, the density of I. scapularis nymphs increased 170%. The number of Lyme disease cases increased by 69 and 106% in the state and 12-town region, respectively. The years that had the highest number of reported cases in Connecticut were 1992, 1994, and 1996, years with the greatest nymphal-tick activity. The number of Lyme disease cases statewide decreased by about 23% in 1993 and 1995 from the previous years, which coincided with 45.4 and 19.6% decreases in nymphal-tick densities for 1993 and 1995, respectively. A similar pattern was observed for the 12-town region (Fig. 4).
FIG. 4.
Lyme disease incidence rate (per 100,000) for Connecticut and the 12-town region for 1989 to 1996; abundance (number per hectare) of nymphal I. scapularis in residential woodlots in the towns of Old Lyme, Lyme, and East Haddam; and the corresponding ERI, 1989 to 1997.
The least-squares linear regression of the incidence of Lyme disease for the state and 12-town region on tick abundance (number per hectare) for the 8-year period from 1989 through 1996 is presented in Table 2. Tick abundance in residential woodlots was significantly correlated with the incidence of Lyme disease in the 12-town area and in Connecticut, especially for the period from 1989 to 1995. Tick abundance increased by only 14% in 1996, but the infection rate was higher, resulting in an increased risk for Lyme disease. The ERI was highly correlated with the number of reported cases in Connecticut during the entire 8-year period (r = 0.944) (Fig. 4). The ERI based upon ticks recovered from the lawn was correlated significantly only with the incidence of Lyme disease in the 12-town area. By contrast, the relationship between tick abundance on the lawn and the incidence of Lyme disease statewide was insignificant (P > 0.05).
TABLE 2.
Regression of nymphal tick abundance and ERI on the number of reported human Lyme disease cases for Connecticut and the 12-town area, 1989 to 1996
| Comparison | F | P | r |
|---|---|---|---|
| Connecticut, woods | |||
| Nymphs/ha | |||
| 1989–96 | 7.326 | 0.035 | 0.741 |
| 1989–95 | 41.610 | 0.001 | 0.945 |
| ERI | |||
| 1989–96 | 48.961 | <0.001 | 0.944 |
| 1989–95 | 12.352 | 0.017 | 0.844 |
| 12 towns | |||
| Nymphs/ha | |||
| Woods | 13.123 | 0.011 | 0.828 |
| Lawn | 4.776 | 0.072 | 0.666 |
| ERI | |||
| Woods | 6.923 | 0.039 | 0.732 |
| Lawn | 7.513 | 0.034 | 0.746 |
DISCUSSION
The incidence of Lyme disease in Connecticut was positively and significantly correlated with long-term trends in the abundance of nymphal I. scapularis and the abundance of spirochete-infected ticks from woodland plots in the communities of Old Lyme, Lyme, and East Haddam. This suggests that recent increases in tick abundance may be responsible, in part, for much of the rise in Lyme disease infection in Connecticut, although the tick is also spreading geographically within the state (11). Trends in tick abundance in these woodlands probably reflected general activity across the state or, at least, in southeastern Connecticut, which has had the highest incidence of Lyme disease. However, in the established foci of Old Lyme, Lyme, and East Haddam, the incidence of Lyme disease increased by 64% from 1995 to 1996, while tick densities increased by only 14%. Annual patterns of tick activity in this study corresponded more closely with Lyme disease in the 12-town area. Unfortunately, the factors that influence the annual abundance of I. scapularis are poorly understood. The population dynamics of the tick population is complex, and future abundance of I. scapularis cannot be predicted (10). Future monitoring of I. scapularis may be required in other regions of the state in order to accurately assess tick trends in relation to the incidence of Lyme disease. Based upon the abundance of I. scapularis per hectare in the 12-town region or the abundance of infected ticks (ERI) in the summer of 1997, 2,329 and 1,708 cases of Lyme disease, respectively, might be expected for 1997. However, increased tick activity and reporting of Lyme disease from other portions of the state could increase the incidence of the disease in 1997 above a rate determined by past trends in the 12-town region. For example, the incidence of reported Lyme disease from Windham and Tolland counties in northern Connecticut has increased from 11 and 27 cases per 100,000, respectively, in 1989 to 257 and 155 cases per 100,000, in 1996, which may be a result of increased tick activity. This could be determined from a broader-based surveillance of the abundance of I. scapularis nymphs in Connecticut.
The relative length of the tick season is another factor in the risk for tick bites. Host-seeking nymphs of I. scapularis in the northeast begin their activity in May, peak in June, and slowly decline in activity through July and August (10, 16, 18). Sampling nymphs of I. scapularis only during the June seasonal peak appears to be an adequate measure of tick abundance in relation to the annual incidence of Lyme disease, but early and late seasonal tick activity will be missed. There was considerable variation in the basic pattern in the level of activity each month through the period of this study. Interestingly, two years, 1992 and 1994, that had a high incidence of Lyme disease not only had a higher peak abundance of nymphal ticks but also had greater tick activity for a longer period. Nymphal-tick activity was extended into August in 1997 as well.
Infection rates were found to vary tremendously among the residential sites in Connecticut, and this may account, in part, for the weak relationship between the lawn ERI and Lyme disease incidence at the local level. The abundance of infected ticks on the lawn more accurately reflected the regional incidence of Lyme disease. Areas of high risk and low risk can be expected between the lawns of individual residences. The lawn is probably where most tick bites are acquired by residents, although only a small proportion (2 to 13%) of the tick population is found there (9, 14, 18).
The risk for Lyme disease is currently assessed from human case reports, isolation from or detection of Lyme disease borrelliae in mice and ticks, serosurveys of animals, collection of host-seeking I. scapularis, or collection of ticks attached to deer, mice, or other animals. Each of these methods has inherent biases and limitations in geographical scale. The number of reported human cases as a measure of the true distribution and incidence of Lyme disease is subject to the surveillance case definition and type of surveillance method used. Despite these limitations, the abundance of host-seeking nymphs was closely associated with the incidence of human Lyme disease based upon case reports over an 8-year period in Connecticut. The incidence of Lyme disease at the state and regional levels closely paralleled the abundance of I. scapularis nymphs and the abundance of spirochete-infected nymphs. The relative abundance of nymphal ticks within the woodland habitat has been a reliable, and independently measured, predictor for Lyme disease at the regional and state levels. Epidemiologically based Lyme disease case reports on regional, state, and national scales appear to reflect real trends in the disease based upon tick activity and spirochetal infection rates, although Lyme disease is clearly underreported (15). The use of active surveillance of human cases and more intensive sampling of I. scapularis nymphs may provide a better assessment of risk within an area of endemicity and for the state.
ACKNOWLEDGMENTS
This study was supported in part by the CDC cooperative agreement U50-CCU106598.
We acknowledge Frank Campbell, Kathryn Gorski, Cynthia Phillips, Patricia Noyes, Collen Moser, Tia Blevins, Shanon Trueman, Vickie Bomba, Cynthia Musante, Curtis Gibson, Michael Harma, Brandon Brei, and Michael Burelle for assistance in collecting ticks over the years. We also thank Alan G. Barbour, University of California—Irvine, for providing murine monoclonal antibody (H3552). The map of Connecticut and the 12-town area was provided by Ellen Cromley, Department of Geography, University of Connecticut.
REFERENCES
- 1.Cartter M L, Mshar P, Hadler J L. The epidemiology of Lyme disease in Connecticut. Conn Med. 1989;53(6):320–323. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Case definitions for public health surveillance. Morbid Mortal Weekly Rep. 1990;39(RR-13):19–21. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Lyme disease—United States, 1993. Morbid Mortal Weekly Rep. 1994;43(31):564–572. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Lyme disease—United States, 1994. Morbid Mortal Weekly Rep. 1995;44(24):459–462. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Lyme disease—United States, 1995. Morbid Mortal Weekly Rep. 1996;45(23):481–484. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Lyme disease—United States, 1996. Morbid Mortal Weekly Rep. 1997;46(23):531–535. [PubMed] [Google Scholar]
- 7.Ciesielski C, Markowitz L, Horsley R, Hightower A, Russell H, Broome C. The geographic distribution of Lyme disease in the United States. Ann N Y Acad Sci. 1988;539:283–288. doi: 10.1111/j.1749-6632.1988.tb31862.x. [DOI] [PubMed] [Google Scholar]
- 8.Dennis D T. Lyme disease. Dermatol Clin. 1995;13(3):537–549. [PubMed] [Google Scholar]
- 9.Falco R C, Fish D. Prevalence of Ixodes dammini near the homes of Lyme disease patients in Westchester County, New York. Am J Epidemiol. 1988;127:826–830. doi: 10.1093/oxfordjournals.aje.a114865. [DOI] [PubMed] [Google Scholar]
- 10.Fish D. Population ecology of Ixodes dammini. In: Ginsberg H S, editor. Ecology and environmental management of Lyme disease. New Brunswick, N.J: Rutgers University Press; 1993. pp. 25–42. [Google Scholar]
- 11.Magnarelli L A, Anderson J F, Cartter M L. Geographic distribution of white-tailed deer with ticks and antibodies to Borrelia burgdorferi in Connecticut. Yale J Biol Med. 1993;66:19–26. [PMC free article] [PubMed] [Google Scholar]
- 12.Magnarelli L A, Anderson J F, Fish D. Transovarial transmission of Borrelia burgdorferi in Ixodes dammini (Acari: Ixodidae) J Infect Dis. 1987;156:234–236. doi: 10.1093/infdis/156.1.234. [DOI] [PubMed] [Google Scholar]
- 13.Mather T N, Nickolson M C, Donnelly E F, Matyas B T. Entomologic index for human risk of Lyme disease. Am J Epidemiol. 1996;144:1066–1069. doi: 10.1093/oxfordjournals.aje.a008879. [DOI] [PubMed] [Google Scholar]
- 14.Maupin G O, Fish D, Zultowsky J, Campos E G, Piesman J. Landscape ecology of Lyme disease in a residential area of Westchester County, New York. Am J Epidemiol. 1991;133:1105–1113. doi: 10.1093/oxfordjournals.aje.a115823. [DOI] [PubMed] [Google Scholar]
- 15.Meek J I, Roberts C L, Smith E V, Jr, Cartter M L. Underreporting of Lyme disease by Connecticut physicians, 1992. J Public Health Man Pract. 1996;2(4):61–65. doi: 10.1097/00124784-199623000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Piesman J, Mather T N, Dammin G J, Telford III S R, Lastavica C C, Spielman A. Seasonal variation of transmission risk of Lyme disease and human babesiosis. Am J Epidemiol. 1987;126:1187–1189. doi: 10.1093/oxfordjournals.aje.a114757. [DOI] [PubMed] [Google Scholar]
- 17.Snedecor G W, Cochran W G. Statistical methods. 6th ed. Ames: Iowa State University Press; 1967. [Google Scholar]
- 18.Stafford K C, III, Magnarelli L A. Spatial and temporal patterns of Ixodes scapularis (Acari: Ixodidae) in southcentral Connecticut. J Med Entomol. 1993;30:762–771. doi: 10.1093/jmedent/30.4.762. [DOI] [PubMed] [Google Scholar]
- 19.Steere A C, Broderick T F, Malawista S E. Erythema chronicum migrans and Lyme arthritis: epidemiologic evidence for a tick vector. Am J Epidemiol. 1978;108:312–321. doi: 10.1093/oxfordjournals.aje.a112625. [DOI] [PubMed] [Google Scholar]