Abstract
Acinetobacter has been reported to be involved in hospital-acquired infections with increasing frequency. However, clinical laboratories still lack simple methods that allow the accurate identification of Acinetobacter strains at the species level. For this study, proteinase K-digested whole-cell lysates from 44 clinical and environmental isolates were investigated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting with hyperimmune rabbit sera to examine the possibility of developing a serotyping scheme based on the O antigen of Acinetobacter lipopolysaccharide (LPS). The antisera, obtained by immunization of rabbits with 13 of the heat-killed isolates investigated, were characterized by Western blotting and enzyme immunoassay by using proteinase K-digested whole-cell lysates and phenol-water-extracted LPS as antigens. In both assays, the antisera were shown to be highly specific for the homologous antigen. In addition, assignment of Acinetobacter LPS to the smooth or the rough phenotype was shown not to be reliable when it was based only on the results obtained with silver-stained gels. O-antigen reactivity, determined by Western blot analysis, was observed with 11 of the 31 isolates, most of which belonged to the species Acinetobacter baumannii (DNA group 2) and the unnamed DNA group 3. Interestingly, some O antigens were found in a DNA group different from that of the strain used for immunization. The results indicate that O serotyping of Acinetobacter strains is feasible and thus may provide a simple method for the routine identification of these opportunistic pathogens.
The genus Acinetobacter belongs to the recently proposed new family Moraxellaceae of the γ subclass of the class Proteobacteria (10, 38, 42). Members of this genus are found in soil, water, and sewage and have also been isolated from clinical specimens of human and animal origin (2, 22, 30). Although initially it was not considered pathogenic, it is now recognized that these organisms play a significant role in the colonization and infection of immunocompromised patients in intensive care units (4, 11, 30, 34), and it seems likely that they will be of increasing epidemiological importance in the future, particularly because of the increased multidrug resistance observed in some strains (4, 12, 13, 34, 44). Despite the reported increase in the significance and the frequency of such Acinetobacter infections, some clinicians still lack appreciation for the importance of these organisms in hospitals, in part because of the confused taxonomic status associated with these bacteria and difficulties in the phenotypic identification of such strains (4, 15, 16, 20, 50). The diversity of the genus is reflected in the different phenotypic and genotypic groups that have been defined (7–9, 45). Since 1986, DNA-DNA hybridization studies have resulted in the identification of at least 18 DNA groups (7, 9, 45). Unfortunately, no single test (or set of tests) other than DNA-DNA hybridization allows the unambiguous identification of some Acinetobacter strains to the species level (15, 20).
Lipopolysaccharide (LPS) is a common constituent of the outer membrane of gram-negative bacteria (28, 40, 41) and has often been used as a taxonomic marker, particularly for those bacteria containing smooth-form (S-form) LPS, i.e., an O-specific side chain or O antigen (1, 31, 35, 40, 41, 43). The different antigens have been shown to correlate with differences in the chemical structures of the repeating units of the LPS (35). We have recently shown that Acinetobacter strains are able to make S-form LPS (23–26, 48, 49) and have therefore started detailed structural investigations of these O antigens, with the aim of providing a molecular basis for an Acinetobacter O-serotyping scheme.
Here, we report on the specificity of rabbit sera against Acinetobacter LPS and show that O-antigen serotyping may be helpful in research as well as in clinical laboratories for the identification of strains belonging to this genus.
MATERIALS AND METHODS
Clinical and environmental isolates.
Forty-four Acinetobacter isolates which had been characterized by DNA-DNA hybridization and by electrophoretic cell envelope protein profiling in a previous study (14) were investigated (Table 1). The strains were preserved at −80°C in Luria-Bertani broth supplemented with 10% (vol/vol) glycerol.
TABLE 1.
Clinical and environmental Acinetobacter isolates investigated in this study
| Straina | DNA groupb | Sourcec |
|---|---|---|
| 2 | 1 | Soil |
| 7d | 1 | Wound |
| ATCC 23055T | 1 | Soil |
| 9d | 2 | Sputum |
| 14 | 2 | Not known |
| 24d | 2 | Urine |
| 34d | 2 | Urine |
| 36 | 2 | Sputum |
| 37 | 2 | Blood |
| 41 | 2 | Catheter tip |
| ATCC 17904 | 2 | Urine |
| NCTC 7844 | 2 | Not known |
| 44d | 3 | Drain |
| 51 | 3 | Not known |
| 53 | 3 | Not known |
| ATCC 19004 | 3 | Cerebrospinal fluid |
| 57d | 4 | Pus |
| 58 | 4 | Not known |
| 59 | 4 | Not known |
| 61d | 4 | Air |
| ATCC 17906Td | 4 | Sputum |
| 64 | 5 | Blood |
| 65d | 5 | Not known |
| 67 | 5 | Water |
| ATCC 17908T | 5 | Urine |
| ATCC 17979 | 6 | Throat |
| 73 | 7 | Urine |
| 76 | 7 | Urine |
| ATCC 17909T | 7 | Gut |
| 80 | 8/9 | Prostate secretion |
| 83 | 8/9 | Skin of a volunteer |
| NCTC 5866T | 8/9 | Not known |
| 90d | 10 | Urine |
| 91 | 10 | Wound |
| ATCC 17924 | 10 | Not known |
| 95 | 11 | Not known |
| 96d | 11 | Urine |
| ATCC 11171d | 11 | Not known |
| 105 | 12 | Urine |
| 107 | 13 | Skin front |
| 108d | 13 | Bronchus |
| ATCC 17903 | 13 | Not known |
| 119 | 14 | Conjunctiva secretion |
| ATCC 17905 | 14 | Conjunctiva secretion |
ATCC, American Type Culture Collection, Rockville, Md.; NCTC, National Collection of Type Cultures, London, United Kingdom; T, type strain.
DNA group designation of Acinetobacter strains classified by DNA-DNA hybridization (45): DNA group 1, A. calcoaceticus; DNA group 2, A. baumannii; DNA group 3, unnamed; DNA group 4, A. haemolyticus; DNA group 5, A. junii; DNA group 6, unnamed; DNA group 7, A. johnsonnii; DNA group 8/9 (considered a single entity), A. lwoffii; DNA group 10, unnamed; DNA group 11, unnamed; DNA group 12, A. radioresistens; DNA group 13, unnamed; DNA group 14, unnamed.
Source or specimen from which the strain was originally isolated.
Strains used to prepare rabbit antisera.
Bacterial LPSs.
The Acinetobacter strains used for immunization (see below) were grown in a fermenter (10 liters), and the cells were subsequently killed with phenol as described previously (48). After centrifugation, LPS was extracted from the bacterial sediment with phenol-water (51) and lyophilized.
Whole-cell lysates and proteinase K digestion.
Preparation of whole-cell lysates and proteinase K digestion were performed as described previously (48), with minor alterations. Briefly, the stored strains were subcultured on solid medium (blood agar), harvested with a sterile swab, suspended in NaCl (5 ml, 0.15 M), and centrifuged (7,200 × g, 10 min). The bacterial pellets (200 to 300 μl) were solubilized in sample buffer (2 to 3 ml; 62.5 mM Tris-HCl [pH 6.8], 2% sodium dodecyl sulfate [SDS], 5% 2-mercaptoethanol, 10% glycerol, 0.01% bromphenol blue) and were subsequently stored at −20°C. For proteinase K digestion, lysates were diluted 1:4 in sample buffer and were then heated (100°C, 5 min). An aliquot (20 μl) of the heated sample was then added to proteinase K, (25 μg in 10 μl of sample buffer; Boehringer Mannheim), and the mixture was incubated at 60°C for 1 h. The digested samples could be stored at −20°C but were heated again (100°C, 5 min) prior to use.
Rabbit antisera.
Thirteen Acinetobacter strains (Table 1) were used to prepare hyperimmune rabbit sera. Rabbits with no detectable antibodies against the LPS of the chosen Acinetobacter strains were immunized with heat-killed bacteria as described previously (48). The sera were stored at −20°C until further use.
EIA.
For enzyme immunoassay (EIA), 50-μl volumes were used, unless stated otherwise. Microtiter polyvinyl plates (Falcon 3911; Becton Dickinson) were coated with LPS (250 ng per well) diluted in phosphate-buffered saline (PBS, pH 7.2) and were incubated overnight at 4°C. All PBS and PBS-containing solutions were supplemented with 0.01% thimerosal. Further incubation steps were performed at 37°C under gentle agitation. The coated plates were washed four times with PBS and were blocked for 1 h with PBS supplemented with 2.5% casein (Sigma) (PBS-C; 200 μl per well). Rabbit antiserum (diluted in PBS-C) was subsequently added, and the mixture was incubated for 1 h. After washing as described above, peroxidase-conjugated goat anti-rabbit immunoglobulin G (heavy and light chains; Dianova) diluted 1:750 in PBS-C was added, and incubation was continued for 1 h. After washing with PBS, two washes were performed with substrate buffer (0.1 M sodium citrate [pH 4.5]), followed by the addition of substrate solution, which was freshly prepared by dissolving azino-di-3-ethylbenzthiazoline-6-sulfonic acid (1 mg) in substrate buffer (1 ml) with sonication in an ultrasound water bath for 1 min and then adding hydrogen peroxide (25 μl of a 0.1% solution). After 30 min, the reaction was stopped by the addition of 2% aqueous oxalic acid, and the plates were read with a microtiter plate reader (Dynatech MR5000) at 405 nm (reference filter, 490 nm). Titers were interpreted as the highest dilution of antiserum yielding an optical density at 405 nm of >0.2.
SDS-PAGE, silver staining, and Western blotting.
Preparations of proteinase K-digested whole-cell lysates were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) with a 5% stacking gel and a 10 or a 15% separating gel in the Laemmli system. After electrophoresis, the gels were stained with alkaline silver nitrate as described previously (48) or were electrotransferred overnight onto polyvinylidene difluoride (PVDF) membranes (pore size, 0.45 μm, Millipore) by tank blotting (Bio-Rad). Prior to use, the membranes were wetted in methanol for 10 s, after which they were washed in distilled water for at least 5 min. Following transfer, the blots were placed in distilled water until further use. They were subsequently immunostained with rabbit antisera as described previously (48).
RESULTS
Reactivity of Acinetobacter LPS with alkaline silver nitrate after SDS-PAGE.
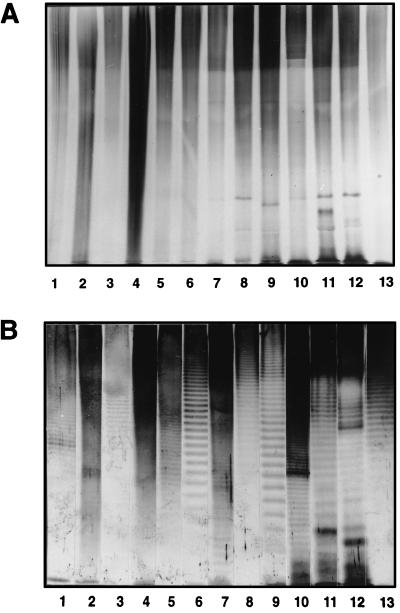
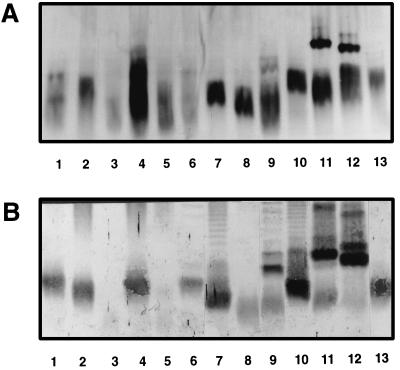
Proteinase K-digested whole-cell lysates of the strains used to prepare rabbit antisera were first investigated by staining with alkaline silver nitrate following SDS-PAGE. No characteristic O-antigen banding patterns were observed for any of the strains on a 10% gel (Fig. 1A). As can be observed in Fig. 2A, only the core lipid A region could be visualized when the samples were separated on a 15% gel.
FIG. 1.
Reactivities of proteinase K-digested whole-cell lysates of Acinetobacter strains used for immunization after separation by SDS-PAGE on a 10% separating gel and staining with alkaline silver nitrate (A) or with homologous rabbit antisera in Western blots (B). Lanes 1, strain 34; lanes 2, strain 57; lanes 3, strain 44; lanes 4, strain 24; lanes 5, strain 7; lanes 6, strain 108; lanes 7, strain 61; lanes 8, strain 65; lanes 9, strain 9; lanes 10, strain ATCC 17906; lanes 11, strain 90; lanes 12, strain ATCC 11171; lanes 13, strain 96.
FIG. 2.
Reactivities of proteinase K-digested whole-cell lysates of Acinetobacter strains used for immunization after separation by SDS-PAGE on a 15% separating gel and staining with alkaline silver nitrate (A) or with homologous rabbit antisera in Western blots (B). Lane numbering is as described in the legend to Fig. 1.
Reactivity of rabbit antisera with Acinetobacter strains used for immunization by Western blotting.
The rabbit antisera were tested by Western blotting with proteinase K-treated bacterial lysates. Whereas no reactivity was observed with preimmune rabbit sera (data not shown), immune sera at dilutions of between 1:300 (serum sample K324) and 1:6,000 (serum sample K330) reacted strongly with the homologous LPS. All sera reacted with the O side chain of the homologous antigen (Fig. 1B). A distinct banding pattern could be observed for most LPSs, although a less distinct pattern was observed with LPSs from strains 57, 24, and 61 (lanes 2, 4, and 7, respectively, in Fig. 1B), which is probably due to the small size of the repeating units in the O antigens of these strains (22a). For most strains, reactivity was also observed with the core lipid A region (Fig. 2B) except in the case of strain 44 and strain 7 (lanes 3 and 5, respectively). A small number of heterologous reactions were also observed (data not shown). However, most of them were with the core-lipid A region of the heterologous LPS. The only heterologous O-antigen reactivity was observed between serum sample K322 and strain 61 and between serum sample K346 and strain 57.
Reactivity of rabbit sera with clinical and environmental isolates by Western blotting.
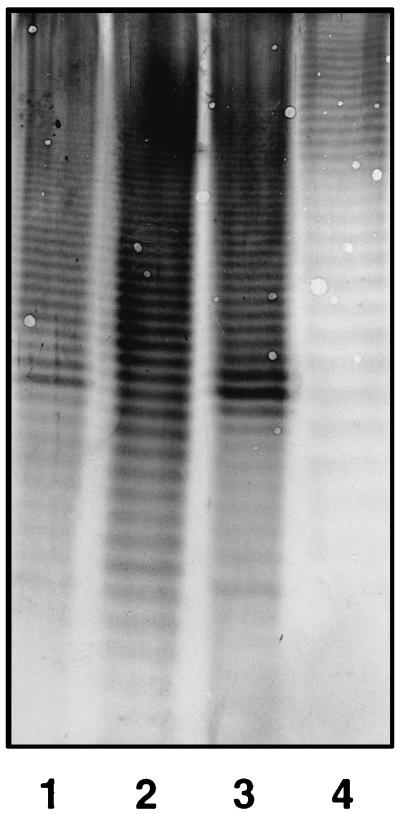
The 13 antiserum samples were then tested by Western blotting with other Acinetobacter strains which had been isolated from different clinical and environmental sources. The observed O-antigen reactions are presented in Table 2. Reactivity was observed with 11 of the 31 isolates tested, and the majority of the reactivity was with strains from DNA groups 2 (Acinetobacter baumannii) and 3 (unnamed species). Interestingly, some sera also reacted with the O antigen of strains not belonging to the same DNA group as that of the strain which was used for immunization. However, in all of these cases the observed banding pattern was different from that of the homologous strain. Such an example is shown in Fig. 3.
TABLE 2.
Reactivity of rabbit antisera in Western blot with the O antigen of LPS from proteinase K-digested whole-cell lysates of clinical and environmental Acinetobacter strains
| Strain | DNA group | Reactivity with the following serum samplea:
|
|||||
|---|---|---|---|---|---|---|---|
| K320 | K322 | K324 | K325 | K351 | K353 | ||
| 36 | 2 | + | − | − | − | − | − |
| 37 | 2 | + | − | − | − | − | − |
| 41 | 2 | − | − | − | + | + | − |
| NCTC 7844 | 2 | − | + | − | − | − | − |
| 51 | 3 | − | − | + | − | − | − |
| 53 | 3 | − | − | + | − | − | − |
| ATCC 19004 | 3 | − | − | + | − | − | − |
| 59 | 4 | − | − | − | − | − | + |
| 64 | 5 | + | − | − | − | − | − |
| 76 | 7 | − | − | + | − | − | − |
| 95 | 11 | − | + | − | − | − | − |
Reactivity with O antigen is indicated as follows: −, no reaction; +, positive reaction; K320, anti-strain 34 (DNA group 2); K322, anti-strain 57 (DNA group 4); K324, anti-strain 44 (DNA group 3); K325, anti-strain 24 (DNA group 2); K351, anti-strain 9 (DNA group 2); K353, anti-strain ATCC 17906 (DNA group 4).
FIG. 3.
Reactivity of rabbit antiserum K320 in Western blots after separation of proteinase K-digested whole-cell lysates of Acinetobacter sp. strains 34 (homologous strain; lane 1), 36 (lane 2), 37 (lane 3), and 64 (lane 4) by SDS-PAGE on a 10% separating gel.
Reactivity of rabbit antisera with Acinetobacter strains used for immunization in EIA.
The rabbit antisera were additionally tested by EIA by using phenol-water-extracted LPS as the antigen (Table 3). As in the Western blots, no reactivity was observed with the respective preimmune sera (data not shown), whereas homologous titers ranged from 128,000 to 4,096,000. Most of the heterologous reactions were negligible (<10% of the homologous titer). The only significant heterologous reactivities were those observed between serum sample K320 and strain 108, between serum sample K322 and strains 61, 96, and ATCC 17906, between serum sample K325 and strains 34 and 108, and between serum sample K346 and strain 57.
TABLE 3.
Reactivity of rabbit antisera in EIA with Acinetobacter LPS
| Serum sample no. | Immunogena | Titer obtained with LPS from the following strainb:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 34 | 57 | 44 | 24 | 7 | 108 | 61 | 65 | 9 | ATCC 17906 | 90 | ATCC 11171 | 96 | ||
| K320 | 34 | 1,024,000 | <1,000 | <1,000 | 2,000 | <1,000 | 512,000 | <1,000 | 16,000 | <1,000 | 16,000 | 2,000 | <1,000 | <1,000 |
| K322 | 57 | <1,000 | 128,000 | <1,000 | <1,000 | <1,000 | <1,000 | 64,000 | 1,000 | <1,000 | 32,000 | <1,000 | <1,000 | 16,000 |
| K324 | 44 | 4,000 | <1,000 | 64,000 | <1,000 | 1,000 | <1,000 | <1,000 | 4,000 | <1,000 | 1,000 | 2,000 | <1,000 | <1,000 |
| K325 | 24 | 64,000 | <1,000 | 2,000 | 256,000 | <1,000 | 128,000 | <1,000 | 4,000 | <1,000 | 16,000 | 2,000 | 1,000 | <1,000 |
| K327 | 7 | 1,000 | <1,000 | <1,000 | <1,000 | 128,000 | <1,000 | <1,000 | <1,000 | <1,000 | 4,000 | 1,000 | 1,000 | <1,000 |
| K330 | 108 | 16,000 | <1,000 | <1,000 | 1,000 | 2,000 | 1,024,000 | 1,000 | 16,000 | 4,000 | 4,000 | 4,000 | 2,000 | 2,000 |
| K346 | 61 | 1,000 | 512,000 | <1,000 | 2,000 | <1,000 | 1,000 | 1,024,000 | 2,000 | <1,000 | 32,000 | 8,000 | 16,000 | 16,000 |
| K349 | 65 | <1,000 | <1,000 | 1,000 | <1,000 | <1,000 | <1,000 | 1,000 | 4,096,000 | <1,000 | 2,000 | 1,000 | <1,000 | <1,000 |
| K351 | 9 | 8,000 | <1,000 | <1,000 | 32,000 | 1,000 | <1,000 | <1,000 | 4,000 | 4,096,000 | 4,000 | <1,000 | 1,000 | <1,000 |
| K353 | ATCC 17906 | 4,000 | 4,000 | <1,000 | 16,000 | <1,000 | 2,000 | <1,000 | 2,000 | <1,000 | 4,096,000 | <1,000 | 2,000 | 4,000 |
| K354 | 90 | <1,000 | <1,000 | <1,000 | <1,000 | <1,000 | 8,000 | <1,000 | 8,000 | <1,000 | 8,000 | 2,048,000 | 8,000 | 8,000 |
| K355 | ATCC 11171 | <1,000 | <1,000 | <1,000 | <1,000 | <1,000 | 8,000 | <1,000 | <1,000 | <1,000 | 1,000 | 2,000 | 2,048,000 | 2,000 |
| K388 | 96 | 2,000 | 2,000 | <1,000 | <1,000 | <1,000 | 4,000 | <1,000 | 4,000 | <1,000 | 4,000 | 8,000 | <1,000 | 1,024,000 |
Rabbits were immunized with heat-killed bacteria (see Materials and Methods).
Highest antibody dilution yielding an optical density at 405 nm of >0.2 with 250 ng of antigen per well; homologous titers are printed in boldface.
DISCUSSION
Over the last few years there has been a dramatic increase in the numbers of nosocomial infections caused by multidrug resistant Acinetobacter spp. in intensive care units (12, 13, 34, 44), and there is no doubt that this trend will continue in the future, in particular because of the increased use of antibiotics and the predominance in these wards of patients susceptible to infection with these organisms. Despite the many identification methods described for Acinetobacter, studies with a large number of strains validated by DNA homology studies have shown that some genomic groups can be unambiguously identified only by DNA-DNA hybridization and not by phenotypic tests (5, 15, 16, 20). The need for species identification in clinical laboratories is questionable, since most strains represent contamination or colonization rather than infection (16, 30, 34, 39). However, in an epidemic situation, detailed identification may be crucial for the tracing of strains and the prevention of spread among patients, since bacteremia caused by these bacteria may be fatal in the case of susceptible patients (3, 11, 34). DNA-DNA hybridization is laborious and time-consuming. New molecular biology-based methods are therefore being evaluated for use in the identification of Acinetobacter (17, 18, 21, 29, 32, 47). Although many of these methods offer ease and simplicity, these advantages must be weighed against the sometimes high costs of the equipment (18, 21), in addition to requiring experience and strict standardization for certain methods (15, 18, 29). Thus, at present, no low-cost, rapid, and reliable method for the routine identification of Acinetobacter genomic species, according to the current taxonomy, is available.
LPS is well suited as a serological marker for gram-negative bacteria, particularly for those possessing an O antigen in which the chemical structure of the repeating units in the O-specific polysaccharide chain is the molecular basis for serotyping schemes (31, 40, 43). Acinetobacter also produces LPS, and for several strains, we have recently shown that this LPS is of the smooth phenotype (23–26, 48, 49). However, a characteristic of most of the Acinetobacter LPSs investigated is that the O-specific side chain is not positive by alkaline silver nitrate staining (24–26, 48, 49), a method often used to determine the LPS phenotypes of bacterial strains (27, 36, 46). This phenomenon, which has also been observed for Campylobacter strains (6, 33) and certain Pseudomonas strains (19), is most likely due to the presence in the polysaccharide chain of the few vicinal diol systems, which give rise to the formation of aldehyde groups upon periodate oxidation during the silver-staining procedure (48). However, a banding pattern characteristic of S-form LPS could be observed following immunostaining of the proteinase K-digested whole-cell lysates of the Acinetobacter strains used for immunization with the homologous polyclonal rabbit serum in Western blots after SDS-PAGE. The antisera were highly specific for the homologous LPS, as demonstrated by EIA with isolated LPS as the solid-phase antigen. The heterologous reactivity observed with some sera could be attributed to common core epitopes (data not shown). Only serum sample K322 (anti-strain 57) and serum sample K346 (anti-strain 61) exhibited O-antigen reactivity with the heterologous strain. The ladder patterns of the two strains were indistinguishable, indicating similar O-antigen structures. The low amount of O-antigen cross-reactivity between the strains used for immunization shows that within the genus Acinetobacter a great O-antigen heterogeneity exists, with only little antigenic relatedness existing among the distinct O types. O-antigen heterogeneity is common to other gram-negative bacteria as well, e.g., in Salmonella (37). In the genus Salmonella, however, there seems to be a much higher degree of common antigenic determinants among serotypes than in the genus Acinetobacter.
The antisera were additionally tested by Western blotting with 31 other Acinetobacter strains of clinical and environmental origin. O-antigen reactivity was observed with 11 strains, whereby an interesting feature was observed. Some sera also reacted with the O antigens of strains belonging a DNA group different from that of the strain whose O antigen was used as the immunizing antigen. However, as can be seen from the example in Fig. 3, the banding patterns of the strains of the same DNA group (lanes 1, 2, and 3) differed from that of the strain which belonged to another genomic species (lane 4), thus indicating structural dissimilarity between the O-polysaccharide chains. In the case of serum sample K324 (anti-strain 44, DNA group 3), reactivity was observed with all of the DNA group 3 strains investigated. This phenomenon was not observed with the other sera and might be due to a greater homogeneity within this group compared to that in the other DNA groups.
From the results obtained in this pilot study, it is evident that an identification scheme based on O antigens is feasible for Acinetobacter strains, especially those belonging to DNA groups 2 and 3. However, although they were highly specific, the rabbit antisera used in this study have certain disadvantages; they contain, in addition to antibodies which react with the O-antigenic polysaccharide, core-reactive antibodies. The antisera additionally contain protein and possible capsular antibodies, which may lead to false-positive reactions when the antisera are used for O-serotyping experiments. Therefore, we will produce monoclonal antibodies against the O antigens of several Acinetobacter strains from clinical as well as environmental sources. In this way, the problem of false-positive reactions due to the presence of core, protein, or capsular antibodies is easily overcome. In addition, monoclonal antibodies allow the use of whole bacteria in colony blots or in agglutination or latex agglutination reactions. Future studies will also show which O antigens or O-antigenic determinants occur in which DNA groups. Therefore, we are starting an O-antigen numbering scheme that includes only those strains that have been characterized by DNA-DNA hybridization and the LPS of which has been investigated by chemical structural analysis.
ACKNOWLEDGMENTS
We thank V. Susott and B. van Harsselaar for skillful technical assistance and S. Haseley for fruitful discussions.
The financial support of the Ministry of Education of Aruba (to R.P.) is gratefully acknowledged.
REFERENCES
- 1.Aucken H M, Pitt T L. Lipopolysaccharide profile typing as a technique for comparative typing of gram-negative bacteria. J Clin Microbiol. 1993;31:1286–1289. doi: 10.1128/jcm.31.5.1286-1289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumann P. Isolation of Acinetobacter from soil and water. J Bacteriol. 1968;96:39–42. doi: 10.1128/jb.96.1.39-42.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergogne-Berezin E. The increasing significance of outbreaks of Acinetobacter spp.: the need for control and new agents. J Hosp Infect. 1995;30:441–452. doi: 10.1016/0195-6701(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 4.Bergogne-Berezin E, Towner K J. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996;9:148–165. doi: 10.1128/cmr.9.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernards A T, van der Toorn J, van Boven C P A, Dijkshoorn L. Evaluation of the ability of a commercial system to identify Acinetobacter genomic species. Eur J Clin Microbiol Infect Dis. 1996;15:303–308. doi: 10.1007/BF01695662. [DOI] [PubMed] [Google Scholar]
- 6.Blake D C, Russell R G. Demonstration of lipopolysaccharide with O-polysaccharide chains among different heat-stable serotypes of Campylobacter jejuni by silver staining of polyacrylamide gels. Infect Immun. 1993;61:5384–5387. doi: 10.1128/iai.61.12.5384-5387.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouvet P J M, Grimont P A D. Taxonomy of the genus Acinetobacter with recognition of Acinetobacter baumannii sp. nov., Acinetobacter haemolyticus sp. nov., Acinetobacter johnsonii sp. nov., Acinetobacter junii sp. nov., and amended descriptions of Acinetobacter calcoaceticus and Acinetobacter lwoffii. Int J Syst Bacteriol. 1986;36:228–240. [Google Scholar]
- 8.Bouvet P J M, Grimont P A D. Identification and biotyping of clinical isolates of Acinetobacter. Ann Microbiol (Inst Pasteur) 1987;138:569–578. doi: 10.1016/0769-2609(87)90042-1. [DOI] [PubMed] [Google Scholar]
- 9.Bouvet P J M, Jeanjean S. Delineation of new proteolytic genomic species in the genus Acinetobacter. Res Microbiol. 1989;140:291–299. doi: 10.1016/0923-2508(89)90021-1. [DOI] [PubMed] [Google Scholar]
- 10.Catlin B W. Branhamaceae fam. nov., a proposed family to accomodate the genera Branhamella and Moraxella. Int J Syst Bacteriol. 1991;41:320–323. [Google Scholar]
- 11.Cisneros J M, Reyes M J, Pachon J, Becerril B, Caballero F J, Garcia-Garmendia J L, Ortiz C, Cobacho A R. Bacteremia due to Acinetobacter baumannii: epidemiology, clinical findings, and prognostic features. Clin Infect Dis. 1996;22:1026–1032. doi: 10.1093/clinids/22.6.1026. [DOI] [PubMed] [Google Scholar]
- 12.Clark R B. Imipenem resistance among Acinetobacter baumannii: association with reduced expression of a 33-36 kDa outer membrane protein. J Antimicrob Chemother. 1996;38:245–251. doi: 10.1093/jac/38.2.245. [DOI] [PubMed] [Google Scholar]
- 13.Crowe M, Towner K J, Humphreys H. Clinical and epidemiological features of an outbreak of Acinetobacter infection in an intensive therapy unit. J Med Microbiol. 1995;43:55–62. doi: 10.1099/00222615-43-1-55. [DOI] [PubMed] [Google Scholar]
- 14.Dijkshoorn L, Tjernberg I, Pot B, Michel M F, Ursing J, Kersters K. Numerical analysis of cell envelope protein profiles of Acinetobacter strains classified by DNA-DNA hybridization. Syst Appl Microbiol. 1990;13:338–344. [Google Scholar]
- 15.Dijkshoorn L. Acinetobacter—Microbiology. In: Bergogne-Berezin E, Joly-Guillou M L, Towner K J, editors. Acinetobacter: microbiology, epidemiology, infections, management. Boca Raton, Fla: CRC Press, Inc.; 1996. pp. 37–69. [Google Scholar]
- 16.Dijkshoorn L, van der Toorn J. Acinetobacter species: which do we mean? Clin Infect Dis. 1992;15:748–749. doi: 10.1093/clind/15.4.748. [DOI] [PubMed] [Google Scholar]
- 17.Dolzani L, Tonin E, Lagatolla C, Prandin L, Monti-Bragadin C. Identification of Acinetobacter isolates in the A. calcoaceticus-A. baumannii complex by restriction analysis of the 16S-23S rRNA intergenic spacer sequences. J Clin Microbiol. 1995;33:1108–1113. doi: 10.1128/jcm.33.5.1108-1113.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehrenstein B, Bernards A T, Dijkshoorn L, Gerner-Smidt P, Towner K J, Bouvet P J M, Daschner F D, Grundmann H. Acinetobacter species identification by using tRNA spacer fingerprinting. J Clin Microbiol. 1996;34:2414–2420. doi: 10.1128/jcm.34.10.2414-2420.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fomsgaard A, Freudenberg M A, Galanos C. Modification of the silver-staining technique to detect lipopolysaccharide in polyacrylamide gels. J Med Microbiol. 1990;16:203–210. doi: 10.1128/jcm.28.12.2627-2631.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerner-Smidt P, Tjernberg I, Ursing J. Reliability of phenotypic tests for identification of Acinetobacter species. J Clin Microbiol. 1991;29:277–282. doi: 10.1128/jcm.29.2.277-282.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerner-Smidt P. Ribotyping of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex. J Clin Microbiol. 1992;30:2680–2685. doi: 10.1128/jcm.30.10.2680-2685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Getchell-White S I, Donowitz L G, Gröschel D H M. The inanimate environment of an intensive care unit as a potential source of nosocomial bacteria: evidence for long survival of Acinetobacter calcoaceticus. Infect Control Hosp Epidemiol. 1989;10:402–407. doi: 10.1086/646061. [DOI] [PubMed] [Google Scholar]
- 22a.Haseley, S. R., and O. Holst. Personal communication.
- 23.Haseley S R, Holst O, Brade H. Structural studies of the O-antigenic polysaccharide of the lipopolysaccharide from Acinetobacter (DNA group 11) strain 94 containing 3-amino-3,6-dideoxy-d-galactose substituted by the previously unknown amide-linked l-2-acetoxypropionic acid or l-2-hydroxypropionic acid. Eur J Biochem. 1997;247:815–819. doi: 10.1111/j.1432-1033.1997.00815.x. [DOI] [PubMed] [Google Scholar]
- 24.Haseley S R, Holst O, Brade H. Structural and serological characterisation of the O-antigenic polysaccharide of the lipopolysaccharide from Acinetobacter haemolyticus strain ATCC 17906. Eur J Biochem. 1997;244:761–766. doi: 10.1111/j.1432-1033.1997.00761.x. [DOI] [PubMed] [Google Scholar]
- 25.Haseley S R, Holst O, Brade H. Structural and serological characterisation of the O-antigenic polysaccharide of the lipopolysaccharide from Acinetobacter strain 90 belonging to DNA group 10. Eur J Biochem. 1997;245:470–476. doi: 10.1111/j.1432-1033.1997.t01-1-00470.x. [DOI] [PubMed] [Google Scholar]
- 26.Haseley S R, Pantophlet R, Brade L, Holst O, Brade H. Structural and serological characterisation of the O-antigenic polysaccharide of the lipopolysaccharide from Acinetobacter junii strain 65. Eur J Biochem. 1997;245:477–481. doi: 10.1111/j.1432-1033.1997.t01-1-00477.x. [DOI] [PubMed] [Google Scholar]
- 27.Hitchcock P J, Brown T M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holst O, Ulmer A J, Brade H, Flad H-D, Rietschel E T. Biochemistry and cell biology of bacterial endotoxins. FEMS Immunol Med Microbiol. 1996;16:83–104. doi: 10.1111/j.1574-695X.1996.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 29.Janssen P, Dijkshoorn L. High resolution DNA fingerprinting of Acinetobacter outbreak strains. FEMS Microbiol Lett. 1996;142:191–194. doi: 10.1111/j.1574-6968.1996.tb08429.x. [DOI] [PubMed] [Google Scholar]
- 30.Juni E. Acinetobacter Brisou and Prevot 1954, 727AL. In: Krieg N R, editor. Bergey’s manual of systematic bacteriology. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 303–307. [Google Scholar]
- 31.Liu P V, Matsumoto H, Kusama H, Bergan T. Survey of heat-stable, major somatic antigens of Pseudomonas aeruginosa. Int J Syst Bacteriol. 1983;33:256–264. [Google Scholar]
- 32.Marcos M A, Jimenez M T, Vila J. Correlation of six methods for typing nosocomial isolates of Acinetobacter baumannii. J Med Microbiol. 1995;42:328–335. doi: 10.1099/00222615-42-5-328. [DOI] [PubMed] [Google Scholar]
- 33.Moran A P, Appelmelk B J, Aspinall G O. Molecular mimicry of host structures by lipopolysaccharides of Campylobacter and Helicobacter spp.: implications in pathogenesis. J Endotox Res. 1996;3:521–531. [Google Scholar]
- 34.Mulin B, Talon D, Viel J F, Vincent C, Leprat R, Thouverez M, Michel-Briand Y. Risk factors for nosocomial colonization with multiresistant Acinetobacter baumannii. Eur J Clin Microbiol Infect Dis. 1995;14:569–576. doi: 10.1007/BF01690727. [DOI] [PubMed] [Google Scholar]
- 35.Orskov I, Orskov F, Jann B, Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977;44:667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palva E T, Mäkelä P H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulphate/polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107:137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- 37.Popoff M Y, Le Minor L. Antigenic formulas of the Salmonella serovars. Paris, France: Institut Pasteur; 1992. pp. 2–145. [Google Scholar]
- 38.Rainey F A, Lang E, Stackebrandt E. The phylogenetic structure of the genus Acinetobacter. FEMS Microbiol Lett. 1994;124:349–354. doi: 10.1111/j.1574-6968.1994.tb07307.x. [DOI] [PubMed] [Google Scholar]
- 39.Reboli A C, Houston E D, Monteforte J S, Wood C A, Hamill R J. Discrimination of epidemic and sporadic isolates of Acinetobacter baumannii by repetitive element PCR-mediated DNA fingerprinting. J Clin Microbiol. 1994;32:2635–2640. doi: 10.1128/jcm.32.11.2635-2640.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rietschel E T, Brade L, Lindner B, Zähringer U. Biochemistry of lipopolysaccharides. In: Morrison D C, Ryan J L, editors. Bacterial endotoxic lipopolysaccharides. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 3–42. [Google Scholar]
- 41.Rietschel E T, Brade H. Bacterial endotoxins. Sci Am. 1992;267:26–33. doi: 10.1038/scientificamerican0892-54. [DOI] [PubMed] [Google Scholar]
- 42.Rossau R, van Landschoot A, Gillis M, de Ley J. Taxonomy of Moraxellaceae fam. nov., a new bacterial family to accommodate the genera Moraxella, Acinetobacter, and Psychrobacter and related organisms. Int J Syst Bacteriol. 1991;41:310–319. [Google Scholar]
- 43.Schable B, Rhoden D I, Hugh R, Weaver R E, Khardori N, Smith P B, Bodey G P, Anderson R L. Serological classification of Xanthomonas maltophilia (Pseudomonas maltophilia) based on heat-stable O antigens. J Clin Microbiol. 1989;27:1011–1014. doi: 10.1128/jcm.27.5.1011-1014.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seifert H, Baginski R, Schulze A, Pulverer G. Antimicrobial susceptibility of Acinetobacter species. Antimicrob Agents Chemother. 1993;37:750–753. doi: 10.1128/aac.37.4.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tjernberg I, Ursing J. Clinical strains of Acinetobacter classified by DNA-DNA hybridization. APMIS. 1989;97:595–605. doi: 10.1111/j.1699-0463.1989.tb00449.x. [DOI] [PubMed] [Google Scholar]
- 46.Tsai C M, Frasch C E. A sensitive silver-stain for detecting lipopolysaccharide in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 47.Vaneechoutte M, Dijkshoorn L, Tjernberg I, Elaichouni A, de Vos P, Claeys G, Verschraegen G. Identification of Acinetobacter genomic species by amplified ribosomal DNA restriction analysis. J Clin Microbiol. 1995;33:11–15. doi: 10.1128/jcm.33.1.11-15.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vinogradov E V, Pantophlet R, Dijkshoorn L, Brade L, Holst O, Brade H. Structural and serological characterization of two O-specific polysaccharides from Acinetobacter. Eur J Biochem. 1996;239:602–610. doi: 10.1111/j.1432-1033.1996.0602u.x. [DOI] [PubMed] [Google Scholar]
- 49.Vinogradov E V, Pantophlet R, Haseley S R, Brade L, Holst O, Brade H. Structural and serological characterization of the O-specific polysaccharide from lipopolysaccharide of Acinetobacter calcoaceticus strain 7 (DNA-group 1) Eur J Biochem. 1997;243:167–173. doi: 10.1111/j.1432-1033.1997.0167a.x. [DOI] [PubMed] [Google Scholar]
- 50.Weaver R E, Actis L A. Identification of Acinetobacter species. J Clin Microbiol. 1994;32:1833. doi: 10.1128/jcm.32.7.1833-.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Westphal O, Jann K. Bacterial lipopolysaccharides. Extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]