Abstract
The presented data are part of a longitudinal within-subject study designed to examine ovulatory shifts in human sexuality in a diverse German sample using validated questionnaires. The final sample consists of 78 individuals (76 female, 2 agender) who declared to be mainly or exclusively attracted to males. Questionnaires were completed anonymously online at three cycle phases. Following the gold standard, the fertile window was calculated through the reverse cycle day method and confirmed via urinary tests detecting luteinizing hormone. The questionnaire included the Sexual Desire Inventory, Dresdner Body Image Inventory, the Revised Sociosexual Orientation Inventory, and an adjective list to measure mate preferences. One hundred eighty-four questionnaires were included in the data analysis using linear mixed models. Findings support previous research reporting heightened sexual desire and an improved body image during the fertile window. No shifts were found for mate preference or sociosexual orientation, thus adding to a growing body of literature contesting parts of the ovulatory shift hypothesis.
Keywords: ovulatory shift, sexuality, body image, sociosexuality, mate preferences, evolutionary psychology
Although a woman’s reproductive capacity is limited to a short period of time within the menstrual cycle, her sexual activity is not constrained by this. Cross-cultural research has shown that women are highly sexually active outside their fertile window (Brewis & Meyer, 2005). This seems to imply that humans do not have an estrus, “a sharp increase in sexual interest and activity that typically occurs at or near ovulation” (Welling & Puts, 2014, p. 244). But research of the last 20 years indicates that also in human females, there are shifts in (sexual) behavior and cognition due to the menstrual cycle.
Mate Preferences
During their fertile window, females of other species prefer males signaling competitiveness, dominance, and other traits that reflect higher levels of testosterone (see Thornhill & Gangestad, 2008). Also human females prefer these characteristics (“good genes” traits [GGT]) during their fertile window compared to other phases (e.g., Brinsmead-Stockham, Johnston, Miles, & Macrae, 2008; Cantú et al., 2014; Gangestad & Thornhill, 1998, 2008; Penton-Voak et al., 1999). GGT are expected to be ancestral indicators of a male’s genetic quality and hence to be a hint for offspring survival (e.g., Thornhill & Gangestad, 2008, but see discussion in Gangestad, Thornhill, & Garver-Apgar, 2015). However, preference for traits reflecting suitability as an investing long-term partner and co-parent (“good provider” traits [GPT]) seems to be stable across the cycle (for a review, see Gangestad & Thornhill, 2008; Gildersleeve, Haselton, & Fales, 2014). For example, Cantú et al. (2014) found that women are flirtier during their fertile window when talking to a male displaying many GGT rather than GPT.
The ovulatory shift hypothesis (Gangestad & Thornhill, 1998, 2008; Gildersleeve et al., 2014) suggests an explanation for this difference in fluctuation: Firstly, that women’s preference for GGT rises during their fertile window compared to other days during their cycle, because only when conception is possible, the genetic quality of a potential mate comes into effect. Secondly, the hypothesis predicts that such cycle shifts in preference are not present for GPT because long-term partners are beneficial for a female and the potential offspring across the entire cycle: On the short run, males may provide material benefits for sex. On the long run, males can be more certain of their paternity if they have sexual access across the entire cycle. Through their paternity certainty, they are more likely to provide parental care.
However, some recent studies with large sample sizes do not support the ovulatory shift hypothesis: For example, Marcinkowska et al. (2016) did not find a link between preference for facial masculinity and women’s hormonal status (see also Jones, Hahn, Fisher, Wang, Kandrik, Han, et al., 2018).
Sociosexual Orientation
Concerning extra-pair copulation and extra-pair attraction, the ovulatory shift hypothesis predicts that these rise during the fertile window, especially if an extra-pair mate possesses many GGT. This view is supported by many studies, among them nonhuman and cross-cultural research (Gangestad & Thornhill, 2008; Pillsworth & Haselton, 2006b; Thornhill & Gangestad, 2008). Gangestad, Thornhill, and Garver (2002) found women to have significantly more sexual interest in and fantasies about nonprimary partners during the fertile window compared to the other phases; sexual interest or fantasies about the primary partner did not shift. However, there are also contradicting studies and meta-analyses (Gildersleeve et al., 2014; Jones, Hahn, Fisher, Wang, Kandrik, & DeBruine, 2018; Wood, Kressel, Joshi, & Louie, 2014) that did not find support for cyclical shifts in extra-pair attraction and copulation. Further research is needed to clarify this.
To be able to include individuals who are not currently in a monogamous relationship and to improve chances of self-disclosure in this delicate topic, we decided to not directly ask for extra-pair attraction or extra-pair copulation. Instead, the concept of sociosexual orientation was measured. Already other studies used indirect measures and found significant cycle shifts in the predicted direction (Gangestad, Thornhill, & Garver-Apgar, 2010; Sheldon, Cooper, Geary, Hoard, & DeSoto, 2006).
Sexual Desire
Women also generally report more sexual fantasies, masturbation, and libido during their fertile window (Bullivant et al., 2004; Caruso et al., 2014; see Motta-Mena & Puts, 2017). This was found in nonheterosexual women as well (Burleson, Trevathan, & Gregory, 2002; Diamond & Wallen, 2011). Conversely, looking at sexual intercourse with a partner, the evidence is ambiguous: Authors who do find an ovulatory shift in sexual behavior with a partner argue that (a) females have a higher sexual motivation during the fertile window and/or (b) a male’s sexual desire shifts synchronically with their partner’s attractiveness, both peaking during the fertile window (Bullivant et al., 2004; Burleson et al., 2002; Wilcox, 2004). Brewis and Meyer (2005) argue that while there may be cyclical shifts in female sexual desire, in their large, cross-cultural study, they did not find sexual intercourse in pair-bonds to shift. The central argument for the lack of cyclical shifts in the frequency of sexual intercourse is the partner’s autonomous pattern of sexual desire (e.g., Burleson et al., 2002). Citing several studies and reviews, Thornhill and Gangestad (2008) conclude that it is still unclear whether there are cyclical shifts in sexual behavior in heterosexual couples.
Body Image
Many magazines targeted at women report about bloated feelings around the time of (pre-) menstruation, and psychological research has indeed shown that body image is affected by the menstrual cycle: Women were found to be less satisfied with their own body and appearance in the premenstrual (Altabe & Thompson, 1990) or perimenstrual (Carr-Nangle, Johnson, Bergeron, & Nangle, 1994) than in the intermenstrual phase. More recent research has found women to feel more attractive and sexy during their fertile window (Arslan, Schilling, Gerlach, & Penke, 2018; Haselton & Gangestad, 2006; Röder, Brewer, & Fink, 2009).
The Current Study
The presented data are part of a study that aims to further investigate within-subject menstrual cycle shifts. In line with previous research, we hypothesize sexual desire to be higher during the fertile window compared to both other cycle phases. Furthermore, body image was found to be most positive during the fertile window. Thus, we expect body image to be more positive during the fertile window compared to both other cycle phases. As predicted by the ovulatory shift hypothesis, we hypothesize sociosexual orientation and the preference for GGT to be highest during the fertile window, while preference for GPT should be stable across the entire cycle.
Method
Participants
Participants were recruited through student mailing lists, social media, and Internet forums, as well as advertisements in local cafés, gynecological practices, and on university campus. Local students could participate in exchange for course credit. There was no financial compensation. Due to the impact on the body’s natural hormone levels and the cycle effects this study is looking at, individuals taking hormonal contraceptives, being pregnant, or currently lactating were not allowed to participate.
If participants indicated to not have had a positive result in the test for luteinizing hormone (LH) or to not have executed it at all, their submitted questionnaire for the fertile window was excluded from data analysis. This left a total of 184 from originally 194 submitted questionnaires and 78 of 81 participants.
The final sample has an age range of 18–40 years (M = 27.72, SD = 5.59). Additional demographic data can be found in Table 1. Length of last menstrual cycle varied between 22 and 57 days (M = 29.59, SD = 4.83).
Table 1.
Demographic Data.
| Variable | N | % |
|---|---|---|
| Education | ||
| School leaving certificate (9 years) | 2 | 2.6 |
| O-levels (10 years) | 4 | 5.1 |
| Specialized A-levels (12–13 years) | 4 | 5.1 |
| A-levels (12–13 years) | 36 | 46.2 |
| University degree | 31 | 39.7 |
| Other | 1 | 1.3 |
| Relationship form | ||
| Single | 12 | 15.4 |
| Dating | 4 | 5.1 |
| Monogamous relationship | 50 | 64.1 |
| Open + polyamorous relationship | 12 | 15.4 |
| Sexual attraction | ||
| Exclusively by males | 42 | 53.8 |
| Mainly by males | 31 | 39.7 |
| Bisexual or pansexual | 5 | 6.4 |
| Mainly by females | 0 | 0 |
| Exclusively by females | 0 | 0 |
| Asexual | 0 | 0 |
| Gender | ||
| Female | 76 | 97.4 |
| Agender | 2 | 2.6 |
| Male | 0 | 0 |
All participants gave written informed consent. This study received ethical approval from the Ethics Committee of the University Hospital Jena, approval code UKJ Nr: 5232-07/17.
Of a total of 78 participants who submitted one questionnaire, 56 persons submitted a second questionnaire and 50 submitted a third one. For the menstrual phase, there were 76 complete questionnaires, 47 for the ovulatory phase and 61 for the luteal phase. Most participants started during the menstrual phase (n = 48, ovulatory phase n = 11, luteal phase n = 19).
Measures
Data were collected from August 20 to December 10, 2017, using the online survey platform www.SoSciSurvey.de.
Demographic data
A brief demographic questionnaire asked participants for age, education, kind of relationship, contraceptive method that is used, length of last menstrual cycle, gender identity, and sexual preference. Also, it asked participants whether and how they track their cycle.
Sexual Desire Inventory (SDI-2)
To measure sexual desire, a short version of the German SDI-2 (Kuhn, Koenig, Donoghue, Hillecke, & Warth, 2014) was used. It is a translation of the English SDI-2 (Spector, Carey, & Steinberg, 1996). It has a high internal consistency (α = .86) and consists of the two factors Desire With Interaction and Desire Without Interaction. Two items can be rated on a 1–8 scale and the other 8 on a 1–9 scale.
Dresdner Body Image Inventory (DKB-35)
To measure body image, the DKB-35 (Pöhlmann, Thiel, & Joraschky, 2008) was used. It is a clinical instrument consisting of five subscales: vitality, self-acceptance, body contact, sexual satisfaction, and self-appreciation. Each of the 35 items is responded to on a 5-point scale (1 = not at all to 5 = completely). Internal consistency of the subscales was between α = .76 (body contact) and α = .94 (sexual satisfaction; nonclinical sample; Matthes, Franke, & Jäger, 2012).
Revised Sociosexual Orientation Inventory (SOI-R)
To measure sociosexual orientation, the 9-point scale version of the German SOI-R (Penke & Asendorpf, 2008) was used. It consists of 9 items belonging to three facets: “behavior,” for example, number of casual sex partners; “attitude” toward uncommitted sex; and sexual “desire” for persons with whom one is not romantically involved. Internal consistency for the facets and the entire questionnaire ranges from α = .83 (facet “attitude,” females only; entire SOI-R, males and females) to α = .87 (facet “attitude,” males only; Penke, 2013).
Internal consistencies for SOI-R, SDI-2, and DKB-35 were computed for our sample. They were comparable to those of the validation samples.
Nonstandardized questions
Two questions with a Visual Analogue Scale (VAS) were used to assess participant’s feelings during the preceding 3 days. One asked for sexual desire and fantasies and the second one asked how sexy the person felt. Additionally, there were six questions concerning frequencies of sexual behavior during the preceding 3 days. They asked for the frequency of masturbation, frequency of sexual desire or fantasies, frequency of sexual intercourse with a partner, and frequency of self-initiated, other-initiated, and mutually initiated sexual intercourse with a partner. Also, an adjective list was used. The list was generated along the lines of Karlestrand (2013). Seven of the adjectives were chosen to represent GGT (e.g., ambitious), and seven were chosen to represent GPT (e.g., faithful). Additionally, the two adjectives masculine and feminine were used. Participants were asked to indicate on a 7-point scale ranging from not at all to precisely (a) whether this item describes their partner or someone they are/were in love with and (b) how important this trait would be in a dream partner.
Quality management
For the purpose of quality management, we included the following questions, which could be responded to with yes or no: “Is the result of today’s LH test positive?” “Did you conduct all required LH tests?” “Did you have any trouble conducting the LH tests?” and “Did you conduct the LH tests according to the instructions?”
Procedure
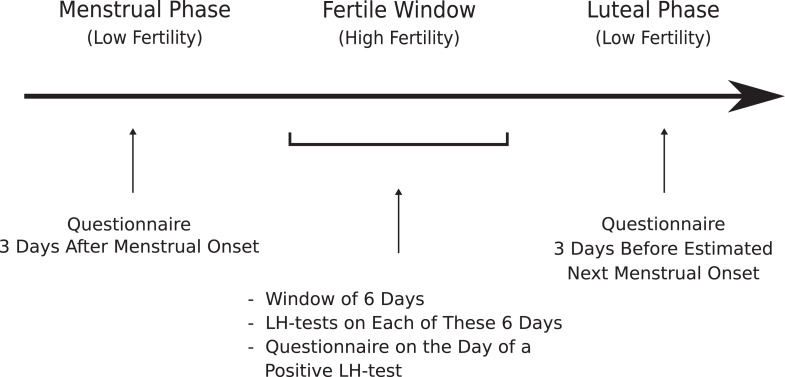
Participants were sent an e-mail with detailed information on the study, instructions for cycle phase calculation, and a link to the online questionnaire. They were asked to calculate specific days according to their cycle on which they were instructed to fill in the questionnaire (see Figure 1). Start of participation was possible regardless of the momentary cycle phase. LH tests were sent to the participants via mail or were fetched by them from the office. In case the LH tests did not indicate ovulation during the given time frame (n = 7 from raw data set), participants were asked to fill in a questionnaire indicating the nonpositive result and to repeat the calculations and LH testing in the next cycle. No such case was part of the final data set.
Figure 1.
Study procedure.
Cycle Phase Estimation
Using the reverse cycle day method (Haselton & Gangestad, 2006), the fertile window was calculated by subtracting the average luteal phase length from the estimated next menstrual onset. Thus, days 17–12 before estimated next menstrual onset were set as the fertile window. As Gangestad et al. (2016) and Haselton and Gildersleeve (2016) recommend to verify the fertile window by LH test, participants were asked to use them during the calculated fertile window to confirm the estimation. The used LH tests had a sensitivity of 10 mIU/ml.
Statistical Analysis
To investigate the effect of cycle phase on the aforementioned measures, linear mixed models were used because they account for repeated measurement as well as missing data. Analyses were conducted using IBM SPSS (Version 25) for Linux.
Cycle phase was defined to be a fixed effect. When including further factors or covariates, these were also defined to be fixed effects. Intercepts were chosen to be random to allow for differing scores and frequencies between participants. Estimations were made using restricted maximum likelihood, and variance components were chosen as covariance structure. In all inferential analyses, the significance threshold was set to .05.
Results
Data were checked for implausibility and obvious misunderstandings (e.g., cycle length below 10 days). As a result, 23 cases were excluded. Descriptive statistics of all used questionnaires and single questions are listed in Table 2.
Table 2.
Descriptive Statistics of Questionnaires and Single Questions.
| Variable | M | SD | Range | |
|---|---|---|---|---|
| Potential | Actual | |||
| SDI-2 | 44.14 | 11.33 | 0–88 | 14–74 |
| DKB-35 | 3.52 | 0.57 | 1–5 | 2.04–4.78 |
| SOI-R | 3.84 | 1.15 | 1–9 | 1.89–6.89 |
| Self-perceived sexiness | 50.30 | 24.77 | 0–100 | 1–98 |
| Intensity of sexual desire and fantasies | 56.52 | 27.68 | 0–100 | 0–100 |
| Dream partner GGT | 5.32 | 0.73 | 1–7 | 3.00–7.00 |
| Actual partner GGT | 5.29 | 0.83 | 1–7 | 3.14–6.86 |
| Dream partner GPT | 6.12 | 0.51 | 1–7 | 4.57–7.00 |
| Actual partner GPT | 5.72 | 0.70 | 1–7 | 3.29–7.00 |
| Frequencya of | ||||
| Sex with a partner in general | 0.94 | 1.24 | 0–5 | |
| Partner-initiated sex | 0.48 | 0.87 | 0–5 | |
| Self-initiated sex | 0.47 | 1.09 | 0–10 | |
| Mutually initiated sex | 0.61 | 1.21 | 0–11 | |
| Masturbation | 0.74 | 1.13 | 0–7 | |
| Sexual desire and fantasies | 3.16 | 2.87 | 0–23 | |
Note. SDI-2 = Sexual Desire Inventory; DKB-35 = Dresdner Body Image Inventory; SOI-R = Revised Sociosexual Orientation Inventory; GGT = good genes traits; GPT = good provider traits.
aGives the frequency of certain sexual activities during the preceding 3 days.
Main Hypotheses
Results concerning the main hypotheses are described in the following section. For numerical results please see Table 3 (main effects) and Table 4 (pairwise comparisons).
Table 3.
Main Effects of Cycle Phase on Scores.
| Variable | Menstrual Phase | Fertile Window | Luteal Phase | dfs | F | p | d | |||
|---|---|---|---|---|---|---|---|---|---|---|
| M (SD) | CI | M (SD) | CI | M (SD) | CI | |||||
| SDI-2 | 43.71 (10.57) | [41.10, 45.94] | 46.09 (12.28) | [42.11, 49.58] | 43.18 (11.50) | [40.24, 46.12] | 2,111 | 7.17 | .001 | .26 |
| DKB-35 | 3.53 (0.57) | [3.41, 3.67] | 3.58 (0.58) | [3.39, 3.73] | 3.48 (0.56) | [3.33, 3.62] | 2,106 | 5.94 | .004 | .18 |
| SOI-R | 3.84 (1.14) | [3.57, 4.09] | 3.75 (1.15) | [3.44, 4.13] | 3.90 (1.18) | [3.60, 4.20] | 2,106 | 0.621 | .539 | |
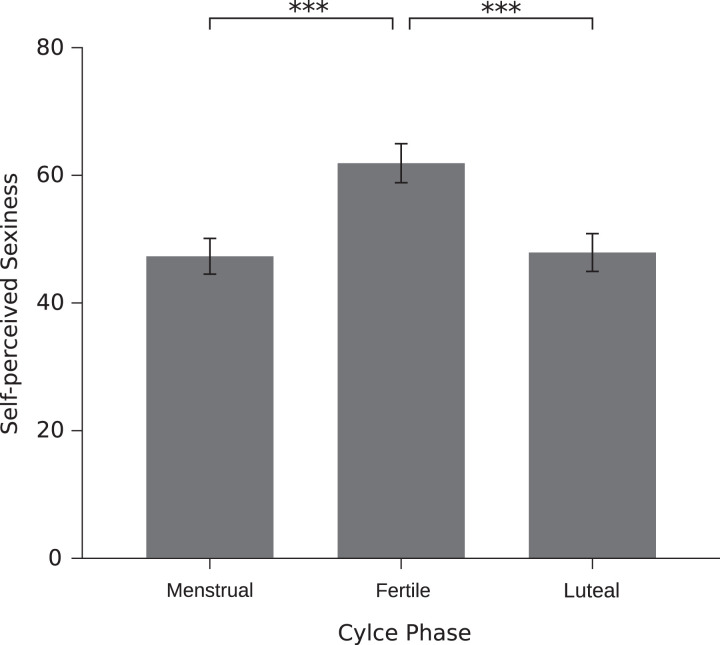
| Self-perceived sexiness | 46.78 (24.67) | [41.68, 52.91] | 59.79 (24.71) | [51.37, 66.28] | 47.39 (23.39) | [41.40, 53.38] | 2,119 | 10.26 | <.001 | .53 |
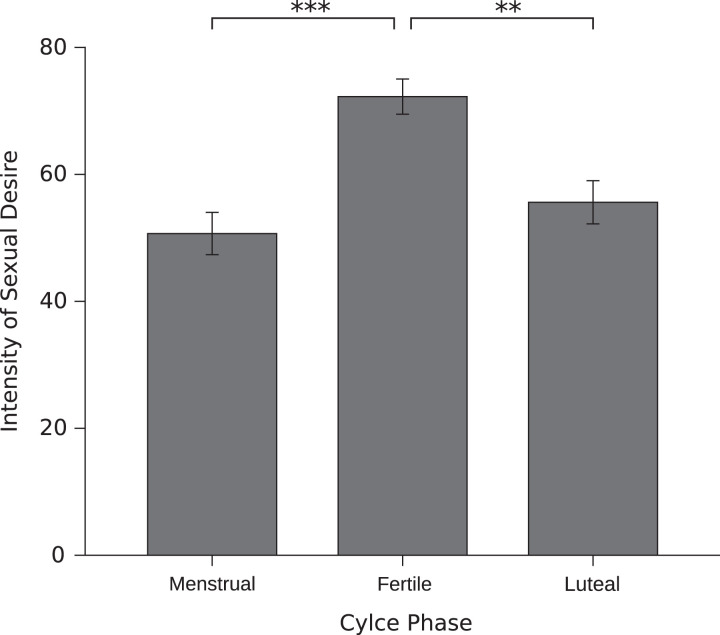
| Intensity of sexual desire and fantasies | 49.68 (29.03) | [43.04, 56.48] | 70.04 (21.99) | [62.90, 76.35] | 54.61 (26.59) | [47.80, 61.42] | 2,134 | 9.77 | <.001 | .74 |
| Dream partner GGT | 5.30 (0.68) | [5.14, 5.46] | 5.34 (0.83) | [5.09, 5.60] | 5.32 (0.73) | [5.14, 5.51] | 2,110 | 0.94 | .392 | |
| Dream partner GPT | 6.11 (0.48) | [6.00, 6.22] | 6.11 (0.57) | [5.95, 6.30] | 6.12 (0.50) | [6.00, 6.25] | 2,110 | 0.53 | .591 | |
| Frequencya of | ||||||||||
| Sex with a partner in general | 0.86 (1.26) | [0.55, 1.13] | 1.13 (1.36) | [0.68, 1.49] | 0.90 (1.12) | [0.61, 1.19] | 2,119 | 1.15 | .320 | |
| Partner-initiated sex | 0.44 (0.92) | [0.23, 0.65] | 0.57 (0.96) | [0.24, 0.78] | 0.48 (0.72) | [0.29, 0.66] | 2,110 | 0.48 | .623 | |
| Self-initiated sex | 0.51 (1.37) | [0.18, 0.81] | 0.60 (0.99) | [0.25, 0.82] | 0.31 (0.70) | [0.13, 0.49] | 2,117 | 1.32 | .272 | |
| Mutually initiated sex | 0.63 (1.50) | [0.27, 0.96] | 0.79 (1.00) | [0.42, 0.96] | 0.46 (0.92) | [0.22, 0.70] | 2,118 | 1.43 | .243 | |
| Masturbation | 0.57 (0.93) | [0.33, 0.74] | 1.11 (1.52) | [0.62, 1.55] | 0.67 (0.96) | [0.43, 0.92] | 2,106 | 3.60 | .031 | .48 |
| Sexual desire and fantasies | 2.86 (3.41) | [2.02, 3.58] | 4.37 (2.85) | [3.47, 5.20] | 2.64 (1.68) | [2.21, 3.07] | 2,119 | 6.79 | .002 | .60 |
| Actual partner GGTb | 5.28 (0.84) | [5.07, 5.49] | 5.40 (0.79) | [5.14, 5.66] | 5.20 (0.85) | [4.95, 5.46] | 2,88 | 2.19 | .118 | |
| Actual partner GPTb | 5.72 (0.66) | [5.55, 5.89] | 5.68 (0.74) | [5.44, 5.93] | 5.74 (0.72) | [5.52, 5.95] | 2,88 | 0.03 | .967 | |
| Single item: actual partner: well-toned | 3.86 (0.19) | [3.45, 4.26] | 4.30 (0.21) | [3.68, 4.58] | 3.97 (0.23) | [3.28, 4.29] | 2,112 | 4.59 | .012 | .27 |
Note. Exploratory results below dashed line. CI = 95% confidence interval; SDI-2 = Sexual Desire Inventory; DKB-35 = Dresdner Body Image Inventory; SOI-R = Revised Sociosexual Orientation Inventory; GGT = good genes traits; GPT = good provider traits.
aGives the frequency of certain sexual activities during the preceding 3 days. bAnalyses include only individuals currently in a relationship.
Table 4.
Pairwise Comparisons of Scores by Cycle Phase.
| Variable | Pairwise Comparison | p | d | |
|---|---|---|---|---|
| SDI-2 | Fertile window | Menstrual phase | .001 | .21 |
| Luteal phase | .001 | .25 | ||
| DKB-35 | Fertile window | Menstrual phase | .001 | .09 |
| Luteal phase | .011 | .18 | ||
| Self-perceived sexiness | Fertile window | Menstrual phase | <.001 | .53 |
| Luteal phase | <.001 | .52 | ||
| Intensity of sexual desire and fantasies | Fertile window | Menstrual phase | <.001 | .77 |
| Luteal phase | .002 | .62 | ||
| Frequencya: Masturbation | Fertile window | Menstrual phase | .011 | .45 |
| Luteal phase | .039 | .37 | ||
| Frequencya: Sexual desire and fantasies | Fertile window | Menstrual phase | .002 | .47 |
| Luteal phase | .001 | .76 | ||
| Single item: Actual partner: Well-toned | Fertile window | Menstrual phase | .004 | .28 |
| Luteal phase | .032 | .35 | ||
Note. Exploratory results below dashed line. Difference between menstrual and luteal phase was not significant in any case. SDI-2 = Sexual Desire Inventory; DKB-35 = Dresdner Body Image Inventory.
aDuring the preceding 3 days.
There was a significant effect of cycle phase on the sum score of the SDI-2, frequency of sexual desire and fantasies, frequency of masturbation, and intensity of sexual desire and fantasies. In all cases, there was a significant increase in the fertile window compared to both other phases. For the menstrual cycle effect on intensity of sexual desire, see Figure 2. No significant effect was found between luteal and menstrual phase. There was no significant cycle shift in general sexual activity with a partner, partner-initiated sexual activity, self-initiated sexual activity, or mutually initiated sexual activity.
Figure 2.
Effect of the menstrual cycle phase on intensity of sexual desire. Error bars represent standard error of mean.
A significant cycle shift in body image was found using the DKB-35 and a VAS asking for self-perceived sexiness. In both cases, there was a significant difference between the fertile window and both other phases, indicating a more positive body image during the fertile window. For the menstrual cycle effect on self-perceived sexiness, see Figure 3. No significant effect was found between luteal and menstrual phase.
Figure 3.
Effect of the menstrual cycle phase on self-perceived sexiness. Error bars represent standard error of mean.
Concerning the mate preference hypothesis, single items were aggregated to two scales: one describing a dream partner along GGT and one describing a dream partner along GPT. Neither the preference for GPT nor the preference for GGT was found to shift significantly across the cycle.
In an additional exploratory analysis on single item basis, no significant cycle effects were found concerning the dream partner. Neither did we find a significant effect for the GGT or GPT scale in descriptions of the actual partner. But, looking at descriptions of the actual partner on single item basis, there was a significant cycle shift of the item “well-toned,” indicating that the actual partner is described as more well-toned during the fertile window compared to both other phases.
There was no significant cycle shift in the SOI-R main score. In additional analyses, the subscales were used as dependent variables in separate models, but neither showed a significant cycle shift.
Discussion
This longitudinal within-subject study investigated the effect of the menstrual cycle on different facets of sexuality and well-being, using an anonymous online questionnaire. Ovulation was assessed via backward counting and LH test. The first hypothesis, stating that sexual desire is strongest during the fertile window, was supported by several indicators: reported frequency of masturbation and sexual fantasies and desire, intensity of sexual fantasies and desire, and sum score of SDI peaked during the fertile window. The second hypothesis was also supported: Measurement with a VAS as well as the DKB-35 yielded a significant improvement of body image during the fertile window. The hypotheses concerning mate preferences and sociosexual orientation were not supported by our findings.
Main Analyses
In contrast to many other studies (Gangestad & Thornhill, 2008; Gildersleeve et al., 2014), we did not find a cyclical shift in preference for GGT. This study used explicit self-reports on specific adjectives. This may be not as sensitive to changes as the spontaneous, less conscious measures used by others (Cantú et al., 2014; Gangestad & Thornhill, 1998; Grammer, 1993; Havlicek, Roberts, & Flegr, 2005; Penton-Voak & Perrett, 2000; Penton-Voak et al., 1999; Roney & Simmons, 2008). Conducting exploratory analyses, we found a significant cycle shift in the preference of one of the adjectives: Participants described their actual partner as more well-toned during the fertile window compared to both other phases. This may be a hint for cycle-related change in perception of GGT, which may be linked to changes in preference. On the other hand, recent studies (Harris, 2013; Jones, Hahn, Fisher, Wang, Kandrik, Han, et al., 2018; Marcinkowska et al., 2016; Wood et al., 2014) did not find a cycle shift in preference for masculinity either. Additionally, a large-scale preregistered study that did find shifts in preference for GGT found shifts for GPT (Jünger, Kordsmeyer, Gerlach, & Penke, 2018). So, although there are studies supporting the ovulatory shift hypothesis, there is a larger body of studies that do not support shifts in masculinity preferences.
To investigate extra-pair attraction, we used sociosexual orientation as a proxy. We expected this to heighten the chances of participants’ self-disclosure and were able to additionally recruit individuals who did not have a primary partner. Previous research has used similar concepts and found a significant rise during the fertile window (Gangestad et al., 2010). A study that also used the SOI-R (Jones, Hahn, Fisher, Wang, Kandrik, & DeBruine, 2018) did not find an effect of hormonal status. These findings and the meta-analyses by Gildersleeve, Haselton, and Fales (2014) and Wood, Kressel, Joshi, and Louie (2014) challenge the part of the ovulatory shift hypothesis that predicts shifts in extra-pair attraction and copulation. Nevertheless, there is a whole body of research that found evidence in favor of this hypothesis (e.g., Gangestad & Thornhill, 2008; Gangestad, Thornhill, & Garver 2002; Grebe, Emery Thompson, & Gangestad, 2016; Havlicek et al., 2005), which is supported by research that did not ask for extra-pair attraction or copulation but, for example, for commitment to relationship (Durante, Li, & Haselton, 2008). Unlike the present study, many of the studies finding an effect took into account the GGT of the primary partner. The rationale behind this is that extra-pair copulation is connected with the risk of losing the primary partner and his investment. Thus, it is expected that the chance for a female to engage in infidelity rises if their primary partner signals few GGT and the extra-pair mate signals many GGT. For instance, Gangestad, Thornhill, and Garver-Apgar (2005) found women with less symmetrical partners to report higher attraction to extra-pair males during their fertile window, relative to women with more symmetrical partners (see also Pillsworth & Haselton, 2006a). However, two recent studies did not find partner’s physical attractiveness to be a significant moderator between hormonal status and extra-pair desire (Arslan et al., 2018; Shimoda, Campbell, & Barton, 2017). Both studies were carefully designed, including several different models to determine the fertile phase, and one of them (Arslan et al., 2018) had a large sample as well as preregistered hypotheses and methods.
It remains to be further investigated whether there is a cycle phase effect on women’s desire for uncommitted sex and extra-pair attraction. Our data suggest that there is no cycle shift in sociosexual orientation, but this may as well be because of this construct being too stable or the response format of the SOI-R being not sensitive enough to measure slight cycle shifts.
Our findings concerning sexual desire and behavior are mostly concordant with previous research. Concerning sexual desire, previous research conjointly draws a picture of higher desire during the fertile window. Our findings replicate those findings, with masturbation frequency and other facets of sexual desire peaking around ovulation (Arslan et al., 2018; Bullivant et al., 2004; Burleson et al., 2002; Caruso et al., 2014; Diamond & Wallen, 2011; Jones, Hahn, Fisher, Wang, Kandrik, & DeBruine, 2018; Roney & Simmons, 2013). Concerning sexual activity with a partner, there are mixed findings: Some studies found a midcycle peak (Bullivant et al., 2004; Burleson et al., 2002; Wilcox, 2004), while others did not (Arslan et al., 2018; Brewis & Meyer, 2005). The latter findings match ours and can be explained by taking into account the partner’s own pattern of sexual desire, which may not be synchronous with the participant’s pattern.
Taken together, our findings concerning sexual desire, mate preferences, and sociosexual orientation do not support the ovulatory shift hypothesis. They can rather be explained by the argumentation of Roney and Simmons (2013, 2017), predicting a shift in general motivation toward more sexual activity when conception is most likely but toward other activities during the rest of the cycle to avoid risks and costs associated with sexual activity.
The improvement of body image and self-perceived sexiness also matched previous results (Altabe & Thompson, 1990; Arslan et al., 2018; Carr-Nangle et al., 1994; Haselton & Gangestad, 2006; Röder et al., 2009). Research on the relationship of own attractiveness and partner preference has found that women high in attractiveness prefer more masculine and symmetrical men (for an overview, see Buss, 2008). In light of these findings, the fertile window shift in body image is discussed to be a rise in self-perceived mate value, which in turn raises mate choice standards (Buss & Schmitt, 2011; Buss & Shackelford, 2008; Haselton & Gangestad, 2006). Other authors interpret the fertile window shift in body image as a by-product rather than an adaptation (e.g., Thornhill & Gangestad, 2008). Other research has found that not only body image but also third-party assessments of women’s attractiveness increase around ovulation. For example, Puts et al. (2013) had men rate women’s facial photographs and voice recordings for attractiveness and found ratings to be most positive when estradiol was high and progesterone low. Using a forced choice task, Roberts et al. (2004) found the same effect: Facial photographs taken during the fertile phase were rated to be more attractive than photographs taken during the luteal phase. Also, Jones et al. (2015) found estradiol level to influence facial coloration. These findings suggest that women’s increased feelings of attractiveness at ovulation have an objective basis.
Effect sizes varied from d = .18 (DKB-35) to d = .74 (intensity of sexual desire). Among the pairwise comparisons, effect sizes varied from d = .09 (DKB 35, fertile window vs. menstrual phase) to d = .77 (intensity of sexual desire, fertile window vs. menstrual phase). Regarding Cohen (1988), some of the effects found in this study are of remarkable size. Higher effect sizes in VAS and single questions can stem from the manner in which questions were asked: We phrased most single questions and VAS to explicitly ask for the last 3 days to get the maximum possible effect of the menstrual cycle.
Limitations
Unlike many other studies in this field, this study used a within-participant design to control for noise (Gangestad et al., 2016). Cycle day estimation was conducted in accordance with the recommendations by Gangestad et al. (2016), and data were collected anonymously and online to assure the participants’ self-disclosure. Our sample size was relatively large and diverse compared to other studies in this field of research. For future research, several aspects should be considered.
In this study, the focus on the menstrual cycle was obvious for participants, thus results could be biased by their expectations. Collecting data on additional physical attributes (e.g., stress) may firstly distract participants from the aims of the study and thus emerge more reliable results and secondly provide further insights. Moreover, more detailed information on participants’ relationships, for example, length of relationship and cohabitation, may be useful for the interpretation of the data.
Also, a daily diary design may be the more suitable way to have consistent insights to sexuality and well-being, especially if data are collected across several months.
Like several other authors (e.g., Gangestad & Thornhill, 2008; Pillsworth & Haselton, 2006b), we criticize that up until now, most studies concerning relationships and sexuality are limited to a small part of the population: well-educated, young, mostly nulliparous cisgender women with short relationship length and from Western societies. These factors limit the knowledge that we can gather from the undertaken research.
Conclusion
In conclusion, the present study found cycle shifts in sexual desire and body image, thus supporting previous research. No such shifts were found for mate preferences and sociosexual orientation, which are presently subject to great controversy among researchers. Understanding the effects of the menstrual cycle could help understand behavior and cognition in modern life, especially in relationship contexts. In light of recent meta-analyses coming to opposing conclusions (Gildersleeve et al., 2014; Wood et al., 2014), future research should aim to preregister their hypotheses and methods to keep researchers’ degrees of freedom to a minimum and to avoid the suspicion of p-hacking or HARKing.
Acknowledgments
The authors would like to thank Dr. Lehmann from Center for Clinical Studies and Institute of Medical Statistics, Computer Sciences and Data Sciences, Jena University Hospital for statistical support. The authors also thank Kalle Hümpfner for helpful comments on the manuscript.
Authors’ Note: The discussed theories make no predictions for nonheterosexual individuals. Thus, if not indicated otherwise, the cited studies are limited to individuals who menstruate and identify as heterosexual females.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: K. R. van Stein  https://orcid.org/0000-0003-0860-8654
https://orcid.org/0000-0003-0860-8654
References
- Altabe M., Thompson J. K. (1990). Menstrual cycle, body image, and eating disturbance. International Journal of Eating Disorders, 9, 395–401. doi:10.1002/1098-108X(199007)9:4<395::AID-EAT2260090405>3.0.CO;2-E [Google Scholar]
- Arslan R., Schilling K., Gerlach T. M., Penke L. (2018). Using 26 thousand diary entries to show ovulatory changes in sexual desire and behavior. Journal of Personality and Social Psychology. doi:10.17605/OSF.IO/JP2YM [DOI] [PubMed] [Google Scholar]
- Brewis A., Meyer M. (2005). Demographic evidence that human ovulation is undetectable (at least in pair bonds). Current Anthropology, 46, 465–471. doi:10.1086/430016 [Google Scholar]
- Brinsmead-Stockham K., Johnston L., Miles L., Neil Macrae C. (2008). Female sexual orientation and menstrual influences on person perception. Journal of Experimental Social Psychology, 44, 729–734. doi:10.1016/j.jesp.2007.05.003 [Google Scholar]
- Bullivant S. B., Sellergren S. A., Stern K., Spencer N. A., Jacob S., Mennella J. A., McClintock M. K. (2004). Women’s sexual experience during the menstrual cycle: Identification of the sexual phase by noninvasive measurement of luteinizing hormone. Journal of Sex Research, 41, 82–93. doi:10.1080/00224490409552216 [DOI] [PubMed] [Google Scholar]
- Burleson M. H., Trevathan W. R., Gregory W. L. (2002). Sexual behavior in lesbian and heterosexual women: Relations with menstrual cycle phase and partner availability. Psychoneuroendocrinology, 27, 489–503. doi:10.1016/S0306-4530(01)00066-X [DOI] [PubMed] [Google Scholar]
- Buss D. M. (2008). Evolutionary psychology: The new science of the mind (3rd ed.). Boston, MA: Pearson/Allyn and Bacon. [Google Scholar]
- Buss D. M., Schmitt D. P. (2011). Evolutionary psychology and feminism. Sex Roles, 64, 768–787. doi:10.1007/s11199-011-9987-3 [Google Scholar]
- Buss D. M., Shackelford T. K. (2008). Attractive women want it all: Good genes, economic investment, parenting proclivities, and emotional commitment. Evolutionary Psychology, 6. doi:10.1177/147470490800600116 [Google Scholar]
- Cantú S. M., Simpson J. A., Griskevicius V., Weisberg Y. J., Durante K. M., Beal D. J. (2014). Fertile and selectively flirty: Women’s behavior toward men changes across the ovulatory cycle. Psychological Science, 25, 431–438. doi:10.1177/0956797613508413 [DOI] [PubMed] [Google Scholar]
- Carr-Nangle R. E., Johnson W. G., Bergeron K. C., Nangle D. W. (1994). Body image changes over the menstrual cycle in normal women. International Journal of Eating Disorders, 16, 267–273. doi:10.1002/1098-108X(199411)16:3<267::AID-EAT2260160307>3.0.CO;2-Y [DOI] [PubMed] [Google Scholar]
- Caruso S., Agnello C., Malandrino C., Lo Presti L., Cicero C., Cianci S. (2014). Do hormones influence women’s sex? Sexual activity over the menstrual cycle. The Journal of Sexual Medicine, 11, 211–221. doi:10.1111/jsm.12348 [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences. New York, NY: Psychology Press. [Google Scholar]
- Diamond L. M., Wallen K. (2011). Sexual minority women’s sexual motivation around the time of ovulation. Archives of Sexual Behavior, 40, 237–246. doi:10.1007/s10508-010-9631-2 [DOI] [PubMed] [Google Scholar]
- Durante K. M., Li N. P., Haselton M. G. (2008). Changes in women’s choice of dress across the ovulatory cycle: Naturalistic and laboratory task-based evidence. Personality and Social Psychology Bulletin, 34, 1451–1460. doi:10.1177/0146167208323103 [DOI] [PubMed] [Google Scholar]
- Gangestad S. W., Haselton M. G., Welling L. L. M., Gildersleeve K., Pillsworth E. G., Burriss R. P.…Puts D. A. (2016). How valid are assessments of conception probability in ovulatory cycle research? Evaluations, recommendations, and theoretical implications. Evolution and Human Behavior, 37, 85–96. doi:10.1016/j.evolhumbehav.2015.09.001 [Google Scholar]
- Gangestad S. W., Thornhill R. (1998). Menstrual cycle variation in women’s preferences for the scent of symmetrical men. Proceedings of the Royal Society B: Biological Sciences, 265, 927–933. doi:10.1098/rspb.1998.0380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangestad S. W., Thornhill R. (2008). Human oestrus. Proceedings of the Royal Society B: Biological Sciences, 275, 991–1000. doi:10.1098/rspb.2007.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangestad S. W., Thornhill R., Garver C. E. (2002). Changes in women’s sexual interests and their partner’s mate-retention tactics across the menstrual cycle: Evidence for shifting conflicts of interest. Proceedings of the Royal Society B: Biological Sciences, 269, 975–982. doi:10.1098/rspb.2001.1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangestad S. W., Thornhill R., Garver-Apgar C. E. (2005). Women’s sexual interests across the ovulatory cycle depend on primary partner developmental instability. Proceedings: Biological Sciences, 272, 2023–2027. Retrieved fromhttp://www.jstor.org/stable/30047909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangestad S. W., Thornhill R., Garver-Apgar C. E. (2010). Fertility in the cycle predicts women’s interest in sexual opportunism. Evolution and Human Behavior, 31, 400–411. doi:10.1016/j.evolhumbehav.2010.05.003 [Google Scholar]
- Gangestad S. W., Thornhill R., Garver-Apgar C. E. (2015). Women’s sexual interests across the ovulatory cycle. In Buss D. M. (Ed.), The handbook of evolutionary psychology (pp. 1–24). Hoboken, NJ: John Wiley. doi:10.1002/9781119125563.evpsych114 [Google Scholar]
- Gildersleeve K., Haselton M. G., Fales M. R. (2014). Do women’s mate preferences change across the ovulatory cycle? A meta-analytic review. Psychological Bulletin, 140, 1205–1259. doi:10.1037/a0035438 [DOI] [PubMed] [Google Scholar]
- Grammer K. (1993). 5a-androst-16en-3cx-on: A male pheromone? A brief report. Evolution and Human Behavior, 14, 8. [Google Scholar]
- Grebe N. M., Emery Thompson M., Gangestad S. W. (2016). Hormonal predictors of women’s extra-pair vs. in-pair sexual attraction in natural cycles: Implications for extended sexuality. Hormones and Behavior, 78, 211–219. doi:10.1016/j.yhbeh.2015.11.008 [DOI] [PubMed] [Google Scholar]
- Harris C. R. (2013). Shifts in masculinity preferences across the menstrual cycle: Still not there. Sex Roles, 69, 507–515. doi:10.1007/s11199-012-0229-0 [Google Scholar]
- Haselton M. G., Gangestad S. W. (2006). Conditional expression of women’s desires and men’s mate guarding across the ovulatory cycle. Hormones and Behavior, 49, 509–518. doi:10.1016/j.yhbeh.2005.10.006 [DOI] [PubMed] [Google Scholar]
- Haselton M. G., Gildersleeve K. (2016). Human ovulation cues. Current Opinion in Psychology, 7, 120–125. doi:10.1016/j.copsyc.2015.08.020 [Google Scholar]
- Havlicek J., Roberts S. C., Flegr J. (2005). Women’s preference for dominant male odour: Effects of menstrual cycle and relationship status. Biology Letters, 1, 256–259. doi:10.1098/rsbl.2005.0332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. C., Hahn A. C., Fisher C. I., Wang H., Kandrik M., DeBruine L. M. (2018). General sexual desire, but not desire for uncommitted sexual relationships, tracks changes in women’s hormonal status. Psychoneuroendocrinology, 88, 153–157. doi:10.1016/j.psyneuen.2017.12.015 [DOI] [PubMed] [Google Scholar]
- Jones B. C., Hahn A. C., Fisher C. I., Wang H., Kandrik M., Han C.…DeBruine L. M. (2018). No compelling evidence that preferences for facial masculinity track changes in women’s hormonal status. Psychological Science, 29, 1–10. doi:10.1177/0956797618760197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. C., Hahn A. C., Fisher C. I., Wincenciak J., Kandrik M., Roberts S. C.…DeBruine L. M. (2015). Facial coloration tracks changes in women’s estradiol. Psychoneuroendocrinology, 56, 29–34. doi:10.1016/j.psyneuen.2015.02.021 [DOI] [PubMed] [Google Scholar]
- Jünger J., Kordsmeyer T. L., Gerlach T. M., Penke L. (2018). Fertile women evaluate male bodies as more attractive, regardless of masculinity. Evolution and Human Behavior, 39, 412–423. doi:10.1016/j.evolhumbehav.2018.03.007 [Google Scholar]
- Karlestrand S. D. (2013). The complexity of romantic relationship: A quantitative study of women’s emotional responses to couple conflict in light of hormones and evolutionary theory (Master’s thesis). Norges Teknisk-Naturvitenskapelige Universitet, Trondheim, Norway. Retrieved fromhttps://brage.bibsys.no/xmlui/handle/11250/271004 [Google Scholar]
- Kuhn W., Koenig J., Donoghue A., Hillecke T., Warth M. (2014). Psychometrische Eigenschaften einer deutschsprachigen Kurzversion des Sexual Desire Inventory (SDI-2) [Psychometric Characteristics of the German Short Version of the Sexual Desire Inventory (SDI-2)]. Zeitschrift für Sexualforschung, 27, 138–149. doi:10.1055/s-0034-1366582 [Google Scholar]
- Marcinkowska U. M., Ellison P. T., Galbarczyk A., Milkowska K., Pawlowski B., Thune I., Jasienska G. (2016). Lack of support for relation between woman’s masculinity preference, estradiol level and mating context. Hormones and Behavior, 78, 1–7. doi:10.1016/j.yhbeh.2015.10.012 [DOI] [PubMed] [Google Scholar]
- Matthes J., Franke G. H., Jäger S. (2012). Psychometrische Prüfung des Dresdner Körperbildfragebogens (DKB-35) in einer nicht-klinischen Stichprobe [Psychometric Test of the Dresden Body Image Inventory (DKB-35) in a non-clinical Sample]. Zeitschrift Für Medizinische Psychologie, 21, 21–30. doi:10.3233/ZMP-2011-2028 [Google Scholar]
- Motta-Mena N. V., Puts D. A. (2017). Endocrinology of human female sexuality, mating, and reproductive behavior. Hormones and Behavior, 91, 19–35. doi:10.1016/j.yhbeh.2016.11.012 [DOI] [PubMed] [Google Scholar]
- Penke L. (2013). The Revised Sociosexual Orientation Inventory. In Fisher T. D., Davis C. M., Yarber W. L., Davis S. L. (Eds.), Handbook of sexuality-related measures (Vol. 3) (pp. 622–625). Oxford, England: Routledge. [Google Scholar]
- Penke L., Asendorpf J. B. (2008). Beyond global sociosexual orientations: A more differentiated look at sociosexuality and its effects on courtship and romantic relationships. Journal of Personality and Social Psychology, 95, 1113–1135. doi:10.1037/0022-3514.95.5.1113 [DOI] [PubMed] [Google Scholar]
- Penton-Voak I. S., Perrett D. I. (2000). Female preference for male faces changes cyclically: Further evidence. Evolution and Human Behavior, 21, 39–48. doi:10.1016/S1090-5138(99)00033-1 [Google Scholar]
- Penton-Voak I. S., Perrett D. I., Castles D. L., Kobayashi T., Burt D. M., Murray L. K., Minamisawa R. (1999). Menstrual cycle alters face preference. Nature, 399, 741–742. doi:10.1038/21557 [DOI] [PubMed] [Google Scholar]
- Pillsworth E. G., Haselton M. G. (2006. a). Male sexual attractiveness predicts differential ovulatory shifts in female extra-pair attraction and male mate retention. Evolution and Human Behavior, 27, 247–258. doi:10.1016/j.evolhumbehav.2005.10.002 [Google Scholar]
- Pillsworth E. G., Haselton M. G. (2006. b). Women’s sexual strategies: The evolution of long-term bonds and extrapair sex. Annual Review of Sex Research, 17, 59–100. doi:10.1080/10532528.2006.10559837 [Google Scholar]
- Pöhlmann K., Thiel P., Joraschky P. (2008). Entwicklung und Validierung des Dresdner Körperbildfragebogens (DKB-35) [Development and Validation of the Dresden Body Image Inventory (DKB-35)]. In Joraschky P., Lausberg H., Pöhlmann K., Röhricht F. (Eds.), Körperorientierte Diagnostik und Psychotherapie bei Essstörungen (pp. 57–72). Gießen, Germany: Psychosozial-Verlag. [Google Scholar]
- Puts D., Bailey D., Cárdenas R., Burriss R., Welling L., Wheatley J., Dawood K. (2013). Women’s attractiveness changes with estradiol and progesterone across the ovulatory cycle. Hormones and Behavior, 63, 13–19. doi:10.1016/j.yhbeh.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Roberts S., Havlicek J., Flegr J., Hruskova M., Little A, Jones B.…Petrie M. (2004). Female facial attractiveness increases during the fertile phase of the menstrual cycle. Proceedings of the Royal Society of London. Series B: Biological Sciences, 271, S270–S272. doi:10.1098/rsbl.2004.0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röder S., Brewer G., Fink B. (2009). Menstrual cycle shifts in women’s self-perception and motivation: A daily report method. Personality and Individual Differences, 47, 616–619. doi:10.1016/j.paid.2009.05.019 [Google Scholar]
- Roney J. R., Simmons Z. L. (2008). Women’s estradiol predicts preference for facial cues of men’s testosterone. Hormones and Behavior, 53, 14–19. doi:10.1016/j.yhbeh.2007.09.008 [DOI] [PubMed] [Google Scholar]
- Roney J. R., Simmons Z. L. (2013). Hormonal predictors of sexual motivation in natural menstrual cycles. Hormones and Behavior, 63, 636–645. doi:10.1016/j.yhbeh.2013.02.013 [DOI] [PubMed] [Google Scholar]
- Roney J. R., Simmons Z. L. (2017). Ovarian hormone fluctuations predict within-cycle shifts in women’s food intake. Hormones and Behavior, 90, 8–14. doi:10.1016/j.yhbeh.2017.01.009 [DOI] [PubMed] [Google Scholar]
- Sheldon M. S., Cooper M. L., Geary D. C., Hoard M., DeSoto M. C. (2006). Fertility cycle patterns in motives for sexual behavior. Personality and Social Psychology Bulletin, 32, 1659–1673. doi:10.1177/0146167206292690 [DOI] [PubMed] [Google Scholar]
- Shimoda R., Campbell A., Barton R. (2017). Women’s emotional and sexual attraction to men across the menstrual cycle. Behavioral Ecology, 29, 51–59. doi:10.1093/beheco/arx124 [Google Scholar]
- Spector I. P., Carey M. P., Steinberg L. (1996). The Sexual Desire Inventory: Development, factor structure, and evidence of reliability. Journal of Sex & Marital Therapy, 22, 175–190. doi:10.1080/00926239608414655 [DOI] [PubMed] [Google Scholar]
- Thornhill R., Gangestad S. W. (2008). The evolutionary biology of human female sexuality. Oxford, England: Oxford University Press. [Google Scholar]
- Welling L. L. M., Puts D. A. (2014). Female adaptations to ovulation. In Weekes-Shackelford V. A., Shackelford T. K. (Eds.), Evolutionary perspectives on human sexual psychology and behavior (pp. 243–260). New York, NY: Springer. doi:10.1007/978-1-4939-0314-6_13 [Google Scholar]
- Wilcox A. J. (2004). On the frequency of intercourse around ovulation: Evidence for biological influences. Human Reproduction, 19, 1539–1543. doi:10.1093/humrep/deh305 [DOI] [PubMed] [Google Scholar]
- Wood W., Kressel L., Joshi P. D., Louie B. (2014). Meta-analysis of menstrual cycle effects on women’s mate preferences. Emotion Review, 6, 229–249. doi:10.1177/1754073914523073 [Google Scholar]