Abstract
Several nucleic acid-based amplification tests are available for the detection of Mycobacterium tuberculosis, but few data are available on their use in the diagnosis of tuberculous meningitis (TBM). We performed a prospective study to assess the Roche AMPLICOR Mycobacterium tuberculosis PCR test (TB AMPLICOR) for use in the diagnosis of TBM and compared it with direct Ziehl-Neelsen staining of smears, radiometric culture for M. tuberculosis, and clinical and cerebrospinal fluid (CSF) findings. Eighty-three CSF specimens collected from 69 patients with suspected meningitis in South Africa were tested by TB AMPLICOR. On the basis of clinical and laboratory findings, 40 of these patients were treated for TBM and 29 patients were not treated for TBM. Ten CSF samples from 10 patients were positive by TB AMPLICOR. Seven of these 10 patients were classified as having definite TBM, 2 were classified as having probable TBM, and 1 was classified as having possible TBM. The sensitivity of TB AMPLICOR for detecting cases of definite and probable TBM in patients from whom CSF specimens had been collected less than 10 days into antituberculosis treatment was 60.0%. Specimens from all 29 patients not treated for TBM were negative by the TB AMPLICOR, giving a 100% specificity. TB AMPLICOR is therefore more sensitive than the combination of Ziehl-Neelsen staining of smears and radiometric culture for M. tuberculosis and is a rapid and highly specific diagnostic test for TBM.
In the past few years there has been a global increase in the incidence of tuberculosis (TB), with an increase in the number of notifications of TB in England and Wales (7), other European countries (20), and the United States (2). Tuberculous meningitis (TBM) is rare in developed countries, but Mycobacterium tuberculosis is an important cause of meningitis in many developing countries, where facilities to confirm it as the cause of meningitis are least available. Without treatment, death occurs in virtually all patients with TBM, while delay in treatment results in a considerable risk of irreversible neurological damage. Therefore, rapid diagnosis is important but difficult, since the spectrum of disease is wide and abnormalities of the cerebrospinal fluid (CSF), although usually present, are very variable. Direct smears of CSF for acid-fast bacilli (AFB), although virtually diagnostic, are usually positive in fewer than 10% of cases of TBM (11, 26), while culture for M. tuberculosis takes up to 8 weeks and is also often negative. A clinical response to antituberculosis treatment is usual, but patients with TBM may deteriorate on appropriate treatment. Hence, a rapid and sensitive test is required for the diagnosis of TBM.
A number of nucleic acid-based amplification tests, most of them based on PCR, have been developed for the detection of M. tuberculosis in clinical specimens. Although considerable data are now available on their use with respiratory specimens in the diagnosis of pulmonary tuberculosis, little is known of their role in diagnosing TBM from specimens of CSF. Only six published studies (in the English-language literature) of M. tuberculosis PCRs for the diagnosis of TBM have included more than 20 patients with suspected or confirmed TBM (Table 1). Those studies used primers targeting either the MPB 64 protein (15, 22, 23) or the insertion sequence IS6110 (12, 16, 19). Overall sensitivities for the diagnosis of TBM ranged from 33% (19) to 90.5% (15), with specificities ranging from 88.2% (23) to 100% (15, 19). From these data it is clear that the role of PCR in the diagnosis of TBM is undefined.
TABLE 1.
Studies of TB PCR for the diagnosis of TBM
| Author, yr (reference no.) | Target | Use of nested PCR | CSF vol (ml) | No. of patients (no. of control subjects) | No. of patients smear (culture) positive | Sensitivity (no. of patients positive/no. of patients tested [%])a | Sensitivity (%)b | Specificity (%) |
|---|---|---|---|---|---|---|---|---|
| Shankar et al., 1991 (23) | MPB 64 | No | 1 | 34 (51) | 0 (4) | 19/27 (70.4) | 64.7 | 88.2 |
| Liu et al., 1994 (15) | MPB 64 | Yes | 0.5 | 21 (79) | 1 (6) | 16/17 (94.1) | 90.5 | 100 |
| Kox et al., 1995 (12) | IS986 | No | <0.2–5 | 24 (18) | 2 (9) | NAc | 48 | NA |
| Miörner et al., 1995 (16) | IS6110 | No | 0.1 | 33 (34) | NA (6) | NA | 54 | 94.1 |
| Seth et al., 1996 (22) | MPB 64 | No | NA | 40 (49) | 0 (0) | 21/24 (87.5) | 85 | 93.1 |
| Nguyen et al., 1996 (19) | IS986 | No | Variable | 97 (39) | 1 (17) | 32/89 (36) | 33 | 100 |
Data for patients with confirmed and probable TBM (note that the classification criteria are not the same for all studies).
Data for all patients with suspected TBM.
NA, not available.
In order to clarify the role of PCR in the diagnosis of TBM we assessed the Roche AMPLICOR Mycobacterium tuberculosis PCR test (TB AMPLICOR), which has been shown to have a sensitivity and a specificity of 66.7 and 99.6%, respectively, for the diagnosis of pulmonary TB from respiratory samples (3).
MATERIALS AND METHODS
Patients.
Cecilia Makiwane Hospital (CMH), a large district general hospital serving a population of about 1.5 million people on the Eastern Cape of the Republic of South Africa, was chosen as the center from which to recruit patients because of the high incidence of TB and lymphocytic meningitis in this area. Samples of CSF and serum were collected prospectively from CMH inpatients with suspected meningitis between February 1995 and August 1996. In total, 114 CSF samples were collected from 99 patients. For this study, 83 CSF specimens from 69 patients were used, together with simultaneous serum samples from 43 patients. There were 31 males and 38 females, and their ages ranged from 1 to 83 years (mean age, 29.7 ± 17.4 [1 standard deviation] years).
Clinical data for each patient were collected on a standardized form by the attending physician. These included each patient’s age, sex, hospital number, hospital ward, dates of admission and discharge (or transfer or death), date(s) of lumbar puncture, initial diagnosis and diagnosis at 2 weeks, chest radiograph and computed tomography brain scan results (when performed), other relevant investigations that were performed (such as viral serology and Treponema pallidum hemagglutination assay-Venereal Disease Research Laboratory test), the dates that treatment commenced, the response to treatment, details of follow-up visits, and all laboratory data mentioned above.
Samples.
Each CSF sample was divided into four portions: one portion was sent to the pathology laboratory at CMH for cytology, biochemistry, Ziehl-Neelsen (ZN) staining of smears, Gram staining, bacterial culture, and Indian ink staining; a second portion was sent for radiometric culture for M. tuberculosis (BACTEC 12B) and cryptococcal antigen (du Buisson and Partners Pathology Laboratory, East London, Republic of South Africa); the third and fourth portions were stored, together with the serum samples, at −35°C until transfer to the United Kingdom. The samples were transported by air to the United Kingdom on frozen CO2 in four separate batches between September 1995 and September 1996, and all samples remained frozen until arrival at the laboratory. The samples were subsequently stored in two freezers, one at −70°C and the other at −20°C, until testing by PCR, which was performed between 3 and 12 months after collection of the samples.
Testing for HIV.
Forty-three patients were tested for human immunodeficiency virus (HIV) antibody as part of their clinical work-up at CMH by two different enzyme-linked immunosorbent assays (Anti HIV-1 and HIV-2 EIA DAGS [Roche Diagnostics Inc.] and the Vidas HIV-1 + 2 new generation [bioMérieux]). Sera from a further 15 patients were tested for HIV antibody by the Bioelisa HIV-1 + 2 kit (BIOKIT S.A., Barcelona, Spain). Approval for the study was granted by the Ethics Committee at CMH.
Criteria for classification of likelihood of TBM.
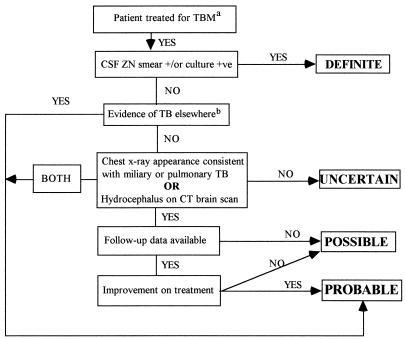
For the purposes of this study, all patients who were treated for TBM, usually on clinical grounds and always by a physician experienced in managing TBM, were classified into one of four categories (definite, probable, possible, or uncertain TBM) by the algorithm shown in Fig. 1. This classification was performed retrospectively when results of culture for M. tuberculosis became available.
FIG. 1.
Algorithm used for classification of likelihood of TBM. a, with a cellular CSF and/or raised CSF protein and/or reduced CSF glucose; b, culture-proven or caseating granulomas on histology.
ZN staining of smears and culture for M. tuberculosis.
Smears for ZN staining were prepared and examined by experienced laboratory technologists at CMH by the technique described by Stewart in 1953 (24). Briefly, CSF samples were concentrated by centrifugation at 15,000 × g for 20 min. A mean CSF volume of 3.8 ± 2.3 ml (calculated as the mean for 58 CSF samples whose volumes were recorded) was used to prepare smears for ZN staining, and each smear was examined for at least 1 h.
Radiometric culture for M. tuberculosis was performed with CSF samples and BACTEC 12B culture medium. Cultures with no growth were reported as having a negative result after 6 weeks of incubation.
PCR.
TB AMPLICOR is a commercially produced PCR test (1994; Roche Diagnostic Systems, Inc., Branchburg, N.J.) that comes in the form of a standardized kit and that is designed primarily for the testing of respiratory specimens. For amplification by PCR, 100-μl CSF samples were processed exactly as recommended for respiratory specimens, according to the instructions of the manufacturer, omitting the liquefaction-decontamination step. An internal control was tested with each sample.
RESULTS
Of the 99 patients from whom 114 CSF specimens were tested by TB AMPLICOR, 30 patients were excluded from the study, leaving 83 CSF specimens from 69 patients. For 10 patients, sufficient clinical and treatment data were unavailable, and all were TB AMPLICOR negative. The other 20 patients were excluded because results of culture for M. tuberculosis were not available, and of these, 9 were treated for TBM and 11 were not treated for TBM. One of nine patients treated for TBM was positive by ZN staining of smears and TB AMPLICOR positive; and eight of nine patients (one classified as having probable TBM, 4 classified as having possible TBM, and 3 classified as having an uncertain diagnosis) were negative by ZN staining of smears and TB AMPLICOR negative. All 11 patients not treated for TBM (including two with confirmed bacterial meningitis, two with presumed viral meningitis, and one with epilepsy) were TB AMPLICOR negative, and the nine patients for whom ZN staining of smears was performed were all smear negative.
Forty patients (58.0%) were treated for TBM, and 29 patients (42.0%) deemed to have an alternative diagnosis (on the basis of clinical findings and laboratory data) were not treated for TBM. Of the 40 patients who were treated for TBM, 8 were classified as having definite TBM (20.0%), 10 were classified as having probable TBM (25.0%), 15 were classified as having possible TBM (37.5%), and 7 were classified as having uncertain TBM (17.5%).
HIV antibody results were available for 58 of 69 patients (84.1%). Of these 58 patients, 22 (37.9%) were HIV antibody positive and 36 (62.1%) were HIV antibody negative. A total of 12 of 35 (34.3%) patients in the treated group and 9 of 23 (39.1%) in the untreated group were HIV antibody positive. Only two of nine (22.2%) TB AMPLICOR-positive patients were HIV positive. Results of ZN staining of smears were available for all patients, and eight of these were smear positive, although only three were positive for M. tuberculosis by culture.
Of the 40 patients (52 samples) who were treated for TBM, 10 CSF samples from 10 patients were TB AMPLICOR positive (Table 2). Seven of these patients were positive by ZN staining of smears (three were culture positive and four were culture negative) and were classified as having definite TBM. The remaining three patients were all negative by ZN staining of smears and culture negative, with two classified as having probable TBM and one classified as having possible TBM. A further sample which was originally TB AMPLICOR negative but which tested positive with a second TB PCR system (automated TB COBAS AMPLICOR PCR) was TB AMPLICOR positive on retesting (the sample was negative by ZN staining of smears and culture negative and the patient was classified as having probable TBM). For the purpose of the analysis, this patient was considered to be TB AMPLICOR negative. Only one patient classified as having definite TBM was TB AMPLICOR negative. On two occasions her CSF was positive by ZN staining of smears, but the only sample available for culture for M. tuberculosis and testing by PCR was obtained 8 days after starting antituberculosis treatment, when the results of both tests were negative. Overall the sensitivity of the TB AMPLICOR (Table 3) was 25.0% (10 of 40 patients), but no CSF samples obtained more than 9 days into treatment were TB AMPLICOR positive. A subanalysis was therefore performed in which data for patients whose first CSF sample was taken more than 9 days into treatment (or data for one patient whose duration of treatment was indeterminate) were excluded, revealing a sensitivity of 28.6% (10 of 35 patients) overall and 60.0% (9 of 15 patients) for the detection of definite and probable cases of TBM. The positive predictive value of the test for CSF samples collected ≤9 days into antituberculosis treatment was 100%, with a negative predictive value of 53.7% (29 true-negative samples/[29 true-negative samples + 25 false-negative samples]). The specificities of ZN staining of smears radiometric culture for M. tuberculosis, and TB AMPLICOR were all 100%.
TABLE 2.
Breakdown of all results of ZN staining of smears, culture for M. tuberculosis, and TB AMPLICOR PCR by treatment for TBM
| Test resulta
|
Treatment for TBM | No. of patients | ||
|---|---|---|---|---|
| ZN staining of smears | Culture for M. tuberculosis | TB AMPLICOR | ||
| + | + | + | Yes | 3 |
| + | − | + | Yes | 4 |
| + | − | − | Yes | 1 |
| − | − | + | Yes | 3 |
| − | − | − | Yes | 29 |
| − | − | − | No | 29 |
+, positive; −, negative.
TABLE 3.
Overall sensitivities of TB AMPLICOR, ZN staining of smears, and radiometric culture for M. tuberculosis for detection of individual categories of TBM and adjusted sensitivities of TB AMPLICOR after excluding patients whose first CSF sample was taken more than 9 days into treatment
| TBM category | Sensitivity (no. of patients positive/total no. of patients tested [%])
|
|||
|---|---|---|---|---|
| TB AMPLICOR | Adjusted TB AMPLICORa | ZN staining of smears | Culture for M. tuberculosis | |
| Definite | 7/8 (87.5) | 7/8 (87.5) | 8/8 (100) | 3/8 (37.5) |
| Probable | 2/10 (20) | 2/7 (28.6) | NAb | NA |
| Possible | 1/15 (6.7) | 1/13 (7.7) | NA | NA |
| Uncertain | 0/7 (0) | 0/7 (0) | NA | NA |
| Definite or probable | 9/18 (50.0) | 9/15 (60.0) | 8/18 (44.4) | 3/18 (16.7) |
| Overall sensitivity | 10/40 (25.0) | 10/35 (28.6) | 8/40 (20) | 3/40 (7.5) |
After excluding patients whose first CSF sample was taken more than 9 days into treatment.
NA, not applicable.
Among the group of 29 patients not treated for TBM, 7 had cryptococcal meningitis, 3 had bacterial meningitis (1 had meningococcal and 2 had pneumococcal meningitis), 1 had presumed viral meningitis, 1 had neurosyphilis (and 1 for whom lumbar puncture excluded neurosyphilis), 2 had a first-ever generalized seizure, 2 had extraneural bacterial infection with confusion, and 2 had pulmonary TB without TBM. The remaining 10 patients were diagnosed as suffering from a wide range of mainly neurological conditions. All 29 patients (31 samples) who were not treated for TBM were TB AMPLICOR negative.
For 23 of 40 patients treated for TBM, the earliest CSF sample available for PCR testing was taken either just before or within 48 h of the commencement of antituberculosis treatment, and 8 of these patients were TB AMPLICOR positive. Of 12 patients whose first CSF sample was obtained 3 to 9 days into treatment, only 2 were positive and none of 4 patients whose first CSF sample was obtained >9 days into treatment were positive (all 4 were negative by ZN staining of smears and culture negative). The treatment time for one patient was unavailable (PCR negative). For four of the TB AMPLICOR-positive patients, follow-up CSF samples were taken between 8 and 23 days after antituberculosis therapy had commenced, and none were TB AMPLICOR positive.
DISCUSSION
This study included a relatively large number of patients (8 of 40 [20.0%] treated patients) whose CSF was positive by ZN staining of smears. Microscopic examination of CSF for AFB is important for a definitive early diagnosis of TBM. The frequency with which AFB are seen on direct smears varies widely between different series, ranging from 0 of 7 patients (18) to 91 of 100 patients (24), and depends on the time devoted to searching for AFB, the number of specimens examined (17), and the experience of the observer. Rates of positivity of ZN staining of smears of CSF of between 10 and 40% are commonly reported (1, 6, 9, 10, 25, 27), although in clinical practice fewer than 10% of smears are probably positive (11, 26). We believe our success with ZN staining of smears in this study is due to a combination of the use of experienced technologists who examined each smear for 1 h, the use of the technique of Stewart (24), and the use of a mean volume of nearly 4 ml of CSF for each smear.
The low rate of positivity for M. tuberculosis by culture (three patients in the entire study) in this study may be related to the commencement of antituberculosis treatment prior to taking a CSF sample for culture. All patients who were culture negative had received prior antituberculosis treatment, with the duration of treatment ranging from only 24 h to up to 3 weeks. Interestingly, two patients who had received only 1 day of treatment and 1 patient who had received 2 days of treatment were also culture negative. A recent South African study of 141 young children with a clinical diagnosis of TBM revealed that only 23 of 141 (16.3%) were CSF culture positive for M. tuberculosis (21). A second contributing factor to the low culture positivity rate is that cultures for M. tuberculosis were reported to be negative after only 6 weeks of incubation and no solid medium was used.
Only 11 of 40 patients (52 samples) treated for TBM (10 of 40 on first test) in this study were found to be TB AMPLICOR positive. There are a number of possible reasons for this. First, there is a relatively high incidence of TBM in the population being studied, and therefore, the local physicians have a high index of suspicion for the disease. Local confirmation of other causes of lymphocytic meningitis (except cryptococcal meningitis) is difficult, and as a result, a proportion of patients who were treated for TBM probably did not have the disease (particularly patients in the possible and uncertain categories). Second, only 100 μl of CSF was used for each PCR. One study (19) noted that 4 of 11 of their CSF samples positive by PCR for M. tuberculosis contained only a few mycobacteria (5 to 100 per ml). Another group of investigators (4) reported that all of their culture-positive CSF samples contained fewer than 100 viable mycobacteria per ml and 88% contained fewer than 10 mycobacteria per ml (equating to one mycobacterium or less per PCR test). Hence, as a result of the “aliquot phenomenon,” culture-positive CSF samples may be false negative by PCR because there are no mycobacteria in the sample, while samples that are culture negative probably contain even fewer mycobacteria. Third, for many patients in the treated group, CSF was available only after starting antituberculosis treatment. No CSF was TB AMPLICOR positive more than 9 days after the commencement of antituberculosis treatment, and for four patients, the first CSF sample available to us was taken 10 days or more after treatment had begun.
Some investigators have reported that the CSF of some patients with TBM remains PCR positive for up to 4 weeks after the start of antituberculosis therapy (5, 8, 14). One study (12) reported a patient whose CSF remained PCR positive for M. tuberculosis 6 weeks after treatment began, although that study used guanidinium thiocyanate extraction of DNA from a 5-ml sample. Another study (13) performed PCR with sequential CSF samples from seven patients with TBM who were initially positive for M. tuberculosis by PCR. Five of seven patients had become PCR negative after day 14 of treatment, and only one patient remained positive at 28 days. In our study, however, of four patients who were initially TB AMPLICOR positive, all had become negative when repeat CSF samples were tested 8 to 23 days after the start of treatment.
In conclusion, the TB AMPLICOR is more sensitive than the combination of staining of smears and radiometric culture for M. tuberculosis and is a rapid and highly specific test for the diagnosis of TBM. The sensitivity, however, is restricted by the low level of mycobacteria within the CSF of patients with TBM. Our data also highlight the importance of obtaining CSF before the commencement of antituberculosis treatment or as soon as possible thereafter in order to maximize the sensitivity of PCR. Furthermore, it may be possible to enhance sensitivity by increasing the volume of CSF used to 1 or 2 ml. Finally, PCR is not an appropriate technology for the Third World, and this study emphasizes the important role of ZN staining of smears for the diagnosis of TBM when it is performed by experienced technologists who use good technique.
ACKNOWLEDGMENTS
We are indebted to P. J. Swift, A. Parrish, and all the junior medical staff at CMH for the collection of CSF and serum samples and clinical data. We also thank J. Damba, H. Stevens, L. Sawyer, G. Potgieter, and A. Hawkes for technical assistance and the medical superintendent of CMH for permission to perform the study.
We also thank Roche Diagnostics Inc. for supplying the TB AMPLICOR kits.
REFERENCES
- 1.Bateman D E, Newman P K, Foster J B. A retrospective survey of proven cases of tuberculous meningitis in the Northern region, 1970–1980. J R College Phys London. 1983;17:106–110. [PMC free article] [PubMed] [Google Scholar]
- 2.Cantwell N F, Snider D E, Cauthen G M, Onorato I M. Epidemiology of tuberculosis in the United States, 1985 through 1992. JAMA. 1994;272:535–539. [PubMed] [Google Scholar]
- 3.D’Amato R F, Wallman A A, Hochstein L H, Colaninno P M, Scardamaglia M, Ardila E, Ghouri M, Kim K, Patel R C, Miller A. Rapid diagnosis of pulmonary tuberculosis by using Roche AMPLICOR Mycobacterium tuberculosis PCR test. J Clin Microbiol. 1995;33:1832–1834. doi: 10.1128/jcm.33.7.1832-1834.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis L E, Rastogi K R, Lambert L C, Skipper B J. Tuberculous meningitis in the southwest United States: a community-based study. Neurology. 1993;43:1775–1778. doi: 10.1212/wnl.43.9.1775. [DOI] [PubMed] [Google Scholar]
- 5.Donald P R, Victor T C, Jordaan A M, Schoeman J F, Van Helden P D. Polymerase chain reaction in the diagnosis of tuberculous meningitis. Scand J Infect Dis. 1993;25:613–617. doi: 10.3109/00365549309008550. [DOI] [PubMed] [Google Scholar]
- 6.Haas E J, Madhavan T, Quinn E L, Cox F, Fisher E, Burch K. Tuberculous meningitis in an urban general hospital. Arch Intern Med. 1977;137:1518–1521. [PubMed] [Google Scholar]
- 7.Hayward A C, Watson J M. Tuberculosis in England and Wales 1982 to 1993: notifications exceeded predictions. CDR Rev. 1995;5:R29–R33. [PubMed] [Google Scholar]
- 8.Kaneko K, Onodera O, Miyatake T, Tsuji S. Rapid diagnosis of tuberculous meningitis by polymerase chain reaction (PCR) Neurology. 1990;40:1617–1618. doi: 10.1212/wnl.40.10.1617. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy D H, Fallon R J. Tuberculous meningitis. JAMA. 1979;241:264–268. [PubMed] [Google Scholar]
- 10.Kent S J, Crowe S M, Yung A, Lucas C R, Mijch A M. Tuberculous meningitis: a 30-year review. Clin Infect Dis. 1993;17:987–994. doi: 10.1093/clinids/17.6.987. [DOI] [PubMed] [Google Scholar]
- 11.Kilpatrick M E, Girgis N I, Yassin M W, Abu El Ella A A. Tuberculosis meningitis—clinical and laboratory review of 100 patients. J Hyg Camb. 1986;96:231–238. doi: 10.1017/s0022172400066006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kox L F F, Kuijper S, Kolk A H J. Early diagnosis of tuberculous meningitis by polymerase chain reaction. Neurology. 1995;45:2228–2232. doi: 10.1212/wnl.45.12.2228. [DOI] [PubMed] [Google Scholar]
- 13.Lin J-J, Harn H-J. Application of the polymerase chain reaction to monitor Mycobacterium tuberculosis DNA in the CSF of patients with tuberculous meningitis after antibiotic treatment. J Neurol Neurosurg Psychiatry. 1995;59:175–177. doi: 10.1136/jnnp.59.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin J-J, Harn H-J, Hsu Y-D, Tsao W-L, Lee H-S, Lee W-H. Rapid diagnosis of tuberculous meningitis by polymerase chain reaction assay of cerebrospinal fluid. J Neurol. 1995;242:147–152. doi: 10.1007/BF00936887. [DOI] [PubMed] [Google Scholar]
- 15.Liu P Y-F, Shi Z-Y, Lau Y-J, Hu B-S. Rapid diagnosis of tuberculous meningitis by a simplified nested amplification protocol. Neurology. 1994;44:1161–1164. doi: 10.1212/wnl.44.6.1161. [DOI] [PubMed] [Google Scholar]
- 16.Miörner H, Sjöbring U, Nayak P, Chandramuki A. Diagnosis of tuberculous meningitis: a comparative analysis of 3 immunoassays, an immunocomplex assay and the polymerase chain reaction. Tubercle Lung Dis. 1995;76:381–386. doi: 10.1016/0962-8479(95)90002-0. [DOI] [PubMed] [Google Scholar]
- 17.Molavi A, LeFrock J L. Tuberculous meningitis. Med Clin N Am. 1985;69:315–331. doi: 10.1016/s0025-7125(16)31045-8. [DOI] [PubMed] [Google Scholar]
- 18.Naughten E, Newton R, Weindling A M, Bower B D. Tuberculous meningitis in children. Lancet. 1981;ii:973–975. doi: 10.1016/s0140-6736(81)91166-1. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen L N, Kox L F F, Pham L D, Kuijper S, Kolk A H J. The potential contribution of the polymerase chain reaction to the diagnosis of tuberculous meningitis. Arch Neurol. 1996;53:771–776. doi: 10.1001/archneur.1996.00550080093017. [DOI] [PubMed] [Google Scholar]
- 20.Raviglione M C, Sudre P, Rieder H L, Spinaci S, Kochi A. Secular trends of tuberculosis in Western Europe. Bull W H O. 1993;71:297–306. [PMC free article] [PubMed] [Google Scholar]
- 21.Schoeman J F, Van Zyl L E, Laubscher J A, Donald P R. Effect of corticosteroids on intracranial pressure, computed tomographic findings, and clinical outcome in young children with tuberculous meningitis. Pediatrics. 1997;99:226–231. doi: 10.1542/peds.99.2.226. [DOI] [PubMed] [Google Scholar]
- 22.Seth P, Ahuja G K, Vijaya N, Bhanu, Behari M, Bhowmik S, Broor S, Dar L, Chakraborty M. Evaluation of polymerase chain reaction for rapid diagnosis of clinically suspected tuberculous meningitis. Tubercle Lung Dis. 1996;77:353–357. doi: 10.1016/s0962-8479(96)90101-x. [DOI] [PubMed] [Google Scholar]
- 23.Shankar P, Manjunath N, Mohan K K, Prasad K, Behari M, Shriniwas, Ahuja G K. Rapid diagnosis of tuberculous meningitis by polymerase chain reaction. Lancet. 1991;337:5–7. doi: 10.1016/0140-6736(91)93328-7. [DOI] [PubMed] [Google Scholar]
- 24.Stewart S M. The bacteriological diagnosis of tuberculous meningitis. J Clin Pathol. 1953;6:241–242. doi: 10.1136/jcp.6.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swart S, Briggs R S, Millac P A. Tuberculous meningitis in Asian patients. Lancet. 1981;ii:15–16. doi: 10.1016/s0140-6736(81)90253-1. [DOI] [PubMed] [Google Scholar]
- 26.Teoh R, Humphries M. Tuberculous meningitis. In: Lambert H P, editor. Kass handbook of infectious diseases: infections of the central nervous system. Philadelphia, Pa: BC Decker; 1991. pp. 189–206. [Google Scholar]
- 27.Traub M, Colchester A C F, Kingsley D P E, Swash M. Tuberculosis of the central nervous system. Q J Med. 1984;209:81–100. [PubMed] [Google Scholar]