Abstract
Conditional reasoning (if p then q) is used very frequently in everyday situations. Conditional reasoning is impaired in brain-lesion patients, psychopathy, alcoholism, and polydrug dependence. Many neurocognitive deficits have also been described in schizophrenia. We assessed conditional reasoning in 25 patients with schizophrenia, 25 depressive patients, and 25 controls, using the Wason selection task in three different domains: social contracts, precautionary rules, and descriptive rules. Control measures included depression, anxiety, and severity of schizophrenia measures as a Verbal Intelligence Scale. Patients with schizophrenia were significantly impaired on all conditional reasoning tasks compared to depressives and controls. However, the social contract and precautions tasks yielded better results than the descriptive tasks. Differences between groups disappeared for social contract but remained for precautions and descriptive tasks when verbal intelligence was used as a covariate. These results suggest that domain-specific reasoning mechanisms, proposed by evolutionary psychologists, are relatively resilient in the face of brain network disruptions that impair more general reasoning abilities. Nevertheless, patients with schizophrenia could encounter difficulties understanding precaution rules and social contracts in real-life situations resulting in unwise risk-taking and misunderstandings in the social world.
Keywords: conditional reasoning, schizophrenia, depression, precautionary, social contract, Wason, evolutionary
Schizophrenia is associated with broad neurocognitive deficits across many cognitive domains (Dickinson, Ramsey, & Gold, 2007; Palmer, Dawes, & Heaton, 2009). Among these deficits, impaired logical reasoning is prominent: patients with schizophrenia often jump to conclusions, and they are too certain of their choices, even if they have too little information at their disposal (Moritz, Woodward, & Hausmann, 2006). These impairments have also been found in individuals with schizotypic characteristics in the general population (Sellen, Oaksford, & Gray, 2005).
Conditional reasoning (if p then q) is used very frequently in everyday situations. Disturbances in appropriate use of conditional reasoning could lead to errors with serious consequences. For example, reasoning about precaution rules (if the hazard exists, then you must take the precaution) is an example of conditional reasoning. It has been argued by evolutionary psychologists that this kind of reasoning was so important that it had led to the development of specialized reasoning mechanisms (Cosmides & Tooby, 1992). The abilities to detect cheaters in cooperative transactions (Cosmides, 1989) and to adhere to appropriate precautions in hazardous situations (Fiddick, Cosmides, & Tooby, 2000) have been proposed as putative candidates of such specific “Darwinian algorithms.” By contrast, it is not clear that would have been an evolutionary advantage to reasoning about abstract rules. These ideas have been tested using the Wason selection task, a standard test of conditional reasoning (Wason, 1968).
In the Wason selection task, participants are given a conditional rule (if P then Q) and four cards bearing information about the antecedent (P or not-P) on one side of the card and the consequent (Q or not-Q) on the other side of the card (see Figure 1). However, only one side of each card is visible to participants. The participants’ task is to determine which of the four cards need to be turned over in order to determine whether or not the rule has been violated. For example, participants might be asked to reason about a conditional, social contract rule of the form: If you take the benefit (P), then you must meet the requirement (Q). The rule is violated when individuals take the benefit (P) without meeting the requirement (not-Q). More concretely, the social contract rule could be “if you borrow my car, then you must fill the tank with gas.” A violation of this rule occurs when the subject borrows the car (P), without filling the tank with gas (not-Q). Therefore, the cards that must be turned over in order to see if the contract has been broken are P card and the not-Q card. Other sorts of rules that have been employed in the Wason selection task are precautions: If the hazard exists (P), then you must take the precaution (Q); abstract rules: If there is a B on one side of the card, then there is a seven on the other side of the card; and descriptive rules: If a train is going to Rochester, then it must be on Track 2.
Figure 1.
Examples of Wason tasks.
Reasoning about contents linked to problems encountered in real-life situations (social contract and precautions) with evolutionary relevance is better solved than purely descriptive problems. Participants perform poorly on the Wason selection task when the rules are descriptive or abstract (10–30%), but they perform well when the rules are social contracts or precautions (65–85%; Cosmides & Tooby, 2005).
Although some have disputed the evolutionary psychologists’ conjecture that reasoning about social contracts and precautions is supported by distinct neurocognitive mechanisms(Cheng & Holyoak, 1989; Manktelow & Over, 1991; Oaksford & Chater, 1994), several neurological studies exploring conditional reasoning have found dissociations supporting the existence of the hypothesized mechanism. In a brain-damaged patient with bilateral lesions in limbic, orbitofrontal, and temporal regions, social contract reasoning was selectively impaired while precautionary reasoning was preserved (Stone, Cosmides, Tooby, Kroll, & Knight, 2002). Several neural imaging studies conducted on neurologically normal participants have also found dissociations between reasoning about social contracts and precautions. These studies found that reasoning about social contracts produced activation in the right frontal and parietal lobes (Canessa et al., 2005); bilateral ventrolateral Prefrontal Cortex (PFC), left angular gyrus, and left orbitofrontal cortex(Laurence Fiddick, Spampinato, & Grafman, 2005); anterior and posterior temporal cortex, the regions associated with reasoning about theory of mind (Ermer, Guerin, Cosmides, Tooby, & Miller, 2006); and right medial frontal gyrus, the temporal lobe, a portion of the occipital cortex and frontal cortex(Reis et al., 2007). Precaution reasoning was associated with activation in bilateral insula, left lentiform nucleus, and cingulate and right postcentral gyrus (Fiddick et al., 2005); activation of middle and ventral prefrontal, middle and posterior temporal, right insula and cingulate for precautions compared to social contract (Ermer et al., 2006), posterior cingulate cortex, the anterior cingulate cortex, and the parahippocampal gyrus for precautions compared to social contracts (Reis et al., 2007). Overall, it seems that thinking about precautions recruits cingulate region, that is, for the detection of potential threats (Fiddick, 2011).
Given the range of brain regions implicated in these neurological studies, dissociations in impairments could theoretically be found in neurological and psychopathological conditions as recruited neuronal circuits vary according to the context involved. Conditional reasoning has been studied in different psychopathological conditions, namely, in psychopathy, alcoholism, and polysubstance dependence patients. Ermer and Kiehl (2010) investigated a group of incarcerated adults. Individuals with psychopathy showed impairment reasoning about social contract and precautionary rules, but not descriptive ones, relative to incarcerated individuals without psychopathy. Their results could not be accounted for by differences in intelligence or motivation. Kornreich et al. (2011) have investigated conditional reasoning in a group of alcoholic patients compared to normal controls. Impairment was present on social contract, precautionary, and descriptive reasoning in alcoholics, but was especially severe with descriptive rules, where alcoholic patients’ performance was at chance. In polysubstance dependence patients (Kornreich et al., 2012), social contract and descriptive reasoning was not above chance level, while precautionary reasoning was relatively preserved—worse than in controls but largely above chance level. All these results could reflect dysfunctions in prefrontal functioning. Chronic alcohol (Uekermann & Daum, 2008) or polysubstance abuse (Verdejo-García, Bechara, Recknor, & Pérez-García, 2006) is certainly detrimental to the functioning of prefrontal areas and prefrontal dysfunction is found in psychopathy (Finger et al., 2008). However, a certain degree of functional sparing is still present in these patients as their results are above chance level for precautionary reasoning in all the psychopathological conditions studied and for social contract conditions in psychopathy and alcoholism.
As schizophrenia is associated with prefrontal dysfunction, we would expect a similar difficulty, solving conditional reasoning problems. We want to explore whether some functional sparing still occurs for conditional reasoning in social or precaution conditions. If not, it could have clinical implications, as detecting cheaters in social exchanges and taking precautions to mitigate hazard have real-world importance.
We have chosen to contrast results in patients with schizophrenia with a population of depressive patients to exclude the possibility that any psychopathological condition could end up with the same results, that is, conditional reasoning difficulties. Although cognitive deficits are present in major depression and include disruption of frontosubcortical pathways (Austin, Mitchell, & Goodwin, 2001), they don’t have the same severity as in schizophrenia (Harvey, 2011).
Method
Subjects
Three groups have been compared: a schizophrenia group and two control groups, a pathological (depressive group) and a normal control group.
The schizophrenia group consisted of 25 inpatients (17men and 8 women) diagnosed with schizophrenia, according to the DSM-IV-TR criteria. Patients who were diagnosed in the acute phase or who were diagnosed with the catatonic subtype were excluded. Patients were recruited from several different psychiatric units: CHU-Brugmann, Erasme Hospital, Clinique Saint Jean, Chêne aux Haies, and Saint-Jean-de-Dieu hospitals. All subjects were medicated for their psychotic symptoms.
The depressive group consisted of 25 patients (14 men and 11 women) hospitalized in a psychiatric unit in Brugmann Hospital. All met the DSM-IV-TR criteria for a major depressive episode. All patients were taking standard antidepressant medication. Exclusion criteria were bipolar 1 or 2 disorder, schizophrenia, organic brain disorder, and substance abuse or dependence assessed during the intake interview.
The healthy control group consisted of 25 volunteers (15 men and 10 women) with no psychiatric record. The healthy group was recruited in the social circle of the investigators.
All groups were similar in gender proportion or education levels. Demographic data about the three groups described above are detailed in Table 1. Written informed consent was obtained from all participants. The Université Libre de Bruxelles ethical board approved this research project.
Table 1.
Characteristics of Groups (Schizophrenics, Depressives, and Normal Controls).
| Characteristics | Schizophrenics (N = 25) | Depressives (N = 25) | Controls (N = 25) |
|---|---|---|---|
| Male/female | 17/8 | 14/11 | 15/10 |
| Age: Mean (SD) | 41.56 (10.56) | 38 (12.06) | 37.28 (12.45) |
| Education level 1/2/3a | 5/9/11 | 3/9/13 | 5/9/11 |
| Paranoid/undifferentiated/Schizo affect | 5/18/2 | — | — |
| Duration: Mean (SD) | 15.52 years (10.40) | 8.12 months (7.17) | — |
| Previous number of hospitalizations: Mean (SD) | 5.36 (3.33) | 0.76 (1.05) | 0 |
| Drug use antecedents (including alcohol, cannabis, cocaine, and opiates) | 9 | 0 | 0 |
| Current medication: Neuroleptics/antidepressive/anxiolytics/thymo stabilizers | 23/14/14/3 | 0/24/15/0 | 0 |
| PANSS positive symptoms | 12.56 (6.44) | — | — |
| PANSS negative symptoms | 16.28 (4.58) | — | — |
| PANSS general psychopathology | 31.16 (9.63) | — | — |
| Mill Hill vocabulary scores | 23 (6.15)*** | 24.68 (3.77) | 27.68 (3.02) |
| BDI | 9.44 (6.52)**** | 25.24 (7.16)**** | 2.12 (2.11) |
| State-Trait Anxiety Inventory part A (STAIA) | 39.2 (11)* | 70.24 (8.04)**** | 31.16 (11.7) |
| State-Trait Anxiety Inventory part B (STAIB) | 46.48 (11.61)**** | 70.12 (6.32)**** | 34.76 (9.4) |
Note. SD = standard deviation; PANSS = Positive and Negative Syndrome Scale; BDI = Beck Depression Inventory; A = State and B = Trait.
aLevel of education was coded as follow: Level 1 = completion of the first 3 years of secondary school or equivalent; Level 2 = completion of secondary school or equivalent; and Level 3 = postsecondary school training.
**p = .01. ***p = .001. ****p < .0001.
Measures
Wason selection tasks
Twenty-four different versions of the Wason selection task were employed in this study. These were comprised of eight social contract tasks (SC; e.g., “If you borrow the car, then you must fill up the tank with gas”), eight precautionary tasks (PRE; e.g., “If you work with TB patients, then you must wear a surgical mask”), and eight social descriptive tasks (DES: describing the habits and traits of people; e.g., “If a person becomes a biologist, then that person enjoys camping”). Scoring was done as follows: 1 point was given for a completely correct response (P and not-Q cards selected, Q and not-P cards not selected) and 0 point for all other responses. The maximum score was 8 for each condition (eight stories per condition). Results are converted into percent correct.
Control measures
We have used three different types of control measures: a measure of the severity of schizophrenia (Positive and Negative Syndrome Scale [PANSS]), measures of the severity of depression and anxiety (Beck Depression Inventory [BDI] Scale and State Trait Anxiety Inventory [STAI-1 and B]), and a verbal intelligence test to ensure that vocabulary processing was not impaired in participants (Mill Hill).
The PANSS (Kay, Fiszbein, & Opler, 1987) is composed by 30 items, coded from 1 (absent) to 7 (extreme). It assesses psychopathological symptoms existing in psychotic syndromes, such as the “schizophrenic state.” Three scores are obtained: positive symptoms (7 items), negative symptoms (7 items), and general psychopathology (16 items).
BDI Scale (BDI short version in 13 items; Beck, Steer, & Carbin, 1988) and STAI for adults (STAI A and B; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983). As schizophrenia is frequently comorbid with depression and anxiety problems, the BDI and the STAI A and B were added as control measures.
Mill Hill Vocabulary Test (Part B; Deltour, 1993).
This test was added to ensure that vocabulary processing was not impaired and to ensure sufficient comprehension of the stories on the Wason selection task. This test consists of 36 items. For each item, the subject must give the best synonym, choosing among six options. The maximum score is 36 (French validation). Results obtained for the control measures are detailed in Table 1.
Results
Reasoning Performance
A two-way analysis of variance was performed on the Wason scores with rule domain (SC versus PRE versus DES) as a within-subjects factor and group (schizophrenia group, depressives, or controls) as a between-subjects factor (Table 2). Post hoc comparisons using a Bonferroni correction were performed when needed.
Table 2.
Wason Selection Tasks Scores.
| Characteristics | Schizophrenics: M (SD) | Depressives: M (SD) | Controls: M (SD) |
|---|---|---|---|
| Wason performance | |||
| Social contract | 0.46 (0.32)a* | 0.59 (0.24) | 0.67 (0.27) |
| Precaution | 0.44 (0.36)a*,b** | 0.73 (0.31) | 0.7 (0.26) |
| Descriptive | 0.12 (0.16)a****,b* | 0.26 (0.18)a* | 0.42 (0.25) |
| Differences of performance between conditions: t test | |||
| Social contract–descriptive | t(24) = 6.742**** | t(24) = 8.222**** | t(24) = 4.201**** |
| Precaution–descriptive | t(24) = 5.628**** | t(24) = 7.784**** | t(24) = 5.584**** |
| Social contract–precaution | t(24) = 0.569 | t(24) = −3.134** | t(24) = −0.663 |
aSignificant level compared to controls.
bSignificant level compared to depressives.
*p < .05. **p < .01. ***p = .001.
Main effects for rule domain
There were significant differences between SC, PRE, and DES: F(2, 144) = 86,997, p < .0001, η2 = .547. Pairwise comparisons showed that both SC (M = 0.57, SD = 0.29) and PRE (M = 0.62, SD = 0.33) elicited higher levels of correct performance than DES (M = 0.26, SD = 0.233), p < .0001, but did not differ between themselves.
Main effects for participant group (schizophrenia versus depression versus controls)
(see Figure 2) There were significant differences between groups: F(2, 72) = 8.710, p < .0001, η2 = .195. Pairwise comparisons showed that participants with schizophrenia (M = 0.34, SD = 0.25) were both worse than depressives (M = 0.52, SD = 0.21), p = .004, and controls (M = 0.59, SD = 0.21), p < .0001.
Figure 2.
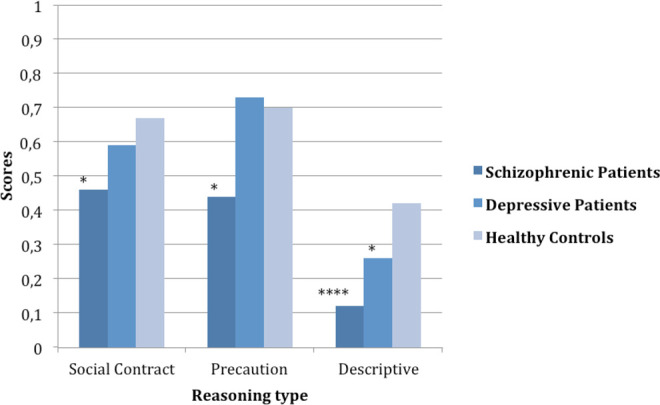
Wason scores according to groups. Significant differences between patient groups (schizophrenics and depressive) and controls: *p < .05, ****p < .0001.
Interaction Rule Domain × Group
Social contract
Differences between groups were significant: F(2, 72) = 3.49, p = .036, η2 = .088. Pairwise comparisons showed that participants with schizophrenia were worse than controls, p = .032.
Precautions
Differences between groups were significant: F(2, 72) = 6.53, p = .002, η2 = .154. Pairwise comparisons showed that participants with schizophrenia were worse than both controls (p = .013) and depressives (p = .005).
Descriptives
Differences between groups were significant: F(2, 72) = 14.007, p < .0001, η2 = .280. Pairwise comparisons showed that participants with schizophrenia performed worse than controls (p < .0001) and depressives (p = .038), while depressives performed worse than controls (p = .024).
There were no Pearson correlations within the schizophrenia group between total PANSS scores and the Wason scores with SC scores: r = −.018, p = .931; with PRE scores: r = −.259, p = .212; and with DES scores: r = −.83, p = .694.
Comparisons were made between the Wason scores in the schizophrenia group comparing positive drug antecedents patients (n = 9; means in percent correct for social contracts: .51; precautions: .56; and descriptives: .13) and negative drug antecedents patients (n = 16; means in percent correct for social contracts: .43; precautions: .37; and descriptives: .11). There were no significant differences in scores between these subgroups.
An analysis of covariance was conducted on the Wason Scores with rule domain as the within-subjects factor and group as the between-subject factor was computed with BDI, STAI A and B, and Mill Hill scores as covariates. The only covariate having a significant influence on the model was the Mill Hill Vocabulary Score. Therefore, we dropped the other variables and retained only Mill Hill scores in the model.
When the Mill Hill score was put as a covariate, the differences between groups were no longer significant for social contract scores, F(2, 71) = 1.053, p = .354, η2 = .029. Differences remained significant for the precaution score: F(2, 71) = 4.372, p = .016, η2 = .110. Pairwise comparisons showed that patients with schizophrenia had lower scores than depressive ones (p = .04).
Differences remained significant for the descriptive scores, F(2, 71) = 8.034, p = .001, η2 = .185. Pairwise comparisons showed that patients with schizophrenia had lower scores than depressive patients (p = .029) and controls (p < .0001), while depressive patients had lower scores than controls (p = .046).
Discussion
Schizophrenia is associated with difficulties in conditional reasoning compared to both depressives and controls. Our results demonstrate that while conditional reasoning is impaired in patients with schizophrenia, their performance improves substantially in specific contexts. Reasoning about descriptive rules was significantly impaired in schizophrenic and control patients compared to healthy controls; this difference remained after controlling for differences in verbal intelligence. Reasoning about social contracts and precautions was impaired in patients with schizophrenia, but not in depressives, compared to controls; however, the differences between patients with schizophrenia and controls disappeared for social contract reasoning but remained for precautionary reasoning after controlling for verbal intelligence.
Verbal intelligence as assessed here does not only reflect verbal comprehension but also requires access to memory and attentional skills. In a previous study of psychopathic subjects (Ermer & Kiehl, 2010), verbal IQ, measured with the Wechsler Adult Intelligence Scale (WAIS), was not responsible for the differences observed in conditional reasoning for social contract or precautionary reasoning, whereas in the present study, verbal IQ was correlated with appropriate social contract reasoning but not reasoning about precautions or descriptive rules. The same assessment of vocabulary scales (Mill Hill) was used in a study on conditional reasoning in alcoholics (Kornreich et al., 2011) and in polydrug abusers (Kornreich et al., 2012) and was not responsible for the differences observed in the Wason test between patients and controls. As verbal IQ reflects not only vocabulary extent but also attentional and memory functioning, further neuropsychological assessments, that is, memory, attention, and more generally executive functions, should be used in future studies to disentangle what kind of precise neuropsychological impairment is responsible here for conditional reasoning scores differences between patients with schizophrenia patients and controls.
More than a third of the patients in the schizophrenic group had drug abuse antecedents but this factor failed to yield different conditional reasoning results between subgroups, although the small size of subgroups warrant to look at the results with caution.
As depressive patients in our study show conditional reasoning difficulties only for descriptive situations, it demonstrates that this ability is not indifferently affected whatever the psychopathological condition. It is plausible although speculative to incriminate prefrontal impairments in the difficulties encountered here. Prefrontal dysfunction must probably be relatively severe to have an impact on conditional reasoning, a level usually not observed in depressive patients (Harvey, 2011). Schizophrenia is also associated with prefrontal dysfunctions, both at the orbitofrontal level with an influence on decision-making (Larquet, Coricelli, Opolczynski, & Thibaut, 2010) and at the dorsolateral level with an influence on working memory performances (Barch & Ceaser, 2011). Patients with schizophrenia show the “classical” improvement usually observed in the Wason task in social contract or precautions situations compared to purely descriptive ones. Nonetheless, conditional reasoning in these particular contexts is impaired, relative to healthy controls, and could have clinical implications: Patients with schizophrenia have difficulties in the social world (Cutting & Murphy, 1990). These difficulties may be linked to impairment in the perception of nonverbal clues and to motivation problems. Difficulties of reasoning in social contract conditions could add an obstacle as they could lead to misunderstandings in very common situations implicating the reciprocation of services.
Similarly, patients with schizophrenia are known to put themselves in risky situations (Brown, 1997; Saha, Chant, & McGrath, 2007). Again, a lack of motivation and difficulties of predicting the consequences of their deeds are probably at work. But a difficult to understand conditional reasoning relating to precautions situations could also contribute to a difficulty to avoid unnecessary risks.
Limitations
Our samples were relatively small and larger studies would be required to confirm our results. Our patients were taking medication and stabilized, but antipsychotic medication was not controlled for. Executive functioning was not controlled for and should be in future studies, as shifting abilities may interfere with conditional reasoning. Duration of illness was different between the schizophrenia group and the depressive groups. Years of living with mental illness, taking medications, and having limited social circles could have contributed to the results. Almost half of our patients had drug abuse antecedents and it would be useful to have patients devoid of any such antecedent although it didn’t make a difference in our results. Future studies could follow the longitudinal course of this kind of reasoning impairment and investigate the effects of disease stage and medication treatment on reasoning performance. Finally, it would have been interesting to measure whether social contract reasoning would correlate with other measures of social cognition, for example, theory of mind.
Conclusion
Conditional reasoning is impaired in patients with schizophrenia compared to depressive patients and to controls. However, reasoning results about social contract and precautionary rules are better than in purely descriptive situations, showing that evolutionary important mechanisms are still working in this population. Differences between groups disappeared for social contracts when verbal IQ was taken into account, which means that general abilities deficits explain at least part of the conditional reasoning difficulties observed in schizophrenic patients. These results suggest that evolved domain-specific reasoning mechanisms display resiliency in the context of brain network disruptions that impair more domain-general reasoning abilities. This resiliency is nonetheless not sufficient enough and schizophrenic patients could put themselves at risk, not reasoning well in precautionary contexts, and display difficulties moving themselves in the social world, as it requires a good comprehension of social contract situations.
Acknowledgment
We are thankful to Stéphanie Dubruille, Carole Gulbis, and Daphné Zoenen for their help in recruiting patients.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present research has been supported by the Laboratoire de Psychologie Médicale, Université Libre de Bruxelles.
References
- Austin M. P., Mitchell P., Goodwin G. M. (2001). Cognitive deficits in depression: Possible implications for functional neuropathology. The British Journal of Psychiatry, 178, 200–206. [DOI] [PubMed] [Google Scholar]
- Barch D. M., Ceaser A. (2011). Cognition in schizophrenia: Core psychological and neural mechanisms. Trends in Cognitive Sciences, 16, 27–34. Retrieved from 10.1016/j.tics.2011.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A. T., Steer R. A., Carbin M. G. (1988). Psychometric properties of the beck depression inventory: Twenty-five years of evaluation. Clinical Psychology Review, 8, 77–100. Retrieved from 10.1016/0272-7358(88)90050-5 [DOI] [Google Scholar]
- Brown S. (1997). Excess mortality of schizophrenia. A meta-analysis. The British Journal of Psychiatry, 171, 502–508. Retrieved from 10.1192/bjp.171.6.502 [DOI] [PubMed] [Google Scholar]
- Canessa N., Gorini A., Cappa S. F., Piattelli-Palmarini M., Danna M., Fazio F., Perani D. (2005). The effect of social content on deductive reasoning: an fMRI study. Human Brain Mapping, 26, 30–43. Retrieved from 10.1002/hbm.20114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P. W., Holyoak K. J. (1989). On the natural selection of reasoning theories. Cognition, 33, 285–313. Retrieved from 10.1016/0010-0277(89)90031-0 [DOI] [PubMed] [Google Scholar]
- Cosmides L. (1989). The logic of social exchange: Has natural selection shaped how humans reason? Studies with the Wason selection task. Cognition, 31, 187–276. [DOI] [PubMed] [Google Scholar]
- Cosmides L., Tooby J. (1992). Cognitive adaptation for social exchange. In Barkow L., Cosmides L., Tooby J. (Eds.), The adapted mind: Evolutionary psychology and the generation of culture. New York, NY: Oxford University Press. [Google Scholar]
- Cosmides L., Tooby J. (2005). Neurocognitive adaptations designed for social exchange. In Buss D. M. (Ed.), Handbook of evolutionary psychology (pp. 584–627). Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Cutting J., Murphy D. (1990). Impaired ability of schizophrenics, relative to manics or depressives, to appreciate social knowledge about their culture. The British Journal of Psychiatry, 157, 355–358. Retrieved from 10.1192/bjp.157.3.355 [DOI] [PubMed] [Google Scholar]
- Deltour J. J. (1993). Mill-Hill Vocabulary Scale of JC Raven. French adaptation and European norms (L’application des techniques modernes) (pp. 163–228). Braine Le Chateau, Belgium. [Google Scholar]
- Dickinson D., Ramsey M. E., Gold J. M. (2007). Overlooking the obvious: A meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Archives of General Psychiatry, 64, 532–542. Retrieved from 10.1001/archpsyc.64.5.532 [DOI] [PubMed] [Google Scholar]
- Ermer E., Guerin S. A., Cosmides L., Tooby J., Miller M. B. (2006). Theory of mind broad and narrow: Reasoning about social exchange engages ToM areas, precautionary reasoning does not. Social Neuroscience, 1, 196. [DOI] [PubMed] [Google Scholar]
- Ermer E., Kiehl K. A. (2010). Psychopaths are impaired in social exchange and precautionary reasoning. Psychological Science, 21, 1399–1405. Retrieved from 10.1177/0956797610384148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiddick L. (2011). There is more than the amygdala: Potential threat assessment in the cingulate cortex. Neuroscience & Biobehavioral Reviews, 35, 1007–1018. Retrieved from 10.1016/j.neubiorev.2010.09.014 [DOI] [PubMed] [Google Scholar]
- Fiddick L., Cosmides L., Tooby J. (2000). No interpretation without representation: The role of domain-specific representations and inferences in the Wason selection task. Cognition, 77, 1–79. [DOI] [PubMed] [Google Scholar]
- Fiddick L., Spampinato M. V., Grafman J. (2005). Social contracts and precautions activate different neurological systems: An fMRI investigation of deontic reasoning. NeuroImage, 28, 778–786. Retrieved from 10.1016/j.neuroimage.2005.05.033 [DOI] [PubMed] [Google Scholar]
- Finger E. C., Marsh A. A., Mitchell D. G., Reid M. E., Sims C., Budhani S.…Blair J. R. (2008). Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Archives of General Psychiatry, 65, 586–594. Retrieved from 10.1001/archpsyc.65.5.586 [DOI] [PMC free article] [PubMed]
- Harvey P. D. (2011). Mood symptoms, cognition, and everyday functioning: In major depression, bipolar disorder, and schizophrenia. Innovations in Clinical Neuroscience, 8, 14–18. [PMC free article] [PubMed] [Google Scholar]
- Kay S., Fiszbein A., Opler L. (1987). The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin, 13, 261–276. [DOI] [PubMed] [Google Scholar]
- Kornreich C., Delle-Vigne D., Campanella S., Noël X., Papageorgiou C., Brown O.…Ermer E. (2012). Conditional reasoning difficulties in polysubstance-dependent patients. Psychology of Addictive Behaviors, 26, 665–671. Retrieved from 10.1037/a0025841 [DOI] [PubMed]
- Kornreich C., Delle-Vigne D., Knittel J., Nerincx A., Campanella S., Noel X.…Ermer E. (2011). Impaired conditional reasoning in alcoholics: A negative impact on social interactions and risky behaviors? Addiction, 106, 951–959. Retrieved from 10.1111/j.1360-0443.2010.03346.x [DOI] [PMC free article] [PubMed]
- Larquet M., Coricelli G., Opolczynski G., Thibaut F. (2010). Impaired decision making in schizophrenia and orbitofrontal cortex lesion patients. Schizophrenia Research, 116, 266–273. [DOI] [PubMed] [Google Scholar]
- Manktelow K. I., Over D. E. (1991). Social roles and utilities in reasoning with deontic conditionals. Cognition, 39, 85–105. Retrieved fromhttp://doi.org/16/0010-0277(91)90039-7 [DOI] [PubMed] [Google Scholar]
- Moritz S., Woodward T. S., Hausmann D. (2006). Incautious reasoning as a pathogenetic factor for the development of psychotic symptoms in schizophrenia. Schizophrenia Bulletin, 32, 327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaksford M., Chater N. (1994). A rational analysis of the selection task as optimal data selection. Psychological Review, 101, 608–631. Retrieved from 10.1037/0033-295X.101.4.608 [DOI] [Google Scholar]
- Palmer B. W., Dawes S. E., Heaton R. K. (2009). What do we know about neuropsychological aspects of schizophrenia? Neuropsychology Review, 19, 365–384. Retrieved from 10.1007/s11065-009-9109-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis D. L., Brackett M. A., Shamosh N. A., Kiehl K. A., Salovey P., Gray J. R. (2007). Emotional intelligence predicts individual differences in social exchange reasoning. NeuroImage, 35, 1385–1391. Retrieved from 10.1016/j.neuroimage.2006.12.045 [DOI] [PubMed] [Google Scholar]
- Saha S., Chant D., McGrath J. (2007). A systematic review of mortality in schizophrenia: Is the differential mortality gap worsening over time? Archives of General Psychiatry, 64, 1123–1131. Retrieved from 10.1001/archpsyc.64.10.1123 [DOI] [PubMed] [Google Scholar]
- Sellen J. L., Oaksford M., Gray N. S. (2005). Schizotypy and conditional reasoning. Schizophrenia Bulletin, 31, 105–116. [DOI] [PubMed] [Google Scholar]
- Spielberger C. D., Gorsuch R. L., Lushene R. L., Vagg P. R., Jacobs G. A. (1983). Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Stone V. E., Cosmides L., Tooby J., Kroll N., Knight R. T. (2002). Selective impairment of reasoning about social exchange in a patient with bilateral limbic system damage. Proceedings of the National Academy of Sciences, 99, 11531–11536. Retrieved from 10.1073/pnas.122352699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uekermann J., Daum I. (2008). Social cognition in alcoholism: A link to prefrontal cortex dysfunction? Addiction, 103, 726–735. Retrieved from 10.1111/j.1360-0443.2008.02157.x [DOI] [PubMed] [Google Scholar]
- Verdejo-García A., Bechara A., Recknor E. C., Pérez-García M. (2006). Executive dysfunction in substance dependent individuals during drug use and abstinence: An examination of the behavioral, cognitive and emotional correlates of addiction. Journal of the International Neuropsychological Society, 12, 405–415. [DOI] [PubMed] [Google Scholar]
- Wason P. C. (1968). Reasoning about a rule. Quarterly Journal of Experimental Psychology, 20, 273–281. Retrieved from 10.1080/14640746808400161 [DOI] [PubMed] [Google Scholar]



