Abstract
Throughout evolutionary history, pathogens have imposed strong selection pressures on humans. To minimize humans’ exposure to pathogens, a behavioral immune system that promotes the detection and avoidance of disease-connoting cues has evolved. Although most pathogens cannot be discerned by our sensory organs, they produce discernable changes in their environment. As a result, a common denominator of many disease-connoting cues is morphological deviance—figurative disparity from what is normal, visual dissimilarity to the prototype stored in memory. Drawing on an evolutionary rationale, we examine the hypothesis that activation of the behavioral immune system renders people more sensitive to morphological deviance and more prone to perceive dissimilarities between stimuli. In Study 1 (N = 343), participants who scored higher on disgust sensitivity demonstrated greater differentiation between normal and disfigured faces, reflecting greater sensitivity to morphological deviance in the bodily domain. In Study 2 (N = 109), participants who were primed with pathogen threat demonstrated greater differentiation between perfect and imperfect geometrical shapes, reflecting greater sensitivity to morphological deviance even in stimuli that have nothing to do with health or disease. In Study 3 (N = 621), participants who scored higher on disgust sensitivity perceived pairs of neutral pictures as less similar (i.e., more dissimilar) to each other. Literature on the relations to social deviance and implications for social perception and for social behavior is discussed.
Keywords: behavioral immune system, deviance, dissimilarity, disgust sensitivity, disease salience, pathogen threat
Introduction
For humans to survive, they need to avoid pathogens. They need to stay clear of elements that pose a threat of physical contamination. In addition to the biological immune system, which protects the body against viruses, parasites, and bacteria, once these are physically encountered, various findings suggest the existence of a first protection line—a behavioral immune system—which helps individuals, from the start, to avoid pathogens altogether (Schaller, 2006, 2011, 2016; Schaller & Park, 2011). The behavioral immune system is assumed to consist of cognitive, affective, and behavioral processes that facilitate detection and avoidance of potential disease carriers (Neuberg, Kenrick, & Schaller, 2011; Schaller & Duncan, 2007; Schaller & Park, 2011). The system is assumed to be activated either temporarily by the presence of pathogen threat (Faulkner, Schaller, Park, & Duncan, 2004) or chronically in people who are, or who perceive themselves to be, vulnerable to disease (Navarrete, Fessler, & Eng, 2007).
Previous research suggests that activation of the behavioral immune system results in cognitive tuning to disease-connoting cues. When the behavioral immune system is activated, people’s attention to disease-connoting cues is heightened, their detection is facilitated (Miller & Manner, 2011), and disengagement from them is difficult (Ackerman et al., 2009). The cognitive component of the behavioral immune system sets in motion its affective and behavioral components. Specifically, once a stimulus is identified as contaminated, people whose behavioral immune system is activated show an increased tendency to experience disgust, an emotional response whose basic function is the expelling of contaminated food from entering an organism’s oral cavity (Fessler, Eng, & Navarrete, 2005; Oaten, Stevenson, & Case, 2009; Rozin, Haidt, & McCauley, 2008; Tybur, Lieberman, Kurzban, & DeScioli, 2013). They further show increased behavioral avoidance away from the contaminating stimuli (Miller & Maner, 2011; Mortensen, Becker, Ackerman, Neuberg, & Kenrick, 2010, Study 2).
Thus, identifying a stimulus as a source of pathogen threat is a first and critical step in defending oneself from the risks that it harbors. In contrast to the biological immune system, which forms a set of reactive processes that are activated after the pathogen invades the body, the behavioral immune system forms a set of preventive processes whose activation necessitates the identification of pathogens in the environment before they contact the body. How then is a stimulus identified as the potential carrier of pathogens? Which processing characteristics promote this process? The present research focuses on the cognitive underpinnings of locating disease-connoting cues and on their consequences for perception in general.
Detecting Disease-Connoting Cues
Most pathogens cannot be discerned by our sensory organs. Their detection presents a real challenge to our cognitive system. What can, however, be detected are the changes that they generate in their environment. Rotten meat looks and smells differently from fresh meat; inflamed skin is characterized by a rash and swelling that make it look different from healthy smooth skin; and contaminated lungs produce coughing sounds that are absent in healthy lungs. Indeed, contamination is consistently coupled in our experience with deviation from what is normal, dissimilarity from the prototype stored in memory and, indeed, from the known and the familiar (Schaller, 2016).
The current research focuses on the visual perception of disease-connoting cues. Many contagious diseases produce visible conspicuous physical features such as lesions, rashes, and swellings (Kurzban & Leary, 2001). Indeed, the mere visual perception of disease symptoms results in activation of both the biological and the behavioral immune systems (Faulkner et al., 2004; Schaller, Miller, Gervais, Yager, & Chen, 2010). The common denominator of most visual symptoms of pathogens is morphological deviance, figurative disparity from what is normal, visual dissimilarity to the prototype stored in memory. Thus, by and large, morphological deviance may serve as a signal for pathogen threat (for a related argument, see Ackerman, Tybur, & Mortensen, 2018; Kurzban & Leary, 2001; Miller & Maner, 2012).
We therefore assume that throughout human evolution, unique adaptive benefits have been associated with increased sensitivity to everything that is morphologically deviant and dissimilar when under pathogen threat. We propose that because the behavioral immune system is designed to help people avoid pathogens, its activation probably entails increased sensitivity to everything that is morphologically deviant and dissimilar, a built-in readiness to perceive morphological deviance and dissimilarity.
In line with this theorizing, people appear to heuristically associate benign physical abnormalities (extreme thinness, obesity, physical disabilities) with contagious disease (Park, Faulkner, & Schaller, 2003; Park, Schaller, & Crandall, 2007). Indeed, findings suggest that facial disfigurement, even when it is not associated with any contagious disease (e.g., crossed eyes), captures the attention of people whose behavioral immune system is activated and is quickly avoided by them (Miller & Maner, 2011). Furthermore, people whose behavioral immune system is either temporarily or chronically activated overperceive bodily deviations (such as obesity) in their environment. They show a bias toward categorizing others as bearing disease-connoting cues (Miller & Maner, 2012). In a related manner, they express greater concern about their own physical appearance and demonstrate stronger behavioral intentions to conceal or to improve imperfections in their appearance (Ackerman et al., 2018). Finally, disgust sensitivity, which is assumed to reflect activation of the behavioral immune system, correlates with traits that involve attention to detail and precision, such as conscientiousness and obsessive–compulsive tendencies (Tolin, Woods, & Abramovitch, 2006; Tybur & De Vries, 2013). Disgust sensitivity is further expressed by narrowing of the eyes, which was shown to improve acuity and discrimination between stimuli (Lee & Anderson, 2017; Lee, Mirza, Flanagan, & Anderson, 2014). Indeed, enhanced discrimination, attention to detail, and precision are prerequisites for the perception of dissimilarity (Förster & Dannenberg, 2010).
We begin by examining the hypothesis that activation of the behavioral immune system involves increased sensitivity to morphological deviance in the context of disease-connoting cues. We then turn to examine the possibility that activation of the behavioral immune system cognitively tunes people to perceive morphological deviance and differences even among completely neutral stimuli that have nothing to do with contamination.
The Present Research
Study 1 examined the hypothesis that activation of the behavioral immune system results in increased sensitivity to morphological deviance in the domain of physical disfigurement. Specifically, we tested whether participants whose behavioral immune system is chronically activated, as indicated by their chronic disgust sensitivity (Curtis, DeBarra, & Aunger, 2011; Oaten et al., 2009; Schaller, 2016) show greater differentiation between normal and disfigured faces. To examine whether people whose behavioral immune system is activated demonstrate biased processing of morphological deviance in domains that are not related to health and sickness, Study 2 explored the perceived deviance of neutral geometrical shapes. We tested whether people who are temporarily worried by disease threat show greater differentiation between perfect and imperfect geometrical shapes than people who are temporarily worried by threat posed by other physical dangers. To examine the hypothesis that activation of the behavioral immune system tunes our processing toward dissimilarities, in Study 3, we asked participants to rate the degree to which pairs of pictures were similar to each other. We expected that participants who are more sensitive to disgust would perceive pairs of pictures as less similar to each other than participants who are less sensitive to disgust.
Activation of the behavioral immune system is assumed to be higher in women (than in men; Curtis, Aunger, & Rabie, 2004; Duncan, Schaller, & Park, 2009; Haidt, McCauley, & Rozin, 1994; Rozin, Haidt, McCauley, Dunlop, & Ashmore, 1999), and in people who were recently ill (than in people who were not recently ill; Miller & Maner, 2011). We thus controlled for these variables in all of our studies. Because disgust sensitivity is associated with degree of religiosity (Berger & Anaki, 2014; Haidt et al., 1994; Inozu, Ulukut, Ergun, & Alcolado, 2014; Olatunji, Tolin, Huppert, & Lohr, 2005), we further measured and controlled for the participants’ degree of religiosity in Studies 1 and 3 (in which activation of the behavioral immune system was indicated by disgust sensitivity).
Study 1
People vary with respect to their predisposition to experience disgust. It is assumed that a tendency to experience disgust reflects chronic activation of the behavioral immune system (Oaten et al., 2009). In line with this theorizing, both factors of the Perceived Vulnerability to Disease Scale (PVD; Duncan et al., 2009), Perceived Infectability (e.g., “If an illness is ‘going around,’ I will get it”) and Germ Aversion, correlate positively with the Disgust Scale–Revised (DS-R; Duncan et al., 2009; Olatunji et al., 2007). Furthermore, various findings suggest that periods characterized by suppression of the biological immune system (e.g., the first trimester of pregnancy and the luteal phase of the menstrual cycle) are also characterized by increased sensitivity to disgust (Conway et al., 2007; Fessler et al., 2005; Fessler & Navarrete, 2003; Fleischman, 2014; Fleischman & Fessler, 2011).
In Study 1, we sought to examine the hypothesis that activation of the behavioral immune system results in increased sensitivity to morphological deviance as it is reflected in physical disfigurement. Specifically, we tested whether participants who are chronically prone to experience disgust perceive greater morphological deviance of disfigured faces when compared to normal faces. We measured participants’ disgust sensitivity and asked them to rate how regular or irregular they perceived each of a series of faces to be. Some of the faces were normal, and some were disfigured. We expected a positive association between participants’ score on the DS-R and the differentiation of disfigured faces from normal faces.
Method
Participants
G*Power software was employed to determine sample size. The analysis (one-tailed) suggested that in order to attain a power of 80% in detecting a small-to-medium effect (|ρ| = .15), we should recruit 270 participants. Because the study was conducted online, we expected “noisy” running conditions and therefore recruited a larger sample of 346 Israeli students (173 females, Mage = 26.26, SD= 3.63, age range: 18–35). Three participants who rated disfigured faces as more regular than normal faces were excluded from the analysis. Participants provided written informed consent for their participation in the study.
Materials and Procedure
The experiment was introduced to participants as a study on “intuitive information processing.” First, participants completed the Disgust Scale-Revised (DS-R; Haidt et al., 1994, modified by Olatunji et al., 2007) which was presented as a measure pertaining to “information processing in everyday life.” Next, they rated the regularity of the faces in a task entitled “intuitive processing of visual information.” Finally, participants reported about how recently they had had a cold, about their degree of religiosity, and demographics.
Disgust Scale-Revised (DS-R)
The scale consists of 25 items assessing sensitivity to a range of disgust elicitors including core, animal reminder, and contamination disgust (see Rozin et al., 1999). Scale items are divided into two sets. In the first 13-item set, participants are asked to indicate their agreement with given statements (e.g., “It bothers me to hear someone clear their throat full of mucus”) on a 5-point Likert-type scale ranging from 0 (strongly disagree) to 4 (strongly agree). In the other 12-item set, respondents are asked to rate how disgusting they find the described experiences (e.g., “You are walking barefoot on concrete, and you step on an earthworm”) on the same scale except for the anchor labeling (0 = not disgusting at all to 4 = extremely disgusting). We used the Hebrew version of the scale translated by Berger and Anaki (2014). The scale’s construct and external validity were confirmed in a heterogeneous Israeli sample. Like the translators, we omitted 2 items due to religious considerations. Cronbach’s α in our sample was .89.
Perceived regularity of faces
Participants were presented with 18 pictures of male and female faces borrowed from Ackerman et al. (2009). Twelve were disfigured (six with strabismus and six with a port-wine stain) and six were normal. The participants’ task was to quickly and intuitively indicate how regular each face is on a scale from 1 (very irregular) to 9 (very regular). On each trial, a picture of a face was first presented, complemented after 3 s by the regularity scale, which was presented underneath it. Both remained on the screen until participants provided their ratings. The pictures were presented in a fixed order. Ratings were averaged to form an index of normal face ratings (α = .86; M = 8.06, SD = 1.02) and disfigured face ratings (α = .96; M = 4.86, SD = 1.66). Our primary dependent measure capturing face differentiation comprised the unstandardized residuals obtained by regressing disfigured faces ratings onto normal faces ratings. For ease of interpretation, the valence of these residuals was reversed so that higher numbers indicate greater differentiation between normal and disfigured faces (i.e., a greater sensitivity to deviance; see Okimoto & Gromet, 2015).
Illness recency and religiosity
Following Miller and Maner (2011), participants indicated the last time they had suffered from a cold by selecting from among the following response options: 1—today, 2—a couple days ago, 3—a week ago, 4—a couple weeks ago, 5—a month ago, 6—a few months ago, and 7—a year or more ago. Finally, participants further indicated how religious they are on a scale from 0—not at all religious to 7—very religious. Except for demographics, no other variables were measured or manipulated. This is true for all the studies.
The ethics committee of the Department of Education and Psychology at the Open University of Israel approved the study (Approval no. 3058).
Results and Discussion
For zero-order correlations among all continuous variables in the study, see Table 1.
Table 1.
Zero-Order Correlations Between Variables Measured in Study 1.
| Measure | 2. | 3. | 4. | 5. | 6. | 7. |
|---|---|---|---|---|---|---|
| 1. DS-R | .109* | −.064 | .047 | .197** | −.113* | −.020 |
| 2. Faces differentiation | −.793** | .100 | −.066 | −.032 | −.017 | |
| 3. Perceived regularity of disfigured faces | .527** | .028 | .042 | −.027 | ||
| 4. Perceived regularity of normal faces | −.047 | .025 | −.068 | |||
| 5. Religiosity | −.144** | .023 | ||||
| 6. Age | .107* | |||||
| 7. Illness recency (single item) |
Note. N = 343.
*p < .05. **p < .01.
We first regressed participants’ face differentiation scores onto their disgust sensitivity scores. As expected, the more disgust-sensitive the participants were, the more they differentiated disfigured from normal faces (β = .10, t = 1.94, p = .05, 95% CI for b [−0.003, 0.45]). The observed association between disgust sensitivity and face differentiation was thus relatively small. We next examined a broader model in which disgust sensitivity, participants’ gender, degree of religiosity, illness recency, and their respective interactions with disgust were entered as predictors in a multiple regression. A significant, stronger, main effect of disgust sensitivity emerged (β = .22, t = 2.69, p = .01, 95% CI for b [0.13, 0.81]), along with an unexpected effect of gender (β = −.18, t = −3.13, p = .002, 95% CI for b [−0.83, −0.19]), such that men tended to differentiate faces (M = 0.15, SD = 1.43) more than women (M = −0.15, SD = 1.38). No other effect was found (see Table S1 in Supplementary Material).
We reran the above analyses, this time using difference scores (obtained by subtracting average regularity ratings of disfigured faces from average regularity ratings of normal faces) as the dependent variable. The analyses yielded highly similar results. Disgust sensitivity predicted face differentiation positively, both when it served as a single predictor in the model (β = .11, t = 2.02, p < .05, 95% CI for b [0.01, 0.46]) and when gender, religiosity, illness recency, and the two-way interaction terms with disgust were additionally entered (β = .20, t = 2.48, p = .01, 95% CI for b [0.09, 0.78]). Here again, a main effect of gender was obtained (β = −.17, t = −2.87, p = .004, 95% CI for b [−0.80, −0.15]) while no other variable was significant. Thus, the more sensitive the participant was to disgust, the greater her or his differentiation between disfigured and normal faces in terms of their regularity, suggesting higher sensitivity to morphological deviance. Since disfigured faces might be considered disgusting, one could wonder whether people highly sensitive to disgust only differentiate disgusting (disfigured faces) from nondisgusting (normal faces) stimuli. The next studies aim to examine whether activation of the behavioral immune system increases sensitivity to morphological deviance even in completely neutral stimuli that have nothing to do with illness, health, or disgust.
Study 2
In Study 2, we aimed to examine the hypothesis that activation of the behavioral immune system results in increased sensitivity to morphological deviance per se, even when it manifests itself in neutral domains that are not related to health or sickness. To temporarily activate the behavioral immune system, half of our participants were primed with the notion of pathogen threat, whereas participants in the control condition were primed with threats posed by other physical dangers. Participants were presented with a series of perfect and imperfect geometrical figures and were asked to identify to what extent they were triangles/circles/squares, and so on (Okimoto & Gromet, 2015). We hypothesized that participants in the pathogen threat condition would demonstrate greater differentiation between perfect and imperfect shapes, indicating greater sensitivity to deviance.
Method
Participants
Sample size computation (one-tailed) aimed at having 80% power to detect a medium effect 1 (d = .5) suggested that 102 participants were required. One hundred and nine students (67 females, Mage = 26.11, SD = 3.95, age range: 21–40) participated. Most were students of Tel Aviv University, and the rest were students of other academic institutions in the greater Tel Aviv area. Compensation for participation was 20 NIS. Participants were randomly allocated to either one of two priming conditions. Participants provided written informed consent for their participation in the study.
Materials and Procedure
The experiment was introduced to participants as a study on “intuitive information processing.” First, participants were either primed or not primed with disease threat in a task entitled “intuitive processing of visual information.” Next, they rated the regularity of geometrical shapes. Finally, participants reported on their current mood, how recently they had had a cold, and their demographics.
Disease salience manipulation
We adopted a manipulation developed by Faulkner, Schaller, Park, and Duncan (2004), which is widely used for priming disease threat. Participants viewed either one of two slide shows; each consists of 10 pictures that would ostensibly be used in a health education program (disease condition) or in a safety education program (accidents condition). The pictures for the disease condition were designed to remind participants of the various ways in which diseases are transmitted (e.g., one slide depicts a strand of hair surrounded by bacteria and is labeled “Hair Bacteria. A microscopic view of a strand of hair and some of the typical bacteria that surround it.” Captions were translated into Hebrew). The pictures for the accidents condition were designed to render physical dangers, unrelated to diseases, especially salient (e.g., a man walking toward an open pit while reading a newspaper. The picture is labeled: “Look where you’re going.”). After looking at each picture, participants were asked to indicate to what extent the picture would be effective if used in a health education program (in the disease condition) or in a safety education program (in the accidents condition) on a 10-point Scale. The participants then looked at the pictures once again. This time they did not rate the pictures but were asked to describe their overall impression of the slide show.
Perceived regularity of geometrical shapes
In a task borrowed from Okimoto and Gromet (2015, Study 2), participants were presented with a series of 45 geometrical shapes. Fifteen were perfect shapes, 15 were ambiguously imperfect, and 15 were clearly imperfect (see Appendix). With respect to each shape, participants were asked “Is this a square?” (or depending on the figure, a circle, square, oval, or rectangle). Participants indicated their ratings on a scale from 1 = definitely not to 6 = definitely yes. Ratings were averaged to form an index of perfect (α = .61; M = 5.97, SD = .08), ambiguously imperfect (α = .96; M = 2.27, SD = 1.24), and clearly imperfect (α = .85; M = 1.55, SD = 0.61) shape ratings.
Mood and illness recency
To control for possible affective differences between the two conditions, participants were asked to indicate their current mood on a scale from 1 = very bad to 11 = very good. They were further asked to indicate the last time they had suffered from a cold (see Study 1).
For exploratory reasons, we also measured participants’ personal values with the short Schwartz’s value survey (Roccas, Sagiv, & Navon, 2017). Initial analyses indicated that participants’ values did not correlate with the DV (shape differentiation) and did not moderate the effect of the experimental condition on the DV, and thus were not included in the report.
The ethics committee of the Department of Education and Psychology at the Open University of Israel approved the study (Approval no. 2996).
Results and Discussion
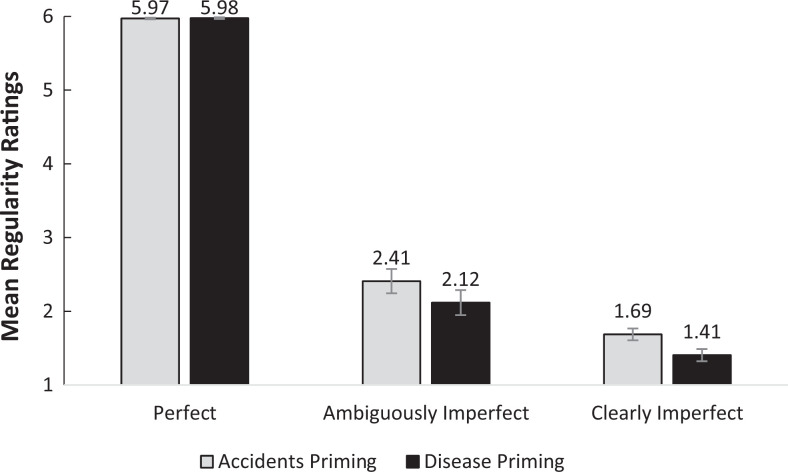
We first conducted a 2 (priming condition: accidents vs. disease) × 3 (shape type: perfect vs. ambiguously imperfect vs. clearly imperfect shapes) mixed analysis of variance (ANOVA) in which priming condition served a between-subject factor, and shape type was a within-subject factor. The analysis revealed that the sphericity assumption was violated (Mauchly’s W = .44, χ2(2) = 68.60, p < .001) with Greenhouse-Geisser’s Epsilon smaller than 0.75 (∊ = .64). Following the recommendation by Field (2013) and by Howell (2002), we turned to examine the multivariate tests. 2 The analysis indicated a significant Priming Condition × Shape Type interaction, F(2, 106) = 3.23, p = .04, η2p = .06, suggesting that regularity ratings of the different types of shapes differed between the experimental conditions. Delving into the interaction, we examined the effect of priming conditions on the differentiation of clearly and ambiguously imperfect shapes separately using two mixed ANOVAs. In the first, we aimed to explore the effect of priming condition on the differentiation of clearly imperfect shapes from perfect shapes. As expected, a significant Priming Condition × Shape Type interaction emerged, F(1, 107) = 5.94, p = .02, η2p = .05, such that the effect of shape type was larger in the disease priming condition, t(52) = 61.27, p < .0001, mean difference = 4.57, 95% CI [4.42, 4.72], than in the accidents priming condition, t(55) = 47.61, p < .0001, mean difference = 4.28, 95% CI [4.10, 4.46]. This interaction suggests a greater differentiation of clearly imperfect shapes in the disease priming condition than in the accident condition. In the second mixed ANOVA, investigating the interaction between the priming condition and the differentiation of ambiguously imperfect shapes from prefect shapes, no interaction emerged, F(1, 107) = 1.55, p = .22, η2p = .01, suggesting that the differentiation of the ambiguously imperfect shapes was not reliably larger in the disease priming condition than in the accidents priming condition (see Figure 1).
Figure 1.
Regularity ratings as a function of priming condition and shape type. Error bars represent 1 standard error (study 2).
To explore whether our disease priming effect persists above and beyond the effects of demographic variables, we reconducted the above three mixed ANOVAs while controlling for mood, gender, illness recency, and their interactions with the experimental condition (i.e., we carried out three analysis of covariance). As in the first analyses, a 2 (priming condition: accidents vs. disease) × 3 (shape type: perfect vs. ambiguously imperfect vs. clearly imperfect shapes) interaction emerged, F(2, 100) = 3.20, p < .05, η2p = .06. Furthermore, when analyzing the differentiation of clearly and ambiguously imperfect shapes individually, the expected Priming Condition × Shape Type interaction was obtained for clearly imperfect shapes, F(1, 101) = 6.22, p = .01, η2p = .06, but not for the ambiguously imperfect ones, F(1, 101) = 2.16, p = .14, η2p = .02, and no additional effects were found.
Thus, when primed with the notion of pathogen threat, participants showed greater differentiation between clearly imperfect shapes and perfect shapes in terms of their regularity, suggesting higher sensitivity to morphological deviance even in completely neutral stimuli. That the effect of activation of the behavioral immune system was observed only for the clearly imperfect shapes but not for the ambiguously imperfect shapes may suggest that perceived deviance is magnified mainly when there is enough objective deviance or dissimilarity. We next turn to examine whether activation of the behavioral immune system renders the cognitive system more tuned to dissimilarities.
Study 3
Tolstoy (1875/1998) noted that All happy families are similar to each other, every unhappy family is miserable in its own way (p. 1). Similarly, all regular stimuli resemble one another; every irregular stimulus is deviant in its own way. This suggests that the tuning of the cognitive system to perceive morphological deviance goes hand in hand with a tuning to perceive dissimilarities.
Indeed, underlying the perception of morphological deviance is the perception of figurative disparity from what is normal or the perception of the target object as dissimilar to the prototype stored in one’s memory. Ample evidence suggests that when comparing objects, the cognitive system may either be tuned to perceive similarities or to perceive dissimilarities and that motivational factors affect this tuning (Gentner & Markman, 1994, 1997; Mussweiler, 2003). We propose that in order to allow for a quick, discerning detection of the morphological deviance characterizing visual disease symptoms, activation of the behavioral immune system results in a tuning to perceive dissimilarities. This biases the cognitive system to quickly locate stimuli that deviate from their prototype and, indeed, from most other stimuli of their kind (Johns & Mewhort, 2002; Mewhort & Johns, 2000; Stewart & Brown, 2005). Such a tuning to the processing of dissimilarities under activation of the behavioral immune system should affect the perceived (dis)similarity of neutral visual stimuli, ones that are not necessarily related to health or sickness.
Study 3a
In Study 3a, we thus tested the hypothesis that when the behavioral immune system is activated, the cognitive system is biased toward processing dissimilarities, such that people are prone to perceive neutral stimuli as more dissimilar. To examine this hypothesis, we measured participants’ disgust sensitivity. We then presented participants with pairs of pictures and had them rate the degree to which pictures in a pair were similar to each other (Nussinson, Seibt, Häfner, & Strack, 2011). The pictures in half the pairs bore some degree of similarity to each other (“related pairs”), whereas the pictures in the rest of the pairs did not bear such similarity (“unrelated pairs”). We expected participants’ score on the DS-R to correlate negatively with the perceived similarity of the pairs of pictures.
Method
Participants
G*Power calculation indicated that in order to achieve a power of 80% in detecting a small-to-medium effect (|ρ| = .15) in a one-directional test, we should recruit 270 participants. The sample consisted of 300 Israeli students recruited by an online survey platform, Hamidgam (152 females, Mage = 26.41, SD = 3.30, age range: 19–35). Participants provided written informed consent for their participation in the study.
Materials and Procedure
The procedure was identical to that of Study 1 except that instead of rating the regularity of faces, participants rated the perceived similarity of pairs of pictures.
Disgust Scale-Revised (DS-R)
See Study 1. Cronbach’s α was .89.
Perceived similarity of pictures
To assess perceived similarity between objects, we adopted the task used by Nussinson et al. (2011). Thirty-six picture pairs depicting landscapes, objects, people, and animals were presented. The pictures in half the pairs (related pairs) bore some degree of similarity to each other (e.g., one picture depicting the profiles of two old people facing each other and the other depicting two old boots facing each other; α = .92, M = 5.69, SD = 1.68). The pictures in the rest of the pairs (unrelated pairs) did not bear such similarity (e.g., one picture depicted two colorful parrots while the other picture was a childish drawing of a girl; α = .91, M = 1.88, SD = 1.04). Participants were instructed to quickly and intuitively indicate how similar the two pictures were. Two thirds of the participants indicated their ratings on a scale from 1 = not at all similar to 11 = very similar, and one third of the participants indicated their ratings on a scale from 1 = very similar to 11 = not at all similar. On each trial, a pair of pictures was presented for 3 s and then a similarity scale appeared underneath the pictures. The pair of pictures remained on the screen until the participants provided their response. Picture pairs were presented in a fixed order. No more than three related or unrelated pairs were displayed successively.
Illness recency and religiosity
We used the same measures used in Study 1. For exploratory reasons, we also included a second measure of illness recency (borrowed from Miller & Maner, 2011) in which participants indicated their agreement with four statements relating to a recent illness (e.g., “Over the past couple days, I have not been feeling well.”). Responses were provided on a 1 strongly disagree to 7 strongly agree Likert-type scale (Cronbach’s α = .84).
The ethics committee of the Department of Education and Psychology at the Open University of Israel approved the study (Approval no. 2995).
Results
Zero-order correlations between variables measured in Study 3a are reported in Table 2.
Table 2.
Zero-Order Correlations Between Variables Measured in Study 3.
| Measure | Study | 2. | 3. | 4. | 5. | 6. | 7. |
|---|---|---|---|---|---|---|---|
| 1. DS-R | a | −.116* | −.048 | .361** | −.095 | −.124* | .129* |
| b | −.053 | −.123* | .251** | −.001 | −.155** | ||
| 2. Perceived similarity of dissimilar objects | a | .602** | .048 | −.071 | .020 | .043 | |
| b | .446** | .152** | −.016 | −.069 | |||
| 3. Perceived similarity of similar objects | a | .019 | −.087 | −.029 | .078 | ||
| b | .036 | −.042 | −.120* | ||||
| 4. Religiosity | a | −.236** | −.076 | .012 | |||
| b | .191** | −.100 | |||||
| 5. Age | a | .086 | .033 | ||||
| b | .107 | ||||||
| 6. Illness recency (single item) | a | −.614** | |||||
| 7. Illness recency (4 items) |
Note. NStudy 3a = 300, NStudy 3b = 321. Four-item measure of illness recency was administered in Study 3a only.
*p < .05. **p < .01.
Moderation regression analyses indicated that scale direction did not interact with disgust sensitivity in predicting the perceived similarity of related and unrelated pairs of pictures (|ts| < 1). We therefore report our analyses across scale direction. To examine our hypothesis, we regressed perceived similarity ratings onto participants’ disgust sensitivity separately for related and unrelated picture pairs. As hypothesized, higher disgust sensitivity predicted lower similarity ratings of unrelated picture pairs (β = −.12, t = −2.02, p < .05, 95% CI for b [−0.34, −0.004]). However, this had no effect on perceived similarity between related pairs of pictures (β = −.05, t = −0.83, p = .41, 95% CI for b [−0.39, 0.16]). We further conducted separate multiple regressions, entering disgust sensitivity, gender, degree of religiosity, illness recency (both the single- and four-item measures), and the two-way interactions with disgust as the predictors, and perceived similarity of unrelated and related picture pairs as the dependent variables. The null finding for the related picture pairs remained unchanged (β = −.02, t = −0.20, p = .85, 95% CI for b [−0.51, 0.42]). For the unrelated pairs, neither of the controlled variables had a reliable effect, but including them in the regression equation turned the effect of disgust sensitivity nonsignificant (β = −.14, t = −1.38, p = .17, 95% CI for b [−0.49, 0.09]). The disgust sensitivity effect reemerged when the interactions were not included in the regression (β = −.15, t = −2.18, p = .03, 95% CI for b [−0.43, −0.02]). See Table S2 in Supplementary Material for full results.
Study 3b
In Study 3b, we sought to increase our confidence in the results of Study 3a by replicating them in an independent sample drawn from a different population and with a different set of pairs of pictures.
Method
Participants
The sample consisted of 321 American and British adults recruited by an online survey platform, Prolific (247 females, Mage = 26.02, SD = 6.07, age range: 18–41). Here again, participants provided written informed consent for their participation in the study.
Materials and Procedure
The procedure was identical to that of Study 3a with two small modifications detailed below:
Disgust Scale-Revised (DS-R)
See Study 1. Cronbach’s α was .86.
Perceived similarity of pictures
Here again, participants rated the perceived similarity of pairs of pictures, half of them (related pairs) bearing some degree of similarity to each other (α = .89, M = 6.14, SD = 1.36) while the rest (unrelated pairs) not bearing such similarity (α = .92, M = 1.64, SD = 0.85). All participants indicated their similarity ratings on a scale from 1 = not at all similar to 11 = very similar.
Illness recency and religiosity
To assess illness recency, we used only the first, single-item measure used in Study 3a (as well as in Studies 1 and 2). To assess religiosity, we used the same measure used in Study 3a (and in Study 1).
The ethics committee of the Department of Education and Psychology at the Open University of Israel approved the study (Approval no. 2995).
Results
Zero-order correlations between variables measured in Study 3b are reported in Table 2.
Here again, we regressed perceived similarity ratings onto participants’ disgust sensitivity separately for related and unrelated picture pairs. As hypothesized, higher disgust sensitivity predicted lower similarity ratings of related picture pairs (β = −.12, t = −2.22, p = .03, 95% CI for b [−.51, −.03]). However, it had no effect on perceived similarity between unrelated pairs of pictures (β = −.05, t = −0.95, p = .34, 95% CI for b [−0.22, 0.08]). We further conducted separate multiple regressions, entering disgust sensitivity, gender, degree of religiosity, illness recency, and the two-way interactions with disgust as the predictors, and perceived similarity of related and unrelated picture pairs as the dependent variables. Disgust sensitivity had a significant negative effect on related pairs ratings (β = −.28, t = −2.27, p = .02, 95% CI for b [−1.16, −0.08]) but not on unrelated pairs ratings (β = −.08, t = −0.63, p = .53, 95% CI for b [−0.44, 0.23]). The analyses further yielded several unexpected main effects and interactions, with religiosity positively predicting the perceived similarity of unrelated pictures (β = .21, t = 3.60, p < .001, 95% CI for b [.04, .14]), illness recency negatively predicting the perceived similarity of related pairs (β = −.15, t = −2.57, p = .01, 95% CI for b [−.24, −.03]), and the interaction between disgust sensitivity and religiosity predicting perceived similarity of both related and unrelated picture pairs (related: β = −.12, t = −2.02, p = .04, 95% CI for b [−.30, −.004], unrelated: β = −.16, t = −2.67, p = .01, 95% CI for b [−.21, −.03]). Specifically, disgust sensitivity predicted similarity perceptions among participants relatively high on religiosity (i.e., 1 SD above the sample’s mean, related: β = −.36, t = −3.54, p < .001, unrelated: β = −.21, t = −3.36, p < .001) while not affecting those relatively low on religiosity (i.e., 1 SD below the mean, related: β = −.06, t = −0.59, p = .56, unrelated: β = .02, t = 0.36, p = .72). The full regression results and a figure presenting the interaction graphically are provided in the Supplementary Materials (see Table S3 and Figure S1).
The negative effect of illness recency on similarity perception can be easily interpreted in light of research showing that recently ill humans exhibit hyperactivity of the behavioral immune system (Miller & Maner, 2011). However, as the main effects and interactions found did not replicate across the studies, it is not clear whether they reflect a robust influence on perceived similarity.
Discussion
That in Study 3a, the expected correlation between perceived similarity and disgust sensitivity was obtained for the unrelated pairs, whereas in Study 3b, it was obtained for the related pairs may reflect differences between the two different sets of stimuli used in the two studies. Indeed, the direction of the correlation between disgust sensitivity and perceived similarity was negative in both studies for both pair types.
Analyzing the data across the two studies, we regressed perceived similarity onto participants’ disgust sensitivity while controlling for study (dummy-coded “0” for Study 1 and “1” for Study 2). As hypothesized, higher disgust sensitivity predicted lower similarity ratings of both related pictures pairs (β = −.08, t = −2.01, p < .05, 95% CI for b [−.37, −.005]) and unrelated pictures pairs (β = −.09, t = −2.21, p = .03, 95% CI for b [−.24, −.01]). Furthermore, when the perceived similarity of picture pairs in general (across pair type) was entered as the dependent variable, it was, as hypothesized, negatively predicted by disgust sensitivity (β = −.10, t = −2.37, p = .02, 95% CI for b [−.29, −.03]).
Thus, the more sensitive the participants were to disgust, the more prone they were to perceive picture pairs as dissimilar, suggesting that activation of the behavioral immune system renders people more sensitive to dissimilarities even between neutral stimuli.
General Discussion
In a series of three studies, we examined the hypothesis that activation of the behavioral immune system renders people more sensitive to morphological deviance and more prone to perceive dissimilarities between stimuli. In Study 1, participants who scored higher on disgust sensitivity demonstrated greater differentiation between normal and disfigured faces, reflecting greater sensitivity to morphological deviance in the physical domain. In Study 2, participants who were primed with pathogen threat demonstrated greater differentiation between perfect and clearly imperfect shapes, reflecting greater sensitivity to morphological deviance even in stimuli that have nothing to do with health or disease. In Study 3, participants who scored higher on disgust sensitivity perceived pairs of pictures as less similar (i.e., more dissimilar) to each other.
Previous studies focusing on the affective and behavioral components of the behavioral immune system have shown that activation of the system results in stronger disgust responses (Fessler et al., 2005) and in increased avoidance to disease-connoting cues (Kurzban & Leary, 2001). More recent findings, focusing on the cognitive component of the system, suggest that its activation results in heightened attention to disease-connoting cues (Ackerman et al., 2009; Miller & Maner, 2011) and in an increased tendency to perceive them in the environment (Miller & Maner, 2012). Our findings contribute to this literature in several ways. First, they suggest that participants whose behavioral immune system is activated experience the deviation of disfigured faces as larger than participants whose behavioral immune system is not activated. Second, they suggest that activation of the behavioral immune system exacerbates the perception of morphological deviance in general, not only that related to disease symptoms. Finally, our results suggest that activation of the behavioral immune system results in an increased tendency to perceive dissimilarities even between neutral stimuli that are not related to health and disease.
Additional Theoretical Perspectives and Underlying Mechanisms
One question that arises is whether our results reflect the effects of general threat or, as we suggest, those of specific tuning. General threat and negative affective states, in general, are associated with the use of narrower categories (Isen & Daubman, 1984; Murray, Sujan, Hirt, & Sujan, 1990). A direct consequence of the use of narrower categories might be the perception of greater morphological deviance in the environment. We believe that our results reflect a unique effect of activation of the behavioral immune system as the effect of pathogen threat on perceived deviance of neutral stimuli (in Study 2) was obtained even in comparison to the threat posed by other physical dangers, and even though the affective state of the participants who were primed with physical dangers was slightly more negative. Although our findings cannot rule out the possibility that at least part of the effect of activation of the behavioral immune system on tuning to morphological deviance is driven by the effects of general threat on category breadth, they do suggest the existence of a specific functional tuning to morphological deviance under pathogen threat (see also Neuberg et al., 2011).
Secondly, disgust sensitivity is known to be positively correlated with intolerance for ambiguity, defined as “the tendency to perceive ambiguous situations as a source of threat” (Budner, 1962; Robinson, Xu, & Plaks, 2017; Rozin & Royzman, 2001; Terrizzi & Goodman, 2018). A morphologically deviant stimulus is not completely the same as the prototype constitutes. Thus, in a sense morphologically deviant stimuli are ambiguous stimuli, rendering people who are intolerant for ambiguity effectively sensitive to morphological deviance. While our results may go hand in hand with a general intolerance for ambiguity, we do not believe that they are best framed in these terms. First, whereas Studies 1 and 3 are of correlational design, and thus cannot exclude the possibility that related individual differences (such as intolerance for ambiguity) contribute to our effects, the results of Study 2, in which we experimentally manipulated pathogen threat suggest that our effects hold even when individual differences vary randomly across conditions. Second, whereas the derivation of the hypothesis of focus on dissimilarity (Study 3) from sensitivity to deviance is relatively straightforward, its derivation from intolerance for ambiguity is not easy. Indeed, there is no evidence in the literature for an association between intolerance for ambiguity and the perception of similarity. Finally, religiosity is known to be positively correlated with intolerance for ambiguity (Hassan & Khalique, 1981). Thus, if our dependent variables predominantly reflect intolerance for ambiguity, one might expect religiosity to correlate with our dependent variables. However, religiosity did not correlate with the differentiation of disfigured faces from normal faces (Study 1, see Table 1), and it either did not correlate or correlated positively with the similarity ratings of the pairs of pictures (Study 3b, see Table S3). Still, future research examining the effect of activation of the behavioral immune system on the perception of morphological deviance and dissimilarity may want to examine the contribution of individual differences in intolerance for ambiguity to this effect.
Finally, disgust sensitivity correlates with obsessive–compulsive tendencies and with traits that involve attention to detail and precision, such as conscientiousness (Tybur & De Vries, 2013; Tolin et al., 2006). Attention to detail is assumed to promote the perception of dissimilarity (Förster & Dannenberg, 2010). The question arises as to whether our results reflect not so much sensitivity to deviance and dissimilarity but rather individual differences in attention to detail.
Although we cannot exclude the possibility that individual differences in attention to detail have contributed to our effects, the results of Study 2 in which we experimentally manipulated pathogen threat suggest that our effects hold even when individual differences in attention to detail vary randomly across conditions. Yet future research focusing on the effect of activation of the behavioral immune system on sensitivity to deviance and dissimilarity would have to control for individual differences in attention to detail and the need to be precise.
Above, we delineated relatively general cognitive characteristics which may underlie our effects. Alternatively, our results may reflect more specific cognitive processes dedicated uniquely toward tuning to deviance and dissimilarity. We elaborate on them below.
Future Research
Identifying a stimulus as morphologically deviant from a category or dissimilar to it is the end product of a categorization process. Categorization usually takes place automatically. It is assumed to involve a first stage in which the stimulus is compared to a category (or to its prototype) in memory. An attempt is made to match the features of the stimulus to those of a potentially matching category. Next, in a second stage, a decision is made as to whether there is enough overlap between the features of the stimulus and of the category to allow for its categorization as a member of the category (Bruner, 1959; Moskowitz, 2005). Our findings are silent as to which of these stages are affected by activation of the behavioral immune system. Could it be that activation of the behavioral immune system affects the comparison process, such that under pathogen threat the cognitive system searches for dissimilarities rather than for similarities between the stimulus and the category (Gentner & Markman, 1994, 1997; Mussweiler, 2003)? Or does the activation of the behavioral immune system affect the criterion to decide that there is or there is not enough overlap between the stimulus and the category (Miller & Maner, 2012)? Our results only demonstrate the end result: an accentuated experience of deviance and dissimilarity. Future research will have to examine the processes underlying these effects.
In this research, we examined the sensitivity of people whose behavioral immune system is activated to morphological deviance. Future research could examine the possibility that their sensitivity to deviance in other modalities is heightened as well. Interestingly, previous findings have already shown that activation of the behavioral immune system is associated with increased sensitivity to social deviance. For example, a recent meta-analysis suggests a moderate positive correlation between a person’s activation level of the behavioral immune system and social conservativism, defined broadly as any belief system that encourages strict adherence to social norms and social exclusivity (such as, Right Wing Authoritarianism, Social Dominance Orientation, vertical collectivism, religious conservatism, ethnocentrism, and political conservatism; Terrizzi, Shook, & McDaniel, 2013). People whose behavioral immune system is activated hold more xenophobic and ethnocentric attitudes (Navarrete & Fessler, 2006; Navarrete et al., 2007; Navarrete, Fessler, Fleischman, & Geyer, 2009) and demonstrate more conformist attitudes (e.g., score higher on items such as “I actively avoid wearing clothes out-of-style”) and behavior (e.g., provide more conforming ratings on works of art; Murray & Schaller, 2012; Wu & Chang, 2012). In addition, recent findings suggest that political conservatism is associated with increased sensitivity to morphological deviance and that this sensitivity partly mediates the link between political conservatism and adherence to harsher policies toward deviant groups (Okimoto & Gromet, 2015). Future research may want to examine whether sensitivity to morphological deviance at least partly explains the correlation between activation of the behavioral immune system and social conservatism (i.e., sensitivity to social deviance; but see Karinen & Chapman, in press).
A final note deals with our control variables. Our results do not support the idea that the assumed higher activation of the behavioral immune system in women (Curtis et al., 2004; Duncan et al., 2009; Haidt et al., 1994; Rozin et al., 1999) results in consistent sex differences in sensitivity to deviance and dissimilarity above and beyond the effects of disgust sensitivity or pathogen threat. They further do not suggest that sex modulates the association between activation of the behavioral immune system and these variables. Similarly, neither the assumed higher activation of the behavioral immune system in the recently ill (Miller & Maner, 2011) nor higher levels of religiosity were consistently associated with sensitivity to deviance and dissimilarity. It should, however, be noted that although we controlled for these variables in our analyses, our studies were not designed to assess their interactions with our variables of interest (e.g., the majority of our participants were women). Future research is needed to shed light on their effects on sensitivity to deviance and to dissimilarity and on their interactions with disgust sensitivity and pathogen threat in affecting the latter.
Implications
That activation of the behavioral immune system is positively correlated with the perception of dissimilarities may have broader implications both for social perception and for social behavior under activation of the behavioral immune system. First, pathogen threat may make others seem more different from ourselves. Indeed, recent data from our lab show that when presented with faces of unknown others, participants who perceive themselves as vulnerable to disease (Duncan et al., 2009) and those who score high on disgust sensitivity (Olatunji et al., 2007) perceive the target persons as less psychologically similar to themselves. Importantly, ample evidence suggests that whereas a focus on similarities between a target and standard (which is the default mode) typically leads to assimilation in judgments, a focus on dissimilarities typically leads to contrast (Mussweiler, 2001, 2003; Mussweiler & Bodenhausen, 2002). The implication might be that when their behavioral immune system is activated, people may show contrast effects when judging themselves on various dimensions when compared to others (e.g., judge themselves as not sportive when primed with the notion of a sportive standard). Finally, the critical determinant of whether automatic behavior is assimilated toward or contrasted away from the typical behavior of primed persons is whether the primed persons are perceived as belonging to the same category as the perceiver or to a different category (i.e., whether they are perceived as similar or different from the perceiver; Schubert & Häfner, 2003). If activation of the behavioral immune system makes others seem more different from the self, people whose behavioral immune system is activated should be more likely to behave in contrast to the typical behavior of others who are on their mind (e.g., walk faster when thinking about old people). In sum, they might be less likely to feel and act “in sync” with whoever is outside their close social environment. These implications, which are at the focus of our current research, are in accordance with recent findings which show that activation of the behavioral immune system inhibits affiliative behavior (Sawada, Auger, Lydon, 2018) and renders people less extravert (Mortensen et al., 2010).
Conclusion
Drawing on an evolutionary rationale, our findings suggest that activation of the behavioral immune system renders people more sensitive to morphological deviance and more prone to perceive dissimilarities between stimuli. They suggest that the activation of the system changes basic categorization and comparison processes even in stimuli that have nothing to do with disease. Given that these processes serve as the fundaments of a wide range of higher order processes in social perception and in social behavior, the implications of the effects that are at the focus of the current research may be numerous. Future research should replicate our findings with different stimuli, examine the contribution of individual differences in attention to detail, and intolerance for ambiguity to our effects as well as examine the latter’s higher order implications.
Supplemental Material
SupplementaryMaterial-R2 for Sensitivity to Deviance and to Dissimilarity: Basic Cognitive Processes Under Activation of the Behavioral Immune System by Ravit Nussinson, Sari Mentser, and Nurit Rosenberg in Evolutionary Psychology
Acknowledgments
We are grateful to the three anonymous reviewers for their invaluable comments and suggestions on an earlier version of this article. We are grateful to Yifat Weiss for her help in the collection of the data. We also thank Barbara Grant for her help in copyediting.
Appendix
Stimuli used in Study 2 (A Modified Version of the Stimuli Designed by Okimoto and Gromet, 2015)
I. Perfect shapes.

II. Ambiguously imperfect shapes.
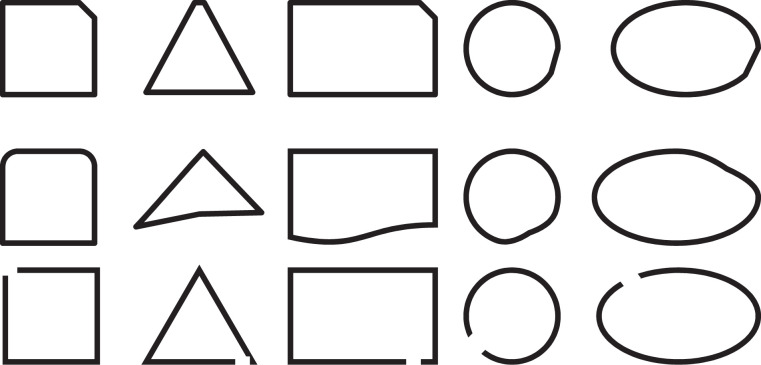
III. Clearly imperfect shapes.
Notes
Although in Study 1, only a small effect emerged, we expected a larger effect in the current study since it was conducted in the lab (rather than on the Internet) and among a more homogeneous population.
Another way to correct for violation of the sphericity assumption is by using the Greenhouse-Geisser correction. Taking this method rendered the 2 × 3 interaction nonsignificant, F(2, 137.33) = 1.69, p = .20, η2p = .06. Of course, however, the focal planned contrasts remained unchanged.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
References
- Ackerman J. M., Becker D. V., Mortensen C. R., Sasaki T., Neuberg S. L., Kenrick D. T. (2009). A pox on the mind: Disjunction of attention and memory in the processing of physical disfigurement. Journal of Experimental Social Psychology, 45, 478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman J. M., Tybur J., Mortensen C. R. (2018). Infectious disease and imperfections of self-Image. Psychological Science, 29, 228–241. [DOI] [PubMed] [Google Scholar]
- Berger U., Anaki D. (2014). Demographic influences on disgust: Evidence from a heterogeneous sample. Personality and Individual Differences, 64, 67–71. [Google Scholar]
- Bruner J. S. (1959). The cognitive consequences of early sensory deprivation. Psychosomatic Medicine, 21, 89–95. [DOI] [PubMed] [Google Scholar]
- Budner S. (1962). Intolerance for ambiguity as a personality variable. Journal of Personality, 30, 29–50. [DOI] [PubMed] [Google Scholar]
- Conway C. A., Jones B. C., Debruine L. M., Welling L. L. M., Law Smith M. J., Perrett D. I.…Al-Dujaili E. A. S. (2007). Salience of emotional displays of danger and contagion in faces is enhanced when progesterone levels are raised. Hormones and Behavior, 51, 202–206. [DOI] [PubMed] [Google Scholar]
- Curtis V., Aunger R., Rabie T. (2004). Evidence that disgust evolved to protect from risk of disease. Proceedings of Royal Society B, 271, S131–S133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis V., de Barra M., Aunger R. (2011). Disgust as an adaptive system for disease avoidance behaviour. Philosophical Transactions of the Royal Society: Series B: Biological Sciences, 366, 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan L. A., Schaller M., Park J. H. (2009). Perceived vulnerability to disease: Development and validation of a 15-item self-report instrument. Personality and Individual Differences, 47, 541–546. [Google Scholar]
- Faulkner J., Schaller M., Park J. H., Duncan L. A. (2004). Evolved disease-avoidance mechanisms and contemporary xenophobic attitudes. Group Processes & Intergroup Relations, 7, 333–353. [Google Scholar]
- Fessler D. M. T., Navarrete C. D. (2003). Domain-specific variation in disgust sensitivity across the menstrual cycle. Evolution and Human Behavior, 24, 406–417. [Google Scholar]
- Fessler D. M. T., Eng S. J., Navarrete C. D. (2005). Elevated disgust sensitivity in the first trimester of pregnancy: Evidence supporting the compensatory prophylaxis hypothesis. Evolution and Human Behavior, 26, 344–351. [Google Scholar]
- Field A. (2013). Discovering Statistics with IBM SPSS. Newbury Park, CA: Sage. [Google Scholar]
- Fleischman D. S. (2014). Women’s disgust adaptations. In Weekes-Shackelford V. A., Shackelford T. K. (Eds.), Evolutionary perspectives on human sexual psychology and behavior (pp. 277–296). New York, NY: Springer. [Google Scholar]
- Fleischman D. S., Fessler D. M. T. (2011). Progesterone’s effects on the psychology of disease avoidance: Support for the compensatory behavioral prophylaxis hypothesis. Hormones and Behavior, 59, 271–275. [DOI] [PubMed] [Google Scholar]
- Förster J., Dannenberg L. (2010). GLOMOsys: A systems account of global versus local processing. Psychological Inquiry, 21, 175–197. [Google Scholar]
- Gentner D., Markman A. B. (1994). Structural alignment in comparison: No difference without similarity. Psychological Science, 5, 152–158. [Google Scholar]
- Gentner D., Markman A. B. (1997). Structure mapping in analogy and similarity. American Psychologist, 52, 45–56. [Google Scholar]
- Haidt J., McCauley C., Rozin P. (1994). Individual differences in sensitivity to disgust: A scale sampling seven domains of disgust elicitors. Personality and Individual Differences, 16, 701–713. [Google Scholar]
- Hassan M. K., Khalique A. (1981). Religiosity and its correlates in college students. Journal of Psychological Researches, 25, 129–136. [Google Scholar]
- Howell D. C. (2002). Statistical methods for psychology (5th ed.). Pacific Grove CA: Duxbury. [Google Scholar]
- Inozu M., Ulukut F. O., Ergun G., Alcolado G. M. (2014). The mediating role of disgust sensitivity and thought-action fusion between religiosity and obsessive compulsive symptoms. International Journal of Psychology, 49, 334–341. [DOI] [PubMed] [Google Scholar]
- Isen A. M., Daubman K. A. (1984). The influence of affect on categorization. Journal of Personality and Social Psychology, 47, 1206–1217. [Google Scholar]
- Johns E. E., Mewhort D. J. K. (2002). What information underlies correct rejections in short-term recognition memory? Memory & Cognition, 30, 46–59. [DOI] [PubMed] [Google Scholar]
- Karinen A. K., Chapman H. A. (in press). Cognitive and personality correlates of trait disgust and their relationship to condemnation of non-purity moral transgressions. Emotion. [DOI] [PubMed] [Google Scholar]
- Kurzban R., Leary M. R. (2001). Evolutionary origins of stigmatization: The functions of social exclusion. Psychological Bulletin, 123, 187–208. [DOI] [PubMed] [Google Scholar]
- Lee D. H., Anderson A. K. (2017). Reading what the mind thinks from how the eye sees. Psychological Science, 28, 494–503. [DOI] [PubMed] [Google Scholar]
- Lee D. H., Mirza R., Flanagan J. G., Anderson A. K. (2014). Optical origins of opposing facial expression actions. Psychological Science, 25, 745–752. [DOI] [PubMed] [Google Scholar]
- Mewhort D. J. K., Johns E. E. (2000). The extralist-feature effect: Evidence against item matching in short–term recognition memory. Journal of Experimental Psychology: General, 129, 262–284. [DOI] [PubMed] [Google Scholar]
- Miller S. L., Maner J. K. (2011). Sick body, vigilant mind. The biological immune system activates the behavioral immune system. Psychological Science, 22, 1467–1471. [DOI] [PubMed] [Google Scholar]
- Miller S. L., Maner J. K. (2012). Overperceiving disease cues: The basic cognition of the behavioral immune system. Journal of Personality and Social Psychology, 102, 1198–1213. [DOI] [PubMed] [Google Scholar]
- Mortensen C. R., Becker D. V., Ackerman J. M., Neuberg S. L., Kenrick D. T. (2010). Infection breeds reticence: The effects of disease salience on self-perceptions of personality and behavioral tendencies. Psychological Science, 21, 440–447. [DOI] [PubMed] [Google Scholar]
- Moskowitz G. B. (2005). Social cognition: Understanding self and others. New York, NY: Guilford Press. [Google Scholar]
- Murray D. R., Schaller M. (2012). Threat (s) and conformity deconstructed: Perceived threat of infectious disease and its implications for conformist attitudes and behavior. European Journal of Social Psychology, 42, 180–188. [Google Scholar]
- Murray N., Sujan H., Hirt E. R., Sujan M. (1990). The influence of mood on categorization: A cognitive flexibility interpretation. Journal of Personality and Social Psychology, 59, 411–425. [Google Scholar]
- Mussweiler T. (2001). Focus of comparison as a determinant of assimilation versus contrast in social comparison. Personality and Social Psychology Bulletin, 27, 38–47. [Google Scholar]
- Mussweiler T. (2003). Comparison processes in social judgment: Mechanisms and consequences. Psychological Review, 110, 472–489. [DOI] [PubMed] [Google Scholar]
- Mussweiler T., Bodenhausen G. (2002). I know you are but what am I? Self-evaluative consequences of judging in-group and out-group members. Journal of Personality and Social Psychology, 82, 19–32. [DOI] [PubMed] [Google Scholar]
- Navarrete C. D., Fessler D. M. (2006). Disease avoidance and ethnocentrism: The effects of disease vulnerability and disgust sensitivity on intergroup attitudes. Evolution and Human Behavior, 27, 270–282. [Google Scholar]
- Navarrete C. D., Fessler D. M. T., Eng S. J. (2007). Elevated ethnocentrism in the first trimester of pregnancy. Evolution and Human Behavior, 28, 60–65. [Google Scholar]
- Navarrete C. D., Fessler D. M. T., Fleischman D. S., Geyer J. (2009). Race bias tracks conception risk across the menstrual cycle. Psychological Science, 20, 661–665. [DOI] [PubMed] [Google Scholar]
- Neuberg S. L., Kenrick D. T., Schaller M. (2011). Human threat management systems: Self-protection and disease-avoidance. Neuroscience & Biobehavioral Reviews, 35, 1042–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussinson R., Seibt B., Häfner M., Strack F. (2011). Cognitive consequences of motivational orientation: Perceived similarity between objects. Acta Psychologica, 138, 39–44. [DOI] [PubMed] [Google Scholar]
- Oaten M., Stevenson R. J., Case T. I. (2009). Disgust as a disease-avoidance mechanism. Psychological Bulletin, 135, 303–321. [DOI] [PubMed] [Google Scholar]
- Okimoto T. G., Gromet D. M. (2015). Differences in sensitivity to deviance partly explain ideological divides in social policy support. Journal of Personality and Social Psychology, 111, 98–117. [DOI] [PubMed] [Google Scholar]
- Olatunji B. O., Tolin D. F., Huppert J. D., Lohr J. M. (2005). The relation between fearfulness, disgust sensitivity and religious obsessions in a non-clinical sample. Personality and Individual Differences, 38, 891–902. [Google Scholar]
- Olatunji B. O., Williams N. L., Tolin D. F., Abramowitz J. S., Sawchuk C., Lohr J. M., Elwood L. S. (2007). The Disgust Scale: Item analysis, factor structure, and suggestions for refinement. Psychological Assessment, 19, 281–297. [DOI] [PubMed] [Google Scholar]
- Park J. H., Faulkner J., Schaller M. (2003). Evolved disease-avoidance processes and contemporary anti-social behavior: Prejudicial attitudes and avoidance of people with physical disabilities. Journal of Nonverbal Behavior, 27, 65–87. [Google Scholar]
- Park J. H., Schaller M., Crandall C. S. (2007). Pathogen-avoidance mechanisms and the stigmatization of obese people. Evolution and Human Behavior, 28, 410–414. [Google Scholar]
- Robinson J. S., Xiaowen X., Plaks J. E. (2017). Disgust and deontology: Trait sensitivity to contamination promotes a preference for order, hierarchy, and rule-based moral judgment. Social Psychological and Personality Science, XX, 1–12. [Google Scholar]
- Roccas S., Sagiv L., Navon M. (2017). Methodological issues in studying personal values. In Roccas S., Sagiv L. (Eds.), Values and behavior: Taking a cross cultural perspective (pp. 15–50). Cham, Switzerland: Springer. [Google Scholar]
- Rozin P., Haidt J., McCauley C. R. (2008). Disgust. In Lewis M., Haviland-Jones J. M., Barrett L. F. (Eds.), Handbook of emotions (3rd ed., pp. 757–776). New York, NY: Guilford Press. [Google Scholar]
- Rozin P., Haidt J., McCauley C., Dunlop L., Ashmore M. (1999). Individual differences in disgust sensitivity: Comparisons and evaluations of paper-and-pencil versus behavioral measures. Journal of Research in Personality, 33, 330–351. [Google Scholar]
- Rozin P., Royzman E. B. (2001). Negativity bias, negativity dominance, and contagion. Personality and Social Psychology Review, 5, 296–320. [Google Scholar]
- Sawada N., Auger E., Lydon J. E. (2018). Activation of the behavioral immune system: Putting the brakes on affiliation. Personality and Social Psychology Bulletin, 44, 224–237. [DOI] [PubMed] [Google Scholar]
- Schaller M. (2006). Parasites, behavioral defenses, and the social psychological mechanisms through which cultures are evoked. Psychological Inquiry, 17, 96–101. [Google Scholar]
- Schaller M. (2011). The behavioural immune system and the psychology of human sociality. Philosophical Transactions of the Royal Society B: Biological Sciences, 366, 3418–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M. (2016). The behavioral immune system. In Buss D. M. (Ed.), The handbook of evolutionary psychology (2nd ed., pp. 206–224). Hoboken, NJ: John Wiley. [Google Scholar]
- Schaller M., Duncan L. A. (2007). The behavioral immune system: Its evolution and social psychological implications. In Forgas J. P., Haselton M. G., von Hippel W. (Eds.), Evolution and the social mind: Evolutionary psychology and social cognition (pp. 293–307). New York, NY: Psychology Press. [Google Scholar]
- Schaller M., Park J. H. (2011). The behavioral immune system (and why it matters). Current Directions in Psychological Science, 20, 99–103. [Google Scholar]
- Schaller M. C., Miller G. E., Gervais W. M., Yager S., Chen E. (2010). Mere visual perception of other people’s disease symptoms facilitates a more aggressive immune response. Psychological Science, 21, 649–652. [DOI] [PubMed] [Google Scholar]
- Schubert T. W., Häfner M. (2003). Contrast from social stereotypes in automatic behavior. Journal of Experimental Social Psychology, 39, 577–584. [Google Scholar]
- Stewart N., Brown G. D. (2005). Similarity and dissimilarity as evidence in perceptual categorization. Journal of Mathematical Psychology, 49, 403–409. [Google Scholar]
- Terrizzi J. A., Goodman R. (2018, July). Tidiness of mind: The evolutionary embodied cognitive consequences of disgust. Poster presented at the 30th Human Behavior and Evolution Society, Amsterdam, The Netherlands.
- Terrizzi J. A., Shook N. J., McDaniel M. A. (2013). The behavioral immune system and social conservatism: A meta-analysis. Evolution and Human Behavior, 34, 99–108. [Google Scholar]
- Tolin D. F., Woods C. M., Abramovitch J. S. (2006). Disgust sensitivity and obsessive–compulsive symptoms in a non-clinical sample. Journal of Behavior Therapy and Experimental Psychiatry, 37, 30–40. [DOI] [PubMed] [Google Scholar]
- Tybur J. M., De Vries R. E. (2013). Disgust sensitivity and the HEXACO model of personality. Personality and Individual Differences, 55, 660–665. [Google Scholar]
- Tybur J. M., Lieberman D., Kurzban R., DeScioli P. (2013). Disgust: Evolved function and structure. Psychological Review, 120, 65–84. [DOI] [PubMed] [Google Scholar]
- Wu P. P., Chang L. (2012). The social impact of pathogen threat: How disease salience influences conformity. Personality and Individual Differences, 53, 50–54. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SupplementaryMaterial-R2 for Sensitivity to Deviance and to Dissimilarity: Basic Cognitive Processes Under Activation of the Behavioral Immune System by Ravit Nussinson, Sari Mentser, and Nurit Rosenberg in Evolutionary Psychology