Abstract
Previous research with hunter-gatherers has found that women perceive men with voices manipulated to be lower in pitch to be better hunters, and men perceive women with lower pitch to be better gatherers. Here, we test if actual voice pitch is associated with hunting and gathering reputations in men and women, respectively. We find that voice pitch does relate to foraging reputation in men, but not in women, with better hunters having a lower voice pitch. In addition, we find that the previously documented relationship between voice pitch and reproductive success no longer holds when controlling for hunting reputation, but hunting reputation remains a significant predictor of reproductive success when controlling for voice pitch. This raises the possibility that voice pitch is being selected for in hunter-gatherers because of the relationship between voice pitch and hunting reputation.
Keywords: Hadza, hunter-gatherers, voice pitch, hunting, reproductive success, sexual dimorphism
Human voice pitch is sexually dimorphic, with males producing a voice pitch approximately 6 standard deviations below females (Puts, Doll, & Hill, 2014). This sex difference is due to males having larger vocal folds (Titze, 1994). Vocal folds begin to sexually differentiate during puberty, when increased testosterone levels in males (Tossi, Postan, & Bianculli, 1976) act upon androgen receptors in vocal folds (Newman, Butler, Hammond, & Gray, 2000; Saez & Sakai, 1976), resulting in larger folds and lower voice pitch. The sex difference in vocal folds is one of the largest anatomical sex differences observed in humans (Rendall, Kollias, Ney, & Lloyd, 2005), yet differences in voice pitch within sex are only weakly related to stature (Collins, 2000; Pisanski et al., 2014; Puts, Apicella, & Cárdenas, 2012). This suggests that sexual dimorphism in voice pitch is not simply the by-product of increased size in males, but rather that lower voice pitch in males was independently selected for, possibly because of its role in mating competition (Puts et al., 2014). Indeed, across a sample of anthropoid primates, increasing mating competition among males is associated with the evolution of greater sexual dimorphism in vocalization pitch (Puts et al., 2016). Also, in an extant population of hunter-gatherers, the Hadza, lower voice pitch is associated with greater reproductive success in men, but no association between voice pitch and fertility outcomes has been found for women (Apicella, Feinberg, & Marlowe, 2007). Together, these findings suggest that dimorphism in voice pitch is the result of selection for lower pitched vocalizations in males.
Sexual Selection and Voice Pitch
One hypothesis is that female mate choice, or intersexual selection, which favors ornaments and showy displays, may have led to sexual dimorphism in voice pitch. Indeed, several lines of research suggest that women are more attracted to men with lower voice pitch (e.g., Collins, 2000; Feinberg, Jones, Little, Burt, & Perrett, 2005; Saxton, Caryl, & Roberts, 2006) and that this preference is more pronounced when women are close to ovulation (Feinberg et al., 2006; Puts, 2005). These results are consistent with the hypothesis that women have evolved conditional strategies for short- and long-term mating, such that during periods of high fecundity women should be more motivated to find partners of high genetic quality because finding a mate who can provide indirect benefits (e.g., good genes) becomes relatively more important than finding a mate who can provide direct benefits (e.g., resources and physical protection). Direct benefits are thought to be important when evaluating prospective mates for long-term partnerships (Buss & Schmitt, 1993). Interestingly, prior work with the Hadza suggests that women do not prefer high or low pitch in men’s voices when selecting for marriage partners; however, this may be due to the high percentage of the sample that was pregnant or lactating (Apicella & Feinberg, 2009). Indeed, Hadza women who were breast-feeding preferred men with voices raised in pitch, though this was an exploratory analysis based on a small sample. Although women were found to prefer lower male voice pitch in the context of short-term versus long-term mating in a Western sample (Puts, 2005), no studies have examined women’s preferences for short-term mates in the Hadza.
One debated explanation for why women prefer men with lower voice pitch is that lower pitch signals heritable immune system efficiency (Feinberg et al., 2006; Puts, 2005, 2006). Androgens, including testosterone, are thought to be immunosuppressant (Bouman, Heineman, & Faas, 2005, but see Roberts, Buchanan, & Evans, 2004, for a critical review), and compromising immune functioning via greater androgen production may be possible only for healthy individuals. Conversely, immune system activation may depress testosterone production (Boonekamp, Ros, & Verhulst, 2008), so that healthy individuals tend to more consistently produce higher testosterone and hence more masculinized traits. The relationship between testosterone and immune function is not entirely clear, as many of the studies conducted are observational (Roberts et al., 2004), and so it is possible that other omitted variables, such as energetic shortages could contribute to a decreased immune system and lower testosterone levels via independent pathways (Ellison, 2011; see also Prall & Muehlenblein, 2014, for review). Nevertheless, it is commonly held that traits associated with greater androgen exposure should influence attractiveness to females because these traits signal genes that confer disease resistance to offspring (Folstad & Karter, 1992).
Cortisol, a glucocorticoid known for its role in metabolic and energy mobilization processes needed during fight and flight responses, also has adverse effects on metabolic and immune processes (Coutinho & Chapman, 2011). Recent evidence indicates that the relationship between the expression of male traits and immune function may be mediated by both testosterone and cortisol, such that men with both high testosterone and low cortisol tend to possess better functioning immune systems (Rantala et al., 2012). Consistent with this, men with lower voice pitch tend to have higher testosterone levels (Dabbs & Mallinger, 1999; Evans, Neave, & Wakelin, 2006; Puts et al., 2012), and this relationship is stronger in men with lower cortisol (Puts et al., 2016). Finally, women with low self-rated health were found to prefer men with lower voice pitch as short-term mates, when the genetic benefit of disease resistance in offspring is higher relative to other components of mate quality, such as investment (Feinberg et al., 2012).
Another hypothesis is that intrasexual selection may have led to increased dimorphism in voice pitch. In many nonhuman animal species, male vocalizations may be used as honest advertisements of competitive ability, particularly in intrasexual aggression (e.g., Clutton-Brock & Albon, 1979; Hauser, 1993). Human males too may have evolved lower voice pitch to advertise competitive ability (Puts et al., 2012). Several studies have found that experimentally lowering voice pitch increases perceived dominance in men (Feinberg et al., 2005; Puts, Gaulin, & Verdolini, 2006; Puts, Hodges, Cárdenas, & Gaulin, 2007; Saxton, Mackey, McCarty, & Neave, 2016; Wolff & Puts, 2010), which is consistent with data suggesting that men with lower voice pitch have more upper body strength and thus, may be more formidable (Puts et al., 2012). Indeed, a cross-cultural study found that individuals could accurately assess upper body strength from men’s voices, even when the voices spoken were from an unfamiliar language (Sell et al., 2009).
Of course, intersexual and intrasexual selection are not mutually exclusive possibilities. Whatever the evolutionary cause of sexual dimorphism in voice pitch, data on Hadza hunter-gatherers suggest that voice pitch is under directional selection in men, but not in women (Apicella et al., 2007). Hadza men with lower voice pitch have greater reproductive success, due largely to having more children born to them rather than their children experiencing less mortality. It is possible that a low pitch elevates Hadza men’s status among men and/or attractiveness to women and thus increases their access to more or higher quality mates. If so, then these findings are consistent with sexual selection accounts for why men have, on average, lower voice pitch.
Hunting reputation in men is another trait that is associated with reproductive success in the Hadza (Apicella, 2014; Marlowe, 2001), and previous research has examined perceptions of foraging reputation in Hadza males and females with manipulated voices (Apicella & Feinberg, 2009). Hadza females perceive males with experimentally manipulated lower voice pitch as better hunters, even when judging the voices of non-Hadza speaking an unfamiliar language. Similarly, Hadza males perceive females with experimentally manipulated lower voice pitch as better gatherers. It is currently unknown to what extent natural variation in voice pitch is correlated with actual hunting and gathering reputations in men and women.
Sexual Selection, Hunting, and Voice Pitch
Hunting has featured heavily in evolutionary accounts of human origins. For example, human’s increased encephalization, lengthened juvenile period, and long life span have all been attributed to the act of hunting (Gurven, Kaplan, & Gutierrez, 2006; Kaplan, Hill, Lancaster, & Hurtado, 2000; Washburn & Lancaster, 1968). Hunting continues to be under selection in extant hunter-gatherers. Hunting success in men is associated with increased reproductive success in several forager populations including the Aché (Hill & Hurtado, 1996), !Kung (Wiessner, 2002), Lamalera (Alvard & Gillespie, 2004), Meriam (Bliege Bird, Smith, & Bird, 2001), and the Hadza (Apicella, 2014; Marlowe, 2001). Successful hunters obtain many reproductive benefits including younger wives and more children (for review, Gurven & von Rueden, 2006). Perhaps unsurprisingly, hunting ability is an important criterion for choosing husbands in the Hadza (Apicella & Crittenden, 2016; Marlowe, 2004). While the reproductive advantages for being a good hunter are clear, the question of what motivates men to hunt is debated. Gathering, on average, brings in more calories than hunting in the Hadza and in warm-climate foragers more generally—calories that can be directed exclusively toward kin (Marlowe, 2010). In contrast, hunted foods tend to be shared widely outside the nuclear family (Hawkes, O’Connell, & Blurton Jones, 2001) possibly because they are more difficult to procure and the packages tend to be larger (for review, see Kaplan, Gurven, Hill, & Hurtado, 2005). For these reasons, traditional explanations suggesting that men hunt to provision their families have been questioned.
Some anthropologists maintain that men are motivated to hunt primarily to advertise their quality to potential mates and allies (Hawkes, 1991; Hawkes & Bliege Bird, 2002). On the one hand, because hunting requires knowledge, strength, and stamina, being a successful hunter may be a reliable indicator of condition (Smith, Bliege Bird, & Bird, 2003). Thus, women may choose successful hunters as mates because hunting success signals underlying male condition including genetic quality. On the other hand, hunted foods may be attractive to women because of the benefits they provide to themselves and their children. Meat is rich in fat, digestible proteins, and essential amino acids, and consequently no plant source in the African Savannah rivals it (Dominguez-Rodrigo et al., 2014). Under this view, hunting may be considered a form of intrasexual competition by which men procure foods that are attractive to women (for overview of the debate, Gurven & von Rueden, 2006).
Interestingly, the best physical predictor of hunting reputation in Hadza men is upper body strength—a physical trait that the Hadza also recognize as important for hunting success (Apicella, 2014). Upper body strength, and in particular strength in the forearm and muscles surrounding the shoulder girdle, is necessary for pulling back on bows (Ertran, Kentel, Tümer, & Korkusuz, 2003; Mann & Littke, 1989) and may increase the distance at which men can successfully strike a target. Since hunting ability is under selection in current hunter-gatherers, sex differences in upper body strength in humans may be due to selection acting on hunting ability and not just fighting ability (Apicella, 2014).
Here we explore the relationships between hunting and gathering reputations, natural voice pitch, and reproductive success in Hadza hunter-gatherers using a data set previously reported in other papers (Apicella, 2014; Apicella et al., 2007; Puts et al., 2012). It is important to examine voice pitch and hunting ability together since lowered voice pitch may have been selected independently or jointly with hunting ability in men. For instance, voice pitch may signal good genes independently of hunting ability and/or through its association with hunting ability. That is, both hunting ability and voice pitch may serve as signals to male quality, and these signals may reflect similar or different aspects of quality. As an example, voice pitch may provide women a means to assess immunocompetence and/or other androgen-related qualities while hunting ability, may, in addition, signal other qualities, such as intelligence. Alternatively, voice pitch dimorphism in humans may have resulted from intrasexual competition—due to its association with men’s ability to acquire resources (i.e., hunt), fight, or both. Here we ask to what extent voice pitch is associated with hunting/foraging reputations in men and women, respectively, and whether the relationship between voice pitch and reproductive success in men persists after controlling for hunting reputation.
Method
Study Population
The Hadza are a traditional population of about 1,000 who subsist on hunted and gathered foods. They occupy a savannah-woodland habitat in Northern Tanzania and live in mobile camps that number approximately 25–30 people. Camps shift location every 6–8 weeks as resources in an area become depleted. Membership in camps is flexible as individuals come and go freely and are welcome in any Hadza camp they choose to live.
The Hadza practice central place foraging where acquired foods are brought back to camp and shared with family and other campmates. They use traditional tools to acquire resources. Men hunt birds and mammals using bow and arrow technology. While bows and arrows are constructed from wood, arrowheads are made with either wooden or metal tips. Metal tips are typically used for larger animals and are often dipped in poison from the panjube plant that acts to hasten the death of the animal (Bartram, 1997).
Women collect water, firewood, fruit, and dig for tubers using sharpened wooden sticks. Women forage for an average of 4.2 hr a day. It too is strenuous and demanding, and women’s ability to acquire resources is also an important factor in mate choice for men (Marlowe, 2005). Compared to men, Hadza women tend to contribute more food to their households, unless they currently have a nursing infant. During this “critical period of nursing,” women’s productivity decreases and their husband’s increases (Marlowe, 2003).
While Hadza men are dominant to women, women have a large degree of autonomy, are free to select their marriage partners, and participate in camp-level decision-making (Marlowe, 2010). Still, Hadza men are, on average, more competitive and more risk-taking than women, as evinced from performance in incentivized economic games (Apicella & Dreber, 2015; Apicella, Crittenden, & Tobolsky, 2017). Other sex differences in economic (e.g., preference for owned items) and social preferences (e.g., cooperation) have not been found (Apicella, 2017; Apicella, Azevedo, Christakis, & Fowler, 2014; Apicella, Marlowe, Fowler, & Christakis, 2012).
Finally, because the Hadza remain relatively isolated from Western culture, do not use birth control, and practice a way of life that more closely approximates the lives of our ancestors compared to agriculturalists, pastoralists, and farmers, they provide a valuable resource for testing evolutionary hypotheses (Apicella & Barrett, 2016). Some have questioned whether the Hadza are atypical because they still practice hunting and gathering when all other surrounding groups have adopted newer modes of subsistence. However, the Hadza are not unusual relative to the full spectrum of contemporary foragers for whom data exist. 1 Nevertheless, evolutionary arguments for evolved traits necessitate some consideration of the challenges faced by our ancestors, and it is difficult to know with certainty the ways in which modern foragers depart from ancestral foragers. To the extent that the problems faced by the Hadza differ from the problems faced by our ancestors, using the Hadza as a model for understanding human evolution may be limited (for a full discussion of this issue, see Apicella & Crittenden, 2016). However, we believe that the traits considered here (e.g., hunting and gathering, mate choice, and reproduction) to still be useful referents for the past. Nevertheless, we urge that prudence be exercised when using single populations to make claims about the past and that each trait’s relevance be considered on a case by case basis.
Procedure
Data were collected over a period of approximately 6 months in 2006 from a sample of Hadza Bushmen in Tanzania. Nine camps were visited for data collection by one of the researchers, and all adults in each camp were invited to participate. The sample included 53 men between the ages of 19 and 59 (M = 37.4, SD = 11.3) and 49 women between the ages of 18 and 53 (M = 31.0, SD = 7.7).
Measures
Voice pitch
To collect voice pitch data, participants were instructed to speak into a microphone the word “hujambo,” which loosely translates from Swahili to “how are you?” in English. Recordings were made in private with only a female experimenter present. The voices were directly encoded in mono onto a computer hard drive using Sonic Foundry’s Sound Forge at 44.1 kHz sampling rate and 16-bit quantization and saved as uncompressed “wav” files. F0, the acoustic correlate of voice pitch, was analyzed using Praat software (Version 4.5) and measured using Pratt’s (Boersma & Weenink, 2007) autocorrelation algorithm using techniques described elsewhere (Feinberg et al., 2005). Voice pitch ranged from 83.64 Hz to 174.28 Hz in men, and from 135.80 Hz to 272.46 Hz in women. Table 1 presents the mean values for voice pitch and other variables for each sex.
Table 1.
Descriptive Statistics by Sex.
| Sex | n | Age (Number of Years) | Reproductive Success (Number of Living Children) | Fertility (Number of Children Born) | Offspring Mortality (Proportion of Children That Died) | Voice Pitch (Hz) |
|---|---|---|---|---|---|---|
| Male | 53 | 37.4 (11.3) | 2.8 (2.6) | 4.6 (3.8) | .43 (.30) | 116.571 (19.790) |
| Female | 49 | 31.0 (7.7) | 2.5 (2.0) | 3.4 (2.4) | .28 (.34) | 205.355 (30.777) |
Note. Values are mean statistics with standard deviations are in parentheses.
Upper body strength
Upper body strength was calculated from upper arm muscle mass and grip strength. Upper arm muscle mass was calculated from a standard formula that estimates the area of the muscle of the upper arm minus the bone from measurements of midupper arm circumference and triceps skinfold measurements (Heymsfield, McManus, Smith, Stevens, & Nixon, 1982). Arm circumference of the left arm was measured using a flexible tape measure. Triceps skinfold measurements were measured in triplicate (Cronbach’s α = .88) using skinfold calipers. Handgrip strength for each hand was measured using a dynamometer. Each measurement was standardized within sex then averaged together. A higher score indicates greater upper body strength.
Foraging reputation
To collect data on hunting and gathering reputation, women were asked to evaluate the hunting ability of men, and men were asked to evaluate the gathering ability of women. To do this, facial photographs were taken of each of the adult men and women in each camp and were displayed simultaneously in a random order to each participant on a computer screen. Men and women were interviewed privately, questioned in their nonnative language, Swahili, and asked: “Which of these men is the best hunter?” or “which of these women is the best gatherer?” After an individual was chosen, his or her picture was removed from the screen. This process was repeated until all men and women were assigned a ranking. Confidentiality was assured during all interviews. This ordinal ranking was used to facilitate data collection because the Hadza lack experience in assigning numerical values. For each individual, the mean rank was calculated from each rater’s ranking and then standardized within camps. A lower value indicates a better reputation.
For each of the nine camps, a different number of men and women participated in ranking hunting and gathering reputation. The number of male participants in each camp ranged from 3 to 7 (M = 5.9, SD = 1.3), and the number of female participants in each camp ranged from 3 to 12 (M = 5.9, SD = 2.9). For women rating men’s hunting ability, Cronbach’s α ranged from .5 to .98 (M = .82, SE = .15). For men rating women’s gathering ability, raters were in less agreement. In two camps—both with three women each—there was a negative correlation between raters. Excluding these camps from the analyses does not change the results. The Cronbach’s α for the other seven camps ranged from .23 to .92 (M = .70, SD = .24). The lower interrater reliability may be due to lower variability between women in gathering returns (Berbesque, Wood, Crittenden, Mabulla, & Marlowe, 2016), making meaningful assessment of ability more difficult, or perhaps men are less motivated to attend to women’s ability to gather resources.
Reproductive success, fertility, and offspring mortality
To collect data on reproductive success, participants were interviewed about the number of children born to them, the number of those children that have died, and the number of children still living. Because Hadza have difficulty counting to large numbers, participants were asked to provide the names of each child born to them sequentially. After the child was named, the participant was then asked if the child was still living. Reproductive success was defined as the number of living children, fertility was defined as the number of children born to the participant, and offspring mortality was defined as the proportion of children born to the participant that died (participants with no children born to them were coded as n/a). Obviously, men do not have the same level of accuracy in assessing their parental status as women. While there are no data available on the rate of misattributed paternity in the Hadza, we have no reason to suspect that it is unusually high compared to other monogamous populations where rates generally hover around 1%. 2
Results
Camp Effects
We tested for differences between camps in voice pitch, upper body strength, age, reproductive success, fertility, and mortality rate using one factor analysis of variances (ANOVAs) for each sex separately. Table 2 presents the output of those analyses. For males, there were significant differences between camps for age, reproductive success, and fertility. For females, there were no significant differences between camps. Because of the significant effects, we control for camps effects in analyses involving age, reproductive success, and fertility.
Table 2.
Camp Effects on Variables by Sex.
| Measure | Male | Female | ||||
|---|---|---|---|---|---|---|
| df | F | p | df | F | p | |
| Voice pitch | 8, 44 | 0.90 | .523 | 8, 40 | 0.85 | .564 |
| Upper body strength | 8, 42 | 1.26 | .290 | 8, 40 | 1.37 | .241 |
| Age | 8, 44 | 3.13 | .007** | 8, 40 | 0.56 | .801 |
| Reproductive success | 8, 44 | 2.24 | .042* | 8, 39 | 1.07 | .407 |
| Fertility | 8, 44 | 2.27 | .040* | 8, 39 | 1.49 | .194 |
| Offspring mortality | 8, 36 | 0.86 | .555 | 8, 36 | 1.48 | .198 |
Note. Output for analysis of variance models testing for camp differences for each variable by sex.
*p < .05. **p < .01.
Voice Pitch and Foraging Reputation
Table 3 presents zero-order correlations between each variable for males. Voice pitch significantly correlated with foraging reputation; males with lower voices were ranked as better hunters (see Figure 1). 3 Voice pitch also correlated with reproductive success; males with lower voices had more living children. Similarly, foraging reputation also correlated with reproductive success; males who were ranked as better hunters had more living children. Table 4 presents zero-order correlations between each variable for females. Voice pitch did not significantly correlate with any other variables including foraging reputation (see Figure 1). Foraging reputation significantly correlated with age; older females were ranked as better gatherers.
Table 3.
Zero-Order Correlations for Males.
| Measure | Foraging Reputation | Upper Body Strength | Age | Reproductive Success | Fertility | Offspring Mortality |
|---|---|---|---|---|---|---|
| Voice pitch | .30* | −.20 | .06 | −.27* | −.20 | .23 |
| Foraging reputation | −.27 | .04 | −.30* | −.18 | .16 | |
| Upper body strength | −.14 | .19 | .08 | −.24 | ||
| Age | .58** | .68** | −.07 | |||
| Reproductive success | .88** | −.59** | ||||
| Fertility | −.22 |
Note. Values are Pearson’s correlation. N = 53, except for analyses involving upper body strength (n = 51) and mortality (n = 45).
*p < .05. **p < .01.
Figure 1.
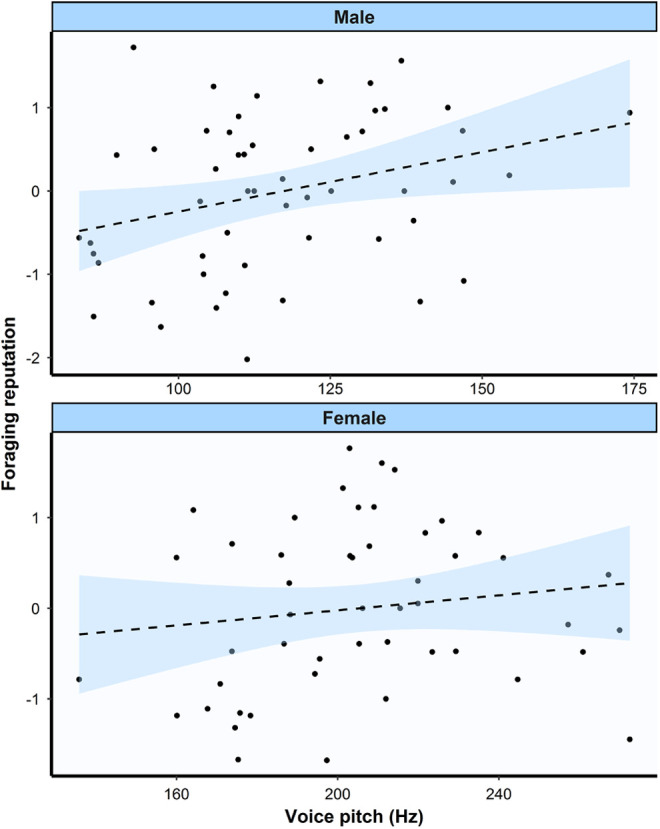
Scatterplot with ordinary least squares regression line of voice pitch and foraging reputation in males and females. Lower reputation scores mean that individuals were ranked as a better hunter/gatherer. Shaded region is 95% confidence interval.
Table 4.
Zero-Order Correlations for Females.
| Measure | Foraging Reputation | Upper Body Strength | Age | Reproductive Success | Fertility | Offspring Mortality |
|---|---|---|---|---|---|---|
| Voice pitch | .14 | .05 | −.17 | −.12 | −.17 | .06 |
| Foraging reputation | −.27 | −.30* | −.15 | −.20 | −.05 | |
| Upper body strength | .15 | .18 | .09 | −.18 | ||
| Age | .38** | .50** | .16 | |||
| Reproductive success | .88** | −.43** | ||||
| Fertility | −.01 |
Note. Values are Pearson’s correlations. N = 49, except for analyses involving reproductive success, fertility (both n = 48), and mortality (n = 45).
*p < .05. **p < .01.
Table 5 presents a series of regression models predicting foraging reputation from voice pitch controlling for camp-fixed effects, separated by sex. For males, voice pitch remained a significant predictor after controlling for camp membership (Model 1) and age (Model 2). For females, voice pitch was still nonsignificant after controlling for camp membership and age. Models 3 and 4 test the hypothesis that voice pitch predicts foraging reputation in males because upper body strength predicts voice pitch (Puts et al., 2012) and hunting reputation (Apicella, 2014). If true, then after controlling for upper body strength, voice pitch should no longer be a significant predictor of hunting reputation. However, contrary to our prediction, for males, voice pitch remained significant when controlling for upper body strength. For females, though upper body strength is a significant predictor of foraging reputation, voice pitch was still a nonsignificant predictor of foraging reputation.
Table 5.
OLS Regression Models Predicting Foraging Reputation.
| Measure | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Male | ||||
| Voice pitch | .36** (.16) | .36** (.16) | .36 (.15)** | |
| Upper body strength | −.40 (.17)** | −.33 (.16)* | ||
| Age | .07 (.18) | −.24 (.20) | −.22 (.19) | |
| Females | ||||
| Voice pitch | .16 (.17) | .09 (.17) | .12 (.17) | |
| Upper body strength | −.30 (.17)* | −.31 (.17) * | ||
| Age | −.31 (.17) * | −.29 (.16) * | −.26 (.16) | |
Note. Values are standardized regression coefficients (SE in parentheses). All regression models control for camp membership. OLS = ordinary least squares.
*p < .10. **p < .05.
Voice Pitch, Foraging Reputation, and Reproductive Success
Both voice pitch and hunting reputation predict reproductive success in males (Apicella, 2014; Apicella et al., 2007; see Table 6), but not in females (see Table 4). We tested if the relationship in males between voice pitch and reproductive success is due, in part, to the relationship between voice pitch and hunting reputation in series of ordinary least squares regression models presented in Table 6. When voice pitch and hunting reputation are entered simultaneously in the model predicting reproductive success, voice pitch is no longer significant, whereas hunting reputation remains significant. Moreover, an ANOVA reveals that adding hunting reputation to the model with just voice pitch significantly improves the variance explained, F(1, 41) = 6.58, p = .014, ΔR2 = .02, whereas adding voice pitch to the model with just hunting reputation does not significantly improve the model, F(1, 41) = 2.48, p = .123, ΔR2 = .01. This suggests that the relationship between voice pitch and reproductive success is indirect and mediated by hunting reputation. To better understand how these variables contribute to reproductive success, we analyzed fertility and offspring mortality separately. We obtain similar results with fertility, with voice pitch and hunting reputation independently predicting fertility, and evidence that the voice pitch’s effect on fertility is mediated by hunting reputation. However, we find that voice pitch and hunting reputation do not predict offspring mortality. Thus, Hadza males with lower voices have better hunting reputations, which lead to having more offspring, but these offspring are no more likely to survive than offspring of fathers with worse hunting reputations. This is consistent with other findings in the literature that status markers increase reproductive success through their effects on fertility rather than offspring mortality (von Rueden & Jaeggi, 2016).
Table 6.
OLS Regression Models Predicting Reproductive Outcomes for Males.
| Measure | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Reproductive success | |||
| Voice pitch | −.27 (.11)** | −.18 (.11) | |
| Hunting reputation | −.32 (.09)** | −.27 (.10)* | |
| Fertility | |||
| Voice pitch | −.20 (.10)* | −.14 (.10) | |
| Hunting reputation | −.21 (.09)** | −.17 (.10)* | |
| Offspring mortality | |||
| Voice pitch | .17 (.17) | .11 (.18) | |
| Hunting reputation | .20 (.16) | .16 (.17) | |
Note. Values are standardized regression coefficients (SE in parentheses) predicting reproductive success, fertility, and offspring mortality for males. All regression models control for camp membership and age. OLS = ordinary least squares.
*p < .10. **p < .05.
Discussion
Hadza men with lower voice pitch have better hunting reputations. This accords with previous research reporting that Hadza women perceived samples of male voices manipulated to be lower in pitch as better hunters (Apicella & Feinberg, 2009). In contrast, we did not find that Hadza women with lower voice pitch have better gathering reputations. This finding conflicts with prior results showing that men rate women’s voices manipulated to be lower in pitch as better gatherers. In addition, though men’s reproductive success is predicted by voice pitch (Apicella et al., 2007) and hunting reputation (Apicella, 2014) separately, when entered in the same regression model only hunting reputation remains a significant predictor of reproductive success, suggesting that voice pitch is related to reproductive success because of its relationship with hunting reputation or another correlate of hunting reputation.
Why do Hadza men with lower voice pitch have better hunting reputations? One possibility is that such men are not in fact better hunters but are merely perceived as such. Studies across several cultures find that people can infer physical formidability from the voice (Puts et al., 2012; Sell et al., 2009). Voice pitch reliably predicts physical size and upper body strength (Bruckert, Liénard, Lacroix, Kreutzer, & Leboucher, 2006; Evans et al., 2006; Hodges-Simeon, Gurven, Puts, & Gaulin, 2014; Puts et al., 2012), current testosterone levels (Bruckert et al., 2006; Dabbs & Mallinger, 1999; Evans et al., 2006; Puts et al., 2012), and pubertal androgen exposure (Harries, Hawkins, Hacking, & Hughes, 1997). Because of its associations with physical formidability, low voice pitch may erroneously suggest hunting ability to perceivers.
Alternatively, men with lower voice pitch may in fact be better hunters. Pubertal testosterone influences both muscle mass (Griggs et al., 1989) and voice pitch (Pedersen, Møller, Krabbe, & Bennett, 1986). Archery involves the use of several major arm muscles including muscles in the forearm and around the shoulder girdle (Ertran et al., 2003; Mann & Littke, 1989); in fact, the best physical predictor of hunting reputation is upper body strength in men (Apicella, 2014). To the extent that voice pitch predicts strength, voice pitch could serve as a cue to at least this component of hunting ability. Though this explanation is plausible, our data do not support it. We find that voice pitch and upper body strength independently predict hunting reputation. However, given our small sample size, it is important that this is tested in other, larger samples before ruling this explanation out.
Finally, it could be that hunting success affects men’s voice pitch through its role in affecting their status and confidence. While there are no clear dominance hierarchies in the Hadza (Marlowe, 2010), men may demonstrate their threat potential (Bliege Bird et al., 2001), as well as gain prestige through nonagonistic sources such as possessing superior aptitude and competency in a valued activity (Henrich & Gil-White, 2001). Certainly, hunting ability is one route by which men in hunter-gatherer societies may gain status (Gurven & von Rueden, 2006) and specifically respect, though other routes exist (von Rueden, Gurven, & Kaplan, 2008). Given the role of voice pitch in status signaling, it would not be surprising if better hunters adopt lower pitch voices and/or worse hunters adopt higher pitch voices. Indeed, much work has suggested that individuals will modulate their voice pitch depending on social context as well as their intentions to signal rank (Cheng, Tracy, Ho, & Henrich, 2016). For instance, individuals have been found to accommodate their voice pitch more if their conversation partner was of higher status (Gregory & Webster, 1996). Similarly, competitors who interact with one another will adjust their voice pitch to match their self-perceived dominance relative to the perceived dominance of their opponent (Puts et al., 2006). That is, men who perceive themselves as less dominant than their competitor will increase their voice pitch and vice versa. Future study may benefit by tracking both changes in hunting success and voice pitch over time to help establish the causal link between voice pitch and hunting reputation. Other work has shown that hunting success increases testosterone in men (Trumble, Smith, O’Connor, Kaplan, & Gurven, 2014).
If voice pitch is related to hunting reputation in men in part because of the influence of pubertal testosterone on both voice pitch and muscle mass, then this could explain why women with lower voices do not have better foraging reputations. Gathering consists mostly of digging up tubers often over a meter underground (Marlowe, 2005) and can thus be arduous labor requiring upper body strength and endurance. Indeed, variation in women’s hormonal profiles across societies may reflect the level of difficulty women face in acquiring resources, such that the more stressful their environment, the more adrenal androgens they produce (Cashdan, 2008). However, while increased testosterone levels cause lower voice pitch in pubescent males (Newman et al., 2000; Tossi et al., 1976), between-women variability in voice pitch may not reflect adult (Puts et al., 2016) or pubertal androgen levels. Thus, gathering reputation in women may relate to upper body strength and/or endurance, but not to voice pitch. Alternatively, women with higher voice pitch are perceived as more attractive in Western (Feinberg, DeBruine, Jones, & Perrett, 2008; Puts, Barndt, Welling, Dawood, & Burriss, 2011) and Hadza (Apicella & Feinberg, 2009) samples, possibly because it is a cue to reproductive potential (Wheatley et al., 2014). If this is the case, then lower voice pitch may not predict better gathering reputation because of competing selection for indicators of fertility. Finally, we may have found no relationship between voice pitch and gathering reputation in women because assessments of reputation were noisy, making it difficult to find a relationship in a small sample.
The fact that there is less agreement on women’s gathering reputation is itself interesting. It is possible that men do not pay as much attention to women’s gathering ability because it is less important to them. When ranking qualities most important in a spouse, Hadza men rank foraging ability only after character and physical attractiveness. This contrasts with Hadza women who rank hunting ability higher (Marlowe, 2004). It may be relatively more difficult to make judgments of women’s gathering ability because there is less disparity between women in their ability to gather. Indeed, gathering returns are markedly less variable than hunting returns (Berbesque et al., 2016). Alternatively, gathering returns may be less conspicuous because they are not widely shared, whereas hunting returns are.
Given that humans are characterized by optical primacy whereby traits are quickly and effortlessly assessed in others based on visual cues (Willis & Todorov, 2006), one might question the usefulness of the voice in providing any new information. However, multiple signaling is relatively common in multisensory, highly social animals like humans. It is thought that multiple signals are beneficial in animal communication because they reduce the risk of errors (e.g., Moller and Pomiankowski, 1993). So even when signals are redundant—providing the same information—they buffer against disruptions in the message due to environmental perturbations (for review, see Partan & Marler, 1999). Redundant signals can thus evoke the same response in a receiver when transmitted independently (i.e., equivalent responses) and sometimes, when transmitted together, they can result in enhanced responses (i.e., multiplicative responses; Partan & Marler, 1999). And finally, when signals are nonredundant, they provide more information per unit of time (Partan & Marler, 1999). For these reasons, we do not think it is unreasonable that voice pitch would be sexually selected even when other signals to mate quality or fighting ability exist. That said, the extent to which vocal and visual cues underlie the same aspects in men is poorly understood and debated. Feinberg (2008) suggests that men’s faces and voices reflect a common trait (i.e., hormone levels) which are cues to dominance and health. While some studies have documented a link between perceived attractiveness of the face and voice (e.g., Saxton, DeBruine, Jones, Little, & Roberts, 2009) and have shown that people make similar judgments independently from faces and voices about masculinity and health (e.g., Smith, Dunn, Baguley, & Stacey, 2016), other studies have not documented such associations (e.g., Lander, 2008; Valentova, Varella, Havlíček, & Kleisner, 2017). Yet other studies have shown that perceptions (Doll et al., 2014; Wheatley et al., 2014) and objective measurements (Hill et al., 2013) of faces and voices provide partly nonredundant information about mate quality and formidability.
The current study has some important limitations. The first is the small sample size and use of a single population. Small samples provide inaccurate estimates of effect size (Fritz, Scherndl, & Kühberger, 2013). Moreover, nonsignificant results, particularly voice pitch failing to predict reproductive success when controlling for hunting reputation, may be significant with a larger sample size. Also, the Hadza represent one of many hunter-gatherer populations and ideally, the findings should be replicated in other societies. Such work is important in moving from “proof-of-concept” demonstrations to generalizable knowledge (Apicella & Barrett, 2016). The second limitation is the use of foraging reputation as a proxy of foraging ability. Hunting returns are highly variable and difficult to measure over a short period (Hawkes, O’Connell, Blurton Jones, Oftedal, & Blumenschine, 1991), making the use of reputation necessary. It could be that reputation assessments are influenced by recent returns (Hill & Kintigh, 2009); however, there is no reason to suspect that error in estimating hunting ability systematically relates to voice pitch. Still, future study would benefit from examining actual caloric returns by both men and women. Finally, it could be the case that hunting reputation assessments are partly conflated with assessments of physical ability, as discussed above. Direct assessments of physical formidability would help pull apart these possibilities.
Voice pitch is one of the most sexually dimorphic features in humans, a result most likely due to sexual selection. Two main explanations for lower voice pitch in males are intrasexual competition—lower voice pitch signals competitive ability to other males—and intersexual selection—lower voice pitch signals mate quality to females, although of course these are not mutually exclusive possibilities. The association of voice pitch with hunting reputation does not distinguish between the two, as hunting success is linked to both competitive ability (Apicella, 2014; Hawkes & Bliege Bird, 2002) and mate choice (Marlowe, 2004, 2005). That is, the practice of hunting has been viewed as subject to intra- and intersexual selection. Given that previous work in this population has found that women do not prefer a more masculine voice pitch, and indeed may prefer a more feminine pitch (Apicella & Feinberg, 2009), it seems unlikely that low pitch is favored directly via female choice. Rather, low pitch may represent a by-product of traits related to hunting proficiency or it may be favored through its influence on perceptions of men’s hunting ability, formidability, and the like, which themselves attract women and/or induce deference from other men. The latter is consonant with many previous findings, for example, that low pitch increases perceptions of hunting ability in this population (Apicella & Feinberg, 2009) and fighting ability and dominance elsewhere (Puts et al., 2007; Saxton et al., 2016), as well as evidence that male anthropoid primates tend to evolve relatively low pitch when they compete more intensely for mates (Puts et al., 2016). Again, we stress that we are using a small sample, from a single population and more work is needed before firm conclusions are drawn.
Notes
Most foragers, including the Hadza, are egalitarian, have a sexual division of labor, practice central place foraging, trace descent bilaterally, mate monogamously, and allow for polygyny (Marlowe, 2010). When comparing the Hadza to 237 warm-climate, nonequestrian foraging societies, Marlowe (2010) showed that the Hadza lie close to the median value on many demographic traits, including calories contributed to the diet by men and women, weaning age, rates of reproduction, infant mortality, and so on.
Recent studies incorporating both genealogical data and Y-chromosome haplotyping suggests the rates for westerners is low—around 1% (Greeff & Erasmus, 2015). Very little data on rates of cuckoldry exist outside Western populations. Scleza (2011) reports some of the highest rates of cuckoldry among Himba pastoralists in Namibia, and Strassmann et al. (2012) estimate rates between 1.3 and 2.9 in different villages among the Dogon of Mali.
Note that when we exclude the male with the highest voice pitch and relatively low hunting reputation the coefficient borders significance (p = .052). However, this small change is more likely due to the decrease in sample size. Indeed, regression diagnostics, as measured by Cook’s D, indicated that this is a nonproblematic point (D = .003).
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was made possible through an NSF REG research grant (C.L.A.) and support from the Department of Anthropology at Harvard University (C.L.A.).
References
- Alvard M. S., Gillespie A. (2004). Good Lamalera whale hunters accrue reproductive benefits. In Alvard M. (Ed.), Socioeconomic aspects of human behavioral ecology (pp. 225–247). Bingley, England: Emerald Group Publishing. [Google Scholar]
- Apicella C. L. (2014). Upper-body strength predicts hunting reputation and reproductive success in Hadza hunter-gatherers. Evolution and Human Behavior, 35, 508–518. doi:10.1016/j.evolhumbehav.2014.07.001 [Google Scholar]
- Apicella C. L. (2017). High levels of rule-bending in a minimally religious and largely egalitarian forager population. Religion, Brain & Behavior. doi:10.1080/2153599X.2016.1267034 [Google Scholar]
- Apicella C. L., Azevedo E. M., Christakis N. A., Fowler J. H. (2014). Evolutionary origins of the endowment effect: Evidence from hunter-gatherers. American Economic Review, 1793–1805. doi:10.1257/aer.104.6.1793 [Google Scholar]
- Apicella C. L., Barrett H. C. (2016). Cross-cultural evolutionary psychology. Current Opinion in Psychology, 7, 92–97. doi:10.1016/j.copsyc.2015.08.015 [Google Scholar]
- Apicella C. L., Crittenden A. N. (2016). Hunter-gatherer families and parenting. In Buss D. M. (Ed.), The handbook of evolutionary psychology (pp. 578–597). Hoboken, NJ: Wiley. [Google Scholar]
- Apicella C. L., Crittenden A. N., Tobolsky V. A. (2017). Hunter-gatherer males are more risk-seeking than females even in late childhood. Evolution and Human Behavior. doi:10.1016/j.evolhumbehav.2017.01.003 [Google Scholar]
- Apicella C. L., Dreber A. (2015). Sex differences in competitiveness: Hunter-gatherer women and girls compete less in gender-neutral and male-centric tasks. Adaptive Human Behavior & Physiology, 1, 247–269. doi:10.1007/s40750-014-0015-z [Google Scholar]
- Apicella C. L., Feinberg D. R. (2009). Voice pitch alters mate-choice-relevant perception in hunter-gatherers. Proceedings of the Royal Society of London B: Biological Sciences, 276, 204–232. doi:10.1037/0033-295X.100.2.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella C. L., Feinberg D. R., Marlowe F. W. (2007). Voice pitch predicts reproductive success in male hunter-gatherers. Biology Letters, 6, 682–684. doi:10.1098/rsbl.2007.0410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella C. L., Marlowe F. W., Fowler J. H., Christakis N. A. (2012). Social networks and cooperation in hunter-gatherers. Nature, 481, 497–501. doi:10.1038/nature10736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartram L. E. (1997). A comparison of Kua (Botswana) and Hadza (Tanzania) bow and arrow hunting. In Knecht H. (Ed.), Projective technology (pp. 321–343). New York, NY: Springer. [Google Scholar]
- Berbesque J. C., Wood B. M., Crittenden A. N., Mabulla A., Marlowe F. W. (2016). Eat first, share later: Hadza hunter-gatherer men consume more while foraging than in central places. Evolution and Human Behavior, 37, 281–286. [Google Scholar]
- Bliege Bird R. L., Smith E. A., Bird D. W. (2001). The hunting handicap: Costly signaling in human foraging strategies. Behavioral Ecology and Sociobiology, 50, 9–19. doi:10.1007/s002650100338 [Google Scholar]
- Bruckert L., Liénard J. S., Lacroix A., Kreutzer M., Leboucher G. (2006). Women use voice parameters to asses men’s characteristics. Proceedings of the Royal Society of London B: Biological Sciences. doi:10.1098/rspb.2005.3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma P., Weenink D. (2007). Praat. Summer Institute of Linguistics. Retrieved fromhttp://www.praat.org
- Boonekamp J. J., Ros A. H., Verhulst S. (2008). Immune activation suppresses plasma testosterone level: A meta-analysis. Biology Letters, 4, 741–744. doi:10.1098/rsbl.2008.0347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouman A., Heineman M. J., Faas M. M. (2005). Sex hormones and the immune response in humans. Human Reproduction Update, 11, 411–423. doi:10.1093/humupd/dmi008 [DOI] [PubMed] [Google Scholar]
- Buss D. M., Schmitt D. P. (1993). Sexual strategies theory: An evolutionary perspective on human mating. Psychological Review, 100, 204–232. doi:10.1037/0033-295X.100.2.204 [DOI] [PubMed] [Google Scholar]
- Cashdan E. (2008). Waist-to-hip ratio across cultures: Trade-offs between androgen-and estrogen-dependent traits. Current Anthropology, 49, 1099–1107. doi:10.1086/593036 [Google Scholar]
- Cheng J. T., Tracy J. L., Ho S., Henrich J. (2016). Listen, follow me: Dynamic vocal signals of dominance predict emergent social rank in humans. Journal of Experimental Psychology: General, 145, 536–547. doi:10.1037/xge0000166 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T. H., Albon S. D. (1979). The roaring of red deer and the evolution of honest advertisement. Behaviour, 69, 145–170. doi:10.1163/156853979X00449 [Google Scholar]
- Collins S. A. (2000). Male voices and women’s choices. Animal Behaviour, 60, 773–780. doi:10.1006/anbe.2000.1523 [DOI] [PubMed] [Google Scholar]
- Coutinho A. E., Chapman K. E. (2011). The anti-inflammatory and immunosuppressive effects of glucocorticoids: Recent developments and mechanistic insights. Molecular and Cellular Endocrinology, 335, 2–13. doi:10.1016/j.mce.2010.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbs J. M., Mallinger A. (1999). High testosterone levels predict low voice pitch among men. Personality and Individual Differences, 27, 801–804. doi:10.1016/S0191-8869(98)00272-4 [Google Scholar]
- Doll L. M., Hill A. K., Rotella M. A., Cardenas R. A., Welling L. L., Wheatley J. R., Puts D. A. (2014). How well do men’s faces and voices index mate quality and dominance? Human Nature, 25, 200–212. doi:10.1007/s12110-014-9194-3 [DOI] [PubMed] [Google Scholar]
- Dominguez-Rodrigo M., Buhn H. T., Mabulla A. Z. P., Baquedano E., Uribelarrea D., Pérez-Gonzáles A.…Ansón M. (2014). On meat eating and human evolution: A taphonomic analysis of BK4b (Upper Bed II, Olduvai Gorge, Tanzania), and its bearing on hominin megafaunal consumption. Quartenary International, 322, 129–152. doi:10.1016/j.quaint.2013.08.015 [Google Scholar]
- Ellison P. T. (2011). On fertile ground. Cambridge, MA: Harvard University Press. [Google Scholar]
- Ertran H., Kentel B., Tümer S. T., Korkusuz F. (2003). Activation patterns in forearm muscles during archery shooting. Human Movement Science, 22, 37–45. doi:10.1016/S0167-9457(02)00176-8 [DOI] [PubMed] [Google Scholar]
- Evans S., Neave N., Wakelin D. (2006). Relationships between vocal characteristics and body size and shape in human males: An evolutionary explanation for a deep male voice. Biological Psychology, 72, 160–163. doi:10.1016/j.biopsycho.2005.09.003 [DOI] [PubMed] [Google Scholar]
- Feinberg D. R. (2008). Are human faces and voices ornaments signaling common underlying cues to mate value? Evolutionary Anthropology: Issues, News, and Reviews, 17, 112–118. [Google Scholar]
- Feinberg D. R., DeBruine L. M., Jones B. C., Little A. C., O’Connor J. J. M., Tigue C. C. (2012). Women’s self-perceived health and attractiveness predict their male vocal masculinity preferences in different directions across short- and long-terms relationship contexts. Behavioral Ecology and Sociobiology, 66, 413–418. doi:10.1007/s00265-011-1287-y [Google Scholar]
- Feinberg D. R., DeBruine L. M., Jones B. C., Perrett D. I. (2008). The role of femininity and averageness of voice pitch in aesthetic judgments of women’s voices. Perception, 37, 615–623. doi:10.1068/p5514 [DOI] [PubMed] [Google Scholar]
- Feinberg D. R., Jones B. C., Law Smith M. J., Moore F. R., DeBruine L. M., Cornwell R. E.…Perrett D. I. (2006). Menstrual cycle, train estrogen level, and masculinity preferences in the human voice. Hormones and Behavior, 49, 215–222. doi:10.1016/j.yhbeh.2005.07.004 [DOI] [PubMed] [Google Scholar]
- Feinberg D. R., Jones B. C., Little A. C., Burt D. M., Perrett D. I. (2005). Manipulations of fundamental and formant frequencies influence the attractiveness of human male voices. Animal Behaviour, 69, 561–568. doi:10.1016/j.anbehav.2004.06.012 [Google Scholar]
- Folstad I., Karter A. J. (1992). Parasites, bright males, and the immunocompetence handicap. The American Naturalist, 139, 603–622. [Google Scholar]
- Fritz A., Scherndl T., Kühberger A. (2013). A comprehensive review of reporting practices in psychological journals: Are effect sizes really enough? Theory and Psychology, 23, 98–122. doi:10.1177/0959354312436870 [Google Scholar]
- Greeff J. M., Erasmus J. C. (2015). Three hundred years of low non-paternity in a human population. Heredity, 115, 396–404. doi:10.1038/hdy.2015.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory S. W., Webster S. (1996). A nonverbal signal in voices of interview partners effectively predicts communication accommodation and social status perceptions. Journal of Personality and Social Psychology, 70, 1231–1240. doi:10.1037/0022-3514.70.6.1231 [DOI] [PubMed] [Google Scholar]
- Griggs R. C., Kingston W., Jozefowicz R. F., Herr B. E., Forbes G., Halliday D. (1989). Effect of testosterone on muscle mass and muscle protein synthesis. Journal of Applied Physiology, 66, 498–503. [DOI] [PubMed] [Google Scholar]
- Gurven M., Kaplan H., Gutierrez M. (2006). How long does it take to be a proficient hunter? Implications for the evolution of extended development and long life span. Journal of Human Evolution, 51, 454–470. doi:10.1016/j.jhevol.2006.05.003 [DOI] [PubMed] [Google Scholar]
- Gurven M., von Rueden C. (2006). Hunting, social status and biological fitness. Biodemography and Social Biology, 53, 81–99. doi:10.1080/19485565.2006.9989118 [DOI] [PubMed] [Google Scholar]
- Harries M., Hawkins S., Hacking J., Hughes I. (1997). Changes in the male voice at puberty. Archives of Disease in Childhood, 77, 445–447. doi:10.1136/adc.77.5.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser M. D. (1993). The evolution of nonhuman primate vocalizations: Effect of phylogeny, body weight, and social context. The American Naturalist, 142, 528–542. [DOI] [PubMed] [Google Scholar]
- Hawkes K. (1991). Showing off: Tests of an hypothesis about men’s foraging goals. Ethology and Sociobiology, 12, 29–54. doi:10.1016/0162-3095(91)90011-E [Google Scholar]
- Hawkes K., Bliege Bird R. L. (2002). Showing off, handicap signaling, and the evolution of men’s work. Evolutionary Anthropology, 11, 58–67. doi:10.1002/evan.20005 [Google Scholar]
- Hawkes K., O’Connell J. F., Blurton Jones N. G. (2001). Hadza meat sharing. Evolution and Human Behavior, 22, 113–142. doi:10.1016/S1090-5138(00)00066-0 [DOI] [PubMed] [Google Scholar]
- Hawkes K., O’Connell J. F., Blurton Jones N. G., Oftedal O. T., Blumenschine R. J. (1991). Hunting income patterns among the Hadza: Big game, common goods, foraging goals, and the evolution of the human diet. Proceedings of the Royal Society of London B: Biological Sciences, 334. doi:10.1098/rstb.1991.0113 [DOI] [PubMed] [Google Scholar]
- Henrich J., Gil-White F. J. (2001). The evolution of prestige: Freely conferred deference as a mechanism for enhancing the benefits of cultural transmission. Evolution and Human Behavior, 22, 165–196. doi:10.1016/S1090-5138(00)00071-4 [DOI] [PubMed] [Google Scholar]
- Heymsfield S. B., McManus C., Smith J., Stevens V., Nixon D. W. (1982). Anthropometric measurement of muscle mass: Revised equations for calculating bone-free arm muscle area. The American Journal of Clinical Nutrition, 36, 680–690. [DOI] [PubMed] [Google Scholar]
- Hill A. K., Hunt J., Welling L. L. M., Cárdenas R. A., Rotella M. A., Wheatley J. R.…Puts D. A. (2013). Quantifying the strength and form of sexual selection on men’s traits. Evolution and Human Behavior, 34, 334–341. doi:10.1016/j.evolhumbehav.2013.05.004 [Google Scholar]
- Hill K., Hurtado A. M. (1996). Ache life history: The ecology and demography of a foraging people. Hawthorne, NY: Aldine de Gruyter. [Google Scholar]
- Hill K., Kintigh K. (2009). Can anthropologists distinguish good and poor hunters? Implications for hunting hypotheses, sharing conventions, and cultural transmission. Current Anthropology, 50, 369–378. doi:10.1086/597981 [Google Scholar]
- Hodges-Simeon C. R., Gurven M., Puts D. A., Gaulin S. J. C. (2014). Vocal fundamental and formant frequencies are honest signals of threat potential in peripubertal males. Behavioral Ecology, 25, 984–988. doi:10.1093/beheco/aru081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H., Hill K., Lancaster J., Hurtado M. (2000). A theory of human life history evolution: Diet, intelligence, and longevity. Evolutionary Anthropology, 9, 156–185. [Google Scholar]
- Kaplan H., Gurven M., Hill K., Hurtado A. M. (2005). The natural history of human food sharing and cooperation: a review and a new multi-individual approach to the negotiation of norms. Moral Sentiments and Material Interests: The Foundations of Cooperation in Economic Life, 6, 75–113. [Google Scholar]
- Lander K. (2008). Relating visual and vocal attractiveness for moving and static faces. Animal Behaviour, 75, 817–822. [Google Scholar]
- Mann D. L., Littke N. (1989). Should injuries in archery. Canadian Journal of Sports Sciences, 14, 85–92. [PubMed] [Google Scholar]
- Marlowe F. W. (2001). Male contribution to diet and female reproductive success among foragers. Current Anthropology, 42, 755–759. doi:10.1086/323820 [Google Scholar]
- Marlowe F. W. (2003). A critical period for provisioning by Hadza men: Implications for pair bonding. Evolution and Human Behavior, 24, 217–229. doi:10.1016/S1090-5138(03)00014-X [Google Scholar]
- Marlowe F. W. (2004). Mate preferences among Hadza hunter-gatherers. Human Nature, 15, 364–375. doi:10.1007/s12110-004-1014-8 [DOI] [PubMed] [Google Scholar]
- Marlowe F. W. (2005). Who tends Hadza children? In Hewlett B. S., Lamb M. E. (Eds.), Hunter-gatherer childhoods (pp. 177–190). New Brunswick, NJ: Aldine. [Google Scholar]
- Marlowe F. W. (2010). The Hadza: Hunter-gatherers of Tanzania. Berkley: University of California Press. [Google Scholar]
- Møller A. P., Pomiankowski A. (1993). Why have birds got multiple sexual ornaments? Behavioral Ecology and Sociobiology, 32, 167–176. [Google Scholar]
- Newman S., Butler J., Hammond E. H., Gray S. D. (2000). Preliminary report on hormone receptors in the human vocal fold. Journal of Voice, 14, 72–81. doi:10.1016/S0892-1997(00)80096-X [DOI] [PubMed] [Google Scholar]
- Partan S., Marler P. (1999). Communication goes multimodal. Science, 283, 1272–1273. [DOI] [PubMed] [Google Scholar]
- Pedersen M. F., Møller S., Krabbe S., Bennett P. (1986). Fundamental voice frequency measured by electroglottography during continuous speech. A new exact secondary sex characteristic in boys in puberty. International Journal of Pediatric Otorhinolaryngology, 11, 21–27. doi:10.1016/S0165-5876(86)80024-6 [DOI] [PubMed] [Google Scholar]
- Pisanski K., Fraccaro P. J., Tigue C. C., O’Connor J. J. M., Röder S., Andrews P. W.…Feinberg D. R. (2014). Vocal indicators of body size in men and women: A meta-analysis. Animal Behaviour, 95, 89–99. doi:10.1016/j.anbehav.2014.06.011 [Google Scholar]
- Prall S. P., Muehlenbein M. P. (2014). Testosterone and immune function in primates: A brief summary with methodological considerations. International Journal of Primatology, 35, 805–824. doi:10.1007/s10764-014-9752-x [Google Scholar]
- Puts D. A. (2005). Mating context and menstrual phase affect women’s preferences for male voice pitch. Evolution and Human Behavior, 26, 388–397. doi:10.1016/j.evolhumbehav.2005.03.001 [Google Scholar]
- Puts D. A. (2006). Cyclic variation in women’s preferences for masculine traits: Potential hormonal causes. Human Nature, 17, 114–127. doi:10.1007/s12110-006-1023-x [DOI] [PubMed] [Google Scholar]
- Puts D. A., Apicella C. L., Cárdenas R. A. (2012). Masculine voices signal men’s threat potential in forager and industrial societies. Proceedings of the Royal Society of London B: Biological Sciences, 279, 601–609. doi:10.1098/rspb.2011.0829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts D. A., Barndt J. L., Welling L. L. M., Dawood K., Burriss R. P. (2011). Intrasexual competition among women: Vocal femininity affects perceptions of attractiveness and flirtatiousness. Personality and Individual Differences, 50, 111–115. doi:10.1016/j.paid.2010.09.011 [Google Scholar]
- Puts D. A., Doll L. M., Hill A. K. (2014). Sexual selection on human voices. In Weekes-Shackelford V. A., Schackelford T. K. (Eds.), Evolutionary perspectives on human sexual psychology and behavior (pp. 69–86). New York, NY: Springer. [Google Scholar]
- Puts D. A., Gaulin S. J. C., Verdolini K. (2006). Dominance and the evolution of sexual dimorphism in human voice pitch. Evolution and Human Behavior, 27, 283–296. doi:10.1016/j.evolhumbehav.2005.11.003 [Google Scholar]
- Puts D. A., Hill A. K., Bailey D. H., Walker R. S., Rendall D., Wheatley J. R.…Jablonski N. G. (2016). Sexual selection on male vocal fundamental frequency in humans and other anthropoids. Proceedings of the Royal Society of London B: Biological Sciences, 283. doi:10.1098/rspb.2015.2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts D. A., Hodges C., Cárdenas R. A., Gaulin S. J. C. (2007). Men’s voices as dominance signals: Vocal fundamental and formant frequencies influence dominance attributions among men. Evolution and Human Behavior, 28, 340–344. doi:10.1016/j.evolhumbehav.2007.05.002 [Google Scholar]
- Rantala M. J., Moore F. R., Skrinda I., Krama T., Kivleniece I., Kecko S., Krams I. (2012). Evidence for the stress-linked immunocompetence handicap hypothesis in humans. Nature Communications, 3, 694–698. doi:10.1038/ncomms1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendall D., Kollias S., Ney C., Lloyd P. (2005). Pitch (F0) and formant profiles of human vowels and vowel-like baboon grunts: The role of vocalizer body size and voice-acoustic allometry. Journal of the Acoustic Society of American, 117, 944–955. doi:10.1121/1.1848011 [DOI] [PubMed] [Google Scholar]
- Roberts M. L., Buchanan K. L., Evans M. R. (2004). Testing the immunocompetence handicap hypothesis: A review of the evidence. Animal Behaviour, 68, 227–239. doi:10.1016/j.anbehav.2004.05.001 [Google Scholar]
- Saez S., Sakai F. (1976). Androgen receptors in human pharyngo-laryngeal mucosa and pharyngo-laryngeal epithelioma. Journal of Steroid Biochemistry and Molecular Biology, 7, 919–921. doi:10.1016/0022-4731(76)90011-X [DOI] [PubMed] [Google Scholar]
- Saxton T. K., Caryl P. G., Roberts C. (2006). Vocal and facial attractiveness judgments of children, adolescents, and adults: The ontogeny of mate choice. Ethology, 112, 1179–1185. doi:10.1111/j.1439-0310.2006.01278.x [Google Scholar]
- Saxton T. K., DeBruine L. M., Jones B. C., Little A. C., Roberts S. C. (2009). Face and voice attractiveness judgments change during adolescence. Evolution and Human Behavior, 30, 398–408. [Google Scholar]
- Saxton T. K., Mackey L. L., McCarty K., Neave N. (2016). A lover or a fighter? Opposing sexual selection pressures on men’s vocal pitch and facial hair. Behavioral Ecology, 27, 512–519. doi:10.1093/beheco/arv178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell A. Bryant G. A. Cosmides L. Tooby J. Sznycer D. von Rueden C.…Gurven M. (2009). Adaptations in humans for assessing physical strength from the voice. Proceedings of the Royal Society in London B: Biological Sciences, 277, 3509–3518. doi:10.1098/rspb.2010.0769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scelza B. A. (2011). Female choice and extra-pair paternity in a traditional human population. Biology Letters, rsbl20110478. doi:10.1098/rsbl.2011.0478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassmann B. I., Kurapati N. T., Hug B. F., Burke E. E., Gillespie B. W., Karafet T. M., Hammer M. F. (2012). Religion to assure paternity. Proceedings of the National Academy of Sciences, 109, 9781–9785. doi:10.1073/pnas.1110442109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. A., Bliege Bird R. L., Bird D. W. (2003). The benefits of costly signaling: Meriam turtle-hunters. Behavioral Ecology, 14, 116–126. doi:10.1093/beheco/14.1.116 [Google Scholar]
- Smith H. M., Dunn A. K., Baguley T., Stacey P. C. (2016). Concordant cues in faces and voices: Testing the backup signal hypothesis. Evolutionary Psychology, 14. doi:10.1177/1474704916630317 [Google Scholar]
- Titze I. R. (1994). Principles of voice production. Englewood Cliffs, NJ: Prentice Hall. [Google Scholar]
- Tossi O., Postan D., Bianculli C. (1976). Longitudinal study of children’s voice at puberty. XVIth International Congress Logopedics and Phoniatrics, 486–490. [Google Scholar]
- Trumble B. C., Smith E. A., O’Connor K. A., Kaplan H. S., Gurven M. (2014). Successful hunting increases testosterone and cortisol in a subsistence population. Proceedings of the Royal Society in London B: Biological Sciences, 281. doi:10.1098/rspb.2013.2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentova J. V., Varella M. A. C., Havlíček J., Kleisner K. (2017). Positive association between vocal and facial attractiveness in women but not in men: A cross-cultural study. Behavioural Processes, 135, 95–100. [DOI] [PubMed] [Google Scholar]
- von Rueden C., Gurven M., Kaplan H. (2008). The multiple dimensions of male social status in an Amazonian society. Evolution and Human Behavior, 29, 402–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Rueden C. R., Jaeggi A. V. (2016). Men’s status and reproductive success in 33 nonindustrial societies: Effects of subsistence, marriage system, and reproductive strategy. Proceedings of the National Academy of Sciences, 113, 10824–10829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn S., Lancaster C. (1968). The evolution of hunting. In Lee R. B., Devore I. (Eds.), Man the hunter (pp. 292–303). Chicago, IL: Aldine. [Google Scholar]
- Wheatley J. R., Apicella C. L., Burriss R. P., Cárdenas R. A., Bailey D. H., Welling L. L., Puts D. A. (2014). Women’s faces and voices are cues to reproductive potential in industrial and forager societies. Evolution and Human Behavior, 35, 264–271. doi:10.1016/j.evolhumbehav.2014.02.006 [Google Scholar]
- Wiessner P. (2002). Hunting, healing, and H’xaro exchange: A long-term perspective on !Kung (Ju/’hoansi) large-game hunting. Evolution and Human Behavior, 23, 407–436. doi:10.1016/S1090-5138(02)00096 [Google Scholar]
- Willis J., Todorov A. (2006). First impressions making up your mind after a 100-ms exposure to a face. Psychological Science, 17, 592–598. [DOI] [PubMed] [Google Scholar]
- Wolff S. E., Puts D. A. (2010). Vocal masculinity is a robust dominance signal in men. Behavioral Ecology and Sociobiology, 64, 1673–1683. doi:10.1007/s00265-010-0981-5 [Google Scholar]


