Abstract
Background
In aesthetic clinical practice, botulinum toxin type A (BoNT-A) is best known for its use as a neuromodulator for the treatment of dynamic facial lines; however, when injected intradermally as microdroplets, BoNT-A can improve skin quality and overall skin appearance.
Objectives
To discuss key aspects of microtoxin use in clinical practice and provide expert guidance on utilization.
Methods
As part of a continuing medical education lecture series and roundtable, the authors discussed key aspects of microtoxin patient selection, injection technique, and safety.
Results
The experiences of expert faculty are shared here. Clinical experience is consistent with reported data. Microtoxin can be used to reduce pore size, sebum production, rosacea, acne, and fine lines, and to improve jawline and neck definition. Intradermal injection can also be employed for the improvement of transverse neck lines as well as for the safe prevention and management of scars and keloids.
Conclusions
Expanding the use of BoNT-A, a predictable, minimally invasive, and affordable treatment to address commonly encountered complaints is appealing. The authors have found that making patients aware of microtoxin as a treatment option results in an increased interest in and utilization of BoNT-A, and high satisfaction among appropriately selected patients.
Resumo
Antecedentes
Na prática clínica estética, a toxina botulínica tipo A (BoNT-A) é mais conhecida por sua utilização como neuromodulador no tratamento de linhas faciais dinâmicas. Entretanto, quando injetada via intradérmica na forma de microgotículas, a BoNT-A pode melhorar a qualidade e o aspecto geral da pele.
Objetivos
Discutir os principais aspectos da utilização de microtoxinas na prática clínica e oferecer orientação especializada sobre a utilização.
Métodos
Como parte de uma série de palestras e mesa redonda de educação médica continuada, os autores discutiram os principais aspectos da seleção de microtoxinas para pacientes, técnicas de injeção e segurança.
Resultados
As experiências de docentes especialistas são compartilhadas aqui. A experiência clínica é consistente com os dados relatados. A microtoxina pode ser utilizada na redução do tamanho dos poros, produção de sebo, rosácea, acne e linhas finas, e para melhorar a definição da mandíbula e do pescoço. A injeção intradérmica também pode ser empregada para alcançar a melhoria das linhas transversais do pescoço, bem como para a prevenção e tratamento seguro de cicatrizes e queloides.
Conclusões
É interessante considerar a expansão da utilização da BoNT-A, um tratamento previsível, minimamente invasivo e acessível, no tratamento de reclamações que ocorrem frequentemente. Os autores constataram que conscientizar os pacientes sobre a microtoxina como uma opção de tratamento resulta em um maior interesse e utilização da BoNT-A, e uma alta satisfação entre os pacientes selecionados adequadamente.
Level of Evidence: 4

In aesthetic medicine, botulinum toxin type A (BoNT-A) is best known for its use as a neuromodulator for the treatment of dynamic facial lines and platysmal bands, for addressing facial asymmetry, and to create lift in the lower face through treatment of depressor muscles. Each of these uses stems from the ability of the BoNT-A toxin to inhibit the release of acetylcholine (ACh) at the neuromuscular junction, causing a loss in cholinergic signaling and resulting in temporary denervation and modulation of muscle activity.1 However, ACh also acts on sympathetic nerves in glandular tissues, where it stimulates the sweat and salivary glands.2 Inhibition of this additional ACh signaling pathway is the basis for BoNT-A treatment of hyperhidrosis and sialorrhea.3 Importantly, BoNT-A receptors and intracellular targets are not isolated to neuronal cells. ACh also has important roles within the nonneuronal cholinergic system, where through complex networks it regulates keratinocyte proliferation, skin homeostasis, sebum production, pigment, microcirculation, and inflammation (such as that found in acne vulgaris or atopic eczema), among other activities.4,5 In addition to these activities, the effect of BoNT-A on fibroblast activity has led to the study of BoNT-A for improving wound healing and decreasing hypertrophic scars, with promising results both in vitro and clinically.5-8
For the aesthetic clinician, this broad range of activities raises the question of whether BoNT-A utilization can be expanded beyond intramuscular injection in the clinic to include intradermal injection for management of issues outside of dynamic lines. Theoretically, when injected intradermally, BoNT-A can exert an effect on the overlying skin through multiple pathways. BoNT-A can act on facial muscles or the platysma where they insert into the skin, adressing fine lines and/or creating lift when acting on depressor muscles; can act on glandular tissues (reducing sebum production); and may exert an effect on other noncholinergic pathways.
Dr Woffles Wu (W.W.) was one of the earliest proponents of the use of BoNT-A injections into the face for cosmetic brow lifting, reduction of glabellar frown and crow's feet lines, and also for facial slimming.9-13 He observed and noted that in many of his cases injected in the glabellar and forehead regions between 1995 and 2000, the forehead skin often became smooth to the touch, glistening, lustrous, and tight. Reasoning that this result was due to the diffusion of intramuscular BoNT-A (placed into the frontalis) into components of the overlying skin such as sweat and sebaceous glands and perhaps other adnexae, he endeavored to pioneer an injection technique that would consistently produce this effect in the forehead skin without total paralysis of the frontalis.
The first logical step was to make the injected droplets of botulinum toxin smaller and more superficial in the dermal-subdermal layer so that diffusion of these tiny droplets into the underlying frontalis muscle would be confined to the upper layer of the muscle. In this way, forehead lines would be decreased because these lines are created by the attachment of the superficial muscle fibers to the undersurface of the skin envelope. At the same time, the elevating function of the frontalis would be largely retained because the deeper fibers of the muscle were not paralyzed.
In 2001, W.W. conceptualized and performed the injection of microdroplets of diluted BoNT-A into the skin and immediate subdermal plane for the purpose of increasing skin sheen and luminosity, reduction of pore size and sebum production, and a reduction of fine lines in the forehead without compromising frontalis muscle action. This microtoxin technique, initially termed Mesobotox, was subsequently changed to Microbotox for scientific accuracy, because W.W. felt that “micro” better described the size of the droplets injected within the dermal-subdermal layer. The inclusion of the Botox brand name (Allergan Aesthetics; Irvine, CA) in this technique was reflective of the fact that at the time Botox was the dominant BoNT-A brand available.
W.W. published his early experience with this technique in 2006, and since that time the technique has been employed successfully to treat rosacea, acne, facial fine lines and laxity; for the improvement of transverse neck lines and jawline and neck definition; for the prevention and management of incisions and scars; and for the treatment of keloids.14-24 This experience was clearly detailed in W.W.’s 2015 paper on Microbotox of the lower face and neck, which further refined the dilution and injection techniques for different indications.21 Today, with so many brands of BoNT-A available, it is more appropriate to utilize the umbrella term “microtoxin treatment,” as any of the avialable products may be used in this technique.
The opportunity to expand use of a predictable, minimally invasive, and affordable treatment such as BoNT-A to address commonly encountered complaints in clinical practice is appealing, especially as patient demographics trend younger and interest in injectables continues to rise. Furthermore, the ability of intradermal BoNT-A to act through multiple mechanisms can give rise to global improvement, and therefore high patient satisfaction.25
As part of this roundtable on the use of microtoxin in clinical practice, the authors discussed key aspects of patient selection, injection technique, and safety. The authors have found that making patients aware of microtoxin as a treatment option has resulted in increased interest, increased employment of BoNT-A in the clinic, and high satisfaction among appropriately selected patients. Their experiences are shared here.
METHODS
As part of a continuing medical education event developed by xMedica (Alpharetta, GA) that took place in June 2022, a team of international experts on BoNT-A consisting of 2 dermatologists, 1 aesthetic and regenerative medicine physician, and 1 plastic surgeon developed a 2-hour virtual webinar including a lecture on microtoxin procedures, a faculty discussion, and patient demonstrations. The lecture included a discussion of the BoNT-A mechanism of action underpinning skin quality improvements (pore size, fine lines, and sebum production, among others) and the clinical research describing procedure outcomes. The activity was concluded by a 1-hour roundtable during which faculty presented case studies and discussed their real-world experience with microtoxin injection in clinical practice. Live demonstration videos were also included. Topics covered included patient selection, microtoxin technique, and special safety considerations. All patients discussed were treated in accordance with the principles outlined in the Declaration of Helsinki, and each patient consented to treatment and photography.
RESULTS
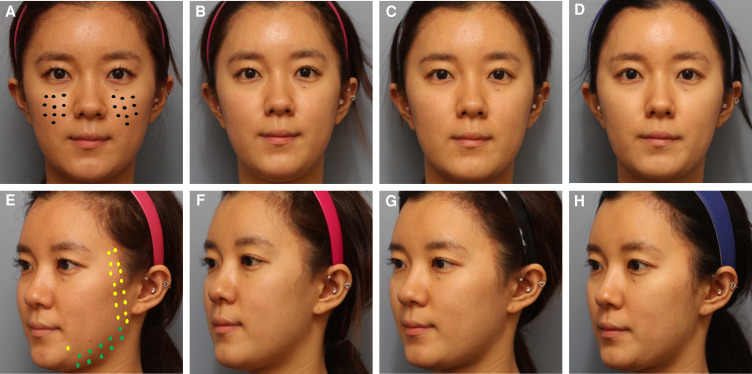
Microtoxin is a fundamentally different treatment approach than standard intramuscular injection. For microtoxin treatment, BoNT-A is diluted and injected in the intradermal layer over the entire surface area where improvement is desired so that the toxin can have an even, wide field of effect on motor nerves, sympathetic nerves, and other cellular pathways. Thus, irrespective of the primary treatment goal, it is critical to have a sufficient number of injections in the correct plane so that the entire area is exposed to BoNT-A. For example, treatment of the neck can require 200 microdroplet injections, whereas treatment of the entire face can require up to 500 injections (5 mL divided into microdroplets). However, this is a flexible technique, and targeting individual facial regions can require fewer injection sites. In Figure 1, baseline images include an example treatment pattern.
Figure 1.
A 30-year-old female patient who presented with concerns related to skin quality (pore size and skin texture) and a desire to narrow her lower face and address excess submental volume. The patient was injected intradermally with 100 U of incobotulinum toxin in 5 mL saline over her entire face. The patient is shown at baseline (A, E); and at postinjection week 1 (B, F); week 4 (C, G); and week 12 (D, H). Closed circles in the midface (Black) indicate 0.5 U, lighter closed circles on the lateral face and DAO (yellow) indicate 1 U, and darker closed circles on the lower lateral face (green) dots indicate 2 U, for a total of 82 U.
In a busy aesthetics practice, microtoxin can develop as a dermatologic treatment employed frequently for a wide range of patients. The learning curve is not steep, and for a practiced injector the procedure takes between 10 and 15 minutes. Given the broad range of effect and the potential to manage a range of issues from fine lines to skin sheen and texture, most patients can benefit from microtoxin treatment. Indeed, for one of the authors, microtoxin has eclipsed traditional injection techniques for treatment of dynamic lines. For surgical practices, microtoxin following surgery is especially helpful for managing incisions and scars. In the sections below, details of injection are included and patient case studies discussed.
DISCUSSION
Patient Selection
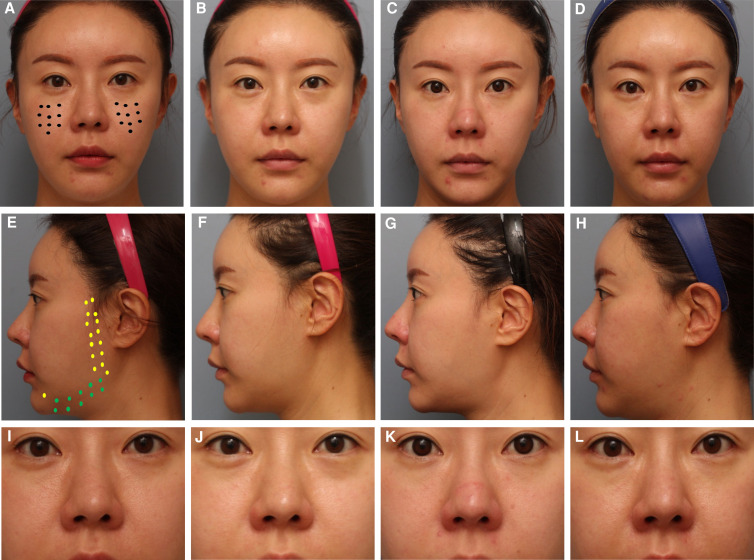
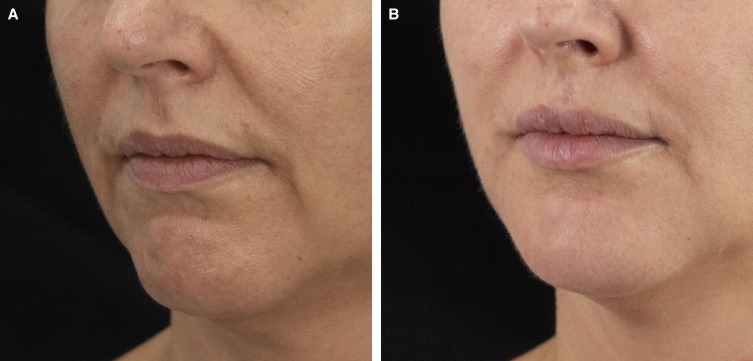
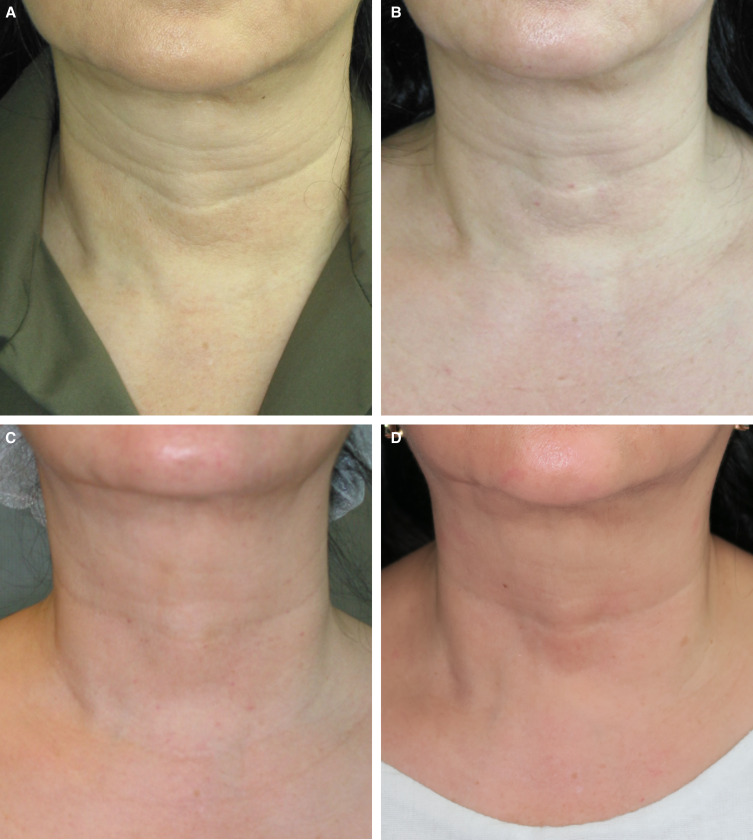
Microtoxin treatment can improve global appearance in a variety of ways. However, in clinical practice, the most pronounced improvements are in younger patients with oily skin and enlarged pores for whom a moderate neuromodulatory effect on the mimetic muscles is sufficient to resolve dynamic lines or to relax depressor muscle such that the contours of the neck are improved and the lower face and jawline are better defined. This type of patient is shown in Figures 1, 2. In each of these cases, the improvement in skin quality is apparent, as is the effect of neuromodulation on the face overall, the width of the lower face, and submental fullness. Although, for these patients, the most apparent improvement in pore size is in the midface, there are multiple areas of the face prone to excess sebum production where neuromodulation is beneficial, including the glabellar complex, forehead, and chin. For the patient shown in Figure 3, improvements in pore size are apparent in the midface, and injection of the chin has led to an improvement in texture and pore size as well as reduced the appearance of acne scars. Importantly, in spite of ethnic variability in pore size and density, reduction in pore appearance is a desired outcome for a wide range of patients from varying ethnic backgrounds.26 The patient in Figure 3 was also treated in the lower face, and improvements in the patient's resting expression are apparent. In Figure 4, the effect of microtoxin on transverse lines is shown. When treating the platysma, patients injected along the mandibular border can expect improvement in the jawline contour. When injected in the neck, transverse neck lines, platysmal banding, and overall smoothness of the neck are improved. Although most patients can benefit from microtoxin treatment for various reasons, microtoxin is unlikely to yield the needed effect in patients with significant laxity in the areas where they would like to see improvement.
Figure 2.
A 36-year-old female patient who presented with concerns related to skin quality (pore size and texture) and a desire to narrow her lower face and address excess submental volume. The patient was injected intradermally with 90 U of incobotulinum toxin in 5 mL saline over her entire face. The patient is shown at baseline (A, E, I); and postinjection week 1 (B, F, J); week 4 (C, G, K); and week 12 (D, H, L). Closed circles on the midface (Black) indicate 0.5 U, lighter closed circles on the lateral face and DAO (yellow) indicate 1 U, and darker closed curcles on the lower lateral face (green) indicate 2 U, for a total of 82 U.
Figure 3.
A 50-year-old female treated in the chin with 1.5 mL of hyaluronic acid filler and microtoxin treatment to the dermis overlying the entire sublabial zone, over the chin and laterally to the marionette line, and superiorly to the lower lip vermillion border with 2.5 U of incobotulinum toxin diluted with 0.6 mL of saline. In addition, the upper lip was treated with 1.0 U of incobotulinum toxin in 0.6 mL of saline and the medial cheeks were treated with a total of 4 U on each side in 0.6 mL of saline. The patient is shown (A) before and (B) 1 month after treatment.
Figure 4.
A 50-year-old female treated for horizontal neck lines. The patient is shown at (A) baseline and post injection at (B) week 2, (C) month 2, and (D) 1 year. Microtoxin treatment consisted of 56 U of onabotulinum toxin A (28 U per side in 1 mL of saline), delivered as 200 total intradermal injections across the surface of the neck.
Microtoxin Injection Technique
BoNT-A product should be diluted for microtoxin treatment. Although a range of dilutions can give rise to excellent results, a final concentration of ∼20 U/mL is well-suited for treatment of the face. This concentration can be attained by reconstituting 100 U of BoNT-A with 5 mL of bacteriostatic saline or by adding 0.5 mL of 100 U BoNT-A in 2.5 mL saline to 0.5 mL of 0.5% lidocaine in a 1-mL syringe. Between 100 and 120 microdroplets can be delivered with each milliliter of solution, which is generally sufficient to treat the forehead, glabellar complex, or lateral canthal areas. To treat the entire face, 100 U in 5 mL is needed. To treat the lower face and neck, a slightly more concentrated solution of 28 U/mL is recommended for each side (total 56 U).
A straightforward method for preparing 3 different dilutions for different applications, described by W.W. in 2015 and in a subsequent 2017 publication, is summarized below and in Table 1:21,22
Table 1.
Overview of Microtoxin Dilutions for Different Aesthetic Concerns
| Microbotox concentration | Amount of 100 U/2.5 mL (40 U/mL) BoNT-A per 1-mL syringe | Preferred application |
|---|---|---|
| 20 U/mL | 0.5 mL | Treating the forehead and cheeks for pore size, controlling sebum, sweating, rosacea, and acne, and smoothing facial skin texture |
| 24 U/mL | 0.6 mL | Treating glabellar, forehead, and crow's feet lines in females and for axillary hyperhidrosis |
| 28 U/mL | 0.7 mL | For neck contouring and smoothing, and for the prevention of postoperative or posttraumatic hypertrophic scar formation Treatment of established keloids Treating glabellar, forehead, and crow's feet lines in males |
BoNT-A, botulinum toxin type A.
Begin with a standard vial of BoNT-A at a concentration of 100 U in 2.5 mL saline (40 U/mL).
In a 1-mL syringe, draw up either 0.5 mL (20 U), 0.6 mL (24 U), or 0.7 mL (28 U), depending on the final concentration needed.
An appropriate amount of saline or lidocaine is then added to this syringe to bring the volume to 1 mL.
Following preparation of the skin with 5% topical lidocaine for 20 minutes (which is subsequently rinsed off), microtoxin can be administered with a 1-mL syringe and a 1-inch, 32- or 34-gauge needle. For the face, microdroplets can range in size from ∼0.01 to 0.05 mL placed in a 1-cm2 grid pattern or closer. Each microdroplet on the neck amounts to ∼0.01 mL and can be placed in a 1-cm2 grid pattern. The needle should be inserted as superficially as possible, and there should be some resistance as the injector pushes the plunger for a very short period. Practice is needed for consistent delivery of microdroplets, and the appearance of small blebs under the skin with some blanching are an indicator that the injection is in the right plane and that delivery of the BoNT-A is consistent. Changing needles in the middle of the procedure can improve patient comfort. An injection is shown in the Video.
Duration of Treatment
Treatment effect begins at 1 week and is apparent by 4 weeks. The durability of microtoxin is about 3 months, and the ideal time for retreatment is 2 to 3 months for most patients. Although this necessitates relatively frequent treatment, the treatment does well in addressing multiple aspects of global appearance and is both an accessible treatment for patients and one that requires no additional equipment for the injector. It should be noted that duration can be far longer for some patients (Figure 4), but this is more the exception than the rule. In the authors’ experience, duration is related to the activity and strength of the underlying muscles.
Scar Management
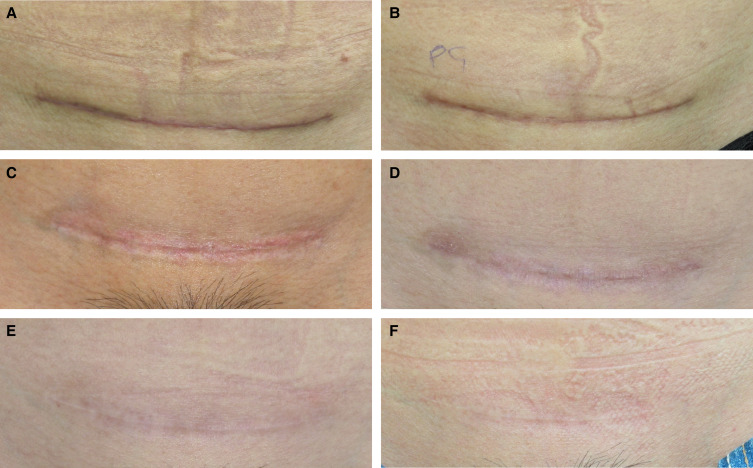
To reduce scarring in postoperative patients, BoNT-A can be injected intradermally within and along the periphery of the scar. Microtoxin can be utilized as a monotherapy or as a steroid adjunct to reduce the need for steroids and therefore protect against undesirable side effects. Microtoxin treatment for postoperative scars is initiated on postoperative day 7 (ie, around the time that stitches are removed) and is repeated every 2 months until resolution of the scar. The patient in Figure 5 was treated for her cesarian scar beginning on day 7 with 32 U of onabotulinumtoxinA in 1.0 mL of saline delivered at each session. At 2 years, the scar is difficult to detect.
Figure 5.
A 31-year-old female with a cesarian scar at (A) baseline (2 weeks following delivery), was treated with 1 session of intense-pulsed light therapy at baseline and with 32 U of onabotulinum toxin A in 1 mL of saline injected intradermally in and around the scar at baseline and at months 1, 2, 4, and 6. The images shown are of the patient at (B) month 1, (C) month 2, (D) month 4, (E) month 6, and (F) 1 year.
Combination Treatments
In practice, it is uncommon for patients to have their concerns addressed by a single therapy. Microtoxin is unique in that it addresses several aspects of appearance. In younger patients, this may be all that is needed to achieve a global aesthetic improvement. However, in situations for which additional tightening is needed with ultrasound or radiofrequency devices, the energy-based treatment is administered first, and microtoxin can be subsequently administered on the same day. Fillers can also be given on the same day, before microtoxin treatment. Lasers or intense-pulsed light therapy can be employed to manage dyschromia or surface issues, but must be used before microtoxin for same-day treatment. If the patient wishes to undergo additional treatments, it is best to wait 2 weeks before doing so.
Safety
In the authors’ experience of collectively treating more than 5000 patients, the safety profile of microtoxin is excellent and is largely restricted to minor injection sequelae. Patients are advised to refrain from massaging their face or applying direct pressures on treated areas for 4 hours.
The muscles and dermis are different immunologically. The dermis contains a higher concentration of dendritic cells than muscles, increasing the likelihood of antigen capture and inflammatory responses. Indeed, comparative studies of vaccination techniques consistently demonstrate higher levels of immunogenicity with intradermal injections when compared with both intramuscular and subcutaneous injections.27 Although there are no controlled long-term studies examining the formation of neutralizing antibodies (nABs) for either traditional or microtoxin treatments, there is an association between toxin dose and duration of treatment with nAb formation.28-31 Given that the patient population for this treatment is relatively young, the treatment interval relatively short, and the dose for a full-face treatment is 100 U, lifetime exposure is a consideration. Hemagglutinin proteins present in the toxin-associated protein complex can increase production of antibodies against the core toxin, raising the question of whether intradermal treatment with BoNT-A preparations lacking these accessory proteins could reduce the formation of nAbs.32,33 Although nAbs in and of themselves are not tightly correlated with nonresponse, the use of BoNT-A as treatment for serious medical conditions (eg, post-stroke spasticity, migraine, and overactive bladder, among others) necessitates the preservation of BoNT-A as a therapeutic option.29,31,34 Furthermore, because the doses utilized in aesthetic treatments for multiple areas of the face approach therapeutic doses for which nAb formation and resistance have been recorded at a somewhat higher rate than individual aesthetic indications, the rate of nAb formation is expected to increase.35 Although robust clinical data, possibly from registries, are still needed on long-term aesthetic use and the relationship between the presence of nontoxin proteins and eventual nonresponse, in the authors’ experience over the past 10 years of treating patients with microtoxin, nonresponse has been exceedingly uncommon, which is supported by results of a recent meta-analysis.36,37 Should patients develop nonresponse or partial nonresponse, switching to a formulation free of complexing or other bacterial proteins, such as incobotulinumtoxinA, may prevent the need for a toxin holiday, which can last 2 to 2.5 years. For one of the authors, the use of incobotulinumtoxinA when treating the dermis is an important preventive strategy.38
Like standard BoNT-A, microtoxin does have the ability to cause ptosis. Once injected, toxin can not only diffuse in the X and Y directions, but can also diffuse in the Z direction. Therefore, it is critical to ensure exact intradermal injection and an even superficial distribution of product to avoid affecting underlying muscles and unnatural-looking results. If the microtoxin volume per injection point is too high or is injected too deep, ptosis and asymmetry are possible adverse events. However, if the injection is more superficial and in the dermis, this is unlikely to be an issue. Although the number of individual injections needed is higher than traditional treatment, the authors recommend against the utilization of microneedling devices to deliver BoNT-A, because there is less control over individual injection depth and volume of toxin delivered. With minimal practice, an injector can develop the skills needed to quickly and consistently deliver the amount needed.
CONCLUSIONS
Although the most common employment of BoNT-A in aesthetic medicine remains a more traditional injection for local neuromodulation, microtoxin treatment is an important tool for neuromodulation at the level of the dermis, where many of the muscles integrate with the dermis to control motion of the overlying skin.39 When injected into this plane as tiny, ∼20 U/mL droplets, rather than into the muscles as larger, more concentrated injections, the toxin has a wider field of effect and is able to act not only on the most superficial motor neurons, but also on sympathetic nerves in glandular tissues and the nonneuronal cholinergic system. This broader activity leads to a global improvement in appearance that is unique to the microtoxin approach. The technique serves a wide range of patients and is an excellent opportunity to expand the use of BoNT-A in clinical practice.
Supplemental Material
This article contains supplemental material located online at www.aestheticsurgeryjournal.com.
Supplementary Material
Acknowledgments
Medical writing assistance was provided by Ginny Vachon, PhD, Principal Medvantage, LLC (Atlanta, GA) under the direction of the authors. This support was provided by xMedica, LLC (Alpharetta, GA) through an educational grant funded by Merz Aesthetics (Frankfurt, Germany) and Abbvie (North Chicago, IL).
Disclosures
Dr Fabi is a consultant for Allergan (Irvine, CA), Galderma (Lausanne, Switzerland), Merz Pharmaceuticals (Frankfurt, Germany), Revance (Nashville, TN), Endo Pharmaceuticals (Malvern, PA), Bausch Health (Laval, Canada), and Exploramed (Mountain View, CA); has received research support from Allergan, Galderma, Merz, Revance, Endo Pharmaceuticals, CROMA (Leobendorf, Austria), Bausch Health, and Exploramed; and holds stock in Allergan and Revance Therapeutics. Dr Park is a consultant for Merz Pharmaceuticals, Allergan, and LG Chem (Seoul, South Korea). Dr Goldie is a consultant for Merz Pharmaceuticals, BTI Aesthetics (Vitoria, Álava, Spain), and Orthogen (Springfield, NJ); and receives research support from Merz and BTI Aesthetics. Dr Wu is a consultant and clinical teacher for Allergan and is the inventor and patent holder of the Woffles Lift Barbed Sutures.
Funding
This activity was paid for by xMedica, LLC (Alpharetta, GA) through an educational grant funded by Merz Aesthetics (Frankfurt, Germany) and Abbvie (North Chicago, IL).
REFERENCES
- 1.Pellizzari R, Rossetto O, Schiavo G, Montecucco C. Tetanus and botulinum neurotoxins: mechanism of action and therapeutic uses. Philos Trans R Soc Lond B Biol Sci. 1999;354(1381):259–268. doi: 10.1098/rstb.1999.0377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sam C, Bordoni B. Physiology, Acetylcholine. StatPearls [Internet]; April 14, 2022. Accessed October 4, 2022. https://www.ncbi.nlm.nih.gov/books/NBK557825
- 3.Botox [prescribing information]. Madison, NJ: Allergan; 2020. [Google Scholar]
- 4.Kurzen H, Wessler I, Kirkpatrick CJ, Kawashima K, Grando SA. The non-neuronal cholinergic system of human skin. Horm Metab Res. 2007;39(2):125–135. doi: 10.1055/s-2007-961816 [DOI] [PubMed] [Google Scholar]
- 5.Grando SA, Zachary CB. The non-neuronal and nonmuscular effects of botulinum toxin: an opportunity for a deadly molecule to treat disease in the skin and beyond. Br J Dermatol. 2018;178(5):1011–1019. doi: 10.1111/bjd.16080 [DOI] [PubMed] [Google Scholar]
- 6.Xiao Z, Qu G. Effects of botulinum toxin type A on collagen deposition in hypertrophic scars. Molecules. 2012;17(2):2169–2177. doi: 10.3390/molecules17022169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong HS, Lee BH, Sung HM, et al. Effect of botulinum toxin type A on differentiation of fibroblasts derived from scar tissue. Plast Reconstr Surg. 2015;136(2):171e–178e. doi: 10.1097/PRS.0000000000001438 [DOI] [PubMed] [Google Scholar]
- 8.Park TH, Park JH, Chang CH, Rah DK. Botulinum toxin A upregulates RAC1, CDC42, and RHOA gene expression in a dose-dependent manner: in vivo and in vitro study. J Craniofac Surg. 2016;27(2):516–520. doi: 10.1097/SCS.0000000000002272 [DOI] [PubMed] [Google Scholar]
- 9.Wu W. Innovative uses of Botox and the Woffles Lift. In: Panfilov D, ed. Aesthetic Surgery of the Facial Mosaic. Springer; 2006:636–649. [Google Scholar]
- 10.Wu W. Facial and lower limb contouring. In: Benedetto AV, ed. Botulinum Toxins in Clinical Aesthetic Practice, 2nd ed. Informa Healthcare; 2011:206–222. [Google Scholar]
- 11.Wu WT. Botox facial slimming/facial sculpting: the role of botulinum toxin-A in the treatment of hypertrophic masseteric muscle and parotid enlargement to narrow the lower facial width. Facial Plast Surg Clin North Am. 2010;18(1):133–140. doi: 10.1016/j.fsc.2009.11.014 [DOI] [PubMed] [Google Scholar]
- 12.Wu W. Facial sculpting and facial slimming with neurotoxins. In: Sundine M and Connell B, eds. Aesthetic Rejuvenation of the Face. Thieme Publishers; 2015:39–44. [Google Scholar]
- 13.Wu W, et al. Botulinum toxin A injections for facial rejuvenation and reshaping. In: Lee PChen YR and Li QF, eds. Aesthetic Plastic Surgery in Asians. CRC Press; 2015:149–169. [Google Scholar]
- 14.Calvisi L, Diaspro A, Sito G. Microbotox: a prospective evaluation of dermatological improvement in patients with mild-to-moderate acne and erythematotelangiectatic rosacea. J Cosmet Dermatol. 2022;21(9):3747–3753. doi: 10.1111/jocd.14692 [DOI] [PubMed] [Google Scholar]
- 15.Casabona GR, Giacomo TB. Improving the appearance of surgical facial scars with incobotulinumtoxinA and microneedling. J Drugs Dermatol. 2020;19(6):611–615. doi: 10.36849/JDD.2020.10.36849 [DOI] [PubMed] [Google Scholar]
- 16.Chang SP, Tsai HH, Chen WY, Lee WR, Chen PL, Tsai TH. The wrinkles soothing effect on the middle and lower face by intradermal injection of botulinum toxin type A. Int J Dermatol. 2008;47(12):1287–1294. doi: 10.1111/j.1365-4632.2008.03895.x [DOI] [PubMed] [Google Scholar]
- 17.Park JY, Cho SI, Hur K, Lee DH. Intradermal microdroplet injection of diluted incobotulinumtoxin-A for sebum control, face lifting, and pore size improvement. J Drugs Dermatol. 2021;20(1):49–54. doi: 10.36849/JDD.5616 [DOI] [PubMed] [Google Scholar]
- 18.Qiao Z, Yang H, Jin L, Li S, Wang X. The efficacy and safety of botulinum toxin injections in preventing postoperative scars and improving scar quality: a systematic review and meta-analysis. Aesthetic Plast Surg. 2021;45(5):2350–2362. doi: 10.1007/s00266-021-02196-5 [DOI] [PubMed] [Google Scholar]
- 19.Wu W. Nonsurgical facial rejuvenation with the 4R principle: innovative uses of Botox and the Woffles Lift. In: Panfilov D, ed. Aesthetic Surgery of the Facial Mosaic. Springer; 2007:636–649. [Google Scholar]
- 20.Wu W. Skin resurfacing with Microbotox and the treatment of keloids. In: Benedetto AV, ed. Botulinum Toxins in Clinical Aesthetic Practice, 2nd ed. Informa Healthcare; 2011:190–205. [Google Scholar]
- 21.Wu WTL. Microbotox of the lower face and neck: evolution of a personal technique and its clinical effects. Plast Reconstr Surg. 2015;136(5 Suppl):92S–100S. doi: 10.1097/PRS.0000000000001827 [DOI] [PubMed] [Google Scholar]
- 22.Wu WTL. The Microbotox technique. In: Tonnard PL, ed. Centrofacial Rejuvenation. Thieme Connect; 2018:289–310. [Google Scholar]
- 23.Yue S, Ju M, Su Z. A systematic review and meta-analysis: botulinum toxin A effect on postoperative facial scar prevention. Aesthetic Plast Surg. 2022;46(1):395–405. doi: 10.1007/s00266-021-02596-7 [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Cai L, Yang M, Li F, Han X. Botulinum toxin to treat horizontal forehead lines: a refined injection pattern accommodating the lower frontalis. Aesthet Surg J. 2020;40(6):668–678. doi: 10.1093/asj/sjz174 [DOI] [PubMed] [Google Scholar]
- 25.Diaspro A, Calvisi L, Manzoni V, Sito G. Microbotulinum: a quantitative evaluation of aesthetic skin improvement in 62 patients. Plast Reconstr Surg. 2020;146(5):987–994. doi: 10.1097/PRS.0000000000007248 [DOI] [PubMed] [Google Scholar]
- 26.Flament F, Francois G, Qiu H, et al. Facial skin pores: a multiethnic study. Clin Cosmet Investig Dermatol. 2015;8:85–93. doi: 10.2147/CCID.S74401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Wang W, Wang S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev Vaccines. 2015;14(11):1509–1523. doi: 10.1586/14760584.2015.1081067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albrecht P, Jansen A, Lee JI, et al. High prevalence of neutralizing antibodies after long-term botulinum neurotoxin therapy. Neurology. 2019;92(1):e48–e54. doi: 10.1212/WNL.0000000000006688 [DOI] [PubMed] [Google Scholar]
- 29.Bellows S, Jankovic J. Immunogenicity associated with botulinum toxin treatment. Toxins (Basel). 2019;11(9):491. doi: 10.3390/toxins11090491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hefter H, Rosenthal D, Bigalke H, Moll M. Clinical relevance of neutralizing antibodies in botulinum toxin long-term treated still-responding patients with cervical dystonia. Ther Adv Neurol Disord. 2019;12:1756286419892078. doi: 10.1177/1756286419892078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lange O, Bigalke H, Dengler R, Wegner F, deGroot M, Wohlfarth K. Neutralizing antibodies and secondary therapy failure after treatment with botulinum toxin type A: much ado about nothing? Clin Neuropharmacol. 2009;32(4):213–218. doi: 10.1097/WNF.0b013e3181914d0a [DOI] [PubMed] [Google Scholar]
- 32.Kukreja R, Chang TW, Cai S, et al. Immunological characterization of the subunits of type A botulinum neurotoxin and different components of its associated proteins. Toxicon. 2009;53(6):616–624. doi: 10.1016/j.toxicon.2009.01.017 [DOI] [PubMed] [Google Scholar]
- 33.Lee JC, Yokota K, Arimitsu H, et al. Production of anti-neurotoxin antibody is enhanced by two subcomponents, HA1 and HA3b, of Clostridium botulinum type B 16S toxin-haemagglutinin. Microbiology (Reading). 2005;151(Pt 11):3739–3747. doi: 10.1099/mic.0.28421-0 [DOI] [PubMed] [Google Scholar]
- 34.Naumann M, Carruthers A, Carruthers J, et al. Meta-analysis of neutralizing antibody conversion with onabotulinumtoxinA (BOTOX®) across multiple indications. Mov Disord. 2010;25(13):2211–2218. doi: 10.1002/mds.23254 [DOI] [PubMed] [Google Scholar]
- 35.Ho WWS, Albrecht P, Calderon PE, et al. Emerging trends in botulinum neurotoxin A resistance: an international multidisciplinary review and consensus. Plast Reconstr Surg Glob Open. 2022;10(6):e4407. doi: 10.1097/GOX.0000000000004407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park JY, Sunga O, Wanitphakdeedecha R, Frevert J. Neurotoxin impurities: a review of threats to efficacy. Plast Reconstr Surg Glob Open. 2020;8(1):e2627. doi: 10.1097/GOX.0000000000002627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahman E, Banerjee PS, Asghar A, Gupta NK, Mosahebi A. Botulinum toxin type A immunogenicity across multiple indications: an overview systematic review. Plast Reconstr Surg. 2022;149(4):837–848. doi: 10.1097/PRS.0000000000008904 [DOI] [PubMed] [Google Scholar]
- 38.Park JY, Corduff N, Frevert J, Wanitphakdeedecha R, Chao YYY. Immunogenicity associated with aesthetic botulinumtoxin A: a survey of Asia-Pacific physicians’ experiences and recommendations. Plast Reconstr Surg Glob Open. 2022;10(4):e4217. doi: 10.1097/GOX.0000000000004217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutto JR, Vattoth S. A practical review of the muscles of facial mimicry with special emphasis on the superficial musculoaponeurotic system. AJR Am J Roentgenol. 2015;204(1):W19–W26. doi: 10.2214/AJR.14.12857 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.