Abstract
Wildlife species are often heavily parasitized by multiple infections simultaneously. Yet research on sylvatic transmission cycles, tend to focus on host interactions with a single parasite and neglects the influence of co-infections by other pathogens and parasites. Co-infections between macro-parasites and micro-parasites can alter mechanisms that regulate pathogenesis and are important for understanding disease emergence and dynamics. Wildlife rodent hosts in the Lyme disease system are infected with macro-parasites (i.e., ticks and helminths) and micro-parasites (i.e., Borrelia spp.), however, there has not been a study that investigates the interaction of all three parasites (i.e., I. pacificus, Borrelia spp., and helminths) and how these co-infections impact prevalence of micro-parasites. We live-trapped rodents in ten sites in northern California to collect feces, blood, ear tissue, and attached ticks. These samples were used to test for infection status of Borrelia species (i.e., micro-parasite), and describe the burden of ticks and helminths (i.e., macro-parasites). We found that some rodent hosts were co-infected with all three parasites, however, the burden or presence of concurrent macro-parasites were not associated with Borrelia infections. For macro-parasites, we found that tick burdens were positively associated with rodent Shannon diversity while negatively associated with predator diversity, whereas helminth burdens were not significantly associated with any host community metric. Ticks and tick-borne pathogens are associated with rodent host diversity, predator diversity, and abiotic factors. However, it is still unknown what factors helminths are associated with on the community level. Understanding the mechanisms that influence co-infections of multiple types of parasites within and across hosts is an increasingly critical component of characterizing zoonotic disease transmission and maintenance.
Keywords: Lyme disease, Macro-parasite, Micro-parasite, Ixodes spp., Borrelia spp., Trichuris spp
Graphical abstract
Highlights
-
•
Ticks, helminths, and Borrelia co-infections of rodent populations in California.
-
•
Neotoma fuscipes are prominent hosts of ticks, helminths, and B. burgdorferi sensu lato.
-
•
Tick and helminth co-infections are not predictive of Borrelia in rodents.
1. Introduction
Wildlife species can be infected with a broad range of macro-parasites and micro-parasites, often at the same time. Yet most zoonotic disease research focuses on a single parasite and neglects the interactions between multiple parasites within the host, leading to an incomplete understanding of pathogen impacts (Vaumourin et al., 2015). The increased pace of disease emergence necessitates consideration of multiple parasitic infections in wildlife hosts to better predict and prevent transmission (Friggens and Beier, 2010; Hahn et al., 2021; Jones et al., 2008; Messina et al., n.d.; Millette et al., 2020; Patz et al., 2000, 2008; Rizzoli et al., 2019; Swei et al., 2020; Tidman et al., 2021; Webster et al., 2016; Wolfe et al., 2005).
Parasites can be classified as micro-parasites (e.g., bacteria and viruses), endo-macroparasites (e.g., helminth worms), or ecto-macroparasites (e.g., ticks and mites). These various parasite types have distinct life history traits such as mechanisms of host infection and tissue tropism. At the same time, different parasite types can interact directly within a single host by competing for space or nutrition (Behnke et al., 1999; Bell et al., 2006; Graham, 2008; Mideo, 2009; Rynkiewicz et al., 2015). Parasites can also interact indirectly through the hosts' immune system. Pressures on hosts such as chronic stress, low resource availability, sex hormone response, and interfering immune responses from multi-parasitic infections; can suppress the immune response in wildlife, leading to the increase of disease morbidity (Cizauskas et al., 2014, 2015; Ezenwa et al., 2012; Ezenwa and Jolles, 2011; Jolles et al., 2008a; Petney and Andrews, 1998). For example, mammalian hosts deploy T-helper 1 cells to combat micro-parasitic infections and deploy T-helper 2 cells when combating a macro-parasite infection. The pathways which initiate the deployment of either of these cells are cross-regulating. Therefore, only one type of infection can be fought by the host's immune system at a time (Morel and Oriss, 1998; van Riet et al., 2007), leading to immune suppression in co-infected hosts. Furthermore, T-helper 2 responses are positively correlated with macro-parasite burdens (Maaz et al., 2016) and as parasites are aggregated across host populations (Shaw et al., 1998) the relative differences in individuals macro-parasite burdens have an impact on the individuals overall health (Jolles et al., 2008b). Research aiming to understand this immune interaction traditionally focuses on the interaction between internal macro-parasites and micro-parasites (Budischak et al., 2012; Ezenwa and Jolles, 2011; Jolles et al., 2008a) but a multi-parasite approach involving ecto-parasites is warranted.
In the United States, ticks are the most common vector of disease and are responsible for transmitting the greatest number of zoonotic pathogens (Swei et al., 2020). They transmit a variety of micro-parasites, most notably, the spirochete bacterium(s) that cause Lyme disease and hard tick relapsing fever (i.e., Borrelia species) (Eisen et al., 2017; Paddock et al., 2016; Rosenberg et al., 2018; Swei et al., 2020). Borrelia burgdorferi sensu lato (i.e., Borrelia burgdorferi and Borrelia bissettiae) and relapsing fever Borreliae (i.e., Borrelia miyamotoi) are naturally maintained in a sylvatic cycle involving Ixodes species tick vectors and wildlife hosts, particularly rodents. These rodent hosts are also commonly parasitized with helminths, thus providing an ideal model system to investigate multi-parasitic interactions within a host. A study in the UK investigated how mice experimentally infected with a commonly isolated helminth from wild rodents impacted the feeding success of ticks or the pathogenicity success of Borrelia afzellii, and found no influence helminth infections on the transmission of Borrelia afzellii (Maaz et al., 2016). Field surveys of wild host populations and their most common co-infections are necessary to document prior to lab experimentation of the molecular mechanisms defining these multi-parasite interactions.
Macro-parasites, particularly ticks, spend a large portion of their life cycle off host in the environment. As ectotherms, ticks are sensitive to abiotic changes and will modify their behavior (e.g., host-seeking questing) to avoid desiccation, while also trying to successfully find a blood meal (Perret et al., 2003; Thomas et al., 2020). Similarly, many species of helminths are environmentally transmitted and spend a great portion of their life cycle exposed to environmental conditions before a host ingests them. Therefore, abiotic conditions are likely to influence the distribution of macro-parasites in general. This study seeks to understand patterns of co-occurrence between these three types of parasites, micro-parasites and two types of macro-parasites, to better understand how they may facilitate or inhibit one another.
We investigated the community dynamics between three parasites: a microparasite (i.e., Borrelia spp.), an ecto-macroparasite (i.e., ticks), and an endo-macroparasite (i.e., helminths) within rodent hosts in a Lyme disease endemic area in the western United States. The goal of this study is to 1) characterize co-infections and burden intensity of micro- and macro-parasites within individual hosts; 2) understand how macro-parasites are associated with host community dynamics and environmental inputs (temperature, precipitation); as well as 3) analyze how macro-parasites are associated with micro-parasite infection within individual hosts.
2. Materials and methods
2.1. Field collection
Field collections were conducted in ten oak woodland fragmented forest patches that were selected in the San Francisco Bay Area. These forest patches were standardized by landscape and vegetation composition but varied in their habitat patch size and fell along a gradient from high fragmented (2.5 ha) to intact (>4000 ha) (Lawrence et al., 2018; Salomon et al., 2021; Sambado et al., 2020). Within each habitat patch, a half hectare sampling grid was established at least 20 m from the edge of the patch, under oak canopy cover, while avoiding north facing slopes (Talleklint-Eisen and Lane, 1999; Tälleklint-Eisen and Lane, 2000).
We conducted three consecutive days of rodent live-trapping at each site between the months of April and May in 2018 to target juvenile Ixodes pacificus activity (MacDonald, 2018). At each site, a total of 49 trapping stations were established in a 7 × 7 grid with each trap station located 11.8 m apart. Two extra-large Sherman Live Traps (7.6 × 9.5 × 30.5 cm; H.B. Sherman Traps, Tallahassee, FL, USA) were placed at each trapping station for a total of 98 traps per grid (Machtinger and Williams, 2020). At five of the sites, 15 tomahawk traps (16L x 5W × 5H in; Tomahawk Live Traps, Hazelhurst, WI) were set at every other trapping station to target larger rodents. Total trapping events totaled to 3165 events across all sites. Traps were baited with a combination of oats and peanut butter, and set out from dusk until dawn. Captured rodents were marked with unique ear tags (National Band & Tag Company Co., KY), weighed, aged, sexed, and identified to species (Machtinger and Williams, 2020). The following samples were collected from each rodent: 2-mm ear biopsies, whole blood from the retro-orbital vein, any and all attached ticks, and feces. Feces were collected directly from the animal's rectum during handling procedures, into a 1.7 mL Eppendorf tube. Animals were anesthetized with a 50% isoflurane and propanediol 3–4 solution prior to retro-orbital bleeding. After processing and recovery from anesthesia, all animals were released at the point of capture. All protocols were approved by institutional animal care and use protocol (#AU16-05). Ear biopsies and attached ticks were each stored in 70% ethanol at 4 °C until further processing. Whole blood was collected in EDTA tubes, held on ice in the field, flash frozen with liquid nitrogen, and then stored at −80 °C until further processing. Rodent feces were brought back to the lab and stored at −20 °C until further lab processing.
To assess the association between questing ticks and ticks attached to hosts, questing ticks were collected using standard dragging methods where a white cotton cloth was dragged within the 0.5 ha sampling grid, totaling 495 m2 at each sampling site (Eisen et al., 2018; Salomon et al., 2020). Drag cloths were checked for ticks every ∼15 m and ticks were transferred to vials containing 70% ethanol. Questing ticks were collected during two different time intervals; first during the three days of rodent trapping in April and early May and then again in the first week of June 2018 (Barbour et al., 1985; MacDonald, 2018) to capture seasonal dynamics of I. pacificus larvae and nymphs.
Two Bushnell Trophy Cam HD (Overland Park, KS) were set up at each site to capture terrestrial vertebrate densities and richness. Cameras were strapped to a tree roughly 60 cm from the forest floor, facing opposite directions along a game trail. Camera motion detection settings were set to ‘normal sensitivity’ to take a series of three photos at a time, with each photo being 1 s apart, and pausing 30 s before another capture (Lawrence et al., 2018). Cameras were active 24 h a day for 40 days of analysis between the months of April 1 through May 10 of 2018.
2.2. Molecular analysis
Tissue and blood samples were extracted using the DNeasy Tissue Extraction kit according to the manufacturer's instructions (Qiagen, Valencia, California, USA). A few adjustments were made to the protocol; for tissue samples, an additional 70% ethanol wash step was added to ear tissue extractions and the final product of both blood and tissue samples were eluted in 100 μL of AE buffer to concentrate the eluate. Once DNA was extracted, microparasite infection was determined using two separate nested PCR protocols. Within hosts there is niche partitioning of Borrelia species where B. burgdorferi sensu lato is found in endothelium, and relapsing fever Borrelia species, including B. miyamotoi, circulate in the hosts' blood (Barbour et al., 2009; Barbour and Hayes, 1986; Sambado et al., 2020). Therefore, one PCR protocol was used on ear tissue samples targeting the 5S–23S rRNA intergenic spacer region to detect Borrelia burgdorferi sensu lato which includes the Lyme disease bacterium, B. burgdorferi, as well as other related genospecies such as B. bissettiae. Another PCR protocol was used on blood samples and targets the 16S–23S rDNA intergenic spacer region for detecting relapsing fevers Borrelia species, such as B. miyamotoi and related bacteria (Barbour et al., 2009; Bunikis et al., 2004; Postic et al., 1994; Sambado et al., 2020). All nested PCR products were tested in triplicate and visualized by gel electrophoresis on a 1.8% agarose gel. Positive samples were then purified using the SeraPure magnetic beads prior to sequencing on an ABI 3730. All positive sequences were edited and aligned using Geneious v 11.15 software and identified to species by aligning to reference sequences on NCBI BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
2.3. Tick species identification and abundance estimates
Collected ticks were identified under a dissecting microscope to species and life stage using taxonomic keys (Furman and Loomis, 1984; Kleinjan and Lane, 2008). Questing tick abundances were quantified by species, life stage, and site. Attached tick abundances (Busht et al., 1997) per species and life stage, were quantified for each animal, which we refer to as ‘tick burden’ throughout the text.
2.4. Helminth detection
Rodent helminth infection was determined by processing rodent feces using a standard protocol to detect helminth eggs. For each sample, 0.1 g of feces was processed from each animal by homogenizing rodent feces in a Sugar-Med solution (Bechtel et al., 2015; Benbrook and Sloss, 1955; Foreyt, 2001; Parkinson et al., 2011). Samples were then centrifuged at 500 RCF for 5 min in a centrifuge with a 161 mm rotor in 15 mL test tubes. After centrifugation, more sugar solution was added to the 15 mL test tube to create a reverse meniscus and coverslips were pressed on top of the solution. After 15 min, coverslips were transferred to glass slides and examined at 40× total magnification under a standard compound microscope. Eggs were identified to genera (Benbrook and Sloss, 1955; Foreyt, 2001) and quantified for each rodent. Total egg abundance (Busht et al., 1997) was quantified per animal, which we refer to as ‘helminth burden’ throughout the text since fecal egg abundance are positively correlated to adult worm burden (Bryan and Kerr, 1989; Kim et al., 2011; Sithithaworn et al., 1991).
2.5. Remotely sensed climate data
When assessing macro-parasite burdens across rodent hosts at the community level (i.e., site), we included abiotic metrics such as temperature, precipitation, and maximum vapor pressure deficit to account for climate impacts on tick populations outside of our host community analysis (i.e., Shannon diversity of vertebrate hosts and predators). Climate data for each site was used from the Oregon State Parameter-elevation Regression on Independent Slopes Model (PRISM) Climate Group (downloaded on 2022-05-01) which provides estimates of primary climate elements such as mean temperature (tmean, °C), precipitation (ppt, mm), and maximum vapor pressure deficit (vpdmax, kPA) (“PRISM Climate Group,” 2014). Data from April 2018 was selected for analysis because peak sampling of all ten sites occurred throughout the month of April (Supplemental Fig. 2). We chose to select the average over a period of one month because ticks are more sensitive to shifts in monthly climate characteristics than yearly averages and their peak activity season (i.e., phenology) occurs between April and May. Climate data for mean temperature is derived as the average of maximum temperature and average minimum temperature that is averaged over all days in the month. To represent precipitation, we selected monthly total precipitation (ppt). Maximum vapor pressure deficit is the daily maximum vapor pressure deficit averaged over all days in the month. Vapor pressure deficit (vpd) is a variable related to humidity and temperature and is calculated as the difference (in units of pressure) between actual vapor pressure and the saturation vapor pressure (e.g., the amount of water air can hold before it becomes liquid). A high vpd can be thought of as an environment with higher temperature and lower humidity levels. Maximum vpd has been shown to be a significant predictor of tick abundance and questing activity, which is why it is incorporated in these analyses (Bacon et al., 2022; Diuk-Wasser et al., 2010; Hacker et al., 2021; Hahn et al., 2016). Single point climate estimates were taken at a 4 km resolution at given site GPS points from Salomon et al., 2021); Lawrence et al. (2018); Salomon et al. (2021); Sambado et al. (2020).
2.6. Community vertebrate metrics
Community vertebrate metrics (i.e., Shannon diversity and abundance estimates) were calculated as done in Lawrence et al., (2018); Salomon et al., (2021); Lawrence et al. (2018); Salomon et al. (2021). Briefly, all captured rodents were marked with unique ear tag numbers in order to calculate mark recapture estimates for site abundances in R (v. 0.99.902) using the ‘Rcapture’ package (Baillargeon and Rivest, 2007). Predator abundance estimates were calculated from camera traps, each captured photo within 30 min of the same species was considered as one individual. From these photographs of activity for each species, we divided by the number of days the camera trap was active (40 days). Using these activity estimates as proxy's for abundance, we were able to calculate a relative Shannon diversity index with the ‘vegan’ package in R for each site (Oksanen, 2016). Similarly the rodent Shannon diversity index was calculated with the ‘Rcapture’ abundance estimates for each site.
2.7. Statistical analysis
Statistical analysis was broken into three parts: 1) characterizing the distribution of macro- and micro-parasites across individual hosts, 2) understanding how macro-parasites are associated with host community dynamics as well as abiotic metrics (negative binomial models), and 3) analyzing how macro-parasites are associated with micro-parasites within individual hosts (binomial models and negative binomial models). Specific model formulation for each part can be found in the statistical analysis subsections. Terminology for all statistical analysis is as follows: micro-parasites examined included a single Borrelia spp. or multiple Borrelia species (i.e., B. burgdorferi, B. bissettiae, B. miyamotoi, or an uncharacterized relapsing fever Borrelia) and the type of micro-parasite included in analyses is stated explicitly in generalized linear mixed effects model interpretation. Macro-parasites included ticks (Ixodes sp. and Dermacentor sp.) and helminths (Trichuris sp., Capillaria sp., Aspiculuris sp., and Hymenolepis sp.) and were evaluated together (all tick spp. and all helminth spp.) or individually as separate groups of ticks or helminths. A complete list of each combination of micro- and macro-parasites evaluated in the analyses can be found in the Supplement (Table 3). All analyses were done in RStudio version 1.4.1717 (“RStudio: Integrated Development Environment for R,” 2021).
Table 3.
Significant regression models for part 2 of the analyses: understand how macro-parasites are influenced by associated with host community dynamics. Levels of significance are indicated with * (*** p-value <0.001; ** p-value <0.01; * p-value <0.05).
| Response | Explanatory | Estimate | Std. Error | z-value | p-value |
|---|---|---|---|---|---|
| Tick burden (negative binomial model) with genera as random effect | |||||
| Rodent Shannon diversity index | 0.796 | 0.177 | 4.51 | <0.001*** | |
| Predator Shannon diversity index | −0.483 | 0.163 | −2.96 | 0.00308 ** | |
| Tick burden (negative binomial model) with genera as random effect | |||||
| Mean temperature | 0.645 | 0.190 | 3.41 | <0.001*** | |
| Tick burden (negative binomial model) with genera as random effect | |||||
| Max vapor pressure deficit | 0.111 | 0.0490 | 2.26 | 0.0238 * | |
| Borrelia spp. infection (binomial model) for N. fuscipes only | |||||
| Rodent Shannon diversity index | −1.157 | 0.600 | −2.07 | 0.0388 * | |
| Predator Shannon diversity index | 0.530 | 0.485 | 1.09 | 0.275 | |
2.8. Characterizing the distribution and host community associations of macro- and micro-parasites
We first calculated the observed infection prevalence (Busht et al., 1997) of each infection type (Borrelia spp., ticks, and helminths) by dividing the number of individuals to be positive for each parasite type by the number of individuals tested for each infection type. We calculated 95% Confidence Intervals using the Agresti-Coull method. Wilcoxon rank sum tests were used to test for significant differences between the two genera of rodent hosts (i.e., Neotoma and Peromyscus spp.) of both the median tick burdens and the median helminth burdens. Additionally, Wilcoxon rank sum tests were used to test for significance between uninfected and Borrelia spp. infected rodents and both their median tick burdens, and their median helminth burdens.
To understand how macro-parasites (ticks and helminths) are associated by individual host demographics, community structure (i.e., vertebrate diversity), and abiotic metrics (i.e., temperature, precipitation, vapor pressure deficit); we fitted a generalized linear mixed effects model (GLMM) with a negative binomial distribution to account for over-dispersed count data of tick and helminth burdens. All models were built and ran separately for ticks and helminths. For each model, the outcome of interest was ticks and helminths burdens on individual hosts. For the host demographic analysis, fixed effects were genera, sex, and age (i.e., adult or juvenile) with the random effect of site. Rodent weight was removed from the analysis due to its high collinearity with age and genera. For the site-level community structure analysis, the fixed effects were rodent Shannon diversity, and predator Shannon diversity, with the random effect of genera. Lastly, for the abiotic metrics in April 2018, negative binomial models were run separately for the fixed effects of mean temperature (°C), total monthly precipitation (mm), and max vapor pressure deficit (kPA) due to their high collinearity with each other, with host genera as the random effect. Additional details of how abiotic metrics were collected and applied can be found in Supplemental Table 2 and Supplemental Fig. 2.
2.9. Macro-parasites and micro-parasites interactions within individual hosts
To understand how micro-parasite infections (Borrelia spp.) are associated with macro-parasites (ticks and helminths), a binomial logit model was fitted with the presence/absence of a Borrelia spp. infection as the response variable with predictors of tick and helminth burden, presence/absence of tick and helminths, and genera of host. Additionally, we ran separate models for only Neotoma spp. as they had higher infection prevalence's of macro-parasites compared to Peromyscus species. For models that just looked at Neotoma spp., we removed genera as a fixed effect. Separate binomial logit models were run for all Borrelia spp., Borrelia burgdorferi sensu lato, relapsing fever Borrelia spp., and individual Borrelia spp. (e.g., B. burgdorferi sensu stricto, B. bissettiae, B. miyamotoi, and an unnamed tick-borne relapsing fever-like organism described in Sambado et al., 2020; Sambado et al., 2020)). Some predictor variables were explored for interactions as well for these models, each model structure is presented in Supplemental Table 2.
For each set of these analyses, the best fit model was selected by the lowest Akaike Information Criterion (AIC) score, and candidate models were compared with and without each predictor variable using one-sided likelihood-ratio tests with the ‘anova' function in R. A list of all model types and structures can be found in Supplemental Table 2.
3. Results
3.1. System characterization
A total of 2812 questing ticks were collected from these ten collection sites, with the majority being I. pacificus larvae (76%, n = 2125). Other life stages of I. pacificus collected consisted of 336 nymphs and 84 adults. We also collected Dermacentor occidentalis, Dermacentor similis (previously known as Dermacentor variabilis) (Lado et al., 2021), Haemaphysalis leporispalustris, and Ixodes spinipalpus, but in much lower numbers.
Rodent sampling totaled 3165 trapping events across 10 field sites and resulted in 313 individual rodents captured. Six rodent species were encountered, included 98 N. fuscipes, 7 Microtus californicus, 30 P. californicus, 35 P. maniculatus, 141 P. truei, and two Reithrodontomys megalotis. Camera trap analysis can be reviewed in depth within Lawrence et al., (2018) and Salomon et al., 2021 (Lawrence et al., 2018; Salomon et al., 2021). But briefly here, eight different predator species were identified across the 10 sites including: Puma concolor (mountain lion), Canis latrans (coyote), Lynx rufus (bobcat), Urocyon cinereoargenteus (gray fox), Procyon lotor (raccoon), Mephitis mephitis (striped skunk), Didelphis virginiana (Virginia opossum), and Felis catus (domestic cat).
3.2. Characterizing the distribution of macro- and micro-parasites across individual hosts
Due to resource constraints and animal safety we could not collect blood, tissue, and feces from all 313 rodents. We examined 184 rodent blood samples and 307 rodent ear tissue samples for pathogen infection with microparasites. A total of 56 samples (hosts) were positive for Borrelia spp. based on 5S–23S IGS and 16S–23S IGS rDNA nested PCR analysis. All but two samples produced sequences that were identified to species and submitted to GenBank (Accession numbers in Supplemental Table 3). We submitted 33 sequences to GenBank, but two samples we determined positive via base pair length on gel electrophoreses (one B. bissettiae and another B. miyamotoi) produced poor quality sequences. Furthermore, 21 samples were identified as an uncharacterized tick-borne relapsing fever Borrelia species (Supplemental Table 3). For statistical analyses, we considered 53 Borrelia positives. We detected 13% of the rodent's blood (n = 24) were infected with Borrelia species that cause relapsing fever, and 10% of rodents were infected with B. burgdorferi sensu lato in ear tissue samples (n = 31). Of the relapsing fever causing species that we isolated, 3 were B. miyamotoi, and 21 were infected with an uncharacterized relapsing fever Borrelia species (Sambado et al., 2020). Of the B. burgdorferi sensu lato group we isolated, 19 were identified as B. burgdorferi sensu stricto and 19 B. bissettiae positive samples. There were two Peromyscus spp. that were co-infected with B. burgdorferi sensu stricto and the uncharacterized relapsing fever Borrelia species (Sambado et al., 2020). Neotoma fuscipes were found to be infected with B. burgdorferi sensu lato at a greater proportion than Peromyscus spp. (Supplemental Table 3). All positive samples are summarized in Supplemental Table 3, with their corresponding Genbank accession numbers. Sequences of the novel uncharacterized relapsing fever Borrelia species isolated from rodent hosts do not have accession numbers, but are first reported in Sambado et al., (2020); Sambado et al. (2020).
From the 312 rodents checked for ticks, we collected 538 attached ticks that consisted mainly of I. pacificus larvae (80%, n = 431) (Supplementary Table 4). Additionally, we collected other life stages of I. pacificus (nymphs = 3, adults = 3), I. angustus (larvae = 7, nymphs = 22, adults = 8), I. spinipalpis (larvae = 28, nymph = 1, adult = 2), Ixodes woodi (nymphs = 6), D. occidentalis (larvae = 20, nymphs = 7) attached to the various rodents. All of these tick species were removed from Neotoma fuscipes, but two individual Peromyscus species (P. maniculatus and P. truei) had tick burdens of 5 and 4 composed of three different species at Windy Hill Open Space Preserve (I. angustus, I. pacificus, and I. spinipalpis). From 99 N. fuscipes we removed 337 I. pacificus, five I. angustus, 27 I. spinipalpis, three D. occidentalis, and 6 I. woodi ticks. While from 207 Peromyscus spp., we removed only 97 I. pacificus, 32 I. angustus, 4 I. spinipalpis, and 20 D. occidentalis.
A total of 107 rodent fecal samples were collected and tested, yielding an overall helminth infection prevalence of 31% (n = 33, Supplemental Table 1). Four different macro-parasite genera, belonging to two different phyla (Nematoda and Platyhelminthes), were identified. The most common helminth infection identified was Trichuris (Nematoda: Trichocephalida) species (n = 32). In addition, co-infections of Trichuris and Aspiculuris tetraptera (Nematoda: Oxyurida) within a P. californicus and Capillaria (Nematoda: Enoplia) within two different Neotoma fuscipes. Lastly, we identified a single occurrence of Hymenolepis (Platyhelminthes: Cyclophyllidea) infection within a Peromyscus californicus.
Of 103 rodents that were tested for all three types of parasites (i.e., ticks, helminths, and Borrelia species), 7% were concurrently infected with them all (n = 7, Table 1 and Fig. 2). Meanwhile, 15% (n = 15) of hosts were not parasitized by any of the infections we examined in this study (Table 1). The most common host co-infection was a combination of ticks and helminths at 18% (n = 19). The second most common co-infection observed was with ticks and Borrelia species, at 11% (n = 32). The least common co-infection was between helminths and Borrelia species, with nine rodents (8.74%) infected with both. Rodent species that most commonly hosted the parasitic infections we examined were N. fuscipes and P. truei (Supplemental Table 1 and Supplemental Fig. 1). However, due to low numbers of certain species (i.e., Peromyscus californicus and Peromyscus maniculatus), hosts were grouped into two genera (i.e., Peromyscus spp. and N. fuscipes) for regression models.
Table 1.
Prevalence of co-infections for all hosts between Borrelia, ticks, and helminths with a 95% Confidence Interval (95% CI).
| Positives | Tested | Observed Infection Prevalence (%) | 95% CI | ||
|---|---|---|---|---|---|
| No infections | 15 | 103 | NA | 8.9–23 | |
| Only Borrelia spp. | 20 | 306 | 6.54 | 4.2–9.9 | |
| Only Ticks | 17 | 309 | 5.50 | 3.4–8.7 | |
| Only Helminths | 13 | 103 | 12.62 | 7.4–21 | |
| Ticks + Helminths | 19 | 107 | 17.76 | 12–26 | |
| Borrelia spp. + Helminths | 9 | 103 | 8.74 | 4.5–16 | |
| Borrelia spp. + Ticks | 32 | 306 | 10.46 | 7.5–14 | |
| Borrelia spp. + Ticks + Helminths | 7 | 103 | 6.80 | 3.1–14 |
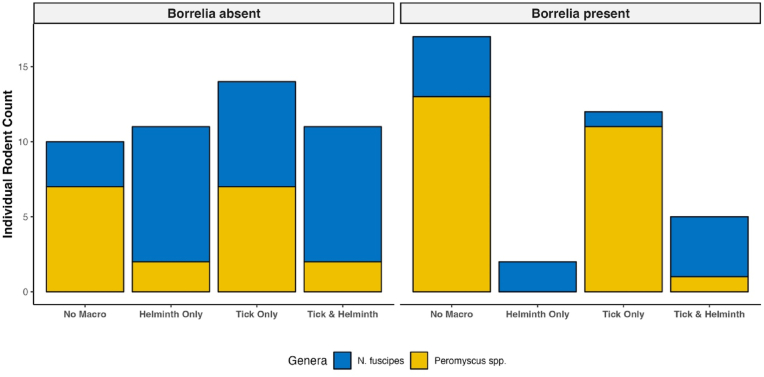
Fig. 2.
A histogram of the counts of individual rodents that were uninfected or infected with B. burgdorferi sensu lato with certain macro-parasite infections. The types of infection were: no tick or helminth burden on an individual rodent (No macro-parasite), helminth burden only (Helminth Only), tick burden only (Tick Only), or both ticks and helminths were found on individual rodent (Tick & Helminth). The colors denote for N. fuscipes (blue) and Peromyscus species (yellow). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The Wilcoxon rank sum test found that tick and helminth median burdens were significantly different between N. fuscipes (W = 12806, p-value <0.001) and Peromyscus spp. (W = 1984.5, p-value <0.001), with N. fuscipes hosting higher burdens of these macro-parasites (Fig. 1). Another Wilcoxon rank sum test found that both tick (W = 5263, p-value <0.05) and helminth (W = 1515, p-value <0.05) median burdens were significantly different for rodents that were uninfected versus Borrelia infected. The mean tick burden was higher in Borrelia infected rodents (infected = 2.4 mean tick burden, uninfected = 1.6 mean tick burden) whereas the helminth burden was higher in Borrelia uninfected rodents (uninfected = 4.6, infected = 1.5).
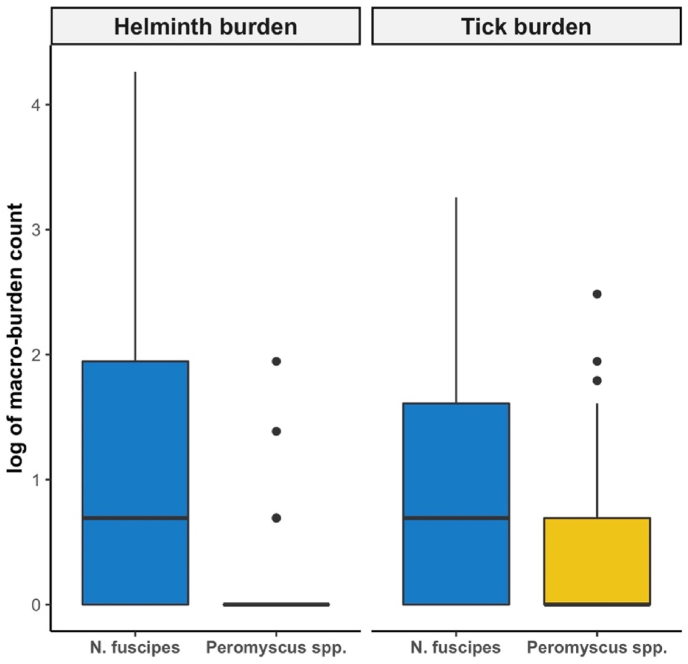
Fig. 1.
A boxplot of the mean macro-parasite burden across N. fuscipes and Peromyscus species. The log of helminth (left panel) and tick (right panel) counts on individual rodents were used for visualization purposes.
Neotoma fuscipes were more likely to be infected with B. burgdorferi sensu lato and have higher burdens of both ticks and helminths compared to Peromyscus species (Table 2). When looking at tick burdens, significant predictors such as Peromyscus spp. (estimate = −1.45 ± 0.21, p-value <0.001), and juvenile status (estimate = −0.79 ± 0.33, p-value = 0.02) were negatively associated with tick burdens, while male rodents had a positive association with tick burdens (estimate = 0.47 ± 0.19, p-value = 0.01). For helminth burdens, significant predictors included a negative association of Peromyscus spp. with helminths counts (estimate = −3.24 ± 0.56, p-value <0.001) and juvenile status (estimate = −2.85 ± 0.94, p-value <0.01).
Table 2.
Significant regression models for part 1 of the analyses: characterize the distribution of macro- and micro-parasites across individual hosts. Levels of significance are indicated with * (*** p-value <0.001; ** p-value <0.01; * p-value <0.05).
| Response | Explanatory | Estimate | Std. Error | z-value | p-value |
|---|---|---|---|---|---|
| Rodent tick burden (negative binomial model) with site as random effect | |||||
| Genus-Peromyscus | −1.45 | 0.206 | −7.03 | <0.001** | |
| Sex-Male | 0.474 | 0.193 | 2.45 | 0.0143 * | |
| Age-Juvenile | −0.786 | 0.330 | −2.38 | 0.0173 * | |
| Rodent helminth burden (negative binomial model) with site as random effect | |||||
| Genus-Peromyscus | −3.06 | 0.575 | −5.32 | <0.001*** | |
| Sex-Male | −0.675 | 0.524 | −1.29 | 0.198 | |
| Age-Juvenile | −2.51 | 0.963 | −2.60 | 0.00922 ** | |
| Rodent B. burgdorferi sensu lato infection (binomial model) | |||||
| Genus-Peromyscus | −2.12 | 0.601 | −3.53 | <0.001*** | |
| Sex-Male | −0.145 | 0.551 | −0.264 | 0.792 | |
| Age-Juvenile | −0.829 | 1.07 | −0.768 | 0.443 | |
3.3. Host community dynamics and abiotic metrics association with parasites
Tick burdens are associated with different host community dynamics such as rodent diversity, predator Shannon diversity, and abiotic metrics (i.e., temperature and vapor pressure deficit), whereas helminths did not have a significant relationship with any of those predictors (Table 3). Tick burdens had a positive association with rodent Shannon diversity (estimate = 0.80 ± 0.18, p-value <0.001) but a negative association with site predator Shannon diversity (estimate = −0.48 ± 0.16, p-value <0.01). Abiotic metrics such as mean temperature (°C; estimate = 0.65 ± 0.19, p-value <0.01) and vapor pressure deficit max (kPA; estimate = 0.11 ± 0.05, p-value = 0.02) were significant predictors of tick burdens while precipitation was not. All Borrelia spp. were investigated separately to identify community level impacts (i.e., Shannon diversity of rodents and predators) on pathogen presence in N. fuscipes. The most fit model included both predator and rodent Shannon diversity, where rodent Shannon diversity has a significant positive association with Borrelia burgdorferi sensu lato (Table 2).
3.4. Macro- and micro-parasite associations within individual hosts
We did not find a significant relationship between micro- and macro-parasite infection in shared rodent hosts. We investigated different combinations of models with macro- or micro-parasite presence/absence as a response to the counterpart parasite burden or presence, but none of the models were significant (Supplemental Table 2).
4. Discussion
Rodents in a Lyme disease endemic region of California experience simultaneous infections of helminths, ticks and Borrelia species (Table 1 and Supplemental Table 1). However, when testing how these three separate infections are associated with each other, there was no significant association observed. This survey supports the findings of Maaz et al., (2016), that helminth infections do not alter Borrelia spp. transmission (Maaz et al., 2016). Patterns of macro-parasite distribution within hosts and across host communities varied for both ticks and helminths. Ticks were the only macro-parasite significantly associated with various community diversity metrics such as rodent Shannon diversity (positive), predator Shannon diversity (negative), and climate variables (i.e., mean temperature and max vapor pressure deficit) (Table 3). We found that adult N. fuscipes were more likely to harbor ticks than sympatric juvenile Peromyscus species, and described higher loads of helminth infections within N. fuscipes than sympatric Peromyscus species (Table 3). The variation in tick and helminth distribution across hosts, along with the different associations within host community structure and abiotic metrics; suggests that ticks are more sensitive to extrinsic host community level factors (i.e., host demographics, host predation, and host diversity) (Table 2, Table 3), than by direct parasite interactions within the host (e.g., immune cross reactivity or resource competition).
We found the distribution of helminths and ticks on individual hosts were significantly different between Neotoma fuscipes and Peromyscus species (Fig. 1). Concordant with other tick findings (Brown and Lane, 1996; Salomon et al., 2021; Swei et al., 2012), N. fuscipes had larger tick burdens than Peromyscus species, and our results identified higher helminths burdens on N. fuscipes, which could be consistent with larger bodied rodents having higher helminth burdens (Froeschke and Matthee, 2014; Mohd Zain et al., 2012; Nunn et al., 2003; Poulin, 1996). Behavioral differences between N. fuscipes and Peromyscus spp. may lead to increased encounter rates with ticks. Examples of behavioral differences between species include a lack of grooming behaviors, larger territories, or use of constructed middens which create favorable microclimates for ecto-parasites (Cranford, 1977; Eisenberg, 1962; Kinsey, 1976; Wallen, 1982; Whitford and Steinberger, 2010). Majority of the helminth infections were of the Trichuris species. Based on lab studies, this helminth has been shown to only reach full development in the presence of certain gut flora (Hayes et al., 2010). The high helminth burdens found in N. fuscipes compared to Peromyscus spp. could be highlighting differences in gut flora between the two hosts or simply different exposure to microenvironments favorable to helminths. While it was not possible to identify the helminths to species, all of the helminth genera identified in this study have zoonotic disease potential and suggests that helminth infections in rodent populations of close proximity to urban spaces require examination in tandem with tick-borne diseases.
Our research identified difference in macro-parasite distribution across an ecological community, with tick burdens responding significantly to extrinsic factors like Shannon diversity of rodents and predators as well as climatic variables, whereas helminth burdens were not significantly associated with any of those variables (Table 2). Looking at the relationships between tick burden and host community structure, we see opposing associations for predator Shannon diversity (negative) and rodent Shannon diversity (positive). The negative relationship between predator Shannon diversity and rodent tick burdens is consistent with earlier work reported in Salomon et al., (2021); Salomon et al. (2021). We hypothesize that this relationship is due to an increase of diverse predator activities that causes rodents to leave their nests less frequently, resulting in a decrease in encounter rates between rodents and questing ticks (Calabrese et al., 2011; Hofmeester et al., 2017; Hudson et al., 1992). With increased predator diversity there is an increase in diversity of behaviors and chemical cues that rodents have to interpret, increasing their fear response and decreasing their behaviors of leaving their nests (Moll et al., 2017, 2020; Suraci et al., 2019a, 2019b). Surprisingly, predator diversity did not impact the helminth burden of these rodent hosts and may represent the different modes of transmission for helminths. For instance, the majority of attached ticks were I. pacificus, therefore since this species is known to display questing behavior, these attached ticks were most likely encountered while the rodent was outside of its nest. Conversely, Trichuris eggs are excreted by hosts in feces and then ingested orally. Neotoma fuscipes are known to create middens (Moore et al., 2020; Whitford and Steinberger, 2010) that are typically within close range of their nests. Therefore, greater predation pressure may not deter N. fuscipes from their midden use nearby their nest, resulting in a higher likelihood of encounter with helminths. Teasing apart the predation of these rodents and reduction of movement due to predator activity by analyzing movement data, would be useful to better understand the negative impact predators have on parasitism patterns of rodent populations. Whereas the positive relationship between tick burdens and rodent Shannon diversity (Table 2) is hypothesized to be an indication of overall habitat quality and environmental conditions that are favorable to rodent populations and tick survivorship (MacDonald, 2018; Macdonald et al., 2017; Williams and Ward, 2010).
As ectoparasites are free-living organisms, ticks spend most of their lives in the environment rather than on hosts. This trait makes them sensitive to climatic parameters impacting their ability to survive off-host and during questing. Considering our last community level metric, climate, we found that tick burdens were positively associated with site level climate variables such as mean temperature and maximum vapor pressure deficit (Table 2 and Supplemental Table 2). In temperate San Francisco Bay Area, higher maximum vapor pressure deficit leads to an increased potential for tick-host encounters due to increased tick habitat suitability, increased tick survivorship, and increase period of host-seeking (Bacon et al., 2022; Diuk-Wasser et al., 2010; Hacker et al., 2021; Hahn et al., 2021). Our analysis shows that higher maximum vapor pressure deficit also increases tick burdens on hosts, providing more evidence that this parameter increases epidemiological risk of tick-borne pathogens.
Extending beyond the macro-parasite relationship with these community level metrics (i.e., Shannon diversity, climate), we found the presence of B. burgdorferi sensu lato infections in N. fuscipes significantly associated with only rodent Shannon diversity (Table 3). Essentially, in less diverse rodent communities there is a higher probability for a N. fuscipes to be infected with B. burgdorferi sensu lato (Table 3). Host diversity has been routinely connected to decreasing the prevalence of B. burgdorferi sensu lato by the literature and is referred to as the dilution effect hypothesis (Schmidt and Ostfeld, 2001). However, we are providing evidence that the host community diversity's impact on pathogen transmission may not be so clear. Our evidence suggests that the higher burden of Ixodes spp. increases probability of B. burgdorferi sensu lato presence in a host (Fig. 1, Fig. 2) (Ostfeld et al., 2018; Salomon et al., 2021). However, our regression results show that tick burdens increase on hosts as rodent host diversity increases, while B. burgdorferi sensu lato decreases as rodent host diversity increases (Table 3). These discordant results suggest that this relationship is surprisingly nonlinear and highlights the need for more investigation surrounding the interactions of host community diversity, macro-parasites, and microparasites.
We sought to detect interactions between Borrelia spp., ticks, and helminths and found simultaneous infections of all three at a higher rate than expected (Table 1) but did not detect any evidence of interaction between them (Supplemental Table 2). However, when we compare uninfected and B. burgdorferi sensu stricto infected N. fuscipes with the Wilcoxon rank sum test, there was a significant difference in burdens of ticks and helminths but in opposite directions. Where B. burgdorferi sensu stricto infected N. fuscipes had a higher burden of ticks but a lower burden of helminths compared to uninfected Neotoma fuscipes. However, when we investigated these differences further with the binomial regression model, the analyses were not powerful enough to detect potential associations of macro-parasites on micro-parasites (Fig. 2). We found that N. fuscipes were more likely to be infected with B. burgdorferi sensu lato than Peromyscus spp. (Table 3), which may reflect the lower tick burdens mice had in comparison to woodrats (W = 12806, p-value <0.001). Since B. burgdorferi sensu lato is a tick-borne pathogen, these results are not surprising (Ostfeld et al., 2018; Salomon et al., 2021). What was unexpected, is that despite finding co-occurrences for all three types of infections, there was no significant associations between all three infections within individual hosts. Future studies with a larger sample size of Borrelia and helminth infected N. fuscipes would give more power to address these questions.
The mechanisms behind these coinfections are indirectly driven by community factors such as rodent diversity and predator diversity. Our results suggest that habitats with dynamic wildlife populations simultaneously facilitate diversity of parasites such as helminths and ticks. Counterintuitively, higher rodent diversity inhibits B. burgdorferi sensu lato infection within N. fuscipes, a rodent host which has been found to be a highly competent reservoir for B. burgdorferi sensu lato (Brown and Lane, 1994; Swei et al., 2012). This study has large implications on disease dynamics while stressing the need for more research so we can better understand how multi-parasitic infections are distributed amongst wildlife reservoirs and are driven by community diversity with consequences for system-wide disease transmission and risk. Changing environmental conditions may alter community interactions between hosts and their parasites and pathogens, it is therefore vital to understand these dynamics to prevent and manage the transmission of human infectious diseases.
Ethics approval and consent to participate
All procedures performed in this study involving animals were in accordance with the ethical standards of the California Department of Fish and Wildlife permit (ID # SC-8407) and the San Francisco State Animal Care and Use Committee (IACUC AU15-06).
Consent for publication
Not applicable.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Code for regression models and figures can be found at https://github.com/sbsambado/MultiParasite.
Funding
This research was funded by grants from CSUPERB, the Bay Area Lyme Disease Foundation, and NSF grants #1427772, 1745411, and 1750037 to AS and NIH MBRS-RISE: R25-GM059298 to JS. SBS was supported by the Training Grant Program of the Pacific Southwest Regional Center of Excellence for Vector-Borne Disease funded by the U.S. Centers for Disease Control and Prevention (Cooperative Agreement 1U01CK000516).
Authors' contributions
JS, AC, SBS collectively conducted field work. JS, AC, SBS, and SS processed all field samples in the lab. JS and SBS analyzed data. JS prepared the initial manuscript. All authors wrote and edited the final manuscript.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
We would like to acknowledge Samira Ghazi, Laura Hughes, Monika Koczela-Stillman, Judith Lindoro, John Navazo, Thor Salvador-Olsen, Eric Seredian, and Grace Shaw for their help processing and collecting samples. We would like to acknowledge that this research was conducted on Native lands of: Ramaytush, Ohlone, Chochenya and Wappo. We thank the City of Belmont, California State Parks, East Bay Municipal Water District, Marin Open Space Trust, Midpeninsula Regional Open Space District, San Mateo County Parks, Sonoma Regional Parks, and the Town of Los Gatos for access to conduct research in their parks.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2023.08.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Bacon E.A., Kopsco H., Gronemeyer P., Mateus-Pinilla N., Smith R.L. Effects of climate on the variation in abundance of three tick species in Illinois. J. Med. Entomol. 2022;59:700–709. doi: 10.1093/jme/tjab189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillargeon S., Rivest L.-P. The Rcapture package: loglinear models for capture-recapture in R. J. Stat. Software. 2007;19:1–31. doi: 10.18637/jss.v019.i05. [DOI] [Google Scholar]
- Barbour A.G., Anderson J.R., Lane R.S., Burgdorfer W., Gresbrink R.A. The western black-legged tick, Ixodes pacificus: a vector of Borrelia burgdorferi. Am. J. Trop. Med. Hyg. 1985;34:925–930. doi: 10.4269/ajtmh.1985.34.925. [DOI] [PubMed] [Google Scholar]
- Barbour A.G., Bunikis J., Travinsky B., Hoen A.G., Diuk-Wasser M.A., Fish D., Tsao J.I. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am. J. Trop. Med. Hyg. 2009;81:1120–1131. doi: 10.4269/ajtmh.2009.09-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A.G., Hayes S.F. Biology of Borrelia species. Microbiol. Rev. 1986;50:381–400. doi: 10.1128/MMBR.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtel M.J., Teglas M.B., Murphy P.J., Matocq M.D. Parasite prevalence and community diversity in sympatric and allopatric populations of two woodrat species (Sigmodontinae: Neotoma) in Central California. J. Wildl. Dis. 2015;51:419–430. doi: 10.7589/2014-04-099. [DOI] [PubMed] [Google Scholar]
- Behnke J.M., Sinski E., Wakelin D. Primary infections with Babesia microti are not prolonged by concurrent Heligmosomoides polygyrus. Parasitol. Int. 1999;48:183–187. doi: 10.1016/S1383-5769(99)00014-8. [DOI] [PubMed] [Google Scholar]
- Bell A.S., Roode J.C., Sim D., Read A.F. Within-host competition in genetically diverse malaria infections: parasite virulence and competitive success. Evolution. 2006;60:1358–1371. doi: 10.1111/j.0014-3820.2006.tb01215.x. [DOI] [PubMed] [Google Scholar]
- Benbrook E., Sloss M. Iowa State College Press; 1955. Veterinary Clinical Parasitology, Veterinary Clinical Parasitology. [DOI] [Google Scholar]
- Brown R.N., Lane R.S. Reservoir competence of four chaparral-dwelling rodents for Borrelia burgdorferi in California. Am. J. Trop. Med. Hyg. 1996;54:84–91. doi: 10.4269/ajtmh.1996.54.84. [DOI] [PubMed] [Google Scholar]
- Brown R.N., Lane R.S. Natural and experimental Borrelia burgdorferi infections in woodrats and deer mice from California. J. Wildl. Dis. 1994;30:389–398. doi: 10.7589/0090-3558-30.3.389. [DOI] [PubMed] [Google Scholar]
- Bryan R., Kerr J. The relation between the natural worm burden of steers and the faecal egg count differentiated to species. Vet. Parasitol. 1989;30:327–334. doi: 10.1016/0304-4017(89)90102-7. [DOI] [PubMed] [Google Scholar]
- Budischak S.A., Jolles A.E., Ezenwa V.O. Direct and indirect costs of co-infection in the wild: linking gastrointestinal parasite communities, host hematology, and immune function. Int. J. Parasitol. Parasites Wildl. 2012;1:2–12. doi: 10.1016/j.ijppaw.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunikis J., Garpmo U., Tsao J., Berglund J., Fish D., Barbour A.G. Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology. 2004;150:1741–1755. doi: 10.1099/mic.0.26944-0. [DOI] [PubMed] [Google Scholar]
- Busht A.O., Laffertyt K.D., Lotz J.M., Shostakll A.W. Parasitology Meets Ecology on Its Own Terms : margolis et al . Revisited Author (s): Albert O . Bush , Kevin D . Lafferty , Jeffrey M . Lotz and Allen W . Shostak Published by : Allen Press on behalf of The American Society of Parasitologists Stable URL. J. Parasitol. 1997;83:575–583. [PubMed] [Google Scholar]
- Calabrese J.M., Brunner J.L., Ostfeld R.S. Partitioning the aggregation of parasites on hosts into intrinsic and extrinsic components via an extended Poisson-gamma mixture model. PLoS One. 2011;6 doi: 10.1371/journal.pone.0029215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizauskas C.A., Turner W.C., Pitts N., Getz W.M. Seasonal patterns of hormones, macroparasites, and microparasites in wild African ungulates: the interplay among stress, reproduction, and disease. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizauskas C.A., Turner W.C., Wagner B., Küstersrs M., Vance R.E., Getz W.M. Gastrointestinal helminths may affect host susceptibility to anthrax through seasonal immune trade-offs. BMC Ecol. 2014;14:27. doi: 10.1186/s12898-014-0027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranford J.A. Home range and habitat utilization by Neotoma fuscipes as determined by radiotelemetry. J. Mammal. 1977;58:165–172. doi: 10.2307/1379573. [DOI] [Google Scholar]
- Diuk-Wasser M.A., Vourc’h G., Cislo P., Hoen A.G., Melton F., Hamer S.A., Rowland M., Cortinas R., Hickling G.J., Tsao J.I., Barbour A.G., Kitron U., Piesman J., Fish D. Field and climate-based model for predicting the density of host-seeking nymphal Ixodes scapularis, an important vector of tick-borne disease agents in the eastern United States. Global Ecol. Biogeogr. 2010;19:504–514. doi: 10.1111/j.1466-8238.2010.00526.x. [DOI] [Google Scholar]
- Eisen R., Eisen L., Graham C., Hojgaard A., Mead P., Kersch G., Karpathy S., Paddock C. Surveillance for Ixodes pacificus and pathogens found in this tick species in the United States. Center for Disease Control. 2018 https://www.cdc.gov/ticks/resources/TickSurveillance_Ipacificus-P.pdf [WWW Document]. URL. [Google Scholar]
- Eisen R.J., Kugeler K.J., Eisen L., Beard C.B., Paddock C.D. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J. 2017;58:319–335. doi: 10.1093/ilar/ilx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg J. Studies on the behavior of Peromyscus maniculatus gambelii and Peromyscus californicus parasiticus. Behaviour. 1962;19:177–207. [Google Scholar]
- Ezenwa V.O., Jolles A.E. From host immunity to pathogen invasion: the effects of helminth coinfection on the dynamics of microparasites. Integr. Comp. Biol. 2011;51:540–551. doi: 10.1093/icb/icr058. [DOI] [PubMed] [Google Scholar]
- Ezenwa V.O., Stefan Ekernas L., Creel S. Unravelling complex associations between testosterone and parasite infection in the wild. Funct. Ecol. 2012;26:123–133. doi: 10.1111/j.1365-2435.2011.01919.x. [DOI] [Google Scholar]
- Foreyt B. Iowa State University Press; 2001. Veterinary Parasitology : Reference Manual. [Google Scholar]
- Friggens M.M., Beier P. Anthropogenic disturbance and the risk of flea-borne disease transmission. Oecologia. 2010;164:809–820. doi: 10.1007/s00442-010-1747-5. [DOI] [PubMed] [Google Scholar]
- Froeschke G., Matthee S. Landscape characteristics influence helminth infestations in a peri-domestic rodent-implications for possible zoonotic disease. 2014. [DOI] [PMC free article] [PubMed]
- Furman D.P., Loomis E.C. Ticks of California (Acari:Ixodida) Bull. Calif. Insect Surv. 1984 [Google Scholar]
- Graham A.L. Ecological rules governing helminth-microparasite coinfection. Proc. Natl. Acad. Sci. USA. 2008;105 doi: 10.1073/pnas.0707221105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker G.M., Jackson B.T., Niemela M., Andrews E.S., Danforth M.E., Pakingan M.J., Novak M.G. A comparison of questing substrates and environmental factors that influence nymphal Ixodes pacificus (Acari: Ixodidae) abundance and seasonality in the Sierra Nevada foothills of California. J. Med. Entomol. 2021;58:1880–1890. doi: 10.1093/jme/tjab037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M.B., Feirer S., Monaghan A.J., Lane R.S., Eisen R.J., Padgett K.A., Kelly M. Modeling future climate suitability for the western blacklegged tick, Ixodes pacificus, in California with an emphasis on land access and ownership. Ticks Tick. Borne. Dis. 2021;12 doi: 10.1016/j.ttbdis.2021.101789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M.B., Jarnevich C.S., Monaghan A.J., Eisen R.J. Modeling the geographic distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the contiguous United States. J. Med. Entomol. 2016;53:1176–1191. doi: 10.1093/jme/tjw076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes K.S., Bancroft A.J., Goldrick M., Portsmouth C., Roberts I.S., Grencis R.K. Exploitation of the intestinal microflora by the parasitic nematode Trichuris muris. Science (80- 2010;328:1391–1394. doi: 10.1126/science.1187703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeester T.R., Jansen P.A., Wijnen H.J., Coipan E.C., Fonville M., Prins H.H.T., Sprong H., van Wieren S.E. Cascading effects of predator activity on tick-borne disease risk. Proc. R. Soc. B Biol. Sci. 2017;284 doi: 10.1098/rspb.2017.0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson P.J., Dobson A.P., Newborn D. Do parasites make prey vulnerable to predation? Red grouse and parasites. J. Anim. Ecol. 1992;61:681. doi: 10.2307/5623. [DOI] [Google Scholar]
- Jolles A.E., Ezenwa V.O., Etienne R.S., Turner W.C., Olff H. Interactions between macroparasites and microparasites drive infection patterns in free-ranging African buffalo. Ecology. 2008;89:2239–2250. doi: 10.1890/07-0995.1. [DOI] [PubMed] [Google Scholar]
- Jolles A.E., Ezenwa V.O., Etienne R.S., Turner W.C., Olff H. Interactions between macroparasites and microparasites drive infection patterns in free- ranging. African Buffalo Author. 2008;89:2239–2250. doi: 10.1890/07-0995.1. [DOI] [PubMed] [Google Scholar]
- Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Choi M.H., Bae Y.M., Oh J.K., Lim M.K., Hong S.T. Correlation between discharged worms and fecal egg counts in human clonorchiasis. PLoS Neglected Trop. Dis. 2011;5:1–5. doi: 10.1371/journal.pntd.0001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey K.P. Social behaviour in confined populations of the Allegheny woodrat, Neotoma floridana magister. Anim. Behav. 1976 doi: 10.1016/S0003-3472(76)80112-1. [DOI] [Google Scholar]
- Kleinjan J.E., Lane R.S. Larval keys to the genera of Ixodidae (Acari) and species of Ixodes (Latreille) ticks established in California. Pan-Pacific Entomol. 2008;84:121–142. doi: 10.3956/2007-38.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lado P., Glon M.G., Klompen H. Integrative taxonomy of Dermacentor variabilis (Ixodida: Ixodidae) with description of a new species, Dermacentor similis n. sp. J. Med. Entomol. 2021;58:2216–2227. doi: 10.1093/jme/tjab134. [DOI] [PubMed] [Google Scholar]
- Lawrence A., O'Connor K., Haroutounian V., Swei A. Patterns of diversity along a habitat size gradient in a biodiversity hotspot. Ecosphere. 2018;9 doi: 10.1002/ecs2.2183. [DOI] [Google Scholar]
- Maaz D., Rausch S., Richter D., Krücken J., Kühl A.A., Demeler J., Blümke J., Matuschka F.-R., von Samson-Himmelstjerna G., Hartmann S. Susceptibility to ticks and Lyme disease spirochetes is not affected in mice coinfected with nematodes. Infect. Immun. 2016;84:1274–1286. doi: 10.1128/IAI.01309-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald A.J. Abiotic and habitat drivers of tick vector abundance, diversity, phenology and human encounter risk in southern California. PLoS One. 2018;13 doi: 10.1371/journal.pone.0201665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald A.J., Hyon D.W., Brewington J.B., Iii, O’connor K.E., Swei A., Briggs C.J. Lyme disease risk in southern California: abiotic and environmental drivers of Ixodes pacificus (Acari: Ixodidae) density and infection prevalence with Borrelia burgdorferi. Parasites Vectors. 2017;10:1–16. doi: 10.1186/s13071-016-1938-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtinger E.T., Williams S.C. Practical guide to trapping Peromyscus leucopus (Rodentia: Cricetidae) and Peromyscus maniculatus for vector and vector-borne pathogen surveillance and ecology. J. Insect Sci. 2020;20 doi: 10.1093/jisesa/ieaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina, J.P., Brady, O.J., Golding, N., Kraemer, M.U.G., Wint, G.R.W., Ray, S.E., Pigott, D.M., Shearer, F.M., Johnson, K., Earl, L., Marczak, L.B., Shirude, S., Davis Weaver, N., Gilbert, M., Velayudhan, R., Jones, P., Jaenisch, T., Scott, T.W., Reiner, R.C., Hay, S.I., n.d. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 10.1038/s41564-019-0476-8. [DOI] [PMC free article] [PubMed]
- Mideo N. Evolutionary parasitology parasite adaptations to within-host competition. 2009. [DOI] [PubMed]
- Millette K.L., Gonzalez A., Cristescu M.E. Breaking ecological barriers: anthropogenic disturbance leads to habitat transitions, hybridization, and high genetic diversity. Sci. Total Environ. 2020;740 doi: 10.1016/j.scitotenv.2020.140046. [DOI] [PubMed] [Google Scholar]
- Mohd Zain S.N., Behnke J.M., Lewis J.W. Parasites & Vectors; Malaysia: 2012. Helminth Communities from Two Urban Rat Populations in Kuala Lumpur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R.J., Eaton J.T., Cepek J.D., Lorch P.D., Dennis P.M., Robison T., Tsao J., Montgomery R.A. Dynamic rodent behavioral response to predation risk: implications for disease ecology. Oecologia. 2020;192:67–78. doi: 10.1007/s00442-019-04565-z. [DOI] [PubMed] [Google Scholar]
- Moll R.J., Redilla K.M., Mudumba T., Muneza A.B., Gray S.M., Abade L., Hayward M.W., Millspaugh J.J., Montgomery R.A. The many faces of fear: a synthesis of the methodological variation in characterizing predation risk. J. Anim. Ecol. 2017;86:749–765. doi: 10.1111/1365-2656.12680. [DOI] [PubMed] [Google Scholar]
- Moore G., Tessler M., Cunningham S.W., Betancourt J., Harbert R. Paleo-metagenomics of North American fossil packrat middens: past biodiversity revealed by ancient DNA. Ecol. Evol. 2020;10:2530–2544. doi: 10.1002/ece3.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel P.A., Oriss T.B. Crossregulation between Th1 and Th2 cells. Crit. Rev. Immunol. 1998;18:275–303. doi: 10.1615/CRITREVIMMUNOL.V18.I4.10. [DOI] [PubMed] [Google Scholar]
- Nunn C.L., Gittleman J.L., Antonovics J. A comparative study of white blood cells and disease risk in carnivores. R. Soc. 2003;270:347–356. doi: 10.1098/rspb.2002.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J. 2016. Vegan: Community Ecology Package. [Google Scholar]
- Ostfeld R.S., Brisson D., Oggenfuss K., Devine J., Levy M.Z., Keesing F. Effects of a zoonotic pathogen, Borrelia burgdorferi , on the behavior of a key reservoir host. Ecol. Evol. 2018;8:4074–4083. doi: 10.1002/ece3.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock C.D., Lane R.S., Staples J.E., Labruna M.B. Chaning paradigms for tick-borne diseases in the Americas. Glob. Heal. impacts vector‐borne Dis. Work. Summ. 2016:221–257. [Google Scholar]
- Parkinson C.M., O'Brien A., Albers T.M., Simon M.A., Clifford C.B., Pritchett-Corning K.R. Diagnosis of ecto- and endoparasites in laboratory rats and mice. J. Vis. Exp. 2011;5–8 doi: 10.3791/2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz J.A., Graczyk T.K., Geller N., Vittor A.Y. Environmental changes & parasitic diseases. Int. J. Parasitol. 2000;30:1395–1405. doi: 10.1016/s0020-7519(00)00141-7. [DOI] [PubMed] [Google Scholar]
- Patz J.A., Olson S.H., Uejio C.K., Gibbs H.K. Disease emergence from global climate and land use change. Med. Clin. 2008;92:1473–1491. doi: 10.1016/j.mcna.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Perret J.L., Guerin P.M., Diehl P.A., Vlimant M., Gern L. Darkness induces mobility, and saturation deficit limits questing duration, in the tick Ixodes ricinus. J. Exp. Biol. 2003;206:1809–1815. doi: 10.1242/jeb.00345. [DOI] [PubMed] [Google Scholar]
- Petney T.N., Andrews R.H. Multiparasite communities in animals and humans: frequency, structure and pathogenic significance. Int. J. Parasitol. 1998;28:377–393. doi: 10.1016/S0020-7519(97)00189-6. [DOI] [PubMed] [Google Scholar]
- Postic D., Assous M.V., Grimont P.A.D., Baranton G. Diversity of Borrelia burgdorfeii sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)-rrl (23S) intergenic spacer amplicons. Int. J. Syst. Bacteriol. 1994;44:743–752. doi: 10.1099/00207713-44-4-743. [DOI] [PubMed] [Google Scholar]
- Poulin R. Ecological Monographs; 1996. Phylogeny, Ecology, and the Richness of Parasite Communities in Vertebrates, Source. [Google Scholar]
- PRISM Climate Group Univ. Oregon state. 2014. https://prism.oregonstate.edu [WWW Document]
- Rizzoli A., Tagliapietra V., Cagnacci F., Marini G., Arnoldi D., Rosso F., Rosà R. Parasites and wildlife in a changing world: the vector-host- pathogen interaction as a learning case. Int. J. Parasitol. Parasites Wildl. 2019 doi: 10.1016/j.ijppaw.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R., Lindsey N.P., Fischer M., Gregory C.J., Hinckley A.F., Mead P.S., Paz-Bailey G., Waterman S.H., Drexler N.A., Kersh G.J., Hooks H., Partridge S.K., Visser S.N., Beard C.B., Petersen L.R. Vital signs: trends in reported vectorborne disease cases — United States and territories, 2004–2016. MMWR Morb. Mortal. Wkly. Rep. 2018;67:496–501. doi: 10.15585/mmwr.mm6717e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudio . 2021. Integrated Development Environment for R. [Google Scholar]
- Rynkiewicz E.C., Pedersen A.B., Fenton A. An ecosystem approach to understanding and managing within-host parasite community dynamics. Trends Parasitol. 2015;31:212–221. doi: 10.1016/j.pt.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Salomon J., Hamer S.A., Swei A. A geginner's guide to collecting questing hard ticks (Acari: Ixodidae): a standardized tick dragging protocol. J. Insect Sci. 2020;20:1–8. doi: 10.1093/jisesa/ieaa073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon J., Lawrence A., Crews A., Sambado S., Swei A. Host infection and community composition predict vector burden. Oecologia. 2021 doi: 10.1007/s00442-021-04851-9. [DOI] [PubMed] [Google Scholar]
- Sambado S., Salomon J., Crews A., Swei A. Mixed transmission modes promote persistence of an emerging tick‐borne pathogen. Ecosphere. 2020;11 doi: 10.1002/ecs2.3171. [DOI] [Google Scholar]
- Schmidt K.A., Ostfeld R.S. Biodiversity and the dilution effect in disease ecology. Ecology. 2001;82:609–619. [Google Scholar]
- Shaw D.J., Grenfell B.T., Dobson A.P. Patterns of macroparasite aggregation in wildlife host populations. Parasitology. 1998;117:597–608. doi: 10.1017/S0031182098003448. [DOI] [PubMed] [Google Scholar]
- Sithithaworn P., Tesana S., Pipitgool V., Kaewkes S., Thaiklar K., Pairojkul C., Sripa B., Paupairoj A. Relationship between faecal egg count and worm burden of Opisthorchis viverrini in human autopsy cases. Parasitology. 1991;102:277–281. doi: 10.1017/S0031182000062594. [DOI] [PubMed] [Google Scholar]
- Suraci J.P., Clinchy M., Zanette L.Y., Wilmers C.C. Fear of humans as apex predators has landscape‐scale impacts from mountain lions to mice. Ecol. Lett. 2019;22:1578–1586. doi: 10.1111/ele.13344. [DOI] [PubMed] [Google Scholar]
- Suraci J.P., Smith J.A., Clinchy M., Zanette L.Y., Wilmers C.C. Humans, but not their dogs, displace pumas from their kills: an experimental approach. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-48742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swei A., Briggs C.J., Lane R.S., Ostfeld R.S. Impacts of an introduced forest pathogen on the risk of Lyme disease in California. Vector Borne Zoonotic Dis. 2012;12:623. doi: 10.1089/vbz.2011.0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swei A., Couper L.I., Coffey L.L., Kapan D., Bennett S. Patterns, drivers, and challenges of vector-borne disease emergence. Vector Borne Zoonotic Dis. 2020;20:159–170. doi: 10.1089/vbz.2018.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talleklint-Eisen L., Lane R.S. Variation in the density of questing Ixodes pacificus (Acari: Ixodidae) nymphs infected with Borrelia burgdorferi at different spatial scales in California. J. Parasitol. 1999;85:824. doi: 10.2307/3285817. [DOI] [PubMed] [Google Scholar]
- Tälleklint-Eisen L., Lane R.S. Efficiency of drag sampling for estimating population sizes of Ixodes pacificus (Acari: Ixodidae) nymphs in leaf litter. J. Med. Entomol. 2000;37:484–487. doi: 10.1093/jmedent/37.3.484. [DOI] [PubMed] [Google Scholar]
- Thomas C.E., Burton E.S., Brunner J.L. Environmental drivers of questing activity of juvenile black-legged ticks (Acari: Ixodidae): temperature, desiccation risk, and diel cycles. J. Med. Entomol. 2020;57:8–16. doi: 10.1093/jme/tjz126. [DOI] [PubMed] [Google Scholar]
- Tidman R., Abela-Ridder B., de Castañeda R.R. The impact of climate change on neglected tropical diseases: a systematic review. Trans. R. Soc. Trop. Med. Hyg. 2021;115:147–168. doi: 10.1093/trstmh/traa192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Riet E., Hartgers F.C., Yazdanbakhsh M. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology. 2007;212:475–490. doi: 10.1016/j.imbio.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Vaumourin E., Vourc’h G., Gasqui P., Vayssier-Taussat M. The importance of multiparasitism: examining the consequences of co-infections for human and animal health. Parasites Vectors. 2015;8:545. doi: 10.1186/s13071-015-1167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen K. Social organization in the dusky-footed woodrat (Neotoma fuscipes): a field and laboratory study. Anim. Behav. 1982;30:1171–1182. doi: 10.1016/S0003-3472(82)80208-X. [DOI] [Google Scholar]
- Webster J.P., Gower C.M., Knowles S.C.L., Molyneux D.H., Fenton A. One health - an ecological and evolutionary framework for tackling neglected zoonotic diseases. Evol. Appl. 2016;9:313–333. doi: 10.1111/eva.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford W.G., Steinberger Y. Pack rats (<i<Neotoma</i> spp.): keystone ecological engineers? J. Arid Environ. 2010;74:1450–1455. doi: 10.1016/j.jaridenv.2010.05.025. [DOI] [Google Scholar]
- Williams S.C., Ward J.S. Effects of Japanese barberry (Ranunculales: Berberidaceae) removal and resulting microclimatic changes on Ixodes scapularis (Acari: Ixodidae) abundances in Connecticut, USA. Environ. Entomol. 2010;39:1911–1921. doi: 10.1603/EN10131. [DOI] [PubMed] [Google Scholar]
- Wolfe N.D., Daszak P., Kilpatrick A.M., Burke D.S. Bushmeat hunting, deforestation, and prediction of zoonotic disease emergence. Emerg. Infect. Dis. 2005;11:1822–1827. doi: 10.3201/eid1112.040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Code for regression models and figures can be found at https://github.com/sbsambado/MultiParasite.