Abstract
Importance
The effects of moderate systolic blood pressure (SBP) lowering after successful recanalization with endovascular therapy for acute ischemic stroke are uncertain.
Objective
To determine the futility of lower SBP targets after endovascular therapy (<140 mm Hg or 160 mm Hg) compared with a higher target (≤180 mm Hg).
Design, Setting, and Participants
Randomized, open-label, blinded end point, phase 2, futility clinical trial that enrolled 120 patients with acute ischemic stroke who had undergone successful endovascular therapy at 3 US comprehensive stroke centers from January 2020 to March 2022 (final follow-up, June 2022).
Intervention
After undergoing endovascular therapy, participants were randomized to 1 of 3 SBP targets: 40 to less than 140 mm Hg, 40 to less than 160 mm Hg, and 40 to 180 mm Hg or less (guideline recommended) group, initiated within 60 minutes of recanalization and maintained for 24 hours.
Main Outcomes and Measures
Prespecified multiple primary outcomes for the primary futility analysis were follow-up infarct volume measured at 36 (±12) hours and utility-weighted modified Rankin Scale (mRS) score (range, 0 [worst] to 1 [best]) at 90 (±14) days. Linear regression models were used to test the harm-futility boundaries of a 10-mL increase (slope of 0.5) in the follow-up infarct volume or a 0.10 decrease (slope of −0.005) in the utility-weighted mRS score with each 20-mm Hg SBP target reduction after endovascular therapy (1-sided α = .05). Additional prespecified futility criterion was a less than 25% predicted probability of success for a future 2-group, superiority trial comparing SBP targets of the low- and mid-thresholds with the high-threshold (maximum sample size, 1500 with respect to the utility-weighted mRS score outcome).
Results
Among 120 patients randomized (mean [SD] age, 69.6 [14.5] years; 69 females [58%]), 113 (94.2%) completed the trial. The mean follow-up infarct volume was 32.4 mL (95% CI, 18.0 to 46.7 mL) for the less than 140–mm Hg group, 50.7 mL (95% CI, 33.7 to 67.7 mL), for the less than 160–mm Hg group, and 46.4 mL (95% CI, 24.5 to 68.2 mL) for the 180–mm Hg or less group. The mean utility-weighted mRS score was 0.51 (95% CI, 0.38 to 0.63) for the less than 140–mm Hg group, 0.47 (95% CI, 0.35 to 0.60) for the less than 160–mm Hg group, and 0.58 (95% CI, 0.46 to 0.71) for the high-target group. The slope of the follow-up infarct volume for each mm Hg decrease in the SBP target, adjusted for the baseline Alberta Stroke Program Early CT score, was −0.29 (95% CI, −0.81 to ∞; futility P = .99). The slope of the utility-weighted mRS score for each mm Hg decrease in the SBP target after endovascular therapy, adjusted for baseline utility-weighted mRS score, was −0.0019 (95% CI, −∞ to 0.0017; futility P = .93). Comparing the high-target SBP group with the lower-target groups, the predicted probability of success for a future trial was 25% for the less than 140–mm Hg group and 14% for the 160–mm Hg group.
Conclusions and Relevance
Among patients with acute ischemic stroke, lower SBP targets less than either 140 mm Hg or 160 mm Hg after successful endovascular therapy did not meet prespecified criteria for futility compared with an SBP target of 180 mm Hg or less. However, the findings suggested a low probability of benefit from lower SBP targets after endovascular therapy if tested in a future larger trial.
Trial Registration
ClinicalTrials.gov Identifier: NCT04116112
Key Points
Question
Are moderately low systolic blood pressure (SBP) targets (<140 and <160 mm Hg) after successful endovascular stroke treatment harmful?
Findings
In this futility-design, randomized clinical trial that included 120 participants, SBP targets of less than either 140 or 160 mm Hg vs the guideline-recommended target of 180 mm Hg or less did not meet prespecified criteria for futility or harm for follow-up infarct volume and 90-day utility-weighted modified Rankin Scale (mRS) score. Based on the utility-weighted mRS score, the predicted probability of success in a future larger clinical trial for an SBP target of less than either 140 mm Hg or 160 mm Hg vs 180 mm Hg or less was 25% and 14%, respectively.
Meaning
Lower SBP targets after successful endovascular therapy for acute ischemic stroke did not meet the criteria for futility, although the findings suggested a low probability of benefit from lower SBP targets if tested in a future larger trial.
This randomized clinical trial evaluates whether moderately lowering systolic blood pressure, compared with a higher target, in the first 24 hours after successful endovascular treatment for acute ischemic stroke is futile.
Introduction
Endovascular clot retrieval treatment has been shown to be a highly effective treatment for patients with acute ischemic stroke with a large cerebral vessel occlusion with an approximate 80% rate of successful vessel recanalization.1 For every 2 to 4 patients treated, an additional patient achieved functional independence.1,2,3 Despite recanalization, 50% to 60% of patients undergoing endovascular therapy for a stroke due to a large cerebral vessel occlusion died or remain disabled at 90 days.1,4,5
In addition to refinement of endovascular therapy technology, optimizing periprocedural management may help improve outcomes. The current American Heart Association/American Stroke Association and European Stroke Organization guidelines recommend targeting systolic blood pressure (SBP) of 180 mm Hg or less after endovascular therapy based on conventions from prior thrombolysis trials.6,7 Although higher systemic SBP is common in the setting of acute ischemic stroke due to cerebral autoregulation,8 observational studies have indicated that lower SBP after successful endovascular therapy may be associated with better functional outcomes.9,10,11 Specifically, a prospective multisite observational study identified that a maximal SBP of approximately 160 mm Hg in the first 24 hours after endovascular therapy distinguished between patients with good vs poor functional outcomes.12 Others showed that moderate SBP levels of between 140 mm Hg and 160 mm Hg were associated with better patient outcomes.13 However, a recent randomized trial among Chinese patients who had undergone successful endovascular therapy found that SBP lowered to less than 120 mm Hg was harmful.14
In a 2017 survey of clinical practice, among 58 US sites, 69% of institutions practiced moderate SBP lowering and only 5% of institutions practiced extreme SBP lowering to less than 120 mm Hg for patients successfully treated with endovascular therapy.15 The safety and efficacy of the more widely practiced SBP targets of less than either 140 mm Hg or 160 mm Hg after endovascular therapy remains to be determined. Herein, we report the results of a randomized trial conducted to evaluate the futility of moderately lowering SBP in the first 24 hours after patients with acute ischemic stroke had undergone endovascular therapy with successful recanalization.
Methods
We conducted a pragmatic, multisite, randomized, open-label, futility-design, clinical trial with blinded end point assessment at 3 comprehensive stroke centers located within the US (South, Midwest, and Northeast regions) to assess the futility of lower SBP targets among patients with stroke after undergoing successful endovascular therapy. We specified a priori that lower SBP targets after endovascular therapy would be considered futile for further testing if (1) there were unequivocal evidence of significant harm with lower targets or (2) the predicted probability of success for a future, pivotal trial with a maximum sample size of 1500 patients would be less than 25%.
The study was approved by the institutional review boards of all participating institutions and the data and safety monitoring board (DSMB) prior to commencement of the study’s enrollment. All participants (or their legally authorized representatives) provided written informed consent. The trial protocol16 was published and is provided, including the prespecified analytic plan, in Supplement 1.
Study Population
The inclusion criteria were adult patients (≥18 years) with ischemic stroke who had undergone successful endovascular therapy (defined as modified Thrombolysis in Cerebral Ischemia, ≥2b; 50%-89% reperfusion; 2c, 90%-99% reperfusion; and 3, complete reperfusion) for an occlusion in an anterior cerebral circulation large vessel (specifically, internal carotid artery and M1 or M2 segments of the middle cerebral artery).
Patients meeting the following criteria were excluded if they had (1) a diagnosis of heart failure with ejection fraction less than 30%; (2) left ventricular assist device; or (3) extracorporeal membrane oxygenation (Figure 1). Additionally, pregnant individuals and patients enrolled in other clinical trials were excluded.
Figure 1. Flow of Patients Through the BEST-II Trial.
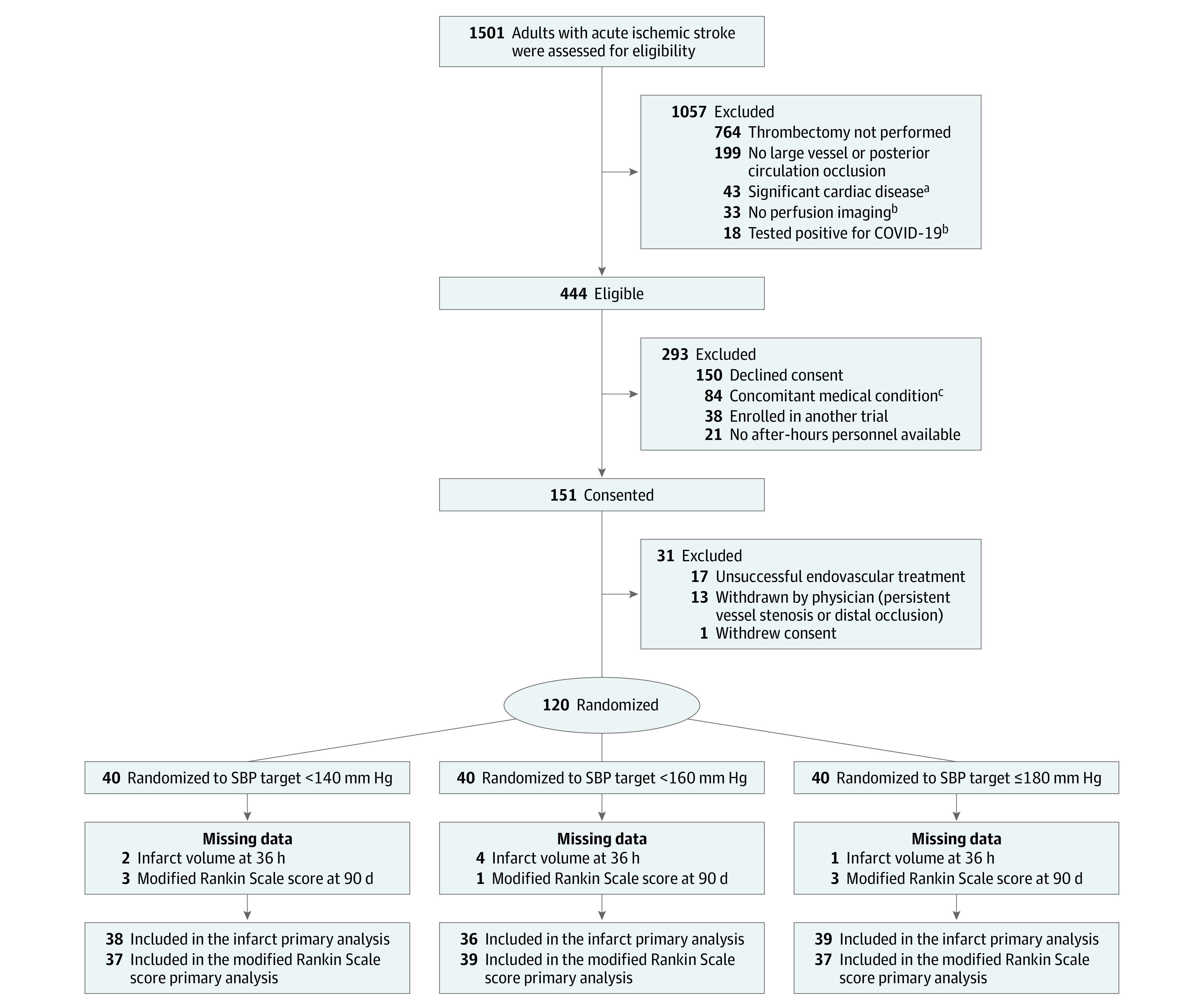
aHeart failure with ejection fraction less than 30%, left ventricular assist device, or extracorporeal membrane oxygenation.
bCriteria were removed in a subsequent protocol amendment.
cConcomitant medical conditions that precluded tight BP management per treating condition.
SBP indicates systolic blood pressure.
Participants of all races and ethnicities were eligible for inclusion. Race and ethnicity information was collected pursuant to the National Institutes of Health (NIH) funding requirements by the study personnel via direct participant interview using fixed categories (Table 1).
Table 1. Baseline Characteristics.
| Systolic blood pressure target, mm Hg | |||
|---|---|---|---|
| <140 (n = 40) | <160 (n = 40) | ≤180 n = 40) | |
| Demographics, No. (%) | |||
| Age, median (IQR), y | 74 (66.8-84.8) | 70.5 (61.8-76) | 68 (58-76.3) |
| Sex, No. (%) | |||
| Female | 28 (70) | 21 (52.5) | 20 (50) |
| Male | 12 (30) | 19 (47.5) | 20 (50) |
| Race and ethnicity, No. (%)a | |||
| Black | 2 (5) | 2 (5) | 5 (12.5) |
| Hispanic or Latino | 0 | 0 | 2 (5.0) |
| Native Hawaiian or Other Pacific Islander | 1 (2.5) | 0 | 0 |
| Not Hispanic or Latino ethnicity | 39 (97.5) | 39 (97.5) | 36 (90) |
| White | 34 (85) | 37 (92.5) | 34 (85) |
| Multiracial | 3 (7.5) | 0 | 0 |
| Other race | 0 | 1 (2.5) | 1 (2.5) |
| Unknown ethnicity | 1 (2.5) | 1 (2.5) | 2 (5) |
| Past medical history, No. (%)b | |||
| Hyperlipidemia | 33.0 (82.5) | 28 (70) | 34 (85) |
| Hypertension | 32 (80) | 28 (70) | 32 (80) |
| Atrial fibrillation | 19 (47.5) | 13 (32.5) | 21 (52.5) |
| Diabetes | 12 (30) | 15 (37.5) | 13 (32.5) |
| Current smoking | 8 (20) | 12 (30) | 10 (25) |
| Prestroke utility-weighted mRS score, mean (95% CI)c | 0.90 (0.86-0.94) | 0.95 (0.90-0.99) | 0.91 (0.86-0.96) [n = 39] |
| Prestroke mRS score, No. (%)d | |||
| 0 | 20 (50) | 30 (75) | 25 (62.5) |
| 1 | 9 (22.5) | 6 (15) | 6 (15) |
| 2 | 7 (17.5) | 1 (2.5) | 3 (7.5) |
| 3 | 4 (10) | 2 (5) | 4 (10) |
| 4 | 0 | 1 (2.5) | 1 (2.5) |
| Medications, No. (%) | |||
| Baseline antihypertensive use | 27 (67.5) | 20 (50) | 27 (67.5) |
| Baseline number of antihypertensives | |||
| 0 | 13 (32.5) | 20 (50.0) | 13 (32.5) |
| 1 | 8 (20) | 13 (32.5) | 12 (30.0) |
| ≥2 | 19 (47.5) | 7 (17.5) | 15 (37.5) |
| Intravenous thrombolysis | 17 (42.5) | 19 (47.5) | 18 (45) |
| Baseline antiplatelet use | 14 (35) | 13 (32.5) | 19 (47.5) |
| Baseline anticoagulant use | 10 (25) | 3 (7.5) | 9 (22.5) |
| Stroke characteristics | |||
| Baseline NIHSS score, median (IQR)e | 16 (11-23) | 18 (15-23) | 14 (11-17) |
| Baseline ASPECT score, median (IQR)f | 8 (7-9) | 7 (6-8) | 8 (7-9) |
| Time last known well to presentation, median (IQR), min | 238 (158-394) | 222 (145-463) | 221 (105-442) |
| Location of large vessel occlusion, No. (%) | |||
| ICA | 7 (17.5) | 9 (22.5) | 5 (12.5) |
| M1 | 31 (77.5) | 25 (62.5) | 23 (57.5) |
| M2 | 6 (15) | 8 (20) | 15 (37.5) |
| Laboratory findings | |||
| Baseline glucose, mean (SD), mg/dL | 150 (116) | 143 (55.5) | 133 (48.9) |
| Baseline platelet count, median (IQR), ×103/μL | 225 (180-270) | 226 (184-268) | 223 (189-274) |
| Imaging findings | |||
| Modified Tan score on baseline CT angiogram, No. (%)g | No. 33 | No. 38 | No. 37 |
| 0 | 0 | 2 (5.3) | 1 (2.7) |
| 1 | 9 (27.3) | 15 (39.5) | 10 (27) |
| 2 | 18 (54.5) | 16 (42.1) | 18 (48.6) |
| 3 | 6(18.2) | 5 (13.2) | 8 (21.6) |
| Baseline CT perfusion brain volume with cerebral blood flow <30% of contralateral side, median (IQR), mL | 5 (0-22) [n = 29] | 14 (1-39.5) [n = 27] | 20.5 (0-39.5) [n = 28] |
| Baseline CT perfusion brain volume with Tmax >6 s, median (IQR), mL | 95 (75-140) [n = 29] | 129 (66.5-169) [n = 27] | 124 (79-177) [n = 28] |
| Endovascular procedural details | |||
| Final mTICI score, No. (%)h | |||
| 2b | 16 (40) | 17 (42.5) | 17 (42.5) |
| 2c | 6 (15) | 5 (12.5) | 7 (17.5) |
| 3 | 18 (45) | 18 (45) | 16 (40) |
| Type of anesthesia used | |||
| General | 8 (20) | 8 (20) | 7 (17.5) |
| Conscious sedation | 28 (70) | 26 (65) | 27 (67.5) |
| Blood pressure prior to endovascular therapy, mean (SD) mm Hg | |||
| Systolic | 150 (22.5) | 151 (25.3) | 146 (23) |
| Diastolic | 82.6 (14.5) | 89 (18.4) | 86.6 (19.6) |
Abbreviations: ICA, internal carotid artery; M1, first segment of the middle cerebral artery; M2, second segment of the middle cerebral artery; Tmax, time to maximum.
SI conversion factors: To convert glucose to mmol/L, multiply by 0.0555.
Race and ethnicity were collected by self-report, as closed categories, and multiple selections were allowed. The “Other” category was available for selection if none of the specific categories best described a participant’s race and/or ethnicity.
Medical history was collected using chart review and direct interview when the medical chart did not contain complete information.
Utility-weighted modified Rankin Scale (mRS) score (range, 0 = worst; 1 = best).
The score ranges from 0 to 6: 0, no symptoms at all; 1, no significant disability despite symptoms; 2, slight disability; 3, moderate disability; 4, moderately severe disability; 5, severe disability; 6, dead (Figure 3).
National Institutes of Health Stroke Scale (NIHSS) score (range, 0-42, higher scores denote more severe neurological deficits).
Alberta Stroke Program Early CT (ASPECT; range 0-10, higher scores denote less severity of stroke on computed tomographic imaging).
Modified Tan score assesses cerebral collaterals (range, 0 to 3; 0 indicates absent collateral supply to the occluded middle cerebral artery territory; 1, collateral supply filling ≤50% but >0% of the occluded middle cerebral artery territory; 2, collateral supply filling >50% but <100% of the occluded middle cerebral artery territory; 3, 100% collateral supply of the occluded middle cerebral artery territory).
Modified Thrombolysis in Cerebral Infarction (mTICI) score denotes the level of angiographic reperfusion (2b indicates 50%-89% reperfusion; 2c, 90%-99% reperfusion; 3, complete reperfusion).
Randomization and Study Interventions
Upon provision of informed consent, eligible patients were randomized within 45 minutes of final recanalization to SBP targets after endovascular therapy of 180 mm Hg or less (current guideline recommendation), less than 160 mm Hg, or less than 140 mm Hg in a 1:1:1 ratio using computer-generated permuted block randomization (block sizes 3, 6, and 9), stratified by site. SBP management was required to be initiated within 60 minutes of recanalization to maintain it at or below the randomly assigned target for 24 hours using nicardipine as the first-line agent. Intravenous labetalol and hydralazine were recommended as second- and third-line agents. If an antihypertensive agent decreased the SBP below the highest number for the next lower target, the study protocol called for the agent to be reduced or stopped.
Study End Points
The trial had 2 primary end points, either of which could independently lead to an assessment of harm: (1) follow-up infarct volume (FIV) at 36 (±12) hours and (2) utility-weighted modified Rankin Scale (mRS) score at 90 (±14) days using standard utility weights ranging from 0 (worst) to 1 (best).17
The minimal clinically important difference for follow-up infarct volume is unknown and ranges from 0.04 to 0.1 for a utility-weighted mRS score based on studies of patients who did not have a stroke and on treatment effects seen in prior large stroke trials.17,18,19,20
Secondary end points included (1) any intracerebral hemorrhage (ICH) on 36-hour computed tomography (CT) or magnetic resonance imaging (MRI) if CT was unavailable, (2) symptomatic intracerebral hemorrhage on the 36-hour CT (MRI if CT is unavailable; any intracerebral hemorrhage associated with ≥4 points increase from the baseline NIH Stroke Scale [NIHSS] score),21 and (3) neurological worsening associated with antihypertensive treatment. The follow-up perfusion core and penumbra volume as secondary outcomes were removed early in the trial due to logistical challenges.
The following exploratory outcomes were assessed: (1) the compliance outcome, the hourly maximum SBP higher than the target from 2 to 24 hours after treatment initiation and (2) the feasibility outcome, separation of hourly maximum SBP values among the 3 SBP target groups 2 to 24 hours after treatment initiation. In-hospital mortality and 24-hour NIHSS score (range, 0-42 with higher scores indicating more severe neurological deficits) were collected as post hoc outcomes.
Study Assessments
Participants’ baseline medical history, neurological assessment, and imaging (CT brain, CT angiogram of head and neck, and CT perfusion) were obtained as the standard of care. A central, blinded neuroradiologist ascertained the Alberta Stroke Program Early CT (ASPECT) score, CT perfusion core, and penumbra volumes, and collateral grade using a modified Tan score on baseline scans and infarct volume on the 36-hour imaging. The infarct was defined as tissue demonstrating restricted diffusion on MRI and was outlined manually. If an MRI was unavailable, tissue demonstrating hypodensity in comparison to the contralateral hemisphere was manually outlined on CT. The follow-up infarct volume was calculated using OsiriX (Pixmeo). Hemorrhage was classified on the 36-hour CT scan according to the third European Cooperative Acute Stroke Study (ECASS-III) criteria.21 At the 24-hour visit, the participant’s NIHSS score was assessed by a certified rater. Patients were followed-up at 90 days by an in-person or telephone interview to ascertain the mRS score by a blinded, certified rater.
Participants’ BP was monitored noninvasively in a recumbent position using a BP cuff with the following minimum frequency: every 5 minutes for the first 15 minutes following nicardipine initiation or dose adjustment, then every 15 minutes for the first hour, followed by at least every 30 minutes until 24 hours after endovascular therapy. Arterial line monitoring was allowed but not required.
Sample-Size Calculations
We prespecified that either a 10-mL increase in the follow-up infarct volume or a 0.10 decrease in the utility-weighted mRS score with each 20-mm Hg decrease in the SBP target were definite, clinically meaningful safety concerns. The harm boundary for the follow-up infarct volume was determined by an expert consensus and the utility-weighted mRS score was based on the minimal clinically important difference.17,18,19,20 Using a linear regression to relate decreasing SBP target on the x-axis with outcome, these harm boundaries equated to a slope of 0.5 for follow-up infarct volume −0.005 for utility-weighted-mRS. A significant finding favoring the alternative hypothesis (ie, slope of follow-up infarct volume >0.5 or utility-weighted-mRS score <−0.005) would be evidence that decreasing SBP increases the follow-up infarct volume or reduces the utility-weighted-mRS score beyond a given level (unequivocal evidence of significant harm). Using the Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3 (DEFUSE-3) trial and our preliminary data,2,12 we estimated 101 participants would allow for 80% power using a 1-sided test with the level of significance of an α of .05 to test these alternative hypotheses. The hypothesis testing was against a nonzero null of the predefined harm boundaries. The final sample size was increased to 120 patients to account for up to 15% loss to follow-up.
Statistical Analysis
General Considerations
The statistical analysis plan has been published (Supplement 1).16 The primary analysis population was defined as all enrolled participants grouped according to their randomly assigned SBP target group with complete outcome data (full analysis data set). An interim analysis was performed after 60 enrolled patients completed follow-up.
Statistical Procedures
R version 4.2.3 was used for primary and secondary outcome analyses and the generation of Figures and Tables. We quantified the slopes of the follow-up infarct volume and utility-weighted mRS score using linear regression models. We adjusted the model for the follow-up infarct volume for the baseline ASPECT score and the model for the utility-weighted mRS score for the prestroke utility-weighted mRS score. We secondarily performed fully adjusted analyses for both primary outcomes using age, baseline NIHSS score, baseline collateral circulation status, and site (where site was treated as random effects) as prespecified covariates using mixed-effects models. Secondary outcomes and differential effect analyses were undertaken per the prespecified analytic plan.16 The differential effect of the SBP group on each outcome was explored according to age, baseline ASPECT score, collateral grade, and reperfusion grade using interaction terms. In case of a significant interaction, a formal subgroup analysis was planned. Additional details are provided in Supplement 2.
We used FACTS software version 6.4 (Berry Consultants LLC) to calculate predicted probability of success for a future, 2-group, superiority-design, phase 3 trial comparing either of the experimental groups (<140 mm Hg or <160 mm Hg) with the 180-mm Hg or less group on the utility-weighted mRS outcome (using a 1-sided α of .025 per convention). The predicted probability of success is the predictive power of the phase 3 trial, calculated as a weighted average over the Bayesian posterior distribution of mean utility-weighted mRS score for each group. FACTS computes this quantity using Markov chain Monte Carlo techniques. We prespecified that a phase 3 trial would be proposed if the predicted probability of success was at least 25% with a sample size of 400, 800, or 1500.
Missing Data
The missingness in the primary outcome data was accounted for in the sample size. To determine if missing data on primary outcomes is not random, a sensitivity analysis was to be conducted after fitting a model to predict the missing follow-up infarct volume and utility-weighted mRS score using baseline variables. However, this analysis could not be reliably conducted because only 7 patients had missing outcome data. We report an alternative, post hoc, sensitivity analysis after using multiple imputations to impute the missing primary outcome data.
Multiple Comparisons
Because the Blood Pressure After Endovascular Stroke Therapy-II (BEST-II) trial was designed to detect harm of lower SBP targets, we did not correct the multiple primary end point analyses for multiple hypothesis testing. In this trial, a type II error, which is failing to detect harm, would have been more detrimental than a type I error. By not correcting for multiplicity, BEST-II tested for harm with greater sensitivity. No hypothesis testing was performed for the secondary, exploratory, and post hoc outcome; thus, results should be considered hypothesis generating.
Results
From January 17, 2020, through February 25, 2022, a total of 120 participants were enrolled (Figure 1); 40 in each group. The DSMB recommended trial continuation without modification after the planned interim analysis. Baseline characteristics are outlined in Table 1. The mean (SD) age was 69.6 (14.5) years, 69 (57.5%) were women, and 92 (76.7%) had a history of hypertension. Generally, the baseline characteristics were comparable across study groups, although the 180-mm Hg or less group had higher anticoagulant use, lower baseline NIHSS scores, and a higher proportion with M2 occlusion. The less than 140-mm Hg group had smaller cerebral infarct volume at baseline. In the overall cohort, conscious sedation was used for 67.5%, and a modified Thrombolysis in Cerebral Infarction score of 3 was achieved among 43.3%. Baseline characteristics were generally comparable among sites (eTable 1 in Supplement 2).
After endovascular therapy, the maximal SBP, defined as the 90th percentile of SBP, was 157 mm Hg in the 180–mm Hg or less group, 153 mm Hg in the less than 160–mm Hg group, and 139 mm Hg in the less than 140-mm Hg group (Figure 2 and eTable 2 in Supplement 2). The mean (SD) 24-hour SBPs were 129 (20) mm Hg in the 180-mm Hg or less group, 130 (18) mm Hg in the less than 160-mm Hg group, and 122 (15) mm Hg in the less than 140-mm Hg group. Additional BP parameters after endovascular therapy are provided in eTable 2 in Supplement 2. An antihypertensive agent was used for 10 patients (25%) in the 180-mm Hg or less group, 22 (55%) in the less than 160-mm Hg group, and 29 (72.5%) in the less than 40-mm Hg group, and 80% received intravenous nicardipine as the first-line agent. Crossover from the randomly assigned SBP target occurred in 3 patients (2.5%; eTable 3 in Supplement 2).
Figure 2. Distribution of Systolic Blood Pressure After Endovascular Treatment According to Randomization Group.
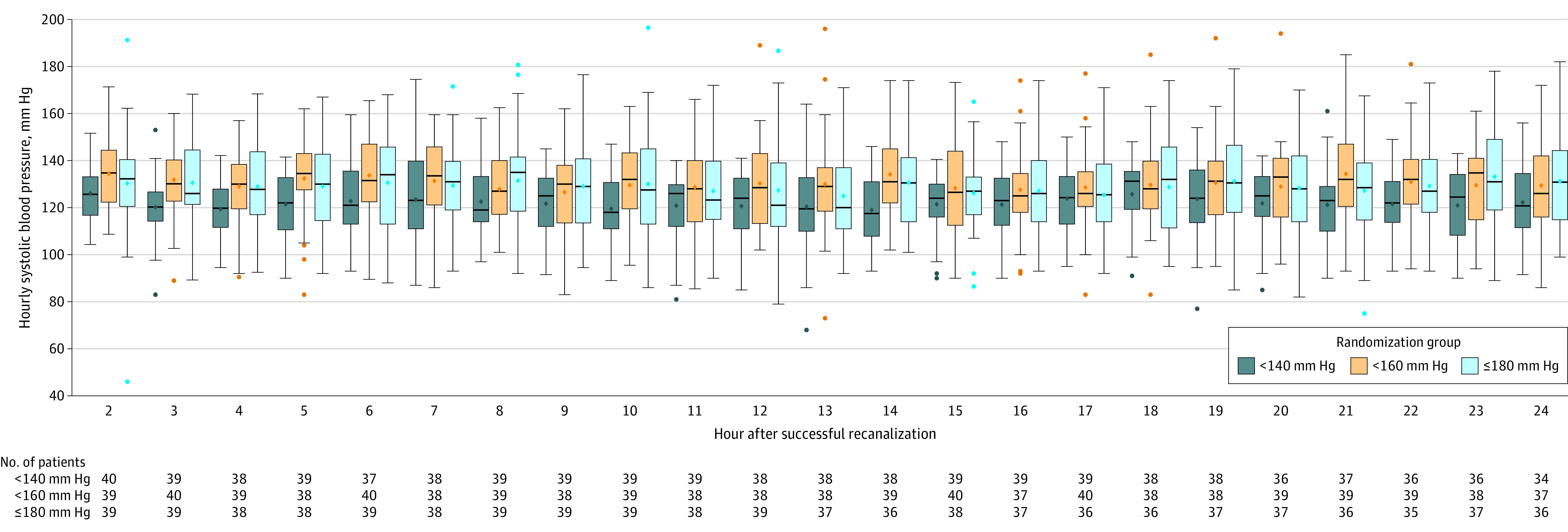
The diamonds indicate means; center bars, medians; boxes, IQRs, and whiskers, highest and lowest values within 1.5 times the IQR for systolic blood pressure from 2 to 24 hours after successful endovascular treatment.
Primary Outcomes
The follow-up infarct volume was available for 113 patients (94.2%) (eTable 4 in Supplement 2; 99 based on MRI and 14 based on CT scan). The mean follow-up infarct volume was 32.4 mL (95% CI, 18 to 46.7 mL) for the less than 140–mm Hg group, 50.7 mL (95% CI, 33.7 to 67.7 mL) for the less than 160–mm Hg group, and 46.4 mL (95% CI, 24.5 to 68.2 mL) for the 180–mm Hg or less group (Figure 3A and eFigure 1A in Supplement 2; no significant linearity concerns). The slope of follow-up infarct volume for each millimeter of mercury decrease in SBP target, adjusted for the baseline ASPECT score, was −0.29 (1-sided 95% CI, −0.81 to ∞; P value for futility = .99; Table 2 and eFigure 2A in Supplement 2; ie, there was a 0.29-mL reduction in follow-up infarct volume for each mm Hg reduction in the SBP target with the possibility of as much benefit as a 0.81-mL reduction). Because the lower bound of the CI was lower than the 0.5 threshold for harm, the results did not conclusively support a finding of futility. In a model fully adjusted for the baseline ASPECT score, age, baseline NIHSS score, and collateral grade, this slope was −0.40 (95% CI, −0.86 to ∞; P value for futility = .99). The sensitivity analysis with multiple imputations rendered a slope of −0.30 (−0.79 to ∞; P value for futility = .99).
Figure 3. Distribution of the 36-Hour Infarct Volume and 90-Day Modified Rankin Scale Score.
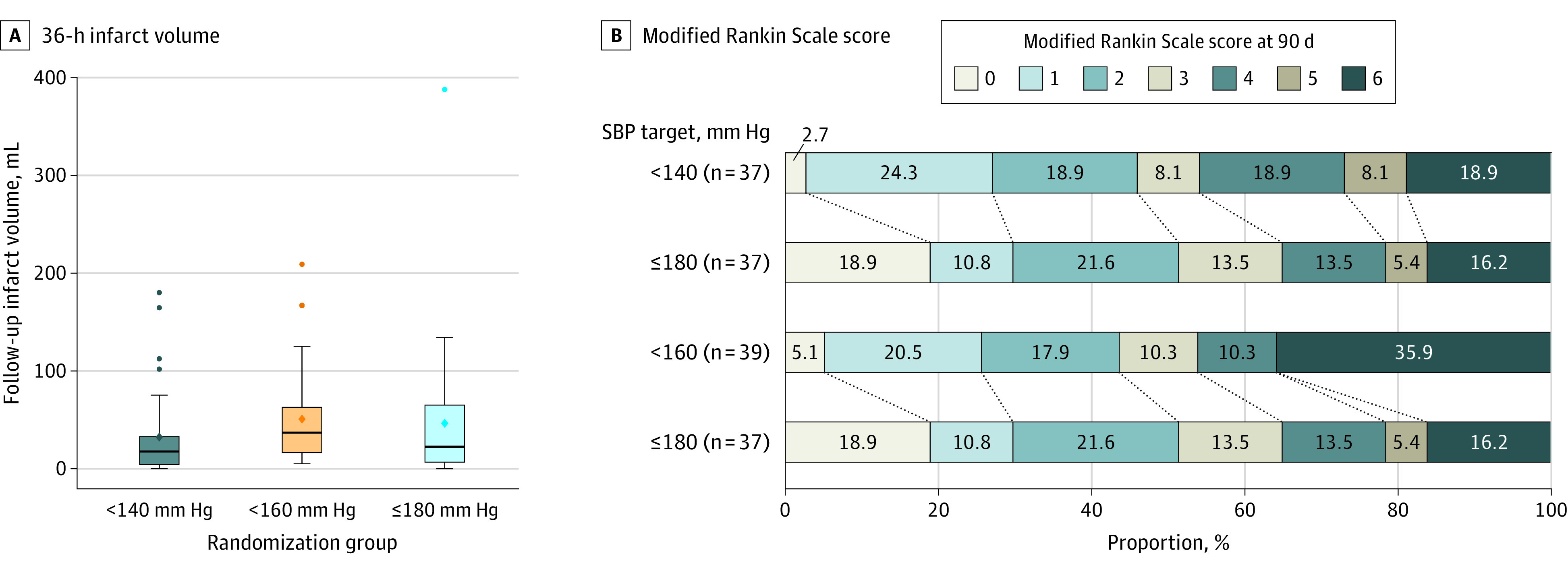
A. Comparative distribution of the 36-hour infarct volume for each target systolic blood pressure group is shown. The mean follow-up infarct volume was 32.4 mL (95% CI, 18.0-46.7 mL) for the less than 140-mm Hg group, 50.7 mL (95% CI, 33.7-67.7 mL) for the less than 160-mm Hg group, and 46.4 mL (95% CI, 24.5-68.2 mL) for the 180-mm Hg or less group. The extreme value in the 180-mm Hg or less group was an older participant with poor collaterals and several risk factors for large infarct volume, which was not attributable to the study treatment per the site investigators. The diamonds indicate means; center bars, medians; boxes, IQRs; and whiskers, highest and lowest values within 1.5 times the IQR.
B. Comparative distribution of the 90-day modified Rankin Scale score (mRS) is shown for each target systolic blood pressure group. The mRS score ranges from 0 to 6 (0, no symptoms at all; 1, no significant disability despite symptoms; able to carry out all usual duties and activities; 2, slight disability; unable to carry out all previous activities, but able to look after own affairs without assistance; 3, moderate disability requiring some help but able to walk without assistance; 4, moderately severe disability, unable to walk without assistance and unable to attend to own bodily needs without assistance; 5, severe disability, bedridden, incontinent, and requires constant nursing care and attention; 6, dead. The median 90-day mRS score was 3 (IQR, 1-5) in the less than 140-mm Hg group, 3 (IQR, 1.5-6) in the less than 160-mm Hg group, and 2 (IQR, 1-4) in the 180-mm Hg or less group.
Table 2. Outcomes by Randomization Group.
| Systolic blood pressure target, mm Hg | Absolute difference (95% CI), mm Hg | Regression coefficient (95% CI) | P value for futility | ||||
|---|---|---|---|---|---|---|---|
| <140 | <160 | ≤180 | <140 vs ≤180 | <160 vs ≤180 | |||
| Primary outcomes, mean (95% CI) | |||||||
| Follow-up infarct volume at 36 h, mL | 32.4 (18.0 to 46.7) [n = 38] | 50.7 (33.7 to 67.7) [n = 36] | 46.4 (24.5 to 68.2) [n = 39] | −14.0 (−39.8 to 11.8) | 4.3 (−22.9 to 31.6) | −0.29 (−0.81 to ∞)a | .99 |
| Utility-weighted mRS score at 90 d | 0.51 (0.38 to 0.63) [n = 37] | 0.47 (0.35 to 0.60) [n = 39] | 0.58 (0.46 to 0.71) [n = 37] | −0.08 (−0.25 to 0.10) | −0.11 (−0.29 to 0.07) | −0.0019 (−∞ to 0.0017)b | .93 |
| Secondary outcomes, No./total (%) c | |||||||
| Any ICH ≤36 h | 14/39 (36) | 12/37 (32) | 12/40 (30) | 5.9 (−14.8 to 26.6) | 2.4 (−18.3 to 23.1) | ||
| Symptomatic ICH ≤36 h | 2/37 (5) | 1/35 (3) | 2/37 (5) | 0 (−10.3 to 10.3) | −2.5 (−11.7 to 6.6) | ||
| Post hoc outcomes c | |||||||
| In-hospital mortality, No./total (%) | 3/40 (7.5) | 6/40 (15) | 3/40 (7.5) | 0 (−11.5 to 11.5) | 7.5 (−6.3 to 21.3) | ||
| 24-hour NIHSS score, mean (95% CI) | 11 (8 to 14) [n = 38] | 12 (9 to 15) [n = 37] | 7 (5 to 10) [n = 37] | 3.9 (−0.2 to 7.9) | 4.6 (0.6 to 8.6) | ||
Abbreviations: ICH, intracerebral hemorrhage; mRS, modified Rankin Scale score; NIHSS, National Institutes of Health Stroke Scale.
Testing across all 3 groups, the regression coefficient denotes a 0.29 mL reduction in follow-up infarct volume with each mm Hg reduction in systolic blood pressure target after endovascular therapy (with a possibility of as much benefit as a 0.81-mL reduction and includes the regions of harm).
Testing across all 3 groups, the regression coefficient denotes a 0.0019 reduction in utility-weighted mRS score with each mm Hg reduction in systolic blood pressure target after endovascular therapy (with a small possible benefit of 0.0017 gain in utility and includes the regions of harm).
Secondary outcomes and in-hospital mortality absolute differences are presented as percentages.
The 90-day utility-weighted mRS score was available for 113 patients (94.2%; eTable 4 in Supplement 2). The mean utility-weighted mRS score was 0.51 (95% CI, 0.38 to 0.63) for the less than 140-mm Hg group, 0.47 (95% CI, 0.35 to 0.60) for the less than 160-mm Hg group, and 0.58 (95% CI, 0.46 to 0.71) for 180-mm Hg or less group (eFigure 1B in Supplement 2; no significant linearity concerns). The slope of the utility-weighted mRS score for each mm Hg decrease the SBP target, adjusted for the baseline utility-weighted mRS score was −0.0019 (95% CI, −∞ to 0.0017; P value for futility = .93; Table 2 and eFigure 2B in Supplement 2; ie, there was a 0.0019 reduction in the utility-weighted mRS score for each mm Hg reduction in the SBP target with small possible benefit of up to 0.0017 gain in utility). Because the upper bound of the CI was higher than the −0.005 threshold of harm, the results did not conclusively support a finding of futility. In a model fully adjusted for prestroke mRS score, age, baseline NIHSS score, and collateral grade, this slope was −0.0006 (95% CI, −∞ to 0.003; P value for futility = .99). The sensitivity analysis with multiple imputations rendered a slope of −0.0018 (95% CI, −∞ to 0.0016; P value for futility = .93). The median 90-day mRS score was 3 (IQR, 1 to 5) in the less than 140-mm Hg group, 3 (IQR, 1.5-6) in the less than 160-mm Hg group, and 2 (IQR, 1-4) in the 180-mm Hg or less group. The distribution of 90-day mRS scores by groups is outlined in Figure 3B.
Secondary Outcomes and Adverse Events
Any ICH was observed in 14 patients (36%) in the less than 140-mm Hg group, 12 (32%) in the less than 160-mm Hg group, and 12 (30%) in the 180-mm Hg or less group. Symptomatic ICH was observed in 2 (5%) in the less than 140-mm Hg group, 1 (3%) in the less than 160-mm Hg group, and 2 (5%) in the 180-mm Hg or less group (Table 2 and eTable 5 in Supplement 2; all assessed on CT). Of the 8 serious adverse events, none were attributed to the study intervention (eTable 6 in Supplement 2). Aspiration pneumonia was most common (n = 2). Specifically, none of the participants had neurological worsening associated with antihypertensive treatment.
Interaction terms did not provide evidence of heterogeneity in treatment effect according to age, baseline ASPECT score, collateral grade, and reperfusion grade (eTable 7 in Supplement 2).
Predicted Probability of Success
The predicted probability of success for a future, 2-group, superiority design trial comparing the efficacy of the less than 140-mm Hg target with the 180-mm Hg or less target using the utility-weighted mRS score outcome was 16%, 21%, and 25% for the maximum prespecified sample sizes of 400, 800, and 1500, respectively. The predicted probability of success of a future trial comparing the less than 160-mm Hg target with the 180-mm Hg or less target was 9%, 12%, and 14%, respectively (eTable 8 in Supplement).
Post Hoc Analyses
The in-hospital mortality rate was 7.5% (3 of 40 patients) in the less than 140-mm Hg group; 15% (6 of 40 patients) in the less than 160-mm Hg group, and 7.5% (3 of 40 patients) in the 180-mm Hg or less group. The mean 24-hour NIHSS scores were 11 (95% CI, 8-14) in the less than 140-mm Hg group, 12 (95% CI, 9-15) in the less than 160-mm Hg group, and 7 (95% CI, 5-10) in the 180-mm Hg or less group (Table 2).
Discussion
In this futility-design, randomized clinical trial that included 120 patients with successful endovascular therapy for acute ischemic stroke, lower SBP targets of less than 140 mm Hg and less than 160 mm Hg did not meet prespecified criteria for futility compared with a target of 180 mm Hg or less. However, the findings suggested a low probability of benefit from lower SBP targets if tested in a future larger trial.
Although the results do not directly support the futility of lower SBP targets, they strongly contribute to a dampened enthusiasm for spending limited research resources on a future, large, pivotal trial for several reasons. First, the point estimate of treatment effect on the patient-centered utility-weighted mRS score outcome was in the direction of harm in both unadjusted and adjusted models. Second, although the trial was not powered to detect benefit, the 1-sided CI included only a marginal benefit of 0.0017 gain in utility-weighted mRS score per mm Hg reduction in the SBP target (or 0.034 per 20 mm Hg). Third, the predicted probability of success of a future superiority trial comparing less than 140 mm Hg and 180 mm Hg or less targets was 25%, which was barely at the prespecified acceptable threshold. Increasing the sample size from 800 to 1500 patients increased predicted probability of success by only 4%, suggesting that this predicted probability of success is likely due to the variability in the underlying data and not true benefit. The predicted probability of success for a trial comparing less than 160 mm Hg with 180 mm Hg or less targets were even lower. Fourth, the suggestion of harm of lower SBP targets is reinforced by the international Enhanced Control of Hypertension and Thrombolysis Stroke Study (ENCHANTED2/MT) trial,14 which was stopped early due to worse 90-day mRS scores among patients in the very low (<120 mm Hg) SBP target group. Although this target is lower than the target studied herein and is uncommon in current clinical practice, the harmful effect is consistent with this study’s findings.14 Fifth, the findings in this current trial are consistent with neutral or harmful effects of lower BP targets in patients who have not undergone endovascular therapy.22,23,24 In totality, the evidence suggests that the current guideline-recommended SBP target of 180 mm Hg or less is most likely to maximize good clinical outcomes.6,7
This trial advances the knowledge regarding the feasibility of achieving SBP targets. Although the achieved SBP remained lower than the randomly assigned target in all 3 groups, the mean SBP was similar between the 180–mm Hg group and the less than 160–mm Hg group. But notably, the less than 160–mm Hg group demonstrated effect estimates in the direction of worse outcomes compared with the 180 mm Hg or less group, although not statistically significant. This suggestion of harm may be due to a key experimental difference in that the less than 160–mm Hg group received antihypertensives more than twice as frequently as the 180–mm Hg or less group, although these findings should be interpreted with caution.
Until now, clarity on the ideal BP management strategies after endovascular therapy have been lacking and clinical practice across the US is heterogeneous.15 Observational studies have shown an association between higher SBP after endovascular therapy and poor functional outcomes and possible hemorrhage, although causality is unclear.9,10,12,13,25 Proponents of causation note that higher BP targets would cause hyperperfusion in the critical period after endovascular therapy, leading to neuroinflammation and cerebral edema. Conversely, lower BP targets are hypothesized to lead to hypoperfusion and increased infarct volume. This trial found similar rates of any type of ICH between the BP target groups, consistent with the findings of the previously published phase 2, Blood Pressure Target in Acute Stoke to Reduce Hemorrhage After Endovascular Therapy (BP-TARGET) trial.26 Notably, immediate SBP reduction after thrombolysis also does not improve patient outcomes.23,27
The less than 140-mm Hg group in this trial demonstrated a lower point estimate for follow-up infarct volume at 36 hours, possibly due to smaller baseline infarct core volumes, but a point estimate of the 90-day utility-weighted mRS score suggested worse functional outcomes. There may be a few reasons for the discrepancy in the direction of treatment effect. First, the long-term biological effects of lower SBP on the brain may not be fully captured by MRI diffusion-based follow-up infarct volume assessment. In fact, follow-up infarct volume only mediates 12% of effect of endovascular therapy on 90-day outcomes.28 The mechanistic effects of SBP on cerebral perfusion may be better captured by dynamic studies such as perfusion imaging. Second, follow-up infarct volume measured at 12 hours after the end of the intervention period may be too early to detect follow-up infarct volume changes.29 Third, lower SBP may cause short-term benefit but long-term harm.
Limitations
This study has several limitations. First, the study is limited by enrollment at only 3 centers and a small sample size. Second, nicardipine was used as the first-line treatment for the majority of enrolled patients. Although nicardipine is commonly used in neurointensive care units within the US, it may not be readily available globally. Third, some baseline characteristics differed among groups. These may impact the generalizability of the results. Fourth, SBP management prior to the endovascular therapy was not addressed nor was the option of allowing SBP targets that were higher than 180 mm Hg after treatment given that there has been no supportive preliminary evidence to support this target.
Conclusions
Among patients with acute ischemic stroke, lower SBP targets after endovascular therapy of less than 140 mm Hg and less than 160 mm Hg did not meet prespecified criteria for futility compared with a target of 180 mm Hg or less. However, the findings suggested a low probability of benefit from a lower SBP target if tested in a future larger trial.
Trial Protocol
eFigure 1. Scatter/Violin plot showing distribution of raw final infarct volume(A) and utility-weighted modified Rankin score (B) by the randomization group
eFigure 2. Multiple Primary Outcome Results
eTable 1. Baseline Characteristics by Site
eTable 2. Details of post-endovascular treatment blood pressure management
eTable 3. BP target modification information
eTable 4. Details of missing outcome data
eTable 5. Details of hemorrhage grade by randomization group
eTable 6. Details of serious adverse events (SAE)
eTable 7. Subgroup analyses
eTable 8. Predicted Probabilities of Success for Future Trials Comparing <140 vs </=180 mmHg and <160 vs </=180 Arms
eStatistical model
Data Sharing Statement
References
- 1.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 2.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 4.Dargazanli C, Consoli A, Barral M, et al. Impact of Modified TICI 3 versus Modified TICI 2b reperfusion score to predict good outcome following endovascular therapy. AJNR Am J Neuroradiol. 2017;38(1):90-96. doi: 10.3174/ajnr.A4968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeCouffe NE, Kappelhof M, Treurniet KM, et al. ; MR CLEAN Registry Investigators* . 2B, 2C, or 3: what should be the angiographic target for endovascular treatment in ischemic stroke? Stroke. 2020;51(6):1790-1796. doi: 10.1161/STROKEAHA.119.028891 [DOI] [PubMed] [Google Scholar]
- 6.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 7.Sandset EC, Anderson CS, Bath PM, et al. European Stroke Organisation (ESO) guidelines on blood pressure management in acute ischaemic stroke and intracerebral haemorrhage. Eur Stroke J. 2021;6(2):XLVIII-LXXXIX. doi: 10.1177/23969873211012133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owens WB. Blood pressure control in acute cerebrovascular disease. J Clin Hypertens (Greenwich). 2011;13(3):205-211. doi: 10.1111/j.1751-7176.2010.00394.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goyal N, Tsivgoulis G, Pandhi A, et al. Blood pressure levels post mechanical thrombectomy and outcomes in large vessel occlusion strokes. Neurology. 2017;89(6):540-547. doi: 10.1212/WNL.0000000000004184 [DOI] [PubMed] [Google Scholar]
- 10.Mistry EA, Mistry AM, Nakawah MO, et al. Systolic blood pressure within 24 hours after thrombectomy for acute ischemic stroke correlates with outcome. J Am Heart Assoc. 2017;6(5):e006167. doi: 10.1161/JAHA.117.006167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anadani M, Arthur AS, Alawieh A, et al. Blood pressure reduction and outcome after endovascular therapy with successful reperfusion: a multicenter study. J Neurointerv Surg. 2020;12(10):932-936. doi: 10.1136/neurintsurg-2019-015561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mistry EA, Sucharew H, Mistry AM, et al. Blood Pressure after Endovascular Therapy for Ischemic Stroke (BEST): a multicenter prospective cohort study. Stroke. 2019;50(12):3449-3455. doi: 10.1161/STROKEAHA.119.026889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anadani M, Arthur AS, Tsivgoulis G, et al. Blood pressure goals and clinical outcomes after successful endovascular therapy: a multicenter study. Ann Neurol. 2020;87(6):830-839. doi: 10.1002/ana.25716 [DOI] [PubMed] [Google Scholar]
- 14.Yang P, Song L, Zhang Y, et al. ; ENCHANTED2/MT Investigators . Intensive blood pressure control after endovascular thrombectomy for acute ischaemic stroke (ENCHANTED2/MT): a multicentre, open-label, blinded-endpoint, randomised controlled trial. Lancet. 2022;400(10363):1585-1596. doi: 10.1016/S0140-6736(22)01882-7 [DOI] [PubMed] [Google Scholar]
- 15.Mistry EA, Mayer SA, Khatri P. Blood pressure management after mechanical thrombectomy for acute ischemic stroke: a survey of the StrokeNet sites. J Stroke Cerebrovasc Dis. 2018;27(9):2474-2478. doi: 10.1016/j.jstrokecerebrovasdis.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 16.Mistry EA, Hart K, Davis T, et al. Design of the BEST-II randomized clinical trial. Stroke Vasc Intervent Neurol. 2022;2(3):e000249. doi: 10.1161/SVIN.121.000249 [DOI] [Google Scholar]
- 17.Chaisinanunkul N, Adeoye O, Lewis RJ, et al. ; DAWN Trial and MOST Trial Steering Committees; Additional contributors from DAWN Trial Steering Committee . Adopting a patient-centered approach to primary outcome analysis of acute stroke trials using a utility-weighted modified Rankin scale. Stroke. 2015;46(8):2238-2243. doi: 10.1161/STROKEAHA.114.008547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le QA, Doctor JN, Zoellner LA, Feeny NC. Minimal clinically important differences for the EQ-5D and QWB-SA in post-traumatic stress disorder (PTSD): results from a Doubly randomized preference trial (DRPT). Health Qual Life Outcomes. 2013;11:59. doi: 10.1186/1477-7525-11-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo N, Johnson J, Coons SJ. Using instrument-defined health state transitions to estimate minimally important differences for four preference-based health-related quality of life instruments. Med Care. 2010;48(4):365-371. doi: 10.1097/MLR.0b013e3181c162a2 [DOI] [PubMed] [Google Scholar]
- 20.Deeds SI, Barreto A, Elm J, et al. The multiarm optimization of stroke thrombolysis phase 3 acute stroke randomized clinical trial: rationale and methods. Int J Stroke. 2021;16(7):873-880. doi: 10.1177/1747493020978345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neuberger U, Möhlenbruch MA, Herweh C, Ulfert C, Bendszus M, Pfaff J. Classification of bleeding events: comparison of ECASS III (European Cooperative Acute Stroke Study) and the New Heidelberg Bleeding Classification. Stroke. 2017;48(7):1983-1985. doi: 10.1161/STROKEAHA.117.016735 [DOI] [PubMed] [Google Scholar]
- 22.Oh MS, Yu KH, Hong KS, et al. ; Valsartan Efficacy on Modest Blood Pressure Reduction in Acute Ischemic Stroke (VENTURE) study group . Modest blood pressure reduction with valsartan in acute ischemic stroke: a prospective, randomized, open-label, blinded-end-point trial. Int J Stroke. 2015;10(5):745-751. doi: 10.1111/ijs.12446 [DOI] [PubMed] [Google Scholar]
- 23.Sandset EC, Bath PM, Boysen G, et al. ; SCAST Study Group . The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet. 2011;377(9767):741-750. doi: 10.1016/S0140-6736(11)60104-9 [DOI] [PubMed] [Google Scholar]
- 24.Wahlgren N, MacMahon D, De Keyser JF, Indredavik B, Ryman T. Intravenous Nimodipine West European Stroke Trial (INWEST) of nimodipine in the treatment of acute ischaemic stroke. Cerebrovasc Dis. 1994;4(3):204-210. doi: 10.1159/000108483 [DOI] [Google Scholar]
- 25.Malhotra K, Goyal N, Katsanos AH, et al. Association of blood pressure with outcomes in acute stroke thrombectomy. Hypertension. 2020;75(3):730-739. doi: 10.1161/HYPERTENSIONAHA.119.14230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazighi M, Richard S, Lapergue B, et al. ; BP-TARGET investigators . Safety and efficacy of intensive blood pressure lowering after successful endovascular therapy in acute ischaemic stroke (BP-TARGET): a multicentre, open-label, randomised controlled trial. Lancet Neurol. 2021;20(4):265-274. doi: 10.1016/S1474-4422(20)30483-X [DOI] [PubMed] [Google Scholar]
- 27.He J, Zhang Y, Xu T, et al. ; CATIS Investigators . Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the CATIS randomized clinical trial. JAMA. 2014;311(5):479-489. doi: 10.1001/jama.2013.282543 [DOI] [PubMed] [Google Scholar]
- 28.Boers AMM, Jansen IGH, Brown S, et al. Mediation of the relationship between endovascular therapy and functional outcome by follow-up infarct volume in patients with acute ischemic stroke. JAMA Neurol. 2019;76(2):194-202. doi: 10.1001/jamaneurol.2018.3661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Federau C, Christensen S, Mlynash M, et al. Comparison of stroke volume evolution on diffusion-weighted imaging and fluid-attenuated inversion recovery following endovascular thrombectomy. Int J Stroke. 2017;12(5):510-518. doi: 10.1177/1747493016677985 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Scatter/Violin plot showing distribution of raw final infarct volume(A) and utility-weighted modified Rankin score (B) by the randomization group
eFigure 2. Multiple Primary Outcome Results
eTable 1. Baseline Characteristics by Site
eTable 2. Details of post-endovascular treatment blood pressure management
eTable 3. BP target modification information
eTable 4. Details of missing outcome data
eTable 5. Details of hemorrhage grade by randomization group
eTable 6. Details of serious adverse events (SAE)
eTable 7. Subgroup analyses
eTable 8. Predicted Probabilities of Success for Future Trials Comparing <140 vs </=180 mmHg and <160 vs </=180 Arms
eStatistical model
Data Sharing Statement


