Key Points
Question
Does early intensive blood pressure management improve outcomes after successful reperfusion with endovascular thrombectomy in acute ischemic stroke?
Findings
In this randomized clinical trial that included 306 patients, 39.4% of patients with intensive blood pressure management (systolic blood pressure target <140 mm Hg) and 54.4% of those with conventional blood pressure management (systolic blood pressure target 140-180 mm Hg) achieved functional independence (modified Rankin Scale score ≤2) at 3 months; this represented a significant difference.
Meaning
Intensive blood pressure lowering during the first 24 hours after successful reperfusion may be harmful in patients with acute ischemic stroke who have undergone endovascular thrombectomy.
Abstract
Importance
Optimal blood pressure (BP) control after successful reperfusion with endovascular thrombectomy (EVT) for patients with acute ischemic stroke is unclear.
Objective
To determine whether intensive BP management during the first 24 hours after successful reperfusion leads to better clinical outcomes than conventional BP management in patients who underwent EVT.
Design, Setting, and Participants
Multicenter, randomized, open-label trial with a blinded end-point evaluation, conducted across 19 stroke centers in South Korea from June 2020 to November 2022 (final follow-up, March 8, 2023). It included 306 patients with large vessel occlusion acute ischemic stroke treated with EVT and with a modified Thrombolysis in Cerebral Infarction score of 2b or greater (partial or complete reperfusion).
Interventions
Participants were randomly assigned to receive intensive BP management (systolic BP target <140 mm Hg; n = 155) or conventional management (systolic BP target 140-180 mm Hg; n = 150) for 24 hours after enrollment.
Main Outcomes and Measures
The primary outcome was functional independence at 3 months (modified Rankin Scale score of 0-2). The primary safety outcomes were symptomatic intracerebral hemorrhage within 36 hours and death related to the index stroke within 3 months.
Results
The trial was terminated early based on the recommendation of the data and safety monitoring board, which noted safety concerns. Among 306 randomized patients, 305 were confirmed eligible and 302 (99.0%) completed the trial (mean age, 73.0 years; 122 women [40.4%]). The intensive management group had a lower proportion achieving functional independence (39.4%) than the conventional management group (54.4%), with a significant risk difference (−15.1% [95% CI, −26.2% to −3.9%]) and adjusted odds ratio (0.56 [95% CI, 0.33-0.96]; P = .03). Rates of symptomatic intracerebral hemorrhage were 9.0% in the intensive group and 8.1% in the conventional group (risk difference, 1.0% [95% CI, −5.3% to 7.3%]; adjusted odds ratio, 1.10 [95% CI, 0.48-2.53]; P = .82). Death related to the index stroke within 3 months occurred in 7.7% of the intensive group and 5.4% of the conventional group (risk difference, 2.3% [95% CI, −3.3% to 7.9%]; adjusted odds ratio, 1.73 [95% CI, 0.61-4.92]; P = .31).
Conclusions and Relevance
Among patients who achieved successful reperfusion with EVT for acute ischemic stroke with large vessel occlusion, intensive BP management for 24 hours led to a lower likelihood of functional independence at 3 months compared with conventional BP management. These results suggest that intensive BP management should be avoided after successful EVT in acute ischemic stroke.
Trial Registration
ClinicalTrials.gov Identifier: NCT04205305
This randomized trial assesses the effect of intensive blood pressure management vs conventional management on functional independence at 3 months among patients with large vessel occlusion acute ischemic stroke treated with endovascular thrombectomy.
Introduction
Although endovascular thrombectomy (EVT) is an established treatment for patients with acute ischemic stroke from large vessel occlusion, many patients do not gain functional independence despite high rates of reperfusion achievement.1,2 Sustained high blood pressure (BP) after EVT may worsen clinical outcomes through increased risks of intracerebral hemorrhage (ICH) and cerebral edema.3,4,5 On the other hand, if the ischemic brain is more susceptible to BP changes from the loss of microvascular integrity,6,7 then BP-lowering treatment may alter perfusion pressure and exacerbate ischemic injury.
Guideline recommendations to maintain BP at less than 180/105 mm Hg for 24 hours after EVT are based on expert opinion.8,9 Observational studies have consistently shown that elevated BP during this period is associated with ICH and worse clinical outcomes.3,4,10,11,12,13 However, 2 multicenter clinical trials of early intensive BP lowering in this patient group have produced conflicting results. The Blood Pressure Target in Acute Stroke to Reduce Hemorrhage After Endovascular Therapy (BP-TARGET) trial14 showed no effect of early intensive BP lowering in 318 patients with successful EVT, whereas the subsequent Enhanced Control of Hypertension and Thrombectomy Stroke Study (ENCHANTED2/MT)15 was stopped early because of harm resulting from intensive BP lowering after 347 of 816 patients with EVT were randomized. Thus, the optimal target for BP control in stroke patients with successful reperfusion following EVT is still unclear.
The Outcome in Patients Treated With Intra-Arterial Thrombectomy–Optimal Blood Pressure Control (OPTIMAL-BP) trial was undertaken to test the hypothesis that intensive BP management during the first 24 hours after successful reperfusion would result in better clinical outcomes than conventional BP management in patients who have achieved successful reperfusion through EVT.
Methods
Trial Design
The OPTIMAL-BP trial was a multicenter, randomized, open-label, blinded end-point clinical trial. This trial was performed at 19 centers throughout South Korea. The protocol is published16 and provided in Supplement 1 along with the statistical analysis plan in Supplement 2. The trial protocol and consent forms were approved by the institutional review board of each participating hospital, and all patients or their legal representatives provided written informed consent. The trial was overseen by an independent data and safety monitoring board (DSMB).
Patient Selection
Adults (aged ≥20 years) who underwent EVT for acute ischemic stroke due to large vessel occlusion were eligible if they had successful reperfusion of the occluded artery, as determined by a modified Thrombolysis in Cerebral Infarction score of 2b or greater,17 and had elevated BP (systolic BP [SBP], ≥140 mm Hg) according to at least 2 measurements within a 2-minute interval within 2 hours of successful reperfusion. Key exclusion criteria were SBP less than 140 mm Hg after EVT, any contraindication to the use of antihypertensive medications, symptomatic ICH evident during or immediately after EVT, serious medical or surgical illness, and prestroke disability (modified Rankin Scale [mRS] score, 3-5). Details about the inclusion and exclusion criteria are provided in eTable 1 in Supplement 3.
Randomization and Masking
The study participants were randomized in a 1:1 ratio within 2 hours of reperfusion and stratified by hospital and degree of neurological impairment (National Institutes of Health Stroke Scale [NIHSS] score at admission, <15 points vs ≥15 points). Patients were randomized using a computerized random sequence generation that was centrally administrated via a password-protected, web-based program. Permuted block randomization was implemented with a block size of 4.
Interventions
Patients were randomly allocated to either intensive (SBP target <140 mm Hg) or conventional (SBP target 140-180 mm Hg) management. All patients received care in a stroke unit or similar facility equipped with continuous BP monitoring and best practice management.8 Blood pressure was continuously monitored using a noninvasive device applied to the nonparalyzed arm or the right arm in cases of coma or tetraparesis. Local treatment protocols for the use of intravenous BP-lowering drugs were available. Nicardipine was the preferred BP-lowering drug; other drugs were used at physician discretion. In the conventional management group, vasopressor drugs were not used to achieve the target if a patient’s SBP decreased to less than 140 mm Hg. However, at physician discretion, intravenous fluids and/or inotropes could be administered to manage any clinically significant hypotension. The goal for both groups was to reach the target SBP within 1 hour following their random assignment.
Radiological follow-up using computed tomography (CT) or magnetic resonance imaging (MRI) was conducted at 24 (±12) hours, and at any other time when neurological symptoms worsened. The mRS scores and adverse events were determined in participants at 1 month and 3 months via telephone or in person by local certified medical staff who were blinded to the treatment allocation. The mRS score was determined by a structured interview using the Korean version of the mRS (http://stroke-edu.or.kr). Health-related quality of life was assessed at 3 months using the 3-level EuroQoL 5-Dimension Self-Report Questionnaire (EQ-5D-3L).18 Two interventional neurologists (J.H. and H.L.) and 1 neuroradiologist (N.-Y.S.) independently performed central adjudication of neuroimaging without knowledge of the treatment allocation, resolving discrepancies by consensus.
Outcomes
The primary efficacy outcome was a dichotomized analysis of the mRS score at 3 months (0-2, indicating functional independence, vs 3-6, indicating dependence or death). The primary safety outcomes were symptomatic ICH within 36 hours and death related to the index stroke within 3 months. Symptomatic ICH was defined per the European Cooperative Acute Stroke Study III as any extravascular blood in the brain or within the cranium that was linked to clinical deterioration, defined by an increase of 4 points or more in the NIHSS score or death, and identified as the main cause of the neurologic deterioration.19 Secondary outcomes included a shift analysis of the distribution of mRS scores, NIHSS score at 24 hours, proportion of patients who achieved excellent recovery (an NIHSS score of 0-1 or an improvement of >8 points at 24 hours), successful reperfusion at 24 hours, frequency of malignant cerebral edema within 36 hours, functional independence at 1 month (mRS score 0-2), and EQ-5D-3L score at 3 months. Successful reperfusion at 24 hours was determined by a modified Thrombolysis in Cerebral Infarction score of 2b or greater using magnetic resonance angiography or CT angiography at 24 hours. Malignant cerebral edema refers to a condition characterized by rapid neurological deterioration accompanied by substantial brain swelling seen on CT or MRI, which often leads to death or poor functional outcomes.20 The determination of malignant cerebral edema was made by local investigators.
The mRS score ranges from 0 to 6, in which 0 denotes no symptoms (best outcome) and 6 represents death (worst outcome). The minimum clinically important difference for the change in proportion of patients achieving functional independence (mRS score 0-2) at 3 months is 1.3%.21 The NIHSS score varies from 0 to 42, with 0 denoting the absence of symptoms (best outcome) and 42 indicating a comatose condition (worst outcome). The minimum clinically important difference of the NIHSS score is not well defined. The EQ-5D-3L score can fall between −0.171 and 1, in which 1 represents a full health state (best outcome) and −0.171 is equivalent to death (worst outcome).22 The minimum clinically important difference of the EQ-5D-3L has been identified as 0.08.23
The mean between-group differences in SBP and diastolic BP were analyzed, and time within the target SBP range was determined based on individual SBP targets (SBP <140 mm Hg, SBP 140-180 mm Hg, and SBP <180 mm Hg). An SBP was considered out of range if it exceeded or fell below any of the following thresholds: >180 mm Hg, >200 mm Hg, or <100 mm Hg. The BP-lowering medications administered during the first 24 hours were analyzed. A post hoc imaging analysis included the Alberta Stroke Programme Early CT Score (ASPECTS),24 modified Tan collateral score,25 immediate reperfusion, 24-hour reperfusion, any ICH, type 2 parenchymal hematoma, and infarction volume. A post hoc exploratory analysis was conducted to reveal the relationship between mean SBP over the 24-hour period and an outcome of dependence or death.
Sample Size Calculation
We performed a systematic review to estimate the necessary sample size.16 A total of 3436 articles were identified, of which 9 met the inclusion criteria. The data showed that a 10–mm Hg increase in mean SBP within 24 hours of successful reperfusion with EVT was associated with worse mRS outcomes at 3 months (odds ratio [OR], 1.45 [95% CI, 1.14-1.83]; P = .002). The estimated rate of an outcome of dependence or death of intensive management was 30% and that of conventional management was 41%, and a single interim analysis was planned. Using the alpha spending function in conjunction with the O’Brien-Fleming boundary method, we estimated that a total of 634 patients (317 per group) would be required.26 Considering a predicted 5% dropout rate, we adjusted the final participant count to 668 patients (334 per group).4
Statistical Analysis
The primary analyses were evaluated in all randomly assigned patients who provided consent, except for those who withdrew informed consent prior to BP control, did not receive the allocated treatment, or were lost to follow-up before 3 months. The per-protocol analysis included patients who underwent the assigned treatment with no major protocol deviations. Missing data were excluded from the analyses. Binary logistic regression analyses were performed for the primary outcome, and treatment effects were presented as ORs with 95% CIs. In addition, risk differences with 95% CIs were calculated. Adjusted OR were calculated using a multivariable logistic regression analysis adjusted for age, sex, time from stroke onset to randomization, and NIHSS score immediately before EVT. For the secondary outcome, the common OR representing a shift in mRS scores was calculated using an ordinal logistic regression analysis. Linear regression analyses were performed for the NIHSS score at 24 hours and the EQ-5D-3L score. A post hoc analysis accounted for enrollment site effects and was carried out using mixed-effects modeling, with the site treated as a random effect. Detailed statistical analyses are provided in the statistical analysis plan (Supplement 2).
A subgroup analysis of the primary outcome was performed in the prespecified subgroups. In the logistic regression analysis, we conducted interaction tests between the groups (intensive or conventional management) and various subgroups (age, sex, etc) to ascertain if the effect of the group on the 3-month mRS score differed across subgroups. The homogeneity of the treatment effect across subgroups was evaluated using a logistic regression model. To show the relationship between mean SBP over the 24-hour period and an outcome of dependence or death, we performed a post hoc 3-knot restricted cubic spline curve analysis. All P values were 2-tailed, and differences were considered significant at P < .05. The results from the analyses of secondary end points were considered exploratory due to the possibility of type I error arising from multiple comparisons. Statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc) and R version 4.1.3 (R Foundation).
Early Trial Termination
While the trial was ongoing, the results of ENCHANTED2/MT were released showing a negative impact on 3-month functional outcomes in the intensive BP treatment group.15 This prompted the OPTIMAL-BP trial’s DSMB to review the accumulated data in a blinded fashion. An interim analysis of data from 247 patients was undertaken by the DSMB on November 17, 2022. This involved group-sequential tests of 2 proportions, yielding a z statistic of −2.811418 and a P value of 0.004932. These values were higher than the recalculated lower boundary of the z statistic (−3.40419) and P value (0.000664). Therefore, according to the interim analysis, the formal stopping rule was not met. However, the DSMB recommended terminating the trial for 2 reasons. First, there was confirmation of safety concerns raised by the ENCHANTED2/MT results. Second, the probability of rejecting the null hypothesis was 1.22%, which was calculated by conditional power. In other words, the futility of intensive management was 98.78% (1 − conditional power). Therefore, the steering committee subsequently accepted the recommendations of the DSMB and terminated patient recruitment on November 29, 2022.
Results
Patients
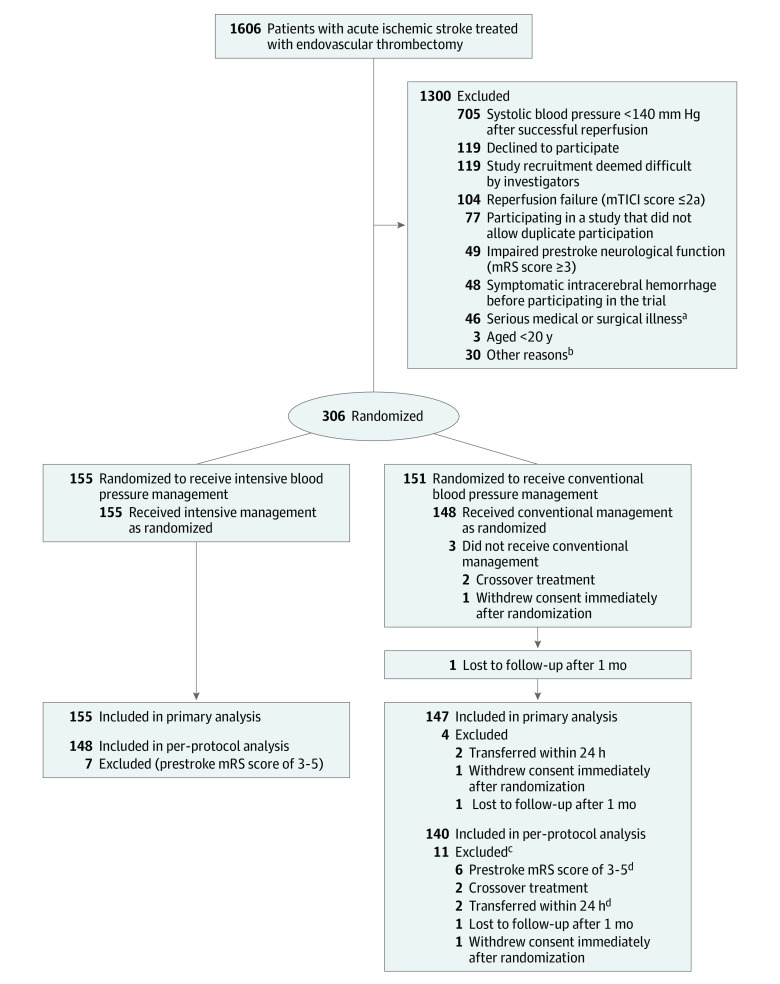
From June 18, 2020, to November 29, 2022, 306 participants were enrolled and randomized to the intensive management (n = 155) and conventional management (n = 151) groups. Four patient in the conventional management group were excluded from the primary analysis because of immediate withdrawal of consent after randomization (n = 1), transfer within 24 hours (n = 2), and loss to follow-up after 1 month (n = 1). The per-protocol analysis included 288 patients after excluding 18 patients for reasons shown in Figure 1.
Figure 1. Participant Flow.
mRS indicates modified Rankin Scale; mTICI, modified Thrombolysis in Cerebral Infarction score.
aFifteen patients were excluded for terminal cancer, 14 with cardiac or aortic disease, 4 with severe anemia and hematologic disease, 4 with chronic kidney disease, 4 with pneumonia, 2 with sepsis, 1 with cholecystitis, 1 with hemothorax, and 1 with radius fracture.
bEleven patients were excluded for COVID-19 infection, 5 due to investigator error, 2 due to non-Korean citizenship, 2 due to immediate transfer to other hospitals, 2 due to no guardian, 2 for advanced dementia, and 6 for unknown reasons.
cOne patient had prestroke disability (mRS score of 3-5) and also was transferred within 24 hours, so numbers below do not add to 11.
dOne patient had prestroke disability (mRS score of 3-5) and also was transferred within 24 hours.
Baseline Characteristics
Baseline demographic, clinical, and imaging characteristics of patients were similar between the groups (primary efficacy analysis in Table 1 and intention-to-treat analysis in eTable 2 in Supplement 3). The mean age was 73.0 (SD, 11.5) years, and 40.4% were women. The mean NIHSS score prior to EVT was 13 (SD, 6). Ninety-eight patients (32.5%) received an intravenous tissue plasminogen activator before EVT. The median time from symptom onset to reperfusion was 418.5 minutes (IQR, 254-764 minutes) and median time from onset to randomization was 480 minutes (IQR, 315-820 minutes). Intracranial stent or angioplasty was performed in 25.2% of the intensive management group and 21.1% of the conventional management group. All patients underwent EVT under conscious sedation.
Table 1. Baseline Characteristics.
| Characteristics | Intensive management (n = 155) | Conventional management (n = 147) |
|---|---|---|
| Demographics | ||
| Age, mean (SD), y | 73.2 (12.1) | 72.9 (10.8) |
| Sex, No. (%) | ||
| Female | 63 (40.6) | 59 (40.1) |
| Male | 92 (59.4) | 88 (59.9) |
| Medical history, No. (%) | ||
| Hypertension | 121 (78.1) | 110 (74.8) |
| Atrial fibrillation | 77 (49.7) | 69 (46.9) |
| Diabetes | 65 (41.9) | 62 (42.2) |
| Hyperlipidemia | 61 (39.4) | 54 (36.7) |
| Smoking | 39 (25.2) | 29 (19.7) |
| Previous stroke | 36 (23.2) | 30 (20.4) |
| Coronary artery obstructive disease | 18 (11.6) | 16 (10.9) |
| Active cancer | 9 (5.8) | 5 (3.4) |
| Congestive heart failure | 7 (4.5) | 7 (4.8) |
| Peripheral artery occlusive disease | 2 (1.3) | 6 (4.1) |
| NIHSS score immediately prior to endovascular thrombectomya | 13 (6) | 12 (7) |
| 0-5 | 14 (9.0) | 18 (12.2) |
| 6-15 | 83 (53.5) | 79 (53.7) |
| ≥16 | 58 (37.4) | 50 (34.0) |
| Intravenous tissue plasminogen activator use | 44 (28.4) | 54 (36.7) |
| TOAST classification, No. (%)b | ||
| Cardioembolism | 76 (49.0) | 76 (51.7) |
| Large vessel atherosclerosis | 41 (26.5) | 43 (29.3) |
| Stroke of other determined etiology | 4 (2.6) | 1 (0.7) |
| Undetermined, negative evaluation | 21 (13.5) | 22 (15.0) |
| Undetermined, ≥2 causes identified | 13 (8.4) | 5 (3.4) |
| Time parameters, median (IQR), min | ||
| Onset to puncture | 388.0 (223.5-692.5) | 357.0 (209.0-725.0) |
| Puncture to reperfusion | 30.0 (22.0-47.0) | 31.0 (20.0-48.0) |
| Onset to reperfusion | 421.0 (265.5-772.0) | 399.0 (250.0-757.5) |
| Onset to randomization | 495.0 (327.5-825.0) | 480.0 (312.5-815.0) |
The National Institutes of Health Stroke Scale (NIHSS) score ranges between 0 and 42; a score of 0 signifies no symptoms (best outcome) and a score of 42 represents a state of coma (worst outcome).
The Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classifications were determined based on a consensus of stroke neurologists in each participating hospital.
Primary Efficacy Outcome
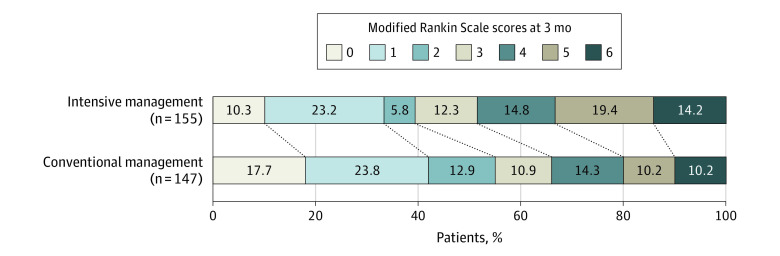
The proportion of patients who achieved functional independence at 3 months was significantly lower in the intensive management group (39.4%) than in the conventional management group (54.4%), with a significant risk difference of −15.1% (95% CI, −26.2% to −3.9%) and an adjusted OR of 0.56 (95% CI, 0.33-0.96; P = .03) (Table 2 and Figure 2).
Table 2. Primary and Secondary Outcomes.
| Outcomes | Intensive management (n = 155) | Conventional management (n = 150) | Risk difference, % (95% CI) | Odds ratio (95% CI) | P value | |
|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | |||||
| Primary efficacy outcome | ||||||
| Functional independence at 3 mo (mRS score 0-2), No./total (%) | 61/155 (39.4) | 80/147 (54.4) | −15.1 (−26.2 to −3.9) | 0.54 (0.34 to 0.86) | 0.56 (0.33 to 0.96) | .03 |
| Primary safety outcomes | ||||||
| Symptomatic intracerebral hemorrhage, No./total (%) | 14/155 (9.0) | 12/149 (8.1) | 1.0 (−5.3 to 7.3) | 1.13 (0.51 to 2.54) | 1.10 (0.48 to 2.53) | .82 |
| Death related to index stroke within 3 mo, No./total (%) | 12/155 (7.7) | 8/147 (5.4) | 2.3 (−3.3 to 7.9) | 1.46 (0.58 to 3.68) | 1.73 (0.61 to 4.92) | .31 |
| Secondary outcomes | ||||||
| mRS score reduction (shift analysis) | 0.59 (0.40 to 0.88) | 0.65 (0.43 to 0.97) | .04 | |||
| NIHSS score at 24 h, mean (SD) | 11.56 (7.48) | 9.59 (7.45) | 1.97 (0.27 to 3.66) | 1.05 (−0.20 to 2.30)b | .10 | |
| Excellent recovery in NIHSS score at 24 h, No./total (%)c | 25/153 (16.3) | 37/148 (25.0) | −8.7 (−17.8 to 0.5) | 0.59 (0.33 to 1.03) | 0.62 (0.34 to 1.11) | .11 |
| Successful reperfusion at 24 h, No./total (%)d | 132/146 (90.4) | 132/141 (93.6) | −3.2 (−9.5 to 3.1) | 0.64 (0.27 to 1.54) | 0.66 (0.26 to 1.65) | .37 |
| Functional independence at 1 mo (mRS score 0-2), No./total (%) | 56/150 (37.3) | 72/144 (50.0) | −12.7 (−23.9 to −1.4) | 0.60 (0.37 to 0.95) | 0.65 (0.38 to 1.11) | .12 |
| EQ-5D-3L score, mean (SD) | 0.50 (0.43) | 0.61 (0.40) | −0.12 (−0.21 to −0.02) | −0.08 (−0.16 to −0.01)b | .05 | |
| Malignant cerebral edema, No./total (%)e | 12/155 (7.7) | 2/149 (1.3) | 6.4 (1.8-11.0) | 6.17 (1.36 to 28.05) | 7.88 (1.57 to 39.39) | .01 |
Abbreviations: EQ-5D-3L, 3-level EuroQoL 5-Dimension Self-Report Questionnaire; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale.
Adjusted for age, sex, onset to randomization time, and NIHSS score immediately prior to endovascular thrombectomy.
Analyzed with linear regression adjusted for age, sex, onset to randomization time, and NIHSS score immediately prior to endovascular thrombectomy.
Indicated by an NIHSS score of 0 to 1 or improvement of more than 8 points.
Successful reperfusion at 24 hours was defined as a modified Thrombolysis in Cerebral Infarction score of 2b or greater by magnetic resonance angiography or computed tomography angiography at 24 hours.
Malignant cerebral edema is a condition characterized by rapid neurological deterioration accompanied by substantial brain swelling seen on computed tomography or magnetic resonance imaging, which often leads to death or poor functional outcomes.
Figure 2. Distribution of mRS Score at 3 Months by Treatment Group.
The modified Rankin Scale (mRS) score measures degree of disability (score range, 0 [symptom free] to 6 [death]). The proportion of patients who achieved functional independence at 3 months (mRS score of 0-2) was lower in the intensive management group (39.4%) than in the conventional management group (54.4%) (adjusted odds ratio, 0.56 [95% CI, 0.33-0.96]; P = .03). The mRS shift analysis also showed that the intensive management group had worse scores than the conventional management group (adjusted odds ratio, 0.65 [95% CI, 0.43-0.97]; P = .04).
Primary Safety Outcomes
The incidence of symptomatic ICH was not significantly different between the intensive management group (9.0%) and the conventional management group (8.1%), with no significant risk difference (1.0% [95% CI, −5.3% to 7.3%]; adjusted OR, 1.10 [95% CI, 0.48-2.53]; P = .82). Similarly, rates of death related to the index stroke within 3 months were 7.7% in the intensive management group and 5.4% in the conventional management group, which did not show a significant risk difference (2.3% [95% CI, −3.3% to 7.9%]; adjusted OR, 1.73 [95% CI, 0.61-4.92]; P = .31) (Table 2).
Secondary Outcomes
The mRS shift analysis also showed that the intensive management group had significantly worse scores than the conventional management group. The adjusted OR for a favorable shift in mRS score was 0.65 (95% CI, 0.43-0.97; P = .04). The intensive management group had a significantly higher rate of malignant cerebral edema (adjusted OR, 7.88 [95% CI, 1.57-39.39]; P = .01). However, no significant differences were found in NIHSS score at 24 hours, rate of excellent recovery at 24 hours, successful reperfusion rate at 24 hours, functional independence at 1 month, or EQ-5D-3L score at 3 months (Table 2).
BP Measurements
At enrollment, the mean SBP was 155.2 mm Hg (SD, 13.4 mm Hg) in the intensive management group and 154.8 mm Hg (SD, 14.4 mm Hg) in the conventional management group. The intensive management group had a significantly lower 24-hour-mean SBP (129.2 mm Hg [SD, 7.7 mm Hg] vs 138.0 mm Hg [SD, 13.6 mm Hg]; P < .001) and 24-hour-mean diastolic BP (72.0 mm Hg [SD, 8.1 mm Hg] vs 77.0 mm Hg [SD, 9.9 mm Hg]; P < .001) than the conventional management group. The mean between-group difference in SBP over 24 hours was −9.6 mm Hg (95% CI, −12.2 to −6.9 mm Hg; P < .001), while the mean between-group difference in DBP over 24 hours was −5.5 mm Hg (95% CI, −7.6 to −3.3 mm Hg; P < .001) (Figure 3; eTable 3 in Supplement 3).
Figure 3. Changes in Mean Systolic and Diastolic Blood Pressure From Randomization to 24 Hours After Randomization.
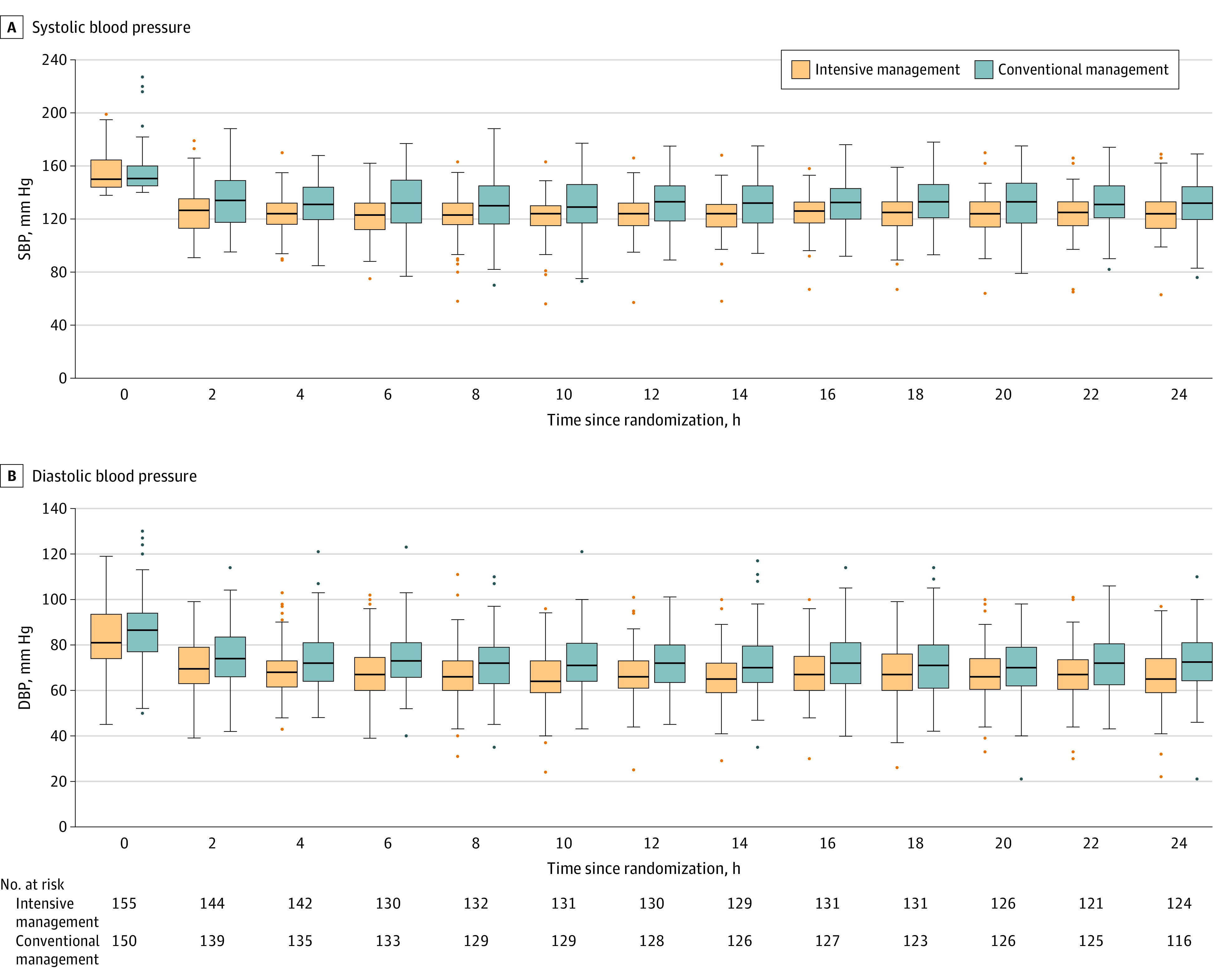
Compared with conventional management, intensive management significantly lowered mean systolic blood pressure (SBP; panel A) over 24 hours (129.2 mm Hg [SD, 7.7 mm Hg] vs 138.0 mm Hg [SD, 13.6 mm Hg]; P < .001) and mean diastolic blood pressure (DBP; panel B) over 24 hours (72.0 mm Hg [SD, 8.1 mm Hg] vs 77.0 mm Hg [SD, 9.9 mm Hg]; P < .001). The mean between-group difference in SBP over 24 hours was −9.6 mm Hg (95% CI, −12.2 to −6.9 mm Hg; P < .001), while the mean between-group difference in DBP over 24 hours was −5.5 mm Hg (95% CI, −7.6 to −3.3 mm Hg; P < .001). The black line within each box is the median blood pressure value at each respective time point; the upper and lower boundaries of the boxes represent the IQRs. Data points outside the boxes either surpass 1.5 times the value of the upper quartile or are less than 1.5 times the value of the lower quartile.
The rates of time spent in the target SBP range were 83.0% in the intensive management group (SBP <140 mm Hg) and 42.1% in the conventional management group (SBP 140-180 mm Hg). The rate of time spent with an SBP below 180 mm Hg was 99.6% in the intensive management group and 99.1% in the conventional management group. Blood pressure drop events (any episode with an SBP <100 mm Hg) occurred more frequently in the intensive management group than in the conventional management group (29.7% vs 17.3%; P = .02). However, the rate of BP overshooting events (any episode with an SBP >180 mm Hg) was not significantly different between groups (3.9% vs 9.3%; P = .09). Intravenous BP-lowering drugs were more frequently used in the intensive management group than in the conventional management group (74.2% vs 18.7%; P < .001). Among 26 patients in the conventional management group who experienced hypotensive events (SBP <100 mm Hg), 2 patients (7.7%) received intravenous BP-lowering drugs. On the other hand, in the intensive management group, 35 (76.1%) of 46 patients received intravenous BP-lowering drugs (eTable 3 in Supplement 3).
Per-Protocol Analysis
The per-protocol analysis results were consistent with those of the primary analysis except for EQ-5D-3L scores. The intensive management group had lower odds of functional independence at 3 months than the conventional management group, with an adjusted OR of 0.52 (95% CI, 0.30-0.90; P = .02). The incidence of symptomatic ICH was not significantly different between the intensive management group (8.1%) and the conventional management group (7.9%) (P = .93). Similarly, rates of death related to the index stroke within 3 months were not significantly different (8.1% in the intensive management group and 5.0% in the conventional management group; P = .36). The mRS shift analysis showed worse scores in the intensive management group (adjusted OR, 0.62 [95% CI, 0.41-0.95]; P = .03). The mean EQ-5D-3L score in the intensive management group was 0.51 (SD, 0.43), significantly lower than in the conventional management group (0.64 [SD, 0.39]; P = .04). Malignant cerebral edema was more frequent in the intensive management group (adjusted OR, 6.01 [95% CI, 1.19-30.27]; P = .03) (eTable 4 in Supplement 3).
Subgroup Analysis and Sensitivity Analyses
No statistically significant interactions were observed in any predetermined subgroup with respect to the outcome of functional independence. Moreover, the point estimates across all strata favored conventional management (eFigure 1 in Supplement 3).
In the post hoc imaging analysis, there were no significant differences in occlusion site, ASPECTS analysis, modified Tan collateral score, immediate reperfusion, and 24-hour reperfusion rates. Any ICH was found in 53.5% of the intensive management group and 52.3% of the conventional management group (P = .93) on MRI (n = 283) or CT (n = 17). Type 2 parenchymal hematomas occurred with similar frequency in the intensive management group (11.0%) and the conventional management group (11.3%; P = .90). The median infarction volumes were not significantly different between the groups (18.6 mL [IQR, 6.7-62.6 mL] in the intensive management group vs 17.1 mL [IQR, 5.4-40.9 mL] in the conventional management group; P = .11) (eTable 5 in Supplement 3).
The post hoc analysis taking into account enrollment site effects largely matched the primary analysis. However, it showed statistically significant differences in functional independence at 1 month and in the EQ-5D-3L score. The intensive management group exhibited less functional independence at 1 month (adjusted OR, 0.65 [95% CI, 0.49-0.87]; P = .003) and inferior EQ-5D-3L scores (adjusted β, −0.08 [95% CI, −0.16 to −0.01]; P = .04) compared with the conventional management group (eTable 6 in Supplement 3).
In another post hoc exploratory analysis, the restricted cubic spline curve revealed a reverse J-shaped association between the mean 24-hour SBP and an outcome of dependence or death (mRS score of 3-6) in the intensive management group, which was consistent with a relationship between lower mean 24-hour SBP and an outcome of dependence or death. However, in the conventional management group, there was a linear association between the mean 24-hour SBP and an outcome of dependence or death, indicating that a higher mean 24-hour SBP was correlated with worse outcomes (eFigure 2 in Supplement 3).
Discussion
This trial showed that intensive BP management was associated with worse functional outcomes than conventional BP management in patients with acute ischemic stroke who achieved successful reperfusion after EVT. Rates of symptomatic ICH and death related to the index stroke were similar between groups, whereas rates of malignant cerebral edema were higher in patients in the intensive BP management group.
The main findings are consistent with those of ENCHANTED2/MT, which showed that death or disability at 3 months occurred more frequently in the more intensive treatment group than in the less intensive treatment group.15 In previous observational studies and meta-analyses, elevated BP was associated with increased risks of ICH and worse outcomes in patients with successful reperfusion after EVT. This evidence suggesting that lowering BP could be beneficial prompted the hypothesis for this trial.3,4,10,11,12,13 However, our findings suggest that actively lowering SBP to levels less than 140 mm Hg in patients with a baseline SBP of 140 mm Hg or greater is harmful. The restricted cubic spline curve showed a sharp increase in the likelihood of an outcome of dependence or death as BP decreased in the intensive management group. Although the occluded artery was recanalized, some areas in the ischemic brain may have already been damaged or were in an oligemic state within an ischemic penumbra zone. Cerebral vessels in these areas may not have a sufficient autoregulatory function to compensate for sudden decreases in BP. We found that median infarction volumes did not differ between the 2 treatment groups, but malignant cerebral edema occurred more often in the intensive management group. Intensive BP lowering might have further decreased blood flow to the oligemic area and exacerbated ischemic injury.
In our trial, the rates of symptomatic ICH, parenchymal hematoma, and any ICH were similar between the groups. Similarly, no significant differences in symptomatic ICH between randomized groups were observed in the ENCHANTED2/MT and BP-TARGET trials. As undertaken in these trials, investigators were required to manage SBP to below 180 mm Hg according to current guidelines,8,9 and this was well achieved in the current trial (the rate of time spent with SBP <180 mm Hg was >99%). The findings of this trial and those of recent trials suggest that the risk of ICH may be small and not significantly different across groups as long as BP is managed below 180 mm Hg based on the current guidelines.
High BP in patients with acute stroke can increase the risk of ICH. However, it may not be completely causal but rather a response to acute stress. Activation of the sympathetic nervous system and the renin-angiotensin axis has been implicated in the increase in BP in acute stroke.27 Brain swelling may compress the blood vessels, leading to subsequent cerebral ischemia. This triggers a compensatory mechanism to increase BP to maintain cerebral perfusion pressure.28 The finding that malignant cerebral edema was more frequent in the intensive management group provides an indication that more intensive BP control can cause harm to the cerebral microcirculation.15 Interestingly, in the conventional management group, in which BP was not actively lowered, the lower the mean 24-hour BP, the better the outcome. This may explain, in part, the discrepancies between our results and the findings of observational studies. Although high BP could increase the risk of ICH, the poor outcomes observed in some patients with high BP during previous observational studies could possibly be explained by their appropriate physiological response to severe stroke.
In the group of patients with hypotensive events (SBP <100 mm Hg), 7.7% in the conventional management group received intravenous BP-lowering drugs, whereas in the intensive management group, 76.1% were administered the same drugs. Our findings suggest that intervention to prevent or manage hypotensive episodes may be necessary. However, the effect of such intervention and optimal BP targets in cases of hypotensive episodes remain unknown. Further studies of this issue are necessary.
The OPTIMAL-BP trial was not designed to test the effect of a specific drug and allowed any available BP-lowering drug. However, nicardipine, a calcium channel blocker, was preferred, and most patients received nicardipine. Similarly, most patients received calcium channel blockers in the BP-TARGET trial, whereas most patients received urapidil, an α-blocker, in ENCHANTED2/MT. Considering different results among trials, the class of BP drug may not be as important as BP lowering itself.
During this trial, most patients were treated according to the current guidelines, and patients with low ASPECTS rarely underwent EVT. Therefore, the role of intensive BP lowering in patients with low ASPECTS could not be determined by our study. Further trials targeting patients with large cores may elucidate the benefits of intensive BP lowering.
In contrast to findings in this trial and ENCHANTED2/MT, the proportion of patients with functional independence was not significantly different between the intensive and conventional management groups in the secondary outcome analysis of the BP-TARGET trial. None of this work has clearly defined an optimal threshold or SBP range, but it may be a narrow window for some patients. Completion and reporting of other trials such as BEST-II (NCT04116112), DETECT (NCT04484350), CRISIS I (NCT04775147), and HOPE (NCT04892511), reactivation of ENCHANTED2/MT with a revised protocol, and pooling of individual patient data meta-analysis results may help define an optimal level of BP control for patients who achieve successful reperfusion after EVT.
Limitations
This study had several limitations. First, the study was terminated early, which may have reduced its statistical power and increased the likelihood of random and significantly large treatment effects. Second, because the study population represented 19.1% of all screened patients, there may be concerns of selection bias in the clinical trial population. Furthermore, nearly half of the patients were excluded due to having an SBP of less than 140 mm Hg. Our study cannot be generalized to all patients with EVT. Third, the rate of time spent in the target SBP range (140-180 mm Hg) was only 42.1% in the conventional management group because of spontaneous BP decline below 140 mm Hg. This might be a natural response after reperfusion.29 However, the low rate of time spent in the target BP range in the conventional management group may have underpowered the trial. Fourth, the adjusted analysis revealed a large OR and a wide 95% CI associated with malignant cerebral edema. This result may be due to the small number of event occurrences, potentially leading to issues with model stability. Fifth, the results should be interpreted while considering potential ethnic differences in the pattern of stroke in South Korea compared with those in other regions. Sixth, the mRS primary end point was ascertained using a patient-reported rather than clinician-rated algorithm.
Conclusions
Among patients who achieved successful reperfusion with EVT for acute ischemic stroke with large vessel occlusion, intensive BP management for 24 hours led to a lower likelihood of functional independence at 3 months compared with conventional BP management. These results suggest that intensive BP management should be avoided after successful EVT in acute ischemic stroke.
Trial Protocol
Statistical Analysis Plan
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Demographic and Clinical Characteristics in Intention-to-Treat Analysis
eTable 3. Changes of Blood Pressure Between Groups
eTable 4. Primary and Secondary Outcomes in Per-Protocol Analysis
eTable 5. Imaging Findings
eTable 6. Post Hoc Sensitivity Analysis Considering Enrollment Site
eFigure 1. Prespecified Subgroup Analysis of the Primary Efficacy Outcome in the Primary Analysis Population
eFigure 2. The Restricted Cubic Spline Curve According to the Treatment Group
Nonauthor Collaborators. OPTIMAL-BP Trial Investigators
Data Sharing Statement
References
- 1.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES Collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 2.Lapergue B, Blanc R, Costalat V, et al. ; ASTER2 Trial Investigators . Effect of thrombectomy with combined contact aspiration and stent retriever vs stent retriever alone on revascularization in patients with acute ischemic stroke and large vessel occlusion: the ASTER2 randomized clinical trial. JAMA. 2021;326(12):1158-1169. doi: 10.1001/jama.2021.13827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maier IL, Tsogkas I, Behme D, et al. High systolic blood pressure after successful endovascular treatment affects early functional outcome in acute ischemic stroke. Cerebrovasc Dis. 2018;45(1-2):18-25. doi: 10.1159/000484720 [DOI] [PubMed] [Google Scholar]
- 4.Goyal N, Tsivgoulis G, Pandhi A, et al. Blood pressure levels post mechanical thrombectomy and outcomes in large vessel occlusion strokes. Neurology. 2017;89(6):540-547. doi: 10.1212/WNL.0000000000004184 [DOI] [PubMed] [Google Scholar]
- 5.Martins AI, Sargento-Freitas J, Silva F, et al. Recanalization modulates association between blood pressure and functional outcome in acute ischemic stroke. Stroke. 2016;47(6):1571-1576. doi: 10.1161/STROKEAHA.115.012544 [DOI] [PubMed] [Google Scholar]
- 6.Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19(6):624-633. doi: 10.1097/00004647-199906000-00005 [DOI] [PubMed] [Google Scholar]
- 7.Heo JH, Han SW, Lee SK. Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med. 2005;39(1):51-70. doi: 10.1016/j.freeradbiomed.2005.03.035 [DOI] [PubMed] [Google Scholar]
- 8.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 9.Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO)–European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg. 2019;11(6):535-538. doi: 10.1136/neurintsurg-2018-014568 [DOI] [PubMed] [Google Scholar]
- 10.Malhotra K, Goyal N, Katsanos AH, et al. Association of blood pressure with outcomes in acute stroke thrombectomy. Hypertension. 2020;75(3):730-739. doi: 10.1161/HYPERTENSIONAHA.119.14230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anadani M, Arthur AS, Tsivgoulis G, et al. Blood pressure goals and clinical outcomes after successful endovascular therapy: a multicenter study. Ann Neurol. 2020;87(6):830-839. doi: 10.1002/ana.25716 [DOI] [PubMed] [Google Scholar]
- 12.Katsanos AH, Malhotra K, Ahmed N, et al. Blood pressure after endovascular thrombectomy and outcomes in patients with acute ischemic stroke: an individual patient data meta-analysis. Neurology. 2022;98(3):e291-e301. doi: 10.1212/WNL.0000000000013049 [DOI] [PubMed] [Google Scholar]
- 13.Samuels N, van de Graaf RA, Mulder MJHL, et al. ; HERMES Collaborators . Admission systolic blood pressure and effect of endovascular treatment in patients with ischaemic stroke: an individual patient data meta-analysis. Lancet Neurol. 2023;22(4):312-319. doi: 10.1016/S1474-4422(23)00076-5 [DOI] [PubMed] [Google Scholar]
- 14.Mazighi M, Richard S, Lapergue B, et al. ; BP-TARGET Investigators . Safety and efficacy of intensive blood pressure lowering after successful endovascular therapy in acute ischaemic stroke (BP-TARGET): a multicentre, open-label, randomised controlled trial. Lancet Neurol. 2021;20(4):265-274. doi: 10.1016/S1474-4422(20)30483-X [DOI] [PubMed] [Google Scholar]
- 15.Yang P, Song L, Zhang Y, et al. ; ENCHANTED2/MT Investigators . Intensive blood pressure control after endovascular thrombectomy for acute ischaemic stroke (ENCHANTED2/MT): a multicentre, open-label, blinded-endpoint, randomised controlled trial. Lancet. 2022;400(10363):1585-1596. doi: 10.1016/S0140-6736(22)01882-7 [DOI] [PubMed] [Google Scholar]
- 16.Nam HS, Kim YD, Choi JK, et al. Outcome in patients treated with intra-arterial thrombectomy: the Optimal Blood Pressure Control (OPTIMAL-BP) trial. Int J Stroke. 2021;17(8):17474930211041213. doi: 10.1177/17474930211041213 [DOI] [PubMed] [Google Scholar]
- 17.Tomsick T, Broderick J, Carrozella J, et al. ; Interventional Management of Stroke II Investigators . Revascularization results in the interventional management of stroke II trial. AJNR Am J Neuroradiol. 2008;29(3):582-587. doi: 10.3174/ajnr.A0843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.EuroQol Group . EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. doi: 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 19.Hacke W, Kaste M, Bluhmki E, et al. ; ECASS Investigators . Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317-1329. doi: 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 20.Wu S, Yuan R, Wang Y, et al. Early prediction of malignant brain edema after ischemic stroke. Stroke. 2018;49(12):2918-2927. doi: 10.1161/STROKEAHA.118.022001 [DOI] [PubMed] [Google Scholar]
- 21.Cranston JS, Kaplan BD, Saver JL. Minimal clinically important difference for safe and simple novel acute ischemic stroke therapies. Stroke. 2017;48(11):2946-2951. doi: 10.1161/STROKEAHA.117.017496 [DOI] [PubMed] [Google Scholar]
- 22.Lee YK, Nam HS, Chuang LH, et al. South Korean time trade-off values for EQ-5D health states: modeling with observed values for 101 health states. Value Health. 2009;12(8):1187-1193. doi: 10.1111/j.1524-4733.2009.00579.x [DOI] [PubMed] [Google Scholar]
- 23.Coretti S, Ruggeri M, McNamee P. The minimum clinically important difference for EQ-5D index: a critical review. Expert Rev Pharmacoecon Outcomes Res. 2014;14(2):221-233. doi: 10.1586/14737167.2014.894462 [DOI] [PubMed] [Google Scholar]
- 24.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet. 2000;355(9216):1670-1674. doi: 10.1016/S0140-6736(00)02237-6 [DOI] [PubMed] [Google Scholar]
- 25.Tan IY, Demchuk AM, Hopyan J, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol. 2009;30(3):525-531. doi: 10.3174/ajnr.A1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeMets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med. 1994;13(13-14):1341-1352. doi: 10.1002/sim.4780131308 [DOI] [PubMed] [Google Scholar]
- 27.Lattanzi S, Silvestrini M, Provinciali L. Elevated blood pressure in the acute phase of stroke and the role of Angiotensin receptor blockers. Int J Hypertens. 2013;2013:941783. doi: 10.1155/2013/941783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alqadri SL, Sreenivasan V, Qureshi AI. Acute hypertensive response management in patients with acute stroke. Curr Cardiol Rep. 2013;15(12):426. doi: 10.1007/s11886-013-0426-7 [DOI] [PubMed] [Google Scholar]
- 29.John S, Hazaa W, Uchino K, Hussain MS. Timeline of blood pressure changes after intra-arterial therapy for acute ischemic stroke based on recanalization status. J Neurointerv Surg. 2017;9(5):455-458. doi: 10.1136/neurintsurg-2016-012369 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Demographic and Clinical Characteristics in Intention-to-Treat Analysis
eTable 3. Changes of Blood Pressure Between Groups
eTable 4. Primary and Secondary Outcomes in Per-Protocol Analysis
eTable 5. Imaging Findings
eTable 6. Post Hoc Sensitivity Analysis Considering Enrollment Site
eFigure 1. Prespecified Subgroup Analysis of the Primary Efficacy Outcome in the Primary Analysis Population
eFigure 2. The Restricted Cubic Spline Curve According to the Treatment Group
Nonauthor Collaborators. OPTIMAL-BP Trial Investigators
Data Sharing Statement