Abstract
Aims
Cryoballoon (CB) ablation is the mainstay of single-shot pulmonary vein isolation (PVI). A radiofrequency balloon (RFB) catheter has recently emerged as an alternative. However, these two technologies have not been compared. This study aims to evaluate the freedom from atrial tachyarrhythmias (ATas) at 1 year: procedural characteristics, efficacy, and safety of the novel RFB compared with CB for PVI in patients with paroxysmal atrial fibrillation (AF).
Methods and results
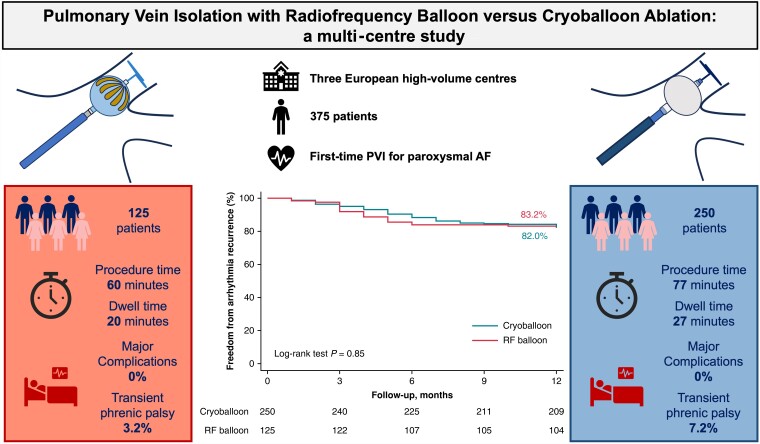
This prospective multi-centre study included consecutive patients with symptomatic drug-resistant paroxysmal AF who underwent PVI with RFB or CB between July 2021 and January 2022 from three European centres. A total of 375 consecutive patients were included, 125 in the RFB group and 250 in the CB. Both groups had comparable clinical characteristics. At 12.33 ± 4.91 months, ATas-free rates were 83.20% and 82.00% in the RFB and CB groups, respectively (P > 0.05). Compared with the CB group, the RFB group showed a shorter procedure time [59.91 (45.80–77.12) vs. 77.0 (35.13–122.71) min (P < 0.001)], dwell time [19.59 (14.41–30.24) vs. 27.03 (17.11–57.21) min (P = 0.04)], time to isolation, and thermal energy delivery in all pulmonary veins (P < 0.001). First-pass isolation was comparable. No major complications occurred in either group, with no stroke, atrio-oesophageal fistula, or permanent phrenic nerve injury. Transient phrenic nerve palsy occurred more frequently with CB than RFB (7.20% vs. 3.20%; P = 0.02). Oesophageal temperature rise occurred in 21 (16.8%) patients in the RFB group, and gastroscopy showed erythema in two of them with complete recovery after 30 days.
Conclusions
The RFB appears to have a safety and efficacy profile similar to that of the CB for PVI. Shorter procedural times appear to be driven by shorter left atrial dwell and thermal delivery times.
Keywords: Pulmonary vein isolation, Radiofrequency balloon, Cryoballoon
Graphical Abstract
Graphical Abstract.
What’s new?
The radiofrequency balloon showed previously promising results in terms of efficacy after pulmonary vein isolation. However, it has never been compared with the standard of care for single-shot thermal catheters, such as the cryoballoon.
This is the first real-life prospective multi-centre study to evaluate the efficacy and safety of the radiofrequency balloon in this setup.
Oesophageal lesions and phrenic nerve injury are known complications of single-shot devices, with a higher risk with radiofrequency. This multi-centre study evaluated these risks.
Introduction
Pulmonary vein isolation (PVI) is the cornerstone of catheter ablation for atrial fibrillation (AF).1,2 In patients with paroxysmal AF, PVI is currently recommended as a rhythm control strategy after a trial with Class I or III anti-arrhythmic drugs (AADs) or, in selected cases, as a first-line therapy to improve quality of life.1,2
Currently, cryoballoon (CB) ablation is the mainstay single-shot technology employed for PVI, with arrhythmia-free survival and procedural complication rates similar to those of point-by-point radiofrequency (RF). Pulmonary vein isolation with CB is faster, more reproducible, and less operator dependent than point-by-point ablation, although it is still limited by higher radiation exposure.3–7
Recently, a multi-electrode RF balloon (RFB) catheter (Biosense Webster, CA, USA) has emerged as a single-shot ablation alternative for PVI. The RFB is a 28 mm compliant balloon, characterized by 10 flexible gold-plated electrodes, each capable of independently delivering irrigated unipolar RF energy to perform focal, segmental, or circumferential ablation to customize energy delivery to different anatomical structures. Moreover, the RFB is compatible with a 3D electroanatomical mapping system (CARTO 3, Biosense Webster, CA, USA), potentially reducing the fluoroscopy time (Table 1). Single-centre and multi-centre studies have demonstrated the feasibility and safety profile of RFB ablation for PVI in paroxysmal AF, with favourable outcomes at the 1-year follow-up.8–11
Table 1.
Radiofrequency and cryoballoon systems characteristics
| Radiofrquency balloon | Cryoballoon | |
|---|---|---|
| Energy | Radiofrequency | Cryo |
| Application time/PV | 60 s | 180 s |
| Real-time signals | Circular catheter + RFB | Circular catheter |
| 3D mapping | (+) | (−) |
| Fluoroscopy guided | Optional | (+) |
| Compliant | (+) | (−) |
| Need for occlusion | ? | (+) |
| Circumferential ablation | (+) | (+) |
| Segmental ablation | (+) | (−) |
PV, pulmonary Vein; RFB, radiofrequency balloon.
To date, no data exist on a direct comparison between CB and RFB for PVI in patients with paroxysmal AF. The aim of this prospective study was to evaluate the freedom of arrhythmias at 1 year out of AAD, procedural characteristics, efficacy, and safety profile of the novel RFB compared with CB for PVI in patients with paroxysmal AF.
Methods
Study population
In this prospective multi-centre study, all consecutive patients with symptomatic drug-resistant paroxysmal AF undergoing PVI with the RFB (Heliostar, Biosense Webster, CA, USA) or the CB [Arctic Front Advance Pro (AFA-Pro), Medtronic, Minneapolis, MN, USA] between July 2021 and January 2022 were included. All procedures were performed in three high volumes by experienced operators in single-shot PVI. To reduce any selection bias, all planned procedures were performed with only one technology (RFB or CB) for the entire day (e.g. 1 day with CB and another with RFB) in all the centres. Baseline characteristics and procedural and follow-up data were collected prospectively. The study protocol was conducted in accordance with the ethical principles established by the Declaration of Helsinki, as revised in 2013, and approved by the local ethics committee of our institution. All the patients provided written informed consent for the ablation procedure and data collection.
Ablation procedure
All procedures were performed under general anaesthesia. Patients taking vitamin K antagonists (VKA) underwent ablation with an international normalized ratio between 2 and 3, while patients taking non-VKA oral anticoagulants skipped the morning dose. All AADs were discontinued 5 half-lives before ablation. Per protocol, a multi-electrode oesophageal temperature probe (S-Cath; Circa Scientific LLC, Englewood, CO, USA) was positioned at the level of the left atrium (LA) in patients undergoing RFB ablation. Two femoral punctures were performed under echo guidance. A single transseptal puncture was performed, followed by bolus dosing with unfractionated heparin to maintain activated clotting time of 250–350 s for CB and 300–350 s for RFB for the entire procedure. A decapolar catheter was used for coronary sinus electrogram recording and phrenic nerve pacing during ablation of the right pulmonary veins (PVs) with CB or RFB. For both technologies, phrenic capture was monitored by palpation; in case of loss of capture or weakening of diaphragmatic contractions, ablation was terminated immediately. In both scenarios, successful PVI was defined as the absence of all PV potentials and exit-block evaluation from the PV. An additional 20 min of waiting time after PVI confirmed the absence of acute reconnection.
Cryoballoon ablation
After obtaining LA access, a steerable 15 Fr sheath (Arctic Front Advance Pro, Medtronic Inc., Minneapolis, MN, USA) and a 28-mm CB (Arctic Front Advance Pro, Medtronic Inc., Minneapolis, MN, USA) were advanced in the LA, and an inner lumen mapping catheter (Achieve, Medtronic Inc., Minneapolis, MN, USA) was positioned in each PV ostium. Baseline electrical information was gathered for each PV ostium. The 28 mm CB ablation (CB-A) was advanced, inflated, and positioned at each PV ostium. Optimal vessel occlusion was defined by selective contrast injection showing total contrast retention with no backflow into the LA. The ablation sequence was as follows: first, the left superior PV (LSPV) was treated, followed by the left inferior PV (LIPV), right inferior PV (RIPV), and right superior PV (RSPV). Once vessel occlusion was deemed satisfactory, delivery of cryoenergy to allow freezing was initiated. The standard cryothermal applications lasted for 180 s. First-pass isolation was defined as a target temperature of −40°C and time to isolation within the first 60 s of freeze. If these objectives were not reached, the freeze was interrupted, and the balloon was repositioned for an extra 180 s.
Radiofrequency balloon ablation
The procedural workflow used has been previously described by our group.12 Briefly, after gaining access to the LA, a pre-ablation 3D electroanatomical map of the PVs and LA was created using a circular mapping catheter (LASSONAV; Biosense Webster, Irvine, CA, USA). The RFB equipped with the intraluminal circular diagnostic catheter (LASSOSTAR, Biosense Webster, Irvine, CA, USA) was then introduced into the LA through a dedicated deflectable sheath (GUIDESTAR, Biosense Webster, Irvine, CA, USA). Optimal RFB positioning, including correct alignment with the PVs and electrode-tissue contact, was assessed with fluoroscopy, mapping system visualization of the balloon, and according to the following baseline parameters: balloon inflation index >0.8, electrode impedance 90–120 Ω with a variability ≤20 Ω across electrodes, and electrode temperature ≤31°C with a variability ≤3°C between electrodes. Pulmonary vein occlusion assessment with contrast injection was performed according to the operator’s discretion. Specific to the RFB, prior to ablation of the right PVs, pacing from the anterior electrodes of the balloon at 10 mA for 2 ms granted no phrenic nerve capture. After confirmation of optimal balloon positioning, ablation was performed in temperature-controlled mode with unipolar RF energy. Typically, three posterior electrodes are identified on the RFB using a 3D electroanatomical map. The power setting was 15 W, and the target electrode temperature was 55°C. The same energy was simultaneously delivered to all electrodes, with a duration of 20 s for the posterior electrode and 60 s for the non-posterior electrodes. In the case of oesophageal temperature rise (defined as >39°C), a shorter application in the posterior electrodes was performed, according to the operator’s preference. During ablation, the PV potentials were monitored using a circular diagnostic catheter to evaluate real-time isolation. First pass was defined as the time to isolation of less than 12 s. In case of a longer time to isolation, an extra application, segmental or circumferential, was achieved.
Post-procedural management and follow-up
All patients underwent continuous telemetry monitoring for at least 24 h after the procedure and were discharged after overnight observation if no complications occurred. Before discharge, transthoracic echocardiography and venous Doppler ultrasound were performed for all patients. Oral anticoagulation was started the same evening after ablation and continued for at least 2 months; thereafter, it was prolonged according to the patient’s thromboembolic risk profile. Anti-arrhythmic drugs were discontinued at the latest 1 month after ablation.
Patients with oesophageal temperature rise of ≥41°C for a cumulative time of ≥10 s during ablation were scheduled for oesophageal endoscopy within 5- to 8-day post-procedure.
The clinical follow-up strategy included in-person outpatient visits 1, 3, 6, and 12 months after ablation for the first year. At each visit, clinical examination and 12-lead electrocardiogram (ECG) were performed. Furthermore, 24-h Holter was recorded at 3 and 6 months, with an additional 7-day Holter monitoring at 12 months. Regular telephone consultations were conducted between scheduled visits.
Study endpoints and definitions
The primary efficacy endpoint was the comparison of arrhythmia-free survival during the 1-year follow-up between RFB and CB for paroxysmal AF. Arrhythmia recurrence was defined as any atrial tachyarrhythmias (ATas) ≥ 30 s. No post-procedural blanking period was considered.
The secondary endpoint was to analyse the differences in procedural characteristics between the balloons.
Procedural failure was defined as the inability to achieve PVI with either balloon catheter with the need for additional focal RF catheter ablation. Device failure was defined as any dysfunction of the system (sheath or balloon catheter).
The total procedure time was defined as the time from the first femoral puncture to catheter removal. The left atrial dwell time was defined as the time at which the balloon catheter was left in the LA.
The primary safety endpoint included any major peri-procedural complications [e.g. death, atrio-oesophageal fistula, stroke/transient ischaemic attack (TIA), pericardial effusion/tamponade with/without surgical treatment, myocardial infarction, and persistent phrenic palsy] occurring within 7-day post-procedure (except for atrio-oesophageal fistula). Minor complications have also been reported, including vascular access complications requiring treatment, pericarditis, and transient phrenic palsy.
Statistical analysis
All variables were tested for normality using the Shapiro–Wilk test. Normally distributed variables were described as mean ± standard deviation, and the groups were compared using analysis of variance (ANOVA) and paired or unpaired t-test as appropriate, while the non-normally distributed variables were described as median (interquartile range) and compared using the Mann–Whitney test or Wilcoxon signed-rank test as appropriate. Categorical variables were described as frequencies (percentages) and compared using the chi-squared test or Fisher’s exact test, as appropriate. Kaplan–Meier plots were used to report arrhythmia-free survival curves for each group, and time-to-event analysis was performed using the log-rank test. Statistical significance was set at P < 0.05.
Statistical analyses were performed using SPSS version 26 (IBM Corporation, Armonk, NY, USA).
Results
Study population
The complete patient characteristics are summarized in Table 2.
Table 2.
Baseline demographic and clinical characteristics
| RFB (n = 125) | CB (n = 250) | P-value | |
|---|---|---|---|
| Age (years) | 64.91 ± 12.01 | 63.23 ± 11.02 | 0.84 |
| Gender (male) | 69 (55.20%) | 148 (59.20%) | 0.62 |
| BMI (kg/m2) | 28.92 ± 5.23 | 30.12 ± 4.94 | 0.33 |
| CHA2DS2-VASc score | 1.91 ± 1.23 | 2.02 ± 1.41 | 0.59 |
| Hypercholesterolaemia (n, %) | 69 (55.20%) | 123 (49.20%) | 0.37 |
| Diabetes (n, %) | 27 (21.60%) | 45 (18.00%) | 0.29 |
| Hypertension (n, %) | 59 (47.20%) | 114 (45.60%) | 0.55 |
| Heart failure (n, %) | 7 (5,60%) | 22 (8.8%) | 0.28 |
| Stroke or TIA (n, %) | 6 (4.80%) | 10 (4.00%) | 0.32 |
| CAD (n, %) | 15 (12.00%) | 37 (14.80%) | 0.11 |
| LVEF (%) | 55.05 ± 5.73 | 54.74 ± 6.81 | 0.71 |
| LAESVI (mL/m2) | 37.12 ± 10.84 | 39.03 ± 11.02 | 0.45 |
| EHRA symptom score | 3.11 ± 0.62 | 2.94 ± 0.32 | 0.77 |
| Prior cardioversion (n, %) | 27 (21.60%) | 60 (24.00%) | 0.29 |
| Time from AF diagnosis (months) | 11.51 ± 7.82 | 12.41 ± 8.64 | 0.26 |
| Drugs | |||
| AADs Class Ic (n, %) | 25 (20.00%) | 50 (20.00%) | 0.81 |
| AADs Class III (n, %) | 52 (41.60%) | 100 (40.00%) | 0.75 |
| OAC (n, %) | 111 (88.89%) | 223 (89.20%) | 0.74 |
| Follow-up | 12.41 ± 4.53 | 12.12 ± 5.32 | 0.31 |
AADs, anti-arrhythmic drugs; AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CB, cryoballoon; EHRA, European Heart Rhythm Association; LAESVI, left atrial end-systolic volume index; LVEF, left ventricular ejection fraction; OAC, oral anticoagulation; RFB, radiofrequency balloon; TIA, transient ischaemic attack.
In total, 375 consecutive patients were included in the study: 125 in the RFB group and 250 in the CB group. The mean age was 64.91 ± 12.01 years vs. 63.23 ± 11.02 (P > 0.05), respectively. Sixty-nine (55.20%) and 148 (59.20%) patients were males in the RFB and CB groups, respectively (P > 0.05).
The mean CHA2DS2-VASc Score, time from diagnosis to ablation, and follow-up were not significantly different between the groups.
Procedural characteristics
The median procedure time was 59.91 (45.80–77.12) vs. 77.02 (35.13–122.71) min (P < 0001), with a median dwell time of 19.59 (14.41–30.24) vs. 27.03 (17.11–57.21) min (P = 0.04) and a median fluoroscopy time of 15.02 (6.01–26.04) vs. 15.83 (5.54–35.04) min (P = 0.26) in the RFB and CB groups, respectively. The complete procedural characteristics of both the groups are summarized in Table 3.
Table 3.
Procedural characteristics
| RFB (n = 125) | CB (n = 250) | P-value | |
|---|---|---|---|
| Procedure time (min) | 59.91 (45.80–77.12) | 77.02 (35.13–122.71) | <0.001 |
| Dwell time (min) | 19.59 (14.41–30.24) | 27.03 (17.11–57.21) | 0.04 |
| Fluoroscopy time (min) | 15.02 (6.01–26.04) | 15.83 (5.54–35.04) | 0.26 |
| LSPV first pass (n, %) | 114 (91.20%) | 228 (91.20%) | 0.10 |
| LSPV applications (n, %) | 0.23 | ||
| 2 | 8 (6.40%) | 20 (8.00%) | |
| >2 | 3 (2.40%) | 1 (0.04%) | |
| LSPV TTI (s) | 10.01 (7.04–11.81) | 41.04 (23.02–57.03) | <0.001 |
| LIPV first pass (n, %) | 113 (90.40%) | 228 (91.20%) | 0.49 |
| LIPV applications (n, %) | 0.12 | ||
| 2 | 10 (8.00%) | 19 (7.60%) | |
| >2 | 2 (1.60%) | 3 (1.20%) | |
| LIPV TTI (s) | 9.02 (6.02–12.01) | 35.04 (23.01–51.04) | <0.001 |
| RIPV first pass (n, %) | 110 (88.00%) | 206 (82.40%) | 0.10 |
| RIPV applications (n, %) | 0.07 | ||
| 2 | 10 (8.00%) | 38 (15.20%) | |
| >2 | 5 (4.00%) | 6 (2.40%) | |
| RIPV TTI (s) | 9.03 (6.04–11.03) | 39.02 (27.03–56.01) | <0.001 |
| RSPV first pass (n, %) | 114 (91.20%) | 213 (85.20%) | 0.08 |
| RSPV applications (n, %) | 0.21 | ||
| 2 | 7 (5.60%) | 14 (5.60%) | |
| >2 | 2 (1.60%) | 9 (3.60%) | |
| RSPV TTI (s) | 9.04 (8.04–10.51) | 33.02 (26.01–58.03) | <0.001 |
| Oesophageal temperature alarm (n, %) | 21 (16.80%) | NA | NA |
CB, cryoballoon; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RF, radiofrequency application; RFB, radiofrequency balloon; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; TTI, time to isolation.
Efficacy endpoints
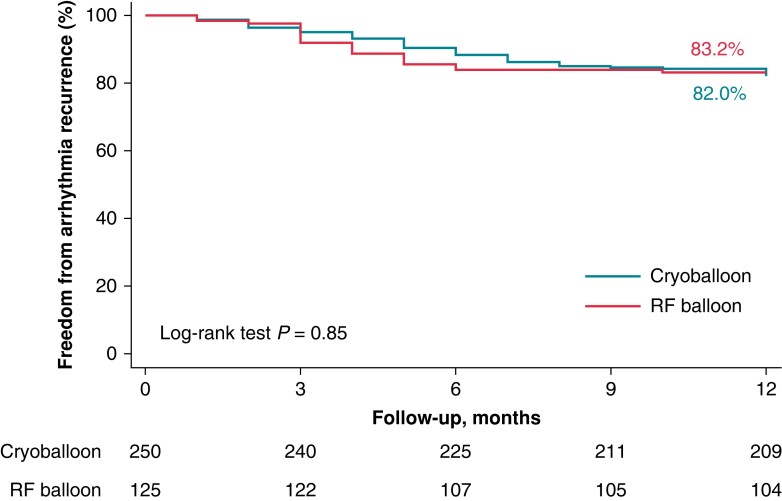
At a mean follow-up of 12.33 ± 4.91 months, the ATas-free rates were 83.20% (104/125) and 82.00% (205/250) in the RFB and CB groups, respectively (P > 0.05; Figure 1). The mean time to recurrence was 3.94 ± 1.32 vs. 4.73 ± 1.91 months (P = 0.09).
Figure 1.
Kaplan–Meier curves of survival free from any ATas recurrence during follow-up. The Kaplan–Meier curve of any ATas-free survival during the follow-up showed that the overall freedom from ATas was 83.2% for the RF balloon and 82.0% for the CB (P = 0.85) at a mean follow-up of 12.1 ± 4.8 months. ATas, atrial tachyarrhythmias; CB, cryoballoon; RF, radiofrequency.
In the RFB group, ATas were characterized as AF in 15 patients (71.43%) and as atrial tachycardia in six patients (28.57%). Repeat ablation was performed in 16 (76.19%) patients. At least one PV reconnection was observed in eight patients (38.09%) for a total of 14 reconnected PV with the following distribution: LSPV in two patients (25.00%), LIPV in two patients (25.00%), RIPV in three patients (37.50%), and RSPV in seven patients (87.50%). A new isolation of all the reconnected PV was performed in these cases.
In the CB group, 34 patients (75.56%) had recurrent AF, and 11 (24.44%) had atrial tachycardia. Repeat ablation using a RF catheter was performed in 29 patients (64.44%). At least one PV reconnection was observed in 12 patients (41.38%) for a total of 16 reconnect PV distributed as follows: LSPV in two patients (12.50%), LIPV in two patients (12.50%), RIPV in nine patients (56.25%), and RSPV in three patients (18.75%).
Secondary endpoints
As depicted in Table 3, compared with the CB group, in the RFB group, the time to isolation and thermal energy delivery were shorter (P < 0.001) in all PVs, namely 10.01 (7.04–11.81) vs. 41.04 (23.02–57.03) s for LSPV; 9.02 (6.02–12.01) vs. 35.04 (23.01–51.04) s for LIPV; 9.04 (8.04–10.51) vs. 33.02 (26.01–58.03) s for RSPV; and 9.03 (6.04–11.03) vs. 39.02 (27.03–56.01) s for RIPV. First-pass isolation rates were comparable in both groups.
In the RFB group, 99.20% (124/125) of PVI was achieved using only RFB. One patient (0.80%) required focal RF ablation due to technical failure of RFB. No device failure was observed in the CB group.
Safety endpoints
No major peri-procedural complications occurred in either of the groups (Table 4). Peripheral vascular access haematoma was observed in two patients in the RFB group and four of the CB group (P = 0.81), whereas uncomplicated pericarditis occurred in two and one patient, respectively (P = 0.07). Per the procedure, phrenic nerve capture was lost in four patients in the RFB group and 18 in the CB group (P = 0.02). All patients recovered phrenic nerve function at the 1-month follow-up visit in the RFB group and 6-month follow-up in the CB group.
Table 4.
Complications
| RFB (n = 125) | CB (n = 250) | P-value | |
|---|---|---|---|
| Major | 0 (0.00%) | 0 (0.00%) | >0.5 |
| Pericarditis with conservative treatment | 2 (1.60%) | 1 (0.40%) | 0.22 |
| Vascular aneurysm with conservative treatment | 1 (0.80%) | 1 (0.40%) | 0.31 |
| Vascular access haematoma | 2 (1.60%) | 4 (1.60%) | 0.81 |
| Phrenic nerve injury | 4 (3.20%) | 18 (7.20%) | 0.02 |
| Recovery before discharge | 0/4 (0.00%) | 2/18 (11.11%) | |
| Recovery at 1-month follow-up visit | 4/4 (100.00%) | 9/18 (50.00%) | |
| Recovery at 6-month follow-up visit | NA | 7/18 (38.89%) |
CB, cryoballoon; RFB, radiofrequency balloon.
Oesophageal temperature rise occurred in 21 (16.80%) patients in the RFB group. Gastroscopy was performed in 18 patients, according to the study protocol. Two of them showed erythema with complete recovery on control gastroscopy 30 days later.
Discussion
The current study is the first to evaluate the 1-year outcome out of AAD, procedural characteristics, efficacy, and safety profile of the novel RFB compared with conventional CB for PVI in paroxysmal AF patients. The major findings are as follows: (i) 83.20% (RFB) vs. 82.00% (CB) freedom of ATas at 1 year out of AAD, both catheters present similar efficacy; (ii) RFB allows faster procedures with shorter dwell time and time to isolation in all PVs for comparable first-pass isolation rates; and (iii) RFB and CB share an equivalent safety profile.
Efficacy of pulmonary vein isolation using radiofrequency balloon vs. cryoballoon
Although electrical isolation of the PV is the pursued goal of AF ablation, recommendations from recent guidelines regarding the use of CB or RFA are not specific.1,2,13 Cryoballoon is available through single-shot catheters, whereas RF is usually delivered via single-tip catheters in point-by-point applications.3,14 Balloon devices promise simple procedures that require less catheter manipulation in the left atrium and allow ablation of a large volume of tissue with single-position energy delivery.5,15–18 While CB operators achieve PVI under fluoroscopy guidance, RF-PVI is mainly performed using a mapping system.3,19–21 These different properties make PVI with RF more complex, operator dependent, and time-consuming. The potential advantage of RF over CB is the available opportunity to achieve additional ablation lines in the left atrium; however, this strategy failed to show significant additional benefits in AF recurrence reduction when compared with PVI only.22–26 Single-shot catheters, such as CB, have been developed to simplify PVI procedures by permitting shorter procedure times, less operator dependency, more consistent outcomes, and improved cost-effectiveness.27,28 The RFB was made available to provide these advantages to RF catheters/users (Table 1). Recent publications have shown that RFB is safe and efficient for isolating PV.10–12,29,30 However, these two single-shot devices have never been prospectively compared in terms of efficacy and safety.
In the current study, at a mid-term follow-up of 12.33 ± 4.91 months, the overall freedom from ATas recurrence out of AAD was identical in both groups (83.20% for RFB and 82.00% for CB), with no difference in recurrence type. This is in line with recent studies on balloons and energy sources. In the first evaluation of the RFB, the RADIANCE study reported ATas freedom at 12 months of 86.4% in a small cohort of 37 patients with paroxysmal AF.10 Subsequently, our group described freedom of ATas at 10.5 ± 5.3 months of 84.1% in patients with paroxysmal AF undergoing PVI with the RFB. In contrast, the largest randomized controlled trial comparing PVI with CB and point-by-point RF in paroxysmal AF was the FIRE AND ICE trial, which revealed similar efficacy.14 Recently, the CIRCA-DOSE study revealed that both procedures resulted in similar efficacy for paroxysmal AF during a 1-year follow-up duration (78.2%).3
In the setting of the actual study, procedure time was shorter in the RFB group, with a median procedure time of 59.91 (45.80–77.12) min (inclusive of 15–20 min of voltage mapping time before and after PVI) vs. 77.02 (35.13–122.71) min, mainly driven by shorter dwell time and energy time delivery per PV (1 min for the RFB vs. 3 min for the CB, Table 3). This is shorter than the first multi-centric reports on RFB, where RADIANCE and SHINE studies reported 102 and 88 min, respectively.8,10 Moreover, it is shorter than that reported in other multi-centric studies regardless of the energy source: 80.6–131.7 min for CB and 76–151 min for RFA.31,32 Since the RFB is a newly available catheter, it is reasonable to expect that with increasing experience, improvement of the ablation system, and the use of a navigation-enabled mapping catheter procedure time may be shortened in the future.
First-pass isolation showed a comparably high rate across all PVs, with a mean of 90.20% for RFB and 87.50% for CB. Given that the latter is considered the reference in one-shot catheters, this can be translated as encouraging results in favour of RFB. Additionally, improved positioning of the RFB at the PV ostium using a 3D-mapping system may lead to a high rate of first-pass isolation.
Safety profile
In this prospective large multi-centre study, no major complications were encountered in any group. Importantly, no pericardial effusion, stroke, TIA, atrio-oesophageal fistulas, or PV stenosis was observed. In comparison, the reported rates of phrenic nerve palsy, PV stenosis requiring intervention, and stroke or TIA with standard RF ablation were 0.4%, 0.29%, and 0.94%, respectively.33
Considering minor complications, phrenic nerve injury was observed more frequently in the CB group than in the RFB group (18 vs. 4 patients, P = 0.002). The hereby reported findings are in line with those reported previously.6,34,35 Importantly, all these injuries were resolved at follow-up visits without any additional treatment. Anatomical proximity to the phrenic nerve where cryoenergy is delivered may lead to phrenic nerve damage that consists of Wallerian degeneration, which, in most cases, is followed by regeneration and eventual recovery, making this complication mostly reversible.36 However, PNP induced by point-by-point RF catheters is usually reported as definite, which is not the case in this series of patients, questioning the physiological lesion growth induced by RFB. Compared with point-by-point RF catheters, unipolar RF delivery and compliance of the RFB architecture may lead to reduced harm and pressure on the myocardial tissue at a specific point. Additionally, ablation was promptly interrupted in cases of loss or weakened capture of the phrenic nerve. This quasi-instantaneous discontinuation of RF delivery may have favoured the recovery of phrenic function. Moreover, the ability of the balloon to pace from its surface electrodes to evaluate phrenic nerve capture before ablation of the right PVs could have prevented injuries by avoiding RF application from specific electrodes. A few other minor complications were reported, with no significant differences between the groups.
In the RFB group, of the 21 patients with a temperature rise on the oesophageal probe (>41°C for >10 s), 18 underwent gastroscopy after a mean of 9 ± 3 days. Two patients showed erythema with complete recovery at repeat gastroscopy 30 days later. Of note, none of the patients had undergone pre-procedural oesophageal protection or deviation. Compared with other technologies and ablation catheters, asymptomatic oesophageal lesions have been observed in 15–20% of patients with RF and CB-A, whereas atrio-oesophageal fistulas have been reported in 0.02–0.11% of patients.37–41
Limitations
Although all patients with paroxysmal AF were prospectively included in the database, this study was not a randomized trial. Good and promising results can be mitigated by the fact that all ablations were performed by highly experienced single-shot PVI operators. Gastroscopy indications were only led by a temperature rise on the oesophageal probe during PVI, representing a relatively small number of patients in the study population. Additionally, although the risk of fistula is probably low for CBs, it might be higher for RF balloons. Therefore, more data are needed in larger cohorts to assess the absence of oesophageal lesions with RFB. Continuous ECG monitoring was not performed in all patients, and sub-clinical ATas may not have been detected during follow-up.
Conclusions
The RFB appears to have a safety and efficacy profile similar to that of the CB for PVI. Shorter procedural times appear to be driven by shorter left atrial dwell and thermal delivery times.
Contributor Information
Alexandre Almorad, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Alvise Del Monte, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Domenico Giovanni Della Rocca, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Luigi Pannone, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Robbert Ramak, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Ingrid Overeinder, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Gezim Bala, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Erwin Ströker, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Juan Sieira, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Aurélie Dubois, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Antonio Sorgente, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Milad El Haddad, Independent Researcher, Helsinki, Finland.
Saverio Iacopino, Arrhythmology Department, Maria Cecilia Hospital SPA, Cotignola, Italy.
Serge Boveda, Département de Rythmologie, Clinique Pasteur, 45 avenue de Lombez, BP 27617, 31076 Toulouse Cedex 3, France.
Carlo de Asmundis, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Gian-Battista Chierchia, Heart Rhythm Management Centre, Postgraduate Program in Cardiac Electrophysiology and Pacing, Universitair Ziekenhuis Brussel—Vrije Universiteit Brussel, European Reference Networks Guard-Heart, Laarbeeklaan 101, 1090 Brussels, Belgium.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The data underlying this article will be shared upon reasonable request by the corresponding author.
References
- 1. Hindricks G, Potpara T, Dagres N, Bax JJ, Boriani G, Dan GAet al. . 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JCet al. . 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation 2019;140:e125–51. [DOI] [PubMed] [Google Scholar]
- 3. Andrade JG, Champagne J, Dubuc M, Deyell MW, Verma A, Macle Let al. . Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation 2019;140:1779–88. [DOI] [PubMed] [Google Scholar]
- 4. Kuck KH, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun Jet al. . Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. J Cardiopulm Rehabil 2016;36:393–4. [DOI] [PubMed] [Google Scholar]
- 5. Kuck KH, Albenque JP, Chun KRJ, Fürnkranz A, Busch M, Elvan Aet al. . Repeat ablation for atrial fibrillation recurrence post cryoballoon or radiofrequency ablation in the FIRE and ICE trial. Circ Arrhythm Electrophysiol 2019;12:e007247. [DOI] [PubMed] [Google Scholar]
- 6. Ravi V, Poudyal A, Pulipati P, Larsen T, Krishnan K, Trohman RGet al. . A systematic review and meta-analysis comparing second-generation cryoballoon and contact force radiofrequency ablation for initial ablation of paroxysmal and persistent atrial fibrillation. J Cardiovasc Electrophysiol 2020;31:2559–71. [DOI] [PubMed] [Google Scholar]
- 7. Sørensen SK, Johannessen A, Worck R, Hansen ML, Hansen J. Radiofrequency versus cryoballoon catheter ablation for paroxysmal atrial fibrillation: durability of pulmonary vein isolation and effect on atrial fibrillation burden: the RACE-AF randomized controlled trial. Circ Arrhythm Electrophysiol 2021;14:e009573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schilling R, Dhillon GS, Tondo C, Riva S, Grimaldi M, Quadrini Fet al. . Safety, effectiveness, and quality of life following pulmonary vein isolation with a multi-electrode radiofrequency balloon catheter in paroxysmal atrial fibrillation: 1-year outcomes from SHINE. Europace 2021;23:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reddy VY, Schilling R, Grimaldi M, Horton R, Natale A, Riva Set al. . Pulmonary vein isolation with a novel multielectrode radiofrequency balloon catheter that allows directionally tailored energy delivery: short-term outcomes from a multicenter first-in-human study (RADIANCE). Circ Arrhythm Electrophysiol 2019;12:e007541. [DOI] [PubMed] [Google Scholar]
- 10. Dhillon GS, Honarbakhsh S, Di MA, Coling AE, Lenka K, Pizzamiglio Fet al. . Use of a multi-electrode radiofrequency balloon catheter to achieve pulmonary vein isolation in patients with paroxysmal atrial fibrillation: 12-month outcomes of the RADIANCE study. J Cardiovasc Electrophysiol 2020;31:1259–69. [DOI] [PubMed] [Google Scholar]
- 11. Del Monte A, Almorad A, Pannone L, Della Rocca DG, Bisignani A, Monaco Cet al. . Pulmonary vein isolation with the radiofrequency balloon catheter: a single centre prospective study. Europace 2023;25:896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Almorad A, Chierchia GB, Pannone L, Osorio TG, Sorgente A, Bisignani Aet al. . The optimized clinical workflow for pulmonary vein isolation with the radiofrequency balloon. J Int Card Electrophysiol 2021;64:531–8. [DOI] [PubMed] [Google Scholar]
- 13. Iliodromitis K, Lenarczyk R, Scherr D, Conte G, Farkowski MM, Marin Fet al. . Patient selection, peri-procedural management, and ablation techniques for catheter ablation of atrial fibrillation: an EHRA survey. Europace 2023;25:667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuck K-H, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KRJet al. . -FIRE and ICE-NEJM (cryoballoon or radiofrequency ablation for paroxysmal AF). N Engl J Med 2016;2016:374. [DOI] [PubMed] [Google Scholar]
- 15. Bordignon S, Fürnkranz A, Perrotta L, Dugo D, Konstantinou A, Nowak Bet al. . High rate of durable pulmonary vein isolation after second-generation cryoballoon ablation: analysis of repeat procedures. Europace 2015;17:725–31. [DOI] [PubMed] [Google Scholar]
- 16. Cheung CC, Deyell MW, MacLe L, Verma A, Champagne J, Leong-Sit Pet al. . Repeat atrial fibrillation ablation procedures in the CIRCA-DOSE study. Circ Arrhythm Electrophysiol 2020;13:e008480. [DOI] [PubMed] [Google Scholar]
- 17. Murray MI, Arnold A, Younis M, Varghese S, Zeiher AM. Cryoballoon versus radiofrequency ablation for paroxysmal atrial fibrillation: a meta-analysis of randomized controlled trials. Clin Res Cardiol 2018;107:658–69. [DOI] [PubMed] [Google Scholar]
- 18. Kawamura I, Neuzil P, Shivamurthy P, Kuroki K, Lam J, Musikantow Det al. . How does the level of pulmonary venous isolation compare between pulsed field ablation and thermal energy ablation (radiofrequency, cryo, or laser)? Europace 2021;23:1757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chun KRJ, Schmidt B, Metzner A, Tilz R, Zerm T, Köster Iet al. . The ‘single big cryoballoon’ technique for acute pulmonary vein isolation in patients with paroxysmal atrial fibrillation: a prospective observational single centre study. Eur Heart J 2009;30:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chierchia GB, Di Giovanni G, Sieira-Moret J, De Asmundis C, Conte G, Rodriguez-Manẽro Met al. . Initial experience of three-minute freeze cycles using the second-generation cryoballoon ablation: acute and short-term procedural outcomes. J Int Card Electrophysiol 2014;39:145–51. [DOI] [PubMed] [Google Scholar]
- 21. Duytschaever M, De Pooter J, Demolder A, El Haddad M, Phlips T, Strisciuglio Tet al. . Long-term impact of catheter ablation on arrhythmia burden in low-risk patients with paroxysmal atrial fibrillation: the CLOSE to CURE study. Heart Rhythm 2020;17:535–43. [DOI] [PubMed] [Google Scholar]
- 22. Chieng D, Sugumar H, Ling LH, Segan L, Azzopardi S, Prabhu Set al. . Catheter ablation for persistent atrial fibrillation: a multicenter randomized trial of pulmonary vein isolation (PVI) versus PVI with posterior left atrial wall isolation (PWI)—the CAPLA study. Am Heart J 2022;243:210–20. [DOI] [PubMed] [Google Scholar]
- 23. Mohanty S, Trivedi C, Horton P, Rocca DGD, Gianni C, Macdonald Bet al. . Natural history of arrhythmia after successful isolation of pulmonary veins, left atrial posterior wall, and superior vena cava in patients with paroxysmal atrial fibrillation: a multi-center experience. J Am Heart Assoc 2021;10:e020563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Verma A, Jiang C-Y, Betts TR, Chen J, Deisenhofer I, Mantovan Ret al. . Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- 25. Nair GM, Birnie DH, Nery PB, Redpath CJ, Sarrazin J-F, Roux J-Fet al. . Standard vs augmented ablation of paroxysmal atrial fibrillation for reduction of atrial fibrillation recurrence: the AWARE randomized clinical trial. JAMA Cardiol 2023;8:475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dixit S, Marchlinski FE, Lin D, Callans DJ, Bala R, Riley MP. Randomized ablation strategies for the treatment of persistent atrial fibrillation: RASTA study. Circ Arrhythm Electrophysiol 2012;5:287–94. [DOI] [PubMed] [Google Scholar]
- 27. Metzner A, Straube F, Tilz RR, Kuniss M, Noelker G, Tebbenjohanns Jet al. . Electrophysiology lab efficiency comparison between cryoballoon and point-by-point radiofrequency ablation: a German sub-analysis of the FREEZE cohort study. BMC Cardiovasc Disord 2023;23:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Monnickendam G, de Asmundis C. Why the distribution matters: using discrete event simulation to demonstrate the impact of the distribution of procedure times on hospital operating room utilisation and average procedure cost. Oper Res Health Care 2018;16:20–8. [Google Scholar]
- 29. Kanthasamy V, Breitenstein A, Schilling R, Hofer D, Tiongco B, Ang Ret al. . Catheter ablation of atrial fibrillation with a multi-electrode radiofrequency balloon; first and early two centre experience in Europe. J Cardiovasc Electrophysiol 2023;34:1350–1359. [DOI] [PubMed] [Google Scholar]
- 30. Bordignon S, My I, Tohoku S, Rillig A, Schaack D, Chen Set al. . Efficacy and safety in patients treated with a novel radiofrequency balloon: a two centres experience from the AURORA collaboration. Europace 2023;25:euad106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Y, Wang W, Yao J, Chen L, Yi S. Second-generation cryoballoon vs. contact-force sensing radiofrequency catheter ablation in atrial fibrillation: a meta-analysis of randomized controlled trials. J Int Card Electrophysiol 2021;60:9–19. [DOI] [PubMed] [Google Scholar]
- 32. Hunter RJ, Baker V, Finlay MC, Duncan ER, Lovell MJ, Tayebjee MHet al. . Point-by-point radiofrequency ablation versus the cryoballoon or a novel combined approach: a randomized trial comparing 3 methods of pulmonary vein isolation for paroxysmal atrial fibrillation (the cryo versus RF trial). J Cardiovasc Electrophysiol 2015;26:1307–14. [DOI] [PubMed] [Google Scholar]
- 33. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L. HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tanese N, Almorad A, Pannone L, Defaye P, Jacob S, Ben KMet al. . Outcomes after cryoballoon ablation of paroxysmal atrial fibrillation with the PolarX or the Arctic front advance pro: a prospective multicentre experience. Europace 2023;25:873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heeger CH, Pott A, Sohns C, Riesinger L, Sommer P, Gasperetti Aet al. . Novel cryoballoon ablation system for pulmonary vein isolation: multicenter assessment of efficacy and safety—ANTARCTICA study. Europace 2022;24:1917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tokuda M, Yamashita S, Sato H, Oseto H, Ikewaki H, Yokoyama Met al. . Long-term course of phrenic nerve injury after cryoballoon ablation of atrial fibrillation. Sci Rep 2021;11:6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yarlagadda B, Deneke T, Turagam M, Dar T, Paleti S, Parikh Vet al. . Temporal relationships between esophageal injury type and progression in patients undergoing atrial fibrillation catheter ablation. Heart Rhythm 2019;16:204–12. [DOI] [PubMed] [Google Scholar]
- 38. Singh SM, D’Avila A, Singh SK, Stelzer P, Saad EB, Skanes Aet al. . Clinical outcomes after repair of left atrial esophageal fistulas occurring after atrial fibrillation ablation procedures. Heart Rhythm 2013;10:1591–7. [DOI] [PubMed] [Google Scholar]
- 39. Fürnkranz A, Bordignon S, Böhmig M, Konstantinou A, Dugo D, Perrotta Let al. . Reduced incidence of esophageal lesions by luminal esophageal temperature-guided second-generation cryoballoon ablation. Heart Rhythm 2015;12:268–74. [DOI] [PubMed] [Google Scholar]
- 40. Wielandts JY, Kyriakopoulou M, Almorad A, Hilfiker G, Strisciuglio T, Phlips Tet al. . Prospective randomized evaluation of high power during CLOSE-guided pulmonary vein isolation: the POWER-AF study. Circ Arrhythm Electrophysiol 2021;14:e009112. [DOI] [PubMed] [Google Scholar]
- 41. Tilz RR, Schmidt V, Pürerfellner H, Maury P, Chun KJ, Martinek Met al. . A worldwide survey on incidence, management and prognosis of oesophageal fistula formation following atrial fibrillation catheter ablation: the POTTER-AF study. Eur Heart J 2023;44:2458–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared upon reasonable request by the corresponding author.