This cohort study examines whether different vaccines are associated with multiple sclerosis flare-ups requiring hospitalization.
Key Points
Question
Is vaccination associated with the risk of severe flare-ups that require hospitalization in patients with multiple sclerosis (MS)?
Findings
Among 35 265 patients with MS who had a flare-up requiring hospitalization and received at least 1 vaccine, including the diphtheria, tetanus, poliomyelitis, pertussis, and Haemophilus influenzae (DTPPHi) vaccine, the influenza vaccine, or the pneumococcal vaccine, no association was found with severe MS flare-ups and vaccine exposure in the 60 days prior to the flare-ups.
Meaning
Findings of this study suggest that there is no association between overall exposure to the DTPPHi, influenza, and pneumococcal vaccines, and MS flare-ups requiring hospitalization; further studies are needed to confirm these results given the number of vaccine subtypes available.
Abstract
Importance
Scientific literature is sparse about the association of vaccination with the onset of multiple sclerosis (MS) flare-ups. Immunization by vaccines of the entire population is crucially important for public health.
Objective
To evaluate the risk of hospitalization for severe MS flare-ups after vaccination in patients with MS.
Design, Setting, Participants
This cohort study included patients diagnosed with MS between January 1, 2007, and December 31, 2017, who were included in the System of National Health Databases, a national health claims database in France. In a nested case-crossover analysis, cases were defined by vaccine exposure prior to the onset of hospitalization due to an MS flare-up, and flare-up rates were compared with those that occurred prior to vaccine exposure in up to 4 control time windows immediately preceding the at-risk time window (ie, the MS flare-up) for each patient. Data were analyzed from January 2022 to December 2022.
Exposure
Receipt of at least 1 vaccination, including the diphtheria, tetanus, poliomyelitis, pertussis, or Haemophilus influenzae (DTPPHi) vaccine, influenza vaccine, and pneumococcal vaccine, during follow-up.
Main Outcomes and Measures
The primary outcome was the risk of hospitalization for an MS flare-up after receipt of a vaccine. Adjusted odds ratios (AORs) and 95% CIs were derived using conditional logistic regression to measure the risk of hospitalization for an MS flare-up associated with vaccination.
Results
A total of 106 523 patients constituted the MS cohort (mean [SD] age, 43.9 [13.8] years; 76 471 females [71.8%]; 33 864 patients [31.8%] had incident MS and 72 659 patients [68.2%] had prevalent MS) and were followed up for a mean (SD) of 8.8 (3.1) years. Of these patients, 35 265 (33.1%) were hospitalized for MS flare-ups during the follow-up period for a total of 54 036 MS-related hospitalizations. The AORs of hospitalization for an MS flare-up and vaccine exposure in the 60 days prior to the flare-up were 1.00 (95% CI, 0.92-1.09) for all vaccines, 0.95 (95% CI, 0.82-1.11) for the DTPPHi, 0.98 (95% CI, 0.88-1.09) for the influenza vaccine, and 1.20 (95% CI, 0.94-1.55) for the pneumococcal vaccine.
Conclusions and Relevance
A nationwide study of the French population found no association between vaccination and the risk of hospitalization due to MS flare-ups. However, considering the number of vaccine subtypes available, further studies are needed to confirm these results.
Introduction
Multiple sclerosis (MS), the most common chronic inflammatory demyelinating disease of the central nervous system (CNS), affects over 2.8 million patients worldwide1 and is the leading cause of permanent nontraumatic neurological disability in young adults.1 It is characterized by multifocal areas of demyelination of oligodendrocytes, astroglial scarring, and, eventually, axonal degeneration, leading to a broad range of focal neurological deficits. The incidence of MS is increasing worldwide (pooled incidence of 2.1 per 100 000 person-years),2 with a higher incidence in the Northern hemisphere.1 Multiple sclerosis is a heterogeneous disease with variable clinical phenotypes and a clinical course that varies over both time and space involved throughout the CNS depending on disease activity.3,4 It is characterized by a combination of flare-ups, which can be either the onset or the recurrence of signs and symptoms and disease progression, when signs and symptoms become persistent and worsen, eventually leading to permanent disabilities.
While the underlying causes of MS remain unknown, hundreds of genetic loci have been identified, explaining about 50% of the disease’s heritability.5 Multiple sclerosis is an immune-mediated disease, and factors interacting with immunity are of particular interest. Immunomodulating agents have been reported to be associated with an improved disease course, despite also increasing the risk of infection.6,7,8,9 Infectious agents may indeed be associated with MS, and there is mounting evidence for the role of the Epstein-Barr virus.10,11,12 A history of infectious mononucleosis and positivity to anti–Epstein-Barr virus antibodies is associated with an increased risk of developing MS.13 Moreover, an association between systemic infections and the risk of MS flare-ups has been reported.11 However, the findings of these studies are subject to debate.14 Therefore, the international guidelines still recommend vaccination in patients with MS.12,15
Vaccines represent one of the greatest advances in medicine for preventing morbidity and mortality. Therefore, it is essential that patients with MS benefit from them. Vaccination is particularly suitable in this population since infections are known to increase the risk of MS flare-ups11,16 and exacerbate the severity of symptoms. However, a possible association between vaccination and the onset of MS is the source of a decade-long debate. Langer-Gould et al17 found that vaccines may accelerate the transition from subclinical to overt autoimmunity in patients with existing disease. Although the evidence remains inconclusive,18 this debate has spurred doubts and potentially detrimental vaccination hesitancy, highlighting the need for well-conducted large-scale studies to examine the association. While several studies have been conducted on the association between vaccination and MS onset, evidence on the association of vaccination with disease activity (flare-ups) and progression among patients with MS remain scarce. Thus, our aim was to evaluate the association between vaccination and the onset of severe flare-ups in an MS population using data from a health care database that includes the entire French population.
Methods
Access to the System of National Health Databases (SNDS) registry19 and the study protocol were submitted to the Ethical Review Committee of Paris-Ile de France III (Comité de Protection des Personnes Ile de France III) and approved by the French Data Protection Authority (Commission Nationale de l’Informatique et des Libertés). Because the data are anonymous, no patient informed consent is required for studies based on SNDS data. All procedures involving human participants were conducted in accordance with the Declaration of Helsinki20 and its amendments. The international regulations for personal data protection (ie, the General Data Protection Regulation) were also followed.21 This cohort study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.22
Data Source and Study Population
The SNDS23 covers more than 99% of French residents, about 65 million people, and links claims to the national hospital discharge database (which records all hospital stays along with the principal, associated, and related diagnoses) and death records.24 Diagnoses are recorded for acute and long-term hospital care as well as emergency hospital visits. In addition, patients with MS have a validated special status of affection de longue dureé (ALD, or long-term disease) with a designated MS-ALD code. The date when this code was assigned establishes the date since the onset of the disease. The MS-ALD code is specific as it is based on the medical information provided by board-certified treating physicians and is audited by the national insurance’s medical services. We obtained an SNDS extract of 133 154 patients with at least 1 MS diagnosis between January 1, 2007, and December 31, 2017, and we collected the lifetime MS-ALD codes for all of the patients included in the analysis. We included only patients who were followed up for at least 12 months and who had no periods of 24 months or more without any record.
The MS cohort included 106 523 patients from the source population of the SNDS who presented at any time during the study period with either (1) an active MS-ALD code plus at least 1 hospital discharge with an International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) code for MS (G35), (2) an active MS-ALD code and at least 2 prescription fills of an MS disease-modifying therapy in a given year (eTable 1 in Supplement 1), (3) at least 1 hospital discharge with an ICD-10 code for MS and at least 2 prescription fills of an MS disease-modifying therapy in any given year, or (4) 2 hospital discharges with an ICD-10 code for MS. Participants were considered as having incident MS when no MS-related event was reported for at least 24 months after their first record in the database and no MS-ALD code was noted in the full database; all other participants were considered part of an MS prevalence group. Events related to MS considered for incidence were hospitalization for a general or a specific MS disorder (eTable 2 in Supplement 1); any MS disease-modifying therapy (eTable 1 in Supplement 1); any visits to a neurologist, an ophthalmologist, or an outpatient clinic followed with a prescription fill of high-dose corticosteroid therapy; and any specific imaging, such as magnetic resonance imaging (MRI) of the brain or spinal cord.
The date of entry in the MS cohort was the date of the first MS-related event in patients with incident MS and January 1, 2007, for patients in the MS prevalence group (ie, the date of the first SNDS data available for this study). The onset of disease was considered to be the first MS-related event in patients with incident MS, in contrast to the date of the first recorded MS-ALD code for patients in the MS prevalence group, when available. The remaining instances were recorded as missing date of onset of disease.
Definition of Flare-Ups
In the main analysis, MS flare-ups were defined by the occurrence of a hospitalization of at least 1 day with an overnight hospital stay with either a specific MS disorder as the principal discharge diagnosis (codes listed in eTable 2 in Supplement 1) or a principal discharge diagnosis of MS (ICD-10 code G35) associated with occurrence of specific MS disorders as associated or related discharge diagnoses. Flare-ups were considered only when they occurred more than 12 months either after disease onset in the incident MS group with known disease onset (to avoid a misclassification of medical procedures related to the new MS diagnosis as flare-ups) or after entry in the cohort for patients in the prevalence group for whom the date of disease onset was not available. At least 120 days between 2 flare-ups had to elapse for the flare-ups to be considered separate events. The index date of a flare-up was shifted if 1 of the following 2 events occurred in the 30 days before the flare-up: consultation with a neurologist or ophthalmologist or performance of MRI of the brain or spinal cord.
Case-Crossover Analysis
To evaluate the association of MS flare-ups with vaccine exposure, a nested case-crossover (CCO) analysis of flare-ups requiring hospitalization was conducted within the cohort of patients with MS. The CCO design25 is an adaptation of the case-control design whereby cases are used as their own controls to study the implications of transient exposures, such as vaccination, for the risk of acute-onset events. The CCO design compares exposure in the at-risk time window immediately preceding an index event with exposure in control time windows before the at-risk window. The advantage of the CCO design is its automatic control of multiple individual confounders not considered as time sensitive.25 The CCO population comprised patients from the MS cohort with at least 12 months of follow-up after entry in the MS cohort.
The date of hospitalization due to an MS flare-up was considered the index date for the outcome analysis. If any of the events considered as prodromic occurred during the 30 days prior to the hospitalization (visit to a neurologist or ophthalmologist, MRI of the brain or spinal cord), we used the date of the event as the index date.
The at-risk time window was defined as the 60-day period preceding and including the index date of the MS flare-up, as it has been reported that an MS flare-up triggered by vaccination is more likely to occur within 2 months after vaccination.26,27 We defined up to 4 control time windows per flare-up to ensure maximum statistical power, with each window lasting 60 days. Control time windows were defined as the most recent eligible 60-day time windows immediately preceding the at-risk time window (−61 to −120 days for the first control time window up to potentially −241 to −300 days for the fourth control time window). Depending on the available time free of flare-ups, 1 to 4 control time windows were used.
In patients with multiple flare-ups, only the time between flare-ups was considered to identify at-risk and control time windows. Flare-ups with an index date occurring less than 120 days after a previous flare-up were excluded from the CCO analysis. All other flare-ups were deemed eligible for the CCO analysis.
Vaccine Exposure
Vaccination was confirmed by recorded claims for vaccines administered in a hospital or a community pharmacy in the SNDS dispensing database, either through the Anatomical Therapeutic Chemical (ATC) code or via vaccine-specific dispensing codes. All vaccines were inactivated because live vaccines as immunosuppressive therapies used in MS are contraindicated.
Vaccine exposure was subsequently classified according to the ATC level: second level (J07 for all vaccines) and fifth level for specific vaccines. The latter were grouped as:
Diphtheria, tetanus, poliomyelitis, pertussis or Haemophilus influenzae vaccines (DTPPHi), alone or in combination, including DTPPHi plus hepatitis B combination vaccine
Hepatitis B virus (HBV) vaccine, excluding DTPPHi plus HBV combination vaccine
Influenza vaccine (any)
Pneumococcal vaccine
Meningococcus vaccine
Measles, mumps, or rubella vaccines, alone or in combination
Others: hepatitis A virus, tuberculosis, varicella virus, or varicella-zoster virus vaccines
The CCO analysis considered administration of any of these vaccines as a vaccine exposure. Vaccines individually studied in the CCO analysis were those administered to at least 1% of patients with MS (ie, DTPPHi, influenza, and pneumococcus). It was assumed that vaccination occurred the day after it was dispensed.
Statistical Analysis
Descriptive statistics were used to characterize the MS cohort using mean and SD and median and range values. To describe the frequency of the onset of outcomes of interest, proportions of patients with events were used for 1-time occurrences, and rates of events per 100 person-years for repeated events were calculated.
Following the CCO design, the association between vaccination and hospitalization for MS flare-ups was evaluated by comparing vaccine exposure in the at-risk time window with that in the control time windows for each patient. Adjusted odds ratios (AORs) and 95% CIs were derived using a generalized estimating equation conditional logistic regression model, which accounted for the correlation between multiple flare-ups experienced by a patient. In each case, a model was fitted overall and for each ATC class of vaccine prescribed. We performed a clustered analysis using the patient as the cluster to control for all time-invariant factors. As patients could have experienced multiple flare-ups and, thus, multiple at-risk time windows, we also considered flare-up subclusters to allow for correlation between at-risk and control time windows. Multivariable models were used to control for time-varying covariates (ie, other vaccinations and hospitalization unrelated to MS and for the number of general practitioner and specialist visits). Vaccination and physician visits are correlated because a health care practitioner typically performs vaccination. Therefore, a linear regression of vaccination by the number of visits was performed, allowing retrieval of residuals to eliminate collinearities between those covariates.
The analysis was stratified by age (<18, 18-34, 35-69, and ≥70 years). Vaccine data were also stratified by sex. Sensitivity analyses evaluated 30-day and 90-day time windows for both the at-risk and the control windows. Statistical analyses were performed from January 2022 to December 2022 using SAS, version 7.15 (SAS Institute Inc) and statistical significance was set at α = .05.
Results
Population Description
A total of 106 523 patients (mean [SD] age, 43.9 [13.8] years; 76 471 [71.8%] females and 30 052 [28.2%] males) met at least 1 of the 4 inclusion criteria and were thus retained in the MS cohort (eFigure in Supplement 1). Table 1 presents the characteristics of the MS cohort; 33 864 patients (31.8%) had incident MS (mean [SD] age, 39.9 [13.6] years at diagnosis); the remaining 72 659 patients (68.2%) had MS at entry in the cohort (the prevalence group; mean [SD] duration of disease, 8.8 [7.0] years). The mean (SD) follow-up after the date of entry in the MS cohort was 8.8 (3.1) years.
Table 1. Characteristics of the Multiple Sclerosis (MS) Cohort and Patients Hospitalized With MS Flare-Ups.
| Characteristic | MS study population, No. (%) (n = 106 523) |
|---|---|
| Sex | |
| Female | 76 471 (71.8) |
| Male | 30 052 (28.2) |
| Age at entry in MS cohort, y | |
| Mean (SD) | 43.9 (13.8) |
| Median (range) | 43.5 (0-97.8) |
| <18 | 1297 (1.2) |
| 18-34 | 28 945 (27.2) |
| 35-69 | 72 188 (67.8) |
| ≥70 | 4093 (3.8) |
| Patients with incident MS | 33 864 (31.8) |
| Duration of follow-up after entry, mean (SD), y | 8.8 (3.1) |
| Hospitalizations, per 100 person-years | 5.78 |
| Flare-ups requiring hospitalization, per 100 person-years | 3.85 |
| Use of at least 1 MS disease-modifying therapya | 74 932 (70.3) |
| Use of at least 1 high-dose corticosteroida | 50 448 (47.4) |
Therapy and corticosteroid listed in eTable 1 in Supplement 1.
A total of 35 265 of 106 523 patients (33.1%) were hospitalized with an MS flare-up at least once during follow-up, accumulating 54 036 hospitalizations (eFigure in Supplement 1); the hospitalization rate for MS flare-ups was 5.78 per 100 person-years of follow-up overall and 3.85 per 100 person-years when only hospitalizations lasting more than 1 day were considered. Two-thirds of patients in the MS cohort (74 932 [70.3%]) used an MS disease-modifying drug at least once, and one-half (50 448 [47.4%]) were treated with high-dose corticosteroids.
Vaccination Exposure
Overall, 58 195 (54.6%) of 106 523 patients in the source population received a vaccine at any time during the 11 years of the study, including 48 232(45.3%) of the MS cohort who received at least 1 vaccine at (same day) or following entry in the MS cohort. The most frequently prescribed vaccines were DTPPHi (30.3%), influenza (19.2%), and pneumococcus (7.0%). Other vaccines were used by less than 1% of patients each. eTable 3 in Supplement 1 provides the frequencies of use of individual vaccines by age groups in the MS cohort.
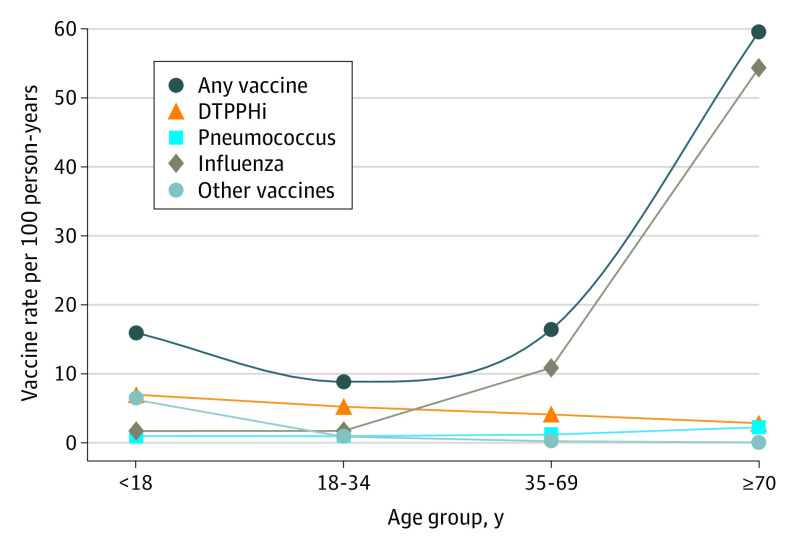
The Figure presents the rates of use of individual vaccines per 100 person-years of follow-up by age group. The DTPPHi vaccination was the most frequently dispensed vaccine among patients aged 34 years or younger; the influenza vaccine was the most frequently dispensed vaccine in other age groups. The pneumococcal vaccine was mainly used in patients aged 70 years or older.
Figure. Rate of Vaccination According to Age Group.
Other vaccines include hepatitis A virus; hepatitis B virus; meningococcus; measles, mumps, and rubella; tuberculosis; varicella virus; and varicella-zoster virus. DTPPHi indicates diphtheria, tetanus, poliomyelitis, pertussis, and Haemophilus influenzae vaccine.
CCO Analysis
The CCO analysis was conducted in patients from the MS cohort with at least 12 months of follow-up after entry into the cohort. Table 2 provides the results of the CCO analysis of 31 712 eligible cases of MS flare-ups that required hospitalization and occurred 120 days or more after a previous flare-up. Among these patients, 736 (2.3%) were vaccinated during a 60-day at-risk time window immediately preceding the index date, and 2683 of 116 901 patients (2.3%) received a vaccine during at least 1 of the 116 901 control time windows. Results are also provided for individual vaccines used by at least 1% of patients in the MS cohort (DTPPHi, influenza, and pneumococcus). The AORs are shown when there are at least 5 patients in the considered time windows and when the multivariable models converge.
Table 2. Association of Multiple Sclerosis (MS) Flare-Ups Requiring Hospitalization and Vaccines Exposure According to Age and 60-Day Time Window.
| Patients, No. (%) | Crude OR (95% CI) | Adjusted OR (95% CI)a | ||
|---|---|---|---|---|
| At-risk time windows | Control time windows | |||
| All age groups | n = 31 712 | n = 116 901 | NA | NA |
| Any vaccine | 731 (2.31) | 2683 (2.30) | 1.00 (0.93-1.09) | 1.00 (0.92-1.09) |
| Any vaccine, femalesb | 488 (2.22) | 1810 (2.23) | 1.00 (0.90-1.10) | 1.00 (0.90-1.10) |
| Any vaccine, malesc | 243/9758 (2.49) | 873/35 796 (2.44) | 1.02 (0.88-1.18) | 1.02 (0.88-1.18) |
| DTPPHi vaccined | 210 (0.66) | 798 (0.68) | 0.97 (0.83-1.13) | 0.95 (0.82-1.11) |
| Influenza vaccine | 431 (1.36) | 1634 (1.40) | 0.97 (0.87-1.08) | 0.98 (0.88-1.09) |
| Pneumococcal vaccine | 81 (0.26) | 244 (0.21) | 1.22 (0.96-1.56) | 1.20 (0.94-1.55) |
| Age group | ||||
| ≤18 y | n = 318 | n = 1081 | NA | NA |
| Any vaccine | 15 (4.72) | 53 (4.90) | 0.96 (0.59-1.57) | 0.96 (0.58-1.61) |
| 18-34 y | n = 6075 | n = 22 414 | NA | NA |
| Any vaccine | 101 (1.66) | 314 (1.40) | 1.19 (0.94-1.51) | 1.19 (0.94-1.51) |
| 35-69 y | n = 23 553 | n = 86 736 | NA | NA |
| Any vaccine | 448 (1.90) | 1760 (2.03) | 0.94 (0.84-1.04) | 0.94 (0.84-1.04) |
| ≥70 y | n = 1766 | n = 6670 | NA | NA |
| Any vaccine | 167 (9.46) | 556 (8.34) | 1.15 (0.95-1.39) | 1.15 (0.95-1.39) |
Abbreviation: DTPPHi, diphtheria, tetanus, poliomyelitis, pertussis, and Haemophilus influenzae.
Adjusted for visits with a general practitioner or specialist, occurrence of any hospitalization for reasons other than MS, and receipt of other vaccines.
Total No. of female patients for at-risk time and control time windows was 21 954 and 81 105, respectively.
Total No. of male patients for at-risk time and control time windows was 9758 and 35 796, respectively.
Any DTPPHi vaccine, including DTPPHi plus hepatitis B combination.
Table 2 shows the results of the main analysis. The AORs for any vaccine and MS flare-ups requiring hospitalization were 1.00 (95% CI, 0.92-1.09) overall, and 1.00 (95% CI, 0.90-1.10) in females and 1.02 (95% CI, 0.88-1.18) in males. The AORs for any vaccine by age group were 0.96 (95% CI, 0.58-1.61) for patients younger than 18 years, 1.19 (95% CI, 0.94-1.51) in those aged 18 to 34 years, 0.94 (95% CI, 0.84-1.04) in those aged 35 to 69 years, and 1.15 (95% CI, 0.95-1.39) in those 70 years old or older. The AOR was 0.95 (95% CI, 0.82-1.11) for the DTPPHi vaccine, 0.98 (95% CI, 0.88-1.09) for the influenza vaccine, and 1.20 (95% CI, 0.94-1.55) for the pneumococcal vaccine.
eTable 4 in Supplement 1 presents the analysis of individual vaccines stratified by age for MS flare-up hospitalizations; the results observed for the vaccines individually according to age groups do not differ from the results observed globally for any vaccine. In sensitivity analyses, using 30-day and 90-day time windows did not substantially alter the observable risk pattern except for an increase in the AOR for pneumococcal vaccine when a 90-day time window was used (AOR, 1.59; 95% CI, 1.27-1.99) (eTable 5 in Supplement 1).
Discussion
To our knowledge, the present study is the most extensive investigation conducted on the risk of the onset of MS flare-ups requiring hospitalization associated with vaccine exposure, with over 36 000 flare-ups studied in more than 100 000 patients with MS documented for up to 11 years (mean follow-up 8.8 years).
We did not observe an association between the risk of hospitalization for an MS flare-up and vaccination, considered overall or individually, regardless of the age group studied. Because health insurance in France is universal and access to care is basically unlimited, we are confident that the study identified virtually all hospitalizations occurring in all patients with MS in France over 11 years (67 million patients registered). The SNDS is a stable database where all residents of France are registered from birth or immigration to emigration or death.
Our results are consistent with those reported in the literature, which found no increased risk of MS flare-ups following vaccination.27 However, the present study had a larger sample size that better represented the entire population of patients with MS. The bulk of the literature on vaccines and the risk of MS flare-ups focuses primarily on the influenza vaccination, whereas this study examined the risk of flare-ups associated with several other vaccines.
To our knowledge, the present study is the only one to investigate the association between the pneumococcal vaccine and the risk of MS flare-ups requiring hospitalization. Information on the pneumococcal vaccine is of paramount importance as it is recommended before initiation of the biotherapies used in MS.12 Considering the number of vaccine subtypes available, further studies are needed to confirm these observed results.
Strengths and Limitations
One strength of the CCO design is that it de facto cancels multiple individual confounders since patients are used as their own controls. Nonetheless, time-varying confounding cannot be ruled out. Minimizing such residual confounding was done in 2 ways: by using visits to physicians and procedures as proxies for changes in health status and by the statistical treatment of the residuals.
Because the study was based on virtually all the hospitalizations for MS flare-ups of patients with MS within an entire country of 67 million inhabitants over 11 years, it has high statistical power to support the conclusions. Indeed, detectable odds ratios with 80% power and α = .05 were 1.12 for exposure to any vaccine, 1.15 for influenza, 1.22 for DTPPHi, and 1.41 for pneumococcal vaccines. Nevertheless, the study cannot completely rule out the existence of a small risk, particularly in the case of the pneumococcal vaccine. Study power was lower for examining MS flare-ups with the human papillomavirus, hepatitis, and measles vaccines, which were used by few patients 18 years or older.
A limitation of the SNDS database is that the clinical data include only hospital diagnosis and drugs dispensed. We are confident that patients retained in the cohort had MS because of the multiple sources of information available over the 11-year study period. The definition of MS flare-up was limited to those flare-ups that led to hospitalizations and were linked to a specific MS diagnosis; however, it is possible that some hospitalizations for MS flare-ups may have been missed if they were not coded with a specific MS diagnosis. Also, if vaccines were associated with milder flare-ups not requiring hospitalization, the present study would not be able to capture those events.
One cannot rule out that some hospitalizations considered in the study were not actual MS flare-ups but possibly other causes or consequences of chronic disease management. Yet they would constitute a minority of cases considering the careful exclusion of any hospitalization without a specific MS diagnosis or with only a procedure code.
Conclusions
No association between overall vaccine exposure and MS flare-ups requiring hospitalization was observed in this large national study. However, given the number of vaccine subtypes available, further studies are needed to confirm these results.
eFigure. Flowchart of the Studied Multiple Sclerosis Cohort from the SNDS
eTable 1. List of SNDS Codes to Capture the Dispensing of a Multiple Sclerosis Disease-Modifying Treatment and High-Dose Corticosteroids in a Given Year
eTable 2. Specific Multiple Sclerosis Diagnosis Codes in the SNDS French Health Care Database
eTable 3. Use of Vaccines in the Multiple Sclerosis Cohort According to Age Group
eTable 4. Association of Multiple Sclerosis Flare-Ups Requiring Hospitalization and Vaccines Exposure According to Age and Type of Vaccine (60-Day Time Window)
eTable 5. Sensitivity Analysis of the Association of Multiple Sclerosis Flare-Ups Requiring Hospitalization and Vaccines Exposure According to 2 Different Time Windows (30-Day Time Window and 90-Day Time Window)
Data Sharing Statement
References
- 1.MS International Federation. Atlas of MS, 3rd ed. Published online 2020. Accessed January 26, 2023. http://www.atlasofms.org
- 2.Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler. 2020;26(14):1816-1821. doi: 10.1177/1352458520970841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breij ECW, Brink BP, Veerhuis R, et al. Homogeneity of active demyelinating lesions in established multiple sclerosis. Ann Neurol. 2008;63(1):16-25. doi: 10.1002/ana.21311 [DOI] [PubMed] [Google Scholar]
- 4.Fiore A, Preziosa P, Tedone N, et al. Correspondence among gray matter atrophy and atlas-based neurotransmitter maps is clinically relevant in multiple sclerosis. Mol Psychiatry. 2023;28(4):1770-1782. doi: 10.1038/s41380-023-01943-1 [DOI] [PubMed] [Google Scholar]
- 5.Patsopoulos NA. Genetics of multiple sclerosis: an overview and new directions. Cold Spring Harb Perspect Med. 2018;8(7):a028951. doi: 10.1101/cshperspect.a028951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tur C, Dubessy AL, Otero-Romero S, et al. The risk of infections for multiple sclerosis and neuromyelitis optica spectrum disorder disease-modifying treatments: Eighth European Committee for Treatment and Research in Multiple Sclerosis Focused Workshop Review. Mult Scler J. 2022;28:1424-1456. doi: 10.1177/13524585211069068 [DOI] [PubMed] [Google Scholar]
- 7.Schweitzer F, Laurent S, Fink GR, et al. Age and the risks of high-efficacy disease modifying drugs in multiple sclerosis. Curr Opin Neurol. 2019;32:305-312. doi: 10.1097/WCO.0000000000000701 [DOI] [PubMed] [Google Scholar]
- 8.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83:278-286. doi: 10.1212/WNL.0000000000000560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Signori A, Schiavetti I, Gallo F, Sormani MP. Subgroups of multiple sclerosis patients with larger treatment benefits: a meta-analysis of randomized trials. Eur J Neurol. 2015;22:960-966. doi: 10.1111/ene.12690 [DOI] [PubMed] [Google Scholar]
- 10.Williamson EML, Chahin S, Berger JR. Vaccines in multiple sclerosis. Curr Neurol Neurosci Rep. 2016;16(4):36. doi: 10.1007/s11910-016-0637-6 [DOI] [PubMed] [Google Scholar]
- 11.Correale J, Fiol M, Gilmore W. The risk of relapses in multiple sclerosis during systemic infections. Neurology. 2006;67(4):652-659. doi: 10.1212/01.wnl.0000233834.09743.3b [DOI] [PubMed] [Google Scholar]
- 12.Lebrun C, Vukusic S; French Group for Recommendations in Multiple Sclerosis (France4MS) and the Société Francophone de la Sclérose En Plaques (SFSEP) . Immunization and multiple sclerosis: recommendations from the French multiple sclerosis society. Mult Scler Relat Disord. 2019;31:173-188. doi: 10.1016/j.msard.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 13.Kuri A, Jacobs BM, Vickaryous N, et al. Epidemiology of Epstein-Barr virus infection and infectious mononucleosis in the United Kingdom. BMC Public Health. 2020;20(1):912. doi: 10.1186/s12889-020-09049-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi: 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 15.Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;93(13):584-594. doi: 10.1212/WNL.0000000000008157 [DOI] [PubMed] [Google Scholar]
- 16.Farez MF, Correale J. Immunizations and risk of multiple sclerosis: systematic review and meta-analysis. J Neurol. 2011;258(7):1197-1206. doi: 10.1007/s00415-011-5984-2 [DOI] [PubMed] [Google Scholar]
- 17.Langer-Gould A, Qian L, Tartof SY, et al. Vaccines and the risk of multiple sclerosis and other central nervous system demyelinating diseases. JAMA Neurol. 2014;71(12):1506-1513. doi: 10.1001/jamaneurol.2014.2633 [DOI] [PubMed] [Google Scholar]
- 18.Frederiksen JL, Topsøe Mailand M. Vaccines and multiple sclerosis. Acta Neurol Scand. 2017;136(suppl 201):49-51. doi: 10.1111/ane.12837 [DOI] [PubMed] [Google Scholar]
- 19.Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: from the système national d’information interrégimes de l’Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65(suppl 4):S149-S167. doi: 10.1016/j.respe.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 20.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 21.The European Parliament and the Council of the European Union . General Data Protection Regulation. Accessed July 24, 2023. https://gdpr-info.eu
- 22.STROBE: Strengthening the Reporting of Observational studies in Epidemiology. Accessed February 10, 2023. https://www.strobe-statement.org/
- 23.Système National des Données de Santé. Accessed January 27, 2023. https://www.snds.gouv.fr/SNDS/Accueil
- 24.Bezin J, Duong M, Lassalle R, et al. The national healthcare system claims databases in France, SNIIRAM and EGB: powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2017;26(8):954-962. doi: 10.1002/pds.4233 [DOI] [PubMed] [Google Scholar]
- 25.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133(2):144-153. doi: 10.1093/oxfordjournals.aje.a115853 [DOI] [PubMed] [Google Scholar]
- 26.Confavreux C, Suissa S, Saddier P, Bourdès V, Vukusic S; Vaccines in Multiple Sclerosis Study Group . Vaccinations and the risk of relapse in multiple sclerosis. N Engl J Med. 2001;344(5):319-326. doi: 10.1056/NEJM200102013440501 [DOI] [PubMed] [Google Scholar]
- 27.Mailand MT, Frederiksen JL. Vaccines and multiple sclerosis: a systematic review. J Neurol. 2017;264(6):1035-1050. doi: 10.1007/s00415-016-8263-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Flowchart of the Studied Multiple Sclerosis Cohort from the SNDS
eTable 1. List of SNDS Codes to Capture the Dispensing of a Multiple Sclerosis Disease-Modifying Treatment and High-Dose Corticosteroids in a Given Year
eTable 2. Specific Multiple Sclerosis Diagnosis Codes in the SNDS French Health Care Database
eTable 3. Use of Vaccines in the Multiple Sclerosis Cohort According to Age Group
eTable 4. Association of Multiple Sclerosis Flare-Ups Requiring Hospitalization and Vaccines Exposure According to Age and Type of Vaccine (60-Day Time Window)
eTable 5. Sensitivity Analysis of the Association of Multiple Sclerosis Flare-Ups Requiring Hospitalization and Vaccines Exposure According to 2 Different Time Windows (30-Day Time Window and 90-Day Time Window)
Data Sharing Statement