Key Points
Question
Can personalized temperature norms be defined to improve the clinical utility of oral temperature measurements?
Findings
In this cross-sectional study, machine learning was applied to 618 306 adult outpatient encounters to define the usual or mean “normal” temperature as 36.64 °C. Using individual and temporal characteristics, the range of mean temperatures for the coolest to the warmest individuals was 36.24 °C to 36.89 °C.
Meaning
These findings suggest that age, sex, height, weight, and time of day are factors that contribute to variations in individualized normal temperature ranges.
This cross-sectional study uses biometric and demographic characteristics of patients to identify factors associated with oral temperature ranges within individuals and thereby generate personalized temperature ranges to assist in clinical decision-making in a population of adults receiving outpatient care.
Abstract
Importance
Although oral temperature is commonly assessed in medical examinations, the range of usual or “normal” temperature is poorly defined.
Objective
To determine normal oral temperature ranges by age, sex, height, weight, and time of day.
Design, Setting, and Participants
This cross-sectional study used clinical visit information from the divisions of Internal Medicine and Family Medicine in a single large medical care system. All adult outpatient encounters that included temperature measurements from April 28, 2008, through June 4, 2017, were eligible for inclusion. The LIMIT (Laboratory Information Mining for Individualized Thresholds) filtering algorithm was applied to iteratively remove encounters with primary diagnoses overrepresented in the tails of the temperature distribution, leaving only those diagnoses unrelated to temperature. Mixed-effects modeling was applied to the remaining temperature measurements to identify independent factors associated with normal oral temperature and to generate individualized normal temperature ranges. Data were analyzed from July 5, 2017, to June 23, 2023.
Exposures
Primary diagnoses and medications, age, sex, height, weight, time of day, and month, abstracted from each outpatient encounter.
Main Outcomes and Measures
Normal temperature ranges by age, sex, height, weight, and time of day.
Results
Of 618 306 patient encounters, 35.92% were removed by LIMIT because they included diagnoses or medications that fell disproportionately in the tails of the temperature distribution. The encounters removed due to overrepresentation in the upper tail were primarily linked to infectious diseases (76.81% of all removed encounters); type 2 diabetes was the only diagnosis removed for overrepresentation in the lower tail (15.71% of all removed encounters). The 396 195 encounters included in the analysis set consisted of 126 705 patients (57.35% women; mean [SD] age, 52.7 [15.9] years). Prior to running LIMIT, the mean (SD) overall oral temperature was 36.71 °C (0.43 °C); following LIMIT, the mean (SD) temperature was 36.64 °C (0.35 °C). Using mixed-effects modeling, age, sex, height, weight, and time of day accounted for 6.86% (overall) and up to 25.52% (per patient) of the observed variability in temperature. Mean normal oral temperature did not reach 37 °C for any subgroup; the upper 99th percentile ranged from 36.81 °C (a tall man with underweight aged 80 years at 8:00 am) to 37.88 °C (a short woman with obesity aged 20 years at 2:00 pm).
Conclusions and Relevance
The findings of this cross-sectional study suggest that normal oral temperature varies in an expected manner based on sex, age, height, weight, and time of day, allowing individualized normal temperature ranges to be established. The clinical significance of a value outside of the usual range is an area for future study.
Introduction
The usual or “normal” oral temperature of 37 °C (to convert to degrees Fahrenheit, multiply by 1.8 and add 32) was established in 1851, based on the mean of reportedly over 1 million individual observations meticulously collected from 25 000 patients in Germany.1,2 This canonical value is a classic example of the use of a population average to define an individual norm; within the standard population, however, individuals differ regarding their temperature. Research has shown that oral temperature, a proxy for core temperature, varies significantly and systematically with age, sex, body composition and metabolism, menstrual cycle, menopausal status, time of day, month, and season, among others.1,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17 Cutoff values for fever, currently used in clinical decision-making, are also likely to differ between individuals when demographic and other characteristics are taken into consideration.4,18,19
Historically, the greatest obstacle to determining standards of normal temperature has been the lack of large data sets of temperature measurements, a problem largely resolved by the now standard use of electronic medical records.20 Within electronic medical records, however, comparable measurements must be identified. Measurements should be obtained with similar, calibrated thermometers from the same body site; axillary measurements, for example, are approximately 0.5 °C lower than oral measurements.21 Finally, defining the underlying health status of the individual is critical, as elevated or low temperatures may be due to illness and, until recently, the filtering out of such patients required expert opinion.
LIMIT (Laboratory Information Mining for Individualized Thresholds) is an unsupervised machine learning algorithm that filters data sets, removing extreme values of a clinical or laboratory measurement in an iterative fashion to generate a representative “normal” reference distribution.20 Using temperature values from all adult outpatient encounters at Stanford Health Care, Stanford, California, over a decade, we applied LIMIT to define normal oral temperature in this population. Biometric and demographic characteristics of patients were then examined to identify factors associated with oral temperature ranges within individuals. We sought to create a model for individual normal oral temperature, with the goal of generating personalized temperature ranges to assist in clinical decision-making.
Methods
This cross-sectional study was approved by the Stanford University Institutional Review Board, which also provided a waiver of informed consent as all regulatory criteria at 45 CFR 46.116.(f)(3) were met. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Population
The Stanford Research Repository22 contains electronic medical record information, including clinical encounters, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) diagnostic codes, laboratory test results, and pharmacy orders, from over 4 million pediatric and adult patients seen at Stanford Health Care. For this study, we identified all adult outpatient encounters that included temperature measurements from April 28, 2008, through June 4, 2017, in the divisions of Internal Medicine and Family Medicine.
Temperature measurements were obtained orally using annually calibrated digital thermometers (SureSigns Vs4; Philips) in predictive mode, which extrapolates a 3-minute measurement from a more rapid one. According to the manufacturer, this predicted measurement has an accuracy of 0.2 °F (0.11 °C) compared with the 3-minute measurement using the same instrument. Temperature, date and time of the temperature measurement, age, sex, weight, height, body mass index (calculated as weight in kilograms divided by height in meters squared), primary reason for the visit, prescribed medications, and all ICD-10 codes were identified from each encounter. An individual patient could have multiple encounters.
Temperature measurements were calculated in degrees Celsius. For encounters where either height or weight values were missing, values from their nearest encounter were used; the encounter was excluded if neither value was ever obtained. Additionally, highly skewed values were excluded based on clinical assessment, including body temperatures less than 34 °C or greater than 40 °C, body mass index less than 10 or greater than 50, height less than 1.37 m or greater than 2.13 m (to convert to feet, divide by 0.3), and weight less than 27.22 kg or greater than 181.44 kg (to convert to pounds, divide by 0.45). Age was limited to 20 through 80 years. We did not evaluate race or ethnicity data because these variables were not routinely included in patient records. Finally, encounters missing any key analysis variable (date or time, temperature, sex, age, height, weight, or primary diagnosis) were excluded.
LIMIT Filtering
The LIMIT algorithm identifies the normal range for a physiologic or laboratory measurement by iteratively removing from the data set patients with diagnoses that are associated with extreme or outlier values.20 Identification of outlier values uses the Hampel method. In brief, patients are split into 2 groups, those with and those without outlier results. A Fisher exact test is then used to find all the ICD-10 codes that are overrepresented in the outlier group. If the most overrepresented ICD-10 code is significantly (eg, P ≤ .05) linked to the outlier group, all patients associated with this ICD-10 code are removed from the data set. The process is repeated iteratively until no further ICD-10 codes are statistically linked to outlier values. The remaining results are then used to create a reference interval by taking the percentiles defining the central 95% of the results. Because there is no oversight in the selection of diagnoses or medications, LIMIT can identify ICD-10 codes associated with outliers that an expert reviewer might not expect.
In applying LIMIT to oral temperature, we used encounters instead of patients as our unit of analysis and considered medications in addition to ICD-10 codes. We specified a standard value of 3 for the t parameter and 2-sided P ≤ .05 to indicate statistical significance and included the Benjamini-Hochberg multiple hypothesis-testing correction. The ICD-10 codes or medications that occurred less than 10 times in the sample were not considered for removal; if a tie occurred, both ICD-10 codes or both medications were removed. We modified the original algorithm by collapsing each ICD-10 code to the base 3 digits that define the disease category, omitting subsequent digits that specify such details as disease etiology, severity, and anatomical site (eTable 1 in Supplement 1). We additionally excluded the parameter “days since diagnosis,” as all temperature measurements were collected on the date of diagnosis. The LIMIT algorithm was run on standard R, version 3.6.2 and greater (R Project for Statistical Computing).23 We identified the final data set of remaining reference range measurements as the analysis set and that of excluded measurements as the outlier set.
Statistical Analysis
Data were analyzed from July 5, 2017, to June 23, 2023. With the LIMIT-generated analysis set, we explored the association between normal body temperature and sex, age, height, weight, and both time of day and month of the temperature measurement. Because of the known difference in normal body temperature between women and men, the analyses were stratified by sex. After a first round of linear regression modeling to explore the data, we performed descriptive mixed-effects modeling with random effects to account for the repeated temperature values from patients having more than 1 encounter. Higher-order effects were evaluated and 95% CIs were estimated using residuals from each model.
We sought to produce a predictive model to generate expected temperature ranges. We split the data into training and testing sets (10% and 90% of patients, respectively), and computed both the overall mean squared error and that per individual patient using a second mixed-effects model. To minimize the effects of individuals with very high mean squared error, we additionally constructed a third subset that removed the top 10% of people with the most extreme errors.24 We compared a semiparametric generalized additive model (GAM) with fixed-knot splines to represent various effects (eg, age, time of day) to the mixed-effects model. Finally, we used a quantile regression program to estimate conditional quantiles from the GAM, which was the basis for an interactive plotting tool.
Because of the very large data sets, statistical significance was expected for almost all comparisons. All analyses including LIMIT used either R or SAS software, version 9.4 (SAS Institute Inc) (eMethods in Supplement 1). The interactive visualization used Javascript and the plotly open-source graphing library.
Results
From 724 199 patient encounters in the Stanford Research Repository occurring between April 28, 2008, and June 4, 2017, a total of 618 306 had complete data, met inclusion criteria, and were considered as our baseline pre-LIMIT data set. The primary reasons for exclusion in this initial round consisted of weight above 181.44 kg, being younger than 20 years, or being older than 80 years (eTable 2 in Supplement 1). After applying the LIMIT algorithm to this baseline sample, 48 diagnoses associated with outlier temperature values were excluded, constituting 35.92% of all original encounters (eTable 1 in Supplement 1). The most common outlier diagnosis was type 2 diabetes, which was associated with 15.71% of all dropped encounters. Of all encounters with type 2 diabetes in our study, 968 (1.69%) had temperatures in the lower tail (26.26% of all low outlier temperature values) and 395 (0.69%) had temperatures in the upper tail (2.87% of all high outlier temperature values). Of the remaining outlier diagnoses, most were related to infectious diseases either directly or indirectly through the use of antimicrobial or antipyretic treatments (76.81% of all dropped encounters and 92.21% of all high outlier temperature values). The 396 195 encounters included in the analysis set consisted of 126 705 individual patients, of whom 72 670 (57.35%) were women and 54 035 (42.65%) were men; the mean (SD) age was 52.7 (15.9) years.
The mean (SD) temperature per encounter was 0.07 °C higher in the outlier set compared with the analysis set, with higher variance (outlier set: 36.71 °C [0.43 °C]; analysis set: 36.64 °C [0.35 °C]; P < .001) (Table and Figure 1). The 396 196 encounters from 126 705 individuals that remained after the LIMIT exclusions had diagnoses unrelated to temperature (eTable 3 in Supplement 1). This analysis set yielded a mean normal oral temperature of 36.64 °C , with the central 95% of these temperatures between 35.95 °C and 37.33 °C (Table and Figure 1).
Table. Characteristics of the Analysis and the Outlier (Excluded) Sets as Generated by LIMIT and the Overall Samplea.
| Characteristic | Patient group | ||
|---|---|---|---|
| Analysis | Outlier | Overall | |
| Temperature, mean (95% CI), °Cb | 36.64 (35.95-37.33) | 36.71 (35.87-37.55) | 36.66 (35.92-37.41) |
| Among men only | 36.59 (35.90-37.28) | 36.67 (35.81-37.53) | 36.62 (35.86-37.38) |
| Among women only | 36.67 (36.00-37.34) | 36.74 (35.92-37.56) | 36.69 (35.97-37.42) |
| No. of encounters | 396 196 | 222 110 | 618 306 |
| No. of patientsc | 126 705 | 86 744 | 151 977 |
| Sex, No. (%) | |||
| Men | 54 035 (42.65) | 34 569 (39.85) | 65 117 (48.85) |
| Women | 72 670 (57.35) | 52 175 (60.15) | 86 860 (57.15) |
| Visits per person, median (IQR) [range]d | 2.0 (1-4) [1-87] | 2.0 (1-3) [1-54] | 2.0 (1-5) [1-111] |
| Age, mean (SD), yb | 52.7 (15.9) | 54.2 (15.6) | 53.2 (15.8) |
| Height, mean (SD), mb,e | 1.68 (0.10) | 1.67 (0.10) | 1.68 (0.01) |
| Weight, median (IQR) [range], kgb,d,e | 75.3 (63.1-88.9) [30.4-181.1] | 77.4 (64.8-92.3) [30.4-181.4] | 76.1 (63.6-90.3) [30.4-181.4] |
| Encounters by department, No. (%) | |||
| Family medicine | 223 194 (56.33) | 115 790 (52.13) | 338 984 (54.82) |
| Internal medicine | 173 002 (43.67) | 106 320 (47.87) | 279 322 (45.18) |
| Time of day, median (IQR) [range], 24-h clockb,d | 11 (9-14) [0-20] | 11 (10-14) [0-23] | 11 (10-14) [0-23] |
Abbreviation: LIMIT, Laboratory Information Mining for Individualized Thresholds algorithm.
Indicates raw data (without adjustment). The distributions of all variables were statistically significantly different between analysis and outlier sets (P < .001 for all).
Indicates per encounter.
The same individual can have encounters in both the analysis and the outlier sets.
Indicated for those variables not demonstrating a normal distribution.
A total of 16 757 (4.2%) encounters had neither weight nor height values; these were approximated by the nearest other encounter if available (eAppendix in Supplement 1). Note that among adults, changes in height between encounters are likely minimal whereas weight can vary significantly. A total of 130 199 (32.9%) and 3412 (0.9%) values were missing for height only or weight only, respectively; these were calculated based on body mass index.
Figure 1. Distribution of Temperature in Both the Analysis and the Outlier Sets.
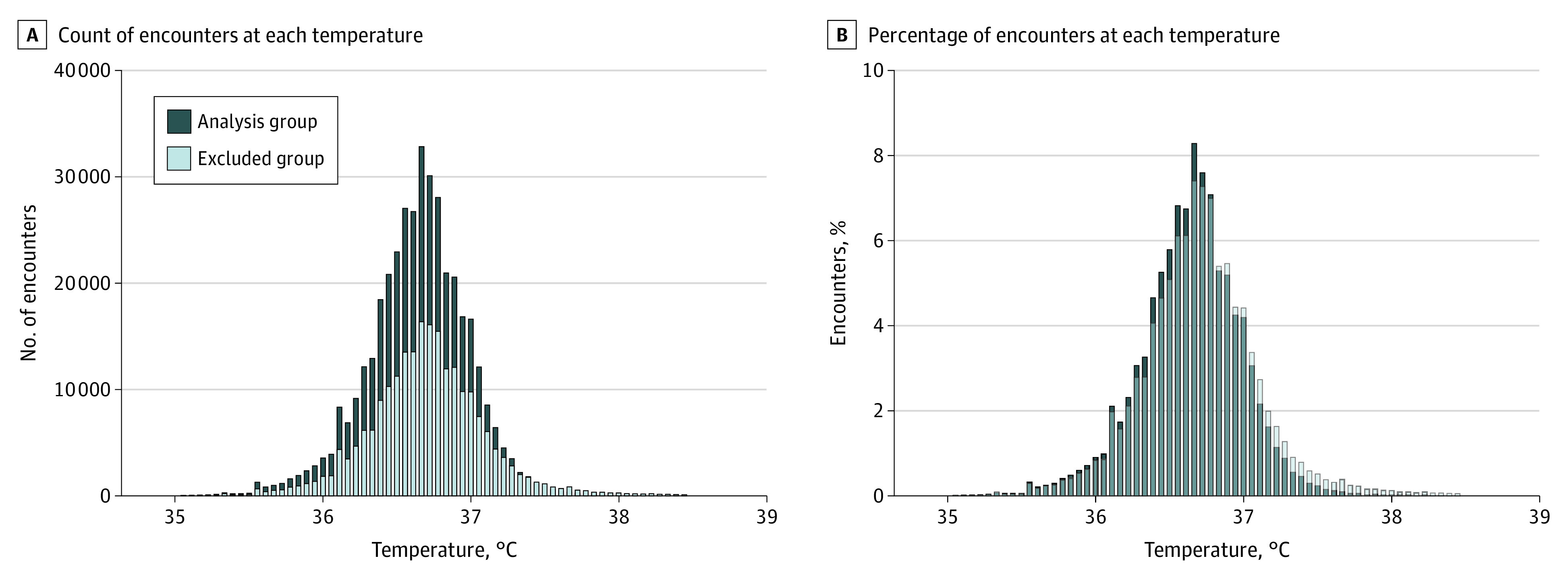
The LIMIT (Laboratory Information Mining for Individualized Thresholds) algorithm determined the analysis and outlier (excluded) groups. To convert temperature to degrees Fahrenheit, multiply by 1.8 and add 32.
Both linear regression and linear mixed-effects modeling separately in men and women identified hour of the day, age, height, weight, and month as independent predictive factors associated with temperature (eTable 4 in Supplement 1). The effect of repeated measures on model SEs was minimal. Visualizing the raw data, men systematically had lower temperatures than women (Figure 2). Temperature was associated with time of day, peaking at approximately 4:00 pm and then decreasing rapidly, for both men and women (Figure 2A). Temperature decreased with age, more consistently in men than women, who experienced a drop in temperature around 45 to 55 years of age (Figure 2B). In men, temperature decreased very slightly with height and increased with weight (Figure 2C and D). (Values were not available for height and weight in 37.09% and 5.09% of encounters, respectively; our findings were not affected by these nonrandomly missing data [eAppendix in Supplement 1].)
Figure 2. Temperature in Men and Women, by Time of Day and Demographic Characteristics.
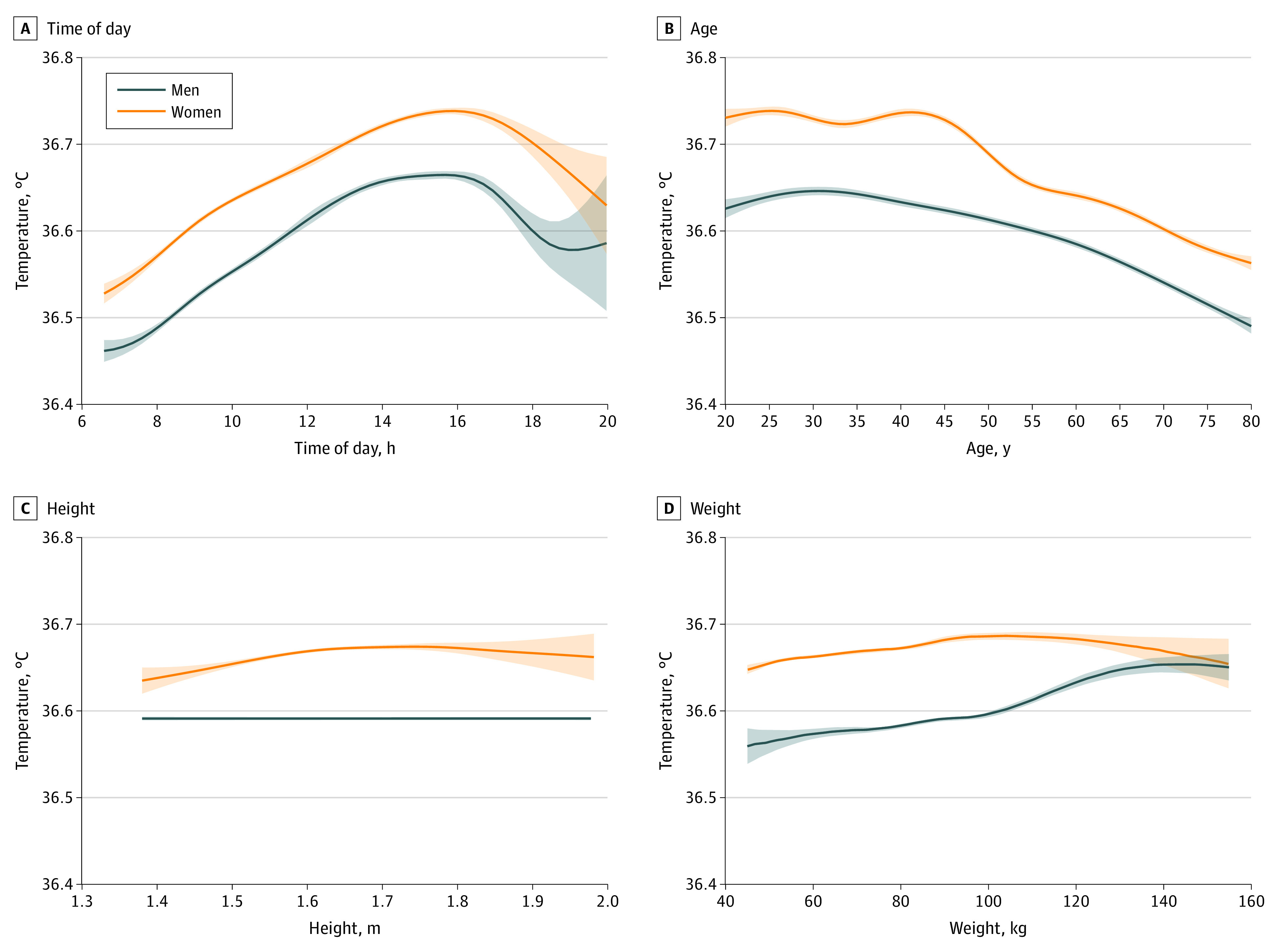
Time of day is measured using a 24-hour clock. Shading marks the 95% CI. Each graph uses unadjusted (raw) data plotted with smoothing. To convert temperature to degrees Fahrenheit, multiply by 1.8 and add 32.
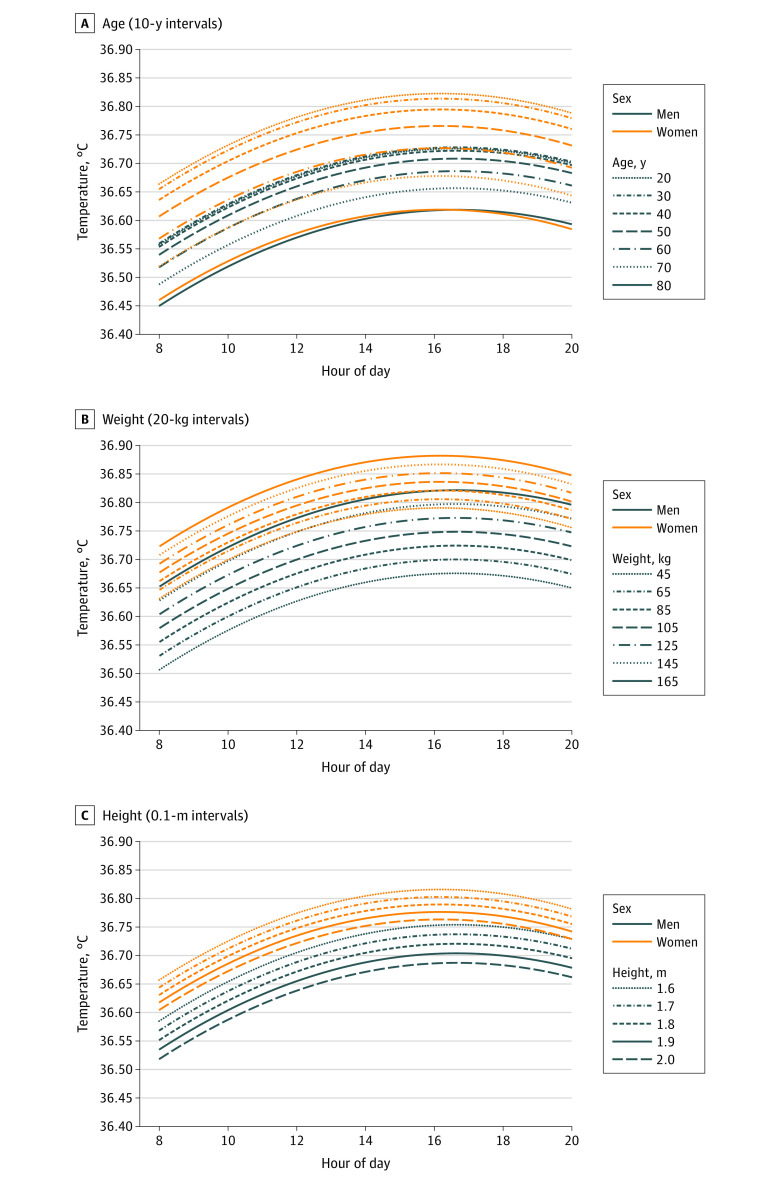
With descriptive mixed-effects modeling adjusting for the effect of demographic characteristics (sex, age, weight, and height) on temperature throughout the day (Figure 3 and eTable 4 in Supplement 1), temperatures decreased linearly by age in both men and women (Figure 3A). Women younger than 50 years had higher oral temperatures than men of any age, with the oldest men aged 70 to 80 years having the lowest temperature. Increasing weight was associated with a linear increase in oral temperature for both sexes (0.001 °C/kg among men and 0.0008 °C/kg among women; P < .001 for both) (Figure 3B). On the other hand, increasing height was associated with decreasing oral temperature in both sexes (−0.17 °C/m for men, −0.13 °C/m for women; P < .001 for both) (Figure 3C), with the visual difference to the raw data in Figure 2C suggesting considerable confounding of height by the other factors in men.
Figure 3. Temperature Throughout the Day, in Men and in Women, by Demographic Characteristics.
Each graph uses output from mixed-effects modeling using age, sex, time of day (on a 24-hour clock), height, and weight. For these specific graphs, temperatures are for an average US person weighing 88.8 kg if male or 76.4 kg if female (A and C), aged 30 years (B and C), and with height 1.75 m if male or 1.62 m if female (A and B). To convert temperature to degrees Fahrenheit, multiply by 1.8 and add 32.
Both the GAM and the predictive mixed-effects model had similar β coefficients and SEs; we used the GAM for our final predictive model. Even for the coolest and warmest demographic characteristic combinations available in our data set (a tall man with underweight aged 80 years at 8:00 am to a short woman with obesity aged 20 years at 2:00 pm, respectively), the 50th percentile for oral temperature remained below 37 °C (36.24 °C to 36.89 °C) even at the warmest time of day (approximately 3:00 pm). The upper 99th percentile value for daily temperature at this time ranged between 36.81 °C and 37.88 °C for these same “extreme” individuals, respectively. (An interactive visualization of individualized temperature is available online25 and an example is shown in eFigure in Supplement 1.)
The GAM had an overall R2 value of 0.0686 (6.68%) and an R2 value by patient of 0.1579 (15.79%). After excluding the top 10% of patients with the largest SEs, the by-patient R2 value improved considerably (overall R2 value: 0.0737 [7.37%]; R2 value by patient: 0.2552 [25.52%]).
Discussion
We used information from 618 306 outpatient encounters over almost a decade from electronic medical records at a single large hospital system and identified a mean usual or “normal” oral temperature of 36.64 °C, with 95% of these normal temperatures ranging from 35.96 °C to 37.32 °C. These findings—which are based on, to our knowledge, the largest outpatient sample examined to date—are in line with numerous studies demonstrating decreased mean normal body temperature among patients in both outpatient and hospitalized encounters (range, 35.4-36.8 °C),1,5,6,7,12,14,15 suggesting that the canonical normal temperature value of 37 °C needs to be retired.
Considering men and women separately, we identified ranges of normal temperature across the day based on factors including age, weight, and height. These findings support an extensive body of literature dating back to Wunderlich and Sequin2 showing that the chief determinants of normal oral temperature are time of day, age, and sex. Oral temperature rises throughout the day and peaks in the late afternoon, is higher in women than in men, and declines with age. Oral temperature is also directly associated with weight (more notably in men) and indirectly with height, though the impact of these variables is less pronounced. The differential effect of weight on temperature between men and women is most likely attributable to higher muscle mass in men26; muscle is highly metabolically active,27 and metabolic rate, the largest component of a typical person’s energy expenditure,28 has a strong association with body temperature.29 Indeed, for a tall man with underweight aged 80 years, mean normal temperature ranged from 36.24 °C at 7:00 am to 36.42 °C at 3:00 pm, with associated upper 99th percentile values of 36.81 °C and 37.09 °C, respectively. Given this case, a temperature greater than 37.0 °C in an older man who feels unwell might require additional workup for causes of temperature elevation, such as infection or malignant neoplasm.
Although this report serves to define normal oral temperature, it does not determine what might be considered abnormal, or fever. On the assumption that clinical decisions are made based on the presence of a fever, then individualized definitions of fever may ultimately be required.4
In addition to revising definitions of fever, studies are needed to determine whether personalized measures of body temperature predict adverse outcomes. In 1 study of 20 718 patients followed up for a year, a 0.149 °C increase in baseline temperature was associated with an 8.4% higher mortality.12 While low metabolic rate is associated with improved aging and increased longevity,30 it remains to be determined whether very low normal temperatures, once adjusted for known predictive factors, also place some individuals at increased risk of morbidity and mortality.
Most of the clinical factors excluded by our filtering algorithm were associated with elevated temperature; these clinical factors included infectious diseases and antibiotics. Coding errors that misclassified excluded factors and instead included them would artificially increase the overall mean normal temperature. Surprisingly, type 2 diabetes was the only excluded diagnosis associated with decreased temperature; of the 51 179 encounters with type 2 diabetes in our study, 1.69% had temperatures in the lower tail whereas 0.69% had temperatures in the upper tail, and the overall mean temperature for the excluded encounters with type 2 diabetes was lower than the mean temperature of all included diagnoses. Although evidence suggests that resting metabolic rate is altered in patients with type 2 diabetes,31 the direction of this change (increased or decreased) is not clearly established, due possibly to confounding by age and weight, among others. For determining “normal” oral temperature in this study, it seemed most prudent to omit type 2 diabetes as performed by the LIMIT algorithm.
Limitations
This study has some limitations. Overall, we found that characteristics known to be associated with body temperature accounted for only a minority—at best 26% from our predictive model—of the observed variability in normal temperature within individuals. Weight is highly variable due to many factors, such as inaccuracy in scales, hydration status of the patient, and stage of menstrual cycle; we expect that adding these features would account for additional variance in our model. Numerous factors not included in the electronic medical record contribute to variation in personal temperature. Although LIMIT removed the effect of some clinical factors documented in the medical record (diagnoses and medications), other unassessed factors not related to health care likely play a significant role (type of clothing worn, hot or cold beverage or food consumption, smoking, light exposure, activity prior to measurement, menstrual cycle, and waking time, among others). Even location of thermometer placement can have a large effect: for example, temperature in the posterior sublingual pocket has been shown to be 0.17 °C (0.3 °F) higher than that measured under the tongue.32
Other limitations to the generalizability of our personalized normal body temperature ranges include the lack of information on race and Hispanic ethnicity, which are not routinely provided in the electronic medical record. Black women have been found to have higher normal temperatures than White women12; historical data suggest the same may be true for Black and White men.13 For women with ovulatory menstrual cycles, core temperature will increase 0.3 °C to 0.7 °C during the luteal compared with the follicular phase33; information on menopausal status or position in the menstrual cycle, however, is not a routine component of the electronic medical record. The geographic catchment area of our study population was very narrow in terms of ambient air temperature and dew point variation; other areas of the US are subject to considerably larger swings in both, which likely affect personalized body temperature ranges. Finally, by design, our analysis population included only ambulatory primary care patients aged 20 to 80 years whose diagnoses were not associated with alterations in temperature; our normal temperature ranges are not generalizable to children, adults younger than 20 years or older than 80 years, or hospitalized people.
Conclusions
Recent analyses have shown that human body temperature has decreased over historical time by approximately 0.03 °C per decade, in both the long term (over almost 200 years in the US13) and the shorter term (over 16 years in Bolivia8). Given that body temperature is a rough marker of metabolic rate, it is reasonable to conclude that metabolic rate has decreased over time. Reasons for such a decrease in metabolic rate remain speculative but likely include a reduction in inflammation, due in part to reduced chronic infections, improved dental care, and access to anti-inflammatory medications such as statins and nonsteroidal anti-inflammatory drugs. Over the same interval, however, the definition of fever has not been well scrutinized. The mining of electronic medical record–based temperature measurements, as well as personal monitoring with wearable technologies, may serve to help us understand abnormal temperature, physiologic changes over time, and their relation to morbidity and longevity.
eTable 1. ICD-10 Codes and Medications Excluded by LIMIT, by Frequency
eTable 2. Encounters That Did Not Meet Initial Inclusion Criteria Into the Pre-LIMIT Sample
eTable 3. Most Common ICD-10 Codes (>0.3%) in the Analysis Set, by Frequency
eTable 4. Linear Regression and Mixed-Effects Modeling of Temperature as a Function of Time of Day, Age, Height, Weight and Month, in Men and Women
eAppendix. Missing Data—Weight and Height
eFigure. View of the Interactive Visualization Tool for Individualized Normal Temperature Ranges With Examples
eMethods. Data and Code (SAS and R)
Data Sharing Statement
References
- 1.Mackowiak PA, Wasserman SS, Levine MM. A critical appraisal of 98.6 °F, the upper limit of the normal body temperature, and other legacies of Carl Reinhold August Wunderlich. JAMA. 1992;268(12):1578-1580. doi: 10.1001/jama.1992.03490120092034 [DOI] [PubMed] [Google Scholar]
- 2.Wunderlich CA, Sequin E. Medical Thermometry, and Human Temperature. William Wood & Co; 1871. [Google Scholar]
- 3.Bastardot F, Marques-Vidal P, Vollenweider P. Association of body temperature with obesity: the CoLaus study. Int J Obes (Lond). 2019;43(5):1026-1033. doi: 10.1038/s41366-018-0218-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamond A, Lye CT, Prasad D, Abbott D. One size does not fit all: assuming the same normal body temperature for everyone is not justified. PLoS One. 2021;16(2):e0245257. doi: 10.1371/journal.pone.0245257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eriksson H, Svärdsudd K, Larsson B, Welin L, Ohlson LO, Wilhelmsen L. Body temperature in general population samples: the study of men born in 1913 and 1923. Acta Med Scand. 1985;217(4):347-352. doi: 10.1111/j.0954-6820.1985.tb02708.x [DOI] [PubMed] [Google Scholar]
- 6.Frazer JS, Barnes GE, Woodcock V, et al. Variability in body temperature in healthy adults and in patients receiving chemotherapy: prospective observational cohort study. J Med Eng Technol. 2019;43(5):323-333. doi: 10.1080/03091902.2019.1667446 [DOI] [PubMed] [Google Scholar]
- 7.Geneva II, Cuzzo B, Fazili T, Javaid W. Normal body temperature: a systematic review. Open Forum Infect Dis. 2019;6(4):ofz032. doi: 10.1093/ofid/ofz032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurven M, Kraft TS, Alami S, et al. Rapidly declining body temperature in a tropical human population. Sci Adv. 2020;6(44):eabc6599. doi: 10.1126/sciadv.abc6599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harding C, Pompei F, Bordonaro SF, McGillicuddy DC, Burmistrov D, Sanchez LD. The daily, weekly, and seasonal cycles of body temperature analyzed at large scale. Chronobiol Int. 2019;36(12):1646-1657. doi: 10.1080/07420528.2019.1663863 [DOI] [PubMed] [Google Scholar]
- 10.Landsberg L, Young JB, Leonard WR, Linsenmeier RA, Turek FW. Is obesity associated with lower body temperatures? core temperature: a forgotten variable in energy balance. Metabolism. 2009;58(6):871-876. doi: 10.1016/j.metabol.2009.02.017 [DOI] [PubMed] [Google Scholar]
- 11.Lu SH, Dai YT. Normal body temperature and the effects of age, sex, ambient temperature and body mass index on normal oral temperature: a prospective, comparative study. Int J Nurs Stud. 2009;46(5):661-668. doi: 10.1016/j.ijnurstu.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 12.Obermeyer Z, Samra JK, Mullainathan S. Individual differences in normal body temperature: longitudinal big data analysis of patient records. BMJ. 2017;359:j5468. doi: 10.1136/bmj.j5468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Protsiv M, Ley C, Lankester J, Hastie T, Parsonnet J. Decreasing human body temperature in the United States since the industrial revolution. Elife. 2020;9:e49555. doi: 10.7554/eLife.49555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Speaker SL, Pfoh ER, Pappas MA, Hu B, Rothberg MB. Oral temperature of noninfected hospitalized patients. JAMA. 2021;325(18):1899-1901. doi: 10.1001/jama.2021.1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sund-Levander M, Forsberg C, Wahren LK. Normal oral, rectal, tympanic and axillary body temperature in adult men and women: a systematic literature review. Scand J Caring Sci. 2002;16(2):122-128. doi: 10.1046/j.1471-6712.2002.00069.x [DOI] [PubMed] [Google Scholar]
- 16.Tatsumi T, Sampei M, Saito K, et al. Age-dependent and seasonal changes in menstrual cycle length and body temperature based on big data. Obstet Gynecol. 2020;136(4):666-674. doi: 10.1097/AOG.0000000000003910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waalen J, Buxbaum JN. Is older colder or colder older? the association of age with body temperature in 18,630 individuals. J Gerontol A Biol Sci Med Sci. 2011;66(5):487-492. doi: 10.1093/gerona/glr001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bush LM. Fever. Modified September 2022. Accessed May 1, 2023. https://www.merckmanuals.com/professional/infectious-diseases/biology-of-infectious-disease/fever
- 19.Mackowiak PA, Chervenak FA, Grünebaum A. Defining fever. Open Forum Infect Dis. 2021;8(6):ofab161. doi: 10.1093/ofid/ofab161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poole S, Schroeder LF, Shah N. An unsupervised learning method to identify reference intervals from a clinical database. J Biomed Inform. 2016;59:276-284. doi: 10.1016/j.jbi.2015.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Bene VE. Temperature. In: Walker HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Butterworths; 1990. https://www.ncbi.nlm.nih.gov/books/NBK331/ [PubMed] [Google Scholar]
- 22.Stanford University. Stanford Medicine Research Data Repository (STARR). 2008. Accessed July 5, 2017. http://starr.stanford.edu
- 23.R Core Team . R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2023. Accessed August 8, 2023. https://www.R-project.org/
- 24.Hastie T, Tibshirani R. Generalized additive models for medical research. Stat Methods Med Res. 1995;4(3):187-196. doi: 10.1177/096228029500400302 [DOI] [PubMed] [Google Scholar]
- 25.Board of Trustees of the Leland Stanford Junior University, Department of Medicine . Personalized temperature ranges. 2023. Accessed August 10, 2023. http://normaltemperature.stanford.edu/
- 26.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985). 2000;89(1):81-88. doi: 10.1152/jappl.2000.89.1.81 [DOI] [PubMed] [Google Scholar]
- 27.Illner K, Brinkmann G, Heller M, Bosy-Westphal A, Müller MJ. Metabolically active components of fat free mass and resting energy expenditure in nonobese adults. Am J Physiol Endocrinol Metab. 2000;278(2):E308-E315. doi: 10.1152/ajpendo.2000.278.2.E308 [DOI] [PubMed] [Google Scholar]
- 28.Heymsfield SB, Harp JB, Rowell PN, Nguyen AM, Pietrobelli A. How much may I eat? calorie estimates based upon energy expenditure prediction equations. Obes Rev. 2006;7(4):361-370. doi: 10.1111/j.1467-789X.2006.00249.x [DOI] [PubMed] [Google Scholar]
- 29.Rising R, Keys A, Ravussin E, Bogardus C. Concomitant interindividual variation in body temperature and metabolic rate. Am J Physiol. 1992;263(4, pt 1):E730-E734. doi: 10.1152/ajpendo.1992.263.4.E730 [DOI] [PubMed] [Google Scholar]
- 30.Bartke A, Brannan S, Hascup E, Hascup K, Darcy J. Energy metabolism and aging. World J Mens Health. 2021;39(2):222-232. doi: 10.5534/wjmh.200112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caron N, Peyrot N, Caderby T, Verkindt C, Dalleau G. Energy expenditure in people with diabetes mellitus: a review. Front Nutr. 2016;3:56. doi: 10.3389/fnut.2016.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erickson R. Oral temperature differences in relation to thermometer and technique. Nurs Res. 1980;29(3):157-164. doi: 10.1097/00006199-198005000-00004 [DOI] [PubMed] [Google Scholar]
- 33.Baker FC, Siboza F, Fuller A. Temperature regulation in women: effects of the menstrual cycle. Temperature (Austin). 2020;7(3):226-262. doi: 10.1080/23328940.2020.1735927 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. ICD-10 Codes and Medications Excluded by LIMIT, by Frequency
eTable 2. Encounters That Did Not Meet Initial Inclusion Criteria Into the Pre-LIMIT Sample
eTable 3. Most Common ICD-10 Codes (>0.3%) in the Analysis Set, by Frequency
eTable 4. Linear Regression and Mixed-Effects Modeling of Temperature as a Function of Time of Day, Age, Height, Weight and Month, in Men and Women
eAppendix. Missing Data—Weight and Height
eFigure. View of the Interactive Visualization Tool for Individualized Normal Temperature Ranges With Examples
eMethods. Data and Code (SAS and R)
Data Sharing Statement