Abstract
Background
Peripheral blood monocyte counts have been associated with poor outcomes in interstitial lung disease (ILD). However, studies are limited by variable biomarker thresholds, analytic approaches and heterogenous populations. This systematic review and meta-analysis characterised the relationship between monocytes and clinical outcomes in ILD.
Methods
Electronic database searches were performed. Two reviewers screened abstracts and extracted data. Pooled estimates (hazard ratios (HRs)) of monocyte count thresholds were calculated for their association with mortality using ≥0.6×109 and >0.9×109 cells·L−1 for unadjusted models and ≥0.95×109 cells·L−1 for adjusted models, using random effects, with heterogeneity and bias assessed. Disease progression associated with monocytes >0.9×109cells·L−1 was also calculated.
Results
Of 3279 abstracts, 13 were included in the systematic review and eight in the meta-analysis. The pooled unadjusted HR for mortality for monocyte counts ≥0.6×109 cells·L−1 was 1.71 (95% CI 1.34–2.19, p<0.001, I2=0%) and for monocyte counts >0.90×109 cells·L−1 it was 2.44 (95% CI 1.53–3.87, p=0.0002, I2=52%). The pooled adjusted HR for mortality for monocyte counts ≥0.95×109 cells·L−1 was 1.93 (95% CI 1.24–3.01, p=0.0038 I2=69%). The pooled HR for disease progression associated with increased monocyte counts was 1.83 (95% CI 1.40–2.39, p<0.0001, I2=28%).
Conclusions
Peripheral blood monocyte counts were associated with an increased risk of mortality and disease progression in patients with ILD.
Tweetable abstract
Systematic review and meta-analysis demonstrate that peripheral blood monocyte counts are associated with increased risk of mortality and disease progression in ILD and ILA, indicating a potential role for blood monocytes as a prognostic biomarker. https://bit.ly/43IGuvC
Introduction
Interstitial lung diseases (ILDs) represent a large and heterogeneous group of disorders characterised by varying degrees of parenchymal inflammation and/or fibrosis [1, 2]. ILDs are often progressive, associated with significant morbidity and early mortality. There are limited treatment options, no curative therapies to date and lung transplantation is not an option for all patients. Research priorities in ILD include improved understanding of disease pathobiology, risk stratification to inform management and prognostication, and identifying novel and effective therapeutic targets [3].
Clinically available biomarkers to predict those at risk of progression would facilitate risk stratification at the time of diagnosis, identifying patients who may benefit from early treatment or referral to lung transplant. Prognostication is also important for patients, to guide decision making and understanding of disease status. The baseline peripheral blood absolute monocyte count has been associated with outcomes in patients with idiopathic pulmonary fibrosis (IPF) [2, 4]. Absolute monocyte count is a component of the complete blood count, a commonly used and widely available routine laboratory test, making this a potentially and readily accessible and inexpensive biomarker.
Monocytes have been suggested to play an important role in the pathogenesis of fibrotic ILD. Aberrantly activated monocyte levels have been found in IPF patients and circulating monocytes can produce profibrotic matricellular proteins [5, 6] leading to progressive pulmonary fibrosis. Previously, a 52-gene signature predicted higher risk in mortality in patients with IPF and was validated in a prospective cohort study [7]; subsequent statistical deconvolution demonstrated that upregulated genes were expressed in monocytes [4]. More recently, peripheral blood mononuclear cells from IPF patients and controls were profiled using single-cell RNA sequencing with classical monocytes increased in both stable and progressive IPF patients, compared to controls [8]. Interestingly, gene enrichment analysis showed increased pro-inflammatory pathways in stable IPF patients compared to those who progressed [8].
The potential role of monocytes as drivers of fibrogenesis may predict disease prognosis in other types of ILD. Previous studies have further described peripheral blood monocyte counts as a potential prognostic biomarker in patients with fibrotic hypersensitivity pneumonitis (fHP) [9], rheumatoid arthritis (RA) associated ILD [10] and interstitial lung abnormalities (ILAs) [11]. However, these studies are limited by variable biomarker thresholds and methodologies and differing outcome measurements and analytic approaches. Given the potential pathophysiologic overlap between different clinical diagnoses of ILD, the potential role of peripheral blood monocyte counts warrants study across different subtypes of fibrotic ILD.
The objective of this systematic review and meta-analysis was to characterise the association between peripheral blood monocyte counts and the clinical outcomes of death or lung transplantation and disease progression in adult patients with ILD or individuals with ILA.
Methods
This systematic review was conducted in accordance with a pre-specified protocol (PROSPERO registration number: CRD42022376916) and has been reported using PRIMSA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines.
Search strategy and study selection
Electronic database searches were conducted in MEDLINE, Embase, SCOPUS and Web of Science from inception to 8 December 2022. Keywords and MESH terms for “interstitial lung disease” and “peripheral blood monocyte count” were applied (supplemental table 1). Studies involving adults ≥18 years of age diagnosed with ILD were included. Studies of children (aged <18 years old), studies reporting on sarcoidosis or coronavirus disease 2019, review articles, case reports, case series and conference abstracts were excluded. Following searches, two reviewers (B.M. and A.G.O.) independently screened titles and abstracts prior to full-text review. Disagreements regarding the eligibility of studies were resolved by consensus or using a third reviewer (K.A.J.).
Data extraction and risk-of-bias assessment
Data were extracted from publications using a standardised, pre-piloted form and verified by a second reviewer. Variables extracted included study characteristics including design, participant characteristics including age, sex, forced vital capacity (FVC) % predicted, monocyte count data, timing of monocyte count measurement and effect estimates for outcomes of interest. Time-to-event outcomes were ascertained from unadjusted and adjusted hazard ratios (HRs), where reported. Where multiple models were presented, effect estimates were taken from the most adjusted models.
Risk-of-bias assessment was conducted independently by two reviewers using the Newcastle–Ottawa Scale (NOS) for assessing nonrandomised studies in meta-analysis. The NOS tool evaluates the risk of bias across three domains, namely selection, comparability and outcome. All studies were included in the analysis regardless of their risk-of-bias rating.
Statistical analysis
The main outcome of interest was mortality and the secondary outcome was disease progression. Monocyte counts were standardised to cells·L−1 for comparison across studies. Given the limited number of studies and varying monocyte counts used by different studies, the HRs for monocyte counts of >0.9×109 cells·L−1 were pooled for unadjusted models and thresholds ≥0.95×109 cells·L−1 were pooled for adjusted models. Additionally, the HRs for monocyte counts of ≥0.6, >0.67 and 0.6–0.8×109 cells·L−1 were combined for unadjusted models due to the low number of studies available. The HR for a monocyte count >0.9×109 cells·L−1 associated with disease progression was also calculated. Pooled HRs were estimated using random-effects meta-analysis through the meta package in RStudio [12]. Unadjusted and adjusted estimates were analysed separately except for the outcome of disease progression, where these were combined. The I2 statistic was used to evaluate heterogeneity between studies. Publication bias was assessed through funnel plot analysis. All statistical analyses were performed using RStudio.
Results
Search results
A total of 3279 articles were identified. Following removal of duplicates and title/abstract screening, 28 studies underwent full-text review, with 13 included in the systematic review (supplemental figure 1). Of these 13 studies, three reported outcomes using odds ratios (ORs) and two reported monocyte count as a continuous variable. A total of eight studies reported effect measures that could be pooled and were included in the meta-analysis.
Study characteristics
Studies included in the systematic review reported on patients with IPF (n=7) [2, 4, 13–17], indeterminate usual interstitial pneumonia (UIP) (n=1) [18], ILA (n=2) [11, 19], fHP (n=1) [9], anti-melanoma differentiation-associated gene 5 (MDA5) positive dermatomyositis (DM)-ILD (n=1) [20] and a pooled cohort of fibrotic ILD (n=1) [21] (table 1). Most were retrospective cohort studies (one a post hoc analysis of randomised controlled trials [2]), except for two, which were prospective cohort studies [14, 15]. All studies were published between 2019 and 2022. Four studies were from the United Kingdom [9, 17–19], two were multinational [11, 13], two from in the United States [2, 4], two from China [14, 20], one from Australia [15] and one from Italy [16]. The number of participants ranged from 32 to 2067, for a total study cohort of 7684. 10 out of 13 studies reported outcomes in HRs, whereas three reported ORs [11, 16, 21] and were therefore excluded from the meta-analysis.
TABLE 1.
Study characteristics
| Study, year | Included in meta-analysis | Country of study | ILD sample size | Type of ILD | Age, years | Male, % | Baseline FVC, % predicted | Baseline DLCO, % predicted | Monocyte parameters | Timing of monocyte measurement | Relevant outcomes reported |
| Achaiah et al. [ 18 ], 2021 | Yes | UK | 32 | Indeterminate UIP | 76.7±6.2 | 66 | 92.6±26.9 | 64.2 (16) | >0.9×109 cells·L−1 | Within 3 months of initial CT | Visual increase in extent of disease or progression of CT to “definite” or “probable” UIP |
| Achaiah et al. [ 19 ], 2022 | Yes | UK | 1259 (mortality) 362 (progression) |
Early fibrotic ILA | 63.4±8.1 | 57.2 | NA | NA | >1×109 cells·L−1 | Closest to CT; median time interval between CT and blood sample 13–30 days | Radiologic progression, all-cause mortality |
| Achaiah et al. [ 17 ], 2022 (NLR) | Yes | UK | 128 | IPF | 74.8±6.9 | 79 | 85.5 (69.9–98.0) | 6.19 (50.9–71.0) | >0.9×109 cells·L−1 | Within 4 months of presentation to ILD clinic | FVC decline >10% per year, all-cause mortality |
| Karampitsakos et al. [ 13 ], 2021 | Yes | Multinational | 300 (discovery) 189 (validation) 489 (pooled) |
IPF | NA | NA | NA | NA | ≥0.95×109 cells·L−1 (pooled) ≥0.6×109 cells·L−1 (discovery and validation) |
Baseline (prior to antifibrotic treatment) | All-cause mortality, 1-year disease progression as assessed by functional decline |
| Kreuter et al. [ 2 ], 2021 | Yes | USA (multicentre) | 2067 | IPF | NA | NA | NA | NA | 0.6–0.8×109 cells·L−1 ≥0.95×109 cells·L−1 |
Baseline | All-cause mortality over 1 year |
| Scott et al. [ 4 ], 2019 | Yes | USA (multicentre) | 130 (Stanford) 36 (COMET) |
IPF | NA | NA | NA | NA | ≥0.95×109 cells·L−1 | Stanford: within 30 days of diagnosis; COMET: baseline | Transplant-free survival (discovery), mortality (validation) |
| Teoh et al. [ 15 ], 2020 | Yes | Australia (multicentre) | 231 | IPF | 69.9±8.3 | 71 | 80.3±22 | 48.2±16.8 | ≥0.95×109 cells·L−1 | Baseline | Survival |
| Zhang et al. [ 14 ], 2022 | Yes | China (multicentre) | 34 | IPF | 64.5±9.46 | 82.69 | 77.39±20.31 | 40.36±18.71 | >0.67×109 cells·L−1 | After admission to hospital | Survival |
| Barratt et al. [ 9 ], 2021 | No | UK | 281 | fHP | 70 (65–80) | 41 | 79 (65–94) | 50 (43–64) | ≥0.95×109 cells·L−1 | At the point of diagnosis | Survival |
| Bernardinello et al. [ 16 ], 2022 | No | Italy | 77 | Newly diagnosed IPF | 70 (53–81) | 83 | 80 (50–125) | 57 (30–106) | Continuous | At diagnosis and within at least 1 year following antifibrotic therapy | FVC decline ≥5% predicted over 1 year |
| Kim et al. [ 11 ], 2021 | No | Multinational | 1659 | ILA | 78±6 | 55 | NA | NA | 1sd increment | At first clinic visit | ILA progression |
| Lv et al. [ 20 ], 2022 | No | China | 351 (pooled) | Anti-MDA5 positive DM-ILD | 53.11±11 | 33.6 | NA | NA | >0.24×109 cells·L−1 | Weekly for first 4 weeks of hospital admission | 6-month all-cause mortality |
| Shao et al. [ 21 ], 2022 | No | Austria | 95 (derivation) | Fibrotic ILD | 70.9±1.5 | 66.6 | 81±3.1 | 54.8±2.7 | ≥0.65×109 cells·L−1 | Baseline | Relative FVC decline ≥10% or DLCO decline ≥15% at 1 year, death or lung transplant |
CT: computed tomography; DLCO: diffusing capacity of the lung for carbon monoxide; DM: dermatomyositis; fHP: fibrotic hypersensitivity pneumonitis; FVC: forced vital capacity; ILA: interstitial lung abnormalities; ILD: interstitial lung disease; IPF: idiopathic pulmonary fibrosis; MDA5: melanoma differentiation-associated gene 5; NA: not applicable/available; UIP: usual interstitial pneumonia.
Monocyte count thresholds
Monocyte counts were measured consistently across different studies using plasma or serum; however, the details of assays used were frequently unavailable. Monocyte counts were measured at varying timepoints, from within 30 days of diagnosis to up to 4 months within presentation to the ILD clinic. Varying monocyte counts were used in analyses; one study used the monocyte count of >0.24×109 cells·L−1 [20], one study used ≥0.6×109 cells·L−1 [13], one study used 0.6–<0.8×109 cells·L−1 [2], one study used ≥0.65×109 cells·L−1 [21], one study used >0.67×109 cells·L−1 [14], two used >0.9×109 cells·L−1 [17, 18], five used ≥0.95×109 cells·L−1 [2, 4, 9, 13, 15] and one study used >1.0×109 cells·L−1 [19]. Two studies reported analyses using monocyte counts defined as a continuous variable [16] or in 1sd increments [11].
Monocyte count and mortality
Data on overall mortality, with or without transplantation, were available for nine out of 13 studies included in the systematic review. One study reported transplant-free survival [4], five studies determined all-cause mortality (specified at 1 year [2, 13], 6 months [20] and an unspecified timeframe [17, 19]) and three studies reported survival as the outcome [14–16]. One study reported a composite outcome of relative FVC decline ≥10% predicted or diffusing capacity of the lung for carbon monoxide (DLCO) decline ≥15% at 1 year, death or lung transplant.
Seven retrospective cohort studies and one prospective cohort study reported an association between elevated monocyte count and overall mortality in patients with ILD. Most of these studies included patients with IPF; however, elevated monocyte counts were also associated with higher risk of all-cause mortality in those with early fibrotic ILA [18] and decreased survival in those with fHP [9].
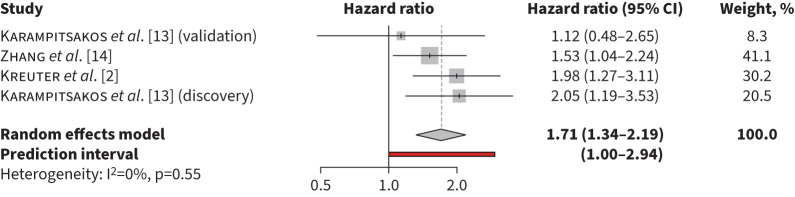
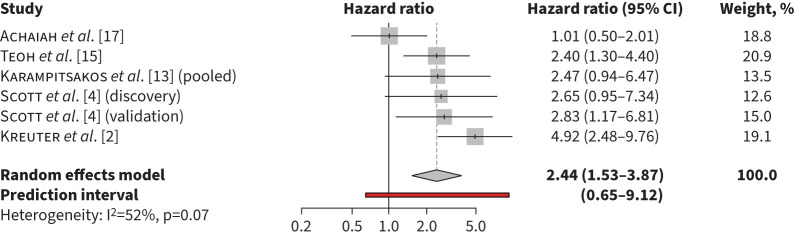
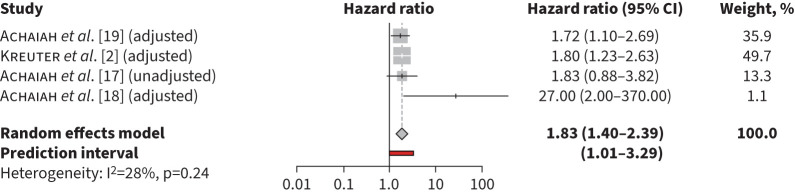
The pooled unadjusted HR for mortality for monocyte counts ≥0.6×109 cells·L−1 was 1.71 (95% CI 1.34–2.19, p<0.001, I2=0%) (figure 1). The pooled unadjusted HR for mortality was 2.44 (95% CI 1.53–3.87, p=0.0002, I2=2%) for monocyte counts >0.9×109 cells·L−1 (figure 2) and in adjusted models was 1.93 (95% CI 1.24–3.01, p=0.0038 I2=69%) for monocyte counts ≥0.95×109 cells·L−1 (figure 3). Heterogeneity was high in the retrospective cohort studies estimating mortality risk with elevated monocyte counts >0.90×109 cells·L−1 in unadjusted models (I2=61%) and ≥0.95×109 cells·L−1 in adjusted models (I2=73%). In studies that used monocyte counts ≥0.6×109 cells·L−1 to estimate mortality, heterogeneity was low (I2=0%), likely due to inclusion of three patient cohorts from only two separate studies, resulting in similar methodology and patient characteristics.
FIGURE 1.
Risk of mortality associated with monocyte counts ≥0.6×109 cells·L−1 in unadjusted models.
FIGURE 2.
Risk of mortality associated with monocyte counts >0.9×109 cells·L−1 in unadjusted models.
FIGURE 3.
Risk of mortality associated with monocyte counts ≥0.95×109 cells·L−1 in adjusted models.
In contrast, one retrospective cohort study investigated patients admitted to hospital with anti-MDA5 antibody positive DM-ILD and found that low monocyte counts (<0.24×109 cells·L−1) predicted higher risk of all-cause mortality at 6 months (pooled HR 2.03, 95% CI 1.42–2.91, p<0.0001). This study utilised the shortest timeframe to determine all-cause mortality.
Monocyte count and disease progression
Seven out of 13 studies in the systematic review assessed disease progression. Definitions of disease progression varied, from radiologic progression to decline in FVC or DLCO. Three of the seven studies investigated IPF and found an association between elevated monocyte count and disease progression [13, 16, 17]. Two studies investigated ILA. Achaiah et al. [19] reported an association between monocyte counts >1.0×109 cells·L−1 and radiological progression in patients with early fibrotic ILA (HR 1.72, 95% CI 1.10–2.69, p=0.018), while Kim et al. [13] reported that higher monocyte counts (analysed in increments of 1sd) were associated with ILA progression when adjusted for age, sex, smoking status, cigarette pack-years and body mass index (OR 1.3, 95% CI 1.1–1.5, p=0.001). Shao et al. [21] determined that in patients with radiographic evidence of fibrosis (defined as the presence of reticular lung abnormalities or honeycombing on high-resolution computed tomography), baseline monocyte counts ≥0.65×109 cells·L−1 were associated with worse prognosis (OR 3.16, 95% CI 1.27–7.88, p=0.014). Achaiah et al. [18] found that monocyte counts >0.9×109 cells·L−1 were associated with radiologic progression in patients with a radiological pattern of indeterminate UIP.
Similarly, disease progression definitions differed across the four studies included in the meta-analysis, including radiographic extent of disease and physiologic parameters (≥10% absolute decline in FVC per year or change in 6-min walk distance). Variable monocyte counts were used, including >0.9×109, ≥0.95×109 and >1.0×109 cells·L−1, which are pooled together here. Three of the four studies reported analysis from adjusted models, whereas one reported results from an unadjusted model, and these were pooled. The pooled HR for disease progression was 1.83 (95% CI 1.40–2.39, p<0.0001, I2=28%) (figure 4).
FIGURE 4.
Risk of disease progression associated with monocyte counts >0.90×109 cells·L−1 in unadjusted and adjusted models.
Risk of bias
A comprehensive risk of bias assessment of the included studies revealed several limitations and possible biases (supplemental table 2), but, overall, the results were similar across the publications included for analysis. Visual inspection of funnel plots implied that some publication bias was present for studies included in the mortality risk and disease progression assessment (supplemental figures 2–5).
Discussion
In this first systematic review and meta-analysis of monocyte counts and outcomes in patients with ILD, elevated baseline peripheral blood monocytes were associated with higher risk of death and disease progression. However, the higher monocyte count threshold of >0.9×109 cells·L−1 demonstrated greater consistency in its association. These data inform the role of peripheral blood monocyte counts to be used as an important and readily available prognostic biomarker in ILD.
These findings suggest a relationship between monocyte counts and ILD prognosis, supporting the possible role of monocytes in the pathogenesis of ILD. Numerous immune cells have been implicated in the development of IPF, including monocytes, neutrophils and lymphocytes [6, 22]. Although immunosuppressive treatments are associated with increased mortality in patients with IPF [23], the innate immune system appears to be involved in establishing fibrosis. Historically, fibrogenesis due to repeated lung injury has been described. Within this model, monocytes migrate to the injured lung, differentiate into macrophages and coordinate a profibrotic and pro-inflammatory response [24, 25].
Monocytes are a plastic cell population with the ability to differentiate into different subtypes, including pulmonary macrophages, depending on the context of their activation [26]. Pulmonary macrophages may be further distinguished by surface markers into alveolar macrophages, interstitial macrophages and monocyte-derived macrophages, with an ability to transition from one phenotype to another [27]. Functionally, pulmonary macrophages have been characterised as classically (M1) or alternatively (M2) activated and may demonstrate dynamic activation depending on disease context [27]. Progression of pulmonary fibrosis has been linked with changes in both the phenotype and function of pulmonary macrophages [22, 28] with alveolar epithelial cells and fibroblasts also playing contributing roles [27, 29, 30]. These alterations in pulmonary macrophages may be partially driven by changes in monocyte subtype populations [2, 22, 26].
Supporting this hypothesis, significant differences in cell and molecular markers involved in monocyte/macrophage activation and recruitment were found between patients with IPF and normal subjects [5, 31]. Immune phenotyping of peripheral blood monocytes of IPF patients showed increased expression of CD64 protein and type 1 interferon response compared to age-matched controls [31].
In murine models, monocyte-derived macrophages appear to drive the development of pulmonary fibrosis [22, 32]. Monocyte-driven changes in airway macrophages have also been associated with aging [33–35]. Pulmonary accumulation of distinct alveolar macrophages has also been associated with worse clinical outcomes [36].
The outcomes reported by each study in this review differed in that variable definitions of disease progression and mortality were used. Only one study reported transplant-free survival [4], whereas others reported all-cause mortality. Importantly, the included studies often had not adjusted for the same potentially confounding variables. Two out of seven studies adjusted for treatment status [2, 14], while one study included treatment-naïve patients with IPF, but the timing of monocyte count measures in relation to drug treatments were not clear. Studies were observational retrospective cohort studies and of small to modest size. Monocyte counts at baseline were measured at different timepoints across different studies, from within 30 days of diagnosis to within 4 months of presentation to an ILD clinic. Finally, out of the seven included studies, only two included cohorts with non-IPF ILDs (indeterminate UIP and early fibrotic ILAs), limiting the generalisability of these findings.
Previously, a change in monocyte count from baseline over 1 year was found to not be associated with increased mortality or disease progression [2]. Further research into the relationship between monocytes and ILD prognosis, particularly in the context of treatment and with longitudinal follow-up, may clarify whether monocyte counts could function as a predictor of therapeutic response. Additionally, it is unknown if the elevated monocyte counts are causal of poor outcomes or reflective of an underlying disease process. This warrants further investigation to understand if targeting of monocytes or their subsets could have positive therapeutic effects.
This study has limitations, largely relating to the low numbers of parent studies included for analysis and quality of the parent data. There was relatively high heterogeneity in the pooled estimates of mortality in adjusted and unadjusted models associated with elevated monocyte count. In addition, due to small study numbers, meta-regression or detailed exploration of heterogeneity was not possible. This heterogeneity was expected, however, given the variability in analytic approaches and reported outcomes of the included studies. Elevated monocyte count thresholds were variably defined across the studies, including 0.9×109, 0.95×109 and 1.0×109 cells·L−1. The HRs of these varying thresholds were pooled due to the few numbers of studies available and residual confounding is possible.
In summary, this systematic review and meta-analysis found that baseline elevated monocyte counts are associated with worse clinical outcomes in patients with ILD and ILA. The absolute monocyte count is promising as a simple and effective biomarker of ILD prognosis. Further prospective studies are needed to better characterise this relationship and determine if it holds true across ILD subtypes and whether it is impacted by ILD-targeted treatment.
Points for clinical practice and questions for future research
Elevated baseline peripheral blood monocyte counts are associated with increased risk of death and disease progression in patients with ILD or ILA.
Prospective studies are needed to further characterise this relationship, accounting for concomitant treatments and ILD subtypes.
Future work should evaluate for specific monocyte cell subsets to characterise the pathobiology of this relationship and determine whether monocytes are pathogenic or a marker of disease activity.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0072-2023.SUPPLEMENT (541.4KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of interest: All authors have nothing to disclose.
Support statement: B. Min is supported by an educational grant from the Canadian Pulmonary Fibrosis Foundation.
References
- 1.Collard HR, Richeldi L. Interstitial Lung Disease. Amsterdam, Elsevier, 2018; p. 190. [Google Scholar]
- 2.Kreuter M, Lee JS, Tzouvelekis A, et al. Monocyte count as a prognostic biomarker in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care 2021; 204: 74–81. doi: 10.1164/rccm.202003-0669OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johannson KA, Collard HR, Richeldi L. Looking ahead: interstitial lung disease diagnosis and management in 2030. Clin Chest Med 2021; 42: 375–384. doi: 10.1016/j.ccm.2021.03.014 [DOI] [PubMed] [Google Scholar]
- 4.Scott M, Quinn K, Li Q, et al. The prognostic value of monocyte count in idiopathic pulmonary fibrosis: a multi-omic cohort study. Am J Respir Crit Care Med 2019; 199: A7342. doi: 10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A7342 [DOI] [Google Scholar]
- 5.Desai B, Mattson J, Paintal H, et al. Differential expression of monocyte/macrophage-selective markers in human idiopathic pulmonary fibrosis. Exp Lung Res 2011; 37: 227–238. doi: 10.3109/01902148.2010.538132 [DOI] [PubMed] [Google Scholar]
- 6.Moore BB, Fry C, Zhou Y, et al. Inflammatory leukocyte phenotypes correlate with disease progression in idiopathic pulmonary fibrosis. Front Med 2014; 1: 56. doi: 10.3389/fmed.2014.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herazo-Maya JD, Sun J, Molyneaux PL, et al. Validation of a 52-gene risk profile for outcome prediction in patients with idiopathic pulmonary fibrosis: an international, multicentre, cohort study. Lancet Respir Med 2017; 5: 857–868. doi: 10.1016/S2213-2600(17)30349-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unterman A, Zhao AY, Neumark N, et al. Single-cell profiling reveals immune aberrations in progressive idiopathic pulmonary fibrosis. medRxiv 2023; preprint [ 10.1101/2023.04.29.23289296]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barratt SL, Creamer AW, Adamali HI, et al. Use of peripheral neutrophil to lymphocyte ratio and peripheral monocyte levels to predict survival in fibrotic hypersensitivity pneumonitis (fHP): a multicentre retrospective cohort study. BMJ Open Respir Res 2021; 8: e001063. doi: 10.1136/bmjresp-2021-001063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saku A, Fujisawa T, Nishimoto K, et al. Prognostic significance of peripheral blood monocyte and neutrophil counts in rheumatoid arthritis-associated interstitial lung disease. Respir Med 2021; 182: 106420. doi: 10.1016/j.rmed.2021.106420 [DOI] [PubMed] [Google Scholar]
- 11.Kim JS, Axelsson GT, Moll M, et al. Associations of monocyte count and other immune cell types with interstitial lung abnormalities. Am J Respir Crit Care Med 2021; 204: 197–208. doi: 10.1164/rccm.202008-3093OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.RStudio Team . RStudio: Integrated Development for R. Boston, RStudio, 2020. [Google Scholar]
- 13.Karampitsakos T, Torrisi S, Antoniou K, et al. Increased monocyte count and red cell distribution width as prognostic biomarkers in patients with idiopathic pulmonary fibrosis. Respir Res 2021; 22: 140. doi: 10.1186/s12931-021-01725-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang XR, Ren YH, Xie BB, et al. Blood monocyte counts as a prognostic biomarker and predictor in Chinese patients with idiopathic pulmonary fibrosis. Front Med 2022; 9: 955125. doi: 10.3389/fmed.2022.955125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teoh AKY, Jo HE, Chambers DC, et al. Blood monocyte counts as a potential prognostic marker for idiopathic pulmonary fibrosis: analysis from the Australian IPF registry. Eur Respir J 2020; 55: 1901855. doi: 10.1183/v13993003.01855-2019 [DOI] [PubMed] [Google Scholar]
- 16.Bernardinello N, Grisostomi G, Cocconcelli E, et al. Lymphocyte to monocyte ratio (LMR) predicts survival in patients with idiopathic pulmonary fibrosis (IPF). Eur Respir J 2021; 58: Suppl. 65, PA392. doi: 10.1183/13993003.congress-2021.PA392 [DOI] [PubMed] [Google Scholar]
- 17.Achaiah A, Rathnapala A, Pereira A, et al. Neutrophil lymphocyte ratio as an indicator for disease progression in idiopathic pulmonary fibrosis. BMJ Open Respir Res 2022; 9: e001202. doi: 10.1136/bmjresp-2022-001202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Achaiah A, Rathnapala A, Pereira A, et al. Monocyte and neutrophil levels are potentially linked to progression to IPF for patients with indeterminate UIP CT pattern. BMJ Open Respir Res 2021; 8: e000899. doi: 10.1136/bmjresp-2021-000899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Achaiah A, Lyon P, Fraser E, et al. Increased monocyte level is a risk factor for radiological progression in patients with early fibrotic interstitial lung abnormality. ERJ Open Res 2022; 8: 00226-2022. doi: 10.1183/23120541.00226-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lv X, Jin Y, Zhang D, et al. Low circulating monocytes is in parallel with lymphopenia which predicts poor outcome in anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis-associated interstitial lung disease. Front Med 2022; 8: 808875. doi: 10.3389/fmed.2021.808875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shao G, Hawle P, Akbari K, et al. Clinical, imaging, and blood biomarkers to assess 1-year progression risk in fibrotic interstitial lung diseases–development and validation of the honeycombing, traction bronchiectasis, and monocyte (HTM)-score. Front Med 2022; 9: 1043720. doi: 10.3389/fmed.2022.1043720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misharin AV, Morales-Nebreda L, Reyfman PA, et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med 2017; 214: 2387–2404. doi: 10.1084/jem.20162152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Idiopathic Pulmonary Fibrosis Clinical Research Network , Raghu G, Anstrom KJ, et al. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med 2012; 366: 1968–1977. doi: 10.1056/NEJMoa1113354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atochina-Vasserman EN, Bates SR, Zhang P, et al. Early alveolar epithelial dysfunction promotes lung inflammation in a mouse model of Hermansky–Pudlak syndrome. Am J Respir Crit Care Med 2011; 184: 449–458. doi: 10.1164/rccm.201011-1882OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck-Schimmer B, Schwendener R, Pasch T, et al. Alveolar macrophages regulate neutrophil recruitment in endotoxin-induced lung injury. Respir Res 2005; 6: 61. doi: 10.1186/1465-9921-6-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyette LB, Macedo C, Hadi K, et al. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS One 2017; 12: e0176460. doi: 10.1371/journal.pone.0176460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byrne AJ, Maher TM, Lloyd CM. Pulmonary macrophages: a new therapeutic pathway in fibrosing lung disease? Trends Mol Med 2016; 22: 303–316. doi: 10.1016/j.molmed.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 28.Gu Y, Lawrence T, Mohamed R, et al. The emerging roles of interstitial macrophages in pulmonary fibrosis: a perspective from scRNA-seq analyses. Front Immunol 2022; 13: 923235. doi: 10.3389/fimmu.2022.923235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selman M, Pardo A. Revealing the pathogenic and aging-related mechanisms of the enigmatic idiopathic pulmonary fibrosis. an integral model. Am J Respir Crit Care Med 2014; 189: 1161–1172. doi: 10.1164/rccm.201312-2221PP [DOI] [PubMed] [Google Scholar]
- 30.Selman M, Pardo A. Idiopathic pulmonary fibrosis: an epithelial/fibroblastic cross-talk disorder. Respir Res 2002; 3: 3. doi: 10.1186/rr175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fraser E, Denney L, Antanaviciute A, et al. Multi-modal characterization of monocytes in idiopathic pulmonary fibrosis reveals a primed type I interferon immune phenotype. Front Immunol 2021; 12: 623430. doi: 10.3389/fimmu.2021.623430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satoh T, Nakagawa K, Sugihara F, et al. Identification of an atypical monocyte and committed progenitor involved in fibrosis. Nature 2017; 541: 96–101. doi: 10.1038/nature20611 [DOI] [PubMed] [Google Scholar]
- 33.Mass E, Nimmerjahn F, Kierdorf K, et al. Tissue-specific macrophages: how they develop and choreograph tissue biology. Nat Rev Immunol 2023; 23: 563–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angelidis I, Simon LM, Fernandez IE, et al. An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat Commun 2019; 10: 963. doi: 10.1038/s41467-019-08831-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byrne AJ, Powell JE, O'Sullivan BJ, et al. Dynamics of human monocytes and airway macrophages during healthy aging and after transplant. J Exp Med 2020; 217: e20191236. doi: 10.1084/jem.20191236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nouno T, Okamoto M, Ohnishi K, et al. Elevation of pulmonary CD163+ and CD204+ macrophages is associated with the clinical course of idiopathic pulmonary fibrosis patients. J Thorac Dis 2019; 11: 4005–4017. doi: 10.21037/jtd.2019.09.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0072-2023.SUPPLEMENT (541.4KB, pdf)