Abstract
Obstructive sleep apnoea is a highly prevalent chronic disorder and has been shown to be associated with disturbed glucose metabolism and type 2 diabetes. However, the evidence from individual clinical trials on the effect of continuous positive airway pressure (CPAP) treatment on glycaemic control in patients with co-existing obstructive sleep apnoea and type 2 diabetes remains controversial. A systematic review of randomised controlled trials assessing the effect of CPAP on glycaemic control in patients with obstructive sleep apnoea and type 2 diabetes was conducted using the databases MEDLINE, Embase, Cochrane and Scopus up to December 2022. Meta-analysis using a random-effect model was performed for outcomes that were reported in at least two randomised controlled trials. From 3031 records screened, 11 RCTs with a total of 964 patients were included for analysis. CPAP treatment led to a significant reduction in haemoglobin A1c (HbA1c) (mean difference −0.24%, 95% CI −0.43– −0.06%, p=0.001) compared to inactive control groups. Meta-regression showed a significant association between reduction in HbA1c and hours of nightly CPAP usage. CPAP therapy seems to significantly improve HbA1c and thus long-term glycaemic control in patients with type 2 diabetes and obstructive sleep apnoea. The amount of improvement is dependent on the hours of usage of CPAP and thus optimal adherence to CPAP should be a primary goal in these patients.
Tweetable abstract
CPAP therapy seems to significantly improve HbA1c in patients with type 2 diabetes and OSA. The amount of improvement is associated with the hours of usage of CPAP; optimal adherence to CPAP should be a primary goal in these patients. https://bit.ly/3CZL9ic
Introduction
Obstructive sleep apnoea (OSA) and type 2 diabetes are highly prevalent diseases that have major clinical, epidemiological and healthcare implications [1]. Epidemiological studies have demonstrated a high prevalence of OSA in patients with type 2 diabetes and vice versa [2]. Despite OSA and type 2 diabetes sharing common risk factors such as advanced age and obesity, several studies recently suggested that the two diseases may influence each other [3]. In patients with OSA, sleep fragmentation and episodes of hypoxaemia, caused by recurrent episodes of partial or complete airway collapse during sleep, lead to pathophysiological sequelae such as activation of the sympathetic nervous system (SNS), systemic inflammation, oxidative stress and changes in hormonal systems (e.g. modulation of the hypothalamic–pituitary–adrenal (HPA) axis) [4]. These OSA-induced alterations likely contribute to derangements in glucose metabolism with development of insulin resistance and glucose intolerance, possibly promoting the development of type 2 diabetes [5, 6]. The results of a recent meta-analysis of different cohort studies including over 60 000 patients with OSA indicate that the presence of OSA has a comparable impact on developing type 2 diabetes as traditional risk factors such as overweight, family predisposition and physical inactivity [7]. In turn, several reports have shown that type 2 diabetes may promote OSA through the effects of peripheral neuropathy, with impaired microvascular regulation and increased nitrosative/oxidative stress, and through the alteration of central respiratory control, but the pathophysiological link between the two diseases is yet to be fully understood [8, 9]. Therefore, investigating the effect of the treatment of one disease on the other could help to unravel the pathophysiological link.
OSA is commonly treated with continuous positive airway pressure (CPAP), which effectively abolishes sleep-disordered breathing and can improve sleep quality, daytime sleepiness and quality of life, and reduce blood pressure [10–12]. Several randomised control trials (RCTs) have investigated the effect of CPAP therapy on glycaemic control in patients with OSA and type 2 diabetes or pre-diabetes [13–15]. Recently published trials are adding new evidence [16–19]. However, the evidence from individual clinical trials on the effect of CPAP treatment on glycaemic control in patients with co-existing OSA and type 2 diabetes is still controversial. Therefore, this review and meta-analysis was performed to assess the cumulative evidence from existing RCTs and clarify the clinical effects of CPAP on glycaemic control.
Methods
This systematic review and meta-analysis was designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The protocol was registered in the international prospective register of systematic reviews (PROSPERO) (registration number: CRD42022372423).
Literature search strategy
Literature search strategies were designed by a medical/healthcare librarian (detailed search strategy in supplementary material: appendix). Systematic literature searches were performed using the databases of MEDLINE, Embase, Cochrane and Scopus up to December 2022. RCTs were identified using the Cochrane Highly Sensitive Search Strategies for identifying RCTs (sensitivity-maximising version in combination with a highly sensitive subject search) [20].
Inclusion criteria
To be eligible for inclusion in the systematic review and meta-analysis, studies had to: 1) use a RCT design; 2) include patients of 18 years or older; 3) include patients with a diagnosis of OSA (defined as an apnoea–hypopnea index (AHI) or oxygen desaturation index of ≥5 events·h−1); 4) include patients with type 2 diabetes (haemoglobin A1c (HbA1c) ≥6.5%); 5) have a comparison of CPAP treatment to an inactive control group (placebo or no treatment); 6) monitor at least one measure of glucose metabolism before CPAP treatment at baseline and with CPAP treatment at follow-up; 7) be published in English.
Selection of studies and data extraction
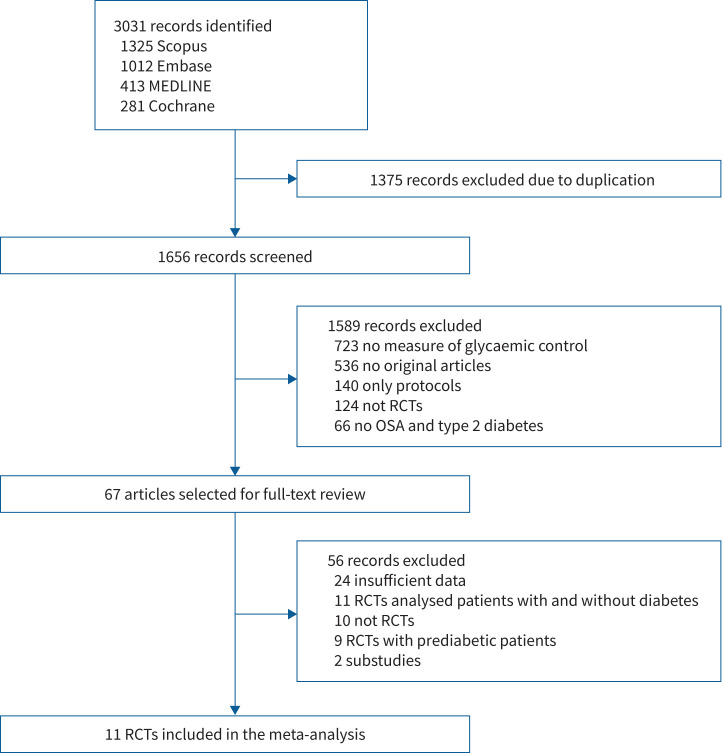
Eligibility of studies found in the literature searches were assessed by two authors independently (JH and FS). Disagreement between the two reviewers was resolved via discussion or by involving a third reviewing author (NAS) to reach consensus. A flow diagram of eligibility screening is shown in figure 1. Data were extracted by one author (JH) and independently verified by another author (NAS) using standardised data extraction forms. The extracted data included study characteristics, participant characteristics and measurements of glycaemic control (primary outcome: HbA1c; secondary outcomes: fructosamine level, homeostasis model of assessment for insulin resistance index (HOMA-IR), fasting blood glucose (FBG) and 24-h plasma glucose level). An attempt was made to obtain missing data by contacting the authors of the respective studies.
FIGURE 1.
Study flow chart. RCT: randomised controlled trial; OSA: obstructive sleep apnoea.
Risk of bias assessment
The included RCTs were evaluated for risk of bias using the Cochrane Collaboration's tool for assessing risk of bias by two reviewers (NAS and FS) independently, with involvement of a third reviewer to obtain consensus [21].
Data synthesis and statistical analysis
Data were analysed using the STATA software package version 16.1 (Stata Corp., College Station, TX, USA) and are presented as mean±sd or median (IQR). For outcomes reported in two or more studies, mean difference (MD) and standard deviation between baseline and follow-up were extracted or calculated from raw values and data were pooled using a random-effects model. Missing standard deviations were imputed by either the standard equation or estimation out of pooled data following the Cochrane guidelines [22]. Statistical significance was set to a two-sided p-value of <0.05. Forest plots were used to present the results of individual studies and the pooled MD±sd. Statistical heterogeneity was tested using the Chi-squared and Higgins I2 tests. In the case of I2≥50%, heterogeneity was further explored by sensitivity analyses. For measures with considerable heterogeneity and a statistically significant MD, univariate meta-regressions with possible moderators (baseline body mass index (BMI), AHI, HbA1c, duration of diabetes and duration of CPAP use per night) that might have contributed to the heterogeneity were performed. Publication bias was evaluated using a funnel plot and the Egger's linear regression test.
Results
From 3031 records identified by the initial search, 11 eligible RCT studies were identified and included in the systematic review and meta-analysis (table 1) [13, 15–19, 23–27]. Of these 11 RCTs, six studies compared CPAP with controls and five studies compared CPAP with inactive sham CPAP. Summaries of characteristics of the included trials are shown in table 1. A total of 964 patients were included: 490 patients were assigned to CPAP treatment and 474 were inactive controls. We reported an overall low risk of bias in five studies, some concerns in four studies and high concerns in two studies (supplementary figure S1). Main reasons to downgrade the evidence were on the basis of the randomisation process and the selection of the reported results. Either methods of randomisation process and allocation concealment were not adequately described or studies did not present a prespecified analysis plan. Furthermore, a relevant dropout rate was observed in various studies and no appropriate analysis to estimate the effect of assignment to intervention was used.
TABLE 1.
Baseline characteristics of trials included in meta-analysis
| Study |
OSA definition
(events·h−1) |
Intervention | Subjects (n) | Follow-up (weeks) |
Age
(years) |
Male (%) | BMI (kg·m−2) |
HbA1c
(%) |
CPAP usage
(h·night−1) |
AHI
(events·h−1) |
Duration DMII
(years) |
Outcomes |
| Banghøj et al., 2020 [19] | AHI ≥15 | CPAP No CPAP |
72 | 12 | 62±7.5 | 75 | 34.6±4.2 | 8.0±0.8 8.1±0.8 |
5.6±1.9 | 32.0 (21–43) 34.0 (22–49) |
16±8.6 | HbA1c, FBG, 24 h glucose |
| Bakker et al., 2020 [27] | AHI ≥10 | CPAP Sham CPAP |
53 | 12 | 56.2±9.3 | 64 | 35.4±6.8 | 6.7±0.5 7.1±0.6 |
4.3±2.4 | 23.5±15.1 | NA | HbA1c, FBG |
| Chasens et al., 2022 [16] | AHI ≥10 | CPAP Sham CPAP |
98 | 6 | 58.7±9.8 | 58 | 36.2±6.6 | 7.7±0.8 8.0±1.0 |
5.0±1.7 4.4±2.1 |
22.5±13.4 25.9±15.9 |
10±9.0 | HbA1c, fructosamine |
| Lam et al., 2017 [23] | AHI ≥15 | CPAP No CPAP |
64 | 12 | 55±9.0 | 81 | 29.9±5.3 | 8.0±1.2 8.3±1.4 |
2.5±2.3 | 43.4±23.1 47.2±23.5 |
9±5.9 | HbA1c, FBG, fructosamine |
| Martínez-Cerón et al., 2016 [15] | AHI ≥5 | CPAP No CPAP |
50 | 12 | 61±9.0 | 60 | 32.5±4.5 | 7.6±1.3 7.6±0.7 |
5.2±1.9 | 35.6±23.4 28.2±17.4 |
5 (3.0–15.0) | HbA1c, FBG, HOMA-IR |
| Mokhlesi et al., 2016 [24] | AHI ≥15 | CPAP Sham CPAP |
19 | 1 | 55±3.3 | 47 | 37.8±2.8 | 7.3±0.4 7.0±0.4 |
7.9±0.1 7.9±0.1 |
NA | 4±2.8 | 24 h glucose |
| Morariu et al., 2017 [25] | AHI ≥10 | CPAP Sham CPAP |
23 | 4 | 55.6±11.6 | 51 | 35.2±5.3 | 6.6±0.5 6.9±1.0 |
4.1±2.9 4.5±2.7 |
56.6±29.8 25.6±11.4 |
NA | 24 h glucose, fructosamine |
| Shaw et al., 2016 [13] | AHI ≥15 | CPAP No CPAP |
298 | 24 | 62.3±9.0 | 65 | 33.0±5.4 | 7.3±0.5 7.3±0.5 |
4.9±NA | 26.2±12.9 28.0±14.1 |
8±6.9 | HbA1c, FBG, |
| West et al., 2007 [26] | ODI ≥10 | CPAP Sham CPAP |
42 | 12 | 56.1±9.9 | 100 | 36.7±4.7 | 8.5±1.8 8.6±1.7 |
3.6±2.8 3.3±3.0 |
NA | NA | HbA1c, FBG, HOMA-IR |
| Zamarron et al., 2022 [17] | AHI ≥10 | CPAP No CPAP |
185 | 52 | 67±9.0 | 76 | 32.0±5.1 | 7.7±1.6 7.5±1.4 |
4.1±2.9 | 37.0±20.0 33.9±18.7 |
16±9.5 | HbA1c, FBG, HOMA-IR, |
| Zhao et al., 2022 [18] | AHI ≥5 | CPAP No CPAP |
60 | 6 | 53.4±10.2 | 58 | 30.5±4.0 | 8.5±2.3 8.7±1.7 |
8.3±2.8 | 28.3±17.8 34.5±20.4 |
7±6.5 | HbA1c, FBG, HOMA-IR |
Values are presented as mean±sd or median (interquartile range) unless otherwise stated. OSA: obstructive sleep apnoea; BMI: body mass index; HbA1c: haemoglobin A1c; CPAP: continuous positive airway pressure; AHI: apnoea–hypopnoea index; DMII: type 2 diabetes; FBG: fasting blood glucose; ODI: oxygen desaturation index; NA: not available; HOMA-IR: homeostasis model of assessment for insulin resistance index.
Changes in HbA1c
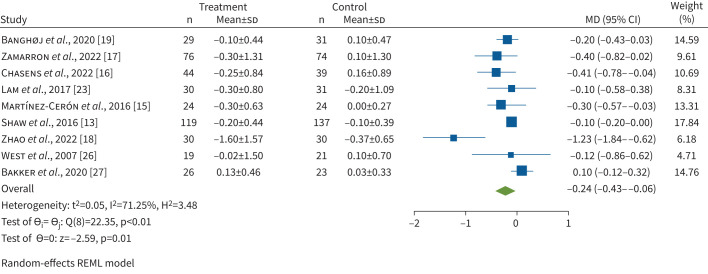
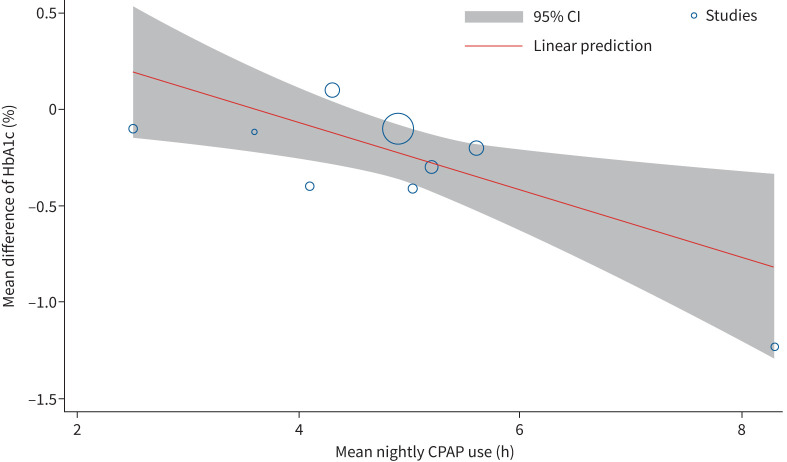
To evaluate the effect of CPAP on HbA1c, data from nine RCTs (total patients n=807) were included. CPAP treatment led to a significant reduction in HbA1c compared to inactive controls (MD −0.24%, 95% CI −0.43– −0.06%, p=0.001) (figure 2). There was significant heterogeneity among the studies (I2=71.25%). Meta-regression showed a significant association between increasing duration of CPAP use per night and reduction in HbA1c (coefficient −0.17, 95% CI −0.31– −0.04, p=0.010) (figure 3) and an association between higher baseline HbA1c values and its reduction with CPAP treatment (coefficient −0.34, 95% CI −0.54– −0.15, p=0.001) (supplementary figure S2). Between-study heterogeneity was not explained by baseline AHI, BMI or duration of diabetes. Egger's test (p=0.0393) and the funnel plot (supplementary figure S3) showed some evidence of publication bias. To investigate the heterogeneity and publication bias due to the presence of an outlier study, a sensitivity analysis was performed. After excluding the study of Zhao et al. [18], who showed the highest CPAP usage hours per night as well as the highest decrease of HbA1c after CPAP therapy, HbA1c still significantly decreased (MD −0.16%, 95% CI −0.28– −0.03%, p=0.013) (supplementary figure S4) with moderate heterogeneity (I2=39.30%) and no evidence of small study effects (p=0.333).
FIGURE 2.
Forrest plot evaluating the effect of continuous positive airway pressure therapy on HbA1c in patients with obstructive sleep apnoea and type 2 diabetes. The sizes of the Forrest plot squares represent the weighting of that trial in the random-effect meta-analysis and the horizontal lines represent the 95% confidence interval. MD: mean difference; REML: restricted maximum likelihood.
FIGURE 3.
Bubble plot evaluating the association of haemoglobin A1c (HbA1c) and mean continuous positive airway pressure (CPAP) usage hours per night. A significant association between increasing CPAP usage hours per night and a reduction of HbA1c was observed.
Change in FBG
Eight studies (total patients n=728) reported the change in FBG and were included in our meta-analysis. Combining the results of all eight studies, there was no change in FBG when comparing the CPAP group with inactive controls (MD −0.48 mmol·L−1, 95% CI −1.31–0.35 mmol·L−1, p=0.255) (supplementary figure S5). There was significant heterogeneity among the studies (I2=86.15%). Egger's test (p=0.384) did not show evidence of publication bias.
Change in fructosamine
Data on fructosamine were available from three RCTs (total patients n=167). Compared with inactive controls, CPAP treatment did not significantly lower fructosamine (MD −5.83 mmol·L−1, 95% CI −16.60–4.95 mmol·L−1, p=0.289) (supplementary figure S6). Heterogeneity assessment among the studies showed no evidence for heterogeneity (I2=0%). Egger's test (p=0.488) did not show evidence of publication bias.
Change in HOMA-IR
Four studies (total patients n=174) were included in the meta-analysis regarding change in HOMA-IR with CPAP treatment. There was no difference in HOMA-IR in the active treatment group compared to the control group (MD −0.82 mmol·L−1, 95% CI −1.67– −0.03 mmol·L−1, p=0.059) (supplementary figure S7). There was no relevant heterogeneity among the studies (I2=25.85%). Egger's test (p=0.177) did not show evidence of publication bias.
Change in 24 h plasma glucose
Three RCTs (total patients n=102) monitored 24 h plasma glucose in patients with OSA and type 2 diabetes. A significant reduction of 24 h plasma glucose with CPAP treatment was observed when compared to inactive controls (MD −0.60 mmol·L−1, 95% CI −0.72– −0.47 mmol·L−1, p=0.001) (supplementary figure S8). No heterogeneity between the studies was detected (I2=0%), with one of the studies counting for 97.6% of the results. Egger's test (p=0.672) did not show evidence of publication bias.
Discussion
This systematic review and meta-analysis assessed the current evidence on the effect of CPAP therapy on glycaemic control in patients with OSA and type 2 diabetes. The main findings from 11 RCTs including a total of 964 patients are that CPAP therapy improves HbA1c and 24 h plasma glucose but seems to have no relevant effect on FBG, HOMA-IR or fructosamine levels. In addition, this meta-analysis found an association between the reduction in HbA1c and nightly CPAP usage hours.
Several potential pathophysiological mechanisms could explain beneficial effects of CPAP treatment on glycaemic control in patients with OSA and type 2 diabetes. CPAP therapy may improve insulin sensitivity by reducing sleep fragmentation and intermittent hypoxia during sleep. Intermittent hypoxia and sleep fragmentation have been shown to cause inflammation and oxidative stress, both of which are known to contribute to insulin resistance and impaired glucose metabolism [28, 29]. Furthermore, SNS activity increases in patients with untreated OSA but can be effectively reduced by CPAP treatment [30–32]. A reduction in SNS activity is important because SNS overdrive can be linked to an increase in glycogenolysis and gluconeogenesis in the liver, a decrease of insulin release in the pancreas and enhanced lipolysis in adipose tissue [33]. OSA has also been associated with activation of the HPA axis and elevated levels of several hormones that can contribute to insulin resistance and hyperglycaemia, including cortisol, growth hormones and catecholamines [34]. Recent meta-analyses showed that CPAP therapy significantly reduced catecholamine levels as well as cortisol secretion by reducing stress induced by OSA [35, 36]. Finally, sleep deprivation and poor sleep quality are associated with disruptions in circadian rhythm, which can have a negative impact on pancreatic β-cell function and insulin sensitivity [37]. It is important to understand the effect of CPAP therapy on glycaemic control given the high prevalence of OSA in patients with type 2 diabetes and vice versa [2]. OSA emerged as a risk factor for alterations in glucose metabolism in patients with type 2 diabetes independent of other risk factors [3].
According to the results of the present meta-analysis, CPAP treatment can effectively reduce HbA1c by 0.24% (95% CI −0.43– −0.06%, p=0.001). Because we detected significant heterogeneity between the studies (I2=71.25%) and Egger's test showed evidence for publication bias, a sensitivity analysis was performed. After excluding the results of Zhao et al. [18] with the highest CPAP usage hours per night as well as the highest decrease of HbA1c after CPAP therapy (MD −1.23%, 95% CI −1.84– −0.62%), HbA1c still significantly decreased (MD −0.16%, 95% CI −0.28– −0.03%, p=0.013) with moderate heterogeneity (I2=39.30%) and no evidence of small study effects (p=0.333). In clinical terms, a reduction in HbA1c of 0.2% could alone reduce the overall mortality in patients with type 2 diabetes by 10% [38]. Therefore, effective treatment of OSA with CPAP could translate to a clinically meaningful reduction of HbA1c. To put this in perspective, carbohydrate-restricted diets showed a short-term (3–6 month) reduction of HbA1c of up to 0.19%, physical activity advice is associated with 0.43% lower HbA1c levels and oral antidiabetic drugs lead to a decrease of HbA1c by 0.3–1.1% [39–41]. Furthermore, the effect size of CPAP therapy on glycaemic control, measured by HbA1c, could be influenced by various factors. The effect of CPAP on OSA is directly linked to treatment compliance and growing evidence suggest better clinical outcomes with increasing nightly CPAP usage [42, 43]. This also seems to be the true for HbA1c. The meta-regression showed a significant association between reduction in HbA1c and nightly CPAP usage hours. Chasens et al. [16] also reported a greater reduction of HbA1c with increasing CPAP adherence; one additional hour of daily CPAP use was associated with a significant decrease of 0.08% in HbA1c in their RCT. This finding is supported by the study of Mokhlesi et al. [24], who assigned patients with OSA and type 2 diabetes to a 1-week in-laboratory full-night CPAP or sham CPAP treatment. Mean 24 h plasma glucose significantly decreased after 1 week of active versus sham CPAP treatment (MD −0.60 mmol·L−1, 95% CI −0.73– −0.47 mmol·L−1), equivalent to a drop of ∼0.4–0.5% of HbA1c, with a mean±sd CPAP adherence of 7.92±0.08 h·night−1 and 7.87±0.11 h·night−1 in the active and sham groups, respectively. The current meta-analysis included three RCTs that investigated 24-h plasma glucose level, and showed a similar reduction among the three; however, the study of Mokhlesi et al. [24] accounted for 97% of the results and therefore these findings must be interpreted with caution.
The effect size of CPAP treatment on glucose metabolism may also be dependent on the patient's baseline glycaemia control. Pharmacological treatment of type 2 diabetes is more effective in patients with worse baseline glycaemic control [44]. Supporting this, our meta-regression showed a significant association between a higher baseline HbA1c and its reduction with CPAP treatment.
Despite the positive effect of CPAP on HbA1c and 24 h plasma glucose level, no effect was observed for FBG, HOMA-IR or fructosamine in our meta-analysis. A possible explanation could be that HbA1c reflects the average blood glucose level over the most recent 2–3 months, while fructosamine and FBG represent short-term glycaemic control and thus are more influenced by short-term variability [45]. It is well known from CPAP withdrawal studies that OSA recurs within 1–2 nights after discontinuation of CPAP therapy and this is associated with a short-term increase of sympathetic activation [46, 47]. Therefore, if patients did not use their CPAP therapy the days before their follow-up visit for glycaemic control assessment, this might have affected their short-term glycaemic control.
Three meta-analyses evaluating RCTs on the effect of CPAP therapy on glycaemic control in OSA patients with type 2 diabetes have been published so far; however, their findings are inconsistent. The most recent meta-analysis [48] showed similar reductions in HbA1c (standardised MD −0.32%, 95% CI −0.60– −0.03, p=0.029), while the other two meta-analyses showed no effects of CPAP treatment on glycaemic control [49, 50]. Given that the previous published meta-analyses did not include the most recent studies and are limited by methodological issues such as inclusion of duplicates, our meta-analysis provides more reliable evidence as well as results of meta-regressions.
Risk of bias assessment of the studies included in our meta-analysis showed mostly low risk or some concerns with two studies rated as high risk. However, two studies [18, 24] did not mention the randomisation process but were classified as RCTs for this systematic review and meta-analysis according to the presented data. Therefore, additional meta-analyses were performed after exclusion of these two studies without changing the conclusion.
Our meta-analysis has some limitations. We excluded non-English studies, which may have biased our findings. Not all of the studies included a sham CPAP group as a control and the follow-up time varied between the trials. Because we did not receive raw data from all the studies included in this meta-analysis, standard equations or estimation from pooled data had to be used to calculate missing data, which may have affected the outcomes. Besides performing a meta-regression, other correlated characteristics could have influenced the outcomes and thus may have affected our results. Finally, the evidence of CPAP therapy on glycaemic control in patients with type 2 diabetes is to some extent still limited. More high-quality RCTs including comparable patient cohorts will help to define the effect of CPAP therapy on different measures of glycaemic control more precisely and determine patient populations that benefit most from this therapy.
Conclusion
CPAP therapy seems to significantly improve HbA1c and thus long-term glycaemic control in patients with type 2 diabetes and OSA. Evidence suggests an association between increasing nightly CPAP use and improved glucose metabolism and an association between higher baseline HbA1c levels and reduction of HbA1c.
Points for clinical practice
CPAP therapy improves HbA1c and thus long-term glycaemic control in patients with co-existing OSA and type 2 diabetes.
The amount of improvement is dependent on the hours of usage of CPAP; optimal adherence to CPAP should be a primary goal in these patients.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0083-2023.SUPPLEMENT (529.4KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Author contributions: J. Herth and M. Kohler developed the idea of the study. Data acquisition was done by J. Herth, F. Schmidt and N.A. Sievi. Statistical analysis was performed by J. Herth and N.A. Sievi. The manuscript was drafted by J. Herth, who had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. M. Kohler and F. Schmidt guaranteed administrative and technical material support. The study was supervised by M. Kohler. All authors contributed to the final manuscript and checked the final manuscript for correctness.
Data sharing: All data were extracted from published sources that are publicly available or were requested from individual trials. Relevant meta-level data and analytical code on which this analysis is based are available on request to the corresponding author (M. Kohler).
Conflict of Interest: M. Kohler reports consulting fees from Bayer, Novartis, GSK, Boehringer Ingelheim, Mundipharma and Astra. All other authors have nothing to disclose.
References
- 1.Garvey JF, Pengo MF, Drakatos P, et al. Epidemiological aspects of obstructive sleep apnea. J Thorac Dis 2015; 7: 920–929. doi: 10.3978/j.issn.2072-1439.2015.04.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doumit J, Prasad B. Sleep apnea in type 2 diabetes. Diabetes Spectr 2016; 29: 14–19. doi: 10.2337/diaspect.29.1.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koren D, O'Sullivan KL, Mokhlesi B. Metabolic and glycemic sequelae of sleep disturbances in children and adults. Curr Diab Rep 2015; 15: 562. doi: 10.1007/s11892-014-0562-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aurora RN, Punjabi NM. Obstructive sleep apnoea and type 2 diabetes mellitus: a bidirectional association. Lancet Respir Med 2013; 1: 329–338. doi: 10.1016/S2213-2600(13)70039-0 [DOI] [PubMed] [Google Scholar]
- 5.Iiyori N, Alonso LC, Li J, et al. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med 2007; 175: 851–857. doi: 10.1164/rccm.200610-1527OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tasali E, Leproult R, Ehrmann DA, et al. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA 2008; 105: 1044–1049. doi: 10.1073/pnas.0706446105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anothaisintawee T, Reutrakul S, Van Cauter E, et al. Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med Rev 2016; 30: 11–24. doi: 10.1016/j.smrv.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 8.Muraki I, Wada H, Tanigawa T. Sleep apnea and type 2 diabetes. J Diabetes Investig 2018; 9: 991–997. doi: 10.1111/jdi.12823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tahrani AA, Ali A, Raymond NT, et al. Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. Am J Respir Crit Care Med 2012; 186: 434–441. doi: 10.1164/rccm.201112-2135OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kakkar RK, Berry RB. Positive airway pressure treatment for obstructive sleep apnea. Chest 2007; 132: 1057–1072. doi: 10.1378/chest.06-2432 [DOI] [PubMed] [Google Scholar]
- 11.Marshall NS, Barnes M, Travier N, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax 2006; 61: 430–434. doi: 10.1136/thx.2005.050583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bratton DJ, Gaisl T, Wons AM, et al. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA 2015; 314: 2280–2293. doi: 10.1001/jama.2015.16303 [DOI] [PubMed] [Google Scholar]
- 13.Shaw JE, Punjabi NM, Naughton MT, et al. The effect of treatment of obstructive sleep apnea on glycemic control in type 2 diabetes. Am J Respir Crit Care Med 2016; 194: 486–492. doi: 10.1164/rccm.201511-2260OC [DOI] [PubMed] [Google Scholar]
- 14.Pamidi S, Wroblewski K, Stepien M, et al. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes. A randomized controlled trial. Am J Respir Crit Care Med 2015; 192: 96–105. doi: 10.1164/rccm.201408-1564OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martínez-Cerón E, Barquiel B, Bezos AM, et al. Effect of continuous positive airway pressure on glycemic control in patients with obstructive sleep apnea and type 2 diabetes. A randomized clinical trial. Am J Respir Crit Care Med 2016; 194: 476–485. doi: 10.1164/rccm.201510-1942OC [DOI] [PubMed] [Google Scholar]
- 16.Chasens ER, Korytkowski M, Burke LE, et al. Effect of treatment of OSA with CPAP on glycemic control in adults with type 2 diabetes: the Diabetes Sleep Treatment Trial (DSTT). Endocr Pract 2022; 28: 364–371. doi: 10.1016/j.eprac.2022.01.015 [DOI] [PubMed] [Google Scholar]
- 17.Zamarron E, Jaureguizar A, Garcia-Sanchez A, et al. CPAP effect on albuminuria progression in patients with obstructive sleep apnea and diabetic kidney disease. A randomized clinical trial. Am J Respir Crit Care Med 2022; 205: 1337–1348. doi: 10.1164/rccm.202206-1091OC [DOI] [PubMed] [Google Scholar]
- 18.Zhao X, Zhang W, Xin S, et al. Effect of CPAP on blood glucose fluctuation in patients with type 2 diabetes mellitus and obstructive sleep apnea. Sleep Breath 2022; 26: 1875–1883. doi: 10.1007/s11325-021-02556-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banghøj AM, Krogager C, Kristensen PL, et al. Effect of 12-week continuous positive airway pressure therapy on glucose levels assessed by continuous glucose monitoring in people with type 2 diabetes and obstructive sleep apnoea; a randomized controlled trial. Endocrinol Diabetes Metab 2020; 4: e00148. doi: 10.1002/edm2.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lefebvre C, Glanville J, Briscoe S, et al. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions, version 6.3 (updated February 2022). London, Cochrane Collaboration, 2022. [Google Scholar]
- 21.Higgins JPT AD, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. London, Cochrane Collaboration, 2011. [Google Scholar]
- 22.Higgins JPT, Li T, Deeks JJ (ed). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions, version 6.3 (updated February 2022). London, Cochrane Collaboration, 2022. [Google Scholar]
- 23.Lam JCM, Lai AYK, Tam TCC, et al. CPAP therapy for patients with sleep apnea and type 2 diabetes mellitus improves control of blood pressure. Sleep Breath 2017; 21: 377–386. doi: 10.1007/s11325-016-1428-7 [DOI] [PubMed] [Google Scholar]
- 24.Mokhlesi B, Grimaldi D, Beccuti G, et al. Effect of one week of 8-hour nightly continuous positive airway pressure treatment of obstructive sleep apnea on glycemic control in type 2 diabetes: a proof-of-concept study. Am J Respir Crit Care Med 2016; 194: 516–519. doi: 10.1164/rccm.201602-0396LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morariu EM, Chasens ER, Strollo PJ, Jr, et al. Effect of continuous positive airway pressure (CPAP) on glycemic control and variability in type 2 diabetes. Sleep Breath 2017; 21: 145–147. doi: 10.1007/s11325-016-1388-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.West SD, Nicoll DJ, Wallace TM, et al. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax 2007; 62: 969–974. doi: 10.1136/thx.2006.074351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bakker JP, Baltzis D, Tecilazich F, et al. The effect of continuous positive airway pressure on vascular function and cardiac structure in diabetes and sleep apnea. a randomized controlled trial. Ann Am Thorac Soc 2020; 17: 474–483. doi: 10.1513/AnnalsATS.201905-378OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamura A, Kawano Y, Watanabe T, et al. Obstructive sleep apnea increases hemoglobin A1c levels regardless of glucose tolerance status. Sleep Med 2012; 13: 1050–1055. doi: 10.1016/j.sleep.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 29.Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol 2019; 11: 45–63. [PMC free article] [PubMed] [Google Scholar]
- 30.Venkataraman S, Vungarala S, Covassin N, et al. Sleep apnea, hypertension and the sympathetic nervous system in the adult population. J Clin Med 2020; 9: 591. doi: 10.3390/jcm9020591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith RP, Veale D, Pepin JL, et al. Obstructive sleep apnoea and the autonomic nervous system. Sleep Med Rev 1998; 2: 69–92. doi: 10.1016/s1087-0792(98)90001-6 [DOI] [PubMed] [Google Scholar]
- 32.Maier LE, Matenchuk BA, Vucenovic A, et al. Influence of obstructive sleep apnea severity on muscle sympathetic nerve activity and blood pressure: a systematic review and meta-analysis. Hypertension 2022; 79: 2091–2104. doi: 10.1161/HYPERTENSIONAHA.122.19288 [DOI] [PubMed] [Google Scholar]
- 33.Carnagarin R, Matthews VB, Herat LY, et al. Autonomic regulation of glucose homeostasis: a specific role for sympathetic nervous system activation. Curr Diab Rep 2018; 18: 107. doi: 10.1007/s11892-018-1069-2 [DOI] [PubMed] [Google Scholar]
- 34.Akset M, Poppe KG, Kleynen P, et al. Endocrine disorders in obstructive sleep apnoea syndrome: a bidirectional relationship. Clin Endocrinol (Oxf) 2023; 98: 3–13. doi: 10.1111/cen.14685 [DOI] [PubMed] [Google Scholar]
- 35.Green M, Ken-Dror G, Fluck D, et al. Meta-analysis of changes in the levels of catecholamines and blood pressure with continuous positive airway pressure therapy in obstructive sleep apnea. J Clin Hypertens (Greenwich) 2021; 23: 12–20. doi: 10.1111/jch.14061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ken-Dror G, Fry CH, Murray P, et al. Changes in cortisol levels by continuous positive airway pressure in patients with obstructive sleep apnoea: meta-analysis of 637 individuals. Clin Endocrinol (Oxf) 2021; 95: 909–917. doi: 10.1111/cen.14573 [DOI] [PubMed] [Google Scholar]
- 37.Mason IC, Qian J, Adler GK, et al. Impact of circadian disruption on glucose metabolism: implications for type 2 diabetes. Diabetologia 2020; 63: 462–472. doi: 10.1007/s00125-019-05059-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khaw KT, Wareham N, Luben R, et al. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of European Prospective Investigation of Cancer and Nutrition (EPIC-Norfolk). BMJ 2001; 322: 15–18. doi: 10.1136/bmj.322.7277.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sainsbury E, Kizirian NV, Partridge SR, et al. Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract 2018; 139: 239–252. doi: 10.1016/j.diabres.2018.02.026 [DOI] [PubMed] [Google Scholar]
- 40.Fang HSA, Gao Q, Tan WY, et al. The effect of oral diabetes medications on glycated haemoglobin (HbA1c) in Asians in primary care: a retrospective cohort real-world data study. BMC Med 2022; 20: 22. doi: 10.1186/s12916-021-02221-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA 2011; 305: 1790–1799. doi: 10.1001/jama.2011.576 [DOI] [PubMed] [Google Scholar]
- 42.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 2007; 30: 711–719. doi: 10.1093/sleep/30.6.711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masa JF, Corral-Penafiel J. Should use of 4 h continuous positive airway pressure per night be considered acceptable compliance? Eur Respir J 2014; 44: 1119–1120. doi: 10.1183/09031936.00121514 [DOI] [PubMed] [Google Scholar]
- 44.Kanatsuka A, Sato Y, Kawai K, et al. Relationship between the efficacy of oral antidiabetic drugs and clinical features in type 2 diabetic patients (JDDM38). J Diabetes Investig 2016; 7: 386–395. doi: 10.1111/jdi.12430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saudek CD, Brick JC. The clinical use of hemoglobin A1c. J Diabetes Sci Technol 2009; 3: 629–634. doi: 10.1177/193229680900300402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agaltsov MV, Drapkina OM. Effect of withdrawing long-term CPAP therapy on the course of obstructive sleep apnea. Russian Journal of Cardiology 2021; 26: 4314. [Google Scholar]
- 47.Thiel S, Haile SR, Peitzsch M, et al. Endocrine responses during CPAP withdrawal in obstructive sleep apnoea: data from two randomised controlled trials. Thorax 2019; 74: 1102–1105. doi: 10.1136/thoraxjnl-2019-213522 [DOI] [PubMed] [Google Scholar]
- 48.Shang W, Zhang Y, Wang G, et al. Benefits of continuous positive airway pressure on glycaemic control and insulin resistance in patients with type 2 diabetes and obstructive sleep apnoea: a meta-analysis. Diabetes Obes Metab 2021; 23: 540–548. doi: 10.1111/dom.14247 [DOI] [PubMed] [Google Scholar]
- 49.Zhu B, Ma C, Chaiard J, et al. Effect of continuous positive airway pressure on glucose metabolism in adults with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Sleep Breath 2018; 22: 287–295. doi: 10.1007/s11325-017-1554-x [DOI] [PubMed] [Google Scholar]
- 50.Labarca G, Reyes T, Jorquera J, et al. CPAP in patients with obstructive sleep apnea and type 2 diabetes mellitus: systematic review and meta-analysis. Clin Respir J 2018; 12: 2361–2368. doi: 10.1111/crj.12915 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0083-2023.SUPPLEMENT (529.4KB, pdf)