Abstract
In the present study, we examined the feasibility of using recombinant antibodies containing murine variable regions and human constant regions as calibrators or controls in immunoassays. As a model system, we chose the Abbott IMx Toxo immunoglobulin M (IgM) and Toxo IgG assays designed to detect antibodies to Toxoplasma gondii. Two mouse monoclonal antibodies were selected based on their reactivity to the T. gondii antigens P30 and P66. Heavy- and light-chain variable-region genes were cloned from both hybridomas and transferred into immunoglobulin expression vectors containing human kappa and IgG1 or IgM constant regions. The constructs were stably transfected into Sp2/0-Ag14 cells. In the IMx Toxo IgG assay, immunoreactivity of the anti-P30 chimeric IgG1 antibody paralleled that of the positive human plasma-derived assay calibrators. Signal generated with the anti-P66 chimeric IgG1 antibody was observed to plateau below the maximal reactivity observed for the assay calibrator. Examination of the IgM chimeric antibodies in the IMx Toxo IgM assay revealed that both the anti-P30 and anti-P66 antibodies matched the assay index calibrator manufactured with human Toxo IgM-positive plasma. When evaluated with patient samples, the correlation between results obtained with the chimeric antibody calibrators and the positive human plasma calibrators was ≥0.985. These data demonstrate that chimeric mouse-human antibodies are a viable alternative to high-titer positive human plasma for the manufacture of calibrators and controls for diagnostic assays.
Serological immunoassays designed to detect specific antibody present in patient samples provide a rapid and sensitive method to monitor for infectious agents, allergy, and autoimmunity (2). Diagnostic immunoassays and kits designed to measure antibodies typically include one or more components containing the specific antibody being measured that function as calibrators (standards) and/or a positive control (3). Calibrators are used to establish calibration curves in quantitative assays for interpolation of antibody concentration in the patient sample, or alternatively, a single index calibrator may be used to establish the assay cutoff in a qualitative assay. The positive control is used to establish assay performance characteristics and is a useful indicator of the integrity of the reagents. Calibrators and positive controls are usually prepared by spiking known quantities of specific antibody derived from seropositive plasma or serum into the negative control reagent.
However, the use of plasma or serum has several significant drawbacks, including increasing difficulty in sourcing large volumes with high titer and specificity, lot-to-lot variability in immunoglobulin G (IgG) or IgM specificity and affinity, limitations with respect to characterization, and cost.
An alternative method for the manufacture of calibrators and controls that circumvents the use of human plasma or serum would represent a significant advance. Hybridoma technology (12) offers an indefinite supply of monoclonal antibodies; however, murine antibodies do not react in the assay format required to measure specific human IgG or IgM and therefore cannot be used to standardize assays to measure human antibodies. With the development of recombinant DNA technology, it has become possible to combine the heavy (H)- and light (L)-chain variable (V) regions of a desired mouse monoclonal antibody with human constant (C) regions creating hybrid (chimeric) antibody molecules (1, 15). Chimeric antibodies can be reproducibly generated in virtually unlimited quantities and are homogeneous in specificity and affinity.
In the present study, we developed mouse-human chimeric IgM and IgG1 antibodies specific for Toxoplasma gondii and evaluated their performance as calibrators in the Abbott IMx Toxo IgM and IMx Toxo IgG assays (13, 16).
MATERIALS AND METHODS
Cell lines.
The hybridomas 1-706-139 (H1) and 5-465-210 (H5) were established by fusion of immunized Swiss Webster spleen cells to Sp2/0-Ag14 cells (Abbott Laboratories, Abbott Park, Ill.; American Type Culture Collection, Rockville, Md.).
Isolation of immunoglobulin V regions.
PCR cloning of immunoglobulin V regions was performed as previously described (6) with primers designed by Jones and Bendig (9) with altered restriction sites (Table 1). M-IgG2b and M-IgG2a were used for specific cDNA synthesis of heavy-chain V regions from H1 and H5, respectively. MK-REV was used for cDNA priming of kappa (κ) light-chain variable regions. H1 VH and Vκ regions were amplified with primer combinations MHV-9/M-IgG2b and MKV-1/MK-REV, respectively. For H5, VH and Vκ regions were amplified with MHV-7/M-IgG2a and MKV-2/MK-REV, respectively. Amplifications consisted of 30 cycles of 94°C for 30 s, 52°C for 30 s, and 72°C for 1 min. PCR-derived products were isolated by the Promega (Madison, Wis.) Magic PCR Preps DNA purification system, digested (SalI and BglII), and cloned into pUC18 (BRL Life Technologies, Gaithersburg, Md.).
TABLE 1.
Oligonucleotides used to generate chimeric antibody constructs
| Oligonucleotide | Sequencea |
|---|---|
| M-IgG2b | 5′ ATTCGGATAGATCTAGTGGATAGACTGATGG 3′ |
| M-IgG2a | 5′ ATTCGGATAGATCTAGTGGATAGACCGATGG 3′ |
| MK-REV | 5′ ATTCGGATAGATCTTGGATGGTGGGAAGATG 3′ |
| MHV-9 | 5′ ACACTAGTCGACATGGMTTGGGTGTGGAMCTTGCTATTCCTG 3′ |
| MKV-1 | 5′ ACACTAGTCGACATGAAGTTGCCTGTTAGGCTGTTGGTGCTG 3′ |
| MHV-7 | 5′ ACACTAGTCGACATGGRATGGAGCKGGRTCTTTMTCTT 3′ |
| MKV-2 | 5′ ACACTAGTCGACATGGAGWCAGACACACTCCTGYTATGGGT 3′ |
| A | 5′ TCAGGTGACTGAACTAGTCCTTGGTGGGGCAGCCACAGCG 3′ |
| B | 5′ CTGATCGAGATATCAAGCCACTGAGGCACGCAGGTGGGTG 3′ |
| VK1-5′ | 5′ TCACGAAGTCTAGACCTCAAATGAAGTTGCCTGTTAGGCTGTTGGTG 3′ |
| VK1-3′ | 5′ GAATCTATGGATCCTGACACACTTACGTTTGATTTCCAGCTTGGTGCCTCC 3′ |
| VH1-5′ | 5′ ACACTATACTCGAGACATCATGGCTTGGGTGTGGACCTTGCTA 3′ |
| VH1-3′ | 5′ TTCAGATCAAGCTTGACACACTTACCTGAGGAGACGGTGACTGAGGTTCC 3′ |
| VK5-5′ | 5′ TCACGAAGTCTAGAGCTCTCAGAGATGGAGTCAGACACACTCCTGCTA 3′ |
| VK5-3′ | 5′ GAATCTATGGATCCTGACACACTTACGTTTTATTTCCAGCTTGGTCCCCG 3′ |
| VH5-5′ | 5′ ACACTATACTCGAGACTCCAACCATGGGATGGAGCTGGATCTTTCTC 3′ |
| VH5-3′ | 5′ TTCAGATCAAGCTTGACACACTTACCTGAGGAGACTGTGAGAGGGGTG 3′ |
Restriction endonuclease sites are underlined.
Chimeric IgM expression constructs.
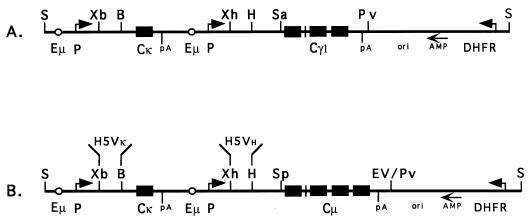
The immunoglobulin expression vector pdHL2 (5) (Fig. 1A) was modified by replacing the human IgG1 constant region (Cγ1) with a human IgM constant region (Cμ). First, the oligonucleotides 5′ GGAACTAGTGGAGC 3′ and 5′ TCCACTAGTTCCGC 3′ were annealed and cloned into the SacII site in pdHL2 (upstream of Cγ1) to introduce a SpeI site. Next, the secretory portion of the Cμ gene (membrane exons excluded) was PCR amplified by using 1 ng of a plasmid containing a genomic clone of human Cμ (pN · χ-μTNP DNA; provided by Marc Shulman, University of Toronto, Toronto, Ontario, Canada) as template, 50 pmol each of primers A (Table 1) (SpeI cloning site) and B (EcoRV cloning site), and 24 cycles of 94°C for 30 s, 63°C for 30 s, and 72°C for 90 s. Gel-isolated Cμ product (spanning from 128 nucleotides [nt] upstream of Cμ1 exon to 152 nt downstream of the polyadenylation signal after Cμ4 [positions 17 to 2278 in reference 20]) was ligated into pGEM-T (Promega) and sequenced. Finally, the SpeI-EcoRV fragment containing Cμ was excised and cloned into SpeI-PvuII-digested pdHL2, replacing Cγ1.
FIG. 1.
Schematic diagram of the IgG1 expression vector pdHL2 (A) and anti-P30 IgM construct pJH2-24-95B1 (B). The locations of the genomic Cκ, Cμ, and Cγ1 genes are indicated. Abbreviations: Eμ, immunoglobulin H-chain enhancer; P, the metallothionein promoter; pA, polyadenylation signal sequence; ori, pBR322 origin of replication; AMP, β-lactamase gene; DHFR, dihydrofolate reductase gene. The positions of Vκ and VH cassettes are shown in panel B. Abbreviations of unique restriction sites: B, BamHI; EV, EcoRV; H, HindIII; Pv, PvuII, S, SalI; Sa, SacII; Sp, SpeI; Xb, XbaI; Xh, XhoI.
Primers used for PCR-mediated transfer of Vκ and VH are shown in Table 1. The H5 Vκ and VH gene fragments were amplified with primer combinations VK5-5′/VK5-3′ and VH5-5′/VH5-3′, respectively. The 100-μl-volume reaction mixtures included 50 pmol of each primer and 1 ng of plasmid containing a V-region gene (see above). Amplification consisted of 22 cycles of 94°C for 30 s, 62°C for 30 s, and 72°C for 1 min. Vκ product was digested with XbaI-BamHI and cloned into the vector. Subsequently, VH product was introduced into the Vκ-containing vector by using XhoI-HindIII, generating pJH2-24-95B1 (Fig. 1B).
The H1 Vκ and VH gene transfer was performed in the same manner by using primer combinations VK1-5′/VK1-3′ and VH1-5′/VH1-3′, respectively, yielding pJH3-19-95A.
Transfer of V regions into the IgG1 expression vector pdHL2.
An XbaI-HindIII fragment containing both Vκ and VH was excised from each of the IgM vectors and introduced into XbaI-HindIII-digested pdHL2, yielding pJH9-14-94/4.1 (H1 set) and pdHL-2/5-465 (H5 set).
Sequencing.
The plasmid template for sequencing was prepared with Magic Miniprep or Maxiprep DNA purification systems (Promega), the EasyPrep Plasmid Prep kit (Pharmacia), or by CsCl banding. Nucleotide sequences were determined manually by using Sequenase version 1.0 with a Sequenase 7-deaza-dGTP DNA sequencing kit (Amersham Life Science, Inc., Arlington Heights, Ill.) or by automated sequencing with the Pharmacia AutoRead sequencing kit and ALF DNA sequencer.
Transfection of murine myeloma cells.
Sp2/0-Ag14 cells were transfected by electroporation as previously described (6). IgM constructs were transfected by using a modified version of the protoplast fusion technique (19). Briefly, Sp2/0-Ag14 cells were resuspended in the protoplast suspension, transferred to a 60-mm-diameter dish, and centrifuged at 650 × g for 8 min. The supernatant was aspirated, and 1.5 ml of 50% (wt/vol) polyethylene glycol (Sigma Hybri-Max) in phosphate-buffered saline (PBS) prewarmed to 37°C was added. The dish was centrifuged at 110 × g until 90 s had elapsed after polyethylene glycol addition. The cells were resuspended by pipetting in two 5-ml volumes and one 10-ml volume of prewarmed wash solution (Dulbecco Modified Eagle medium [DMEM] supplemented with 1% fetal bovine serum, 50 U of penicillin per ml, and 50 μg of streptomycin per ml), which were added to a 50-ml centrifuge tube containing 15 ml of the wash solution. After centrifugation at 225 × g for 7.5 min, the cells were resuspended in plating medium (DMEM supplemented with 10% fetal bovine serum, 50 U of penicillin per ml, 50 μg of streptomycin per ml, and 100 μg of kanamycin per ml [Sigma]), plated in one 96-well dish, and incubated at 37°C. After 24 h (day 1), 100 μl of selective medium (plating medium supplemented with 0.1 μM methotrexate [MTX; Adria Laboratories, Columbus, Ohio]) was added. On day 5, 50 μl of the selective medium was added per well. After 2 days, 100 μl was removed from each well and 100 μl of fresh selective medium was added per well. MTX-resistant colonies were tested for secretion of chimeric antibody by an enzyme-linked immunosorbent assay (ELISA). Transfectants secreting chimeric antibody were cloned by limiting dilution and passaged in medium containing 0.1 μM MTX.
Assays for chimeric antibody production.
Transfectants were assayed by ELISA for production of chimeric IgG1 as previously described (6). To assay for chimeric IgM, plates were coated with 0.36 μg of goat anti-human IgM (Fc5μ-specific; Jackson ImmunoResearch, West Grove, Pa.) per μl in Dulbecco’s phosphate-buffered saline (D-PBS). Peroxidase-labeled goat anti-human IgM (Fc5μ; Jackson ImmunoResearch) at 0.8 μg/ml was used as the enzyme antibody conjugate, with ChromPure human IgM (Jackson ImmunoResearch) as a standard.
Antibody concentrations were determined by radial immunodiffusion (RID). Seven- to 14-day cell culture supernatants were vortexed, and then 5- and 10-μl samples for IgG and IgM, respectively, were applied to each well of human IgG or human IgM RID plates (The Binding Site, San Diego, Calif.). Standards (25, 50, 75, 100, and 150 μg/ml) were run on each plate. The IgM and IgG1 standards were ChromPure human IgM and protein A-purified anti-P66 IgG1, respectively. Plates were incubated in an inverted position at 35 to 37°C for 16 to 22 h. Ring diameter was determined by using a calibrated RID electronic plate reader (The Binding Site).
Purification of chimeric antibodies.
To purify chimeric IgG1 antibodies, culture supernatants were dialyzed overnight against 0.1 M sodium phosphate (pH 8.2) with 0.1% sodium azide, filtered (0.2-μm-pore-size filter), and passed through a column containing PerSeptive Biosystems (Cambridge, Mass.) Poros 50A resin by using a Bio-Rad low-pressure chromatography system. Antibody was eluted with 0.1 M citrate–0.15 M NaCl (pH 3.0), and pooled fractions were dialyzed against PBS (10 mM sodium phosphate, 150 mM NaCl [pH 7.2]).
To purify chimeric IgM antibodies, culture supernatants were diluted 1:1 with H2O and loaded on a column containing 175 ml of PBS-equilibrated DEAE FastFlow resin (Pharmacia). Antibody was eluted with 10 mM sodium phosphate–300 mM NaCl at pH 7.2.
IMx evaluation of chimeric calibrators.
Reactivity of chimeric IgG1 and IgM antibodies was measured with the Abbott IMx Toxo IgG 2.0 antibody assay (Abbott Laboratories) and the Abbott IMx Toxo IgM 2.0 antibody assay, respectively. Testing was done as described in the assay package insert, except where chimeric antibodies were substituted for calibrators in the assay. Reactivity rates are expressed as counts per second per second.
Chimeric IgG and IgM antibodies were diluted into anti-Toxoplasma antibody-negative plasma to evaluate their reactivity in the assays and to prepare calibrator sets. Point-to-point data reduction was used to generate calibration curves and determine patient specimen IgG values. Index values in the IgM assay are generated by dividing the patient specimen assay rate by the index calibrator assay rate.
Nucleotide sequence accession numbers.
GenBank accession numbers for V region sequences are as follows: for 1-706-139 Vκ, AF031633; for 1-706-139 VH, AF031634; for 5-465-210 Vκ, AF031635; and for 5-465-210 VH, AF031636.
RESULTS
Selection of monoclonal antibodies.
A panel of murine monoclonal antibodies specific for a variety of T. gondii antigens was established. Two with potential utility as calibrators and controls were selected based on a combination of antigen specificity and performance in competitive binding studies with pooled human plasma reactive to T. gondii. The monoclonal antibody H5 is specific for the major surface antigen of T. gondii, P30, which is thought to be a key antigen for identification of individuals infected with T. gondii. A second monoclonal antibody, H1, reactive with P66, was also evaluated since this appears to be a potentially important protein for measurement of the IgM response to T. gondii (unpublished observations). In competitive binding experiments with the IMx Toxo IgM index calibrator, each of these monoclonal antibodies showed some inhibition of human IgM antibody binding (data not shown).
Cloning and sequencing of antibody variable regions.
To generate chimeric mouse-human antibodies, the H- and L-chain V regions were cloned from H5 and H1. The V genes were amplified by PCR using a combination of degenerate primers annealing to conserved VH and Vκ gene leader sequences and C region-specific primers (9). Multiple clones of each V gene product were sequenced to monitor for AmpliTaq DNA polymerase-induced errors.
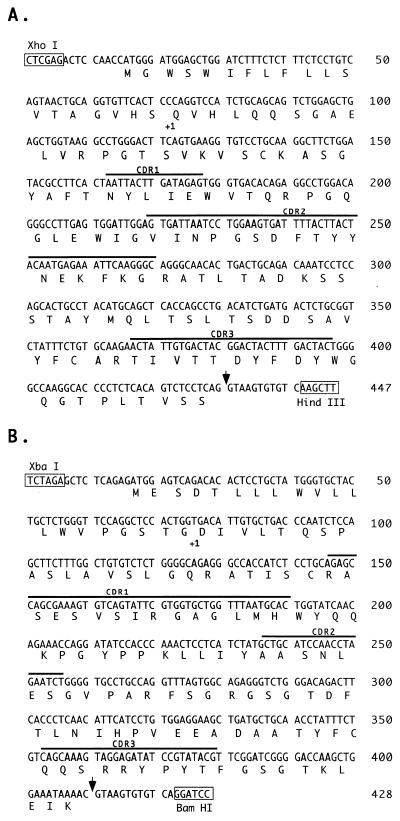
The VH and VL cDNA sequences isolated from H5 are shown in Fig. 2A and B, respectively. The VH cDNA carries a functionally rearranged V region gene consisting of a leader, a VH gene, a DH region, and a JH2 minigene. The VH gene is a member of murine subgroup IIB (10). The functional VL cDNA consists of a leader, a Vκ gene from the murine subgroup III (10), and a Jκ2 minigene. The Jκ2 segment contains four mismatches relative to the germ line sequence of Jκ2 isolated from the BALB/c mouse strain (10). The observed mismatches may reflect somatic mutations or represent differences in the germ line sequence since the hybridoma was generated from a Swiss Webster strain mouse.
FIG. 2.
Nucleotide and deduced amino acid sequences of the VH cassette (A) and the Vκ cassette (B) derived from H5. The first amino acid of mature protein is indicated by +1; the splice points of JH2 to the C region gene (A) and of Jκ2 to Cκ (B) are indicated by the arrows; complementarity-determining regions (CDRs) as defined in reference 10 are indicated. Features of panel A: nt 1 to 39, sense primer; 16 to 72, leader; 73 to 366, V gene; 367 to 384, D segment; 385 to 429, JH2; 430 to 447, splice and cloning sites. Features of panel B: nt 1 to 40, sense primer; 17 to 76, leader; 77 to 373, V gene; 374 to 409, Jκ2; 410 to 428, splice and cloning sites.
The VH and VL cDNA sequences isolated from H1 are shown in Fig. 3A and B, respectively. The functional VH region includes a leader, a VH gene from murine subgroup IIA (10), a DH segment, and a JH4 minigene. The exact boundary between the rearranged DH segment and the JH4 minigene could not be determined. The functional VL cDNA consists of a leader, a Vκ gene from the murine subgroup II (10), and a Jκ1 minigene.
FIG. 3.
Nucleotide and deduced amino acid sequences of VH cassette (A) and Vκ cassette (B) derived from H1. The first amino acid of mature protein is indicated by +1; the splice point of JH4 to the C region gene (A) and the splice junction of Jκ1 to Cκ (B) are marked with arrows; complementarity-determining regions (CDRs) are indicated. Features of panel A: nt 1 to 35, sense primer; 12 to 68, leader; 69 to 362, V gene; 363 to 380, D segment (precise D-J junction is unclear); 381 to 434, JH4; 434 to 452, splice and cloning sites. Features of panel B: nt 1 to 39, sense primer; 13 to 69, leader; 70 to 368, V gene; 369 to 405, Jκ1; 406 to 424, splice and cloning sites.
Construction of mouse-human chimeric antibodies.
The VH and VL gene fragments were modified to form cassettes (Fig. 2 and 3) suitable for expression with any C region containing a functional splice acceptor site. As a result of RNA processing, the V and C regions are fused in the cDNA clones. Therefore, adaptation to the cassette format involved recreation of a splice donor site at the end of the V region and addition of flanking cloning sites. A leader sequence was already present in the cDNA clones. However, in cases where degenerate positions were present in the amplification primers, the sequence was modified, if necessary, based on a comparison to leader sequences in the database.
To produce chimeric mouse-human IgG1, the V gene cassettes were cloned into the immunoglobulin expression vector pdHL2 (5) (Fig. 1A). This vector contains genomic clones of the human kappa (Cκ) and IgG1 (Cγ1) C-region genes. Both loci are controlled by a metallothionein I promoter and a mouse immunoglobulin H-chain enhancer. An altered dihydrofolate reductase gene serves as a selectable marker. Introduction of the murine VH and Vκ regions results in expression of chimeric mouse-human IgG1 antibody.
To produce chimeric mouse-human IgM antibodies, pdHL2 was modified by replacement of the Cγ1 gene with a genomic clone of the human IgM (Cμ) gene. Sequence analysis of the Cμ clone revealed only a single base change relative to the published germ line sequence (20), the insertion of a T between residues 1583 and 1584 in the Cμ3-Cμ4 intron. This alteration would not be expected to affect expression of the Cμ gene since it is in the intron. The H1 and H5 VH and Vκ cassettes were cloned into the IgM expression vector to generate chimeric IgM antibody specific for P66 and P30, respectively.
Expression of IgM and IgG chimeric antibodies.
The chimeric antibody constructs were transfected by electroporation into Sp2/0-Ag14 cells. Multiple MTX-resistant colonies from each transfection were screened for production of chimeric antibody by using an anti-human antibody ELISA. Transfectants that tested positive were expanded and cloned. Clones secreting high levels of chimeric antibody were further subcloned. The observed production levels for the anti-P30 and anti-P66 chimeric IgG1 antibodies were 90 μg/ml or greater. In contrast, IgM transfectants established by electroporation secreted very low levels of antibody. Protoplast fusion is an alternative method for transfection (1, 19) that often results in introduction of higher copy numbers of vector. The IgM constructs were introduced into Sp2/0-Ag14 cells by protoplast fusion in an effort to enhance expression. A clone secreting anti-P30 chimeric IgM antibody that produces 115 μg/ml was isolated. Transfection of the anti-P66 chimeric IgM construct met with more limited success. Transfectants secreting chimeric IgM were obtained, but secretion levels were low (<1 μg/ml). In all but one case, production of recombinant chimeric antibody by the transfectants greatly exceeded that of the hybridomas from which they were derived. Analysis of the IgM chimeric antibody revealed that it is pentameric.
Assay performance of the chimeric antibodies.
Purified chimeric IgG1 and IgM antibodies were examined for immunoreactivity to T. gondii in the IMx Toxo IgG and IMx Toxo IgM assays. These assays utilize microparticles coated with formalin-treated T. gondii as the solid phase to capture T. gondii-specific antibodies. The captured antibodies are detected with anti-human IgG or IgM antibody-labeled conjugates.
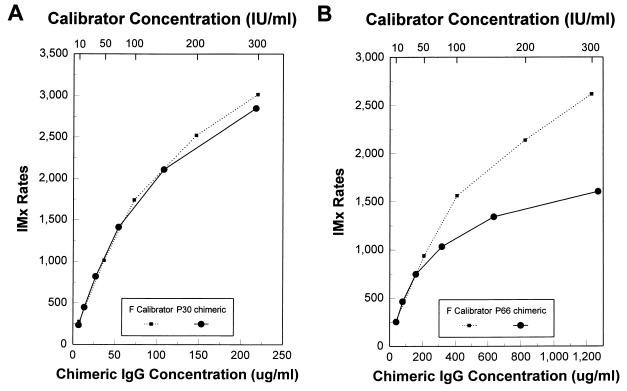
Reactivity of purified anti-P30 chimeric IgG1 was compared to the reactivity of human anti-T. gondii antibody in the IMx Toxo IgG assay. The calibrators used in this assay are manufactured from pooled anti-T. gondii IgG-positive plasma and are matched to the World Health Organization International Standard for anti-T. gondii antibody at the following levels: 0, 10, 50, 100, 200, and 300 IU/ml. The F calibrator (300 IU/ml) marks the upper end of the dynamic range of the assay. The anti-P30 chimeric IgG1 antibody at 218 μg/ml was found to give the same signal as the F calibrator in the IMx assay. Twofold serial dilutions of the anti-P30 chimeric IgG1 antibody into negative human serum were tested in duplicate. The observed calibration curve for the chimeric IgG1 antibody was similar to the dilution profile of the human plasma-derived assay calibrator (Fig. 4A). At a concentration of 870 μg/ml, the anti-P30 chimeric IgG1 antibody generated a signal of 4,261 cps/s in the assay.
FIG. 4.
Evaluation of IgG chimeric antibodies in the IMx Toxo IgG assay. The curve of a twofold dilution of chimeric IgG1 antibodies is shown in relation to the calibration curve of the IMx Toxo IgG assay generated with human plasma. (A) Anti-P30 chimeric IgG antibody; (B) anti-P66 chimeric IgG1 antibody.
The dilution curve for the anti-P66 chimeric IgG1 antibody, compared to the human plasma-derived assay calibration curve, is shown in Fig. 4B. The starting concentration of the purified anti-P66 chimeric IgG1 antibody was 1.27 mg/ml, and a signal of only 1,600 cps/s was achieved. The signal begins to plateau at antibody concentrations between 300 and 600 μg/ml and never reaches the level of the F calibrator (300 IU/ml). Concentration of the antibody to 11.59 mg/ml did not result in a significant increase in the IMx assay signal generated.
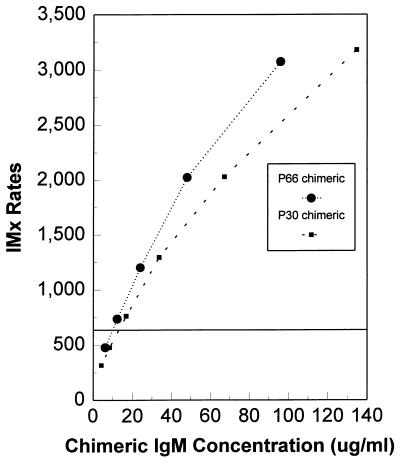
The IMx Toxo IgM assay differs from the IMx Toxo IgG assay in that it is a qualitative assay. The purpose of the calibrator in the IgM assay is to establish the assay cutoff based on the rate value generated with a consistent concentration of anti-T. gondii IgM antibodies. Purified anti-P30 chimeric IgM and anti-P66 chimeric IgM antibodies were diluted by twofold serial dilutions into negative human plasma, and the resulting values were compared to that of the IMx Toxo IgM index calibrator. The anti-P30 chimeric IgM and anti-P66 chimeric IgM antibodies gave signals equivalent to that of the index calibrator at concentrations of ∼13 and 10 μg/ml, respectively (Fig. 5).
FIG. 5.
Evaluation of IgM chimeric antibodies in the IMx Toxo IgM assay. Twofold dilutions of the anti-P30 and anti-P66 chimeric IgM antibodies were assayed. The horizontal line represents the index calibrator rate value.
Evaluation of patient samples by using kits calibrated with chimeric antibody-derived calibrators.
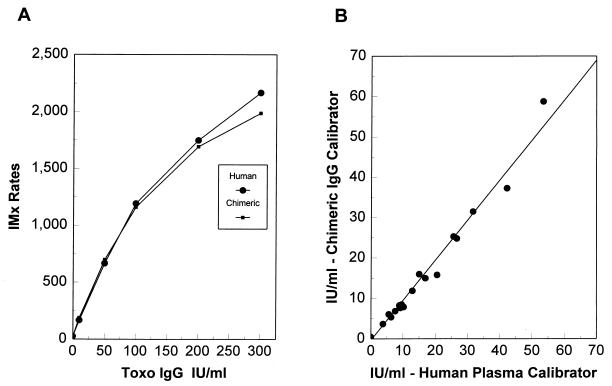
The anti-P30 chimeric IgG1 and IgM antibodies were diluted to match human plasma-derived calibrators to demonstrate the utility of the chimeric antibody as a source of calibrators for the IMx Toxo IgG and IMx Toxo IgM assays. Human plasma-derived calibrators for the IMx Toxo IgG assay ranged from 0 to 300 IU/ml. The anti-P30 chimeric IgG1 antibody was diluted in negative human plasma to match each level of the human plasma calibrators within 2% of IMx assay calibrator rates. Calibration curves obtained for each of the kit calibrators and anti-P30 chimeric IgG1-derived calibrators were similar (Fig. 6A). Thirty-six serum specimens with anti-Toxoplasma IgG concentrations less than 60 IU/ml were evaluated in the IMx Toxo IgG assay using the chimeric antibody calibrators as the source of the standard curve, and the results were compared with those of the same specimens run in the assay using human plasma-derived calibrators. The correlation exhibited an r2 value of 0.985 and a slope of 0.991 (Fig. 6B).
FIG. 6.
Comparison of IgG1 chimeric calibrator to human plasma-derived calibrator in the IMx Toxo IgG assay. (A) Calibration curve generated with IMx Toxo IgG kit calibrators compared to curve of anti-P30 chimeric IgG1 antibody calibrators matched to each level; (B) specimen correlation comparing calibration with anti-P30 chimeric IgG1 calibrators to calibration with the kit human plasma calibrators (n = 36; r2, 0.985; slope, 0.991).
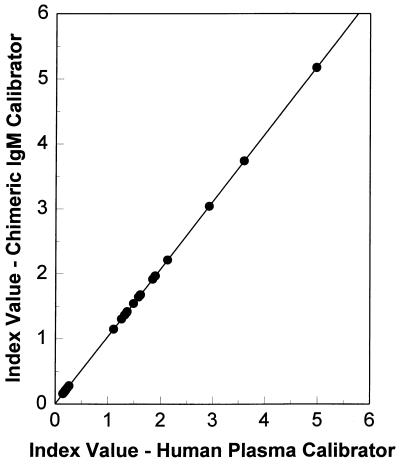
The anti-P30 chimeric IgM antibody was diluted in negative human plasma to match within 2% the rate level of the human plasma IgM index calibrator used in the IMx Toxo IgM assay. The specimen results are expressed as index values relative to the index calibrator. Specimens with index values equal to or greater than 0.600 are considered reactive for IgM antibody to T. gondii. Thirty-one serum specimens were evaluated in the IMx Toxo IgM assay using the anti-P30 chimeric antibody index calibrator, and the results were compared with those of the same specimens run in the assay using human plasma-derived calibrators. The correlation exhibited an r2 value of 0.999 and a slope of 1.039 (Fig. 7).
FIG. 7.
Comparison of IgM chimeric calibrator to human plasma-derived calibrator in the IMx Toxo IgM assay. Shown is specimen correlation comparing calibration with the anti-P30 chimeric IgM index calibrator to calibration with the IMx Toxo IgM assay human plasma-derived index calibrator (n = 31; r2, 0.999; slope, 1.039).
DISCUSSION
In the present report, we describe the development and utility of mouse-human chimeric antibodies as calibrating reagents in automated immunoassays measuring human antibodies to T. gondii. Murine monoclonal antibodies specific for P30 (11) and P66 (18) were selected for conversion to mouse-human chimeric antibodies. Functional immunoglobulin V region genes were cloned from the hybridoma cells and transferred into expression vectors containing human constant-region genes to generate recombinant mouse-human chimeric IgM and IgG1 antibodies. In all cases, the recombinant chimeric antibodies retained their native specificity.
Analysis of the anti-P30 IgG1 chimeric antibody revealed that it was fully functional in the Toxo IgG immunoassay designed to measure human IgG antibody to T. gondii. The signal generated by the anti-P30 IgG1 chimeric antibody at a concentration of 218 μg/ml matched that of the F calibrator which is set at the high end of the dynamic range of the Toxo IgG assay. Moreover, the calibration curve obtained with serial dilutions of the anti-P30 chimeric IgG1 antibody was parallel to that of the human plasma-derived assay calibrator (Fig. 4A). Such parallelism between dilution curves of the test and reference (calibrator) antibodies is required to provide accurate quantitation of specific antibody (3, 8). To establish the utility of the chimeric calibrator, 36 T. gondii-reactive clinical specimens were run in the IMx Toxo IgG assay using the kit calibrators and the resulting values were compared to the values obtained with the anti-P30 IgG1 chimeric antibody calibrators. The observed correlation between the two types of calibrators was excellent. Based on these data, the anti-P30 IgG1 chimeric antibody provides an excellent alternative to the use of positive human plasma in the manufacture of calibrators.
In contrast, although the anti-P66 IgG1 chimeric antibody was reactive in the IMx Toxo IgG assay, it did not reach the maximum rate read of the positive human plasma calibrators. Attempts to increase the signal generated by the chimeric antibody by increasing antibody input concentration were unsuccessful. This suggests that the P66 antigen on the surface of the microparticles is limiting. Unless the available P66 epitope were to be increased, the anti-P66 IgG1 chimeric antibody alone does not appear to be a viable alternative for manufacture of calibrators.
Since the Toxo IgG calibrator is made with polyclonal T. gondii-reactive human plasma, it would be expected to contain antibodies against many different T. gondii proteins (17, 18) and to multiple epitopes on each antigen. Thus, the observation that the signal of a monoclonal reagent specific for one epitope of a single T. gondii protein is unable to match the cumulative signal generated by the polyclonal calibrator reagent is not necessarily unexpected. It is conceivable that blending of two or more monoclonal reagents may better mimic the polyclonal human antibody calibrator and more closely approximate the routine human test samples. In preliminary tests where the anti-P66 IgG1 chimeric antibody was combined with the anti-P30 chimeric IgG1, a signal exceeding the high end of the dynamic range (F calibrator) was achievable and the dilution profile was similar to that of the human plasma-derived Toxo IgG calibrator (data not shown). The concept of utilizing more than one chimeric antibody to generate a calibrator might become particularly important if one is measuring a response to more than one epitope of an antigen or to more than one antigen when considering issues such as the differential stability of antigen reagents in immunoassay kits. If the epitope recognized by one of the chimeric antibodies was impaired, a second epitope may be required to accurately calibrate the assay. Determination of whether it is advantageous to pool more than one chimeric antibody reagent will be dependent upon the desired performance characteristics for the calibrator and can be determined empirically.
As opposed to the quantitative Toxo IgG assay, the Toxo IgM assay is a qualitative assay that utilizes a single index calibrator to determine the cutoff value. When examined in the Toxo IgM assay, acceptable calibration curves were obtained independently for both the anti-P30 and anti-P66 IgM chimeras. The feasibility of using a chimeric antibody calibrator was examined by comparing the results obtained for 31 T. gondii-reactive specimens in the Toxo IgM assay to those obtained by using the anti-P30 chimeric IgM index calibrator. There was a high correlation between the results generated with the human plasma-derived and chimeric IgM antibody calibrators. These data demonstrate that the anti-P30 IgM chimeric antibody can be substituted for T. gondii-reactive human plasma in the manufacture of an index calibrator.
An advantage of chimeric antibody technology in the manufacture of calibrating reagents is its flexibility. One can readily screen candidate monoclonal antibodies for desired properties such as specificity and affinity prior to the more labor-intensive steps of cloning the V regions and production of chimeric antibody. Binding properties of the chimeric antibody should reflect those of the original monoclonal antibody. In the present study, the choice of antibody specificity was based on competitive inhibition studies using T. gondii-reactive human plasma in an effort to identify monoclonal antibodies reacting with immunodominant epitopes. Presumably, the more closely the epitope reactivity of the chimeric antibodies and human test samples are matched, the better the calibrator. In some cases, this may require the use of more than one chimeric antibody recognizing different antigens or of multiple epitopes on the same antigen. Ideally, the affinity of the chimeric antibodies would be similar to that of the antibodies being monitored in the test samples. Other factors such as epitope stability (i.e., after heat stress or prolonged storage) can also be examined prior to conversion of the monoclonal antibody to the chimeric format.
In addition to epitope specificity, proper selection of heavy-chain isotype is also important. In most cases, immunoassays that monitor specific antibody are designed to detect antibody classes or subclasses with in vivo or diagnostic relevance. In patients acutely infected with T. gondii, IgM is the predominant class of antibody, whereas in patients with acquired or reactivated toxoplasmosis, IgG1, IgG2, IgG3, and IgA antibodies can be detected (4). The IMx Toxo IgG assay uses a polyclonal anti-human IgG as the conjugate reagent. We found that the chimeric IgG1 antibody calibrates the IMx assay accurately over multiple lots of the polyclonal anti-human IgG reagent (data not shown).
The use of chimeric antibodies in preparation of calibrators and positive controls for immunoassays offers several substantial advantages over traditional methods utilizing high-titer plasma or serum, as well as other alternatives such as chemical coupling of human IgG Fc′ fragments to a murine monoclonal antibody (14). First, the chimeric antibody can be produced continuously with the same affinity and specificity. Reduction in lot-to-lot variability over time with respect to antibody class composition, titer, specificity, and affinity would be expected to yield a consistent calibrator that should give the most reproducible patient results. Second, virtually unlimited quantities of the chimeric antibodies can be generated. Recombinant cell lines can stably produce chimeric antibodies at manufacturable levels and at a reasonable cost. Finally, the homogeneous nature of the chimeric antibodies allows for better characterization.
Mouse-human chimeric antibodies have previously been shown to be useful as quality control reagents and for quantitation of specific antibody in reference standards by heterologous interpolation (7, 8). The present study establishes the feasibility of using mouse-human chimeric antibodies as calibrator reagents in automated immunoassays for the measurement of human antibodies specific for T. gondii. This is the first demonstration of the utility of chimeric antibody calibrators for homologous interpolation of specific human antibody levels. Chimeric antibody calibrators should be applicable to any diagnostic assay or kit designed to detect the presence of human antibodies specific for a given antigen (e.g., infectious agent, autoantigen, allergen, pharmaceutical).
ACKNOWLEDGMENTS
We thank Robert Ziemann, Jeffrey Alder, Joan Tyner, Lawrence Howard, and Michael Sheu for generation, screening, and characterization of the monoclonal antibodies; Odin Cabal for assistance with the protoplast fusions; and Richard Thomas for purification of the chimeric antibodies.
REFERENCES
- 1.Boulianne G L, Hozumi N, Shulman M J. Production of functional chimaeric mouse/human antibody. Nature. 1984;312:643–646. doi: 10.1038/312643a0. [DOI] [PubMed] [Google Scholar]
- 2.Butler J E, editor. Immunochemistry of solid-phase immunoassay. Boca Raton, Fla: CRC Press, Inc.; 1991. [Google Scholar]
- 3.Butler J E, Hamilton R G. Quantitation of specific antibodies: methods of expression, standards, solid-phase considerations, and specific applications. In: Butler J E, editor. Immunochemistry of solid-phase immunoassay. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 173–198. [Google Scholar]
- 4.Derouin F, Sulcebe G, Ballet J J. Sequential determination of IgG subclasses and IgG specific antibodies in primary and reactivating toxoplasmosis. Biomed Pharmacother. 1987;41:429–433. [PubMed] [Google Scholar]
- 5.Gillies S D, Lo K-M, Wesolowski J. High-level expression of chimeric antibodies using adapted cDNA variable region cassettes. J Immunol Methods. 1989;125:191–202. doi: 10.1016/0022-1759(89)90093-8. [DOI] [PubMed] [Google Scholar]
- 6.Hackett J, Jr, Hoff-Velk J, Golden A, Dealwis C, Ostrow D, Mandecki W. The effect of site-specific mutagenesis of a cysteine residue on the stability of a monoclonal antibody. In: Hori W, editor. Antibody engineering II. 2. New technology, application and commercialization. Southborough, Mass: International Business Communications, Inc., USA; 1997. pp. 133–157. [Google Scholar]
- 7.Hamilton R G. Engineered human antibodies as immunologic quality control reagents. Ann Biol Clin. 1990;48:473–477. [PubMed] [Google Scholar]
- 8.Hamilton R G. Application of engineered chimeric antibodies to the calibration of human antibody standards. Ann Biol Clin. 1991;49:242–248. [PubMed] [Google Scholar]
- 9.Jones S T, Bendig M M. Rapid PCR-cloning of full-length mouse immunoglobulin genes. Bio/Technology. 1991;9:88–89. doi: 10.1038/nbt0191-88. [DOI] [PubMed] [Google Scholar]
- 10.Kabat E A, Wu T T, Perry H M, Gottesman K S, Foeller C. Sequences of proteins of immunological interest. 5th ed. Bethesda, Md: U.S. Department of Health and Human Services; 1991. [Google Scholar]
- 11.Kasper L H, Crabb J H, Pfefferkorn E R. Purification of a major membrane protein of Toxoplasma gondii by immunoabsorption with a monoclonal antibody. J Immunol. 1983;130:2407–2412. [PubMed] [Google Scholar]
- 12.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 13.Liesenfeld O, Press C, Flanders R, Ramirez R, Remington J S. Study of Abbott Toxo IMx system for detection of immunoglobulin G and immunoglobulin M toxoplasma antibodies: value of confirmatory testing for diagnosis of acute toxoplasmosis. J Clin Microbiol. 1996;34:2526–2530. doi: 10.1128/jcm.34.10.2526-2530.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyachi J, Doi K, Kitamura K, Jitsukawa T, Watanabe H. Chemically humanized murine monoclonal antibody against a cell nuclear antigen: usefulness in autoimmune diagnostics. J Clin Lab Anal. 1992;6:343–350. doi: 10.1002/jcla.1860060602. [DOI] [PubMed] [Google Scholar]
- 15.Morrison S L, Johnson M J, Herzenberg L A, Oi V T. Chimeric human antibodies: mouse antigen binding domains with human constant regions. Proc Natl Acad Sci USA. 1984;81:6851–6855. doi: 10.1073/pnas.81.21.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Safford J W, Abbott G G, Craine M C, MacDonald R G. Automated microparticle enzyme immunoassays for IgG and IgM antibodies to Toxoplasma gondii. J Clin Pathol. 1991;44:238–242. doi: 10.1136/jcp.44.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santoro F, Charif H, Capron A. The immunodominant epitope of the major membrane tachyzoite protein (P30) of Toxoplasma gondii. Parasite Immunol. 1986;8:631–639. doi: 10.1111/j.1365-3024.1986.tb00876.x. [DOI] [PubMed] [Google Scholar]
- 18.Sharma S D, Mullenax J, Araujo F G, Erlich H A, Remington J S. Western blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J Immunol. 1983;131:977–983. [PubMed] [Google Scholar]
- 19.Shin S, Morrison S L. Production and properties of chimeric antibody molecules. Methods Enzymol. 1989;178:459–476. doi: 10.1016/0076-6879(89)78034-4. [DOI] [PubMed] [Google Scholar]
- 20.Word C J, White M B, Kuziel W A, Shen A L, Blattner F R, Tucker P W. The human immunoglobulin Cμ-Cγ locus: complete nucleotide sequence and structural analysis. Int Immunol. 1989;1:296–309. doi: 10.1093/intimm/1.3.296. [DOI] [PubMed] [Google Scholar]