Abstract
Background
The absence of distinct symptoms in the majority of individuals with metabolic dysfunction-associated fatty liver disease (MAFLD) poses challenges in identifying those at high risk, so we need simple, efficient and cost-effective noninvasive scores to aid healthcare professionals in patient identification. While most noninvasive scores were developed for the diagnosis of nonalcoholic fatty liver disease (NAFLD), consequently, the objective of this study was to systematically assess the diagnostic ability of 12 noninvasive scores (METS-IR/TyG/TyG-WC/TyG-BMI/TyG-WtHR/VAI/HSI/FLI/ZJU/FSI/K-NAFLD) for MAFLD.
Methods
The study recruited eligible participants from two sources: the National Health and Nutrition Examination Survey (NHANES) 2017-2020.3 cycle and the database of the West China Hospital Health Management Center. The performance of the model was assessed using various metrics, including area under the receiver operating characteristic curve (AUC), net reclassification index (NRI), integrated discrimination improvement (IDI), decision curve analysis (DCA), and subgroup analysis.
Results
A total of 7398 participants from the NHANES cohort and 4880 patients from the Western China cohort were included. TyG-WC had the best predictive power for MAFLD risk in the NHANES cohort (AUC 0.863, 95% CI 0.855–0.871), while TyG-BMI had the best predictive ability in the Western China cohort (AUC 0.903, 95% CI 0.895–0.911), outperforming other models, and in terms of IDI, NRI, DCA, and subgroup analysis combined, TyG-WC remained superior in the NAHANES cohort and TyG-BMI in the Western China cohort.
Conclusions
TyG-BMI demonstrated satisfactory diagnostic efficacy in identifying individuals at a heightened risk of MAFLD in Western China. Conversely, TyG-WC exhibited the best diagnostic performance for MAFLD risk recognition in the United States population. These findings suggest the necessity of selecting the most suitable predictive models based on regional and ethnic variations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-023-01902-3.
Keywords: Metabolic dysfunction-associated fatty liver disease, External validation of prediction models, Receiver operating characteristic curve, Net reclassification index, Integrated discrimination improvement, Decision curve analysis
Introduction
Nonalcoholic fatty liver disease (NAFLD) has emerged as the predominant etiology of chronic liver disease on a global scale, affecting approximately one-third of the world’s population [1]. Additionally, NAFLD is intricately linked with comorbidities such as diabetes, hypertension, insulin resistance (IR), dyslipidemia, and heightened susceptibility to cardiovascular disease [2–4]. As research and comprehension of NAFLD have advanced, the initial “exclusionary” concept and diagnostic criteria are no longer deemed suitable for directing clinical practice and scientific investigation. Consequently, the international hepatology community has recommended the adoption of a new disease name, metabolic dysfunction-associated fatty liver disease (MAFLD), and corresponding diagnostic criteria to supersede the original NAFLD disease name and diagnostic criteria [5, 6]. Nevertheless, the majority of MAFLD patients remain asymptomatic, underscoring the necessity to explore an efficacious tool for predicting and diagnosing fatty liver at an early stage. Noninvasive diagnostic scoring has garnered significant clinical attention in recent years, owing to its noninvasive nature, ease of use, reproducibility, and minimal operator skill requirements, which is particularly useful for early screening and assessment of MAFLD [7].
IR has been identified as a crucial factor in the pathogenesis of fatty liver disease [8, 9]. The triglyceride glucose (TyG) index, which comprises fasting plasma glucose (FPG) and triglycerides (TG), has emerged as a dependable alternative marker of IR [10, 11]. Furthermore, numerous investigations have demonstrated that TyG-related indices derived from TyG (TyG-BMI/TyG-WC/TyG-WtHR) exhibit superior predictive capability for IR, owing to the robust correlation between IR and obesity, waist circumference (WC), and waist-to-height ratio (WtHR) [12, 13]. Consequently, subsequent investigations have evaluated the diagnostic potential of TyG-related indices for NAFLD in light of these discoveries, but there were significant differences in diagnostic efficacy between articles, and the metrics assessed by these studies stopped at comparing the area under the receiver operating characteristic curve (AUC) [14–19]. Similarly, the metabolic score for insulin resistance (METS-IR) [20], a recently proposed alternative index to IR, has also been shown to have good predictive value for NAFLD [21, 22]. The predictive value of TyG-related indices and METS-IR in MAFLD should be further validated in all aspects.
Additionally, there are previously constructed noninvasive diagnostic models for NAFLD that should also be validated in MAFLD. The hepatic steatosis index (HSI) [23], developed by Lee et al. in Korea in 2010, utilizes ultrasonography and incorporates the ALT/AST ratio, BMI, and presence of diabetes as its components. Similarly, the visceral adiposity index (VAI) [24], developed in Italy in 2010 by Amato et al., employs ultrasonography as a diagnostic criterion for fatty liver and includes WC, BMI, TG, and HDL. The lipid accumulation product (LAP) [25], introduced in 2005, is an index that utilizes the National Health and Nutrition Examination Survey (NHANES) III data and comprises WC and TG as its components. The fatty liver index (FLI) is a widely utilized diagnostic model for fatty liver disease that was established in Italy in 2006 by Bedogni et al. [26]. This model employs ultrasonography as a diagnostic criterion and incorporates variables such as body mass index (BMI), waist circumference (WC), triglycerides (TG), and γ-glutamyl transferase (GGT). In 2015, the Zhejiang University index (ZJU) [27] was developed in China, which includes BMI, FPG, TG, and the ALT/AST ratio. Additionally, the Framingham steatosis index (FSI) [28] was constructed by Long et al. in 2016 in the United States based on computed tomography (CT) and includes variables such as age, sex, BMI, ALT/AST ratio, presence of hypertension, and diabetes. Jeong et al. [29] employed a sample size of 3,634 individuals from the Korean National Health and Nutrition Examination Survey (KNHANES) conducted between 2008 and 2010. They developed the KNHANES NAFLD (K-NAFLD) score, incorporating variables such as sex, WC, systolic blood pressure (SBP), FPG, TG, and ALT.
However, the majority of the noninvasive indices and models mentioned above were developed for the diagnosis of NAFLD, and their applicability to MAFLD requires further validation. Consequently, this study aims to systematically validate the diagnostic accuracy of 12 noninvasive scores for MAFLD, utilizing the NHANES dataset and the dataset from the Health Management Center of West China Hospital at Sichuan University. Upon reviewing the pertinent literature, we discovered that this study represents the most extensive investigation of noninvasive models, providing a more comprehensive evaluation.
Materials and methods
Data sources
The present study sourced its data from NHANES 2017-2020.3, a research initiative that employs a sophisticated, multistage, probability sampling technique to gather a representative sample for evaluating the health and nutritional status of both adults and children in the United States. The NHANES study protocol was backed by the National Center for Health Statistics (NCHS). Moreover, an additional Western China cohort was sourced from the Health Management Center of West China Hospital at Sichuan University. The study protocol was approved by the Ethics Committee of West China Hospital at Sichuan University and was conducted in accordance with the ethical principles delineated in the Declaration of Helsinki. Furthermore, the present investigation adhered to the identical methodology as delineated in the Transparent Reporting of a Multivariable Predictive Model for Individual Prognosis or Diagnosis (TRIPOD) guidelines [30].
Laboratory measurement and clinical data
The NHANES dataset and the Health Management Center dataset of West China Hospital at Sichuan University were utilized as the primary sources for all variables, encompassing demographic parameters, anthropometric parameters, comorbidities, and laboratory factors, as expounded upon in the Supplementary information. The online supplement provides definitions of demographics, lifestyle, and comorbidities, including racial status, diabetes [31], and hypertension [32]. Formulas for calculating METS-IR [20], TyG [11], TyG-BMI [13], TyG-WC [33], TyG-WtHR [12], HSI [23], VAI [24], FLI [26], LAP [25], ZJU [27], FSI [28], and K-NAFLD [29] are shown in the online supplement as well.
Definition of MAFLD
The utilization of the controlled attenuation parameter (CAP) via VCTE proves advantageous in identifying individuals afflicted with hepatic steatosis [34, 35]. The current investigation incorporates a threshold of CAP ≥ 258 dB/m to indicate the presence of substantial hepatic steatosis, which is based on prior research [36]. The most recent expert consensus delineates the diagnosis of MAFLD as the presence of hepatic steatosis in conjunction with one or more of the following: overweight/obesity, diabetes, or metabolic dysfunction (details are available in the Supplementary Materials) [5, 6].
Statistical analyses
R (version 4.2.2) was used for statistical analyses. Statistical significance was defined as P < 0.05. Continuous variables are shown as the mean ± standard deviation (SD) and were compared by Student’s t test or the Mann‒Whitney U test. Categorical values are shown as % and were compared using the χ2 test.
To evaluate the predictive value of noninvasive indices and models, the investigation generated receiver operating characteristic (ROC) curves and contrasted specific parameters, such as AUC, sensitivity (SEN), specificity (SPE), positive predictive value (PPV), and negative predictive value (NPV). The Delong approach was employed to ascertain whether there were statistically significant disparities in AUC between noninvasive scores [37]. Furthermore, the present study determined optimal cutoff values utilizing the Youden index. Additionally, subgroup analysis was conducted based on demographic characteristics such as age, sex, and race, as well as health indicators including overweight status, hypertension, and diabetes. Moreover, given the nonintuitive nature of the significance of AUC increments, the study also employed integrated discrimination improvement (IDI), net weight classification index (NRI) [38, 39], and decision curve analysis (DCA) [40] to further evaluate the findings.
Results
Characteristics of the study population
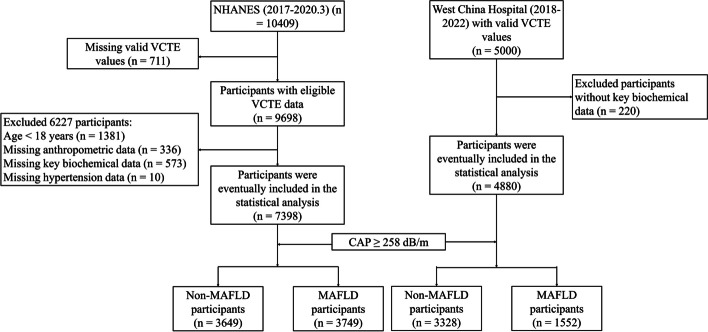
Excluding participants without significant variables, a total of 7398 subjects were included in the NHANES cohort from the 10,409 subjects in the 2017-2030.3 NHANES cycle, as depicted in Fig. 1. Likewise, a Western China cohort comprising 4880 patients with valid VCTE and key variables was recruited between 2018 and 2022 at the West China Hospital of Sichuan University. Both cohorts were grouped according to whether they met the diagnostic criteria for MAFLD [5, 6].
Fig. 1.
Flow diagram of study design
Table 1 presents the demographic and clinical features of the two cohorts, namely, the NHANES cohort and the Western China cohort. The NHANES cohort exhibited a MAFLD prevalence of 50.68%, with a mean age of 48.96 ± 18.06 years (45.70 ± 18.99 in the non-MAFLD group and 52.12 ± 16.49 in the MAFLD group). Notably, significant differences in baseline characteristics were observed between participants with and without MAFLD, except for creatinine (CRE). In contrast, the Western China cohort demonstrated a MAFLD prevalence of 31.80%, with a mean age of 44.15 ± 12.14 years (43.64 ± 12.22 in the non-MAFLD group and 45.23 ± 11.89 in the MAFLD group). Similarly, statistically significant differences in all baseline characteristics were observed between participants with and without MAFLD in this cohort.
Table 1.
Baseline characteristics of participants with or without MAFLD assessed by VCTE in the NHANES cohort and the Western China cohort
| Variables | NHANES cohort | Western China cohort | ||||
|---|---|---|---|---|---|---|
| Non-MAFLD (n = 3649) | MAFLD (n = 3749) | P value | Non-MAFLD (n = 3328) | MAFLD (n = 1552) | P value | |
| Demographic parameters | ||||||
| Age (years) | 45.70 ± 18.99 | 52.12 ± 16.49 | < 0.001 | 43.64 ± 12.22 | 45.23 ± 11.89 | < 0.001 |
| Sex (%) | < 0.001 | < 0.001 | ||||
| Female | 1996 (54.70%) | 1750 (46.68%) | 1639 (49.25%) | 303 (19.52%) | ||
| Male | 1653 (45.30%) | 1999 (53.32%) | 1689 (50.75%) | 1249 (80.48%) | ||
| Race (%) | < 0.001 | - | ||||
| Non-Hispanic Black | 1028 (28.17%) | 828 (22.09%) | - | - | ||
| Non-Hispanic White | 1235 (33.84%) | 1359 (36.25%) | - | - | ||
| Other Hispanic | 362 (9.92%) | 409 (10.91%) | - | - | ||
| Non-Hispanic Asian | 513 (14.06%) | 378 (10.08%) | - | - | ||
| Mexican American | 331 (9.07%) | 596 (15.90%) | - | - | ||
| Other races | 180 (4.93%) | 179 (4.77%) | - | - | ||
| Anthropometric parameters | ||||||
| WC (cm) | 90.51 ± 13.43 | 109.80 ± 15.01 | < 0.001 | 60.33 ± 9.85 | 75.30 ± 10.65 | < 0.001 |
| WtHR | 0.55 ± 0.08 | 0.66 ± 0.09 | < 0.001 | 0.47 ± 0.05 | 0.54 ± 0.04 | < 0.001 |
| BMI (kg/m2) | 26.06 ± 5.44 | 33.35 ± 7.02 | < 0.001 | 22.17 ± 2.55 | 26.60 ± 2.51 | < 0.001 |
| VCTE parameters | ||||||
| CAP (dB/m) | 213.26 ± 35.47 | 314.24 ± 39.31 | < 0.001 | 221.48 ± 25.34 | 287.23 ± 23.95 | < 0.001 |
| LSM (kPa) | 5.15 ± 4.10 | 6.76 ± 5.84 | < 0.001 | 4.93 ± 1.01 | 5.62 ± 1.11 | < 0.001 |
| Serum test | ||||||
| ALT (U/L) | 18.83 ± 19.02 | 25.73 ± 18.38 | < 0.001 | 21.04 ± 15.46 | 35.72 ± 23.75 | < 0.001 |
| AST (U/L) | 21.19 ± 15.43 | 22.56 ± 13.28 | < 0.001 | 21.58 ± 11.37 | 25.94 ± 12.13 | < 0.001 |
| ALP (U/L) | 74.46 ± 26.03 | 81.16 ± 25.37 | < 0.001 | 72.56 ± 21.16 | 81.03 ± 22.04 | < 0.001 |
| GGT (U/L) | 26.22 ± 55.77 | 36.89 ± 46.53 | < 0.001 | 26.56 ± 33.27 | 48.19 ± 50.57 | < 0.001 |
| CRE (umol/L) | 78.62 ± 39.12 | 79.77 ± 41.76 | 0.223 | 74.10 ± 15.93 | 81.50 ± 26.99 | < 0.001 |
| eGFR (ml/min/m2) | 98.05 ± 23.95 | 93.09 ± 23.18 | < 0.001 | 98.82 ± 14.96 | 95.56 ± 14.50 | < 0.001 |
| UA (umol/L) | 298.94 ± 79.40 | 342.24 ± 87.21 | < 0.001 | 321.70 ± 81.35 | 387.55 ± 88.18 | < 0.001 |
| FPG (umol/L) | 5.17 ± 1.27 | 6.07 ± 2.36 | < 0.001 | 4.93 ± 0.96 | 5.46 ± 1.52 | < 0.001 |
| TG (umol/L) | 1.21 ± 0.78 | 1.86 ± 1.29 | < 0.001 | 1.31 ± 1.25 | 2.20 ± 1.58 | < 0.001 |
| TC (umol/L) | 4.70 ± 1.02 | 4.87 ± 1.06 | < 0.001 | 4.79 ± 0.90 | 4.96 ± 0.94 | < 0.001 |
| HDL (umol/L) | 1.50 ± 0.41 | 1.26 ± 0.36 | < 0.001 | 1.49 ± 0.38 | 1.18 ± 0.27 | < 0.001 |
| Noninvasive indices and models | ||||||
| TyG | 8.37 ± 0.54 | 8.90 ± 0.64 | < 0.001 | 8.36 ± 0.59 | 8.98 ± 0.62 | < 0.001 |
| TyG-BMI | 218.59 ± 50.10 | 296.81 ± 65.24 | < 0.001 | 186.00 ± 29.41 | 239.05 ± 29.79 | < 0.001 |
| TyG-WC | 759.51 ± 135.51 | 977.98 ± 153.37 | < 0.001 | 652.72 ± 102.74 | 815.28 ± 98.47 | < 0.001 |
| TyG-WtHR | 4.58 ± 0.84 | 5.85 ± 0.91 | < 0.001 | 3.96 ± 0.58 | 4.86 ± 0.56 | < 0.001 |
| METS-IR | 36.99 ± 9.39 | 51.46 ± 12.33 | < 0.001 | 31.40 ± 5.79 | 41.69 ± 6.19 | < 0.001 |
| HSI | 34.30 ± 6.45 | 43.78 ± 8.06 | < 0.001 | 30.70 ± 4.13 | 37.71 ± 4.80 | < 0.001 |
| VAI | 1.52 ± 1.60 | 2.81 ± 2.58 | < 0.001 | 1.53 ± 2.79 | 2.92 ± 2.89 | < 0.001 |
| LAP | 37.39 ± 33.12 | 88.53 ± 65.59 | < 0.001 | 23.41 ± 29.28 | 60.81 ± 50.99 | < 0.001 |
| FLI | 34.73 ± 28.07 | 75.70 ± 22.75 | < 0.001 | 18.15 ± 19.20 | 53.45 ± 24.11 | < 0.001 |
| ZJU | 36.15 ± 6.24 | 45.55 ± 7.91 | < 0.001 | 32.21 ± 3.60 | 38.61 ± 4.03 | < 0.001 |
| FSI | -2.00 ± 1.38 | 0.23 ± 1.67 | < 0.001 | -2.71 ± 1.24 | -0.86 ± 1.42 | < 0.001 |
| K-NAFLD | -2.24 ± 2.80 | 1.14 ± 3.08 | < 0.001 | -3.31 ± 2.33 | 0.01 ± 3.24 | < 0.001 |
| Metabolic diseases | ||||||
| Hypertension | < 0.001 | < 0.001 | ||||
| No | 2701 (74.02%) | 2015 (53.75%) | 3036 (91.23%) | 1226 (78.99%) | ||
| Yes | 948 (25.98%) | 1734 (46.25%) | 292 (8.77%) | 326 (21.01%) | ||
| Diabetes | < 0.001 | < 0.001 | ||||
| No | 3324 (91.09%) | 2639 (70.39%) | 3251 (97.69%) | 1429 (92.07%) | ||
| Yes | 325 (8.91%) | 1110 (29.61%) | 77 (2.31%) | 123 (7.93%) | ||
Continuous variables are shown as mean ± SD and compared by Student’s t-test or Mann-Whitney U-test. Categorical values are shown as % and compared using the χ2 test
Abbreviations: MAFLD Metabolic-associated fatty liver disease, NHANES National Health and Nutrition Examination Survey, VCTE Vibration-controlled transient elastography, CAP Controlled attenuation parameter, LSM Liver stiffness measurements, BMI Body mass index, WC Waist circumference, WtHR Waist to height ratio, FPG Fasting plasma glucose, ALT Alanine aminotransferase, AST Aspartate aminotransferase, GGT γ-glutamyl transpeptidase, ALP Alkaline phosphatase, TC Total cholesterol, TG Triglyceride, HDL High-density lipoprotein cholesterol, UA Uric acid, CRE Creatinine, eGFR Estimated glomerular filtration rate
In each of the studied cohorts, it was observed that individuals diagnosed with MAFLD exhibited a greater tendency toward advanced age, male sex, and a higher prevalence of hypertension and diabetes. Additionally, these subjects demonstrated elevated levels of a range of noninvasive indices and models, including METS-IR, TyG, TyG-BMI, TyG-WC, TyG-WtHR, HSI, VAI, FLI, LAP, ZJU, FSI, and K-NAFLD.
Performances of noninvasive indices and models in predicting MAFLD risk in the NHANES cohort
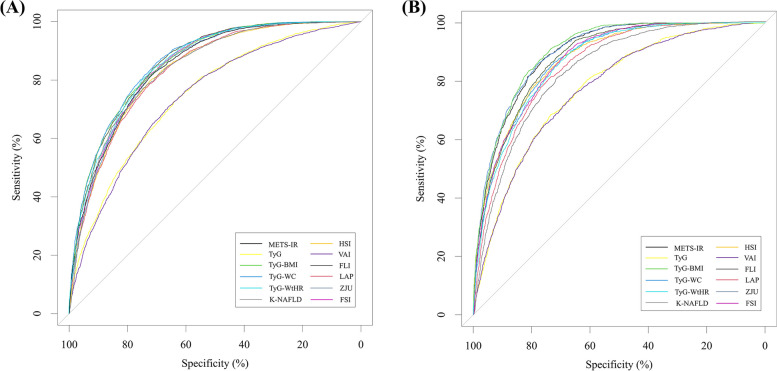
The performance of noninvasive indices or models in predicting MAFLD risk in the NHANES cohort was evaluated using ROC curves (Fig. 2A), with AUCs ranging from 0.741 to 0.863. As presented in Table 2, the TyG-WC exhibited the largest AUC (0.863, 95% CI: 0.855–0.871), followed by FLI (0.859 (0.851–0.867)), FSI (0.858 (0.850–0.867)), TyG-WtHR (0.853 (0.845–0.862)), TyG-BMI (0.850 (0.841–0.859)), METS-IR (0.846 (0.837–0.854)), ZJU (0.845 (0.836–0.854)), K-NAFLD (0.838 (0.829–0.847)), HSI (0.835 (0.826–0.844)), LAP (0.834 (0.825–0.843)), TyG (0.746 (0.735–0.757)), and VAI (0.741 (0.730–0.752)). Moreover, an evaluation of 12 noninvasive scores in the NHANES cohort through pairwise comparison demonstrated that TyG-WC exhibited superior predictive performance, as evidenced by a statistically significant difference in AUC compared to the remaining 11 noninvasive scores (all P < 0.05) (Supplementary Table 1). There was 70.2% specificity (SPE), 85.9% sensitivity (SEN), 82.9% negative predictive value (NPV), and 74.8% positive predictive value (PPV) for TyG-WC, while the cutoff value was 822.332 Table 3.
Fig. 2.
ROC curves for predicting MAFLD in the NHANES cohort (A) and Western China cohort (B). The x-axis is the specificity; the y-axis is the sensitivity
Table 2.
Performance assessment of the noninvasive indices and models for the prediction of MAFLD in the NHANES cohort
| Variables | AUC (95% CI) | SEN (95% CI) | SPE (95% CI) | PPV (95% CI) | NPV (95% CI) | Cutoff value |
|---|---|---|---|---|---|---|
| METS-IR | 0.846 (0.837–0.854) | 0.818 (0.805–0.830) | 0.717 (0.703–0.732) | 0.748 (0.735–0.761) | 0.793 (0.779–0.807) | 40.887 |
| TyG | 0.746 (0.735–0.757) | 0.727 (0.713–0.741) | 0.640 (0.625–0.656) | 0.675 (0.661–0.689) | 0.695 (0.680–0.711) | 8.497 |
| TyG-BMI | 0.850 (0.841–0.859) | 0.819 (0.807–0.831) | 0.727 (0.712–0.741) | 0.755 (0.741–0.768) | 0.796 (0.782–0.810) | 240.222 |
| TyG-WC | 0.863 (0.855–0.871) | 0.859 (0.848–0.870) | 0.702 (0.687–0.717) | 0.748 (0.735–0.760) | 0.829 (0.816–0.842) | 822.332 |
| TyG-WtHR | 0.853 (0.845–0.862) | 0.810 (0.798–0.823) | 0.740 (0.726–0.754) | 0.762 (0.749–0.775) | 0.791 (0.778–0.805) | 5.066 |
| HSI | 0.835 (0.826–0.844) | 0.799 (0.787–0.812) | 0.722 (0.708–0.737) | 0.747 (0.734–0.761) | 0.778 (0.764–0.792) | 37.131 |
| VAI | 0.741 (0.730–0.752) | 0.711 (0.697–0.726) | 0.658 (0.643–0.673) | 0.681 (0.667–0.696) | 0.689 (0.674–0.705) | 1.494 |
| FLI | 0.859 (0.851–0.867) | 0.839 (0.828–0.851) | 0.714 (0.700-0.729) | 0.751 (0.738–0.764) | 0.812 (0.799–0.826) | 49.405 |
| LAP | 0.834 (0.825–0.843) | 0.787 (0.773-0.800) | 0.725 (0.711–0.740) | 0.746 (0.733–0.760) | 0.768 (0.754–0.782) | 45.180 |
| ZJU | 0.845 (0.836–0.854) | 0.826 (0.814–0.838) | 0.711 (0.696–0.726) | 0.746 (0.733–0.759) | 0.799 (0.786–0.813) | 38.522 |
| FSI | 0.858 (0.850–0.867) | 0.768 (0.755–0.782) | 0.775 (0.761–0.789) | 0.778 (0.765–0.792) | 0.765 (0.751–0.779) | -1.068 |
| K-NAFLD | 0.838 (0.829–0.847) | 0.771 (0.757–0.784) | 0.748 (0.734–0.762) | 0.759 (0.745–0.772) | 0.760 (0.746–0.774) | -1.023 |
Abbreviations: AUC Area under the receiver operating characteristic curve, SPE Specificity, SEN Sensitivity, NPV Negative predictive value, PPV Positive predictive value
Table 3.
Performance assessment of the noninvasive indices and models for the prediction of MAFLD in the Western China cohort
| Variables | AUC (95% CI) | SEN (95% CI) | SPE (95% CI) | PPV (95% CI) | NPV (95% CI) | Cutoff value |
|---|---|---|---|---|---|---|
| METS-IR | 0.896 (0.888–0.905) | 0.876 (0.859–0.892) | 0.760 (0.745–0.774) | 0.630 (0.609–0.650) | 0.929 (0.919–0.939) | 35.223 |
| TyG | 0.776 (0.762–0.789) | 0.685 (0.662–0.708) | 0.736 (0.721–0.751) | 0.547 (0.525–0.570) | 0.834 (0.820–0.847) | 8.680 |
| TyG-BMI | 0.903 (0.895–0.911) | 0.836 (0.817–0.854) | 0.811 (0.798–0.825) | 0.674 (0.653–0.695) | 0.914 (0.904–0.924) | 211.515 |
| TyG-WC | 0.873 (0.864–0.883) | 0.865 (0.848–0.882) | 0.724 (0.709–0.739) | 0.594 (0.574–0.614) | 0.920 (0.909–0.930) | 714.871 |
| TyG-WtHR | 0.866 (0.856–0.876) | 0.904 (0.889–0.919) | 0.667 (0.651–0.683) | 0.559 (0.539–0.578) | 0.937 (0.927–0.947) | 4.198 |
| HSI | 0.873 (0.863–0.883) | 0.842 (0.824–0.860) | 0.736 (0.721–0.751) | 0.598 (0.578–0.619) | 0.909 (0.898–0.920) | 33.032 |
| VAI | 0.773 (0.759–0.786) | 0.755 (0.733–0.776) | 0.657 (0.641–0.673) | 0.506 (0.486–0.527) | 0.852 (0.838–0.865) | 1.426 |
| FLI | 0.879 (0.869–0.888) | 0.848 (0.830–0.866) | 0.751 (0.736–0.765) | 0.613 (0.593–0.634) | 0.914 (0.903–0.924) | 25.876 |
| LAP | 0.854 (0.843–0.864) | 0.810 (0.790–0.829) | 0.740 (0.725–0.755) | 0.592 (0.571–0.613) | 0.893 (0.882–0.905) | 28.720 |
| ZJU | 0.900 (0.891–0.908) | 0.865 (0.848–0.882) | 0.773 (0.759–0.787) | 0.640 (0.620–0.661) | 0.925 (0.915–0.935) | 34.549 |
| FSI | 0.872 (0.863–0.882) | 0.904 (0.889–0.919) | 0.676 (0.660–0.692) | 0.565 (0.546–0.585) | 0.938 (0.928–0.948) | -2.408 |
| K-NAFLD | 0.836 (0.825–0.847) | 0.810 (0.790–0.829) | 0.715 (0.700-0.731) | 0.570 (0.550–0.591) | 0.890 (0.878–0.902) | -2.589 |
Abbreviations: AUC Area under the receiver operating characteristic curve, SPE specificity, SEN Sensitivity, NPV Negative predictive value, PPV Positive predictive value
To enhance the evaluation of the potential of noninvasive scores in identifying MAFLD risk, NRI and IDI were computed due to the nonintuitive and intricate nature of AUC increments. The outcomes revealed that the NRI and IDI values between TyG-WC and METS-IR/TyG/TyG-BMI/HSI/VAI/LAP/ZJU/FSI/K-NAFLD were greater than 0 and significantly different (all P < 0.05). Conversely, the NRI and IDI between TyG-WC and TyG-WtHR/FLI were not statistically significant, as indicated in Supplementary Table 1.
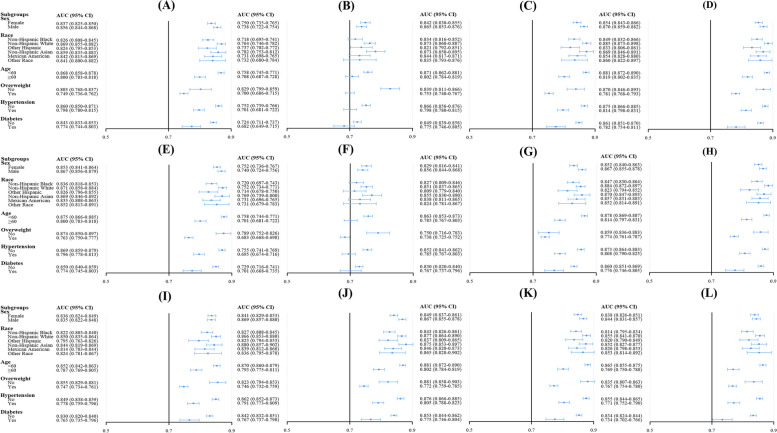
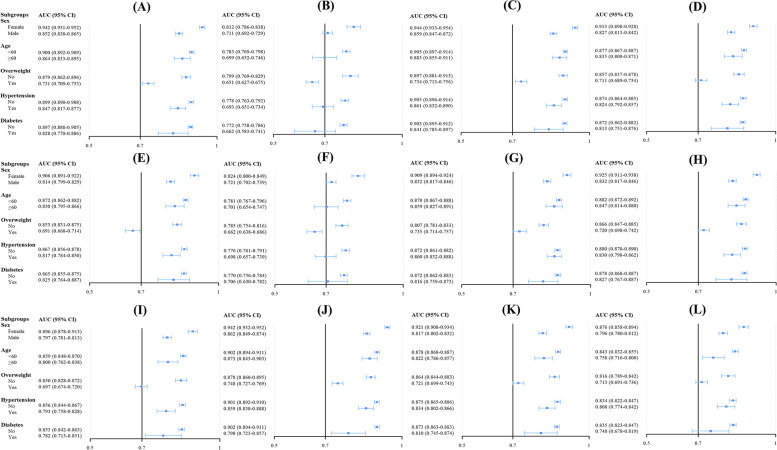
The study conducted subgroup analyses by stratifying the participants into distinct subgroups according to variables such as sex, race, age, overweight status, and the presence of diabetes or hypertension. The results, as shown in Fig. 3 and Supplementary Tables 3–14, indicate that the TyG-WC demonstrated the highest AUC among subgroups of male, female, non-Hispanic Black, non-Hispanic White, individuals aged < 60 years, those aged ≥ 60 years, individuals with overweight, individuals with hypertension, individuals without diabetes, and individuals with diabetes. However, in subgroups of Other Hispanic, non-Hispanic Asian, Mexican American, other races, individuals with nonoverweight, and nonhypertensive individuals, the AUC of TyG-WC was not the highest, although the difference was not statistically significant when compared to the noninvasive score with the highest AUC in each subgroup.
Fig. 3.
AUC and 95% CI for noninvasive scores to detect MAFLD risk in different subgroups of the NHANES cohort. A for METS-IR, (B) for TyG, (C) for TyG-BMI, (D) for TyG-WC, (E) for TyG-WtHR, (F) for VAI, (G) for HSI, (H) for FLI, (I) for LAP, (J) for ZJU , (K) for FSI, and (L) for K-NAFLD
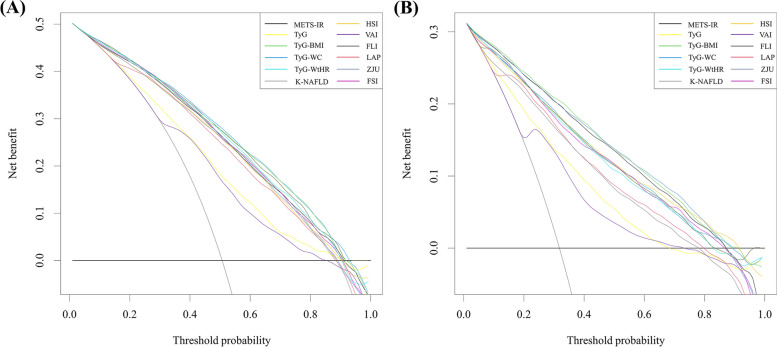
Furthermore, this study employed DCA to evaluate the clinical usefulness of noninvasive scores by quantifying the probability of net benefit across thresholds ranging from 0.0 to 1.0. The results, as depicted in Fig. 4A, indicate that TyG-WC exhibited a superior net benefit compared to other models within a threshold range of approximately 0.02–0.92, with a maximum net benefit of 0.50.
Fig. 4.
The Clinical utility of the indices were evaluated by decision curves in the NHANES cohort (A) and Western China cohort (B). The x-axis measures the threshold probability. The y-axis represents net benefits , calculated by subtracting the relative harms (false positive) from the benefits (true positives)
In summary, the aforementioned findings indicate that the combined predictive value of TyG-WC for MAFLD risk in the NHANES cohort is superior to other indices.
Performances of noninvasive indices and models in predicting MAFLD risk in the western China cohort
Figure 2B depicts the ROC curves of 12 noninvasive scores for predicting MAFLD risk in the Western China cohort, with AUCs ranging from 0.773 to 0.903. Notably, unlike the NHANES cohort, the TyG-BMI score exhibited the largest AUC (0.903; 95% CI: 0.895–0.911), followed by ZJU (0.900 (0.891–0.908)), METS-IR (0.896 (0.888–0.905)), FLI (0.879 (0.869–0.888)), TyG-WC (0. 873 (0.864–0.883)), HSI (0.873 (0.863–0.883)), FSI (0.872 (0.863–0.882)), TyG-WtHR (0.866 (0.856–0.876)), LAP (0.854 (0.843–0.864)), K-NAFLD (0.836 (0.825–0.847)), TyG (0.776 (0.762–0.789)), and VAI (0.773 (0.759–0.786)). Upon conducting additional pairwise comparisons of AUC differences, it was determined that TyG-BMI exhibited statistically significant differences from the remaining 10 noninvasive scores (all P < 0.05), with the exception of ZJU (P = 0.145) (Supplementary Table 2). The SPE for TyG-BMI was 81.1%, SEN was 83.6%, NPV was 91.4%, PPV was 67.4%, and the critical value was 211.515. Furthermore, the NRI and IDI values of TyG-BMI in comparison to the remaining nine noninvasive scores were observed to be greater than 0 and exhibited a significant difference (P < 0.05), with the exception of METS-IR and ZJU, as evidenced in Supplementary Table 2. Consistently, the results of DCA curves, as shown in Fig. 4B, indicated that TyG-BMI possessed greater net benefits compared to the other models within the threshold range of approximately 0.01 to 0.91, with a maximum net benefit of 0.31.
In the subgroup analysis, as shown in Fig. 5 and Supplementary Tables 15–26, TyG-BMI exhibited the highest AUC in the Western China cohort across various subgroups, including women, individuals under the age of 60, those aged 60 years or older, individuals with nonoverweight, and those who were nonhypertensive and hypertensive, nondiabetic and diabetic. In the male subgroup, ZJU demonstrated the highest AUC, although the difference in AUC between ZJU and TyG-BMI was not statistically significant (P = 0.504). Additionally, ZJU exhibited the highest AUC in the overweight subgroup, without a statistically significant difference in AUC compared to TyG-BMI (P = 0.308).
Fig. 5.
AUC and 95% CI for noninvasive scores to detect MAFLD risk in different subgroups of the Western China cohort. A for METS-IR, (B) for TyG, (C) for TyG-BMI, (D) for TyG-WC, (E) for TyG-WtHR, (F) for VAI, (G) for HSI, (H) for FLI, (I) for LAP, (J) for ZJU, (K) for FSI, and (L) for K-NAFLD
Overall, the above results suggest that TyG-BMI was superior to other indices in terms of its combined ability to predict MAFLD risk in the Western China cohort.
Discussion
MAFLD is the most prevalent chronic liver disease globally, and its disease burden is on the rise. Consequently, numerous studies are investigating noninvasive, pragmatic, and dependable disease predictive models to identify and manage individuals at high risk of MAFLD, ultimately mitigating the disease burden. The present study evaluated the predictive capacity of 12 widely employed, noninvasive indices or models for estimating the risk of MAFLD in both U.S. and Chinese cohorts. The predictive capacity of TyG and VAI in determining individual risk for MAFLD was found to be limited in both the NHANES and Western China cohorts, whereas the remaining 10 noninvasive scores demonstrated superior predictive performance. It is noteworthy that although variations in predictive performance were observed across different populations and subgroups, the TyG-related indices exhibited superior performance in predicting MAFLD overall. Specifically, the TyG-WC demonstrated the best performance in the NHANES cohort, while the TyG-BMI exhibited the best performance in the Western China cohort. Despite not achieving the highest AUC in certain subgroups, the lack of statistical significance in the difference between the AUC of TyG-WC/TyG-BMI and the highest AUC within the corresponding subgroups, combined with the straightforward calculation formula, minimal variables needed, and cost-effectiveness, suggests that TyG-WC/TyG-BMI outperformed other methods overall.
MAFLD is a multifactorial disease of a complex nature that encompasses genetics, environment, and metabolism [41]. Among them, genetic susceptibility genes associated with MAFLD have received attention from researchers in recent years, especially transmembrane 6 superfamily member 2 (TM6SF2) [42]. TM6SF2 plays a crucial role in hepatocellular lipid metabolism, regulates very low-density lipoprotein (VLDL) secretion and is implicated in hepatocyte inflammation [43]. The nonsynonymous variant in TM6SF2 (E167K, rs58542926) results in protein dysfunction, leading to an excessive buildup of TG in the liver, thereby contributing to the onset of fatty liver disease [44]. However, the pathogenesis of MAFLD remains incompletely understood, with the “double-hit” and “multiple-hit” theories being the most widely accepted [45, 46]. Regardless of the “double-hit” theory or “multiple-hit” theory, IR is a crucial component in the development of MAFLD [8, 47–51]. At present, the hyperinsulinemic-euglycemic clamp (HEC) [52, 53] technique stands as the foremost method for directly assessing IR. However, owing to its operational intricacy, noninvasive fasting insulin (FINS)-based indices are commonly employed in clinical settings to evaluate IR. Nonetheless, the clinical utility of FINS is constrained due to its nonroutine nature. Consequently, a plethora of fasting insulin-independent indices, including TyG-related indices and METS-IR, have been devised to accurately reflect IR and serve as indirect substitutes [10–13, 20]. The mechanism may be attributed to the two primary constituents of TyG-WC/TyG-BMI (TG and FPG), which are associated with “glucotoxicity” and “lipotoxicity” that play a key role in the development of IR [54, 55]. TGs are predominantly derived from the uptake of free fatty acids (FFAs) by the liver. In the presence of IR, the breakdown of adipose tissue in the periphery is augmented, resulting in an excessive production of FFA that enters the liver via the portal vein system and accumulates aberrantly within the hepatic tissue. Consequently, this abnormal accumulation leads to an upregulation of intrahepatic TG synthesis. Moreover, elevated concentrations of FFAs exhibit lipotoxic properties, impeding insulin signaling and impairing insulin utilization in various target organs across the body, thereby exacerbating IR. It is noteworthy that the excessive influx of FFAs into skeletal muscle and hepatocytes hampers glucose uptake in these tissues by inhibiting insulin, thereby disrupting glucose metabolism [56, 57]. It is evident that IR significantly influences the onset and progression of NAFLD/MAFLD by disrupting glucose and lipid metabolism and promoting excessive fat accumulation in hepatocytes. This reciprocal relationship between IR and hepatocellular fat storage establishes a detrimental cycle that constantly promotes the development and advancement of NAFLD/MAFLD. Since the two components of TyG-BMI/TyG-WC are important components of glucose metabolism and lipid metabolism, respectively, TyG-WC/TyG-BMI is closely related to the occurrence of MAFLD, which is not only a risk factor for MAFLD but also a reliable indicator for the prediction of MAFLD. Considering the favorable predictive capabilities of TyG-BMI/TyG-WC in assessing the risk of MAFLD, it would be advantageous to prioritize the inclusion of metrics pertaining to IR, lipid metabolism, and glucose metabolism, such as FPG, TG, and the emerging focus on remnant cholesterol (RC), in the development of future MAFLD prediction models. RC predominantly signifies cholesterol content within VLDL remnants and exhibits a significant association with the risk of MAFLD/NAFLD [58, 59].
Prior research has demonstrated the favorable predictive capacity of TyG-related indices in relation to NAFLD/MAFLD [14–19, 60–62]. Khamseh et al. [60] found that TyG-WC and TyG-BMI were significantly associated with NAFLD in an overweight/obese cohort and could reliably predict the risk of NAFLD in this population. In addition, Sheng et al. [61] conducted a comparative analysis of 15 indices related to obesity and lipid levels, revealing that TyG-related parameters exhibited the most robust association with NAFLD. Specifically, in the female subgroup, TyG-WC demonstrated a predictive capacity for NAFLD with an AUC of 0.905, while TyG-BMI exhibited an AUC of 0.908. In the male subgroup, TyG-WC displayed an AUC of 0.836, and TyG-BMI displayed an AUC of 0.843. Furthermore, Chang and colleagues [62] investigated the prognostic efficacy of TyG-associated indices for MAFLD in 20,922 Chinese participants. The results indicated that TyG-BMI exhibited the highest predictive capacity, with an AUC of 0.933 (0.927–0.938) in the female subgroup and 0.870 (0.864–0.876) in the male subgroup. TyG-WC followed with AUCs of 0.922 (0.915–0.928) and 0.847 (0.841–0.854), respectively. A paucity of research has conducted comparisons between predictive models developed for fatty liver and indirect indices that reflect IR. A study utilizing the NHANES database conducted a comparison between the TyG-related indices and VAI and LAP, revealing that the former exhibited superior predictive capabilities for both MAFLD and NAFLD risk [19]. The studies mentioned earlier were assessed using a singular approach, relying solely on the AUC to determine predictive value. In contrast, the current study stands out as the most extensive investigation of predictive models for fatty liver and noninvasive indices that indirectly reflect IR in a cross-sectional analysis. Furthermore, this study systematically and comprehensively evaluates the predictive capacity of these models.
In the NHANES cohort, the AUC of FLI was found to be greater than that of other noninvasive scores, with the exception of TyG-WC. Similarly, in the Chinese cohort, the AUC of ZJU was observed to be higher than that of other noninvasive scores, except for TyG-BMI. The findings were consistent with prior external validation studies of fatty liver prediction models. Li et al. conducted an external validation of ZJU, FLI, HSI, LAP, and VAI for NAFLD risk in a cohort of 19,804 individuals in western China and reported that ZJU exhibited an AUC of 0.925 (95% CI: 0.919–0.931) with a cutoff value of 35.29, surpassing the performance of the other four noninvasive models. Furthermore, ZJU demonstrated superior sensitivity, specificity, positive predictive value, and negative predictive value compared to the other four models [63]. Fu et al. conducted a study on 107 severely obese Western women with NAFLD and found that ZJU outperformed HSI, LAP, and VAI, with an AUC of 0.742 (95% 0.647–0.837) [64]. A Japanese study revealed that ZJU and FLI had similar AUCs of 0.886 and 0.884, respectively. Further analysis by sex indicated that ZJU had a higher AUC than FLI in both the male and female groups, while FLI performed better in the diabetes subgroup [65]. However, in a study from eastern China, FLI demonstrated a superior AUC of 0.852 (95% 0.839–0.864) for NAFLD risk compared to ZJU, LAP, and VAI, and DCA showed a higher net benefit [66].
After the renaming of NAFLD to MAFLD, a limited number of studies have investigated the efficacy of predictive models in diagnosing MAFLD. Notably, a recent study utilizing the NHANES III database found that FLI exhibited the highest diagnostic value for MAFLD diagnosed by ultrasonography, with an AUC of 0.793 (0.786-0.800) [67]. Additionally, an external validation article assessing MAFLD diagnosed by VCTE, also based on the NHANES database, reported that FLI had a superior AUC of 0.840 (95% 0.822–0.858) compared to ZJU (0.826 (0.808–0.845)), FSI (0.833 (0.815–0.852)), HSI (0.814 (0.795–0.834)), LAP (0.826 (0.807–0.844)), and VAI (0.747 (0.723–0.770)) [68]. Furthermore, Han and colleagues conducted an analysis of noninvasive prediction models for the diagnosis of MAFLD using CT and determined that FLI exhibited the most effective diagnostic ability, with the highest AUC of 0.791 (95% 0.766–0.816) and an optimal cutoff value of 29.9, which was better than HSI, VAI, ZJU, and LAP [69]. The ZJU algorithm, developed in China, comprises BMI, FPG, TG, and ALT/AST ratio, while the FLI algorithm, first developed in Italy in 2006, is the first predictive model applied to the diagnosis of NAFLD, consisting of BMI, TG, GGT, and WC. Both algorithms incorporate variables that reflect metabolic conditions, which are a crucial aspect emphasized in the diagnosis of MAFLD. This may explain the superior performance of FLI and ZJU in identifying MAFLD risk. In the Western China population, ZJU exhibited superior performance compared to FLI. This trend was further substantiated in the subgroup analysis of the study, wherein the NHANSE cohort indicated that although FLI outperformed ZJU overall, ZJU exhibited the highest AUC of 0.880 (95% 0.857–0.902) among the non-Hispanic Asian group (Supplement Table 12).
Recently, several hepatology societies have gone through several rounds of investigation and discussion to form a consensus [70]. Specifically, they have proposed the adoption of a novel terminology for the precise categorization and nomenclature of fatty liver disease. Under this proposed framework, steatotic liver disease (SLD) would serve as a comprehensive term encompassing the diverse etiologies of steatosis, which include metabolic dysfunction-associated steatotic liver disease (MASLD), metabolic and alcohol-related steatotic liver disease (MetALD), alcohol-associated liver disease (ALD), etiology-specific SLD, and cryptogenic SLD. The diagnosis of MASLD bears striking resemblance to MAFLD but requires only the presence of one cardiovascular-related metabolic disorder and hepatic steatosis to be diagnosed. Overall, the release of the consensus is only a first step, and the impact of the new disease name and diagnostic criteria on other populations and organizations needs to be further evaluated. Irrespective of the alteration in nomenclature, it is critical that noninvasive methods identify people at high risk for fatty liver disease early and determine the point at which specialized treatment is needed.
Study strengths and limitations
Several advantages of this study are worth mentioning. First, in this research, liver steatosis was measured by VCTE, which is more accurate than ultrasonography [71]. Second, this study comprised two validation cohorts consisting of 12,278 participants from the United States and China. The inclusion of such a substantial sample size and data from two different centers enhances the reliability of the study’s findings. Third, this study aimed to assess and compare noninvasive indices and models in terms of their AUC, subgroup analysis, NRI, IDI, and DCA, thus providing a more comprehensive analysis compared to previous literature.
On the other hand, there are some limitations. First, due to its impracticality and invasiveness in a sample of thousands, liver biopsy, the diagnostic gold standard, was not performed in this study. Second, this study found that the optimal noninvasive model for MAFLD risk identification was different across regions and races; therefore, external validation is needed in regions other than China and the United States.
Conclusion
In summary, TyG-BMI demonstrated satisfactory diagnostic efficacy in identifying individuals at high risk of MAFLD in the western Chinese population, surpassing other noninvasive scores or models. Conversely, TyG-WC exhibited optimal diagnostic value and satisfactory diagnostic performance for high-risk MAFLD in the US population. These indices necessitate fewer variables, possess straightforward calculation formulas, are cost-effective, and can be applied across various medical institutions to facilitate early identification, treatment, and mitigation of the disease burden. In light of the findings obtained from the present study, it may be advisable to discontinue the pursuit of a “perfect” noninvasive model and instead employ the most appropriate model tailored to different regions and ethnicities.
Supplementary Information
Acknowledgements
We thank all participants in the present study. This article is supported by the National Clinical Key Specialty Construction Project.
Abbreviations
- MAFLD
Metabolic-associated fatty liver disease
- NAFLD
Nonalcoholic fatty liver disease
- SLD
Steatotic liver disease
- MASLD
Metabolic dysfunction associated steatotic liver disease
- MetALD
Metabolic and alcohol-related steatosis liver disease
- ALD
Alcohol-associated liver disease
- APASL
Asia-Pacific Association for the Study of the Liver
- KNHANES
Korean National Health and Nutrition Examination Survey
- NHANES
National Health and Nutrition Examination Survey
- NCHS
National Center for Health Statistics
- TRIPOD
Transparent Reporting of a Multivariable Predictive Model for Individual Prognosis or Diagnosis
- CT
Computed tomography
- VCTE
Vibration-controlled transient elastography
- CAP
Controlled attenuation parameter
- LSM
Liver stiffness measurements
- IR
Insulin resistance
- HEC
Hyperinsulinemic-euglycemic clamp
- FINS
Fasting insulin
- BMI
Body mass index
- WC
Waist circumference
- WtHR
Waist to height ratio
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- GGT
γ-glutamyl transpeptidase
- ALP
Alkaline phosphatase
- TC
Total cholesterol
- TG
Triglyceride
- TM6SF2
Transmembrane 6 superfamily member 2
- FFA
Free fatty acids
- VLDL
Very low-density lipoprotein
- RC
Remnant cholesterol
- HDL
High-density lipoprotein cholesterol
- UA
Uric acid
- CRE
Creatinine
- eGFR
Estimated glomerular filtration rate
- ROC
Receiver operating characteristic curve
- SEN
Sensitivity
- SPE
Specificity
- PPV
Positive predictive value
- NPV
Negative predictive value
- AUC
Area under receiver operating characteristic curve
- NRI
Net reclassification index
- IDI
Integrated discrimination improvement
- DCA
Decision curve analysis
- OR
Odds ratio
- CI
Confidence interval
- FLI
Fatty liver index
- FSI
Framingham steatosis index
- HSI
Hepatic steatosis index
- VAI
Visceral adiposity index
- ZJU
Zhejiang University index
- LAP
Lipid accumulation product
- TyG
Triglyceride glucose index
- TyG-BMI
Triglyceride glucose-body mass index
- TyG-WC
Triglyceride glucose-waist circumference index
- TyG-WtHR
Triglyceride glucose-waist to height ratio index
- METS-IR
Metabolic score for insulin resistance
Authors' contributions
The present study involved the conception and design of the research protocol by HZ and YX, while data collection was performed by HZ, XM, and FZ. Subsequently, HZ and XM conducted data analysis, and HZ, YX, XM, and FZ collaborated on data interpretation. Finally, manuscript drafting and revision were carried out by HZ, YX, and XM.
Funding
Supported by Health Commission of Sichuan Provincial science and technology project (universal application project), grant number: 21PJ028 and Sichuan Science and Technology Program, grant number: 2023YFS0282.
Availability of data and materials
Data pertaining to NHANES can be accessed on its official website (https://www.cdc.gov/nchs/nhanes/).
Declarations
Ethics approval and consent to participate
The NCHS Ethics Review Board approved the NHANES, and the Ethics Committee of West China Hospital of Sichuan University approved the study protocol (Approval No. 558 in 2023).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fan Zhang, Email: 45701759@qq.com.
Yan Xie, Email: xieyan@wchscu.cn.
References
- 1.Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, Swain MG, Congly SE, Kaplan GG, Shaheen AA. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851–861. doi: 10.1016/S2468-1253(22)00165-0. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American association for the study of liver diseases, american college of gastroenterology, and the american gastroenterological association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, Fujii H, Wu Y, Kam LY, Ji F, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4:389–398. doi: 10.1016/S2468-1253(19)30039-1. [DOI] [PubMed] [Google Scholar]
- 4.Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5:1544–60. doi: 10.3390/nu5051544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour J-F, Schattenberg JM, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 6.Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, Zheng MH, Shiha G, Yilmaz Y, Gani R, et al. The Asian pacific association for the study of the liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889–919. doi: 10.1007/s12072-020-10094-2. [DOI] [PubMed] [Google Scholar]
- 7.Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1264–1281e1264. doi: 10.1053/j.gastro.2018.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan RS, Bril F, Cusi K, Newsome PN. Modulation of insulin resistance in nonalcoholic fatty liver disease. Hepatology. 2019;70:711–24. doi: 10.1002/hep.30429. [DOI] [PubMed] [Google Scholar]
- 9.Wang XJ, Malhi H. Nonalcoholic fatty liver disease. Ann Intern Med. 2018;169:Itc65–itc80. doi: 10.7326/AITC201811060. [DOI] [PubMed] [Google Scholar]
- 10.Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MaG, Hernández-González SO, Jacques-Camarena O, Rodríguez-Morán M. The product of Triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metabolism. 2010;95:3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 11.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 12.Lim J, Kim J, Koo SH, Kwon GC. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in korean adults: an analysis of the 2007–2010 korean National Health and Nutrition Examination Survey. PLoS ONE. 2019;14:e0212963. doi: 10.1371/journal.pone.0212963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, Ko YL. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS ONE. 2016;11:e0149731. doi: 10.1371/journal.pone.0149731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo W, Lu J, Qin P, Li X, Zhu W, Wu J, Xu N, Zhang Q. The triglyceride-glucose index is associated with the severity of hepatic steatosis and the presence of liver fibrosis in non-alcoholic fatty liver disease: a cross-sectional study in chinese adults. Lipids Health Dis. 2020;19:218. doi: 10.1186/s12944-020-01393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitae A, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. The triglyceride and glucose index is a predictor of incident nonalcoholic fatty liver disease: a population-based cohort study. Can J Gastroenterol Hepatol. 2019;2019:5121574. doi: 10.1155/2019/5121574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim J. Validation of fatty liver index in a healthy korean population and its comparison with triglyceride glucose index and its related parameters. Clin Chim Acta. 2019;493:374. [Google Scholar]
- 17.Simental-Mendía LE, Simental-Mendía E, Rodríguez-Hernández H, Rodríguez-Morán M, Guerrero-Romero F. The product of triglycerides and glucose as biomarker for screening simple steatosis and NASH in asymptomatic women. Ann Hepatol. 2016;15:715–720. doi: 10.5604/16652681.1212431. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, Du T, Zhang J, Lu H, Lin X, Xie J, Yang Y, Yu X. The triglyceride and glucose index (TyG) is an effective biomarker to identify nonalcoholic fatty liver disease. Lipids Health Dis. 2017;16:15. doi: 10.1186/s12944-017-0409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng H, Pan L, Ran S, Wang M, Huang S, Zhao M, Cao Z, Yao Z, Xu L, Yang Q, Lv W. Prediction of MAFLD and NAFLD using different screening indexes: a cross-sectional study in U.S. adults. Front Endocrinol (Lausanne) 2023;14:1083032. doi: 10.3389/fendo.2023.1083032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bello-Chavolla OY, Almeda-Valdes P, Gomez-Velasco D, Viveros-Ruiz T, Cruz-Bautista I, Romo-Romo A, Sánchez-Lázaro D, Meza-Oviedo D, Vargas-Vázquez A, Campos OA, et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. 2018;178:533–544. doi: 10.1530/EJE-17-0883. [DOI] [PubMed] [Google Scholar]
- 21.Lee JH, Park K, Lee HS, Park HK, Han JH, Ahn SB. The usefulness of metabolic score for insulin resistance for the prediction of incident non-alcoholic fatty liver disease in korean adults. Clin Mol Hepatol. 2022;28:814–826. doi: 10.3350/cmh.2022.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai X, Gao J, Hu J, Wen W, Zhu Q, Wang M, Liu S, Hong J, Wu T, Yang S, et al. Dose-response Associations of metabolic score for insulin resistance index with nonalcoholic fatty liver disease among a nonobese Chinese population: retrospective evidence from a population-based cohort study. Dis Markers. 2022;2022:4930355. doi: 10.1155/2022/4930355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J, Kim D, Kim H, Lee C, Yang J, Kim W, Kim Y, Yoon J, Cho S, Sung M, Lee HJ. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Disease. 2010;42:503–508. doi: 10.1016/j.dld.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, Galluzzo A. Group ftAS: visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33:920–922. doi: 10.2337/dc09-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahn HS. The lipid accumulation product is better than BMI for identifying diabetes: a population-based comparison. Diabetes Care. 2006;29:151–3. doi: 10.2337/diacare.29.1.151. [DOI] [PubMed] [Google Scholar]
- 26.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Xu C, Xun Y, Lu Z, Shi J, Yu C, Li YJ. ZJU index: a novel model for predicting nonalcoholic fatty liver disease in a chinese population. Sci Rep. 2015;5:16494. doi: 10.1038/srep16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long MT, Pedley A, Colantonio LD, Massaro JM, Hoffmann U, Muntner P, Fox CS. Development and validation of the Framingham Steatosis Index to identify persons with hepatic steatosis. Clin Gastroenterol Hepatol. 2016;14:1172–1180e1172. doi: 10.1016/j.cgh.2016.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeong S, Kim K, Chang J, Choi S, Kim SM, Son JS, Lee G, Kim W, Park SM. Development of a simple nonalcoholic fatty liver disease scoring system indicative of metabolic risks and insulin resistance. Ann Transl Med. 2020;8:1414. doi: 10.21037/atm-20-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, Vickers AJ, Ransohoff DF, Collins GS. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1–73. doi: 10.7326/M14-0698. [DOI] [PubMed] [Google Scholar]
- 31.Classification and Diagnosis of Diabetes Standards of medical care in diabetes-2020. Diabetes Care. 2020;43:14–s31. doi: 10.2337/dc20-S002. [DOI] [PubMed] [Google Scholar]
- 32.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 33.Zheng S, Shi S, Ren X, Han T, Li Y, Chen Y, Liu W, Hou PC, Hu Y. Triglyceride glucose-waist circumference, a novel and effective predictor of diabetes in first-degree relatives of type 2 diabetes patients: cross-sectional and prospective cohort study. J Transl Med. 2016;14:260. doi: 10.1186/s12967-016-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cassinotto C, Boursier J, de Lédinghen V, Lebigot J, Lapuyade B, Cales P, Hiriart JB, Michalak S, Bail BL, Cartier V, et al. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817–27. doi: 10.1002/hep.28394. [DOI] [PubMed] [Google Scholar]
- 35.Ferraioli G, Soares Monteiro LB. Ultrasound-based techniques for the diagnosis of liver steatosis. World J Gastroenterol. 2019;25:6053–62. doi: 10.3748/wjg.v25.i40.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, Kumar M, Lupsor-Platon M, Han KH, Cardoso AC, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022–1030. doi: 10.1016/j.jhep.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 37.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 38.Kerr KF, McClelland RL, Brown ER, Lumley T. Evaluating the incremental value of new biomarkers with Integrated discrimination improvement. Am J Epidemiol. 2011;174:364–374. doi: 10.1093/aje/kwr086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 40.Van Calster B, Wynants L, Verbeek JFM, Verbakel JY, Christodoulou E, Vickers AJ, Roobol MJ, Steyerberg EW. Reporting and interpreting decision curve analysis: a guide for investigators. Eur Urol. 2018;74:796–804. doi: 10.1016/j.eururo.2018.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu Y, Cai J, She Z, Li H. Insights into the Epidemiology, Pathogenesis, and therapeutics of nonalcoholic fatty liver Diseases. Adv Sci (Weinh) 2019;6:1801585. doi: 10.1002/advs.201801585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo F, Oldoni F, Das A. TM6SF2: a novel genetic player in nonalcoholic fatty liver and cardiovascular disease. Hepatol Commun. 2022;6:448–60. doi: 10.1002/hep4.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo F, Smagris E, Martin SA, Vale G, McDonald JG, Fletcher JA, Burgess SC, Hobbs HH, Cohen JC. Hepatic TM6SF2 is required for Lipidation of VLDL in a Pre-Golgi compartment in mice and rats. Cell Mol Gastroenterol Hepatol. 2022;13:879–899. doi: 10.1016/j.jcmgh.2021.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjærg-Hansen A, Vogt TF, Hobbs HH, Cohen JC. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernando DH, Forbes JM, Angus PW, Herath CB. Development and progression of non-alcoholic fatty liver disease: the role of Advanced Glycation End Products. Int J Mol Sci. 2019;20(20):5037. doi: 10.3390/ijms20205037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 47.Rosso C, Kazankov K, Younes R, Esmaili S, Marietti M, Sacco M, Carli F, Gaggini M, Salomone F, Møller HJ, et al. Crosstalk between adipose tissue insulin resistance and liver macrophages in non-alcoholic fatty liver disease. J Hepatol. 2019;71:1012–1021. doi: 10.1016/j.jhep.2019.06.031. [DOI] [PubMed] [Google Scholar]
- 48.Hwang JH, Stein DT, Barzilai N, Cui MH, Tonelli J, Kishore P, Hawkins M. Increased intrahepatic triglyceride is associated with peripheral insulin resistance: in vivo MR imaging and spectroscopy studies. Am J Physiol Endocrinol Metab. 2007;293:E1663–1669. doi: 10.1152/ajpendo.00590.2006. [DOI] [PubMed] [Google Scholar]
- 49.Najjar SM, Perdomo G. Hepatic insulin clearance: mechanism and physiology. Physiol (Bethesda) 2019;34:198–215. doi: 10.1152/physiol.00048.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qureshi K, Clements RH, Saeed F, Abrams GA. Comparative evaluation of whole body and hepatic insulin resistance using indices from oral glucose tolerance test in morbidly obese subjects with nonalcoholic Fatty liver disease. J Obes. 2010;2010:741521. doi: 10.1155/2010/741521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santoleri D, Titchenell PM. Resolving the Paradox of hepatic insulin resistance. Cell Mol Gastroenterol Hepatol. 2019;7:447–456. doi: 10.1016/j.jcmgh.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borai A, Livingstone C, Kaddam I, Ferns G. Selection of the appropriate method for the assessment of insulin resistance. BMC Med Res Methodol. 2011;11:158. doi: 10.1186/1471-2288-11-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 54.Lair B, Laurens C, Van Den Bosch B, Moro C. Novel insights and mechanisms of lipotoxicity-driven insulin resistance. Int J Mol Sci. 2020;21:6358. doi: 10.3390/ijms21176358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mota M, Banini BA, Cazanave SC, Sanyal AJ. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. 2016;65:1049–1061. doi: 10.1016/j.metabol.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heeren J, Scheja L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol Metab. 2021;50:101238. doi: 10.1016/j.molmet.2021.101238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–2564. doi: 10.1016/j.cell.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 58.Chen J, Su Y, Su X, Luo F. Remnant cholesterol has a non-linear association with non-alcoholic fatty liver disease. Diabetes Res Clin Pract. 2023;201:110733. doi: 10.1016/j.diabres.2023.110733. [DOI] [PubMed] [Google Scholar]
- 59.Huang H, Guo Y, Liu Z, Zeng Y, Chen Y, Xu C. Remnant cholesterol predicts long-term mortality of patients with metabolic dysfunction-associated fatty liver disease. J Clin Endocrinol Metab. 2022;107:e3295–303. doi: 10.1210/clinem/dgac283. [DOI] [PubMed] [Google Scholar]
- 60.Khamseh ME, Malek M, Abbasi R, Taheri H, Lahouti M, Alaei-Shahmiri F. Triglyceride glucose index and related parameters (triglyceride glucose-body Mass Index and triglyceride Glucose-Waist circumference) identify nonalcoholic fatty liver and liver fibrosis in individuals with Overweight/Obesity. Metab Syndr Relat Disord. 2021;19:167–173. doi: 10.1089/met.2020.0109. [DOI] [PubMed] [Google Scholar]
- 61.Sheng G, Lu S, Xie Q, Peng N, Kuang M, Zou Y. The usefulness of obesity and lipid-related indices to predict the presence of non-alcoholic fatty liver disease. Lipids Health Dis. 2021;20:134. doi: 10.1186/s12944-021-01561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang M, Shao Z, Shen G. Association between triglyceride glucose-related markers and the risk of metabolic-associated fatty liver disease: a cross-sectional study in healthy chinese participants. BMJ Open. 2023;13:e070189. doi: 10.1136/bmjopen-2022-070189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li L, You W, Ren W. The ZJU index is a powerful index for identifying NAFLD in the general chinese population. Acta Diabetol. 2017;54:905–911. doi: 10.1007/s00592-017-1024-8. [DOI] [PubMed] [Google Scholar]
- 64.Fu CP, Ali H, Rachakonda VP, Oczypok EA, DeLany JP, Kershaw EE. The ZJU index is a powerful surrogate marker for NAFLD in severely obese north american women. PLoS ONE. 2019;14:e0224942. doi: 10.1371/journal.pone.0224942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murayama K, Okada M, Tanaka K, Inadomi C, Yoshioka W, Kubotsu Y, Yada T, Isoda H, Kuwashiro T, Oeda S, et al. Prediction of nonalcoholic fatty liver disease using noninvasive and non-Imaging procedures in Japanese health checkup examinees. Diagnostics (Basel) 2021;11(1):132. doi: 10.3390/diagnostics11010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang L, Zhang M, Wang M, Wang M, Zhang R, Wang H, Zhang W, Ding Y, Wang J. External validation and comparison of simple tools to screen for nonalcoholic fatty liver disease in chinese community population. Eur J Gastroenterol Hepatol. 2022;34:865–872. doi: 10.1097/MEG.0000000000002399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y, Liu S, Huang J, Zhu Y, Lin S. Validation of five hepatic steatosis algorithms in metabolic-associated fatty liver disease: a population based study. J Gastroenterol Hepatol. 2022;37:938–45. doi: 10.1111/jgh.15799. [DOI] [PubMed] [Google Scholar]
- 68.Chen J, Mao X, Deng M, Luo G. Validation of nonalcoholic fatty liver disease (NAFLD) related steatosis indices in metabolic associated fatty liver disease (MAFLD) and comparison of the diagnostic accuracy between NAFLD and MAFLD. Eur J Gastroenterol Hepatol. 2023;35:394–401. doi: 10.1097/MEG.0000000000002497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han AL, Lee HK. Comparison of the diagnostic performance of steatosis indices for discrimination of CT-Diagnosed metabolic Dysfunction-Associated fatty liver disease. Metabolites. 2022;12(7):664. doi: 10.3390/metabo12070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol 2023;40:47–51.
- 71.Li Q, Dhyani M, Grajo JR, Sirlin C, Samir AE. Current status of imaging in nonalcoholic fatty liver disease. World J Hepatol. 2018;10:530–542. doi: 10.4254/wjh.v10.i8.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data pertaining to NHANES can be accessed on its official website (https://www.cdc.gov/nchs/nhanes/).