Abstract
Background
One of five global deaths are attributable to sepsis. Hyperferritinemic sepsis (> 500 ng/mL) is associated with increased mortality in single-center studies. Our pediatric research network’s objective was to obtain rationale for designing anti-inflammatory clinical trials targeting hyperferritinemic sepsis.
Methods
We assessed differences in 32 cytokines, immune depression (low whole blood ex vivo TNF response to endotoxin) and thrombotic microangiopathy (low ADAMTS13 activity) biomarkers, seven viral DNAemias, and macrophage activation syndrome (MAS) defined by combined hepatobiliary dysfunction and disseminated intravascular coagulation, and mortality in 117 children with hyperferritinemic sepsis (ferritin level > 500 ng/mL) compared to 280 children with sepsis without hyperferritinemia. Causal inference analysis of these 41 variables, MAS, and mortality was performed.
Results
Mortality was increased in children with hyperferritinemic sepsis (27/117, 23% vs 16/280, 5.7%; Odds Ratio = 4.85, 95% CI [2.55–9.60]; z = 4.728; P-value < 0.0001). Hyperferritinemic sepsis had higher C-reactive protein, sCD163, IL-22, IL-18, IL-18 binding protein, MIG/CXCL9, IL-1β, IL-6, IL-8, IL-10, IL-17a, IFN-γ, IP10/CXCL10, MCP-1/CCL2, MIP-1α, MIP-1β, TNF, MCP-3, IL-2RA (sCD25), IL-16, M-CSF, and SCF levels; lower ADAMTS13 activity, sFasL, whole blood ex vivo TNF response to endotoxin, and TRAIL levels; more Adenovirus, BK virus, and multiple virus DNAemias; and more MAS (P-value < 0.05). Among these variables, only MCP-1/CCL2 (the monocyte chemoattractant protein), MAS, and ferritin levels were directly causally associated with mortality. MCP-1/CCL2 and hyperferritinemia showed direct causal association with depressed ex vivo whole blood TNF response to endotoxin. MCP-1/CCL2 was a mediator of MAS. MCP-1/CCL2 and MAS were mediators of hyperferritinemia.
Conclusions
These findings establish hyperferritinemic sepsis as a high-risk condition characterized by increased cytokinemia, viral DNAemia, thrombotic microangiopathy, immune depression, macrophage activation syndrome, and death. The causal analysis provides rationale for designing anti-inflammatory trials that reduce macrophage activation to improve survival and enhance infection clearance in pediatric hyperferritinemic sepsis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-023-04628-x.
Keywords: Severe sepsis, Multiple organ failure, Immunoparalysis, Thrombocytopenia-associated multiple organ failure, Macrophage activation syndrome, Hyperferritinemic sepsis
Introduction
An autopsy audit from 1992 to 2017 estimated that one of five deaths worldwide were attributable to sepsis, with 3 million occurring in children and 8 million occurring in adults [1]. Hyperferritinemia has been associated with increased sepsis mortality in single-center studies [2–8]. Brazilian pediatric investigators were first to report that hyperferritinemic sepsis defined by a ferritin level > 500 ng/ml had the highest mortality [2, 3]. Investigators in Washington State reported incremental increases in need for intensive care and also mortality in hospitalized children with ferritin levels > 1,000 and > 3,000 ng/mL [8]. In adults, Rosario et al. used the term Hyperferritinemic Syndrome to describe this condition in adult patients with macrophage activation syndrome (MAS) related to Still’s disease, septic shock, and catastrophic antiphospholipid with ferritin levels > 3,000 ng/mL [9]. Lachman et al. reported extreme hyperferritinemia (> 9,083 ng / mL) was most commonly seen in adults with septic shock and poor outcomes [10].
Macrophage activation has been linked to sepsis and multiple organ failure in adult autopsies. In three single-center autopsy studies [11–13], investigators reported that hyperplasia of activated, hemophagocytic histiocytes/macrophages was associated with a multiple organ failure pattern characterized by hepatobiliary dysfunction and disseminated intravascular coagulation with increased IL-10, IL-6, IL-1, and IL-8 levels. They correlated this condition to the presence of sepsis and numbers of blood transfusions received. Shakoory et al. performed a post hoc analysis of a multicenter trial and reported that a 3-day infusion of the anti-inflammatory IL-1 receptor antagonist protein reduced mortality in a dose-dependent manner in adults with septic shock and features of macrophage activation syndrome including hepatobiliary dysfunction plus disseminated intravascular coagulation, or hyperferritinemia > 2,000 ng/mL [14]. Two multicenter cohorts have corroborated that adults with hyperferritinemic sepsis and the multiple organ failure pattern of combined hepatobiliary dysfunction and disseminated intravascular coagulation have circulating cytokine response profiles reflective of macrophage activation [15–17]. Kernan et al. reported that hyperferritinemic sepsis and MAS can have pathogenic variants related to treatable inherited errors of immunity [18, 19]. There remains a gap in knowledge as to whether therapeutically targeting macrophage activation in personalized clinical trials might benefit patients with hyperferritinemic sepsis and MAS.
To gain important background data, rationale, and impetus for designing and performing anti-inflammatory clinical trials targeting pediatric hyperferritinemic sepsis in our multicenter research network, we herein test the hypothesis that children with hyperferritinemic sepsis (> 500 ng/mL) would demonstrate increased inflammatory cytokinemia and immune depression [20], viral DNAemia [21], thrombotic microangiopathy [22], MAS [14] and death [15–17]. We hypothesized that mortality rates would increase with increasing hyperferritinemia level categories (ferritin 500–999, 1,000–2,999, 3000–9,999, and ≥ 10,000 ng/mL) [2, 3, 8–10]. To gain insights into which modifiable inflammatory cytokines might be optimal therapeutic targets in anti-inflammatory clinical trials designed to improve hyperferritinemic sepsis survival, we applied causal inference analysis to 32 inflammatory cytokines, two biomarkers of immune depression and thrombotic microangiopathy, seven viral DNAemias, five categorical ferritin levels, MAS, and death.
Materials and methods
The present study analyzes clinical data and bio-banked samples available from children previously enrolled in our nine center Eunice Kennedy Shriver National Institutes of Child Health and Human Development Collaborative Pediatric Critical Care Research Network Phenotyping Pediatric Sepsis-induced Multiple Organ Failure (PHENOMS) study [23], approved by the central Institutional Review Board (IRB) of the University of Utah, IRB #70,976. Written informed consent was obtained from one or more parents/guardians for each child. Assent was garnered when the child was able. Patients were enrolled from 2015 to 2017. The details of the clinical protocol have been published [23]. Children qualified for enrollment if they (1) were between the ages of 44 weeks gestation to 18 years; (2) were suspected of having infection and two or more of four systemic inflammatory response criteria [24]; (3) had one or more organ failures defined by modified Proulx et al. criteria [25]; and (4) had an indwelling arterial line or central venous catheter for blood drawing. Children were excluded from enrollment if there was a lack of commitment to aggressive care.
Clinical data were assessed daily until 28 days or discharge from the PICU. These included demographic variables (age, sex, ethnicity, previously healthy status, post-op status), Pediatric Risk of Mortality Score 3 (PRISM-3) to assess severity of illness at admission, and organ failures (Central Nervous System = Glasgow Coma Scale score < 12 not explained by use of sedation; Cardiovascular = requirement for vasoactive agents for systolic blood pressure < 5th percentile for age; Respiratory = PaO2/FiO2 ratio < 300 requiring mechanical ventilation; Renal = oliguria and serum creatinine > 1 mg/dL; Hepatic = ALT > 100 and bilirubin > 1 mg/dL; Hematologic = platelet count < 100 K and INR > 1.5) to assess organ failure index or the number of organ failures. Blood samples were obtained after 24 h of sepsis and twice a week thereafter. The blood samples were analyzed for measurement of ferritin as well as 32 additional cytokine biomarkers, ADAMTS13 activity, whole blood ex vivo TNF response to endotoxin, and seven viral DNAemias.
Thrombocytopenia-associated multiple organ failure (TAMOF) was defined by ADAMTS13 activity < 57% with new onset thrombocytopenia < 100,000, plus acute kidney injury measured by creatinine > 1 mg/dL with oliguria or need for continuous renal replacement therapy or dialysis [26]. Immunoparalysis was defined as whole blood ex vivo TNFα response to endotoxin < 200 pg/mL beyond day three of sepsis [27, 28]. Macrophage activation syndrome was defined by platelet count < 100,000 + INR > 1.5 + ALT > 100 IU/L + bilirubin > 1 mg/dL [14–16, 23]. Death was defined by death in the hospital.
Plasma for cytokine measurement was divided into three assays. IL-18, IL-18BP, and CXCL9 were measured at 25-fold dilution [29]. IFNβ, sCD163, and IL-22 were measured by BioPlex inflammatory flex-set assay per manufacturer’s instructions (Bio-Rad). The remainder were measured by BioPlex Group I/II flex-set assay (Bio-Rad). All cytokines were measured on a BioPlex 200 System (Bio-Rad). The functional assays were measured as previously described. DNA was extracted from frozen plasma samples using the NuclieSENS easyMag automated nucleic acid extractor (bioMerieux) and tested using quantitative real-time polymerase chain reactions (qPCR) for cytomegalovirus (CMV), Epstein-Barr virus (EBV), herpes simplex virus (HSV), human herpesvirus-6 (HHV-6), parvovirus B-19, BK virus, adenovirus, and torque teno virus (TTV) [30].
We compared admission characteristics, outcomes, and 32 cytokines and functional biomarkers across 397 patients in five ferritin groups (< 500, 500–999, 1,000–2,999, 3,000–9,999, and ≥ 10,000 ng/mL), MAS, and mortality groups. We presented continuous variables with median (IQR) or mean (sd) and categorical variables with the percentage value (%). To compare patients visually, we utilized heatmaps with hierarchical clustering as well as bar plots and violin plots to illustrate the characteristics, outcomes, and cytokine differences across the groups. To compare patients statistically, we used Kruskal–Wallis tests for continuous data and the chi-square test for categorical data. Fisher exact tests were applied for groups containing less than five individuals. The threshold for statistical significance was 0.05 for two-sided tests after adjustment for multiple testing. Holm–Bonferroni (BH) correction was applied to correct for multiple testing. Odds ratios were presented with 95% confidence intervals. Adjusted odds ratios for ferritin’s association with mortality were modeled by adding baseline epidemiologic characteristics as well as variables associated with univariate analysis with increasing ferritin and / or death. All analyses were performed with R version 3.6.2.
Causal inference method
We performed causal inference to observe potential causal associations in the data. With the same set of variables as above as input, we learned causality in a two-step approach. First, associations were identified between variables using a mixed graphical model (MGM) approach coupled with sparsity parameters defined separately for different types of data, named stable edge-specific penalty selection (MGM-StEPS) [31]. Then, we normalized the variables and determined the causal direction of each association using degenerate Gaussian (DG) score (see Additional file 1) [32]. For both the MGM-StEPS algorithm and DG score calculation, we set all parameters to default values. Under fundamental causal assumptions (see Additional file 1), the resulting directed acyclic graph (DAG) allowed us to identify potential causal associations as well as third variables including confounding, mediators, collider bias, and M-bias among the causal associations [33]. By presenting the causal pathways in directed acyclic graph (DAG) [34–36], we could identify not only the causal relationships, but also the third variables that would affect each causal relationship. To facilitate correct interpretations of the potential causal findings, we explicitly reported our findings as “causal association” not “causal effect.” Since our inference was based on observational data that are subject to biases from confounding, selection, and measurement, our inference can result in an underestimate or overestimate of the effect of interest and thus we use the term causal association.
Results
Of the 401 patients enrolled and reported in the Phenotyping Pediatric Sepsis-induced Multiple Organ Failure study (PHENOMS), 397 had ferritin levels, 32 cytokines, whole blood ex vivo TNF response to endotoxin, and ADAMTS13 activity assayed for this analysis (CONSORT diagram, Additional file 1: eFigure 1). Individual patient ferritin values ranged from < 10 ng/mL to > 100,000 ng/mL with marked skewness. The continuous variable ferritin displays a quadratic relationship with death in Additional file 1: eFigure 2 [2]. The odds ratio of mortality increases as the continuous integer value of ferritin increases within this range of ferritin values (Odds Ratio 1.0000099 [95% CI 1.0000071–1.000013]; P-value = 0.00000003808).
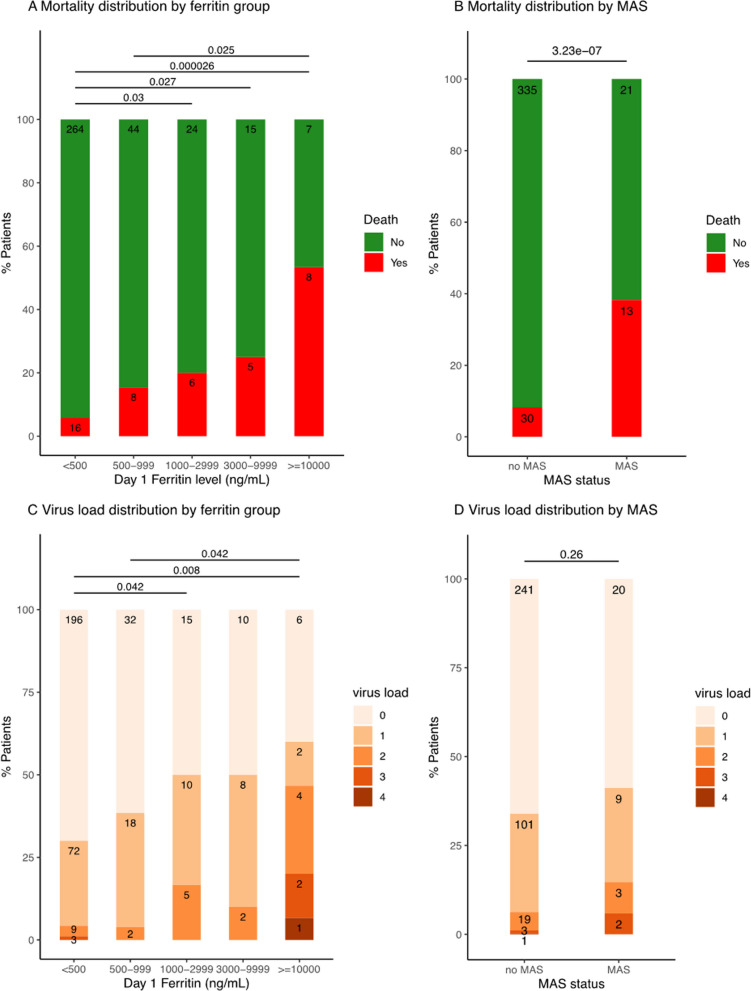
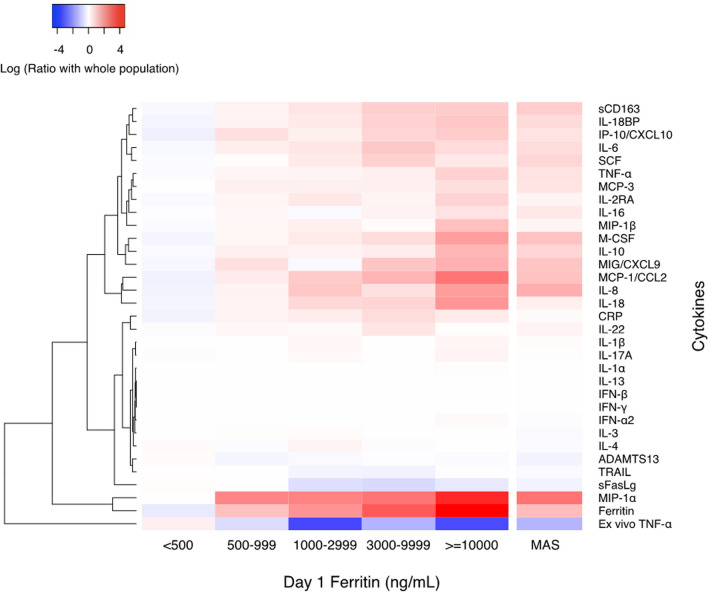
Of these 397 children, 117 (29%) had hyperferritinemia > 500 ng / mL and 280 (71%) did not (Table 1, Fig. 1). Admission characteristics associated with hyperferritinemia > 500 ng/mL included chronic illness, immunocompromise, increased severity of illness, increased number of organ failures, fungal infection, red blood cell transfusion, and platelet transfusion (P-value < 0.05) (Table 1). Children with hyperferritinemia > 500 ng / mL had higher levels of C-reactive protein, sCD163, IL-22, IL-18, IL-18 binding protein, MIG/CXCL9, IL-1β, IL-6, IL-8, Il-10, IL-17A, IFN-γ, IP10/CXCL10, MCP-1/CCL2, MIP-1α, MIP-1β, TNF, MCP-3, IL-2RA, IL-16, M-CSF, and SCF; lower ADAMTS13 activity, sFasL levels, whole blood ex vivo TNF response to endotoxin, and TRAIL levels (P-value < 0.05) (Fig. 2, Additional file 1: eTable 1, eFigure 3); and more Adenovirus, BK virus, and multiple virus DNAemias (P-value < 0.05) (Table 2, Fig. 1). Macrophage activation syndrome developed in 24/117 (21%) of children with hyperferritinemic sepsis and 10/280 (4%) of children with sepsis without hyperferritinemia (Odds Ratio 6.95; 95% CI 3.21–15.12; z = 4.91; P-value < 0.0001) (Table 3). Frequency of MAS increased as ferritin levels increased, from 4% (ferritin < 500 ng/mL) to 17% (ferritin 500 to 999 ng/mL), to 17% (ferritin > 1,000 to 2,999 ng/mL), to 10% (ferritin 3,000 to 9,999 ng/mL), and to 53% (ferritin ≥ 10,000 ng/mL) (Table 3).
Table 1.
Day 1 admission characteristics of ferritin categories and MAS status
| Admission Characteristica | Ferritin Level | no MAS | MAS | ||||
|---|---|---|---|---|---|---|---|
| < 500 | 500–999 | 1000–2999 | 3000–9999 | ≥ 10,000 | |||
| No. of patients, N (%) | 280 (71) | 52 (13) | 30 (8) | 20 (5) | 15 (4) | 363(91) | 34 (9) |
| Age, median (IQR), y | 5.1(1.3,11.7) | 4.7(0.5, 13) | 5.5(3.8,11.5) | 12.2(7.8,14.5)a | 8.9(6.3–11.2) | 5.7(1.5,12.0) | 5.6(1.1,13.2) |
| Sex, N (%) | |||||||
| Female*** | 129 (46) | 19 (37) | 14(47) | 8 (40.0) | 7 (47) | 171(47) | 6(18) |
| Male*** | 151 (54) | 33 (63) | 16(53) | 12 (60.0) | 8 (53) | 192(53) | 28(82) |
| Race, N (%) | |||||||
| White | 189 (68) | 34 (65) | 23(77) | 12 (60) | 11 (73) | 243(67) | 26 (77) |
| Black | 60 (21) | 10 (19) | 6 (20) | 3 (15) | 2 (13) | 77 (21) | 4 (12) |
| Asian | 11 (4) | 5 (10) | 1 (3) | 2 (10) | 0 (0) | 19 (5) | 0 (0) |
| Other | 20 (7) | 3 (6) | 0 (0) | 3 (15) | 2 (13) | 24 (7) | 4 (12) |
| Ethnicity, N (%) | |||||||
| Non-Hispanic | 220 (79) | 44 (85) | 25 (83) | 15 (75) | 15 (100) | 290 (80) | 29 (85) |
| Hispanic | 48 (17) | 7 (14) | 4 (13) | 5 (25.0) | 0 (0.0) | 60 (17) | 4 (12) |
| Previous healthy, N (%)*** | 142 (51) c,d | 25 (48)c | 4 (13) | 3 (15) | 3 (20) | 169 (46) | 8 (23)* |
| Immunocompromised, N (%)*** | 38 (14) | 16 (31)a | 23 (77)a,b | 14 (70)a | 11 (73)a | 85 (23) | 17 (50)** |
| PRISM Score, median (IQR)*** | 7 (3–13) | 10 (6–15) | 12 (9–16) | 9 (5–15.0) | 13 (8–19) | 8 (3–14) | 13 (10- 19)*** |
| OFI, median (IQR)* | 2 (1,2) | 2 (1,2) | 2 (1,2) | 2 (1, 3) | 3 (1, 3) | 2 (1, 2) | 2 (2,4) *** |
| Bacterial infection, N (%) | 94 (34) | 23 (44) | 10(33) | 10 (50) | 3 (20) | 127(35) | 13 (38) |
| Viral infection, N (%) | 88 (31) c | 13 (25) | 2 (7) | 4 (20) | 6 (40) | 105(29) | 8 (23.5) |
| Co-infection, N (%) | 21(8) | 4 (8) | 0 (0) | 2 (10) | 3 (20) | 27 (7) | 3 (9) |
| Fungal infection, N (%)** | 0 (0) | 2 (4) | 1 (3) | 0 (0) | 1 (7) | 2 (1) | 2 (6)* |
| PRBC Transfusion, N (%)*** | 42 (15) | 14 (27) | 19 (63)a,b | 9 (45)a | 5 (33) | 71 (19) | 18 (53)*** |
| Platelet Transfusion, N (%)*** | 13 (5) | 8 (15) a | 16 (53) a,b | 9 (45)a | 9 (60) a,b | 37 (10) | 18 (53)*** |
Comparisons were performed between the group with ferritin < 500 and ferritin ≥ 500, and the group with MAS and without MAS. Kruskal–Wallis test was used for continuous variables, and the χ2 test or the Fisher’s exact test (group sample size < 10) was used for discrete variables. ***: P-value < 0.001 **: P-value < 0.01 *: P-value < 0.05
IQR interquartile range, PRISM Pediatric Risk of Mortality Index, OFI organ failure index, is an integer score reflecting the number of organ failures. OFI Scores are either 0 or 1 for cardiovascular, hepatic, hematologic, respiratory, neurological, and renal, and summed for total range of 0 to 6. Co-infection = bacteria + virus infections. Immunocompromise – Cancer, transplantation, use of immune suppressant therapies
aThe outcome characteristic is significantly higher than ferritin < 500 group (P-value < 0.05)
bThe outcome characteristic is significantly higher than ferritin 500–999 group (P-value < 0.05)
cThe outcome characteristic is significantly higher than ferritin 1000–2999 group (P-value < 0.05)
dThe outcome characteristic is significantly higher than ferritin 3000–9999 group (P-value < 0.05)
Fig. 1.
Mortality and virus DNAemia count distribution by ferritin level and MAS category A Mortality distribution according to ferritin category; B Mortality distribution by MAS (macrophage activation syndrome) category; C Number of circulating DNA viruses (count) by ferritin categories; D Number of circulating DNA viruses by MAS (macrophage activation syndrome) category
Fig. 2.
Cytokine heatmap of ferritin categories and MAS—The heatmap shows the log ratio of the median biomarker values for various markers of the host response and their hierarchical cluster relationships. Red represents a greater median biomarker value for that phenotype compared with the median for the entire study cohort, whereas blue represents a lower median biomarker value compared with the median for the entire study cohort
Table 2.
Viral DNAemia according to ferritin categories and MAS status
| Outcomea | Ferritin Level | No MAS | MAS | ||||
|---|---|---|---|---|---|---|---|
| < 500 | 500–999 | 1000–2999 | 3000–9999 | ≥ 10,000 | |||
| No. of patients, N (%) | 280 (71) | 52 (13) | 30 (8) | 20 (5) | 15 (4) | 363 (91) | 34 (9) |
| EBV,N (%) | 14 (5) | 5 (9) | 0 (0) | 1 (5) | 1 (7) | 19 (5) | 2 (6) |
| CMV, N (%) | 19 (7) | 3 (6) | 7 (23) | 1 (5) | 4 (27) | 32 (9) | 2 (6) |
| HSV1, N (%) | 4 (1) | 1 (2) | 1 (3) | 0 (0) | 0 (0) | 5 (1) | 1 (3) |
| Adeno, N (%)** | 11 (4) | 6 (12) | 3 (10) | 3 (15) | 1 (7) | 22 (6) | 2 (6) |
| BK, N (%)* | 7 (3) | 0 (0.0) | 2 (7) | 2 (10) | 6 (40)a,b | 14 (4) | 3 (9) |
| Parvo, N (%) | 13 (5) | 2 (4) | 1 (3) | 1 (5) | 2 (13) | 18 (5) | 1 (3) |
| HHV-6, N (%) | 31 (11) | 5 (10) | 6 (20) | 4 (20) | 6 (40) | 42 (12) | 10 (29)* |
| No. of viruses | |||||||
| Mean (sd)*** | 0.35(0.60) | 0.42(0.57) | 0.67(.76)a | 0.6(0.68) | 1.33 (1.35)a,b | 0.42 (.66) | 0.62 (0.88) |
| Median (IQR)*** | 0 (0, 1) | 0 (0, 1) | 0.5 (0,1)a | 0.5 (0,1) | 1(0,2)a,b | 0 (0,1) | 0 (0,1) |
Comparisons were performed between the group with ferritin < 500 and ferritin ≥ 500, and the group with MAS and without MAS. Kruskal–Wallis test was used for continuous variables, and the χ2 test or the Fisher’s exact test (group sample size < 10) was used for discrete variables. ***: P-value < 0.001 **: P-value < 0.01 *: P-value < 0.05
aThe outcome characteristic is significantly higher than ferritin < 500 group (P-value < 0.05)
bThe outcome characteristic is significantly higher than ferritin 500–999 group (P-value < 0.05)
cThe outcome characteristic is significantly higher than ferritin 1000–2999 group (P-value < 0.05)
Table 3.
Outcomes according to ferritin category and MAS status
| Outcome | Ferritin level | No MAS | MAS | ||||
|---|---|---|---|---|---|---|---|
| < 500 | 500–999 | 1000–2999 | 3000–9999 | ≥ 10,000 | |||
| No. of patients, N (%) | 280 (71) | 52 (13) | 30 (8) | 20 (5) | 15 (4) | 363 (91) | 34 (9) |
| Lymphopenia, N (%)*** | 140 (50)c | 25 (48) | 5 (17) | 5 (25) | 4 (27) | 162 (45) | 17 (50) |
| MechVent, N (%)** | 252 (90)c | 42 (81) | 20 (67) | 18 (90) | 10 (67) | 311 (86) | 31 (91) |
| ECMO, N (%)*** | 8 (3) | 4 (8) | 1 (3) | 0 (0) | 2 (13) | 9 (3) | 6 (18)*** |
| CRRT, N (%)*** | 5 (2) | 5 (10)a | 4 (13) | 1 (5)a | 1 (7)a | 10 (3) | 6 (18)*** |
| PLEX, N (%)*** | 1 (0.3) | 1 (2) | 1 (3) | 0 (0) | 2 (13)a | 2 (0.6) | 3 (9)** |
| Mortality, N (%)*** | 16 (6) | 8 (15) | 6 (20)a | 5 (25)a | 8 (53)a,b | 30 (8) | 13 (38)*** |
| IPMOF, N (%)*** | 41 (15) | 16 (31) | 8 (27) | 10 (50)a | 9 (60)a | 64 (18) | 20 (59)*** |
| TAMOF, N (%)*** | 12 (4) | 9 (17)a | 5 (17) | 4 (20)a | 7 (47)a | 16 (4) | 21 (62)*** |
| MAS, N (%)*** | 10 (4) | 9 (17)a | 5 (17) | 2 (10) | 8 (53)a | – | – |
Lymphopenia = Absolute Lymphocyte Count < 1,000/mm3
Comparisons were performed between the group with ferritin < 500 and ferritin ≥ 500, and the group with MAS and without MAS. Kruskal–Wallis test was used for continuous variables, and the χ2 test or the Fisher’s exact test (group sample size < 10) was used for discrete variables. ***: P-value < 0.001 **: P-value < 0.01 *: P-value < 0.05
IQR interquartile range, MechVent Mechanical Ventilation, ECMO Extracorporeal Membrane Oxygenation, CRRT Continuous Renal Replacement Therapies, PLEX Plasma Exchange, IPMOF immunoparalysis-associated multiple organ failure, TAMOF thrombocytopenia-associated multiple organ failure, MAS macrophage activation syndrome
aThe outcome characteristic is significantly higher than ferritin < 500 group (P-value < 0.05)
bThe outcome characteristic is significantly higher than ferritin 500–999 group (P-value < 0.05)
cThe outcome characteristic is significantly higher than ferritin 1000–2999 group (P-value < 0.05)
Mortality occurred in 27/117 (23%) of children with hyperferritinemic sepsis > 500 ng/mL compared to 16/280 (6%) of children with sepsis without hyperferritinemia (Odds Ratio 4.85; 95% CI [2.55–9.60]; z = 4.728; P-value < 0.0001) (Table 3, Fig. 1). Mortality increased as ferritin category levels increased from 5.7% (ferritin < 500 ng/mL) to 15.4% (ferritin 500 to 999 ng/mL), to 20% (ferritin > 1,000 to < 2,999 ng/mL), to 25% (ferritin > 3,000 to 9,999 ng/mL), and to 53.3% (ferritin ≥ 10,000 ng/mL) (Table 3, Fig. 1). After adjustment for epidemiologic factors including age, sex, race, and ethnicity and admission variables associated with ferritin levels (Table 1) and / or death including previously healthy status, PRISM score, preexisting immunocompromise status, maximum organ failures, presence of fungal infection, or receipt of packed red blood cell transfusion and/or platelet transfusion (Additional file 1: eTables 2–4), these ferritin category levels remained independently associated with increasing mortality odds in a stepwise manner (adjusted odds ratio of mortality 1.044, 95% CI: [1.012–1.078], P-value = 0.006483 for each increase in ferritin category level; Additional file 1: eTable 5). Children with hyperferritinemic sepsis also developed more immunoparalysis, TAMOF, and requirement for extracorporeal support (P-value < 0.05) but less lymphopenia (Table 3).
Admission characteristics associated with MAS included male sex, chronic illness, immunocompromise, increased severity of illness, increased number of organ failures, fungal infection, red blood cell transfusion, and platelet transfusion (P-value < 0.05) (Table 1). Children who developed MAS showed increased ferritin, sCD163, IL-22, IL-18, IL-18 BP, MIG/CXCL9, IL-1β, IL-6, IL-8, IL-10, MCP-1/CCL2, MIP-1α, MIP-1β, TNF, IL-16, M-CSF, and SCF; decreased ADAMTS13 activity, whole blood ex vivo TNF response to endotoxin, and IFN-β (P-value < 0.05) (Fig. 2, Additional file 1: eTable 6, eFigure 4); and more Human Herpes Virus 6 DNAemias (P-value < 0.05) (Table 2). Mortality occurred in 13/34 (38.2%) of children with MAS compared to 30/363 (8.3%) of children without (Odds Ratio 6.87; 95% CI [3.13–15.08]; z = 4.81; P-value < 0.0001) (Fig. 1, Table 3). Children with MAS also developed more immunoparalysis, TAMOF, and requirement for extracorporeal support (P-value < 0.05) (Table 3).
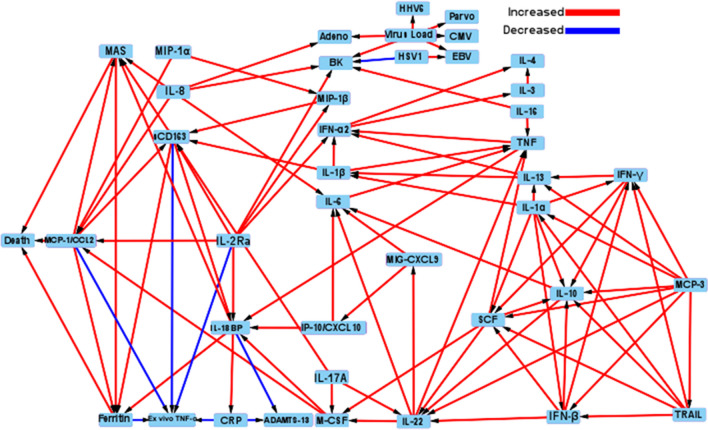
Figure 3 shows the pediatric sepsis causal pathway DAG analysis demonstrating all MGM-derived causal associations. Among the 41 identified causal nodes, only increased MCP-1/CCL2, MAS, and hyperferritinemia levels were directly causally associated with death (Figs. 3 and 4, Additional file 1: Table S7). Increased MCP-1/CCL2 levels were also causally associated with increased MAS, hyperferritinemia, and sCD163 levels but decreased whole blood ex vivo TNF response to endotoxin. Macrophage activation syndrome was also causally associated with hyperferritinemia levels, while hyperferritinemia levels were also causally associated with decreased whole blood ex vivo TNF response to endotoxin. Confounders, mediators, and colliders are identified among MCP-1/CCL2, MAS, and hyperferritinemia, with death in Additional file 1: Table S7.
Fig. 3.
Full causal pathway directed acyclic graph analysis of all causal associations found among cytokines, viral DNAemia, hyperferritinemia, MAS, and death. Causal analysis revealed the associations among all variables/outcomes. Red arrows denote a positive causal association (increased variable/outcome: increased variable/outcome), and blue arrows denote a negative causal association (increased variable/outcome: decreased variable outcome). Arrows denote direction of causality
Fig. 4.
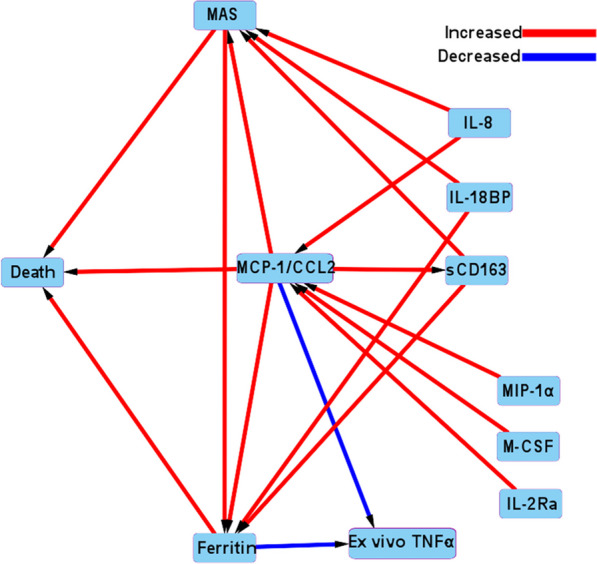
Abridged causal pathway directed acyclic graph analysis of eight cytokines with MAS, hyperferritinemia, and death. Causal analysis revealed the associations among variables/ outcomes. Red arrows denote a positive causal association (increased variable/outcome: increased variable/outcome), and blue arrows denote a negative causal association (increased variable/outcome: decreased variable outcome). Arrows denote direction of causality
Increased IL-8, IL-18BP, and sCD163 levels were causally associated with MAS; increased IL-8, MIP-1 alpha, M-CSF, and sCD25/IL-2Ra levels were causally associated with increased MCP-1/CCL2 levels; and increased IL-18BP and sCD163 levels were causally associated with hyperferritinemia (Figs. 3 and 4). All identified causal associations are depicted in Fig. 3 to allow inference of potential cytokine modulation strategies.
Whole blood ex vivo TNF response was lower in children with preexisting immunocompromised status (P = e2.9e-07) (Additional file 1: eFigure 5) as well as in children with bacterial infection (P < 0.05) (Additional file 1: eFigure 6) but higher in children with viral infection (P < 0.05) (Additional file 1: eFigure 6). Causal associations of increasing hyperferritinemia category and MCP-1 / CCL2 levels with death and reduced whole blood ex vivo TNF response remained with the addition of preexisting immunocompromise status to the DAG.
Discussion
Our multicenter study establishes pediatric hyperferritinemic sepsis as a common high-risk condition characterized by hypercytokinemia, viral DNAemia, thrombotic microangiopathy, immune depression, MAS, and death [37]. Nearly, one of three children in our sepsis cohort had ferritin levels > 500 ng/mL. Nearly, two of three children who required continuous renal replacement therapy, one of two children who required extracorporeal membrane oxygenation, three of four who developed MAS, and two of three children who died had ferritin levels > 500 ng/mL. Death increased in a stepwise manner with increasing category levels of hyperferritinemia [8]. Consistent with adult studies, children with hyperferritinemic sepsis had a hypercytokinemia profile reflective of macrophage activation without a commensurate Th2 response (low IL-4 and IL-13) [15–17]. Decreased ADAMTS13 activity with TAMOF reflective of thrombotic microangiopathy [22] and increased circulating IL-10 reduced whole blood ex vivo TNF response to endotoxin, and immunoparalysis reflective of immune depression with reduced ability to kill infection [27, 28, 37] was also observed with increasing ferritin levels [20].
We applied causal analysis to gain inference into potential anti-inflammatory strategies that might reduce inflammation and mortality in these children. Monocyte chemoattractant protein 1 (MCP-1)/CCL 2, MAS, and hyperferritinemia were identified as the only three variables directly causally associated with death. Hyperferritinemia and MCP-1/CCL2 were also causally associated with reduced ex vivo whole blood TNF response to endotoxin implying that targeting hyperferritinemic sepsis and macrophage activation could also improve infection clearance.
Clinical drugs specifically targeting ferritin-related ferritinophagy and ferroptosis such as dexrazoxane and dexmedetomidine have benefit in experimental sepsis models [38–40]. Similarly, the selective MCP-1/CCL2 inhibitor Bindarit is beneficial in experimental sepsis [41]. However, visualization of the causal pathways with third factor analysis in the DAG implies that therapies which target MCP-1/CCL2, MAS, and hyperferritinemia all together will be more efficacious than therapies that target any one of these alone. Demirkol previously reported that the combined therapies of methylprednisolone, IVIG, and plasma exchange therapy reversed pediatric hyperferritinemic sepsis and MAS, improved outcome, and facilitated infection clearance in the low middle-income PICU setting [42]. Methylprednisolone and IVIG are anti-inflammatory therapies commonly used to treat MAS [43, 44], while plasma exchange has been shown to remove M-CSF, IL-8, and ferritin [44]. Our DAG analysis shows M-CSF and IL-8 were causally associated with MCP-1/CCL2.
Our DAG analysis further identified causal associations between IL-18BP, sCD163, M-CSF, IL-8, MIP-1 alpha, and IL-2Ra/sCD25 with increased MCP-1/CCL2, MAS, and hyperferritinemia. This provides rationale for testing combinations of specific inhibitors and/or signaling pathway inhibitors with activity against many or all of these cytokines. For practical reasons, our network has chosen interleukin 1 receptor antagonist protein (Anakinra) for our first placebo controlled clinical trial. It is already FDA approved for use in neonatal onset multisystem inflammatory disease (NOMID) with a proven excellent safety profile. This biologic blocks MCP-1/CCL2 production during experimental radiation injury [45] reduces sCD163, IL-2RA/sCD25, and ferritin production in COVID19 patients [46, 47], inhibits IL-8 and MIP-1 alpha production in mixed lymphocyte cultures without affecting t-cell function [48], and reverses MAS in children [49]. Interleukin 1β was causally associated with TNF and sCD163 in our DAG. In turn, TNF had causal association with IL-18BP which had causal association with MAS and hyperferritinemia. Similarly, sCD163 had causal association with hyperferritinemia and MAS. From this, we infer that IL-1β inhibition by Anakinra might decrease hyperferritinemia and MAS. Our research network is presently enrolling children in the targeted reversal of inflammation in pediatric sepsis-induced MODS trial (TRIPS: NCT05267821) testing the ability of seven days of Anakinra to improve 28-day MODS resolution in hyperferritinemic sepsis [50].
The major limitation of our study is that the numbers of hyperferritinemia / MAS / mortality patients are small. We are unable to fully evaluate the role of preexisting immunocompromised status. Even though we found increasing ferritin category levels remained associated with mortality after controlling for preexisting immunocompromised conditions and increasing ferritin categories and MCP-1/CCL2 levels remained causally associated with death and low whole blood ex vivo TNF response after adding preexisting immunocompromise status, only five of our 401 patients had hematopoietic stem cell transplantation which is the severest form of immunocompromise. Concern about generalizability of our findings will remain pending further investigations in independent pediatric cohorts.
Because our study was designed to corroborate previous findings of single-center pediatric and multicenter adult studies in our independent multicenter pediatric cohort, the biomarkers and ferritin category levels were pre-selected as a confirmatory list rather than an agnostic one. Specimen collections occurred after 24 h of severe sepsis giving time for ferritin levels to reach their peak; however, cytokines expected to peak in the first 6 h of sepsis such as IL-1β and TNF may have been higher if sampled earlier. Causal relationships might differ at different time points. Further, observations that specific cytokines show causal associations with mortality do not mean that their blockade will improve survival. Rather these findings are hypothesis generating. Clinical trials such as TRIPS are needed to confirm proposed in vivo effects. Findings in pediatric hyperferritinemic sepsis-induced MODS are not generalizable to all critical illness as hyperferritinemia is less predictive of poor outcomes in adult community-acquired pneumonia unaccompanied by hematologic cancers [51–54]. Findings are also expected to differ in resource-poor settings where malnutrition leads to lower baseline ferritin levels, and tropical infections including dengue virus, hemorrhagic fevers, severe malarial anemia, Ebola virus, Leishmaniasis, and scrub typhus lead to higher ferritin levels during sepsis [55–63]. Whereas hyperferritinemia is linked to mortality in COVID19 patients [64], it is important to emphasize that our cohort is pre-COVID19 pandemic.
Conclusions
This multicenter investigation establishes hyperferritinemic sepsis as a high-risk hyperinflammatory condition in children. The causal inference pathway analysis provides rationale for designing clinical trials testing anti-inflammatory therapies that modulate reticuloendothelial system activation to improve outcome and enhance infection clearance in children with hyperferritinemic sepsis.
Supplementary Information
Additional file 1. Supplemental Digital Content.
Acknowledgements
Clinical Research Investigation and Systems Modeling of Acute illness center: Ali Smith, BS; Octavia Palmer, MD; Vanessa Jackson, AA; Renee Anderko, BS, MS. Children’s Hospital of Pittsburgh: Jennifer Jones, RN; Luther Springs. Children’s Hospital of Philadelphia: Carolanne Twelves, RN, BSN, CCRC; Mary Ann Diliberto, BS, RN, CCRC; Martha Sisko, BSN, RN, CCRC, MS; Pamela Diehl, BSN, RN; Janice Prodell, RN, BSN, CCRC; Jenny Bush, RNC, BSN; Kathryn Graham, BA; Kerry Costlow, BS; Sara Sanchez. Children’s National Hospital: Elyse Tomanio, BSN, RN; Diane Hession, MSN, RN; Katherine Burke, BS. Children’s Hospital of Michigan, Central Michigan University: Ann Pawluszka, RN, BSN; Melanie Lulic, BS. Nationwide Children’s Hospital: Lisa Steele, RN, CCRC; Andrew R. Yates, MD; Josey Hensley, RN; Janet Cihla, RN; Jill Popelka, RN; Lisa Hanson-Huber, BS. Children’s Hospital Los Angeles and Mattel Children’s Hospital: Jeni Kwok, JD; Amy Yamakawa, BS. Children’s Hospital of Washington University of Saint Louis: Michelle Eaton, RN. Mott Children’s Hospital: Frank Moler, MD; Chaandini Jayachandran, MS, CCRP. University of Utah Data Coordinating Center: Teresa Liu, MPH, CCRP; Jeri Burr, MS, RN-BC, CCRC, FACRP; Missy Ringwood, BS, CMC; Nael Abdelsamad, MD, CCRC; Whit Coleman, MSRA, BSN, RN, CCRC. For information regarding this article, E-mail: carcilloja@ccm.upmc.edu and the Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network
Abbreviations
- MOF
Multiple organ failure
- PICU
Pediatric intensive care unit
- IPMOF
Immunoparalysis MOF
- TAMOF
Thrombocytopenia-associated MOF
- MAS
Macrophage activation syndrome
- SMOF
Sequential MOF
- IVIG
Intravenous immune globulin
- NIGMS
National Institutes of General Medical Sciences
- PHENOMS
PHENOtyping pediatric sepsis-induced Mof Study
- ADAMTS13
A disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13
- PRISM
Pediatric RISk of Mortality
- SIRS
Systemic Inflammatory Response Syndrome
- CRRT
Continuous renal replacement therapy
- ECMO
Extracorporeal membrane oxygenator
- NICHD
National Institutes of Child Health and Human Development
- DAG
Directed acyclic graph
- MGM
Mixed graphical model
- StEPS
Stable edge-specific penalty selection
Author contributions
HJP and JAC contributed equally as corresponding authors this work. ZF, KFK, YQ, SC, HJP, and JAC performed conceptualized this study, performed and interpreted the analyses. RAB, DW, MMP, KM, MWH, CN, JCL, AD, TS, TC, REH, AFZ, KS, and JMD enrolled children and collected and collated clinical data and specimens for the analysis. All authors critically edited the manuscript and approve and take responsibility for the validity of its contents.
Funding
Supported, in part, by grant R01GM108618 (to Dr. Carcillo and Dr Park) and 1K23GM148827-01 (to Dr Kernan) from the National Institute of General Medical Sciences, and by 5U01HD049934-10S1 (to Dr Carcillo), from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services and the following cooperative agreements: U10HD049983, U10HD050096, U10HD049981, U10HD063108, U10HD63106, U10HD063114, U10HD050012, and U01HD049934.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its Additional file 1. The datasets used and /or analyzed during the current study are available from the corresponding author on reasonable request. The PHENOMS database can also be uploaded from the NICHD sponsored DASH website.
Declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board at University of Utah Central IRB #70976.
Consent for publication
Not applicable.
Competing interests
The authors had no competing interest but they did have the following. Authors Carcillo, Berg, Wessel, Pollack, Meert, Hall, Newth, Doctor, Shanley, Cornell, Harrison, Zuppa, and Dean received support for article research from the NICHD. Dr. Carcillo and Dr Park’s institutions also received funding from the National Institute of General Medical Sciences. Dr. Pollack disclosed that his research is supported by philanthropy from Mallinckrodt Pharmaceuticals. Dr. Hall received funding from LaJolla Pharmaceuticals (service as a consultant), unrelated to the current submission. Dr. Newth received funding from Philips Research North America. Dr. Doctor’s institution received funding from the Department of Defense and Kalocyte. Dr. Shanley received funding from Springer publishing, International Pediatric Research Foundation, and Pediatric Academic Societies. Dr. Cornell disclosed he is co-founder of Pre-Dixon Bio. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia PC, Longhi F, Branco RG, Piva JP, Lacks D, Tasker RC. Ferritin levels in children with severe sepsis and septic shock. Acta Paediatr. 2007;96:1829–1831. doi: 10.1111/j.1651-2227.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- 3.Tonial CT, Costa CAD, Andrades GRH, et al. Prediction of poor outcomes for septic children according to ferritin levels in a middle-income setting. Pediatr Crit Care Med. 2020;21:e259–e266. doi: 10.1097/PCC.0000000000002273. [DOI] [PubMed] [Google Scholar]
- 4.Carcillo JA, Sward K, Halstead ES, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative pediatric critical care research network investigators: a systemic inflammation mortality risk assessment contingency table for severe sepsis. Pediatr Crit Care Med. 2017;18:143–150. doi: 10.1097/PCC.0000000000001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horvat CM, Bell J, Kantawala S, et al. C-reactive protein and ferritin are associated with organ dysfunction and mortality in hospitalized children. Clin Pediatr (Phila) 2019;58:752–760. doi: 10.1177/0009922819837352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carcillo JA, Simon DW, Podd BS. How we manage hyperferritinemic sepsis-related multiple organ dysfunction syndrome/macrophage activation syndrome/secondary hemophagocytic lymphohistiocytosis histiocytosis. Pediatr Crit Care Med. 2015;16:598–600. doi: 10.1097/PCC.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo L, Carcillo J. Secondary hemophagocytic lymphohistiocytosis and severe sepsis/systemic inflammatory response syndrome/multiorgan dysfunction syndrome/macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. Pediatr Crit Care Med. 2009;10:387–392. doi: 10.1097/PCC.0b013e3181a1ae08. [DOI] [PubMed] [Google Scholar]
- 8.Bennett TD, Hayward KN, Farris RW, Ringold S, Wallace CA, Brogan TV. Very high serum ferritin levels are associated with increased mortality and critical care in pediatric patients. Pediatr Crit Care Med. 2011;12:e233–236. doi: 10.1097/PCC.0b013e31820abca8. [DOI] [PubMed] [Google Scholar]
- 9.Rosario C, Zandman-Goddard G, Meyron-Holtz EG, et al. The Hyperferritinemic Syndrome: macrophage activation syndrome, Still’s disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013;11:185. doi: 10.1186/1741-7015-11-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lachmann G, Knaack C, Vorderwülbecke G, et al. Hyperferritinemia in critically Ill patients. Crit Care Med. 2020;48:459–465. doi: 10.1097/CCM.0000000000004131. [DOI] [PubMed] [Google Scholar]
- 11.Suster S, Hilsenbeck S, Rywlin AM. Reactive histiocytic hyperplasia with hemophagocytosis in hematopoietic organs: a reevaluation of the benign hemophagocytic proliferations. Hum Pathol. 1988;19:705–712. doi: 10.1016/s0046-8177(88)80177-1. [DOI] [PubMed] [Google Scholar]
- 12.Strauss R, Neureiter D, Westenburger B, et al. Multifactorial risk analysis of bone marrow histiocytic hyperplasia with hemophagocytosis in critically ill medical patients–a postmortem clinicopathologic analysis. Crit Care Med. 2004;32:1316–1321. doi: 10.1097/01.ccm.0000127779.24232.15. [DOI] [PubMed] [Google Scholar]
- 13.Inai K, Noriki S, Iwasaki H, Naiki H. Risk factor analysis for bone marrow histiocytic hyperplasia with hemophagocytosis: an autopsy study. Virchows Arch. 2014;465:109–118. doi: 10.1007/s00428-014-1592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyriazopoulou E, Leventogiannis K, Norrby-Teglund A, et al. Hellenic Sepsis Study Group: Macrophage activation-like syndrome: An immunological entity associated with rapid progression to death in sepsis. BMC Med. 2017;15:172. doi: 10.1186/s12916-017-0930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karakike E, Giamarellos-Bourboulis EJ. Macrophage activation-like syndrome: a distinct entity leading to early death in sepsis. Front Immunol. 2019;10:55. doi: 10.3389/fimmu.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderko RR, Gómez H, Canna SW, et al. Sepsis with liver dysfunction and coagulopathy predicts an inflammatory pattern of macrophage activation. Intensive Care Med Exp. 2022;10:6. doi: 10.1186/s40635-022-00433-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kernan KF, Ghaloul-Gonzalez L, Shakoory B, Kellum JA, Angus DC, Carcillo JA. Adults with septic shock and extreme hyperferritinemia exhibit pathogenic immune variation. Genes Immun. 2019;20:520–526. doi: 10.1038/s41435-018-0030-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kernan KF, Ghaloul-Gonzalez L, Vockley J, et al. Prevalence of pathogenic and potentially pathogenic inborn error of immunity associated variants in children with severe sepsis. J Clin Immunol. 2022;42:350–364. doi: 10.1007/s10875-021-01183-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29:401–409. doi: 10.1093/intimm/dxx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon DW, Halstead ES, Davila S, et al. DNA viremia is associated with hyperferritinemia in pediatric sepsis. J Pediatr. 2019;213:82–87. doi: 10.1016/j.jpeds.2019.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bashir DA, Da Q, Pradhan S, et al. Secretion of von willebrand factor and suppression of ADAMTS-13 activity bu markedly high concentration of Ferritin. Clin Appl Thromb Hemost. 2021;27:1076029621992128. doi: 10.1177/1076029621992128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carcillo JA, Berg RA, Wessel D, et al. A multicenter network assessment of three inflammation phenotypes in pediatric sepsis-induced multiple organ failure. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2019;20:1137–1146. doi: 10.1097/PCC.0000000000002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc 2005;6:2–8. [DOI] [PubMed]
- 25.Villeneuve A, Joyal JS, Proulx F, Ducruet T, Poitras N, Lacroix J. Multiple organ dysfunction syndrome in critically ill children: clinical value of two lists of diagnostic criteria. Ann Intensive Care. 2016;6:40. doi: 10.1186/s13613-016-0144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen TC, Han YY, Kiss JE, et al. Intensive plasma exchange increases a disintegrin and metalloprotease with thrombospondin motifs-13 activity and reverses organ dysfunction in children with thrombocytopenia-associated multiple organ failure. Crit Care Med. 2008;36:2878–2887. doi: 10.1097/ccm.0b013e318186aa49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muszynski JA, Nofziger R, Moore-Clingenpeel M, et al. Early immune function and duration of organ dysfunction in critically III children with sepsis. Am J Respir Crit Care Med. 2018;198:361–369. doi: 10.1164/rccm.201710-2006OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall MW, Knatz NL, Vetterly C, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011;37:525–532. doi: 10.1007/s00134-010-2088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss ES, Girard-Guyonvarch C, Holzinger D, et al. Interleukin-18 diagnostically distinguishes and pathogenically promotes human and murine macrophage activation syndrome. Blood. 2018;131:1442–1455. doi: 10.1182/blood-2017-12-820852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mallet F, Diouf L, Meunier B, et al. Herpes DNAemia and TTV viraemia in intensive care unit critically Ill patients: a single-centre prospective longitudinal study. Front Immunol. 2021;12:698808. doi: 10.3389/fimmu.2021.698808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sedgewick AJ, Shi I, Donovan RM, Benos PV. Learning mixed graphical models with separate sparsity parameters and stability-based model selection. BMC Bioinform. 2016;17:175. doi: 10.1186/s12859-016-1039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrews B, Ramsey J, Cooper GF. Learning high-dimensional directed acyclic graphs with mixed data-types. Proc Mach Learn Res. 2019;104:4–21. [PMC free article] [PubMed] [Google Scholar]
- 33.Lederer DJ, Bell SC, Branson RD, Chalmers JD, Marshall R, Maslove DM, et al. Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc. 2019;16(1):22–28. doi: 10.1513/AnnalsATS.201808-564PS. [DOI] [PubMed] [Google Scholar]
- 34.Lu YY, Fan Y, Lv J, Noble WS. Deeppink: Reproducible feature selection in deep neural networks. Adv Neural Inform Process Syst. 2018;2018:8676–8686. [Google Scholar]
- 35.Kormaksson M, Kelly LJ, Zhu X, Haemmerle S, Pricop L, Ohlssen D. Sequential knockoffs for continuous and categorical predictors: With application to a large psoriatic arthritis clinical trial pool. Stat Med Wiley Online Library. 2021;40(14):3313–3328. doi: 10.1002/sim.8955. [DOI] [PubMed] [Google Scholar]
- 36.Andrews B, Ramsey J, Cooper GF. Learning high-dimensional directed acyclic graphs with mixed data-types. In: Le TD, Li J, Zhang K, Cui EKP, Hyvärinen A, editors. Proceedings of Machine Learning Research. Anchorage: PMLR; 2019. pp. 4–21. [PMC free article] [PubMed] [Google Scholar]
- 37.Carcillo JA, Podd B, Aneja R, Weiss SL, Hall MW, Cornell TT, et al. Pathophysiology of pediatric multiple organ dysfunction syndrome. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2017;18:S32–45. doi: 10.1097/PCC.0000000000001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li N, Wang W, Zhou H, et al. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic Biol Med. 2020;160:303–318. doi: 10.1016/j.freeradbiomed.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 39.She H, Hu Y, Zhou Y, et al. Protective effects of dexmedetomidine on sepsis-induced vascular leakage by alleviating ferroptosis via regulating metabolic reprogramming. J Inflamm Res. 2021;14:6765–6782. doi: 10.2147/JIR.S340420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C, Yuan W, Hu A, Lin J, Xia Z, Yang CF, Li Y, Zhang Z. Dexmedetomidine alleviated sepsis-induced myocardial ferroptosis and septic heart injury. Mol Med Rep. 2020;22(1):175–184. doi: 10.3892/mmr.2020.11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramnath RD, Ng SW, Guglielmotti A, Bhatia M. Role of MCP-1 in endotoxemia and sepsis. Int Immunopharmacol. 2008;8(6):810–818. doi: 10.1016/j.intimp.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 42.Demirkol D, Yildizdas D, Bayrakci B, et al. Turkish Secondary HLH/MAS Critical Care Study Group: Hyperferritinemia in the critically ill child with secondary hemophagocytic lymphohistiocytosis/ sepsis/multiple organ dysfunction syndrome/macrophage activation syndrome: What is the treatment? Crit Care. 2012;16:R52. doi: 10.1186/cc11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emmenegger U, Frey U, Reimers A, et al. Hyperferritinemia as indicator for intravenous immunoglobulin treatment in reactive macrophage activation syndromes. Am J Hematol. 2001;68:4–10. doi: 10.1002/ajh.1141. [DOI] [PubMed] [Google Scholar]
- 44.Kodama K, Kuno H, Koide M. T Matsuo Virus-associated haemophagocytic syndrome responsive to steroid pulse therapy and double filtration plasmapheresis. Clin Lab Haematol. 2000;22(3):179–181. doi: 10.1046/j.1365-2257.2000.00297.x. [DOI] [PubMed] [Google Scholar]
- 45.Christersdottir T, Pirault J, Gisterå A, et al. Prevention of radiotherapy-induced arterial inflammation by interleukin-1 blockade. Eur Heart J. 2019;40(30):2495–2503. doi: 10.1093/eurheartj/ehz206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kyriazopoulou E, Panagopoulos P, Metallidis S, et al. An open label trial of anakinra to prevent respiratory failure in COVID-19. Elife. 2021;10:e66125. doi: 10.7554/eLife.66125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naveed Z, Sarwar M, Ali Z, et al. Anakinra treatment efficacy in reduction of inflammatory biomarkers in COVID-19 patients: a meta-analysis. J Clin Lab Anal. 2022;36(6):e24434. doi: 10.1002/jcla.24434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lukacs NW, Kunkel SL, Burdick MD, Lincoln PM, Strieter RM. Interleukin-1 receptor antagonist blocks chemokine production in the mixed lymphocyte reaction. Blood. 1993;82:3668–3674. [PubMed] [Google Scholar]
- 49.Charlesworth JEG, Wilson S, Qureshi A, et al. Continuous intravenous anakinra for treating severe secondary haemophagocytic lymphohistiocytosis/macrophage activation syndrome in critically ill children. Pediatr Blood Cancer. 2021;68(9):e29102. doi: 10.1002/pbc.29102. [DOI] [PubMed] [Google Scholar]
- 50.Hall M. Targeted Reversal of Inflammation in Pediatric Sepsis-induced MODS (TRIPS) [Internet]. clinicaltrials.gov; 2022 May [cited 2022 May 30]. Report No.: NCT05267821. Available from: https://clinicaltrials.gov/ct2/show/NCT05267821
- 51.Xanthe Brands X, de Vries FMC, et al. Ferritin as marker of macrophage activation-like syndrome in critically Ill patients with community-acquired pneumonia. Crit Care Med. 2021;49:1901–1911. doi: 10.1097/CCM.0000000000005072. [DOI] [PubMed] [Google Scholar]
- 52.Brands X, van Engelen TSR, de Vries FMC, et al. Association of hyperferritinemia with distinct host response aberrations in patients with community-acquired pneumonia. J Infect Dis. 2022;225:2023–2032. doi: 10.1093/infdis/jiac013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldhaber G, Segal G, Dagan A. Hyperferritinemia in the elderly can differentiate the bad from the worst: a retrospective cohort analysis. Medicine (Baltimore) 2020;99:e21419. doi: 10.1097/MD.0000000000021419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Annous Y, Manning S, Khoujah D. Ferritin, fever, and frequent visits: Hyperferritinemic syndromes in the emergency department. Am J Emerg Med. 2021;48:249–254. doi: 10.1016/j.ajem.2021.04.088. [DOI] [PubMed] [Google Scholar]
- 55.Shrivastava G, Valenzuela Leon PC, Calvo E. Inflammasome fuels dengue severity. Front Cell Infect Microbiol. 2020;10:489. doi: 10.3389/fcimb.2020.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McElroy AK, Erickson BR, Flietstra TD, et al. Ebola hemorrhagic fever: Novel biomarker correlates of clinical outcome. J Infect Dis. 2014;210:558–566. doi: 10.1093/infdis/jiu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ab-Rahman HA, Rahim H, AbuBakar S, et al. Macrophage activation syndrome-associated markers in severe dengue. Int J Med. 2016;13:179–186. doi: 10.7150/ijms.13680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ansuini V, Rigante D, Esposito S. Debate around infection-dependent hemophagocytic syndrome in paediatrics. BMC Infect Dis. 2013;13:15. doi: 10.1186/1471-2334-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nandy A, Mondal T, Datta D, et al. Serum Ferritin as a Diagnostic Biomarker for Severity of Childhood Sepsis. Indian Pediatr. 2021;58:1143–1146. [PubMed] [Google Scholar]
- 60.Williams V, Menon N, Bhatia P, et al. Hyperferritinemia in children hospitalized with scrub typhus Trop. Med Health. 2021;49:15. doi: 10.1186/s41182-021-00304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams V, Menon N, Bhatia P, et al. Serum Ferritin predicts neither organ dysfunction nor mortality in pediatric sepsis due to tropical infections. Front Pediatr. 2020;8:607673. doi: 10.3389/fped.2020.607673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Awasthi PR, Angurana SK. Ferritin levels in children with sepsis in low-middle income countries: do we need lower threshold? Pediatr Crit Care Med. 2020;21:923. doi: 10.1097/PCC.0000000000002443. [DOI] [PubMed] [Google Scholar]
- 63.Ghosh S, Baranwal AK, Bhatia P, Nallasamy K. Suspecting hyperferritinemic sepsis in iron-deficient population: do we need a lower plasma ferritin threshold? Pediatr Crit Care Med. 2018;19:e367–e373. doi: 10.1097/PCC.0000000000001584. [DOI] [PubMed] [Google Scholar]
- 64.Colafrancesco S, Alessandri C, Conti F, Priori R. COVID-19 gone bad: A new character in the spectrum of the hyperferritinemic syndrome? Autoimmun Rev. 2020;19:102573. doi: 10.1016/j.autrev.2020.102573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplemental Digital Content.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Additional file 1. The datasets used and /or analyzed during the current study are available from the corresponding author on reasonable request. The PHENOMS database can also be uploaded from the NICHD sponsored DASH website.