Abstract
Cancer remains a significant global health challenge, necessitating the exploration of novel and more precise therapeutic options beyond conventional treatments. In this regard, clustered regularly interspaced short palindromic repeats (CRISPR) systems have emerged as highly promising tools for clinical gene editing applications. The CRISPR family encompasses diverse CRISPR-associated (Cas) proteins that possess the ability to recognize specific target sequences. The initial CRISPR system consisted of the Cas9 protein and a single-guide RNA, which guide Cas9 to the desired target sequence, facilitating precise double-stranded cleavage. In addition to the traditional cis-cleavage activity, the more recently discovered Cas12 and Cas13 proteins exhibit trans-cleavage activity, which expands their potential applications in cancer diagnosis. In this review, we provide an overview of the functional characteristics of Cas9, Cas12, and Cas13. Furthermore, we highlight the latest advancements and applications of these CRISPR systems in cancer gene therapy and molecular diagnosis. We also emphasize the importance of understanding the strengths and limitations of each CRISPR system to maximize their clinical utility. By providing a comprehensive overview of the current state of CRISPR technology in cancer research, we aim to inspire further exploration and innovation in this rapidly evolving field.
Graphical abstract
Keywords: Cas9, Cas12, Cas13, Cancer, Gene therapy, Diagnostic tools, Clinical trials
Introduction
Cancer, characterized by an unrestrained and invasive proliferation of abnormal cells [1], has long been the leading cause of death worldwide and remained a vital public health concern [2] (https://gco.iarc.fr/today/fact-sheets-cancers). Despite being the cornerstone of cancer treatment, traditional therapies such as surgery [3], chemotherapy [4], and radiation therapy [5] have limitations including lack of specificity, significant side effects, and challenges in preventing disease progression or recurrence [6]. Therefore, there is an urgent need to develop therapeutic strategies that offer enhanced precision, effectiveness, and tolerability. One such advancement is gene therapy, which involves manipulating genetic material to correct cancer-causing genetic mutations [7]. With the growing understanding of cancer genomics, gene editing technologies like CRISPR are revolutionizing the potential for precision cancer medicine [8].
The discovery of clustered regularly interspaced palindromic repeats (CRISPR) in Escherichia coli in 1987 laid the foundation for understanding its functional role in bacteria [9], which was elucidated in 2003 when Mojica suggested their contribution to microbial adaptive immunity [10]. The turning point in the field occurred when the first CRISPR-associated protein (Cas), Cas9, was used to cleave the DNA in vitro in 2012 [11, 12]. Following this groundbreaking discovery, researchers demonstrated its programmable ability and showcased its application in editing eukaryotic genes both in vitro and in vivo [13–15].
CRISPR-Cas systems are classified into two major classes [16]. Class 1 systems, found primarily in bacteria and archaea, constitute approximately 90% of known CRISPR-Cas loci and are composed of heteromeric multiprotein effectors [17]. Class 2 systems, accounting for about 10% of CRISPR-Cas loci, are more frequently used due to their single multidomain effector, which is easier to manipulate [18]. Class 2 CRISPR-Cas systems can be divided into three types (type II, V, and VI), each with distinct properties and mechanisms [19]. Type II, exemplified by Cas9, is the most well-known and extensively studied system, responsible for double-stranded DNA cleavage [19]. Type V, represented by Cas12, also targets DNA, while type VI, represented by Cas13, has a different structure and targets single-stranded RNA (ssRNA) [20, 21].
Among CRISPR-based editing systems, CRISPR-Cas9 has been extensively investigated both in vitro and in vivo. Over the years, researchers have made various modifications to improve its specificity and efficiency, leading to promising preliminary results in ongoing clinical trials [22]. Recently discovered Cas12 and Cas13 systems have also shown potential in gene therapy, with the additional advantage of collateral cleavage activity, which enables their use as sensitive and specific diagnostic tools [23, 24].
This review provides a comprehensive summary of the structural and functional characteristics of signature Class 2 Cas proteins, including Cas9, Cas12, and Cas13, as well as their applications in cancer gene therapy and molecular diagnosis. The inclusion of up-to-date research and clinical trials highlights the current progress in the field, while also addressing the key limitations of different CRISPR tools. Although CRISPR-based therapies and diagnostics offer great promise, further rigorous trials and long-term follow-up are needed to establish their safety and efficacy.
DNA editing mechanism of CRISPR-Cas9
CRISPR-Cas9 is a genome editing system composed of two essential components: guide RNA (gRNA) and Cas9. The Cas9 protein exhibits a bi-lobed structure, comprising the recognition lobe (REC) with three Helical domains and a Bridge Helix, and the nuclease lobe (NUC) containing a split RuvC domain, an HNH domain, a Topo domain, and a C-terminal domain (CTD) (Fig. 1A). These structural elements play crucial roles in target recognition, DNA binding, and cleavage, guided by the sequence of the gRNA [12]. The gRNA is artificially synthesized by combining CRISPR RNA (crRNA) and trans-activating crRNA (tracrRNA), where the crRNA identifies the DNA target while the tracrRNA binds to the Cas9 protein [12]. The editing process initiates with Cas9 recognizing the protospacer adjacent motif (PAM) sequence, typically 5'-NGG-3' (N representing any nucleotide) [25]. The gRNA then pairs with the complementary target sequence [26, 27], leading to the activation of the RuvC and HNH domains of the Cas9, resulting in the cleavage of the non-target and target DNA strands, respectively [28]. This generates a blunt double-stranded break (DSB) at three base pairs upstream of PAM site (Fig. 2A).
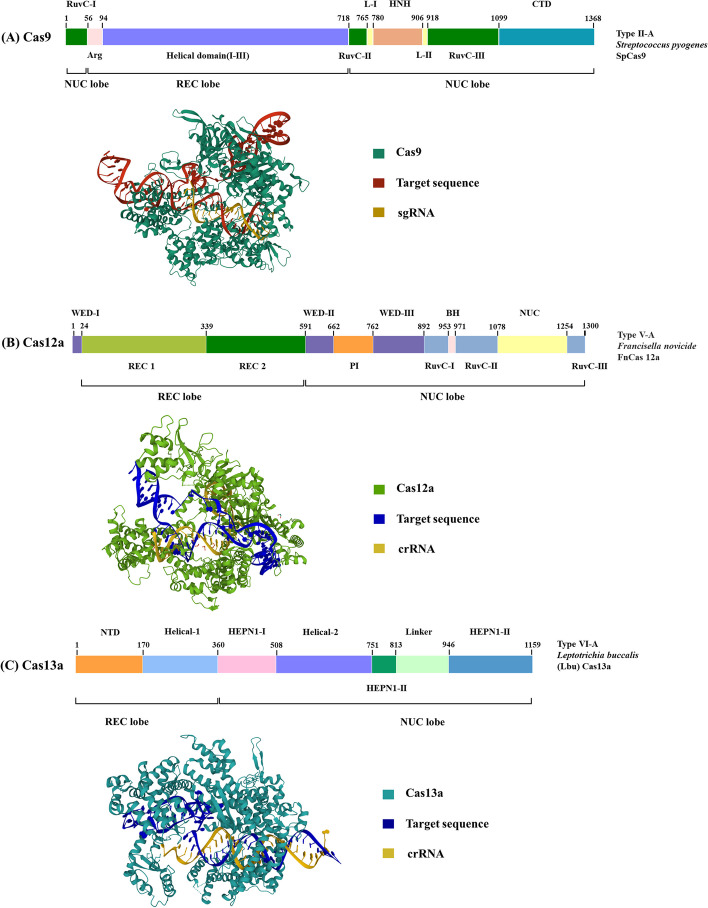
Fig. 1.
Schematics of the domain organization of Cas proteins and crystal structures of Cas protein-sgRNA-target sequence complex. A SpCas9 (PDB: 4OO8) (B) FnCas12a (PDB: 6I1K) (C) LbuCas13a (PDB: 5WXP). REC recognition lobe, NUC nuclease lobe, BH bridge helix, Arg arginine-rich bridge helix, CTD C-terminal domain, WED Wedge, PI protospacer adjacent motif (PAM) interacting, NTD N-terminal domain, HEPN higher eukaryotes and prokaryotes nucleotide-binding domains
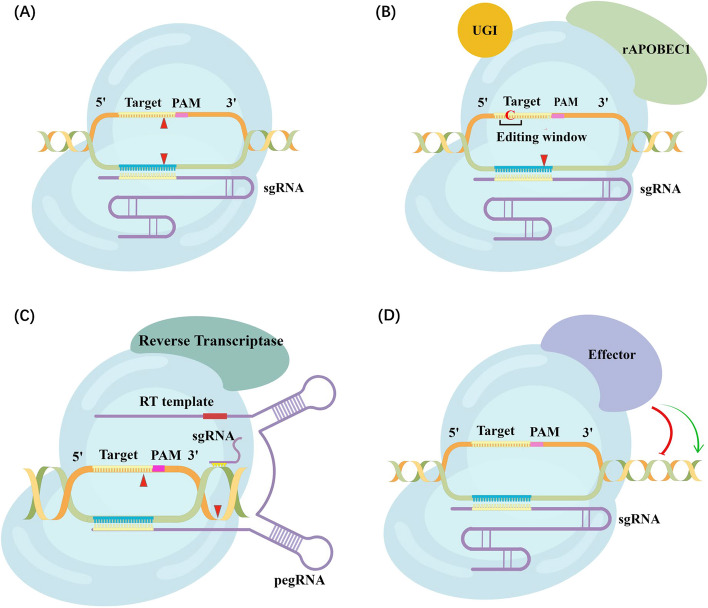
Fig. 2.
Schematic of various CRISPR-Cas9 functions. A Classical CRISPR/Cas9 works as genetic scissors for a specific edition. The Cas9 nuclease (light blue) recognizes 3′ PAM sequence (NGG, NAG) (pink) and target DNA pairs with complementary sgRNA (purple), followed by the formation of blunt DNA double-stranded breaks at the target site (red). Cas9 was fused with selected proteins to induce distinct biological effects (B–D). B dCas9 protein fuses to the cytosine deaminase APOBEC1 (light green) and the uracil DNA glycosylase inhibitor (UGI, orange) to form cytosine base editor. The dCas9 component enables the guidance to its target sequence, then the APOBEC1 component induces C to T conversion. C Cas9 nickase fuse with reverse transcriptase (RT, green) to form prime editors. The prime-editing guide RNA (pegRNA, purple) binds to its complementary target DNA, and the unbound DNA of the PAM-containing strand is cleaved by Cas9. This creates a complementary primer for the desired sequence on the pegRNA, and the RT actively extended the unbound DNA using the pegRNA as a template. D dCas9 fuses to transcriptional activator or repressor (purple) to create CRISPRa and CRISPRi tools and enable target gene transcription regulation
Upon DSB formation, DNA repair mechanisms are triggered, primarily via two pathways: homology-directed repair (HDR) and nonhomologous end joining (NHEJ) [29]. HDR requires a homologous DNA template and is most efficient during the late S and G2 phases of the cell cycle [30]. It allows the introduction of specific point mutations or the insertion of desired sequences through DNA template recombination [31]. Although this pathway is preferred for restoring cellular function, it occurs less frequently than NHEJ. NHEJ operates throughout the cell cycle and repairs DSBs by directly joining DNA fragments without the need for external homologous DNA [30]. Despite its higher occurrence rate, it often results in small, random insertions or deletions that cause frameshift mutations or premature stop codons [32–34].
Having elucidated the mechanism of CRISPR-Cas9, researchers have explored its diverse applications across various fields, including disease model generation, gene therapy, and high-throughput screening platforms for comprehensive gene analysis [35]. Additionally, numerous Cas9-based therapeutics are currently undergoing clinical trials, showcasing promising results and highlighting the immense potential for future gene therapy endeavors.
Application of CRISPR-Cas9 in cancer modelling
The CRISPR-Cas9 system has played a pivotal role in the development of disease models, particularly for cancer and genetic diseases [36, 37]. For instance, Platt et al. established a Cas9 knock-in mouse model by delivering the CRISPR system to the lung via adeno-associated virus (AAV). They established adenocarcinoma pathology through targeted mutations in frequently altered genes such as Tumor Protein P53 (TP53), Liver kinase B1 (LKB1), or Kirsten ratsarcoma viral oncogene homolog (KRAS) [38].
In addition to mouse models, the advent of organoids has expanded the scope of gene therapy research. Lo et al. used CRISPR/Cas9 to generate a knockout (KO) of the tumor suppressor gene AT-rich interaction domain 1A (ARID1A) in primary TP53(−/−) human gastric organoids. This led to morphologic dysplasia, tumorigenicity, and mucinous differentiation [39]. Zhao et al. reported the generation of a Cas9-edited TP53 and cyclin-dependent kinase inhibitor 2A (CDKN2A) KO gastro-esophageal junction (GEJ) organoid model. This study revealed the important role of TP53/CDKN2A inactivation in early GEJ neoplasia, which may aid in early diagnosis and prevention [40]. The efficient and straightforward nature of CRISPR-Cas9 as a method for creating disease models has significantly advanced our understanding of disease pathologies.
Functional gene screening and epigenetic regulation in cancer cells via CRISPR-Cas9
Moreover, the CRISPR-Cas9 system has revolutionized high-throughput screening of gene activities in various biological processes, including tumor growth, metastasis, therapeutic resistance, and response to immunotherapy [41, 42]. For example, Shalem et al. developed a genome-scale CRISPR-Cas9 knockout (GeCKO) library and identified that numerous genes, both previously validated and newly discovered, that contribute to vemurafenib resistance in a melanoma model [43]. Similarly, Wei et al. utilized the CRISPR-Cas9 screening tool to identify phosphoglycerate dehydrogenase (PHGDH) as a driver of resistance in hepatocellular carcinoma (HCC) treatment. The use of the PHGDH inhibitor NCT-503 successfully overcame the resistance, resulting in the abolition of in vivo HCC growth [44]. Additionally, Chen et al. identified Haspin (GSG2) as targetable kinases that displayed synthetic lethal interactions with selective Aurora-A inhibitor alisertib (MLN8237) in breast cancer cells. They also discovered the Haspin inhibitor CHR-6494, which synergistically reduced tumor growth, enhancing the therapeutic effects of MLN8237 in a combinational therapy [45]. These findings underscore the significant impact of the CRISPR-Cas9 screening platform in expediting new drug development and benefiting the pharmaceutical industry.
In addition to its role in gene editing, extensive research has explored the transcriptional regulatory capabilities of CRISPR-Cas9. Qi et al. first introduced the concept of dCas9, which refers to an endonuclease-deficient mutant Cas9 that retains its target recognition capability but lacks nuclease activity [46]. By fusing dCas9 with transcriptional regulators, the CRISPR activation (CRISPRa) and interference (CRISPRi) tools were developed [47–49]. These tools enable the epigenetic regulation of target gene expression (Fig. 2D). Building upon these tools, Cui et al. established a dual CRISPR interference and activation (CRISPRi/a) system that simultaneously silenced the X-inactive specific transcript (XIST) and activated Forkhead box P3 (FOXP3) in breast cancer cells. This manipulation led to increased H4 acetylation and decreased DNA methylation, highlighting the potential of CRISPRi/a for transcriptional and epigenetic modifications [50].
The regulatory capacity of CRISPR-Cas9 has sparked the development of screening platforms based on CRISPRa and CRISPRi for functional studies [51–53]. For example, Myacheva et al. utilized this screening strategy in human lung adenocarcinoma (LUAD) and identified CASP8AP2 as a key regulator influencing the viability of all eight examined LUAD cell lines [54]. Another research group conducted a super enhancer screening in leukemic cells, revealing the specific expression of receptors associated with cell growth and survival in acute leukemia [55]. The development of dCas9 and its fusion with regulatory domains holds great promise for precise regulation of target gene expression. Furthermore, CRISPRa and CRISPRi screening platforms offer significant opportunities for genome-wide functional studies.
Enhancing cancer therapy through CAR-T cell engineering by CRISPR-Cas9
CRISPR-Cas9 has been extensively investigated for its potential in gene therapy, particularly in the context of cancer treatment, which presents complex challenges and high mortality rates. The application of CRISPR-Cas9-mediated knock-out or knock-in strategies offers a valuable approach to interfere with oncogene expression and evaluate its impact on tumor progression [41]. Encouraging results have been obtained from clinical trials employing CRISPR-Cas9 in patients with refractory cancer, particularly in the realm of T cell therapy, where synthetic chimeric antigen receptor (CAR)-T cells have been programmed using CRISPR-Cas9 [56]. CAR-T cells are genetically engineered to specifically target tumor antigen-expressing cells, independent of the major histocompatibility complex (MHC), which is often implicated in immune evasion by tumors. While the production of individual autologous CAR-T cells is costly and complex, the simplicity of CRISPR-Cas9 technology allows for the development of allogeneic CAR-T cells, including T-cell receptor β (TCRβ), programmed cell death protein 1 (PD-1), cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), and more [57, 58]. These modified T cells have demonstrated both in vitro and in vivo efficacy without causing graft-versus-host disease (GVHD), a major complication of transplantation therapy [57, 58].
Recent clinical trials utilizing CAR-T cells have shown promising outcomes in cancer therapy. For instance, You et al. reported improvements in median progression-free survival and median overall survival in advanced non-small-cell lung cancer (NSCLC) patients treated with PD-1 edited T cells (NCT02793856) (Table 1) [59]. Other groups have targeted multiple genes in T cell. Stadtmauer et al. used CRISPR-Cas9 to remove the endogenous TCRα, TCRβ, and the PD-1 from CAR-T cells specific to New York esophageal squamous cell carcinoma 1(NY-ESO-1). This approach successfully reduced TCR mispairing and improved antitumor immunity [60]. Zhang et al. administered anti-CD19 CAR-T cells with integrated PD-1 into patients with relapsed/refractory aggressive B cell non-Hodgkin lymphoma (NCT04213469) (Table 1). The researchers reported a substantial rate of complete remission (87.5%) and long-term response, without any serious adverse events [61]. In addition, Hu et al. infused CRISPR-edited CD19/CD22 dual-targeted CAR-T cells to six patients with relapsed/refractory acute lymphoblastic leukemia, achieving a relatively high complete remission rate without genotoxicity or chromosomal translocation [62]. Trial focusing on solid tumours have also been conducted, Foy et al. designed transgenic T cells with knockout of the endogenous TCR α constant (TRAC) and TCR β constant (TRBC), and the insertion of two chains of a patient-specific neoantigen-specific TCR (neoTCR) into the TRAC locus. Five out of the sixteen participants demonstrated stable disease, while the others experienced disease progression [63]. These clinical trials of CRISPR-Cas9 have laid a solid foundation for the future widespread clinical application of gene therapy.
Table 1.
Summarization of ongoing CRISPR related clinical trials
| Classification | Identifier | Targeted disease | Targeted gene | Start date | Intervention/treatment | Estimated enrollment | Phase |
|---|---|---|---|---|---|---|---|
| CRISPR/Cas9 | NCT02793856 | Metastatic non-small cell lung cancer | Programmed cell death protein 1(PDCD1) gene | August 28, 2016 | Autologous CRISPR Cas9 mediated PD-1 Knockout T cells | 12 | Phase 1 |
| NCT03044743 | Advanced Stage EBV Associated Malignancies | PD-1 | April 7, 2017 | CRISPR-Cas9 mediated PD-1 knockout-T cells from autologous origin | 20 | Phase 1/2 | |
| NCT03164135 | HIV-infected Subjects With Hematological Malignances | CCR5 | May 30, 2017 | CD34 + hematopoietic stem/progenitor cells from donor are treated with CRISPR/Cas9 targeting CCR5 gene | 5 | – | |
| NCT03166878 | Relapsed or Refractory CD19 + Leukemia and Lymphoma | CD19 | June 2017 | Universal CRISPR-Cas9 Gene-Editing CAR-T Cells Targeting CD19 | 80 | Phase 1/2 | |
| NCT03398967 | Relapsed or Refractory B Cell Leukemia and Lymphoma | CD19 and CD20 or CD22 | January 2, 2018 | Universal CRISPR-Cas9 Gene-Editing CAR-T Cells Targeting CD19 and CD20 or CD22 | 80 | Phase 1/2 | |
| NCT03545815 | Mesothelin Positive Multiple Solid Tumors | PD-1 and TCR | March 19, 2018 | CRISPR-Cas9 Mediated PD-1 and TCR Gene-knocked Out Mesothelin-directed CAR-T Cells | 10 | Phase 1 | |
| NCT03747965 | Mesothelin Positive Multiple Solid Tumors | PD-1 | November 2018 | CRISPR-Cas9 Mediated PD-1 Gene-knocked Out Mesothelin-directed CAR-T Cells | 10 | Phase 1 | |
| NCT04417764 | Advanced Hepatocellular Carcinoma | PD-1 | June 20, 2019 | Autologous PD-1 Knockout CRISPR-Cas9-Engineered T Cells | 10 | Phase 1 | |
| NCT04035434 | Relapsed or Refractory B-Cell Malignancies | CD19 | July 22, 2019 | CD19-directed Allogeneic CRISPR-Cas9-Engineered T Cells (CTX110) | 143 | Phase 1 | |
| NCT04244656 | Relapsed or Refractory Multiple Myeloma | BCMA (B-cell maturation antigen) | January 22, 2020 | Anti-BCMA(B-cell maturation antigen) Allogeneic CRISPR-Cas9-Engineered T Cells (CTX120) | 80 | Phase 1 | |
| NCT04213469 | Relapse/Refractory B-cell Lymphoma | PD1 and CD19 | March 13, 2020 | PD1 specific integrated anti-CD19 Chimeric Antigen Receptor T Cells | 20 | – | |
| NCT04426669 | Metastatic Gastrointestinal Epithelial Cancer | CISH (Cytokine-induced SH2 protein) | May 15, 2020 | Autologous CISH (Cytokine-induced SH2 protein) inactivated CRISPR-Cas9-Engineered Tumor-Infiltrating Lymphocytes | 20 | Phase 1/2 | |
| NCT04438083 | Relapsed or Refractory Renal Cell Carcinoma With Clear Cell Differentiation | CD70 | June 16, 2020 | CD70-directed Allogeneic CRISPR-Cas9-Engineered T Cells (CTX130) | 107 | Phase 1 | |
| NCT04502446 | Relapsed or Refractory T or B Cell Malignancies | CD70 | July 31, 2020 | Anti-CD70 Allogeneic CRISPR-Cas9-Engineered T Cells (CTX130) | 45 | Phase 1 | |
| CRISPR/Cas12 | NCT05447169 | Nasopharyngeal Carcinoma | Epstein-Barr virus DNA | July 10, 2022 | Quantitative polymerase chain reaction and Cas 12a target sequencing of EBV DNA in nasopharyngeal brushing and plasma | 11,625 | – |
Off-target editing of CRISPR-Cas9
While CRISPR-Cas9 editing holds great potential for clinical research, it faces challenges that impact its specificity and efficiency. Alanis-Lobato et al. observed off-target editing in approximately 16% of human embryonic cells [58], while Boutin et al. detected megabase-scale losses of heterozygosity (LOH) [64]. Liu et al. found unwanted repairing byproducts such as deletions, vector integrations, and chromosomal translocations [65], and Tuladhar et al. noticed the production of functional foreign mRNAs and aberrant proteins in approximately 50% of CRISPR-Cas9 edited cell lines [66]. Until these fundamental issues are resolved, the use of CRISPR-Cas9 in gene therapy remains limited in terms of safety and feasibility.
To address some of the limitations of CRISPR-Cas9 editing, novel CRISPR-based tools such as base editors and prime editors have been developed. These tools aim to avoid the induction of double-stranded breaks (DSBs) and the risk of deletions, insertions, and frameshift mutations during DNA repair processes [67]. Base editors enable highly efficient and precise single-base substitutions [68, 69]. Prime editors allow the induction of transitions, transversions, small insertions, and deletions without the need for an exogenous donor template [70]. These tools allow for more specific donor-free editing in a programmable manner.
Expanding therapeutic opportunities with novel CRISPR tools
The first CRISPR-based base editor was developed by Komor et al. in 2016 [68]. They fused the cytosine deaminase Apolipoprotein B MRNA Editing Enzyme Catalytic Subunit 1 (APOBEC1) to the amino terminus of dead Cas9 (dCas9). The dCas9 component enables target sequence guidance, while the APOBEC1 component induces C to T conversion (Fig. 2B) [68]. Subsequently, Adenine base editors inducing A to G conversions [69], and C-to-G base editors (CGBE) inducing C-to-G base transversions[71] were developed. Numerous enhanced base editors with improved efficiency and specificity have since been developed [68, 72–74]. In 2021, Wang et al. constructed a new transformer base editing (tBE) system that increases on-target specificity while reducing unintended mutations [75]. Later, Zhang et al. developed a dual adenine and cytosine base editor system by combining both deaminases with a Cas9 nickase, thereby expanding the scope of base editors to enable C-to-T and A-to-G conversions at the same target [74]. Though research efforts have significantly improved the efficiency, targeting scope, and product purity of base editors [67, 76], drawbacks including limitations in targetable sites and unanticipated off-target editing hinder their feasibility in therapeutic use.
Base editors have proven to be highly valuable in large-scale genome screening to identify gain- and loss-of-function variants [77, 78], as well as pathogenic variants of cancer [79]. These applications enhance our understanding of the functional implications of cancer-associated variants. The screening system has also been used to investigate the impact of protein modifications, such as phosphorylation, on chemotherapy response. For instance, Li et al. revealed the involvement of P21-activated Kinase 4/Extracellular Regulated Protein Kinases (PAK4/ERK) and Ribosomal S6 Kinase 2/Recombinant Tumor Protein p53 Binding Protein 1/ Phosphorylated H2A Histone family member X (RSK2/TP53BP1/γ-H2AX) signaling pathways in anti-chemoresistance [80].
Base editors have shown significant therapeutic potential in directly modifying oncogenes or tumor suppressor genes. Annunziato et al. employed CBEs to modify oncogenic mutations in a triple-negative breast cancer model [81]. Sayed et al. used ABEs to correct KRAS and TP53 mutations in cancer organoids from patients [82]. Both studies provided proof of concept for extending the use of flexible base editing tools to other tumor types. Additionally, base editors have been utilized in the development of CAR-T platforms, demonstrating the disruption of multiple genes with high efficiency and minimal double-strand breaks [83]. Diorio et al. achieved promising results by developing a CD7-directed allogeneic CAR-T (7CAR8) using CBEs, which showed high effectiveness against T-cell acute lymphoblastic leukemia (T-ALL) in various in vitro and in vivo models [84]. These advancements hold significant translational potential for cancer treatment.
Another breakthrough in precise gene editing is prime editing, which incorporates an engineered reverse transcriptase (RT) with Cas9 nickase [70]. Together with a prime-editing guide RNA (pegRNA), they can precisely modify target sequences in a programmable manner. The pegRNA specifically binds to the complementary target DNA, and Cas9 cleaves the unbound DNA of the PAM-containing strand. This generates a complementary primer for the desired sequence on the pegRNA, and the RT actively extends the unbound DNA utilizing the pegRNA as a template. The resulting edited 3' flap aims to integrate into the genome while the original 5' flap is vulnerable to cellular endonucleases during lagging-strand DNA synthesis (Fig. 2C) [70].
Prime editing represents a revolutionary advancement in precise gene editing. The initial version, Prime Editor 1 (PE1), integrated features of both CRISPR and reverse transcriptase but displayed suboptimal efficiency for mass application [70]. Subsequent versions, Prime Editor 2 (PE2) and Prime Editor 3 (PE3), showed steady progress, with PE2 improving editing efficacy by merging a designed reverse transcriptase with Cas9 nickase, and PE3 further enhancing efficiency by inducing strand replacement [85]. However, prime editing has certain limitations compared to other technologies such as CRISPR-Cas9, including lower efficiency, challenges in cell delivery due to its large size, and the requirement of a longer time to implement genetic changes [86, 87]. Additionally, it can introduce unwanted byproducts like indels, posing risks of side effects and complications [88, 70].
While the clinical safety of prime editors is still being evaluated, current applications have mainly focused on cancer modeling. Ely et al. utilized prime editing to model lung and pancreatic cancer. This system introduced clinically relevant mutations, such as KRAS mutations associated with drug resistance and TP53 mutations in pancreatic cancer [89]. Another group established in vivo tumor models by inducing the S45 deletion of cadherin-associated protein beta 1 (CTNNB1) in somatic cells, achieving effective editing rates of over 80% at the target site [90]. The establishment of efficient disease models facilitates basic research and enhances our understanding of disease pathology and mechanisms.
Dual role of Cas12 and Cas13 as gene editing and diagnostic tools
In recent years, extensive research has been conducted on the Cas12 and Cas13 systems, complementing the well-established Cas9 system. Cas12 has demonstrated high specificity in recognizing and cleaving double-stranded DNA [91, 92]. In contrast, Cas13 is distinguished by its unique recognition and targeted cleavage of single-stranded RNA [93]. Both Cas12 and Cas13 possess the characteristic of collateral cleavage, leading to unintended cleavage of non-targeted sequences [94, 95]. While this feature may not be ideal for therapeutic editing, it proves incredibly valuable in the development of CRISPR-based diagnostic tools. Consequently, several diagnostic approaches have been devised utilizing the Cas12a and Cas13a systems [23, 24].
DNA editing mechanism of CRISPR-Cas12
Cas12 protein shares similar functions with Cas9 in recognizing and editing double-stranded DNA, but it relies solely on CRISPR RNA (crRNA) for guidance. Unlike the Cas9 system, which requires the host endonuclease RNase III for processing pre-crRNA into mature crRNA, Cas12 can independently carry out this step [96]. The Cas12 family can be further classified into Cas12a-i, but only Cas12a, b, f, and g have demonstrated potential in disease diagnosis [94]. Cas12a, the first identified type V CRISPR protein, consists of the REC and NUC lobes, which are further divided into the REC1 and REC2 domains, the RuvC, PAM-interacting (PI), and the Wedge (WED) domains, respectively (Fig. 1B) [97, 98]. The RuvC domain houses a single catalytic site responsible for the DNase activity [99], enabling sequential cleavage of the double-stranded DNA, with the non-target strand being cleaved first. This would result in a staggered-end DNA break with 5’ overhangs (Fig. 3A) [97].
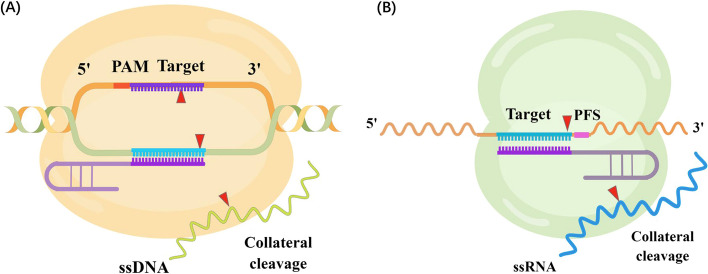
Fig. 3.
Schematic of CRISPR-Cas12a and CRISPR-Cas13a functions. A Cas12a nuclease recognizes DNA targets complementary to the crRNA spacer. Target recognition results in cleavage activities which leads to a staggering DNA double-strand break. In addition, activated Cas12a could nonspecifically cleave nearby ssDNA. B Cas13a is a crRNA-guided RNA-targeting nuclease. Upon binding to the target RNA, it could perform the target cleavage downstream of the protospacer flanking site (PFS, pink). In addition, activated Cas13a also possesses a nonspecific ribonuclease activity. The cleavage sites are marked by red arrows
Previous knowledge on Cas9 has emphasized the significance of the PAM region, which is also crucial for Cas12a. The PI, REC1, and WED domains of Cas12a enzymes collectively facilitate PAM recognition [100]. Cas12a typically recognizes a 5′-TTTV-3′ PAM [101, 102]. In addition, it exhibits a more flexible recognition capability, allowing for suboptimal PAM binding [103], such as 5′-TCTA-3′, 5′-TCCA-3′, and 5′-CCCA-3′. Under these circumstances, it retains the cleavage activity but at a lower efficiency [104]. Similar to Cas9, various Cas12a variants have been engineered to recognize alternative PAM sequences without compromising efficacy, broadening their range of applications [105].
Application of the Cas12a in cancer therapy via target gene modification
Extensive investigations have been carried out to explore the application of Cas12a in human cells for genetic editing and diagnostics. Studies evaluating various Cas12a homologs have demonstrated their effective genome editing capabilities. In comparison to the well-established Cas9 editing system, Cas12a has exhibited slightly reduced efficiency but improved accuracy in editing [92]. This can be attributed to the longer spacer region of the CRISPR RNA (crRNA), which enhances specificity. Notably, the development of dead Cas12a has revealed its potential for target gene repression, surpassing the performance of the dCas9 system [106]. Another advantageous feature of the Cas12a system is its ability to autonomously process pre-mature crRNA, facilitating simultaneous multiplex gene regulation when multiple crRNAs are encoded on the plasmid [107].
Cas12a has emerged as a powerful tool for genome editing that could be applied in cancer therapy. Specifically, it has been designed to disrupt commonly mutated genes such as vrafmurine sarcoma viral oncogene homolog B (BRAF)-V600E [108, 109]. In addition, Choi et al. leveraged the multiplex gene targeting capability of Cas12a by designing systems that target three frequently mutated genes (TP53, Adenomatous polyposis coli (APC), and Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha gene (PIK3CA)), in colorectal cancer patients [110]. These studies have highlighted the unique advantages of Cas12a in therapeutic editing for cancer cells.
Application of the Cas12a in cancer diagnosis utilizing collateral cleavage activity
Apart from its potential in gene therapy, Cas12a is widely employed as a diagnostic tool. This is accomplished through its collateral cleavage activity upon binding to the target. Upon binding, Cas12a undergoes a conformational change in the lid region, exposing the catalytic residue and enabling indiscriminate cleavage of single-stranded DNA (ssDNA) [111]. This collateral cleavage activity is a multiple turnover process, rendering it highly sensitive for detection purposes. Commonly mutated genes, such as TP53, Breast Cancer gene 1 (BRCA1), and KRAS, serve as popular tumor biomarkers [112, 113]. Cas12a has achieved attomolar sensitivity in detecting specific targets [23], and further studies have shown its superior sensitivity compared to droplet digital PCR (ddPCR) while offering the advantage of faster detection [114].
These features underscore the feasibility of Cas12a as an excellent diagnostic tool. Ongoing clinical trials primarily focus on genetic diseases or infections, with a single trial aiming to test the detection of Epstein-Barr virus (EBV) DNA in nasopharyngeal brushing and patient plasma as an indicator for nasopharyngeal carcinoma (NCT05447169) (Table 1). Further trials should be conducted to evaluate the effectiveness, safety, and feasibility of implementing Cas12a in a clinical setting. Additionally, expanding the range of target genes and diseases for therapeutic editing could provide new avenues for exploration.
RNA editing mechanism of CRISPR-Cas13
Cas13 is a distinct CRISPR effector that operates through crRNA-guided RNA targeting and does not share structural homology with the other types of CRISPR proteins [115]. It consists of the REC and NUC lobes, and its target RNA cleavage is mediated by the two higher eukaryotes and prokaryotes nucleotide-binding (HEPN) domains (Fig. 1C) [115]. Cas13 can be categorized into four subtypes, including Cas13a, b1, b2, c, d, X, and Y [116]. Among these subtypes, Cas13a was the first to be discovered and extensively studied for its potential in gene editing and diagnostics. As an RNA-targeting nuclease, Cas13a interacts with the protospacer flanking site (PFS) instead of PAM recognition [117]. Notably, while bacterial cells have exhibited collateral cleavage of random single-stranded RNAs (ssRNAs) by Cas13a [95], this activity has not been observed in eukaryotic cells [93].
Application of the Cas13a in cancer therapy via post-transcriptional regulation
Cas13a, a crRNA-guided RNA-targeting CRISPR effector, displays structural dissimilarity to other CRISPR proteins [115]. It exhibits diverse mechanisms for regulating gene expression, including gene knockdown [118], transcript tracking [93], and RNA editing [119, 120]. Previous studies have demonstrated its post-transcriptional knockdown ability on endogenous genes such as KRAS, C-X-C Motif Chemokine Receptor 4 (CXCR4), and Peptidylprolyl Isomerase B (PPIB) [93]. This form of regulation avoids direct alterations to genomic DNA, leading to more controlled outcomes. Other studies proved the potential of Cas13a gene therapy in lung cancer and chronic myelogenous leukemia [121, 122]. Recently, high-fidelity Cas13 variants, such as Cas13d and Cas13X, have been proposed to further enhance their therapeutic potential [123]. Compared to RNA interference (RNAi) systems, CRISPR-Cas13 exhibits improved specificity and efficacy, achieving over 90% knockdown efficiency. Moreover, off-target effects observed in RNAi systems are substantially reduced with CRISPR-Cas13 [124]. Both processes are highly efficient, with significant post-transcriptional suppression observed within a single day. However, the clinical application of the CRISPR-Cas13 system is hindered by challenges associated with in vivo delivery, limiting its potential in clinical settings [125].
Abudayyeh et al. proposed a modified version of Cas13a in which the catalytic activity is rendered inactive by mutating the arginine residues in the HEPN domains [93]. This modified Cas13a functions in a similar way to dCas9. It can be directed to specific targets but loses its editing capability. This expanded functionality allows for its application not only in transcriptome analysis but also in therapeutic approaches. However, despite these advancements, none of these approaches have progressed from the laboratory to clinical settings, emphasizing the need for more clinical studies to validate their potential in real-world applications.
Application of the Cas13a in cancer diagnosis utilizing collateral cleavage activity
Cas13a exhibits collateral cleavage of adjacent non-target ssRNAs, enabling the design of diagnostic tools similar to Cas12a (Fig. 3B). When the crRNA-target RNA duplex binds to the NUC lobe of Cas13a, a conformational change occurs, activating its catalytic function. As the HEPN domains are exposed on the surface, free ssRNAs in the surrounding solution can be readily accessed and cleaved, resulting in collateral cleavage activity [126]. One notable example of such a diagnostic tool is the specific high-sensitivity enzymatic reporter unlocking (SHERLOCK) system. In this system, the target sequence is first amplified using techniques such as recombinase polymerase amplification (RPA) or reverse transcriptase (RT)-RPA to generate target DNA sequences. Subsequently, T7 transcription is employed to transcribe the target sequence into RNA. The resulting RNA sample then activates the Cas13a-gRNA complex, leading to the cleavage of fluorophore quencher labeled (FQ-labeled) ssRNA reporters. The intensity of the fluorescence signal can be used as a reference to determine the initial target concentration [24]
Previous studies have demonstrated the attomolar sensitivity of Cas13a in detecting low-frequency cancer mutations in cell-free DNA fragments (cfDNA), comparable to the sensitivity by ddPCR and quantitative PCR (qPCR) [127]. Gootenberg et al. successfully detected common mutations, such as Epidermal growth factor receptor (EGFR) L858R, T790M and BRAF V600E, in samples from patients with non-small cell lung cancer (NSCLC) [24]. More recently, researchers found Cas13a could also detect mRNA, miRNAs and even exosomal protein markers. Similar to cfDNA, mRNA of mutated genes, such as EGFR mRNA, could serve as an effective biomarker for NSCLC [128]. miRNAs have garnered significant attention in cancer biomarker research, and studies have demonstrated the ability of the Cas13a system to detect specific miRNA biomarkers. For example, miR-19b has been identified as a potential marker for medulloblastoma [129], while miR-17 and miR-21 have been identified as potential markers for breast cancer [130]. Other than its sensitivity, the specificity of Cas13a has been investigated. It has demonstrated the capability to distinguish between miR-17, miR-106a, miR-20a, and miR-20b, proving a single-nucleotide resolution [131].
Furthermore, research groups have designed more complexed circuits that allow Cas13a to detect protein markers, such as Programmed cell death 1 ligand 1 (PD-L1), Interleukin-6 (IL-6), and Vascular endothelial growth factor (VEGF), in patient serum [132, 133]. The system involved a target-specific aptamer, which would be amplified upon binding to the target. The amplified aptamer sequence can be transcribed by T7 polymerase, which simultaneously activates the Cas13a-crRNA complex and triggers its collateral cleavage activity [132]. These recent developments expand the scope of Cas13a-based detection and present promising potential for these tools to enter clinical trials.
Benefits of Cas-based detection methods over conventional techniques
Cas-based detection methods offer several advantages compared to conventional detection methods such as real-time PCR (RT-PCR) or qPCR. RT-PCR and qPCR are labor-intensive, prone to human error, time-consuming, and expensive [134]. They require specialized equipment and lab environments. In contrast, CRISPR-based detections are portable, and commercial lateral flow strips enable rapid detection with results available within two hours [135]. The high sensitivity of Cas-based methods allows for single-copy viral detection, which is a significant advantage [23, 24]. Moreover, they are cost-friendly, particularly when combined with newly developed diagnostic tools [136].
In 2020, the introduction of Combinatorial Arrayed Reactions for Multiplexed Evaluation of Nucleic acids (CARMEN) further expanded the detection range and efficiency of the SHERLOCK by incorporating numerous optimized crRNAs [137]. CARMEN can accommodate more than 1000 samples or detect 169 pathogens for five samples in each chip, reducing the cost of SHERLOCK by 300-fold. Other groups have proposed innovative designs that combine CRISPR-Cas13 with microfluidic electrochemical biosensors, creating simple, easily scalable, and amplification-free diagnostic tools [129]. These advantages have generated significant interest in CRISPR-based diagnostic tools for infectious diseases and cancers with specific biomarkers.
Conclusion
Over the years, the landscape of cancer treatment has witnessed remarkable progress, transitioning from traditional interventions to more innovative approaches such as immunotherapy and gene therapy [138]. Traditional cancer treatments have long been the mainstays of cancer management. However, updated approaches such as immunotherapy, which harnesses the body’s immune system to target and eliminate cancer cells, has revolutionized the field [139]. Additionally, gene therapies, including the use of CRISPR-Cas9 technology, hold promise for targeted genetic modifications and precision medicine.
The past decade has witnessed significant advancements in the understanding and application of CRISPR systems. Their simplicity, robustness, and high efficiency have opened up possibilities for the treatment and diagnosis of various diseases, including cancer [28]. This review has provided an overview of the structural and functional characteristics of Cas9, Cas12, and Cas13, as well as their applications in gene therapy and molecular diagnosis.
Despite the rapid development of CRISPR, which provides new hope for once-believed incurable diseases, there are still significant hurdles that limit its widespread application. These challenges include unstable editing efficiency, off-target editing concerns, and limitations in vivo delivery.
Acknowledgements
Parts of graphic abstract of this article was drawn by using pictures from Servier Medical Art by that was licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/) and by Figdraw (www.figdraw.com). The Figs. 2 and 3 of this article were drawn by Figdraw (www.figdraw.com).
Abbreviations
- CRISPR
Clustered regularly interspaced short palindromic repeats
- Cas
CRISPR-associated protein
- DNA
Deoxyribonucleic acid
- RNA
Ribonucleic acid
- pre-crRNAs
Precursor-CRISPR RNAs
- crRNA
CRISPR RNA
- tracrRNA
Trans-activating crRNA
- ssRNA
Single-stranded RNA
- gRNA
Guide RNA
- REC
Recognition lobe
- NUC
Nuclease lobe
- CTD
C-terminal domain
- PAM
Protospacer Adjacent Motif
- DSB
Double-stranded break
- HDR
Homology-directed repair
- NHEJ
Nonhomologous end joining
- AAV
Adeno-associated virus
- TP53
Tumor Protein P53
- LKB1
Liver kinase B1
- KRAS
Kirsten ratsarcoma viral oncogene homolog
- KO
Knockout
- ARID1A
AT-rich interaction domain 1A
- CDKN2A
Cyclin-dependent kinase inhibitor 2A
- TP53/CDKN2A(KO)
TP53 and cyclin-dependent kinase inhibitor 2A (CDKN2A) knockout
- GEJ
Gastro-esophageal junction
- GeCKO
Genome-scale CRISPR-Cas9 knockout
- PHGDH
Phosphoglycerate dehydrogenase
- HCC
Hepatocellular carcinoma
- CAR
Chimeric antigen receptors
- MHC
Major histocompatibility complex
- TCRβ
T-cell receptor β
- PD-1
Programmed cell death protein 1
- CTLA-4
Cytotoxic T lymphocyte-associated antigen-4
- GVHD
Graft-versus-host disease
- TCRα
T-cell receptor α
- NY-ESO-1
New York esophageal squamous cell carcinoma 1
- TRAC
T-cell receptor alpha constant
- TRBC
T-cell receptor beta constant
- neoTCR
Neoantigen-specific T-cell receptor
- LOH
Losses of heterozygosity
- BEs
Base editors
- CBEs
Cytosine base editors
- ABEs
Adenine base editors
- PEs
Prime editors
- APOBEC1
Apolipoprotein B MRNA Editing Enzyme Catalytic Subunit 1
- dCas9
Dead CRISPR-associated protein 9
- CGBE
C-to-G base editors
- tBE
Transformer base editing
- dCDI
Deoxycytidine deaminase inhibitor
- PAK4/ERK
P21-activated Kinase 4/Extracellular Regulated Protein Kinases
- RSK2/TP53BP1/γ-H2AX
Ribosomal S6 Kinase 2/Recombinant Tumor Protein p53 Binding Protein 1/Phosphorylated H2A Histone family member X
- 7CAR8
CD7-directed allogeneic CART
- T-ALL
T-cell acute lymphoblastic leukemia
- RT
Reverse transcriptase
- pegRNA
Prime-editing guide RNA
- CTNNB1
Cadherin-associated protein beta 1
- CRISPRa
CRISPR activation
- CRISPRi
CRISPR interference
- XIST
X-inactive specific transcript
- FOXP3
Forkhead box P3
- PI
PAM-interacting
- WED
Wedge
- BRAF
Vrafmurine sarcoma viral oncegene homolog B
- APC
Adenomatous polyposis coli
- PIK3CA
Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha gene
- BRCA1
Breast Cancer gene 1
- ddPCR
Droplet digital polymerase chain reaction
- EBV
Epstein-Barr virus
- HEPN
Higher eukaryotes and prokaryotes nucleotide-binding
- PFS
Protospacer flanking site
- CXCR4
C-X-C Motif Chemokine Receptor 4
- PPIB
Peptidylprolyl Isomerase B
- RNAi
RNA interference
- SHERLOCK
Specific high-sensitivity enzymatic reporter unlocking
- RPA
Recombinase Polymerase Amplification
- RT-RPA
Reverse transcriptase-RPA
- FQ-labeled
Fluorophore quencher labeled
- cfDNA
Cell-free DNA fragments
- NSCLC
Non-small cell lung cancer
- PD-L1
Programmed cell death 1 ligand 1
- IL-6
Interleukin-6
- VEGF
Vascular endothelial growth factor
- RT-PCR
Real-time polymerase chain reaction
- qPCR
Quantitative polymerase chain reaction
- CARMEN
Combinatorial arrayed reactions for multiplexed evaluation of nucleic acids
- NGS
Next-generation sequencing
- FLASH
Finding low abundance sequences by hybridization
Author contributions
BY and XH designed the manuscript. XW, MW and MC searched the literature. MW and MC wrote the manuscript. BY and XH provided the funding. All authors read and approved the final manuscript.
Funding
This work was supported by a grant from the Shenzhen Sanming Project (No. SZSM201812059) and Shenzhen Key Medical Discipline Construction Fund (No. SZXK040.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mingxia Wang and Menghui Chen contributed equally to this work and considered as co-first authors.
Contributor Information
Xinbo Huang, Email: huangxinbo92@pku.edu.cn.
Bo Yu, Email: drboyu_derm@126.com.
References
- 1.Braakhuis BJ, Tabor MP, Kummer JA, Leemans CR, Brakenhoff RH. A genetic explanation of Slaughter's concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63(8):1727–1730. [PubMed] [Google Scholar]
- 2.Soerjomataram I, Bray F. Planning for tomorrow: global cancer incidence and the role of prevention 2020–2070. Nat Rev Clin Oncol. 2021;18(10):663–672. doi: 10.1038/s41571-021-00514-z. [DOI] [PubMed] [Google Scholar]
- 3.Wyld L, Audisio RA, Poston GJ. The evolution of cancer surgery and future perspectives. Nat Rev Clin Oncol. 2015;12(2):115–124. doi: 10.1038/nrclinonc.2014.191. [DOI] [PubMed] [Google Scholar]
- 4.Galmarini D, Galmarini CM, Galmarini FC. Cancer chemotherapy: a critical analysis of its 60 years of history. Crit Rev Oncol Hematol. 2012;84(2):181–199. doi: 10.1016/j.critrevonc.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Schaue D, McBride WH. Opportunities and challenges of radiotherapy for treating cancer. Nat Rev Clin Oncol. 2015;12(9):527–540. doi: 10.1038/nrclinonc.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farber S, Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med. 1948;238(23):787–793. doi: 10.1056/NEJM194806032382301. [DOI] [PubMed] [Google Scholar]
- 7.Brody H. Gene therapy. Nature. 2018;564(7735):S5. doi: 10.1038/d41586-018-07639-9. [DOI] [PubMed] [Google Scholar]
- 8.van der Oost J, Westra ER, Jackson RN, Wiedenheft B. Unravelling the structural and mechanistic basis of CRISPR-Cas systems. Nat Rev Microbiol. 2014;12(7):479–492. doi: 10.1038/nrmicro3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishino YSH, Makino K, Amemura M, Nakata A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol. 1987;169(12):5429–5433. doi: 10.1128/jb.169.12.5429-5433.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mojica FJ, Díez-Villaseñor C, García-Martínez J, Soria E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol. 2005;60(2):174–182. doi: 10.1007/s00239-004-0046-3. [DOI] [PubMed] [Google Scholar]
- 11.Gasiunas G, Barrangou R, Horvath P, Siksnys V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci USA. 2012;109(39):E2579–E2586. doi: 10.1073/pnas.1208507109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jinek M, East A, Cheng A, Lin S, Ma E, Doudna J. RNA-programmed genome editing in human cells. Elife. 2013;2:e00471. doi: 10.7554/eLife.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol. 2013;31(3):227–229. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makarova KS, Wolf YI, Iranzo J, Shmakov SA, Alkhnbashi OS, Brouns SJJ, et al. Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. Nat Rev Microbiol. 2020;18(2):67–83. doi: 10.1038/s41579-019-0299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shmakov S, Smargon A, Scott D, Cox D, Pyzocha N, Yan W, et al. Diversity and evolution of class 2 CRISPR-Cas systems. Nat Rev Microbiol. 2017;15(3):169–182. doi: 10.1038/nrmicro.2016.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.JolanyVangah S, Katalani C, Booneh HA, Hajizade A, Sijercic A, Ahmadian G. CRISPR-based diagnosis of infectious and noninfectious diseases. Biol Proced Online. 2020;22:22. doi: 10.1186/s12575-020-00135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shmakov S, Abudayyeh OO, Makarova KS, Wolf YI, Gootenberg JS, Semenova E, et al. Discovery and functional characterization of diverse class 2 CRISPR-Cas systems. Mol Cell. 2015;60(3):385–397. doi: 10.1016/j.molcel.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strecker J, Jones S, Koopal B, Schmid-Burgk J, Zetsche B, Gao L, et al. Engineering of CRISPR-Cas12b for human genome editing. Nat Commun. 2019;10(1):212. doi: 10.1038/s41467-018-08224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swarts DC, Jinek M. Mechanistic Insights into the cis- and trans-Acting DNase Activities of Cas12a. Mol Cell. 2019;73(3):589–600.e4. doi: 10.1016/j.molcel.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frangoul H, Altshuler D, Cappellini MD, Chen YS, Domm J, Eustace BK, et al. CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. N Engl J Med. 2021;384(3):252–260. doi: 10.1056/NEJMoa2031054. [DOI] [PubMed] [Google Scholar]
- 23.Chen JS, Ma E, Harrington LB, Da Costa M, Tian X, Palefsky JM, et al. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science. 2018;360(6387):436–439. doi: 10.1126/science.aar6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gootenberg JS, Abudayyeh OO, Lee JW, Essletzbichler P, Dy AJ, Joung J, et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science. 2017;356(6336):438–442. doi: 10.1126/science.aam9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirano S, Nishimasu H, Ishitani R, Nureki O. Structural basis for the altered PAM specificities of engineered CRISPR-Cas9. Mol Cell. 2016;61(6):886–894. doi: 10.1016/j.molcel.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Sternberg SH, Redding S, Jinek M, Greene EC, Doudna JA. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature. 2014;507(7490):62–67. doi: 10.1038/nature13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cong L, Zhang F. Genome engineering using CRISPR-Cas9 system. Methods Mol Biol. 2015;1239:197–217. doi: 10.1007/978-1-4939-1862-1_10. [DOI] [PubMed] [Google Scholar]
- 28.Nishimasu H, Ran FA, Hsu PD, Konermann S, Shehata SI, Dohmae N, et al. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell. 2014;156(5):935–949. doi: 10.1016/j.cell.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hustedt N, Durocher D. The control of DNA repair by the cell cycle. Nat Cell Biol. 2016;19(1):1–9. doi: 10.1038/ncb3452. [DOI] [PubMed] [Google Scholar]
- 30.Branzei D, Foiani M. Regulation of DNA repair throughout the cell cycle. Nat Rev Mol Cell Biol. 2008;9(4):297–308. doi: 10.1038/nrm2351. [DOI] [PubMed] [Google Scholar]
- 31.Lin S, Staahl BT, Alla RK, Doudna JA. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. Elife. 2014;3:e04766. doi: 10.7554/eLife.04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musunuru K. The hope and hype of CRISPR-Cas9 genome editing: a review. JAMA Cardiol. 2017;2(8):914–919. doi: 10.1001/jamacardio.2017.1713. [DOI] [PubMed] [Google Scholar]
- 33.Román-Rodríguez FJ, Ugalde L, Álvarez L, Díez B, Ramírez MJ, Risueño C, et al. NHEJ-mediated repair of CRISPR-Cas9-induced DNA breaks efficiently corrects mutations in HSPCs from patients with fanconi anemia. Cell Stem Cell. 2019;25(5):607–621.e7. doi: 10.1016/j.stem.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 34.Fu YW, Dai XY, Wang WT, Yang ZX, Zhao JJ, Zhang JP, et al. Dynamics and competition of CRISPR-Cas9 ribonucleoproteins and AAV donor-mediated NHEJ, MMEJ and HDR editing. Nucleic Acids Res. 2021;49(2):969–985. doi: 10.1093/nar/gkaa1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selvakumar SC, Preethi KA, Ross K, Tusubira D, Khan MWA, Mani P, et al. CRISPR/Cas9 and next generation sequencing in the personalized treatment of Cancer. Mol Cancer. 2022;21(1):83. doi: 10.1186/s12943-022-01565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torres-Ruiz R, Rodriguez-Perales S. CRISPR-Cas9 technology: applications and human disease modelling. Brief Funct Genomics. 2017;16(1):4–12. doi: 10.1093/bfgp/elw025. [DOI] [PubMed] [Google Scholar]
- 37.Barazesh M, Mohammadi S, Bahrami Y, Mokarram P, Morowvat MH, Saidijam M, et al. CRISPR/Cas9 technology as a modern genetic manipulation tool for recapitulating of neurodegenerative disorders in large animal models. Curr Gene Ther. 2021;21(2):130–148. doi: 10.2174/1566523220666201214115024. [DOI] [PubMed] [Google Scholar]
- 38.Platt RJ, Chen S, Zhou Y, Yim MJ, Swiech L, Kempton HR, et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell. 2014;159(2):440–455. doi: 10.1016/j.cell.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lo YH, Kolahi KS, Du Y, Chang CY, Krokhotin A, Nair A, et al. A CRISPR/Cas9-engineered ARID1A-deficient human gastric cancer organoid model reveals essential and nonessential modes of oncogenic transformation. Cancer Discov. 2021;11(6):1562–1581. doi: 10.1158/2159-8290.CD-20-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao H, Cheng Y, Kalra A, Ma K, Zheng Y, Ziman B, et al. Generation and multiomic profiling of a TP53/CDKN2A double-knockout gastroesophageal junction organoid model. Sci Transl Med. 2022;14(673):eabq6146. doi: 10.1126/scitranslmed.abq6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H, Qin C, An C, Zheng X, Wen S, Chen W, et al. Application of the CRISPR/Cas9-based gene editing technique in basic research, diagnosis, and therapy of cancer. Mol Cancer. 2021;20(1):126. doi: 10.1186/s12943-021-01431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang SW, Gao C, Zheng YM, Yi L, Lu JC, Huang XY, et al. Current applications and future perspective of CRISPR/Cas9 gene editing in cancer. Mol Cancer. 2022;21(1):57. doi: 10.1186/s12943-022-01518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343(6166):84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei L, Lee D, Law CT, Zhang MS, Shen J, Chin DW, et al. Genome-wide CRISPR/Cas9 library screening identified PHGDH as a critical driver for Sorafenib resistance in HCC. Nat Commun. 2019;10(1):4681. doi: 10.1038/s41467-019-12606-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen A, Wen S, Liu F, Zhang Z, Liu M, Wu Y, et al. CRISPR/Cas9 screening identifies a kinetochore-microtubule dependent mechanism for Aurora-A inhibitor resistance in breast cancer. Cancer Commun (Lond) 2021;41(2):121–139. doi: 10.1002/cac2.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152(5):1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gilbert LA, Horlbeck MA, Adamson B, Villalta JE, Chen Y, Whitehead EH, et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell. 2014;159(3):647–661. doi: 10.1016/j.cell.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, Barcena C, et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517(7536):583–588. doi: 10.1038/nature14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dominguez AA, Lim WA, Qi LS. Beyond editing: repurposing CRISPR-Cas9 for precision genome regulation and interrogation. Nat Rev Mol Cell Biol. 2016;17(1):5–15. doi: 10.1038/nrm.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cui X, Zhang C, Xu Z, Wang S, Li X, Stringer-Reasor E, et al. Dual CRISPR interference and activation for targeted reactivation of X-linked endogenous FOXP3 in human breast cancer cells. Mol Cancer. 2022;21(1):38. doi: 10.1186/s12943-021-01472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adamson B, Norman TM, Jost M, Cho MY, Nuñez JK, Chen Y, et al. A multiplexed single-cell CRISPR screening platform enables systematic dissection of the unfolded protein response. Cell. 2016;167(7):1867–1882.e21. doi: 10.1016/j.cell.2016.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian R, Gachechiladze MA, Ludwig CH, Laurie MT, Hong JY, Nathaniel D, et al. CRISPR interference-based platform for multimodal genetic screens in human iPSC-derived neurons. Neuron. 2019;104(2):239–255.e12. doi: 10.1016/j.neuron.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Semesta KM, Tian R, Kampmann M, von Zastrow M, Tsvetanova NG. A high-throughput CRISPR interference screen for dissecting functional regulators of GPCR/cAMP signaling. PLoS Genet. 2020;16(10):e1009103. doi: 10.1371/journal.pgen.1009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Myacheva K, Walsh A, Riester M, Pelos G, Carl J, Diederichs S. CRISPRi screening identifies CASP8AP2 as an essential viability factor in lung cancer controlling tumor cell death via the AP-1 pathway. Cancer Lett. 2023;552:215958. doi: 10.1016/j.canlet.2022.215958. [DOI] [PubMed] [Google Scholar]
- 55.Benbarche S, Lopez CK, Salataj E, Aid Z, Thirant C, Laiguillon MC, et al. Screening of ETO2-GLIS2-induced Super Enhancers identifies targetable cooperative dependencies in acute megakaryoblastic leukemia. Sci Adv. 2022;8(6):eabg9455. doi: 10.1126/sciadv.abg9455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 57.Zhao J, Lin Q, Song Y, Liu D. Universal CARs, universal T cells, and universal CAR T cells. J Hematol Oncol. 2018;11(1):132. doi: 10.1186/s13045-018-0677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alanis-Lobato G, Zohren J, McCarthy A, Fogarty NME, Kubikova N, Hardman E, et al. Frequent loss of heterozygosity in CRISPR-Cas9-edited early human embryos. Proc Natl Acad Sci U S A. 2021;118(22):e2004832117. doi: 10.1073/pnas.2004832117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu Y, Xue J, Deng T, Zhou X, Yu K, Deng L, et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat Med. 2020;26(5):732–740. doi: 10.1038/s41591-020-0840-5. [DOI] [PubMed] [Google Scholar]
- 60.Stadtmauer EA, Fraietta JA, Davis MM, Cohen AD, Weber KL, Lancaster E, et al. CRISPR-engineered T cells in patients with refractory cancer. Science. 2020;367(6481):eaba7365. doi: 10.1126/science.aba7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang J, Hu Y, Yang J, Li W, Zhang M, Wang Q, et al. Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL. Nature. 2022;609(7926):369–374. doi: 10.1038/s41586-022-05140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu Y, Zhou Y, Zhang M, Ge W, Li Y, Yang L, et al. CRISPR/Cas9-engineered universal CD19/CD22 dual-targeted CAR-T cell therapy for relapsed/refractory B-cell acute lymphoblastic leukemia. Clin Cancer Res. 2021;27(10):2764–2772. doi: 10.1158/1078-0432.CCR-20-3863. [DOI] [PubMed] [Google Scholar]
- 63.Foy SP, Jacoby K, Bota DA, Hunter T, Pan Z, Stawiski E, et al. Non-viral precision T cell receptor replacement for personalized cell therapy. Nature. 2023;615(7953):687–696. doi: 10.1038/s41586-022-05531-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boutin J, Rosier J, Cappellen D, Prat F, Toutain J, Pennamen P, et al. CRISPR-Cas9 globin editing can induce megabase-scale copy-neutral losses of heterozygosity in hematopoietic cells. Nat Commun. 2021;12(1):4922. doi: 10.1038/s41467-021-25190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu M, Zhang W, Xin C, Yin J, Shang Y, Ai C, et al. Global detection of DNA repair outcomes induced by CRISPR-Cas9. Nucleic Acids Res. 2021;49(15):8732–8742. doi: 10.1093/nar/gkab686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tuladhar R, Yeu Y, Tyler Piazza J, Tan Z, Rene Clemenceau J, Wu X, et al. CRISPR-Cas9-based mutagenesis frequently provokes on-target mRNA misregulation. Nat Commun. 2019;10(1):4056. doi: 10.1038/s41467-019-12028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kantor A, McClements ME, MacLaren RE. CRISPR-Cas9 DNA base-editing and prime-editing. Int J Mol Sci. 2020;21(17):6240. doi: 10.3390/ijms21176240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533(7603):420–424. doi: 10.1038/nature17946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, et al. Programmable base editing of A⋅T to G⋅C in genomic DNA without DNA cleavage. Nature. 2017;551(7681):464–471. doi: 10.1038/nature24644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, Levy JM, et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576(7785):149–157. doi: 10.1038/s41586-019-1711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kurt IC, Zhou R, Iyer S, Garcia SP, Miller BR, Langner LM, et al. CRISPR C-to-G base editors for inducing targeted DNA transversions in human cells. Nat Biotechnol. 2021;39(1):41–46. doi: 10.1038/s41587-020-0609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grünewald J, Zhou R, Lareau CA, Garcia SP, Iyer S, Miller BR, et al. A dual-deaminase CRISPR base editor enables concurrent adenine and cytosine editing. Nat Biotechnol. 2020;38(7):861–864. doi: 10.1038/s41587-020-0535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sakata RC, Ishiguro S, Mori H, Tanaka M, Tatsuno K, Ueda H, et al. Base editors for simultaneous introduction of C-to-T and A-to-G mutations. Nat Biotechnol. 2020;38(7):865–869. doi: 10.1038/s41587-020-0509-0. [DOI] [PubMed] [Google Scholar]
- 74.Zhang X, Zhu B, Chen L, Xie L, Yu W, Wang Y, et al. Dual base editor catalyzes both cytosine and adenine base conversions in human cells. Nat Biotechnol. 2020;38(7):856–860. doi: 10.1038/s41587-020-0527-y. [DOI] [PubMed] [Google Scholar]
- 75.Wang L, Xue W, Zhang H, Gao R, Qiu H, Wei J, et al. Eliminating base-editor-induced genome-wide and transcriptome-wide off-target mutations. Nat Cell Biol. 2021;23(5):552–563. doi: 10.1038/s41556-021-00671-4. [DOI] [PubMed] [Google Scholar]
- 76.Anzalone AV, Koblan LW, Liu DR. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat Biotechnol. 2020;38(7):824–844. doi: 10.1038/s41587-020-0561-9. [DOI] [PubMed] [Google Scholar]
- 77.Hanna RE, Hegde M, Fagre CR, DeWeirdt PC, Sangree AK, Szegletes Z, et al. Massively parallel assessment of human variants with base editor screens. Cell. 2021;184(4):1064–1080.e20. doi: 10.1016/j.cell.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 78.Cuella-Martin R, Hayward SB, Fan X, Chen X, Huang JW, Taglialatela A, et al. Functional interrogation of DNA damage response variants with base editing screens. Cell. 2021;184(4):1081–1097.e19. doi: 10.1016/j.cell.2021.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Coelho MA, Cooper S, Strauss ME, Karakoc E, Bhosle S, Gonçalves E, et al. Base editing screens map mutations affecting interferon-γ signaling in cancer. Cancer Cell. 2023;41(2):288–303.e6. doi: 10.1016/j.ccell.2022.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li J, Lin J, Huang S, Li M, Yu W, Zhao Y, et al. Functional phosphoproteomics in cancer chemoresistance using CRISPR-mediated base editors. Adv Sci (Weinh) 2022;9(30):e2200717. doi: 10.1002/advs.202200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Annunziato S, Lutz C, Henneman L, Bhin J, Wong K, Siteur B, et al. In situ CRISPR-Cas9 base editing for the development of genetically engineered mouse models of breast cancer. Embo j. 2020;39(5):e102169. doi: 10.15252/embj.2019102169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sayed S, Sidorova OA, Hennig A, Augsburg M, Cortés Vesga CP, Abohawya M, et al. Efficient correction of oncogenic KRAS and TP53 mutations through CRISPR base editing. Cancer Res. 2022;82(17):3002–3015. doi: 10.1158/0008-5472.CAN-21-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Webber BR, Lonetree CL, Kluesner MG, Johnson MJ, Pomeroy EJ, Diers MD, et al. Highly efficient multiplex human T cell engineering without double-strand breaks using Cas9 base editors. Nat Commun. 2019;10(1):5222. doi: 10.1038/s41467-019-13007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Diorio C, Murray R, Naniong M, Barrera L, Camblin A, Chukinas J, et al. Cytosine base editing enables quadruple-edited allogeneic CART cells for T-ALL. Blood. 2022;140(6):619–629. doi: 10.1182/blood.2022015825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen PJ, Hussmann JA, Yan J, Knipping F, Ravisankar P, Chen PF, et al. Enhanced prime editing systems by manipulating cellular determinants of editing outcomes. Cell. 2021;184(22):5635–5652.e29. doi: 10.1016/j.cell.2021.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao Z, Shang P, Mohanraju P, and Geijsen N. Prime editing: advances and therapeutic applications. Trends Biotechnol. 2023. [DOI] [PubMed]
- 87.Chen PJ, Liu DR. Prime editing for precise and highly versatile genome manipulation. Nat Rev Genet. 2023;24(3):161–177. doi: 10.1038/s41576-022-00541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Habib O, Habib G, Hwang GH, Bae S. Comprehensive analysis of prime editing outcomes in human embryonic stem cells. Nucleic Acids Res. 2022;50(2):1187–1197. doi: 10.1093/nar/gkab1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ely Z A, Mathey-Andrews N, Naranjo S, Gould S I, Mercer K L, Newby G A, et al. A prime editor mouse to model a broad spectrum of somatic mutations in vivo. Nat Biotechnol. 2023. [DOI] [PMC free article] [PubMed]
- 90.Liu P, Liang SQ, Zheng C, Mintzer E, Zhao YG, Ponnienselvan K, et al. Improved prime editors enable pathogenic allele correction and cancer modelling in adult mice. Nat Commun. 2021;12(1):2121. doi: 10.1038/s41467-021-22295-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim D, Kim J, Hur JK, Been KW, Yoon SH, Kim JS. Genome-wide analysis reveals specificities of Cpf1 endonucleases in human cells. Nat Biotechnol. 2016;34(8):863–868. doi: 10.1038/nbt.3609. [DOI] [PubMed] [Google Scholar]
- 92.Kleinstiver BP, Tsai SQ, Prew MS, Nguyen NT, Welch MM, Lopez JM, et al. Genome-wide specificities of CRISPR-Cas Cpf1 nucleases in human cells. Nat Biotechnol. 2016;34(8):869–874. doi: 10.1038/nbt.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abudayyeh OO, Gootenberg JS, Essletzbichler P, Han S, Joung J, Belanto JJ, et al. RNA targeting with CRISPR-Cas13. Nature. 2017;550(7675):280–284. doi: 10.1038/nature24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yan WX, Hunnewell P, Alfonse LE, Carte JM, Keston-Smith E, Sothiselvam S, et al. Functionally diverse type V CRISPR-Cas systems. Science. 2019;363(6422):88–91. doi: 10.1126/science.aav7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abudayyeh OO, Gootenberg JS, Konermann S, Joung J, Slaymaker IM, Cox DB, et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science. 2016;353(6299):aaf5573. doi: 10.1126/science.aaf5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471(7340):602–607. doi: 10.1038/nature09886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163(3):759–771. doi: 10.1016/j.cell.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stella S, Alcón P, Montoya G. Structure of the Cpf1 endonuclease R-loop complex after target DNA cleavage. Nature. 2017;546(7659):559–563. doi: 10.1038/nature22398. [DOI] [PubMed] [Google Scholar]
- 99.Paul B, Montoya G. CRISPR-Cas12a: functional overview and applications. Biomed J. 2020;43(1):8–17. doi: 10.1016/j.bj.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yamano T, Nishimasu H, Zetsche B, Hirano H, Slaymaker IM, Li Y, et al. Crystal structure of Cpf1 in complex with guide RNA and target DNA. Cell. 2016;165(4):949–962. doi: 10.1016/j.cell.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Karvelis T, Gasiunas G, Young J, Bigelyte G, Silanskas A, Cigan M, et al. Rapid characterization of CRISPR-Cas9 protospacer adjacent motif sequence elements. Genome Biol. 2015;16:253. doi: 10.1186/s13059-015-0818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fonfara I, Le Rhun A, Chylinski K, Makarova KS, Lécrivain AL, Bzdrenga J, et al. Phylogeny of Cas9 determines functional exchangeability of dual-RNA and Cas9 among orthologous type II CRISPR-Cas systems. Nucleic Acids Res. 2014;42(4):2577–2590. doi: 10.1093/nar/gkt1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yamano T, Zetsche B, Ishitani R, Zhang F, Nishimasu H, Nureki O. Structural basis for the canonical and non-canonical PAM recognition by CRISPR-Cpf1. Mol Cell. 2017;67(4):633–645.e3. doi: 10.1016/j.molcel.2017.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim HK, Song M, Lee J, Menon AV, Jung S, Kang YM, et al. In vivo high-throughput profiling of CRISPR-Cpf1 activity. Nat Methods. 2017;14(2):153–159. doi: 10.1038/nmeth.4104. [DOI] [PubMed] [Google Scholar]
- 105.Nishimasu H, Yamano T, Gao L, Zhang F, Ishitani R, Nureki O. Structural basis for the altered PAM recognition by engineered CRISPR-Cpf1. Mol Cell. 2017;67(1):139–147.e2. doi: 10.1016/j.molcel.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim SK, Kim H, Ahn WC, Park KH, Woo EJ, Lee DH, et al. Efficient transcriptional gene repression by Type V-A CRISPR-Cpf1 from Eubacterium eligens. ACS Synth Biol. 2017;6(7):1273–1282. doi: 10.1021/acssynbio.6b00368. [DOI] [PubMed] [Google Scholar]
- 107.Campa CC, Weisbach NR, Santinha AJ, Incarnato D, Platt RJ. Multiplexed genome engineering by Cas12a and CRISPR arrays encoded on single transcripts. Nat Methods. 2019;16(9):887–893. doi: 10.1038/s41592-019-0508-6. [DOI] [PubMed] [Google Scholar]
- 108.Lee JG, Ha CH, Yoon B, Cheong SA, Kim G, Lee DJ, et al. Knockout rat models mimicking human atherosclerosis created by Cpf1-mediated gene targeting. Sci Rep. 2019;9(1):2628. doi: 10.1038/s41598-019-38732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang H, Huang G, Tan Z, Hu Y, Shan L, Zhou J, et al. Engineered Cas12a-Plus nuclease enables gene editing with enhanced activity and specificity. BMC Biol. 2022;20(1):91. doi: 10.1186/s12915-022-01296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Choi E, Hwang HY, Kwon E, Kim D, Koo T. Expanded targeting scope of LbCas12a variants allows editing of multiple oncogenic mutations. Mol Ther Nucleic Acids. 2022;30:131–142. doi: 10.1016/j.omtn.2022.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim S, Ji S, Koh HR. CRISPR as a diagnostic tool. Biomolecules. 2021;11(8):1162. doi: 10.3390/biom11081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Teng F, Guo L, Cui T, Wang XG, Xu K, Gao Q, et al. CDetection: CRISPR-Cas12b-based DNA detection with sub-attomolar sensitivity and single-base specificity. Genome Biol. 2019;20(1):132. doi: 10.1186/s13059-019-1742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu R, He Y, Lan T, Zhang J. Installing CRISPR-Cas12a sensors in a portable glucose meter for point-of-care detection of analytes. Analyst. 2021;146(10):3114–3120. doi: 10.1039/d1an00008j. [DOI] [PubMed] [Google Scholar]
- 114.Tsou JH, Leng Q, Jiang F. A CRISPR test for rapidly and sensitively detecting circulating EGFR mutations. Diagnostics (Basel). 2020;10(2). [DOI] [PMC free article] [PubMed]
- 115.Kordyś M, Sen R, Warkocki Z. Applications of the versatile CRISPR-Cas13 RNA targeting system. Wiley Interdiscip Rev RNA. 2022;13(3):e1694. doi: 10.1002/wrna.1694. [DOI] [PubMed] [Google Scholar]
- 116.Liu L, Pei D S. Insights gained from RNA editing targeted by the CRISPR-Cas13 family. Int J Mol Sci. 2022;23(19). [DOI] [PMC free article] [PubMed]
- 117.Khan MZ, Amin I, Hameed A, Mansoor S. CRISPR-Cas13a: prospects for plant virus resistance. Trends Biotechnol. 2018;36(12):1207–1210. doi: 10.1016/j.tibtech.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 118.Konermann S, Lotfy P, Brideau NJ, Oki J, Shokhirev MN, Hsu PD. Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell. 2018;173(3):665–676.e14. doi: 10.1016/j.cell.2018.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cox DBT, Gootenberg JS, Abudayyeh OO, Franklin B, Kellner MJ, Joung J, et al. RNA editing with CRISPR-Cas13. Science. 2017;358(6366):1019–1027. doi: 10.1126/science.aaq0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang F, Wang L, Zou X, Duan S, Li Z, Deng Z, et al. Advances in CRISPR-Cas systems for RNA targeting, tracking and editing. Biotechnol Adv. 2019;37(5):708–729. doi: 10.1016/j.biotechadv.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 121.Singh A, Bhatia P. Effective downregulation of BCR-ABL tumorigenicity by RNA targeted CRISPR-Cas13a. Curr Gene Ther. 2021;21(3):270–277. doi: 10.2174/1566523221666210217155233. [DOI] [PubMed] [Google Scholar]
- 122.Saifullah, Sakari M, Suzuki T, Yano S, Tsukahara T. Effective RNA knockdown using CRISPR-Cas13a and molecular targeting of the EML4-ALK transcript in H3122 lung cancer cells. Int J Mol Sci. 2020;21(23). [DOI] [PMC free article] [PubMed]
- 123.Tong H, Huang J, Xiao Q, He B, Dong X, Liu Y, et al. High-fidelity Cas13 variants for targeted RNA degradation with minimal collateral effects. Nat Biotechnol. 2023;41(1):108–119. doi: 10.1038/s41587-022-01419-7. [DOI] [PubMed] [Google Scholar]
- 124.Granados-Riveron JT, Aquino-Jarquin G. CRISPR-Cas13 precision transcriptome engineering in cancer. Cancer Res. 2018;78(15):4107–4113. doi: 10.1158/0008-5472.CAN-18-0785. [DOI] [PubMed] [Google Scholar]
- 125.Oude Blenke E, Evers MJ, Mastrobattista E, van der Oost J. CRISPR-Cas9 gene editing: delivery aspects and therapeutic potential. J Control Release. 2016;244(Pt B):139–148. doi: 10.1016/j.jconrel.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 126.Liu L, Li X, Ma J, Li Z, You L, Wang J, et al. The molecular architecture for RNA-guided RNA cleavage by Cas13a. Cell. 2017;170(4):714–726.e10. doi: 10.1016/j.cell.2017.06.050. [DOI] [PubMed] [Google Scholar]
- 127.Puig-Serra P, Casado-Rosas MC, Martinez-Lage M, Olalla-Sastre B, Alonso-Yanez A, Torres-Ruiz R, et al. CRISPR approaches for the diagnosis of human diseases. Int J Mol Sci. 2022;23(3). [DOI] [PMC free article] [PubMed]
- 128.Sheng Y, Zhang T, Zhang S, Johnston M, Zheng X, Shan Y, et al. A CRISPR/Cas13a-powered catalytic electrochemical biosensor for successive and highly sensitive RNA diagnostics. Biosens Bioelectron. 2021;178:113027. doi: 10.1016/j.bios.2021.113027. [DOI] [PubMed] [Google Scholar]
- 129.Bruch R, Baaske J, Chatelle C, Meirich M, Madlener S, Weber W, et al. CRISPR/Cas13a-powered electrochemical microfluidic biosensor for nucleic acid amplification-free miRNA diagnostics. Adv Mater. 2019;31(51):e1905311. doi: 10.1002/adma.201905311. [DOI] [PubMed] [Google Scholar]
- 130.Tian T, Shu B, Jiang Y, Ye M, Liu L, Guo Z, et al. An ultralocalized Cas13a assay enables universal and nucleic acid amplification-free single-molecule RNA diagnostics. ACS Nano. 2021;15(1):1167–1178. doi: 10.1021/acsnano.0c08165. [DOI] [PubMed] [Google Scholar]
- 131.Shan Y, Zhou X, Huang R, Xing D. High-fidelity and rapid quantification of miRNA combining crRNA programmability and CRISPR/Cas13a trans-cleavage activity. Anal Chem. 2019;91(8):5278–5285. doi: 10.1021/acs.analchem.9b00073. [DOI] [PubMed] [Google Scholar]
- 132.He YWY, Wang Y, Wang X, Xing S, Li H. Applying CRISPR/Cas13 to construct exosomal PD-L1 ultrasensitive biosensors for dynamic monitoring of tumor progression in immunotherapy. Adv Biomed. 2021.
- 133.Chen Q, Tian T, Xiong E, Wang P, Zhou X. CRISPR/Cas13a signal amplification linked immunosorbent assay for femtomolar protein detection. Anal Chem. 2020;92(1):573–577. doi: 10.1021/acs.analchem.9b04403. [DOI] [PubMed] [Google Scholar]
- 134.Barnes KG, Lachenauer AE, Nitido A, Siddiqui S, Gross R, Beitzel B, et al. Deployable CRISPR-Cas13a diagnostic tools to detect and report Ebola and Lassa virus cases in real-time. Nat Commun. 2020;11(1):4131. doi: 10.1038/s41467-020-17994-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kellner MJ, Koob JG, Gootenberg JS, Abudayyeh OO, Zhang F. SHERLOCK: nucleic acid detection with CRISPR nucleases. Nat Protoc. 2019;14(10):2986–3012. doi: 10.1038/s41596-019-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gootenberg JS, Abudayyeh OO, Kellner MJ, Joung J, Collins JJ, Zhang F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science. 2018;360(6387):439–444. doi: 10.1126/science.aaq0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ackerman CM, Myhrvold C, Thakku SG, Freije CA, Metsky HC, Yang DK, et al. Massively multiplexed nucleic acid detection with Cas13. Nature. 2020;582(7811):277–282. doi: 10.1038/s41586-020-2279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Roth JA, Cristiano RJ. Gene therapy for cancer: what have we done and where are we going? J Natl Cancer Inst. 1997;89(1):21–39. doi: 10.1093/jnci/89.1.21. [DOI] [PubMed] [Google Scholar]
- 139.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.