Abstract
The randomized controlled trial (RCT) research design assumes that a drug’s “specific” effect can be isolated, added, and subtracted from the “nonspecific” effect of context and person. While RCTs are helpful in assessing the added benefit of a novel drug, they tend to obscure the curative potential of extra-pharmacological variables, known as “the placebo effect.” Ample empirical evidence suggests that person/context-dependent physical, social, and cultural variables not only add to, but also shape drug effects, making them worth harnessing for patient benefits. Nevertheless, utilizing placebo effects in medicine is challenging due to conceptual and normative obstacles. In this article, we propose a new framework inspired by the field of psychedelic science and its employment of the “set and setting” concept. This framework acknowledges that drug and nondrug factors have an interactive and synergistic relationship. From it, we suggest ways to reintegrate nondrug variables into the biomedical toolbox, to ethically harness the placebo effect for improved clinical care.
Keywords: Placebo, placebo effect, set and setting, psychedelics, additive model, interactive model
Introduction
Despite a tumultuous history, the placebo effect has been recognized as a legitimate biopsychosocial phenomenon that is an integral part of the overall treatment response (Roth, 2003; Zion and Crum, 2018). For instance, research finds that placebo effects can be of a comparable magnitude to treatment-specific effects in randomized controlled trials (RCTs) (Howick et al., 2013). For these reasons, there have been several compelling calls to harness the placebo effect for patient benefits, as part of routine clinical care (Bishop et al., 2017; Chavarria et al., 2017; Colloca and Miller, 2011; Evers et al., 2018; Petrie and Rief, 2019; Zion and Crum, 2018). Unfortunately, the translation of these ideas into practice has been largely unfruitful. The impasse may have to do with (a) the placebo effect’s contradictory theoretical background (Benedetti, 2014; Bishop et al., 2014) and (b) a lack of roadmaps to guide its future use.
This article aims to palliate these shortcomings in two ways. First, we will explore the conceptual evolution of the “placebo effect” in the clinic and research, to shed light on its paradoxical nature and to debunk assumptions that have hindered its ethical use. Second, we will turn to related literature—that of “set and setting” in psychedelic research—to gain inspiration from a field that has long been making use of placebo ingredients for therapeutic outcomes. Based on the psychedelic literature, we will provide concrete recommendations for the reintegration of placebo effects into the biomedical toolbox.
Historical background
Placebos in the clinic
The first record of the term “placebo” in a medical context dates to 1772, when Scottish doctor William Cullen reported prescribing a remedy to a patient despite believing it to be inefficacious. He explains that sometimes “it is necessary to give a medicine and [this is] what I call a placebo” (Cullen, 1772). The term was readily adopted into medical jargon, and the use of placebos remained widespread and largely unquestioned until the mid-20th century. The role of placebos was to provide hope to patients and make doctors’ work easier in difficult or desperate cases (Annoni, 2020). Richard Cabot, a physician at Harvard Medical School, describes how “he was brought up, as I suppose every physician is, to use placebos, bread pills, water subcutaneously, and other devices” (Cabot, 1903). In short, placebos were considered as inert substances with “fake” (psychological) but benign (“cannot harm but may relieve”) effects (Pepper, 1945). It is only in the 1950s, with the rise of informed consent and autonomy as pillars of medical ethics, that the practice of deceiving patients “for their own good” was put into question (Annoni, 2020).
Today, the deceptive use of placebos in the clinic is stigmatized as a “relic of a bygone age of medical paternalism” (Annoni and Miller, 2016). Nonetheless, a recent meta-analysis of 16 studies from 13 countries revealed that 46%–95% of general practicioners (GPs) had prescribed “pure” and “impure” placebos within the last year (Linde et al., 2018). The former are completely inert substances, the latter are drugs that are not indicated for the problem in question. Some quoted motives are: to increase therapeutic effectiveness, to treat nonspecific symptoms, and to meet the patient’s request for treatment (Fässler et al., 2009). This begs the question: what counts as “treatment?” In certain scenarios, GPs may be prescribing placebos instead of recommending psychological support, watchful waiting, or simple behavioral changes, because they are aware of the expectation that a medical encounter ends with a prescription. Indeed, by current biomedical standards, receiving a prescription for a pill or a cream may feel more “real” to patients than being told to go for a walk every evening or to wait and see if their rash goes away in a few days. While this “treatment” may meet patient expectations, it does so through the use of deception. A qualitative study finds that GPs are grappling with ethical concerns about placebo interventions and request guidance on acceptable practice around placebo use (Bishop et al., 2014).
Placebos in research
The history of placebo took a turn in the 20th century. Placebos began being used in research as epistemic tools to demonstrate the added benefit of specific interventions, and to discard ineffective or harmful treatments (Kaptchuk and Miller, 2015). Placebos and the “placebo effect” gained legitimacy in this context. In a study investigating the treatment of angina pectoris with xanthines, Gold et al. (1937) used placebos as a means to control for potential confounds, such as “spontaneous variations in the course of the pain,” “change of diet,” “confidence aroused in the treatment,” “encouragement afforded by any new procedure,” “change in medical advisor,” and the value of “reassurance alone.” Controlling for these sources of error, the authors found no difference in the pain relief afforded by the drug compared to the placebo (Gold et al., 1937). Placebos thus helped refute the popular but unfounded idea that xanthines have any “specific usefulness” in the treatment of cardiac pain.
By 1946, the comparative experimental framework had gained momentum. During Conferences on Therapy at Cornell University, participants explicitly advocated for the use of placebos in clinical trials, to permit the comparison between “an allegedly potent agent and a blank of such [agent]” (Gold et al., 1954). The purpose of placebo was now to isolate the true “drug effect” from “the rest”, to determine whether the drug provided added benefit or not.
The placebo effect
While the “drug effect” was of main interest, the “placebo effect” was acknowledged in its own right when Beecher published his seminal article entitled The Powerful Placebo (Beecher, 1955). Beecher conducted a proto-systematic review of 15 placebo-controlled trials that covered a wide variety of ailments (wound pain, cardiac pain, headache, nausea, the common cold, etc.) and found that, on average, a third of participants in placebo groups were experiencing relief. In addition, the effects could involve “gross physical change,” meaning that they could “mimic drug action” (Beecher, 1955). Beecher advanced that the placebo effect’s ubiquity and ability to mimic drug action might obscure “true” drug effects and threaten to confound scientific research (Annoni, 2018). He concluded that placebo effects (a) are worthy of scientific investigation and (b) must be controlled for through appropriate experimental design.
Beecher’s ideas became widely popular and influential. They formed the basis of how placebos and “placebo effects” came to be understood in biomedical research (Annoni, 2018), offering a stark contrast to how they were conceptualized in the clinic (see Table 1). Indeed, whereas the placebo effect was discredited in the clinic for being purely psychological, it gained legitimacy in research when it was discovered to be physiologically grounded too. Although in the clinic, placebos served the pragmatic function of appeasing patients; in research they held the epistemological function of differentiating between useful and non-useful treatments. Finally, although the rise of medical ethics discouraged the clinical use of placebos, the concurrent push for evidence-based medicine turned placebos into a pillar of modern clinical research. Indeed, by 1962, the RCT design comparing effects in treatment and placebo groups became the gold standard by which pharmaceutical manufacturers could demonstrate efficacy and safety to regulators (Muthukumaraswamy et al., 2021). However influential, it should be noted that some of Beecher’s arguments were based on the unproven but highly consequential theoretical assumptions, which are worth rectifying.
Table 1.
Historical conceptualization of “placebo” and “placebo effects” in the clinic and research.
| Clinic | Research |
|---|---|
| The placebo has “fake” effects a | The placebo has “real” effects |
| - They are psychological (product of the patient’s imagination) | - Some effects are even physical and can mimic the action of a drug |
| The placebo effect is “benign” | The placebo effect is “dangerous” |
| - It is “better than nothing” and is unlikely to harm | - It threatens to obscure the effects of legitimate biochemical compounds |
| The placebo pill’s purpose is practical | The placebo pill’s purpose is epistemological |
| - To please the patient and make the doctor’s life easier | - To control for this precisely real and powerful effect |
| By the 1950s, the use of placebo was unethical | By 1950s, the use of placebo was ethical |
| - The deceptive use of placebos violates patient autonomy, shared decision-making, and informed consent | - The use of placebos for blinding is justified and necessary in research |
The terms “fake” and “real” here are used as a hyperbole to situate historical thinking about the mind versus the body. Nowadays, it is understood that psychological and biological forces are two sides of the same coin, the former being just as “real” as the latter.
Assumption of causality
The observation of a “placebo effect” following the administration of a “placebo” in research or practice is effectively a correlation, but it makes a causal assumption intuitive. Indeed, Beecher was not the first nor the last to assume that the placebo agent causes the placebo effect (Wolff and Dubois, 1946). Beecher wrote that the “powerful placebo” itself “[. . .] has an average effectiveness of 35.2 ± 2.2%” (Beecher, 1955). Still today, placebo effects are often understood as “those accruing from taking dummy pills or inactive treatments” (e.g., McQueen and Smith, 2012). This notion underlies the false paradox or “mystery” that placebos are “fake” substances with “real” effects (Frenkel, 2008; Moerman, 2002). Referring back to Gold’s rationale for the use of placebos in research helps identify the mistake in logic. The placebo does not cause the placebo effect; it controls for it. Indeed, by acting as the blank—or an inactive placeholder—of the drug, the placebo controls for the “nonspecific effect” of everything that is not the drug (i.e., “the rest”). What we can safely conclude is that the sum of ingredients that underlie the placebo effect can be found in “the rest.”
Assumption of additivity
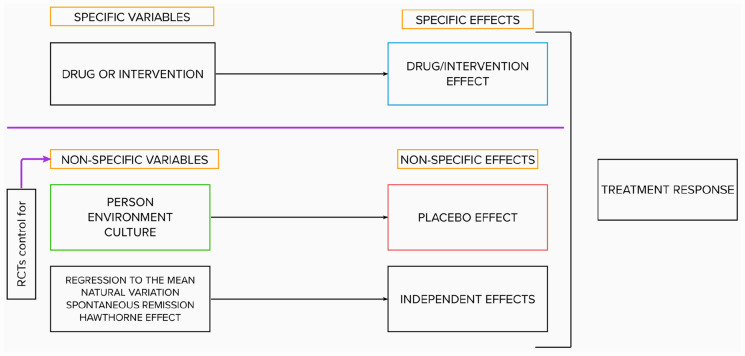
The additive model argues that specific and nonspecific effects can be separated, added, and subtracted. Suggested by Gold and refined by Beecher, it consists today of three elements, with varying nomenclature: specific effects, placebo effects, and independent effects (Finniss, 2018). These three effects can be measured in research trials with three arms (experimental, placebo, and no treatment) (Aday et al., 2022). Independent effects, which include regression to the mean, natural variation in the course of treatment, Hawthorne effects, and so on, and placebo effects fall under the umbrella of nonspecific effects. The additive model rests on two assumptions: (a) that nonspecific effects are constant and of equal size in both the experimental and placebo arms of studies and (b) that specific and nonspecific variables do not interact (Kube and Rief, 2017). This permits the isolation of the specific efficacy of a medical intervention through simple arithmetic: by subtracting the improvements measured in the placebo group from those measured in the experimental group (Boehm et al., 2017).
The additive model’s simplicity is both its strength and its weakness. On the one hand, it has made the RCT the cornerstone of evidence-based medicine (Sackett et al., 1996)—as a tool that can challenge dubious treatments and prove new medical procedures’ efficacy (Crum et al., 2017). On the other hand, it is harmfully reductive: it severs mind from body and treatment from context (Barrett et al., 2006; Schleim, 2022). In the additive model, specific and nonspecific effects are conceptualized along the lines of Cartesian dualism. The former effects are considered “objective,” that is, measurable physiological events that arise in the patient’s body, while the latter are considered “subjective,” that is, intangible effects rooted in the patient’s mind. Furthermore, the model artificially dissociates the therapeutic agent from the therapeutic situation (Sullivan, 1993). It purports that a drug, or procedure, no matter what context it is given in, should have the same effect (Hartogsohn, 2017). Nevertheless, both in practice and research, medical treatments are never isolated from their context; rather, they are embedded within it (Crum et al., 2017; McQueen et al., 2013). That is to say: person, treatment, and context invariably interact.
Unsurprisingly, the additivity assumption was challenged as soon as it was formulated (Dinnerstein and Halm, 1970; Kast and Loesch, 1961; Lyerly et al., 1964; Modell and Garrett, 1960; Uhlenhuth et al., 1959)—and a recent review finds very little experimental evidence to support it (Boussageon et al., 2022). On the other hand, the balanced-placebo design (Rohsenow and Marlatt, 1981), which crosses intervention (drug vs placebo) with instructions (told drug vs told placebo), has provided robust evidence for drug × placebo interaction effects for a variety of model drugs, including caffeine (Hammami et al., 2010; Lotshaw et al., 1996), alcohol (George et al., 2012), marijuana (Metrik et al., 2012), nicotine (Kelemen and Kaighobadi, 2007), and hydroxyzine (Hammami et al., 2016). Hammami et al. (2016) actually compared the results they obtained when they modeled the effects of an innocuous drug (hydroxyzine) using a balanced-placebo design versus a conventional RCT design. With the balanced design, they found a clinically significant positive drug × placebo interaction effect. They also found that the RCT-estimated drug effect was larger than the balanced-placebo-estimated drug effect. In short, their work empirically demonstrates that treatment effects cannot be reduced to drug + placebo effects; these two elements can yield a whole that is greater than the sum of its parts. Today, experts agree that placebo effects can substantially modulate the efficacy and tolerability of active pharmacological or other medical treatments (Evers et al., 2018), and interactive models have been proposed to better account for the sum of these findings (Kube and Rief, 2017; Muthén and Brown, 2009). Despite this all, the additive (RCT) model—which spotlights the drug and relegates “the rest” to the shadows (Sullivan, 1993)—remains at the core of today’s biomedical paradigm, as depicted in Figure 1. The status quo favors a knowledge of compartimentalized “things” over an understanding of “relationships.” We may need a new source of inspiration to get out of this rut.
Figure 1.
The additive biomedical model.
Contemporary placebo theory meets “set and setting”
The historical evolution of the “placebo effect” explains why it remains such a nebulous concept. How can one phenomenon be at once “fake” and benign (in the clinic), but also “real” and threatening (in research)? And, if we agree on the power of placebo, how can the ends of eliciting therapeutic benefits justify the means of deceiving patients? The ethical dilemma is illusory: the placebo effect is not caused by a “dummy pill,” but rather by everything that surrounds the administration of the said pill. That is, the power of the placebo does not reside in the sham treatment itself but in the psychosocial forces that shape the treatment context (Zion and Crum, 2018). Thus, eliciting placebo effects does not require patient deception; rather it requires the careful leveraging of the psychological and environmental forces that shape the therapeutic encounter. The question that remains is: how can this be done?
Based on the extensive placebo literature, Zion and Crum (2018) propose a framework that accounts for the placebo effect’s biopsychosocial scaffolding. They suggest that the placebo effect is driven by disease-specific neurobiological mechanisms (e.g., the endogenous opioid system), which are evoked and modulated by psychological processes (implicit learning, expectations, and mindsets), which in turn, are shaped by environmental factors (patient–provider relationship, treatment characteristics, culture and development). Interestingly, the concept of “set and setting,” which emerged and gained centrality within the field of psychedelic science, designates “nonspecific” psychological and environmental variables that are very similar to those cited in the placebo literature (Gukasyan and Nayak, 2022; Hartogsohn, 2016). “Set” refers to the psychological state and mindset of a person (expectations, mood, and intentions) taking a psychedelic drug, and “setting” refers to the physical, social, and cultural environment in which a psychedelic experience takes place (Alpert et al., 1964). Drawing parallels between placebo and psychedelic research (see Table 2), we may understand “set and setting” as the ingredients that underlie placebo effects, insofar as they are extra-pharmaceutical variables that surround drug administration and that hold healing potential. Among these variables, expectancy is discussed extensively in both the placebo and psychedelic literature (e.g., Aday et al., 2022; Colloca and Barsky, 2020). It is defined as the specific cognitions about the likelihood of future events, pertaining to the course of illness, the response to treatment, the likelihood of side effects, or the ability to influence these outcomes (Petrie and Rief, 2019). In the biomedical and psychological literature, expectancy has been found to moderate the strength of the placebo effect (Bjorkedal and Flaten, 2011; Howe et al., 2017) and to be a determinant of treatment outcomes (Constantino et al., 2018), including of the effects of active pharmaceutical agents (Flaten et al., 1999; Rutherford and Roose, 2013). In the psychedelic literature, a proof of concept study demonstrated that an intensive manipulation of expectancy via “set and setting” was sufficient to produce a “psychedelic-like” placebo effect (Olson et al., 2020). Likewise, participants in ayahuasca ceremonies who received placebos reported psychedelic effects and improvements in mental health, suggesting that these were driven by non-pharmacological “set and setting” factors (Uthaug et al., 2021). Given that “set and setting” theory is at the vanguard of putting contemporary placebo theory into practice, this burgeoning field may serve as a way out of the placebo’s pejorative past, and as a way forward for the ethical reintegration of extra-pharmaceutical variables into the biomedical toolkit.
Table 2.
Parallels in the conceptualization of how biology, psychology, and environment interact in placebo and “set and setting” research.
| Placebo effect = Neurobiological effect modulated by | Psychedelic effect = Psychedelic drug effect modulated by |
|---|---|
| Psychological processes | Set = psychological state of a person |
| - Expectations - Mindsets - Implicit learning |
- Expectations - Mood - Intentions |
| Environmental factors | Setting |
| - Treatment characteristics - Patient–provider relationship - Development and culture |
- Physical environment - Social environment - Cultural environment |
Historical evolution of “set and setting”
While “set and setting” shape placebo effects (as per Olson et al., 2020), the psychedelic literature emphasizes how these extra-drug variables also shape drug effects. This reveals a break from the biomedical paradigm: instead of dichotomizing drug and extra-drug variables, psychedelic science revolves around their interaction. The distinct theoretical orientation may stem from psychedelics’ unique properties as “mind-manifesting” and “suggestibility-enhancing” agents (Hartogsohn, 2018). Psychedelic compounds are thought of as amplifiers of consciousness (Osmond, 1957) that make users particularly suggestible to sensory/emotional/cognitive cues (Carhart-Harris et al., 2015; Carhart-Harris et al., 2018). Thus, psychedelic effects are described as being “radically malleable” (Becker, 1967), and intensely “plastic and responsive” to internal and external conditions (Hartogsohn, 2017). The concept of “set and setting” has indeed been used to explain the extreme diversity of experiences elicited by psychedelics, from paranoïa and fear to mystical, creative, and therapeutic—depending on the context of use (Hartogsohn, 2020).
Modern set and setting research
Following a decades-long hiatus in psychedelic research, the turn of the millennium witnessed a resurgent interest in the clinical application of psychedelic substances, both in the scientific and popular press (Yaden et al., 2021). This was accompanied by new investigations into the principles of “set and setting” (Aday et al., 2021; Hartogsohn, 2017). Although the field remains relatively young, and empirical studies on the the optimization of set and setting variables do remain limited (Golden et al., 2022), findings have started to accumulate.
For instance, Griffiths et al. (2018) found interactive (and positive) effects of psilocybin dose and added support for spiritual practice on a wide range of longitudinal measures, including interpersonal closeness, life meaning, and death transcendence (Griffiths et al., 2018). Recent survey studies provide further granularity into what specific ingredients of “set and setting” contribute to therapeutic outcomes. Regarding set—positive mood, low anxiety, preparedness, and openness toward the upcoming experience have been found to predict positive experiences (Aday et al., 2021; Haijen et al., 2018). Conversely, negative mood, apprehension, confusion, and psychological distress leading up to the dosing session have been found to predict more acute adverse reactions (Aday et al., 2021; Studerus et al., 2012). Regarding setting—“antiseptic” environments (e.g., that of a positron emission tomography (PET) laboratory) have been found to predict anxious reactions (Studerus et al., 2012), whereas feeling comfortable in the environment (Haijen et al., 2018; Pontual et al., 2022), experiencing an intense sense of togetherness (“communitas”) (Kettner et al., 2021), and liking the music played during a session (Kaelen et al., 2018) predicted better session and subsequent improvements.
Finally, a thematic analysis of psychedelic use provides qualitative insight into predictors of psychedelic experiences (McCartney et al., 2022). The analysis revealed three “internal” predictors (set) and three “external” predictors (setting). The former are understanding (informed or uninformed), mindset (surrendered or resistant), and motivation (escapism or self-exploration), and the latter are nature, music, and environment (atmosphere and safety).
Discussion and recommendations
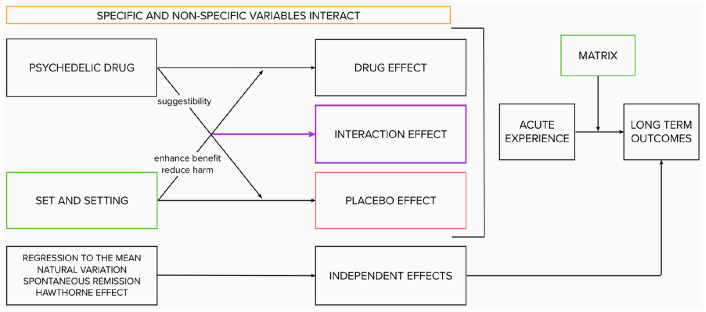
To summarize, the psychedelic paradigm offers an alternative to the traditional biomedical paradigm (Schenberg, 2018). Rather than dichotomizing drug and extra-drug variables, it considers them indissociable. Rather than characterizing the relationship between these variables as additive, it assumes it to be interactive and synergistic: The drug influences the effect of “set and setting,” and “set and setting” influences the drug effect (see Figure 2, inspired by Carhart-Harris et al., 2018). Carefully curating “set and setting” parameters maximize potential for benefit, whereas disregarding them increases potential for harm (Johnson et al., 2008). As such, extra-pharmaceutical variables are not marginal but central to psychedelic science; they are not “noise” but fundamental to the safe and ethical investigation and use of psychedelic compounds. In the final part of this article, we will draw inspiration from the psychedelic paradigm for ways to leverage extra-drug factors for patient benefits.
Figure 2.
The interactive psychedelic model.
Psychedelic science: Lessons for the clinic
Since the start of the 21st century, psychedelic-assisted psychotherapy has become the mainstay framework for the clinical investigation and use of psychedelics. Psychedelic-assisted psycho therapy is a brief intervention model, lasting for 1–3 months in total and including 1–3 moderate to high-dose administrations of psychedelics, with preparation and integration sessions pre- and post-administration (Garcia-Romeu and Richards, 2018). The basic idea is that drug dosing does not happen in a vacuum; patients are supported before, during, and after to ensure and sustain beneficial drug effects. Thus, the model broadens the notion of “drug efficacy” to the wider notion of “experience efficacy” (Roseman et al., 2017). Healthcare professionals may draw inspiration from psychedelic-assisted psychotherapy preparation, intervention, and integration protocols to enhance the “experience efficacy” of the treatments they provide, as indicated and summarized in Table 3.
Table 3.
A summary of extra-pharmaceutical tools that healthcare professionals may employ prior, during, and following pharmacological treatments to enhance “experience efficacy.”
| Step | Target | Tool |
|---|---|---|
| Preparation | Set | • Therapeutic alliance |
| ○ Obtain general life overview ○ Get to know patient as a person ○ Elicit patient questions, concerns, hopes and fears ○ Use engaged body language and active listening | ||
| • Patient empowerment | ||
| ○ Help patients set intentions/motivation ○ Establish patient-relevant treatment rationale ○ Provide information for patient to understand treatment and navigate its challenges | ||
| Intervention | Setting | • Sense of warmth and safety |
| ○ Leverage social context (human relations) ○ Leverage physical context (artifacts, decoration, clothing, spatial layout) | ||
| • Sense of ritual/ceremony | ||
| ○ Performance in medical context For example, open administration ○ Rituals outside of medical context ○ For example, “taking the pill” | ||
| Integration | Matrix | • Support with difficulties |
| ○ Schedule follow-up appointments ○ Monitor side effects ○ Elicit patient’s perspective on treatment progress | ||
| • Maintenance of beneficial outcomes | ||
| ○ Support lifestyle changes via the matrix |
Preparation
Psychedelic-assisted psychotherapy typically involves 2–4 dedicated preparation meetings prior to dosing days. Preparation serves to optimize “set,” such that individuals feel safe and comfortable with their therapist(s)/guides, and well prepared for what is to come (Garcia-Romeu and Richards, 2018). To this end, preparation involves building therapeutic alliance between the patient and clinician and fostering patient empowerment.
Therapeutic alliance, or patient-practitioner relationship, is a major predictor of patient outcomes across healthcare. In medicine, it has been found to improve subjective and objective outcomes in a variety of disease states (Howick et al., 2018; Kelley et al., 2014). In psychiatry, variance in depression scores has been found to be nearly 3x more influenced by psychiatrists (9.1%) than by medication regimens (3.4%) (McKay et al., 2006). In psychotherapy, a meta-analysis including 30,000 patients from 295 studies found that therapeutic alliance had an effect size of d = 0.579 on treatment outcomes (Flückiger et al., 2018). Finally, in psychedelics research, pre-dosing session therapeutic alliance was found to predict the intensity of the acute psychedelic experience, which in turn predicted depressive symptomatology 6 weeks post-dosing (Murphy et al., 2021).
Alliance is built very consciously in psychedelic-assisted psychotherapy. First, clinicians/facilitators obtain a general life review of the patient, including major life events, potential traumatic experiences, current and past relationships, and worldview (Garcia-Romeu and Richards, 2018). Second, they explore patients’ questions, concerns, and fears, validate them and provide reassurance. Time pressure, increasing numbers of patients per physician, and increasing reliance on technology may shorten interaction time and hinder the maturation of patient-physician relationships in medical settings (Abuqayyas et al., 2021). Nonetheless, clinicians can be intentional with their time. When taking patients’ history, medical professionals can mirror the psychedelic protocol by asking some personal questions, to engage the patient about their life and forge connections. Further, clinicians can use active listening and nonverbal communication, including eye contact and seating at patient level (which is encouraged in psychedelic-assisted psychotherapy), to convey understanding and empathy toward their patients’ concerns (Fassaert et al., 2007). Regarding patient worldview, spiritual beliefs have been found to impact placebo effects independently of—and with greater effects than—treatment expectancies (Hyland et al., 2006). Clinicians need not agree with these beliefs, but they can still acknowledge them as an intrinsic part of patients’ internal and external resources for healing (Green and Wright, 2017). Overall, the goal is to let the patient know that they have been seen and understood in their humanity. This need not take hours, only care and intentionality.
“Patient empowerment” during psychedelic-assisted psychotherapy encompasses a few practices. First, the therapist(s)/facilitators often help patients elucidate their intentions and motivations for treatment. Second, they inform patients regarding potential drug effects, including challenging experiences and ways to navigate them. Third, therapist(s)/facilitators and patients establish a conceptual scheme or rationale for treatment. This is particularly obvious in traditional psychedelic ceremonies, whereby a shaman “gives the patient a language in which unspoken mind-states find a verbal expression and explanation” (Apud and Romani, 2020). Outside of the psychedelic literature, studies suggest that patients’ acceptance of the myth or rationale for psychological treatment is more important for outcomes than the actual contents or scientific validity of the interventions (Wampold and Imel, 2015). In medicine, a patient’s explanatory model (EM)—that is, the culturally determined understanding of an illness, its causes, available treatments, and prognosis (Kleinman et al., 1978)—has been found to have powerful consequences on treatment adherence and outcomes (Galli et al., 2010; Weinman et al., 2000). Thus, in preparation for treatments, clinicians can explore their patients’ EMs, find a treatment rationale that resonates with their patients, and help patients set intentions for treatment outcomes.
Evidence that “patient empowerment” interventions are feasible and worthwhile in medicine is found in the PSY-HEART study (Rief et al., 2017). The investigators randomized 124 patients who were scheduled for coronary artery bypass graft surgery into three arms: a control group that received “standard medical care” (SMC) and two intervention groups that received social support. In addition, one of the intervention groups received guidance to reflect on the benefits and challenges of surgery, and to set intentions for what they wanted to achieve post-recovery (e.g., gardening). At 6 months post-surgery, patients in the intention-setting group showed significantly less work-related disability than those in the two other groups, and both social support groups showed better cardiovascular health indices than the SMC group. The PSY-HEART study demonstrates that social support and intention-setting (nonspecific variables) can enhance specific surgical treatment effects.
Dosing day
On the dosing day of psychedelic-assisted psychotherapy, elements of the setting (physical/social/cultural) are harnessed to (a) foster a safe space (to minimize the drug’s potential for harm) and (b) foster a sense of ceremony and ritual (to maximize the drug’s potential for benefit). For the first aim, the setting of dosing sessions is intentionally curated to feel reassuring (e.g., comfortable living room-like space; presence of a male–female therapist dyad; hand-holding in the case of challenging experiences) and safe (e.g., trained clinicians present at all times; physician on call; rescue medication at disposal; blood-pressure monitoring) (Johnson et al., 2008). Interestingly, the concepts of reassurance and safety are analogous to that of clinician warmth and competence, which were found to potentiate placebo effects (Howe et al., 2017). Based on this work, Howe et al. (2019) proposed that the healing potential of patient–physician interactions can be dissected into patients’ perceptions of whether a doctor “gets it” (i.e., displays efficiency, knowledge, and skill) and “gets me” (i.e., displays personal engagement, connection, and care for the patient) (Howe et al., 2019). The “set and setting” literature serves as a reminder that patients can derive feelings of reassurance and safety not only from the social context, but also the physical context. Indeed, the clinical environment is replete with evocative artifacts—whether paintings, pictures, and flowers in the psychedelic setting; or lab coats, stethoscopes, and diplomas in the medical one. These artifacts are powerful cues with powerful consequences (Wager and Atlas, 2015). Various studies in health and social psychology demonstrate that medical artifacts can influence patients’ perceptions about the quality of care, and in turn, health outcomes (Bernstein et al., 2020). It may be interesting for healthcare professionals and institutions to explore how they can curate clinical environments for patient benefits. For inspiration: nicely furnished, well lit, and decorated waiting rooms have been associated with higher perceived quality of care (Arneill and Devlin, 2002); physicians who don a white coat are rated more favorably by patients (Petrilli et al., 2018); and surgery patients were found to require less analgesia when their hospital room window overlooked trees compared to a wall (Ulrich, 1984). Softening the “sterile” esthetic of the traditional medical environment with plants, paintings, and warmer lighting could alleviate some of the anxiety that patients feel when at the doctor’s office (see the “white coat syndrome”), and place patients in a better mindset for ensuing medical care.
Although providing a sense of reassurance and safety mainly serves to minimize psychedelic harm, the sense of ceremony and ritual that is involved in psychedelic dosing potentiates these compounds’ benefits. For example, shamanic rituals that accompany ayahuasca sessions can be thought of as performances that unite various props (e.g., songs, smoke blowing, whistles) that amplify drug effects (Hartogsohn, 2017). Medical procedures can be thought of as performances too (Kaptchuk, 2002). Indeed, research finds that the open application of analgesics (in patient full view) substantially enhances analgesic effects compared to hidden applications (Benedetti et al., 2011), and that powerful opioids lose at least 30% of their efficacy when administered unbeknownst to patients (Bingel et al., 2011). Like in a shamanic ritual, the performativity of the medical procedure and its accompanying props (medical apparatus, patient–physician communication, bedside presence of caregivers, seeing a medicine being delivered) enhance the drugs’ standalone effects. Outside of the hospital context, patients engage in their own healing rituals (Bishop et al., 2017). For example, the “taking of a pill” has been suggested to evoke the dual meaning of taking care of oneself (active) and being taken care of (passive) (Barrett et al., 2006) and several large studies report that patients who adhere to medication protocols do better than those who do not, regardless of whether they are taking active or inert substances (e.g., Irvine et al., 1999). Physicians can thus emphasize the importance of the action itself and encourage patients to create a “pill taking” ritual, to make the experience less automatic and more salient. In sum, medical procedures, like shamanic rituals, can be conceptualized as multisensory “dramas,” with “sensory, affective, moral and esthetic components” that lie outside of the pharmacological scope but are just as important in helping the patient move from “brokenness to intactness” (Kaptchuk, 2011).
Integration
Integration sessions tap into the “matrix,” a concept introduced by Eisner (1997) that refers to the larger environment from which a subject comes and to which a subject returns (Eisner, 1997). The aim of integration is to bridge the powerful but transient psychedelic experience with everyday life (Garcia-Romeu and Richards, 2018), particularly during the fertile “afterglow” period that follows psychedelic consumption, in which the brain is thought to be in a more plastic state (Majic et al., 2015). This step in the process highlights that the end of the “trip” does not mark the end of the treatment; there is still much work to be done in its aftermath.
In the medical context, patients may similarly struggle with challenging side effects or adverse reactions following a medical prescription or procedure. It is essential to provide structured opportunities for follow-up, to monitor and manage these eventualities. In fact, a major issue with contemporary pharmacology is the gap between research and practice regarding drug safety. Clinical trials testing new pharmacological agents rarely last longer than 6 months (Downing et al., 2017), whereas the medications that are approved based on these trials are often prescribed for daily use spanning years, sometimes decades (Schenberg, 2018). Prolonged and unmonitored use can result in many adverse consequences such as toxicity (Kukreja et al., 2013), addiction (Novak et al., 2007), and countless unpleasant side effects (Bet et al., 2013). Physicians should solicit patient reports of drug (side)-effects post-prescription, and take them as seriously as the science that supported their decision to prescribe the drug in the first place. The concept of “matrix” further serves as a reminder that patients can only comply with treatment regimens, enact healthy lifestyle changes, and heal insofar as they are enabled to by their environment. Rather than referring to patients as having “failed treatment,” we may start asking why treatment “failed them.”
Strengths and limitations
This article builds on the theoretical work by Hartogsohn (2016) and Gukasyan and Nayak (2022), which outlined the parallels between placebo, “set and setting,” and “common factors in psychotherapy” literature. It offers a unique set of practical recommendations for harnessing placebo in a biomedical context, inspired by the psychedelic paradigm. These recommendations are the result of translational work; they reconcile two fields that are normally siloed. Instead of simply criticizing the additive biomedical paradigm, this article highlights a conceptual alternative in the interactive psychedelic model, and illustrates how it can be put into practice. We intend for these recommendations to be accessible to psychedelic researchers and medical practitioners alike, and hope they may foster novel conversations between parties in both fields. This article has some limitations worth mentioning. First, the practical recommendations we offer were not based on a systematic review of the placebo or “set and setting” literature. We had to limit ourselves to a narrative review format for the sake of time and parsimony. Second, this article did not discuss the “nocebo” effect and how it relates to “set and setting.” Finally, we anticipate some challenges in implementing “set and setting” principles to medical care. Medical providers are already stretched incredibly thin in terms of their time, and some of these recommendations may feel like an added burden. It is worth noting, however, that we do not expect medical practitioners to become therapists or shamans, or to spend additional hours doing everything outlined in Table 3. Rather, we seek to offer inspiration for ways to make routine medical encounters and their surrounding environments more therapeutic; that is, for simple ways to potentiate treatment effectiveness via biopsychosocial forces. This could, ultimately, make doctors’ lives easier, and patients’ lives better.
Conclusion
The placebo effect has long been recognized in medicine as a powerful yet underutilized element of the healing process. Psychedelic medicine provides a potentially fruitful model for integrating the lessons of placebo into medical practice. It delivers a framework which challenges traditional boundaries between subjective and objective, mind and body, treatment and context, person and surroundings, and art and science. While these concepts’ dichotomization may have been conducive to scientific progress, acknowledging their interaction is crucial for patient benefits. The central importance of “set and setting” in psychedelic science places this field at the vanguard of harnessing extra-drug variables for the sake of patient healing, and offers a way out of the harmfully reductive biomedical status quo.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Chloé Pronovost-Morgan  https://orcid.org/0000-0002-8386-4925
https://orcid.org/0000-0002-8386-4925
References
- Abuqayyas S, Yurosko C, Ali A, et al. (2021) Bedside manner 2020: An inventory of best practices. South Med J 114: 156–160. [DOI] [PubMed] [Google Scholar]
- Aday JS, Davis AK, Mitzkovitz CM, et al. (2021) Predicting reactions to psychedelic drugs: A systematic review of states and traits related to acute drug effects. ACS Pharmacol Transl Sci 4: 424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aday JS, Heifets BD, Pratscher SD, et al. (2022). Great expectations: Recommendations for improving the methodological rigor of psychedelic clinical trials. Psychopharmacology (Berl) 239: 1989–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert R, Leary T, Metzner R. (1964) The Psychedelic Experience. New York: University Books. [Google Scholar]
- Annoni M. (2018) The ethics of placebo effects in clinical practice and research. Int Rev Neurobiol 139: 463–484. [DOI] [PubMed] [Google Scholar]
- Annoni M. (2020) Better than nothing: A historical account of placebos and placebo effects from modern to contemporary medicine. Int Rev Neurobiol 153: 3–26. [DOI] [PubMed] [Google Scholar]
- Annoni M, Miller FG. (2016) Placebo effects and the ethics of therapeutic communication: A pragmatic perspective. Kennedy Inst Ethics J 26: 79–103. [DOI] [PubMed] [Google Scholar]
- Apud I, Romani O. (2020). Medical anthropology and symbolic cure: From the placebo to cultures of meaningful healing. Anthropol Med 27: 160–175. [DOI] [PubMed] [Google Scholar]
- Arneill AB, Devlin AS. (2002) Perceived quality of care: The influence of the waiting room environment. J Environ Psychol 22: 345–360. [Google Scholar]
- Barrett B, Muller D, Rakel D, et al. (2006) Placebo, meaning, and health. Perspect Biol Med 49: 178–198. [DOI] [PubMed] [Google Scholar]
- Becker HS. (1967) History, culture and subjective experience: An exploration of the social bases of drug-induced experiences. J Health Soc Behav 8: 163–176. [PubMed] [Google Scholar]
- Beecher HK. (1955) The powerful placebo. J Am Med Assoc 159: 1602–1606. [DOI] [PubMed] [Google Scholar]
- Benedetti F. (2014) Placebo effects: From the neurobiological paradigm to translational implications. Neuron 84: 623–637. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Carlino E, Pollo A. (2011) Hidden administration of drugs. Clin Pharmacol Ther 90: 651–661. [DOI] [PubMed] [Google Scholar]
- Bernstein MH, Locher C, Kube T, et al. (2020) Putting the ‘art’ into the ‘art of medicine’: The under-explored role of artifacts in placebo studies. Front Psychol 11: 1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bet PM, Hugtenburg JG, Penninx BW, et al. (2013) Side effects of antidepressants during long-term use in a naturalistic setting. Eur Neuropsychopharmacol 23: 1443–1451. [DOI] [PubMed] [Google Scholar]
- Bingel U, Wanigasekera V, Wiech K, et al. (2011) The effect of treatment expectation on drug efficacy: Imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med 3: 70ra14. [DOI] [PubMed] [Google Scholar]
- Bishop FL, Coghlan B, Geraghty AW, et al. (2017) What techniques might be used to harness placebo effects in non-malignant pain? A literature review and survey to develop a taxonomy. BMJ Open 7: e015516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop FL, Howick J, Heneghan C, et al. (2014) Placebo use in the UK: A qualitative study exploring GPs’ views on placebo effects in clinical practice. Fam Pract 31: 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkedal E, Flaten MA. (2011) Interaction between expectancies and drug effects: An experimental investigation of placebo analgesia with caffeine as an active placebo. Psychopharmacology (Berl) 215: 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm K, Berger B, Weger U, et al. (2017) Does the model of additive effect in placebo research still hold true? A narrative review. JRSM Open 8: 2054270416681434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussageon R, Howick J, Baron R, et al. (2022) How do they add up? The interaction between the placebo and treatment effect: A systematic review. Br J Clin Pharmacol 88: 3638–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabot RC. (1903) The Use of Truth and Falsehood in Medicine: An Experimental Study. p. 348. Am Medicine 5:344–349. [Google Scholar]
- Carhart-Harris RL, Kaelen M, Whalley MG, et al. (2015) LSD enhances suggestibility in healthy volunteers. Psychopharmacology (Berl) 232: 785–794. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Roseman L, Haijen E, et al. (2018) Psychedelics and the essential importance of context. J Psychopharmacol 32: 725–731. [DOI] [PubMed] [Google Scholar]
- Chavarria V, Vian J, Pereira C, et al. (2017) The placebo and nocebo phenomena: Their clinical management and impact on treatment outcomes. Clin Ther 39: 477–486. [DOI] [PubMed] [Google Scholar]
- Colloca L, Barsky AJ. (2020) Placebo and nocebo effects. N Engl J Med 382: 554–561. [DOI] [PubMed] [Google Scholar]
- Colloca L, Miller FG. (2011) Harnessing the placebo effect: The need for translational research. Philos Trans R Soc B Biol Sci 366: 1922–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino MJ, Visla A, Coyne AE, et al. (2018) A meta-analysis of the association between patients’ early treatment outcome expectation and their posttreatment outcomes. Psychotherapy (Chic) 55: 473–485. [DOI] [PubMed] [Google Scholar]
- Crum AJ, Leibowitz KA, Verghese A. (2017) Making mindset matter. BMJ 356: j674. [DOI] [PubMed] [Google Scholar]
- Cullen W. (1772) Clinical Lecture, Edinburgh. February-April, 218f. [Google Scholar]
- Dinnerstein AJ, Halm J. (1970) Modification of placebo effects by means of drugs: Effects of aspirin and placebos on self-rated moods. J Abnorm Psychol 75: 308. [DOI] [PubMed] [Google Scholar]
- Downing NS, Shah ND, Aminawung JA, et al. (2017) Postmarket safety events among novel therapeutics approved by the US Food and Drug Administration between 2001 and 2010. JAMA 317: 1854–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner B. (1997) Set, setting, and matrix. J Psychoactive Drugs 29: 213–216. [DOI] [PubMed] [Google Scholar]
- Evers AWM, Colloca L, Blease C, et al. (2018) Implications of placebo and nocebo effects for clinical practice: Expert consensus. Psychother Psychosom 87: 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassaert T, van Dulmen S, Schellevis F, et al. (2007) Active listening in medical consultations: Development of the Active Listening Observation Scale (ALOS-global). Patient Educ Couns 68: 258–264. [DOI] [PubMed] [Google Scholar]
- Fässler M, Gnädinger M, Rosemann T, et al. (2009) Use of placebo interventions among Swiss primary care providers. BMC Health Serv Res 9: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finniss DG. (2018) Placebo effects: Historical and modern evaluation. Int Rev Neurobiol 139: 1–27. [DOI] [PubMed] [Google Scholar]
- Flaten MA, Simonsen T, Olsen H. (1999) Drug-related information generates placebo and nocebo responses that modify the drug response. Psychosom Med 61: 250–255. [DOI] [PubMed] [Google Scholar]
- Flückiger C, Del Re AC, Wampold BE, et al. (2018) The alliance in adult psychotherapy: A meta-analytic synthesis. Psychotherapy 55: 316. [DOI] [PubMed] [Google Scholar]
- Frenkel O. (2008) A phenomenology of the ‘placebo effect’: Taking meaning from the mind to the body. J Med Philos 33: 58–79. [DOI] [PubMed] [Google Scholar]
- Galli U, Ettlin DA, Palla S, et al. (2010) Do illness perceptions predict pain-related disability and mood in chronic orofacial pain patients? A 6-month follow-up study. Eur J Pain 14: 550–558. [DOI] [PubMed] [Google Scholar]
- Garcia-Romeu A, Richards WA. (2018) Current perspectives on psychedelic therapy: Use of serotonergic hallucinogens in clinical interventions. Int Rev Psychiatry 30: 291–316. [DOI] [PubMed] [Google Scholar]
- George WH, Gilmore AK, Stappenbeck CA. (2012) Balanced placebo design: Revolutionary impact on addictions research and theory. Addict Res Theory 20: 186–203. [Google Scholar]
- Gold H, Grace W, Ferguson F, et al. (1954) How to evaluate a new drug. Am J Med 17: 722–727. [DOI] [PubMed] [Google Scholar]
- Gold H, Kwit NT, Otto H. (1937) The xanthines (theobromine and aminophylline) in the treatment of cardiac pain. J Am Med Assoc 108: 2173–2179. [Google Scholar]
- Golden TL, Magsamen S, Sandu CC, Lin S, Roebuck GM, Shi KM, Barrett FS. (2022) Effects of Setting on Psychedelic Experiences, Therapies, and Outcomes: A Rapid Scoping Review of the Literature. Curr Top Behav Neurosci 56: 35–70. [DOI] [PubMed] [Google Scholar]
- Green J, Wright H. (2017) From bench to bedside: Converting placebo research into belief activation. J Altern Complement Med 23: 575–580. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, et al. (2018) Psilocybin-occasioned mystical-type experience in combination with meditation and other spiritual practices produces enduring positive changes in psychological functioning and in trait measures of prosocial attitudes and behaviors. J Psychopharmacol 32: 49–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gukasyan N, Nayak SM. (2022) Psychedelics, placebo effects, and set and setting: Insights from common factors theory of psychotherapy. Transcult Psychiatry 59: 652–664. [DOI] [PubMed] [Google Scholar]
- Haijen E, Kaelen M, Roseman L, et al. (2018) Predicting responses to psychedelics: A prospective study. Front Pharmacol 9: 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammami MM, Al-Gaai EA, Alvi S, et al. (2010) Interaction between drug and placebo effects: A cross-over balanced placebo design trial. Trials 11: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammami MM, Hammami S, Al-Swayeh R, et al. (2016) Drug*placebo interaction effect may bias clinical trials interpretation: Hybrid balanced placebo and randomized placebo-controlled design. BMC Med Res Methodol 16: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartogsohn I. (2016) Set and setting, psychedelics and the placebo response: An extra-pharmacological perspective on psychopharmacology. J Psychopharmacol 30: 1259–1267. [DOI] [PubMed] [Google Scholar]
- Hartogsohn I. (2017) Constructing drug effects: A history of set and setting. Drug Sci Policy Law 3: 2050324516683325. [Google Scholar]
- Hartogsohn I. (2018) The meaning-enhancing properties of psychedelics and their mediator role in psychedelic therapy, spirituality, and creativity. Front Neurosci 12: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartogsohn I. (2020) American Trip: Set, Setting, and the Psychedelic Experience in the Twentieth Century. Cambridge, Massachusetts: MIT Press. [Google Scholar]
- Howe LC, Goyer JP, Crum AJ. (2017) Harnessing the placebo effect: Exploring the influence of physician characteristics on placebo response. Health Psychol 36: 1074–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe LC, Leibowitz KA, Crum AJ. (2019) When your doctor “gets it” and “gets you”: The critical role of competence and warmth in the patient-provider interaction. Front Psychiatry 10: 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howick J, Moscrop A, Mebius A, et al. (2018) Effects of empathic and positive communication in healthcare consultations: A systematic review and meta-analysis. J R Soc Med 111: 240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howick J, Friedemann C, Tsakok M, et al. (2013) Are treatments more effective than placebos? A systematic review and meta-analysis. PLoS One 8: e62599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland ME, Geraghty AW, Joy OE, et al. (2006) Spirituality predicts outcome independently of expectancy following flower essence self-treatment. J Psychosom Res 60: 53–58. [DOI] [PubMed] [Google Scholar]
- Irvine J, Baker B, Smith J, et al. (1999) Poor adherence to placebo or amiodarone therapy predicts mortality: Results from the CAMIAT study. Canadian Amiodarone Myocardial Infarction Arrhythmia Trial. Psychosom Med 61: 566–575. [DOI] [PubMed] [Google Scholar]
- Johnson M, Richards W, Griffiths R. (2008) Human hallucinogen research: Guidelines for safety. J Psychopharmacol 22: 603–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelen M, Giribaldi B, Raine J, et al. (2018) The hidden therapist: Evidence for a central role of music in psychedelic therapy. Psychopharmacology 235: 505–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptchuk TJ. (2002) The placebo effect in alternative medicine: Can the performance of a healing ritual have clinical significance? Ann Intern Med 136: 817–825. [DOI] [PubMed] [Google Scholar]
- Kaptchuk TJ. (2011) Placebo studies and ritual theory: A comparative analysis of Navajo, acupuncture and biomedical healing. Philos Trans R Soc B Biol Sci 366: 1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptchuk TJ, Miller FG. (2015) Placebo effects in medicine. N Engl J Med 373: 8–9. [DOI] [PubMed] [Google Scholar]
- Kast EC, Loesch J. (1961) Influence of the doctor-patient relationship on drug action. Ill Med J 119: 390–393. [PubMed] [Google Scholar]
- Kelemen WL, Kaighobadi F. (2007) Expectancy and pharmacology influence the subjective effects of nicotine in a balanced-placebo design. Exp Clin Psychopharmacol 15: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley JM, Kraft-Todd G, Schapira L, et al. (2014) The influence of the patient-clinician relationship on healthcare outcomes: A systematic review and meta-analysis of randomized controlled trials. PLoS One 9: e94207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettner H, Rosas FE, Timmermann C, et al. (2021) Psychedelic communitas: Intersubjective experience during psychedelic group sessions predicts enduring changes in psychological wellbeing and social connectedness. Front Pharmacol 12: 623985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman A, Eisenberg L, Good B. (1978) Culture, illness, and care: Clinical lessons from anthropologic and cross-cultural research. Ann Intern Med 88: 251–258. [DOI] [PubMed] [Google Scholar]
- Kube T, Rief W. (2017) Are placebo and drug-specific effects additive? Questioning basic assumptions of double-blinded randomized clinical trials and presenting novel study designs. Drug Discov Today 22: 729–735. [DOI] [PubMed] [Google Scholar]
- Kukreja S, Kalra G, Shah N, et al. (2013) Polypharmacy in psychiatry: A review. Mens Sana Monogr 11: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde K, Atmann O, Meissner K, et al. (2018) How often do general practitioners use placebos and non-specific interventions? Systematic review and meta-analysis of surveys. PLoS One 13: e0202211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotshaw SC, Bradley JR, Brooks LR. (1996) Illustrating caffeine’s pharmacological and expectancy effects utilizing a balanced placebo design. Journal of Drug Education 26: 13–24. [DOI] [PubMed] [Google Scholar]
- Lyerly SB, Ross S, Krugman AD, et al. (1964) Drugs and placebos: The effects of instructions upon performance and mood under amphetamine sulfate and chloral hydrate. J Abnorm Soc Psychol 68: 321. [DOI] [PubMed] [Google Scholar]
- Majic T, Schmidt TT, Gallinat J. (2015) Peak experiences and the afterglow phenomenon: When and how do therapeutic effects of hallucinogens depend on psychedelic experiences? J Psychopharmacol 29: 241–253. [DOI] [PubMed] [Google Scholar]
- McCartney A, McGovern H, De Foe A. (2022) Predictors of psychedelic experience: A thematic analysis. J Psychoactive Drugs. Epub ahead of print 5 October 2022. DOI: 10.1080/02791072.2022.2129885. [DOI] [PubMed] [Google Scholar]
- McKay KM, Imel ZE, Wampold BE. (2006) Psychiatrist effects in the psychopharmacological treatment of depression. J Affect Disord 92: 287–290. [DOI] [PubMed] [Google Scholar]
- McQueen D, Cohen S, St John-Smith P, et al. (2013) Rethinking placebo in psychiatry: How and why placebo effects occur. Adv Psychiatr Treat 19: 171–180. [Google Scholar]
- McQueen D, Smith PSJ. (2012) Placebo effects: A new paradigm and relevance to psychiatry. Int Psychiatry 9: 1–3. [PMC free article] [PubMed] [Google Scholar]
- Metrik J, Kahler CW, Reynolds B, et al. (2012) Balanced placebo design with marijuana: Pharmacological and expectancy effects on impulsivity and risk taking. Psychopharmacology 223: 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modell W, Garrett M. (1960) Interactions between pharmacodynamic and placebo effects in drug evaluations in man. Nature 185: 538–539. [DOI] [PubMed] [Google Scholar]
- Moerman DE. (2002) Meaning, Medicine, and the ‘Placebo Effect’, vol. 28. Cambridge: Cambridge University Press. [Google Scholar]
- Murphy R, Kettner H, Zeifman R, et al. (2021). Therapeutic alliance and rapport modulate responses to psilocybin assisted therapy for depression. Front Pharmacol 12: 788155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén B, Brown HC. (2009) Estimating drug effects in the presence of placebo response: Causal inference using growth mixture modeling. Stat Med 28: 3363–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Forsyth A, Lumley T. (2021) Blinding and expectancy confounds in psychedelic randomized controlled trials. Expert Rev Clin Pharmacol 14: 1133–1152. [DOI] [PubMed] [Google Scholar]
- Novak SP, Kroutil LA, Williams RL, et al. (2007) The nonmedical use of prescription ADHD medications: Results from a national Internet panel. Subst Abuse Treat Prev Policy 2: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JA, Suissa-Rocheleau L, Lifshitz M, et al. (2020) Tripping on nothing: Placebo psychedelics and contextual factors. Psychopharmacology 237: 1371–1382. [DOI] [PubMed] [Google Scholar]
- Osmond H. (1957) A review of the clinical effects of psychotomimetic agents. Ann N Y Acad Sci 66: 418–434. [DOI] [PubMed] [Google Scholar]
- Pepper OH. (1945) A note on the placebo. Am J Pharm 117: 409–412. [Google Scholar]
- Petrie KJ, Rief W. (2019) Psychobiological mechanisms of placebo and nocebo effects: Pathways to improve treatments and reduce side effects. Annu Rev Psychol 70: 599–625. [DOI] [PubMed] [Google Scholar]
- Petrilli CM, Saint S, Jennings JJ, et al. (2018) Understanding patient preference for physician attire: A cross-sectional observational study of 10 academic medical centres in the USA. BMJ Open 8: e021239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontual AAD, Tofoli LF, Corradi-Webster CM, et al. (2022) The influence of ceremonial settings on mystical and challenging experiences occasioned by ayahuasca: A survey among ritualistic and religious ayahuasca users. Front Psychol 13: 857372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rief W, Shedden-Mora MC, Laferton JA, et al. (2017) Preoperative optimization of patient expectations improves long-term outcome in heart surgery patients: Results of the randomized controlled PSY-HEART trial. BMC Med 15: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Marlatt GA. (1981) The balanced placebo design: Methodological considerations. Addict Behav 6: 107–122. [DOI] [PubMed] [Google Scholar]
- Roseman L, Nutt DJ, Carhart-Harris RL. (2017) Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment-resistant depression. Front Pharmacol 8: 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RS. (2003) A biopsychosocial perspective on the placebo effect: Comment on Benedetti et al.(2003). Prev Treat 6: 8c. [Google Scholar]
- Rutherford BR, Roose SP. (2013) A model of placebo response in antidepressant clinical trials. Am J Psychiatry 170: 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett DL, Rosenberg WM, Gray JM, Haynes RB, Richardson WS. (1996) Evidence based medicine: what it is and what it isn’t. British Medical Journal Publishing Group, 312: 71–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenberg EE. (2018) Psychedelic-assisted psychotherapy: A paradigm shift in psychiatric research and development. Front Pharmacol 9: 733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleim S. (2022) Grounded in biology: Why the context-dependency of psychedelic drug effects means opportunities, not problems for anthropology and pharmacology. Front Psychiatry 13: 906487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studerus E, Gamma A, Kometer M, et al. (2012) Prediction of psilocybin response in healthy volunteers. PLoS One 7: e30800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MD. (1993) Placebo controls and epistemic control in orthodox medicine. J Med Philos 18: 213–231. [DOI] [PubMed] [Google Scholar]
- Uhlenhuth E, Canter A, Neustadt JO, et al. (1959) The symptomatic relief of anxiety with meprobamate, phenobarbital and placebo. Am J Psychiatry 115: 905–910. [DOI] [PubMed] [Google Scholar]
- Ulrich RS. (1984) View through a window may influence recovery from surgery. Science 224: 420–421. [DOI] [PubMed] [Google Scholar]
- Uthaug MV, Mason NL, Toennes SW, et al. (2021) A placebo-controlled study of the effects of ayahuasca, set and setting on mental health of participants in ayahuasca group retreats. Psychopharmacology (Berl) 238: 1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Atlas LY. (2015) The neuroscience of placebo effects: Connecting context, learning and health. Nat Rev Neurosci 16: 403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wampold BE, Imel ZE. (2015) The Great Psychotherapy Debate: The Evidence for What Makes Psychotherapy Work. New York: Routledge. [Google Scholar]
- Weinman J, Petrie KJ, Sharpe N, et al. (2000) Causal attributions in patients and spouses following first-time myocardial infarction and subsequent lifestyle changes. Br J Health Psychol 5: 263–273. [Google Scholar]
- Wolff H, Dubois E. (1946) The use of placebos in therapy. N Y State J Med 46: 1718–1727. [PubMed] [Google Scholar]
- Yaden DB, Yaden ME, Griffiths RR. (2021) Psychedelics in psychiatry-keeping the renaissance from going off the rails. JAMA Psychiatry 78: 469–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zion SR, Crum AJ. (2018) Mindsets matter: A new framework for harnessing the placebo effect in modern medicine. Int Rev Neurobiol 138: 137–160. [DOI] [PubMed] [Google Scholar]