Abstract
BACKGROUND.
Understanding of dynamic changes of MRI findings in response to intracranial pressure (ICP) changes in idiopathic intracranial hypertension (IIH) is limited. Brain stiffness, as assessed by MR elastography (MRE), may reflect changes in ICP.
OBJECTIVE.
The purpose of this study was to compare pituitary height, ventricular size, and brain stiffness between patients with IIH and control individuals and to evaluate for changes in these findings in patients with IIH after interventions to reduce ICP.
METHODS.
This prospective study included 30 patients (28 women, two men; median age, 29.9 years) with IIH and papilledema and 21 control individuals (21 women, 0 men; median age, 29.1 years), recruited from January 2017 to July 2019. All participants underwent 3-T brain MRI with MRE; patients with IIH underwent additional MRI examinations with MRE after acute intervention (lumbar puncture with normal closing pressure; n = 11) and/or chronic intervention (medical management or venous sinus stenting with resolution or substantial reduction in papilledema; n = 12). Pituitary height was measured on sagittal MP-RAGE images. Ventricular volumes were estimated using unified segmentation, and postintervention changes were assessed by tensor-based morphometry. Stiffness pattern score and regional stiffness values were estimated from MRE.
RESULTS.
In patients with IIH, median pituitary height was smaller than in control individuals (3.1 vs 4.9 mm, p < .001) and was increased after chronic (4.0 mm, p = .05), but not acute (2.3 mm, p = .50), intervention. Ventricular volume was not different between patients with IIH and control individuals (p = .33) and did not change after acute (p = .83) or chronic (p = .97) intervention. In patients with IIH, median stiffness pattern score was greater than in control individuals (0.25 vs 0.15, p < .001) and decreased after chronic (0.23, p = .11) but not acute (0.25, p = .49) intervention. Median occipital lobe stiffness was 3.08 kPa in patients with IIH versus 2.94 kPa in control individuals (p = .07) and did not change after acute (3.24 kPa, p = .73) or chronic (3.10 kPa, p = .83) intervention.
CONCLUSION.
IIH is associated with a small pituitary and increased brain stiffness pattern score; both findings may respond to chronic interventions to lower ICP.
CLINICAL IMPACT.
The “partially empty sella” sign and brain stiffness pattern score may serve as dynamic markers of ICP in IIH.
Keywords: brain stiffness, idiopathic intracranial hypertension, IIH, MRE, MR elastography, pituitary gland, ventricular size
Idiopathic intracranial hypertension (IIH) is a syndrome of increased intracranial pressure (ICP) in the absence of CNS pathology and occurs primarily in young obese women [1, 2]. Patients may present with headache, pulsatile tinnitus, and vision changes. MRI is required to exclude CNS pathology and often shows indirect signs of raised ICP, supporting the diagnosis of IIH. Such features include a partially empty sella, transverse sinus stenosis, optic nerve sheath dilatation, posterior globe flattening, slitlike ventricles, Meckel cave enlargement, optic nerve head diffusion abnormality, and inferior cerebellar tonsillar descent [3–7]. Some of these findings are nonspecific or uncommon and are therefore of limited clinical utility. Nonetheless, some indirect signs of raised ICP (e.g., partially empty sella, transverse sinus stenosis, optic nerve sheath dilatation, and flattening of the posterior globe) have been incorporated into proposed diagnostic criteria for IIH, whereby a diagnosis of IIH can be suggested even without papilledema if several indirect signs of ICP are present [8]. However, in one study, 39.5% of patients referred to a neuroophthalmology clinic for suspected IIH did not have IIH, with some of these incorrect referrals attributed to incidental indirect neuroimaging signs of raised ICP [9]. In addition, lumbar puncture (LP) opening pressures are variable, and healthy individuals can have ICP above the cutoff of 250 mm H2O that is typically used for defining normal ICP [10]; therefore, an elevated LP opening pressure alone is insufficient to confirm a diagnosis of IIH. For these reasons, it is important to understand the specificity of the indirect imaging signs of raised ICP as well as their dynamic nature in response to changes in ICP.
Partially empty sella can be evaluated by standard noncontrast MRI sequences and is thus a readily assessed feature of IIH. In comparison, optimal imaging of the venous sinuses requires IV contrast material administration, and imaging of the optic nerve sheath requires high-resolution orbital sequences, which may be degraded by motion. Partially empty sella has thus been consistently applied as an MRI feature of IIH in the peer-reviewed literature [3, 4, 6]. However, the finding’s dynamic nature with clinical improvement remains unclear given conflicting results of prior studies [11–13].
The ventricles have been reported to be smaller and slitlike in patients with IIH [11, 14], providing an additional potential MRI marker of IIH. Qualitative or linear quantitative (i.e., Evans index) description of ventricular size may be performed on standard noncontrast MRI sequences, but these metrics are insensitive to small temporal changes. Volumetric measurements using 3D sequences and coregistration of serial MRI examinations are more sensitive for detecting small changes [15, 16]. Nonetheless, a limited number of studies have described changes in ventricular size after acute and chronic intervention for increased ICP [11].
A property that has been less well studied in patients with IIH is brain stiffness, which may be assessed noninvasively by MR elastography (MRE). MRE measures mechanical properties by first introducing small strains into the tissue using an external driver, imaging the resulting displacement field using a phase-contrast sequence, and finally inverting the displacements to create a map of estimated tissue stiffness [17, 18]. An animal study showed changes in MRI measurements of brain stiffness in association with acute pressure changes in the brain [19]. This observation raises the possibility that brain stiffness may be increased in patients with IIH and could potentially be used to noninvasively detect ICP and monitor ICP changes.
The aim of this study was to compare pituitary height, ventricular size, and brain stiffness between patients with IIH and control individuals and to evaluate for changes in these findings in patients with IIH after acute and chronic interventions to reduce ICP.
Methods
Participants
This prospective HIPAA-compliant study was performed under institutional review board approval. All participants provided written informed consent.
The study was conducted at a tertiary care center with a dedicated IIH referral clinic. All clinic patients with papilledema and clinical suspicion of IIH from January 2017 to July 2019 were potentially eligible and were referred for study participation. Potentially eligible patients were screened for the 2013 revised diagnostic criteria for IIH [8], including absence on neuroimaging of a CNS lesion to explain increased ICP. Those meeting the diagnostic criteria were recruited for study participation. Control individuals were recruited during the same period from the local community via flyers, online postings, and word of mouth. Control individuals were eligible for participation if having no neurologic or psychiatric condition or symptoms of raised ICP. Both patients with IIH and control individuals were also considered ineligible and were not approached for consent if they were less than 18 years old, were pregnant, or had an MRI contraindication.
All recruited patients with IIH underwent an investigational brain MRI examination after consenting to participate (here-after, the baseline MRI). For purposes of clinical workup unrelated to this investigation, patients with IIH may have undergone a large-volume (30 mL) diagnostic LP after study enrollment and the baseline investigational brain MRI. LP was performed in patients who had not undergone an LP at an outside institution before presentation to the clinic and was also performed in some patients who had undergone prior LP at the discretion of the treating neuroophthalmologist. Among patients who underwent large-volume diagnostic LP, if the LP closing pressure was less than 250 mm H2O (i.e., normal closing LP), then the patient underwent an additional investigational brain MRI examination later the same day as the LP, and these patients form the acute intervention subgroup. Also for clinical purposes unrelated to this investigation, patients with IIH typically underwent medical management and/or therapeutic venous sinus stenting after the investigational baseline MRI examination. Patients who returned for clinical follow-up after such interventions underwent an additional investigational brain MRI examination. Those who experienced resolution or substantial improvement of papilledema (i.e., a decrease by two or more Frisen papilledema grades) were included in the chronic intervention subgroup. Follow-up MRI in the chronic intervention subgroup was always performed at least 6 months after treatment initiation; if patients in the chronic intervention subgroup underwent multiple posttreatment MRI examinations, then the last available examination was used for analysis. Patients with IIH could have been included in both the acute intervention and chronic intervention subgroups if meeting the criteria for both.
A board-certified neuroophthalmologist with 8 years of posttraining experience (J.J.C.) clinically assessed patients with IIH at the time of each brain MRI examination to assign a Frisen papilledema grade based on a combination of dilated fundoscopy, optic disk photos, and optic coherence tomography. In patients who underwent diagnostic LP after enrollment, the LP opening pressure was recorded.
Brain MRI Examinations
Brain MRI examinations were performed on a compact 3T system, which uses a high-performance gradient system and has a 37-cm bore [20], using an 8-channel head coil and without IV contrast medium administration. The MRI examination included anatomic assessment, MRE, and MR venography (MRV). Anatomic assessment was performed using a whole-brain T1-weighted MP-RAGE acquisition with parameters of TR/TE, 6.1/2.5; inversion time, 600 ms; flip angle, 8°; FOV, 160 × 160 mm; matrix, 256 × 256; slice thickness, 1.2 mm; and acquisition time, 4 minutes 18 seconds. For MRE, shear waves were introduced via a commercially available pneumatic actuator at a frequency of 60-Hz vibration, and images were acquired using a flow-compensated spin-echo echo-planar imaging sequence with the following parameters: TR/TE, 4000/59; FOV, 240 × 240 mm; matrix, 80 × 80; 48 contiguous 3-mm-thick axial slices; one 16.7-millisecond 8-G/cm motion- encoding gradient on each side of the refocusing pulse; x-, y-, and z-motion-encoding directions; 8 phase offsets spaced evenly over one period of 60-Hz motion; and acquisition time, 5 minutes 20 seconds [21]. MRV was performed using a 3D phase-contrast sequence with the following parameters: TR/TE, 8/3.4; flip angle, 8°; FOV, 240 × 240 mm; matrix, 384 × 256; slice thickness, 1.2 mm; velocity encoding, 30 cm/s; and acquisition time, 5 minutes 30 seconds.
Assessment of Pituitary Gland Height and Other Qualitative Findings
A fellowship-trained neuroradiologist with 3 years of posttraining experience (P.M.C.) reviewed the MRI examinations without being informed of any clinical information. In patients with IIH in the acute and/or chronic intervention subgroups, the multiple MRI examinations were reviewed concurrently in a coregistered fashion. Examinations were reviewed using a PACS (Visage, version 7.1.14, Visage Imaging) with the same magnification, window, and level settings applied for all examinations.
The observer measured the height of the pituitary gland in the middle third portion of the gland on a midsagittal image of the MP-RAGE sequence. The observer also assessed examinations for the presence versus absence of transverse sinus stenosis and inferior cerebellar tonsillar descent. Transverse sinus stenosis was defined as focal narrowing of the lateral aspect of the transverse sinuses on MRV and was viewed using multiplanar reconstructions (MPRs) and 3D maximum intensity projections. Inferior cerebellar tonsillar descent was defined as cerebellar tonsillar descent to 5 mm or more below the foramen magnum on MPRs of the MP-RAGE sequence.
An additional fellowship-trained neuroradiologist with 1 year of posttraining experience (A.A.M.) independently reviewed the examinations in a similar fashion to assess pituitary gland height and the presence of transverse sinus stenosis and inferior cerebellar tonsillar descent. These assessments were used only for the purposes of determining interobserver agreement; all subsequent analyses used the first observer’s observations.
Automated MR Elastography Processing
Brain stiffness maps were generated from the MRE acquisitions in an automated fashion using open-source software (SPM, version 12 [22], Functional Imaging Laboratory), programmed in MATLAB (version R2018a, MathWorks), as described in the Supplemental Methods (available in the online supplement). The time required to generate the stiffness map for each examination was approximately 5 minutes including all preprocessing steps. Two categories of summary measurements were automatically extracted from the stiffness maps. First, a pattern analysis was performed, as previously described [23, 24], to generate a stiffness pattern score. This pattern analysis used a leave-one-out approach to compute the spatial correlation between each participant’s stiffness map (corrected for age and sex) and the expected effect of IIH (computed from the other participants) [24]. Second, the mean stiffness in kilopascals was computed in nine regions of the brain using an in-house lobar atlas (frontal lobe, occipital lobe, parietal lobe, temporal lobe, gray matter–white matter junction, corpus callosum, cerebellum, brainstem, and global region). For lobar regions, right and left lobes were combined, given anticipated symmetry. The cerebellum included white and gray matter, and the global region included all regions. Images were smoothed for display purposes.
Automated Determination of Ventricular Volumes
An automated unified segmentation algorithm was used to estimate baseline ventricular volume, using the same software as was used for MRE analysis (i.e., SPM) [22]. Tensor-based morphometry with symmetric normalization was used to evaluate for changes in ventricular volumes after treatment in patients with IIH. For tensor-based morphometry with symmetric normalization, all MRI examinations performed in a given patient were intensity-matched, coregistered, and warped to each other to estimate change over time, reducing measurement variability and improving accuracy in longitudinal measurement compared with the use of independently determined atlas volumes [16, 25, 26].
Statistical Analyses
Data were summarized descriptively using counts, percentages, medians, and IQR; MRI measures were compared between patients with IIH at baseline and control individuals. Age and BMI were compared between groups using the Wilcoxon rank sum test, and sex was compared using the chi-square test. A linear regression model, adjusted for age and sex, was used to compare groups in terms of pituitary height, ventricular size, MRE pattern score, and stiffness in the nine brain regions. The Wilcoxon signed rank test was used to assess for changes in MRI features after intervention in the acute and chronic intervention subgroups, comparing postinterventional MRI examinations with baseline examinations only for patients in the given subgroup. Comparisons involving brain stiffness were performed separately for the nine regions, and the associated p values were corrected for the false-discovery rate [27] to account for multiple comparisons. In patients with IIH, baseline occipital lobe stiffness was compared between patients with versus without baseline transverse sinus stenosis using the Wilcoxon signed rank test. In patients with IIH, Spearman correlation was used to assess for an association of LP opening pressure with baseline pituitary height, stiffness pattern score, and occipital lobe stiffness. Finally, interreader agreement of the pituitary height was assessed by the intraclass correlation coefficient (ICC) [28], and interreader agreement of the qualitative MRI measures was calculated by Cohen kappa coefficient [29].
Statistical significance was defined as p < .05. Statistical analysis was performed using MATLAB (version R2018a).
Results
Study Participants and Interventions
During the study period, 44 patients with papilledema and clinically suspected IIH were screened for study eligibility. Nine patients were deemed ineligible (not meeting 2013 revised diagnostic criteria in four patients, age < 18 years in three, pregnancy in one, MRI contraindication in one). The remaining 35 patients were approached for consent. Of these, two patients declined participation, and 33 were enrolled. Of those who enrolled, one patient was later excluded because the diagnosis changed from papilledema in the context of IIH to pseudopapilledema without an IIH diagnosis, and two patients were excluded because they were too large to be imaged by the available MRI scanner. After these exclusions, the IIH group included 30 patients (28 [93%] women, two [7%] men). In addition, 21 individuals (21 [100%] women, 0 [0%] men) met all eligibility criteria for and were enrolled into the control group. Figure 1 shows the flow of participant recruitment.
Fig. 1—

Flowchart shows patient selection. Patients with idiopathic intracranial hypertension (IIH) in acute intervention subgroup underwent large-volume diagnostic lumbar puncture after enrollment with closing pressure of less than 250 mm H2O. Patients with IIH in chronic intervention subgroup underwent medical management or venous sinus stenting with resolution or substantial improvement in papilledema. ICP = intracranial pressure.
Median age was not significantly different between patients with IIH (29.2 years [IQR, 27.0–38.3 years]) and control individuals (29.1 years [IQR, 22.5–43.3 years]) (p = .60). Median BMI was significantly greater in patients with IIH (37.8 [IQR, 33.5–44.6]) than in control individuals (26.6 [IQR, 23.7–31.4]) (p < .001). Median baseline Frisen papilledema score in patients with IIH was 2.0 (IQR, 1.3–3.0). In patients with IIH, the baseline MRI was performed at a median of 5 months (IQR, 2–19 months) after symptom onset. Further demographic and clinical characteristics of the control individuals and patients with IIH are detailed in Table 1.
TABLE 1:
Baseline Demographic and Clinical Characteristics in Study Groups and Subgroups
| Characteristic | Control Individuals (n = 21) | All Patients With IIH at Baseline (n = 30) | Acute Interventiona Subgroup at Baseline (n = 11) | Chronic Interventionb Subgroup at Baseline (n = 12) |
|---|---|---|---|---|
|
| ||||
| Sex | ||||
| Female | 100 (21/21) | 93 (28/30) | 91 (10/11) | 92 (11/12) |
| Male | 0 (0/21) | 7 (2/30) | 9 (1/11) | 8 (1/12) |
| Age (y) | 29.1 (22.5–43.3) | 29.9 (27.0–38.3) | 34.9 (27.0–38.3) | 29.8 (25.5–39.7) |
| BMI | 26.6 (23.7–31.4) | 37.8 (33.5–44.6) | 35.8 (33.1–38.5) | 37.8 (32.4–44.9) |
| Frisen grade of papilledema | — | 2.0 (1.3–3.0) | 2.0 (1.0–3.0) | 2.3 (1.0–3.0) |
| Lumbar puncture opening pressure (mm H2O)c | — | 361 (278–498) | 361 (270–420) | 448 (310–502) |
Note—Data are reported as percentage with numerator and denominator in parentheses or as median with IQR in parentheses. Dash (—) indicates data not available. IIH = idiopathic intracranial hypertension.
Large-volume diagnostic lumbar puncture.
Medical management or venous sinus stenting.
Available in 22 patients with IIH at baseline, including 10 in the acute intervention subgroup and 10 in the chronic intervention subgroup.
Large-volume diagnostic LP was performed in 12 patients with IIH, resulting in a closing pressure of less than 250 mm H2O in 11 patients; those 11 patients underwent MRI after the LP and formed the acute intervention subgroup. Chronic intervention was performed in 16 patients with IIH, resulting in resolution or substantial improvement in papilledema in 12; those 12 patients formed the chronic intervention subgroup. Five patients underwent both acute and chronic intervention; 18 unique patients underwent acute and/or chronic intervention. Of the 12 patients in the chronic intervention subgroup, six (50%) received medical management, and six (50%) underwent venous sinus stenting. In the chronic intervention subgroup, MRI was performed at a median of 10.5 months (IQR, 8.0–14.5 months) after initiation of medical management or venous sinus stenting.
Comparisons of MRI Findings Between Groups and Subgroups
Table 2 compares the MRI features between patients with IIH and control individuals, and Tables 3 and 4 compare the features between patients with IIH at baseline and after acute and chronic intervention, respectively. Figure 2 shows the distributions of quantitative MRI metrics across the study groups and subgroups.
TABLE 2:
Comparison of MRI Features Between Patients With IIH at Baseline and Control Individuals
| MRI Feature | Patients With IIH (n = 30) | Control Individuals (n = 21) | p a |
|---|---|---|---|
|
| |||
| Pituitary height (mm) | 3.1 (2.3–3.9) | 4.9 (3.9–5.9) | < .001 |
| Ventricular volume (mm3) | 15,430 (13,195–19,078) | 12,533 (10,276–17,810) | .33 |
| Stiffness pattern score | 0.25 (0.18–0.33) | 0.15 (0.09–0.18) | < .001 |
| Regional stiffness (kPa) | |||
| Frontal lobe | 3.04 (2.91–3.14) | 3.05 (2.95–3.23) | .18 |
| Occipital lobe | 3.08 (2.96–3.26) | 2.94 (2.85–3.14) | .07 |
| Parietal lobe | 2.86 (2.73–3.00) | 2.88 (2.75–2.95) | .11 |
| Temporal lobe | 3.11 (3.02–3.21) | 3.10 (3.01–3.23) | .11 |
| GMWM junction | 3.41 (3.21–3.57) | 3.36 (3.25–3.54) | .16 |
| Corpus callosum | 2.85 (2.63–3.23) | 3.07 (2.77–3.20) | .14 |
| Cerebellum | 2.04 (1.97–2.12) | 2.01 (1.97–2.09) | .12 |
| Brainstem | 3.77 (3.59–4.08) | 3.92 (3.72–4.11) | .14 |
| Global regionb | 2.91 (2.83–3.04) | 2.95 (2.80–3.03) | .13 |
| Transverse sinus stenosisc | 89 (24/27) | 5 (1/19) | — |
| Inferior cerebellar tonsillar descent | 7 (2/30) | 0 (0/21) | — |
Note—Data are reported as median with IQR in parentheses or as percentage with numerator and denominator in parentheses. Dash (—) indicates not calculated. IIH = idiopathic intracranial hypertension, GMWM = gray matter–white matter.
All values were adjusted for age and sex. Values for regional stiffness were corrected for the false-discovery rate.
Includes all eight regions.
The denominator is less than the total number of patients in a given group due to the lack of MR venography sequence in the MRI examinations of some patients.
TABLE 3:
Comparison of MRI Features at Baseline and After Acute Intervention in 11 Patients With IIH
| MRI Feature | Baseline | After Acute Interventiona | p b |
|---|---|---|---|
|
| |||
| Pituitary height (mm) | 2.4 (1.9–3.1) | 2.3 (1.7–3.6) | .50 |
| Ventricular volume (mm3) | 13,958 (8194–17,161) | 12,270 (8036–15,340) | .83 |
| Stiffness pattern score | 0.22 (0.17–0.26) | 0.25 (0.17–0.32) | .49 |
| Regional stiffness (kPa)b | |||
| Frontal lobe | 2.94 (2.91–3.14) | 2.98 (2.86–3.15) | .97 |
| Occipital lobe | 3.13 (2.98–3.34) | 3.24 (2.95–3.43) | .73 |
| Parietal lobe | 2.89 (2.83–3.04) | 2.93 (2.86–3.08) | > .99 |
| Temporal lobe | 3.17 (3.03–3.26) | 3.10 (3.03–3.32) | > .99 |
| GMWM junction | 3.36 (3.21–3.57) | 3.37 (3.32–3.62) | .70 |
| Corpus callosum | 2.85 (2.64–3.07) | 2.92 (2.77–3.17) | .96 |
| Cerebellum | 2.04 (1.98–2.09) | 2.08 (2.03–2.19) | .93 |
| Brainstem | 3.80 (3.59–4.23) | 3.74 (3.33–4.22) | > .99 |
| Global regionc | 2.96 (2.85–3.04) | 2.95 (2.88–3.14) | .97 |
| Transverse sinus stenosisd | 88 (7/8) | 88 (7/8) | — |
| Inferior cerebellar tonsillar descent | 9 (1/11) | 9 (1/11) | — |
Note—Data are reported as median with IQR in parentheses or as percentage with numerator and denominator in parentheses. Dash (—) indicates not calculated. IIH = idiopathic intracranial hypertension, GMWM = gray matter–white matter.
Large-volume diagnostic lumbar puncture.
Calculated using the Wilcoxon signed rank test. Values for regional stiffness were corrected for the false-discovery rate.
Includes all eight regions.
The denominator is less than the total number of patients in a given group due to the lack of MR venography sequence in the MRI examinations of some patients.
TABLE 4:
Comparison of MRI Features at Baseline and After Chronic Intervention in 12 Patients With IIH
| MRI Feature | Baseline | After Chronic Interventiona | p b |
|---|---|---|---|
|
| |||
| Pituitary height (mm) | 3.2 (2.5–3.5) | 4.0 (2.5–4.6) | .05 |
| Ventricular volume (mm3) | 14,840 (13,489–19,765) | 15,119 (13,602–19,549) | .97 |
| Stiffness pattern score | 0.25 (0.20–0.34) | 0.23 (0.19–0.28) | .11 |
| Regional stiffness (kPa) | |||
| Frontal lobe | 3.04 (2.90–3.11) | 3.02 (2.96–3.20) | .46 |
| Occipital lobe | 3.03 (2.96–3.28) | 3.10 (3.00–3.22) | .83 |
| Parietal lobe | 2.89 (2.76–2.99) | 2.85 (2.79–2.98) | > .99 |
| Temporal lobe | 3.09 (3.00–3.17) | 3.05 (2.99–3.17) | > .99 |
| GMWM junction | 3.41 (3.27–3.62) | 3.30 (3.26–3.48) | .52 |
| Corpus callosum | 2.85 (2.66–3.02) | 2.90 (2.65–3.18) | .87 |
| Cerebellum | 1.98 (1.92–2.03) | 2.05 (1.96–2.08) | .68 |
| Brainstem | 3.65 (3.35–4.01) | 3.76 (3.31–4.24) | .87 |
| Global regionc | 2.91 (2.82–3.02) | 2.95 (2.82–3.02) | .51 |
| Transverse sinus stenosisd | 100 (11/11) | 36 (4/11) | — |
| Inferior cerebellar tonsillar descent | 17 (2/12) | 17 (2/12) | — |
Note—Data are reported as median with IQR in parentheses or as percentage with numerator and denominator in parentheses. Dash (—) indicates not calculated. IIH = idiopathic intracranial hypertension, GMWM = gray matter–white matter.
Medical management or venous sinus stenting.
Calculated using the Wilcoxon signed rank test. Values for regional stiffness were corrected for the false-discovery rate.
Includes all eight regions.
The denominator is less than the total number of patients in a given group due to the lack of MR venography sequence in the MRI examinations of some patients.
Fig. 2—
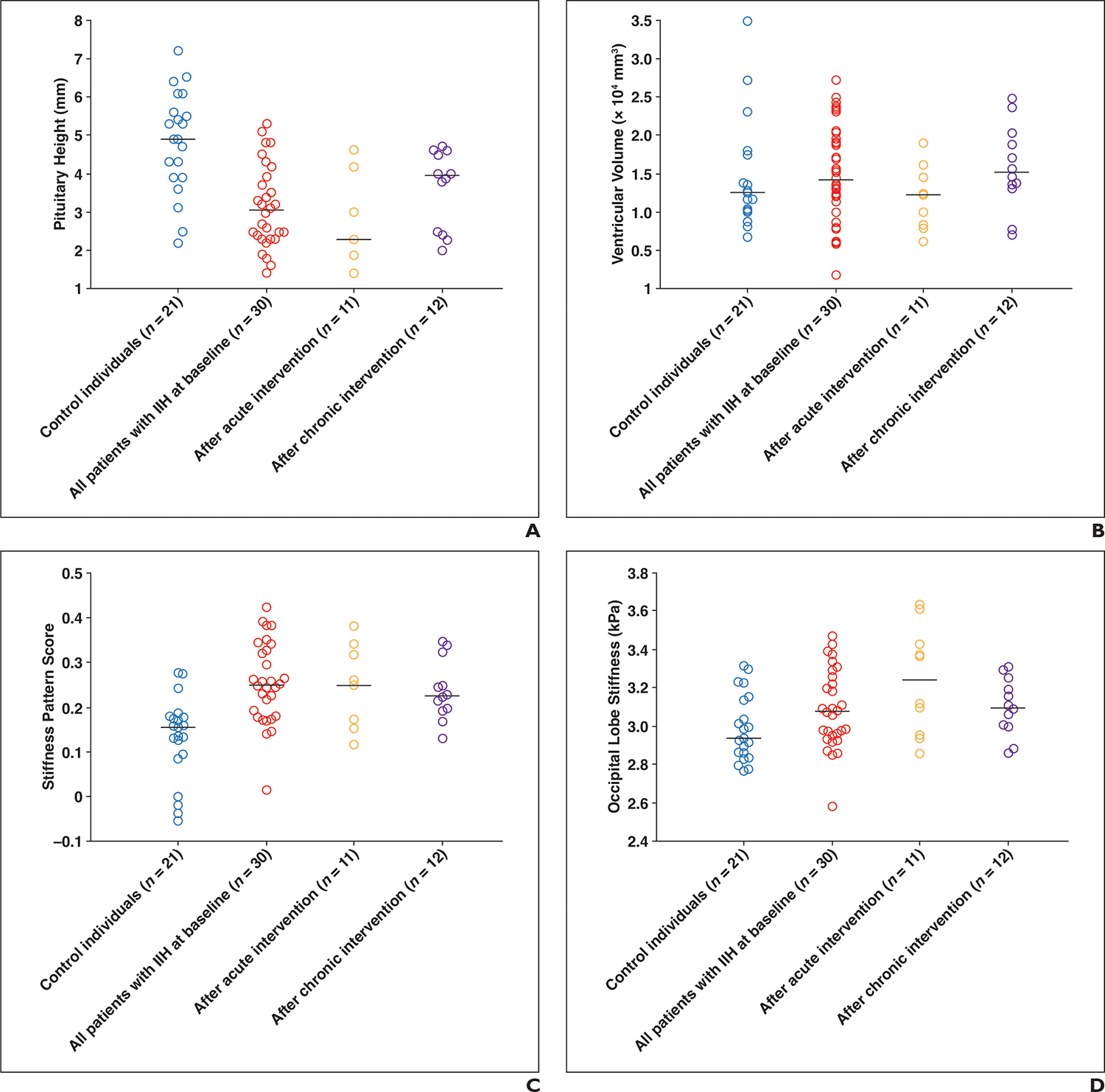
Dot plots of quantitative MRI metrics across study groups and subgroups.
A–D, Dot plots of pituitary height (A), ventricular size (B), MR elastography (MRE) stiffness pattern (C), and MRE occipital region stiffness (D). Each dot represents one participant, and horizontal lines mark medians of groups and subgroups. Acute intervention refers to large-volume diagnostic lumbar puncture, and chronic intervention refers to medical management or venous sinus stenting. IIH = idiopathic intracranial hypertension.
The median pituitary height was significantly lower in patients with IIH at baseline than in control individuals (3.1 mm [IQR, 2.3–3.9 mm] vs 4.9 mm [IQR, 3.9–5.9 mm]) (p < .001). In patients with IIH, the median pituitary gland height increased from 3.2 mm (IQR, 2.5–3.5 mm) at baseline to 4.0 mm (IQR, 2.5–4.6 mm) after chronic treatment (p = .05) but did not significantly change between baseline (2.4 mm [IQR, 1.9–3.1 mm]) and after acute intervention (2.3 mm [IQR, 1.7–3.6 mm]) (p = .50). Although the pituitary height increased after chronic intervention, it remained significantly smaller than in control individuals (p = .01).
The median ventricular volume was not significantly different between patients with IIH at baseline and control individuals (15,430 mm3 [IQR, 13,195–19,078 mm3] vs 12,533 mm3 [IQR, 10,276–17,810 mm3]) (p = .33) and did not significantly change after acute (p = .83) or chronic (p = .97) intervention.
On MRE, the median stiffness pattern score was significantly greater in patients with IIH than in control individuals (0.25 [IQR, 0.18–0.33] vs 0.15 [IQR, 0.09–0.18]) (p < .001). In patients with IIH, the median stiffness pattern score did not significantly change from baseline to after acute intervention (0.22 [IQR, 0.17–0.26] vs 0.25 [IQR, 0.17–0.32]) (p = .49) but decreased from 0.25 (IQR, 0.20–0.34) at baseline to 0.23 (IQR, 0.19–0.28) after chronic intervention (p = .11). The occipital lobe stiffness was greater in patients with IIH than in control individuals (3.08 kPa [IQR, 2.96–3.26 kPa] vs 2.94 kPa [IQR, 2.85–3.14 kPa]), although this difference was not statistically significant (p = .07). In patients with IIH, the occipital region stiffness did not significantly change from baseline (3.13 kPa [IQR, 2.98–3.34 kPa]) to acute intervention (3.24 kPa [IQR, 2.95–3.43 kPa]) (p = .73) or from baseline (3.03 kPa [IQR, 2.96–3.28 kPa]) to chronic intervention (3.10 kPa [IQR, 3.00–3.22 kPa]) (p = .83). Other regions showed variable nonsignificant differences in stiffness between patients with IIH and control individuals and among patients with IIH after acute and chronic interventions (all p > .05) (Tables 3 and 4).
Baseline MRV was not available for three patients and two control individuals due to technical error or inability to complete the full MRI examination. Transverse sinus stenosis was present in 24 of 27 (89%) patients with IIH at baseline compared with one of 19 (5%) control individuals. In patients with IIH, the median stiffness pattern score was greater in patients with versus those without transverse sinus stenosis, although this difference was not statistically significant (0.25 [IQR, 0.20–0.33] vs 0.17 [IQR, 0.01–0.42]) (p = .56). The median occipital lobe stiffness did not differ between patients with versus those without transverse sinus stenosis (3.07 kPa [IQR, 2.94–3.24 kPa] vs 3.09 kPa [IQR, 2.97–3.10 kPa]) (p > .99). Transverse sinus stenosis was present in seven of eight (88%) patients with IIH after acute intervention and in four of 11 (36%) after chronic intervention, resolving after medical management in one in five (20%) patients and after venous sinus stenting in six of six (100%) patients. In the six patients with transverse sinus stenosis who underwent venous sinus stenting, the median stiffness pattern score was 0.28 (IQR, 0.19–0.34) at baseline and 0.20 (IQR, 0.19–0.32) after chronic intervention, and the median occipital lobe stiffness was 3.09 (IQR, 2.98–3.26) at baseline and 3.10 (IQR, 3.06–3.25) after chronic intervention.
The baseline frequency of inferior cerebellar descent among patients with IIH was two of 30 (7%) patients, including one of 11 (9%) patients in the acute intervention and two of 12 (17%) patients in the chronic intervention subgroups and was unchanged after intervention in all patients. No control individual showed inferior cerebellar tonsillar descent.
Baseline opening pressure measurements were available for 22 of 30 patients with IIH. In these patients, the opening pressure did not show a significant correlation with pituitary height (ρ = −0.01; p = .98), stiffness pattern score (ρ = 0.27; p = .22), or occipital lobe stiffness (ρ = 0.02; p = .93).
Figures 3–5 and Figure S1 (available in the online supplement) show patients with IIH in whom the pituitary height increased after venous sinus stenting, occipital lobe stiffness decreased after venous sinus stenting, transverse sinus stenosis resolved after medical management, and transverse sinus stenosis persisted after medical management, respectively.
Fig. 3—
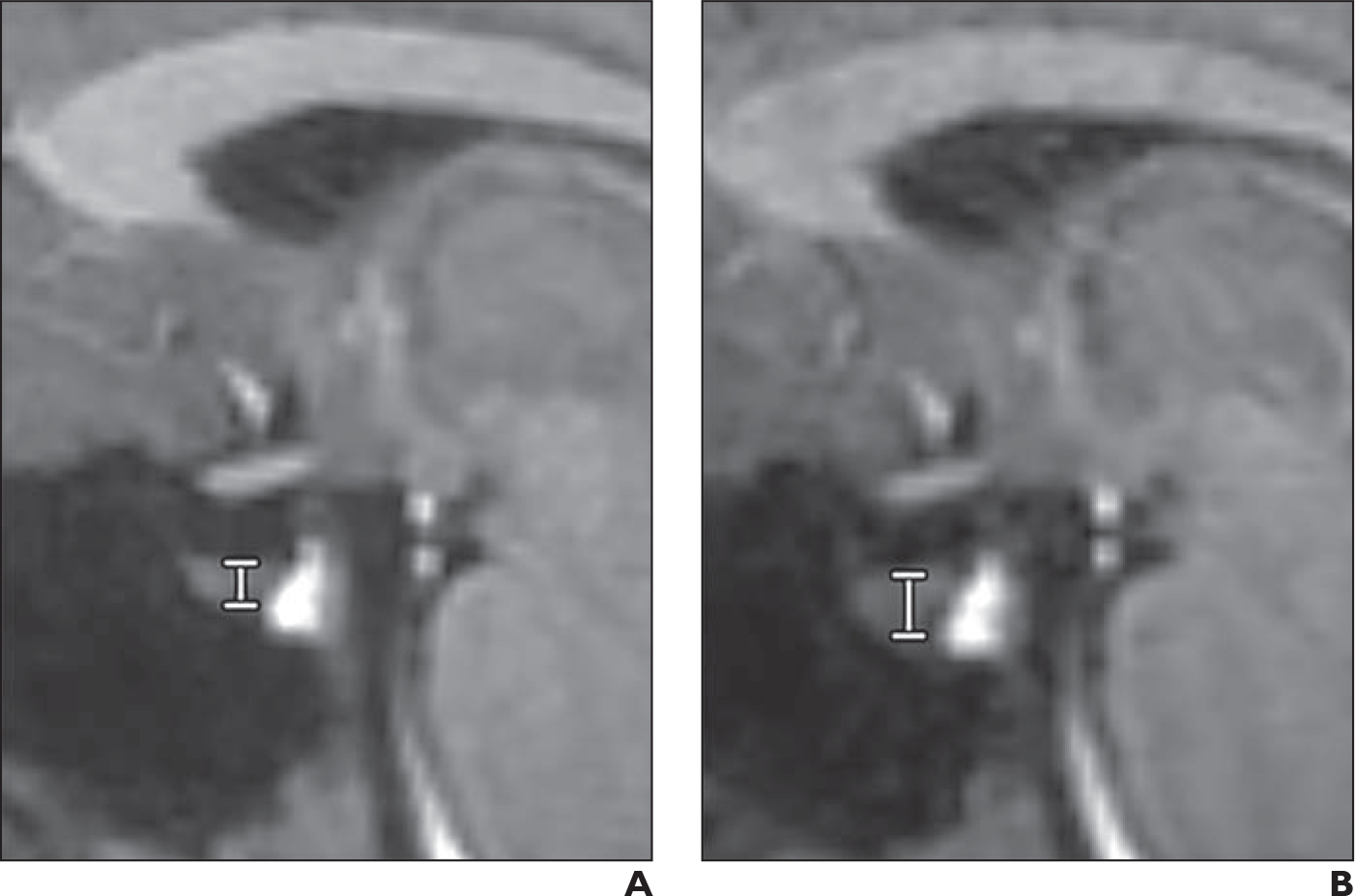
28-year-old woman with idiopathic intracranial hypertension.
A and B, Sagittal T1-weighted MP-RAGE images at baseline (A) and after chronic intervention by venous sinus stenting (B). Measurement of pituitary height (vertical line between horizontal lines marking top and bottom of pituitary) is depicted on both images. Pituitary height increased from 3.2 mm at baseline to 4.6 mm after chronic intervention.
Fig. 5—

26-year-old woman with idiopathic intracranial hypertension.
A and B, Baseline MRI. Axial source image (A) and 3D maximum-intensity-projection (MIP) image (B) from phase-contrast MR venography (MRV) show marked narrowing of lateral aspect of transverse sinuses bilaterally (arrows), consistent with transverse sinus stenosis.
C and D, Postintervention MRI. Patient underwent medical management with substantial improvement of papilledema. Axial source image (C) and 3D MIP image (D) from phase-contrast MRV show resolution of transverse sinus stenosis (arrows).
Interreader Agreement
Interreader agreement was 0.94 (ICC) for pituitary height, 0.91 (κ) for transverse sinus stenosis, and 0.66 (κ) for inferior cerebellar tonsillar descent.
Discussion
In this study, we evaluated established MRI features of IIH of pituitary height and ventricular size as well as a less studied feature of brain stiffness. The pituitary gland was smaller in patients with IIH compared with control individuals and increased in size after chronic but not acute intervention with reduction in ICP. However, ventricular size was not different between patients with IIH and control individuals and did not change after intervention. Moreover, the MRE stiffness pattern score and occipital lobe stiffness were greater in patients with IIH than in control individuals, but only the stiffness pattern score decreased after intervention and only after chronic intervention.
As previously reported, pituitary gland height was smaller in patients with IIH compared with control individuals [3, 4]. This difference has been hypothesized to represent gland deformation from chronically increased ICP. The increase in pituitary height after chronic but not acute intervention to reduce ICP supports the concept that changes in gland deformation are slow to manifest [30], such that time is needed for the gland to rebound from the effects of raised ICP. The finding suggests that an increase in pituitary height may serve as a measure of successful IIH treatment. However, despite the increase in pituitary height after chronic intervention to lower ICP, the gland remained smaller than in control individuals. Similar findings have been previously reported [13, 31, 32] and have been attributed to permanent sellar expansion after resolution of the direct effects of increased ICP. Thus, follow-up MRI after normalization of a previously elevated ICP may still show a partially empty sella. On the other hand, one study showed no detectable change in pituitary gland size after IIH treatment [11], possibly due to a shorter follow-up period (median, 6 months in that study [11] vs 10.5 months in the current study) or differences in pituitary measurement technique.
Although small slitlike ventricles have been described as an MRI feature of IIH, in the current study the ventricular size, adjusted for age and sex, was not significantly different between patients with IIH and control individuals and did not significantly change after treatment. Prior studies found similar results [11, 14, 33]. The lack of a difference in ventricular size may reflect the small scale of disease-related changes in ventricular size, being of a magnitude similar to that of variation in normal ventricular size.
The baseline frequencies of transverse sinus stenosis and inferior cerebellar tonsillar descent were similar to those reported in prior studies [34–36]. Inferior cerebellar tonsillar descent was infrequent in patients with IIH and was not present in any control individual. This MRI finding is not sensitive or specific and is not included in current IIH diagnostic guidelines [8]. Transverse sinus stenosis was present in most patients with IIH and in only one control individual, similar to this finding’s distribution in other studies [35, 36]. Although transverse sinus stenosis has been reported to improve after large-volume LP [37], in the current study transverse sinus stenosis persisted in all but one patient after acute intervention and in all but one patient after medical management. In one patient, transverse sinus stenosis improved after medical management, supporting the possibility that transverse sinus stenosis is a result, rather than a cause, of raised ICP [38, 39]. MRV was performed using a velocity-dependent phase-contrast technique that may show apparent stenosis that in fact represents slow flow or arachnoid granulations. The transverse sinus narrowing in the one control individual may have been due to such phenomena or due to uncommon transverse venous sinus stenosis without raised ICP [36, 40].
MRE showed a difference in stiffness pattern between patients with IIH and control individuals and a nonsignificant increase in regional stiffness in the occipital lobe of patients with IIH. Kolipaka et al. [41] also reported greater brain stiffness in patients with IIH compared with control individuals, although their study found a greater global stiffness in IIH, which was not observed in the current study. The difference in findings may relate to differences in the studied populations or in acquisition and processing techniques. The regional increase in occipital lobe stiffness in IIH suggests nonuniformity in disease-related changes. Similarly, nonuniformity or asymmetry of elevated ICP may be suggested clinically by asymmetric papilledema and through diagnostic testing by focal narrowing of the lateral aspect of the transverse sinuses and asymmetric pressure gradients across transverse sinus stenoses [42, 43]. The regional proximity of the stiffer occipital lobes and the localized narrowing of the lateral aspect of the transverse sinuses in IIH raise the possibility of associated mechanisms for these findings, as well as a predilection of the posterior inferior aspects of the brain to the effects of raised ICP.
The lack of change in occipital lobe stiffness after chronic intervention with resolution of papilledema suggests that increased occipital stiffness may be a predisposing factor to IIH rather than a consequence of it. Indeed, if increased pressure in veins draining the occipital lobes were to be the cause of increased stiffness, then stiffness would have been anticipated to decrease after stenting in patients with transverse sinus stenosis, which was not observed. On the other hand, stiffness pattern scores decreased after chronic intervention, supporting increased ICP as a cause of elevated stiffness. It is possible that the elevated occipital lobe stiffness had originally been caused by elevated ICP but was no longer reversible or would have required additional time to normalize beyond the follow-up period. Whether regional brain stiffness changes in IIH are a cause or effect of chronically increased ICP remains unclear and will likely require larger studies with longer durations of monitoring before and after treatment.
Brain stiffness did not change after acute intervention, similar to a prior study’s result [41]. However, acute increases in ICP have been associated with increased brain stiffness in animals and in humans with normal baseline ICP [19, 44]. Thus, the brain may exhibit different mechanical responses to acute versus chronic changes in ICP.
There are limitations to this study. The acute and chronic intervention subgroups were small, limiting the ability to detect statistically significant differences. In addition, the chronic intervention subgroup included patients treated by either medical management or venous sinus stenting; an insufficient number of patients underwent these individual treatments to support more granular subgroup analyses by type of chronic intervention. Despite the difference in treatment, all patients in the chronic intervention subgroup showed resolution or substantial improvement of papilledema. Furthermore, the pituitary gland was measured using simple linear assessments of height. Volumetric measurements may have provided greater sensitivity to subtle changes in pituitary deformation. Multiple examinations in individual patients were also reviewed concurrently, indicating that such individuals had IIH (rather than being in the control group). Also, the MRE measurements were performed using a standard clinical technique. In a previous study of acute ICP changes in animals, MRE performed using relatively higher frequencies was more sensitive to the nonlinear mechanical effects of tissue compression [19]. Thus, use of comparable higher frequencies and resolutions for MRE in clinical settings may help detect additional IIH- and treatment-related effects.
Conclusion
The pituitary gland was smaller in patients with IIH than in control individuals. In patients with IIH, the pituitary increased in size after chronic, but not acute, intervention with improvement or substantial reduction in ICP, likely representing a gradual recovery from chronic deformation. Ventricular size was not different between patients with IIH and control individuals and did not change in patients with IIH after acute or chronic intervention, indicating that slitlike ventricles is not a reliable feature of IIH. Based on MRE findings, patients with IIH showed greater regional stiffness in the occipital lobes compared with control individuals, representing either a sequela of or a predisposing factor to raised ICP. The findings support pituitary height and the MRE stiffness pattern score as established and novel indirect indicators, respectively, of raised ICP that change after interventions to lower ICP. Further evaluation of regional stiffness changes is warranted to improve understanding of the pathophysiologic mechanisms underlying IIH and treatment response.
Supplementary Material
Fig. 4—

28-year-old patient with idiopathic intracranial hypertension.
A and B, Stiffness maps from MR elastography at baseline (A) and 6 months after venous sinus stenting (B) visually show increased occipital lobe stiffness bilaterally at baseline and reduction in occipital lobe stiffness after intervention. Occipital lobe stiffness measured 3.20 kPa at baseline and 3.06 kPa after chronic intervention.
Key Finding
Pituitary height was smaller in patients with IIH than control individuals (3.1 vs 4.9 mm, p < .001) and increased after chronic intervention (4.0 mm, p = .05). Brain stiffness pattern score was also greater in IIH than control individuals (0.25 vs 0.15, p < .001) and decreased after chronic intervention (0.23, p = .05).
Importance
Pituitary height and the MRE-derived brain stiffness pattern score are established and novel indicators, respectively, of elevated ICP that change after interventions to lower ICP.
Acknowledgments
Supported by the NIH (R01 EB027064 and R37 EB001981) and the Theodore W. Batterman Family Foundation, Inc.
Footnotes
M. C. Murphy, J. Huston III, and Mayo Clinic have a financial interest in MRE technology. The remaining authors declare that there are no other disclosures relevant to the subject matter of this article.
Based on a presentation at the Association for Research in Vision and Ophthalmology 2019 annual meeting, Vancouver, BC, Canada.
References
- 1.Wall M Idiopathic intracranial hypertension. Neurol Clin 2010; 28:593–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J, Wall M, Epidemiology and risk factors for idiopathic intracranial hypertension. Int Ophthalmol Clin 2014; 54:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agid R, Farb RI, Willinsky RA, Mikulis DJ, Tomlinson G. Idiopathic intracranial hypertension: the validity of cross-sectional neuroimaging signs. Neuroradiology 2006; 48:521–527 [DOI] [PubMed] [Google Scholar]
- 4.Brodsky MC, Vaphiades M. Magnetic resonance imaging in pseudotumor cerebri. Ophthalmology 1998; 105:1686–1693 [DOI] [PubMed] [Google Scholar]
- 5.Viets R, Parsons M, Van Stavern G, Hildebolt C, Sharma A. Hyperintense optic nerve heads on diffusion-weighted imaging: a potential imaging sign of papilledema. AJNR 2013; 34:1438–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degnan AJ, Levy LM . Pseudotumor cerebri: brief review of clinical syndrome and imaging findings. AJNR 2011; 32:1986–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aiken AH, Hoots JA, Saindane AM, Hudgins PA. Incidence of cerebellar tonsillar ectopia in idiopathic intracranial hypertension: a mimic of the Chiari I malformation. AJNR 2012; 33:1901–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 2013; 81:1159–1165 [DOI] [PubMed] [Google Scholar]
- 9.Fisayo A, Bruce BB, Newman NJ, Biousse V. Overdiagnosis of idiopathic intracranial hypertension. Neurology 2016; 86:341–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang F, Lesser ER, Cutsforth-Gregory JK, et al. Population-based evaluation of lumbar puncture opening pressures. Front Neurol 2019; 10:899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belachew NF, Almiri W, Encinas R, et al. Evolution of MRI findings in patients with idiopathic intracranial hypertension after venous sinus stenting. AJNR 2021; 42:1993–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zagardo MT, Cail WS, Kelman SE, Rothman MI. Reversible empty sella in idiopathic intracranial hypertension: an indicator of successful therapy? AJNR 1996; 17:1953–1956 [PMC free article] [PubMed] [Google Scholar]
- 13.Batur Caglayan HZ, Ucar M, Hasanreisoglu M, Nazliel B, Tokgoz N. Magnetic resonance imaging of idiopathic intracranial hypertension: before and after treatment. J Neuroophthalmol 2019; 39:324–329 [DOI] [PubMed] [Google Scholar]
- 14.Kwee RM, Kwee TC. Systematic review and meta-analysis of MRI signs for diagnosis of idiopathic intracranial hypertension. Eur J Radiol 2019; 116:106–115 [DOI] [PubMed] [Google Scholar]
- 15.Crook JE, Gunter JL, Ball CT, et al. Linear vs volume measures of ventricle size: relation to present and future gait and cognition. Neurology 2020; 94:e549–e556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vemuri P, Senjem ML, Gunter JL, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Accelerated vs. unaccelerated serial MRI based TBM-SyN measurements for clinical trials in Alzheimer’s disease. Neuroimage 2015; 113:61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mariappan YK, Glaser KJ, Ehman RL. Magnetic resonance elastography: a review. Clin Anat 2010; 23:497–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy MC, Huston J 3rd, Ehman RL. MR elastography of the brain and its application in neurological diseases. Neuroimage 2019; 187:176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arani A, Min H-K, Fattahi N, et al. Acute pressure changes in the brain are correlated with MR elastography stiffness measurements: initial feasibility in an in vivo large animal model. Magn Reson Med 2018; 79:1043–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foo TKF, Laskaris E, Vermilyea M, et al. Lightweight, compact, and high-performance 3T MR system for imaging the brain and extremities. Magn Reson Med 2018; 80:2232–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy MC, Huston J 3rd, Jack CR Jr, et al. Decreased brain stiffness in Alzheimer’s disease determined by magnetic resonance elastography. J Magn Reson Imaging 2011; 34:494–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005; 26:839–851 [DOI] [PubMed] [Google Scholar]
- 23.Murphy MC, Manduca A, Trzasko JD, Glaser KJ, Huston J 3rd, Ehman RL. Artificial neural networks for stiffness estimation in magnetic resonance elastography. Magn Reson Med 2018; 80:351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy MC, Cogswell PM, Trzasko JD, et al. Identification of normal pressure hydrocephalus by disease-specific patterns of brain stiffness and damping ratio. Invest Radiol 2020; 55:200–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashburner J, Ridgway GR. Symmetric diffeomorphic modeling of longitudinal structural MRI. Front Neurosci 2013; 6:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cash DM, Frost C, Iheme LO, et al. Assessing atrophy measurement techniques in dementia: results from the MIRIAD atrophy challenge. Neuroimage 2015; 123:149–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storey JD. A direct approach to false discovery rates. J R Stat Soc Series B Stat Methodol 2002; 64:479–498 [Google Scholar]
- 28.Matthew RP. f_ICC. github.com/robertpetermatthew/f_ICC. GitHub website. Accessed June 23, 2022 [Google Scholar]
- 29.Layden E Cohen’s kappa. github.com/elayden/cohensKappa. GitHub website. Accessed June 23, 2022 [Google Scholar]
- 30.Yuh WT, Zhu M, Taoka T, et al. MR imaging of pituitary morphology in idiopathic intracranial hypertension. J Magn Reson Imaging 2000; 12:808–813 [DOI] [PubMed] [Google Scholar]
- 31.Suzuki H, Takanashi J, Kobayashi K, Nagasawa K, Tashima K, Kohno Y. MR imaging of idiopathic intracranial hypertension. AJNR 2001; 22:196–199 [PMC free article] [PubMed] [Google Scholar]
- 32.Ranganathan S, Lee SH, Checkver A, et al. Magnetic resonance imaging finding of empty sella in obesity related idiopathic intracranial hypertension is associated with enlarged sella turcica. Neuroradiology 2013; 55:955–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maralani PJ, Hassanlou M, Torres C, et al. Accuracy of brain imaging in the diagnosis of idiopathic intracranial hypertension. Clin Radiol 2012; 67:656–663 [DOI] [PubMed] [Google Scholar]
- 34.Banik R, Lin D, Miller NR. Prevalence of Chiari I malformation and cerebellar ectopia in patients with pseudotumor cerebri. J Neurol Sci 2006; 247:71–75 [DOI] [PubMed] [Google Scholar]
- 35.Riggeal BD, Bruce BB, Saindane AM, et al. Clinical course of idiopathic intracranial hypertension with transverse sinus stenosis. Neurology 2013; 80:289–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farb RI, Vanek I, Scott JN, et al. Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology 2003; 60:1418–1424 [DOI] [PubMed] [Google Scholar]
- 37.Scoffings DJ, Pickard JD, Higgins NP. Resolution of transverse sinus stenoses immediately after CSF withdrawal in idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry 2007; 78:911–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King JO, Mitchell PJ, Thomson KR, Tress BM. Manometry combined with cervical puncture in idiopathic intracranial hypertension. Neurology 2002; 58:26–30 [DOI] [PubMed] [Google Scholar]
- 39.Corbett JJ, Digre K. Idiopathic intracranial hypertension: an answer to, “the chicken or the egg?” Neurology 2002; 58:5–6 [DOI] [PubMed] [Google Scholar]
- 40.Kelly LP, Saindane AM, Bruce BB, et al. Does bilateral transverse cerebral venous sinus stenosis exist in patients without increased intracranial pressure? Clin Neurol Neurosurg 2013; 115:1215–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolipaka A, Wassenaar PA, Cha S, et al. Magnetic resonance elastography to estimate brain stiffness: measurement reproducibility and its estimate in pseudotumor cerebri patients. Clin Imaging 2018; 51:114–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larson A, Rinaldo L, Chen JJ, Cutsforth-Gregory J, Theiler AR, Brinjikji W. Reductions in bilateral transverse sinus pressure gradients with unilateral transverse venous sinus stenting for idiopathic intracranial hypertension. J Neurointerv Surg 2021; 13:187–190 [DOI] [PubMed] [Google Scholar]
- 43.Ahmed RM, Wilkinson M, Parker GD, et al. Transverse sinus stenting for idiopathic intracranial hypertension: a review of 52 patients and of model predictions. AJNR 2011; 32:1408–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hatt A, Cheng S, Tan K, Sinkus R, Bilston LE. MR elastography can be used to measure brain stiffness changes as a result of altered cranial venous drainage during jugular compression. AJNR 2015; 36:1971–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


