Abstract
We have developed a PCR-based method for the subspecific discrimination of Aspergillus fumigatus types by using two primers designed to amplify the intergenic spacer regions between ribosomal DNA transcription units. The method permitted the reproducible discrimination of 11 distinct DNA types among a total of 119 isolates of A. fumigatus collected from patients and from the environment of a bone marrow transplantation (BMT) unit over a three-year period. Ten DNA types of A. fumigatus were isolated from patients in the BMT unit; eight of these types were also found in the hospital environment, and six of these were present in the unit itself. Thirteen BMT patients developed infection with one of three DNA types some months after these had first been found in the environment of the unit. In other instances, the same DNA types of A. fumigatus were isolated from BMT patients that were later recovered from the environment of the unit. Several DNA types of A. fumigatus were found in the hospital environment over an 18-month period. Molecular typing of multiple isolates of A. fumigatus, obtained from postmortem tissue samples, showed that one patient was infected with a single DNA type, but two others had up to three different DNA types. Our findings suggest that A. fumigatus infection in BMT recipients may be nosocomial in origin and underline the need for careful environmental monitoring of units in which high-risk patients are housed.
Aspergillus fumigatus is a ubiquitous saprobic fungus which can cause lethal infection in neutropenic individuals. The lung is the commonest site of human infection, most cases being the result of inhalation of spores. It is well recognized that nosocomial outbreaks of aspergillosis often occur in association with hospital demolition or construction works (3, 15, 30), and it has been assumed that this is due to the release of large numbers of spores into the air. Housing neutropenic patients in a protective environment to prevent exposure to aspergillus spores has reduced the incidence of aspergillosis (6, 25), but infection can still occur if air filtration systems fail or are inadequate (17, 24). Moreover, aspergillosis can develop if patients are colonized before their admission to a protective environment (23) or if they are moved to other parts of a hospital for irradiation (13) or for insertion of catheters (1).
The evidence incriminating different environmental sources of aspergillus infection has always been circumstantial because only recently have reliable molecular typing methods been developed for tracing the spread of particular subspecific types of A. fumigatus. Among the methods that have been applied to the typing of this organism are restriction endonuclease analysis (7, 9, 18, 29) and the detection of restriction fragments by Southern hybridization and probing with ribosomal or other repetitive sequences (2, 10, 11, 22, 26). The application of randomly amplified polymorphic DNA (RAPD) analysis as a genotyping method has also been reported (2, 4, 16, 18, 19, 22, 27–29).
This report describes the development of a simple PCR-based method for the subspecific discrimination of A. fumigatus strains by using primers which amplify intergenic spacer regions (IGS) between ribosomal DNA transcription units. The method was used to characterize isolates of A. fumigatus collected from patients and from the environment of a bone marrow transplantation (BMT) unit over a three-year period.
MATERIALS AND METHODS
Isolates.
A total of 119 isolates of A. fumigatus were collected between March 1994 and January 1997 (Table 1). These comprised 52 isolates from 25 patients undergoing BMT at the Bristol Royal Hospital for Sick Children, 7 isolates from 4 patients in other parts of the hospital, 19 isolates from the environment of the BMT unit, and 41 isolates from other parts of the hospital. Isolates were confirmed as A. fumigatus on the basis of their macroscopic and microscopic characteristics in culture. Isolates were stored on slopes of Oxoid Sabouraud dextrose agar (Unipath Ltd., Basingstoke, England) at −20°C and in vials of sterile water at room temperature.
TABLE 1.
DNA types of 119 clinical and environmental A. fumigatus isolatesa
| Isolate no. | Source | Isolation date (day-mo-yr) | DNA type |
|---|---|---|---|
| AF1 | Patient 1: nasal swab | 8-3-94 | F |
| AF2 | Environment | 24-5-95 | F |
| AF3 and 4 | Environment: BMT | 13-12-95 | F |
| AF5 and 6 | Environment | 12-1-96 | F |
| AF7 | Environment: BMT | 4-3-96 | F |
| AF8 | Environment | 12-7-96 | F |
| AF9 | Environment | 24-7-96 | F |
| AF10 | Patient 2: BF | 22-3-94 | H |
| AF11 | Patient 3: nasal swab | 30-3-94 | H |
| AF12 | Patient 1: nasal swab | 5-4-94 | H |
| AF13 | Patient 4: nasal swab | 5-4-94 | H |
| AF14 | Patient 5: nasal swab | 27-4-94 | H |
| AF15 | Patient 6: BF | 19-5-94 | H |
| AF16 | Patient 7: PeF (non-BMT) | 15-6-94 | H |
| AF17 | Patient 8: sputum | 22-6-94 | H |
| AF18 | Environment | 17-5-95 | H |
| AF19 and 20 | Environment | 28-6-95 | H |
| AF21 | Environment: BMT | 7-7-95 | H |
| AF22 and 23 | Environment: BMT | 27-9-95 | H |
| AF24 | Patient 23: BF | 8-12-95 | H |
| AF25 | Patient 25: PM kidney | 28-12-95 | H |
| AF26 and 27 | Patient 25: PM spleen | 28-12-95 | H |
| AF28 | Patient 25: PM lung | 28-12-95 | H |
| AF29 | Patient 22: BF | 9-1-96 | H |
| AF30 | Environment | 1-3-96 | H |
| AF31 and 32 | Environment: BMT | 1-3-96 | H |
| AF33 | Environment | 12-7-96 | H |
| AF34 | Patient 7: PeF (non-BMT) | 15-6-94 | B |
| AF35 | Patient 9: sputum (non-BMT) | 30-11-94 | D |
| AF36 | Environment: BMT | 4-12-94 | D |
| AF37 | Patient 12: BF | 4-4-95 | D |
| AF38 | Patient 13: PF | 11-4-95 | D |
| AF39 | Environment | 24-5-95 | D |
| AF40 | Environment | 28-6-95 | D |
| AF41 | Environment | 14-7-95 | D |
| AF42 | Patient 20: nasal swab | 11-9-95 | D |
| AF43 | Environment: BMT | 27-10-95 | D |
| AF44 | Environment | 12-1-96 | D |
| AF45 | Patient 26: sputum | 2-2-96 | D |
| AF46 | Patient 27: sputum | 14-2-96 | D |
| AF47 | Patient 26: sputum | 15-2-96 | D |
| AF48 | Environment: BMT | 4-3-96 | D |
| AF49 | Environment: BMT | 12-7-96 | D |
| AF50 | Environment | 12-7-96 | D |
| AF51 to 53 | Environment | 24-7-96 | D |
| AF54 | Patient 28: BF | 6-9-96 | D |
| AF55 | Patient 29: sputum (non-BMT) | 26-9-96 | D |
| AF56 | Patient 10: BF | 6-12-94 | A |
| AF57 | Patient 15: PM esophagus | 9-5-95 | A |
| AF58 | Patient 15: PM lung | 9-5-95 | A |
| AF59 to 62 | Environment | 17-5-95 | A |
| AF63 | Environment | 24-5-95 | A |
| AF64 to 66 | Environment | 28-6-95 | A |
| AF67 | Environment: BMT | 7-7-95 | A |
| AF68 | Patient 19: BF | 25-7-95 | A |
| AF69 | Environment: BMT | 4-8-95 | A |
| AF70 | Environment | 12-1-96 | A |
| AF71 to 73 | Environment | 1-3-96 | A |
| AF74 | Environment | 24-7-96 | A |
| AF75 | Environment: BMT | 13-1-97 | A |
| AF76 and 77 | Patient 14: AF (non-BMT) | 3-5-95 | E |
| AF78 | Environment | 5-5-95 | E |
| AF79 | Patient 17: BF | 18-5-95 | E |
| AF80 | Environment | 24-5-95 | E |
| AF81 to 83 | Patient 18: PM lung | 16-6-95 | E |
| AF84 to 87 | Patient 18: PM kidney | 16-6-95 | E |
| AF88 to 89 | Patient 18: PM brain | 16-6-95 | E |
| AF90 to 93 | Patient 18: PM gall bladder | 16-6-95 | E |
| AF94 | Patient 18: PM heart | 16-6-95 | E |
| AF95 | Patient 21: sputum | 30-10-95 | E |
| AF96 | Environment: BMT | 13-12-95 | E |
| AF97 | Environment | 12-1-96 | E |
| AF98 | Environment | 1-3-96 | E |
| AF99 | Environment | 24-7-96 | E |
| AF100 | Environment | 21-4-95 | C |
| AF101 | Patient 16: CSF | 16-5-95 | C |
| AF102 | Patient 21: sputum | 30-10-95 | G |
| AF103 | Patient 11: nasal swab | 20-2-95 | I |
| AF104 | Patient 18: PM brain | 16-6-95 | I |
| AF105 | Environment: BMT | 7-7-95 | I |
| AF106 | Environment: BMTb | 4-8-95 | I |
| AF107 | Patient 22: BF | 1-12-95 | I |
| AF108 | Patient 24: PM lung | 21-12-95 | I |
| AF109 | Patient 25: TA | 23-12-95 | I |
| AF110 | Patient 25: PM lung | 28-12-95 | I |
| AF111 | Patient 25: PM trachea | 28-12-95 | I |
| AF112 | Environment: BMT | 1-3-96 | I |
| AF113 | Environment | 12-7-96 | I |
| AF114 and 115 | Environment | 24-7-96 | I |
| AF116 | Patient 25: PM lung | 28-12-95 | J |
| AF117 | Patient 18: sputum | 19-6-95 | K |
| AF118 | Patient 29: sputum (non-BMT) | 22-4-96 | K |
| AF119 | Environment | 12-7-96 | K |
AF, ascitic fluid; BF, bronchoalveolar lavage fluid; CSF, cerebrospinal fluid; PeF, pericardial fluid; PF, pleural fluid; PM, postmortem; TA, tracheal aspirate.
Recovered from an isolation room.
Nineteen isolates (AF1, AF7, AF25, AF32, AF34, AF38, AF49, AF58, AF67, AF80, AF82, AF100, AF101, AF102, AF106, AF109, AF116, AF118, and AF119) have been deposited with the United Kingdom National Collection of Pathogenic Fungi, held at the Mycology Reference Laboratory, Bristol, as NCPF 7255 to 7258 and NCPF 7260 to 7274.
Hospital setting.
The BMT unit, which was opened in March 1994, is supplied with filtered air under positive pressure and consists of 10 isolation rooms around a central nursing station, a changing room, a small kitchen, and a sluice room. About 60 transplants were performed per annum, between 1994 and 1996.
Environmental sampling.
The environment of the BMT unit was sampled at 6-week intervals starting in March 1994. Other parts of the hospital, including two wards in which neutropenic patients were housed, were sampled at intervals of 1 to 6 months starting in April 1995. Sterile swabs, moistened in sterile distilled water, were used to collect dust from selected inanimate surfaces within each of the rooms used by the patients, the adjoining nurses’ station, the kitchen, the sluice room, and the changing room. The swabs were then spread onto plates of Sabouraud dextrose agar which were incubated at 35°C and assessed for fungal growth after 48 h of incubation.
Isolation of fungal DNA.
Isolates were subcultured onto Sabouraud dextrose agar slopes and incubated at 35°C for 3 to 5 days to allow profuse sporulation. Aliquots of Sabouraud dextrose broth (3 ml) in 5-cm petri dishes were inoculated with spores so that there was an even distribution over the surface of the broth. The plates were incubated for 16 h at 37°C after which time a fine mycelial mat was visible on the surface of the broth. The mycelial mats were removed with sterile forceps, blotted to remove excess medium, and placed in 1.5-ml centrifuge tubes. Four glass beads (5 to 7 mm in diameter) were added to each tube, and they were then immersed in liquid nitrogen for 30 s, removed, and allowed to thaw. Then 500 μl of lysis buffer, pH 8.0 (200 mM Tris-HCl, 0.5 M NaCl, 10 mM EDTA, 1% [wt/vol] sodium dodecyl sulfate), was added, and the tubes were vortexed for 30 s. The tubes were then immersed again in liquid nitrogen for 30 s, thawed, and revortexed for 30 s. Genomic DNA was purified from the lysate by repeated phenol-chloroform extractions as described by Aufauvre-Brown et al. (4). The DNA was precipitated with 0.1 volume of 5 M ammonium acetate and 1 volume of isopropanol and washed with 70% (vol/vol) ethanol. The DNA was suspended in 40 μl of Tris-EDTA buffer (100 mM Tris HCl, 10 mM EDTA) with a 2-μl volume of 10 mg of RNase A (Sigma Chemical Co., St. Louis, Mo.) per ml. The DNA concentration was assessed by spectrophotometry (Genequant; Pharmacia Biotech, St. Albans, England), and the samples were stored at −20°C until used in PCRs.
PCR amplification.
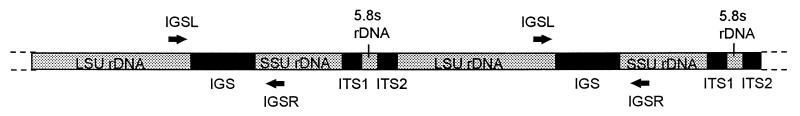
Two oligonucleotide primers targeting conserved regions near the 3′ end of the large ribosomal subunit and the 5′ end of the small ribosomal subunit of the ribosomal DNA gene sequences were designed (Fig. 1). The primer sequences were IGSL (5′-TAGTACGAGAGGAACCGT-3′) and IGSR (5′-GCATATGACTACTGGCAG-3′) and correspond to bases 120 to 137 and 2285 to 2302 of the Aspergillus nidulans sequence (EMBL accession number Z27114).
FIG. 1.
The ribosomal RNA gene complex (rDNA). Diagrammatic representation of rDNA in filamentous fungi to illustrate the gene order and positions of the spacer regions. LSU rDNA is the large subunit ribosomal RNA and SSU rDNA is the small subunit ribosomal RNA and ITS1 and ITS2 are the two internal transcribed spacer regions.
The PCRs were performed in 50-μl volumes. The reaction mixture contained 0.5 U of Super TAQ polymerase (HT Biotechnology Ltd.), buffer (10 mM Tris-HCl [pH 9.0], 1.5 mM MgCl2, 50 mM KCl, 0.1% [vol/vol] Triton X-100, 0.01% [wt/vol] gelatin) with an additional 1 mM concentration of MgCl2 (25 mM; Advanced Biotechnologies Ltd., Epsom, England), 60 μM concentrations of each deoxynucleoside triphosphate (dNTP) (Pharmacia), and 0.4 μM concentrations of each of the two primers. A 2-μl sample of DNA suspension in TE buffer was added to the reaction mixture (about 20 ng of DNA). The reaction mixtures were overlaid with 2 drops of paraffin oil. In preliminary studies the optimum reaction conditions were established by testing different DNA, primer, enzyme, and dNTP concentrations as well as a range of cycling conditions.
PCR with primers IGSL and IGSR was carried out with the following temperature profile: an initial denaturation step of 94°C for 3 min followed by 40 cycles of alternating denaturation (30 s, 94°C), primer annealing (1 min, 45°C), and primer extension (2 min, 72°C). After the last cycle there was a final extension step of 7 min at 72°C.
Control samples that had each of the reaction constituents except genomic DNA were prepared. All PCRs were performed in a thermal cycler (Omnigene; Hybaid Ltd., Teddington, England). Amplification products were visualized by electrophoresis through 1.8% agarose gels and detected by staining with ethidium bromide. Gels were photographed, and the banding patterns were compared visually by comparison to a 100-bp-fragment molecular-size marker (Gibco BRL, Glasgow, Scotland). The resulting banding patterns were indexed by capital lettering, and even a single band difference led to a different letter code. Differences in intensities of fluorescence were ignored. Each isolate was analyzed on at least two occasions, by using separate subcultures, to verify the reproducibility of the method. Each time an isolate was found to give a novel banding pattern indicating a new DNA type, the PCR and DNA extraction stages were repeated three times to confirm the existence of the new type.
RESULTS
The PCR conditions used in this work were chosen on the basis of initial tests with a range of primer, magnesium, enzyme, and dNTP concentrations. The PCR conditions were also optimized for maximum band intensity and reproducibility by changing the annealing temperature (45 to 55°C), the duration of individual steps (denaturation, annealing, and synthesis), and the number of amplification cycles. The clearest, most consistent, and most differential banding patterns were obtained with an annealing temperature of 45°C and the reaction conditions described in Materials and Methods. The banding patterns were relatively resistant to changes in the amount of DNA suspension used (1 to 3 μl) and to changes in polymerase concentration (0.25 to 0.75 U/50 μl), with the same basic bands being present but with the larger bands (≥700 bp) showing slight differences in intensity (data not shown).
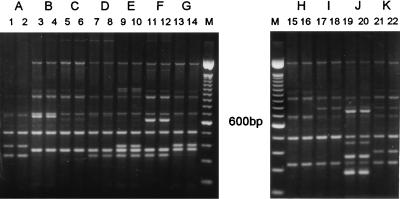
The two primers, IGSL and IGSR, differentiated the 119 isolates of A. fumigatus used in this study into 11 distinct DNA types, coded A to K (Fig. 2). The DNA banding patterns, which were obtained with the optimum reaction conditions, were reproducible both on repeat PCR of the same DNA suspension and with repeated DNA extraction and PCR from different subcultures of particular isolates.
FIG. 2.
PCR banding patterns (types A to K) generated from the genomic DNA of duplicate subcultures of isolates of A. fumigatus by using the oligonucleotide primers IGSL and IGSR. Negative reagent control amplifications without DNA added (not shown) resulted in no bands. Lanes 1 and 2, isolate AF58; lanes 3 and 4, AF34; lanes 5 and 6, AF101; lanes 7 and 8, AF38; lanes 9 and 10, AF82; lanes 11 and 12, AF1; lanes 13 and 14, AF102; lanes 15 and 16, AF25; lanes 17 and 18, AF110; lanes 19 and 20, AF116; lanes 21 and 22, AF118. M, 100-bp ladder. Material in each lane was prepared from separate DNA extractions.
The sources, dates of isolation, and DNA types of the 119 isolates of A. fumigatus, collected from patients and from the environment of the BMT unit itself and from other parts of the hospital, are summarized in Table 1. Most of the isolates fell into one of five types: type H (24 isolates), type E (24 isolates), type D (21 isolates), type A (20 isolates), or type I (13 isolates). Three DNA types (types B, G, and J) were isolated on only a single occasion. Two of these isolates were from BMT patients, and the third was from a non-BMT patient. In all three cases, at least one other DNA type was recovered from the same clinical sample.
Of the eight distinct DNA types of A. fumigatus that were obtained on more than one occasion, all were isolated from both patients and the environment (Table 1). Six DNA types were recovered from BMT patients and the environment of the unit (types F, H, D, A, E, and I). All of these types were also found in the environment of other parts of the hospital. Two of the less common DNA types (types C and K), isolated from BMT patients, were also recovered from the hospital environment, although not from the environment of the BMT unit itself.
Seven of the eight DNA types of A. fumigatus that were isolated from both patients and the environment were obtained from the patients first (Table 1). The exception was type C, which was recovered from the hospital environment 3 weeks before it was first isolated from a patient on the BMT unit. Although the original isolate of A. fumigatus type D came from a non-BMT patient, this DNA type was recovered from the environment of the BMT unit 4 months before it was first isolated from a BMT patient.
The shortest time interval between the first patient and the first environmental isolation was 2 days for type E (from a non-BMT patient and the general hospital environment). The shortest interval between the first BMT patient isolation and the first isolation from the environment of the unit was 5 months (type I). The longest interval between the first isolation from a BMT patient and the first isolation from the environment of the unit was 16 months (type H). This DNA type had been isolated from seven patients on the BMT unit and one other non-BMT patient by the time it was first recovered from the hospital environment. It was subsequently isolated from three more patients and persisted in the environment for a total of 14 months. The two most persistent DNA types were types H and D, which were isolated over a 22-month period from BMT and non-BMT patients, respectively.
One patient harbored the same DNA type of A. fumigatus in two sputum samples taken 2 weeks apart (patient 26, type D) (Table 1). Three others harbored different DNA types in similar samples taken on different occasions (patient 1, types F and H; patient 22, types H and I; patient 29, types D and K). Two patients harbored two different types in the same antemortem sample (patient 7, types H and B; patient 21, types E and G).
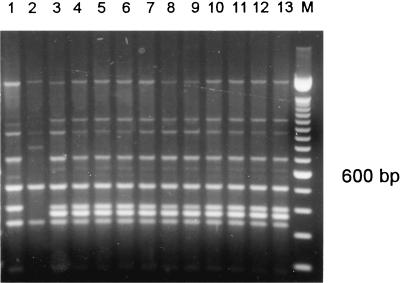
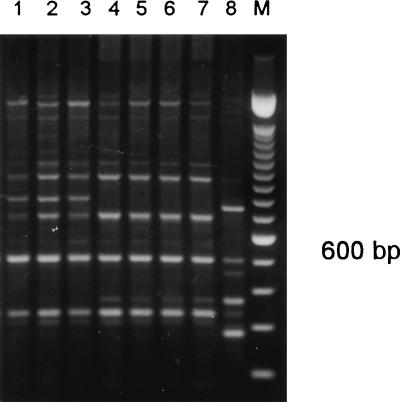
One patient harbored 3 different DNA types of A. fumigatus in one antemortem and 15 postmortem samples (patient 18, types E, I, and K) (Table 1 and Fig. 3). Another patient harbored 3 DNA types in eight postmortem samples (patient 25, types H, I, and J) (Table 1 and Fig. 4). A third patient harbored the same DNA type in two different postmortem samples (patient 15, type A).
FIG. 3.
PCR banding patterns generated from the genomic DNA of A. fumigatus isolates from patient 18 by using primers IGSR and IGSL. Lane 1, isolate AF117; lane 2, AF104; lane 3, AF81; lane 4, AF82; lane 5, AF84; lane 6, AF85; lane 7, AF86; lane 8, AF88; lane 9, AF87; lane 10, AF90; lane 11, AF91; lane 12, AF92; lane 13, AF94. M, 100-bp ladder. Isolates in lanes 3 to 13 were considered indistinguishable.
FIG. 4.
PCR banding patterns generated from the genomic DNA of A. fumigatus isolates from patient 25 by using primers IGSR and IGSL. The marker is a 100-bp fragment of which the prominent 600-bp fragment is indicated. Lane 1, isolate AF109; lane 2, AF110; lane 3, AF111; lane 4, AF25; lane 5, AF26; lane 6, AF27; lane 7, AF28; lane 8, AF116.
DISCUSSION
Invasive aspergillosis is a serious nosocomial infection, affecting 10 to 20% of neutropenic cancer patients and 0.5 to 10% of patients following BMT (8). A. fumigatus is the predominant cause of this infection, being implicated in over 80% of cases (8). Prevention of aspergillosis is difficult because of the ubiquitous nature of aspergillus spores in the environment (21). It has often been assumed that nosocomial clusters of infection occur during periods of building works in and around hospitals, because an overall increase in spore concentrations in the air leads to an increased likelihood of infection. However, tracing the sources of nosocomial aspergillus infection has been difficult, because of the lack of reliable typing methods for subspecific discrimination of the organism.
A number of DNA-based typing methods have now been developed for A. fumigatus. These include RAPD, restriction fragment length polymorphism (RFLP) detection, and Southern hybridization with various repetitive sequence-based probes. However, many of the reports that have so far been published have largely been concerned with the application of novel methods to groups of unrelated isolates which would be expected to differ irrespective of the criteria used (4, 9, 10, 18, 19, 26). Less commonly, DNA typing has been applied to the investigation of nosocomial clusters of A. fumigatus isolates to ascertain whether patients have acquired the same type and to attempt to trace the source of the infection (7, 12, 16, 20, 27). In some instances a link has been demonstrated between isolates from individual patients and potential environmental sources (7, 12, 16, 20), but in others it has not (27).
The fungal ribosomal DNA complex consists of about 100 highly conserved copies of the ribosomal gene sequences, which are tandemly repeated head to tail with spacer regions in between (5). The intergenic spacer region is the most variable part of the ribosomal DNA complex, and a number of RFLP methods have their origin here (5, 26). Previous work with Aspergillus spp., with probes and RFLPs, has indicated that the IGS region between ribosomal DNA operon repeat units is variable in length and that different copies of the complex in different isolates contain variable numbers of a 200-bp repeat unit (5).
Our results suggest that PCR amplification of the IGS region of A. fumigatus is a reproducible method for subspecific typing of this organism. We have not sequenced the amplicons to confirm that the primers are binding specifically to the intended target, but several observations suggest that this might be the case. No PCR products were observed when either one of the IGS primers was omitted; such products might have been expected if low-stringency primer binding was responsible for the IGS patterns observed. Furthermore, IGS PCR was unaffected by slight changes in amplification conditions; use of an annealing temperature of 50°C resulted in products similar to but less intense than those obtained with an annealing temperature of 45°C. We cannot be certain that some random annealing was not occurring, given that none of the IGS sequences of A. fumigatus have been determined and that some of the PCR products observed were shorter than might have been anticipated from the published details of a corresponding A. nidulans sequence (EMBL accession number Z27114). However, we found IGS PCR typing to be considerably more reproducible than were a number of RAPD and related nonspecific-primer-based analyses (data not shown).
The IGS PCR method used in this investigation permitted us to distinguish 11 distinct DNA types among a total of 119 clinical and environmental isolates of A. fumigatus. This enabled us to investigate the persistence of individual types in a hospital environment and assess their possible role in the development of nosocomial aspergillus infection. Of the eight distinct DNA types of A. fumigatus that were isolated on more than one occasion, six were recovered from the environment of the BMT unit itself as well as from other parts of the hospital (types F, H, D, A, E, and I). Two DNA types (types C and K) were recovered from the hospital environment but not from the BMT unit. A number of DNA types of A. fumigatus persisted in the hospital environment for long periods: four types (types F, D, A, and E) were found on two occasions 14 months apart, and two of these (types D and A) were recovered from the BMT unit itself on two occasions 18 months apart. Long-term persistence of particular DNA types of A. fumigatus in the hospital environment has been reported elsewhere (12).
Of the 10 DNA types of A. fumigatus that were isolated from patients on the BMT unit, 8 were also present in the hospital environment, and 6 of these were found in the unit itself. In most instances, each new DNA type was isolated from one or more patients before it was recovered from the environment for the first time. The long intervals between environmental sampling might well account for this. Moreover, environmental sampling is by its nature a rather inexact method of detecting fungal contamination, and the failure to detect A. fumigatus on a particular occasion does not mean that the organism was not present in the environment. It is notable that 13 BMT patients developed infection with type H, I, or D some months after these types had first been found in the environment of the unit. These findings suggest that some patients may have become infected with A. fumigatus from the hospital environment.
Although six different types of A. fumigatus were recovered from different locations within the environment of the BMT unit on 10 different occasions over a 25-month period, only 1 of the 19 isolates (AF106) came from one of the isolation rooms in which patients were housed. Most of the isolates were recovered from the kitchen, the sluice room, or a ventilation grill above the nursing station. However, it is possible that the spores were transmitted to the patients via staff, visitors, contaminated fomites, or air currents when doors were opened or closed.
Several of the patients studied in this investigation died with aspergillosis. Multiple isolates of A. fumigatus, obtained from postmortem tissue samples, showed that it was not unusual for more than one DNA type to be present. One patient harbored one DNA type of A. fumigatus in 14 postmortem samples and a second type in another tissue sample (patient 18, types E and I) (Table 1 and Fig. 3). Another patient was found to have three DNA types in eight postmortem samples (patient 25, types H, I, and J) (Table 1 and Fig. 4). Molecular typing of A. fumigatus isolates from patients with various forms of aspergillosis has demonstrated that many individuals are infected with a particular DNA type (9, 11). In contrast, typing of sputum isolates from cystic fibrosis patients has shown that these individuals may be colonized with many different types of A. fumigatus (29).
The results of this study show that IGS PCR typing of A. fumigatus has a potential role in the investigation of hospital outbreaks and should be of use in answering some epidemiological questions. Kostman et al. (14) have suggested that PCR ribotyping is a suitable method for typing many diverse bacterial species. The PCR ribotyping method described in this paper may also be applicable to the strain differentiation of a range of fungal species.
Our results confirm and extend those of previous molecular typing studies which demonstrated possible links between the isolates of A. fumigatus recovered from hospitalized patients with aspergillosis and potential environmental sources within the hospital (7, 12, 16, 20). We have shown that some BMT recipients became infected with particular DNA types of A. fumigatus some months after these types had first been detected in the environment of the unit in which the patients had been housed following transplantation. These findings, which suggest that A. fumigatus infection in BMT recipients may be nosocomial in origin, highlight the need for careful environmental monitoring in hospital units in which high-risk patients are housed.
ACKNOWLEDGMENT
This study was supported by a grant from the Special Trustees for the United Bristol Hospitals.
REFERENCES
- 1.Allo M D, Miller J, Townsend T, Tan C. Primary cutaneous aspergillosis associated with Hickman intravenous catheters. N Engl J Med. 1987;317:1105–1108. doi: 10.1056/NEJM198710293171802. [DOI] [PubMed] [Google Scholar]
- 2.Anderson M J, Gull K, Denning D W. Molecular typing by random amplification of polymorphic DNA and M13 Southern hybridization of related paired isolates of Aspergillus fumigatus. J Clin Microbiol. 1996;34:87–93. doi: 10.1128/jcm.34.1.87-93.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnow P M, Andersen R L, Mainous P D, Smith E J. Pulmonary aspergillosis during hospital renovation. Am Rev Respir Dis. 1978;118:49–53. doi: 10.1164/arrd.1978.118.1.49. [DOI] [PubMed] [Google Scholar]
- 4.Aufauvre-Brown A, Cohen J, Holden D W. Use of randomly amplified polymorphic DNA markers to distinguish isolates of Aspergillus fumigatus. J Clin Microbiol. 1992;31:2991–2993. doi: 10.1128/jcm.30.11.2991-2993.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bainbridge B W. Modern approaches to the taxonomy of Aspergillus. In: Powell K A, Renwick A, Peberdy J F, editors. The genus Aspergillus. New York, N.Y: Plenum Press; 1994. pp. 229–301. [Google Scholar]
- 6.Barnes R A, Rogers T R. Control of an outbreak of nosocomial aspergillosis by laminar air-flow isolation. J Hosp Infect. 1989;14:89–94. doi: 10.1016/0195-6701(89)90110-2. [DOI] [PubMed] [Google Scholar]
- 7.Birch M, Nolard N, Shankland G S, Denning D W. DNA typing of epidemiologically-related isolates of Aspergillus fumigatus. Epidemiol Infect. 1995;114:161–168. doi: 10.1017/s0950268800052018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denning D W. Invasive aspergillosis in immunocompromised patients. Curr Opin Infect Dis. 1994;7:456–462. [Google Scholar]
- 9.Denning D W, Clemons K V, Hanson L H, Stevens D A. Restriction endonuclease analysis of total cellular DNA of Aspergillus fumigatus isolates of geographically and epidemiologically diverse origin. J Infect Dis. 1990;162:1151–1158. doi: 10.1093/infdis/162.5.1151. [DOI] [PubMed] [Google Scholar]
- 10.Girardin H, Latge J P, Srikantha T, Morrow B, Soll D R. Development of DNA probes for fingerprinting Aspergillus fumigatus. J Clin Microbiol. 1993;31:1547–1554. doi: 10.1128/jcm.31.6.1547-1554.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girardin H, Sarfati J, Kobayashi H, Bouchara J P, Latge J P. Use of DNA moderately repetitive sequence to type Aspergillus fumigatus isolates from aspergilloma patients. J Infect Dis. 1994;169:683–685. doi: 10.1093/infdis/169.3.683. [DOI] [PubMed] [Google Scholar]
- 12.Girardin H, Sarfati J, Traore F, Dupouy Camet J, Derouin F, Latge J P. Molecular epidemiology of nosocomial invasive aspergillosis. J Clin Microbiol. 1994;32:684–690. doi: 10.1128/jcm.32.3.684-690.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hopkins C C, Weber D J, Rubin R H. Invasive aspergillus infection: possible non-ward common source within the hospital environment. J Hosp Infect. 1989;13:19–25. doi: 10.1016/0195-6701(89)90091-1. [DOI] [PubMed] [Google Scholar]
- 14.Kostman J R, Alden M B, Mair M, Edlind T D, LiPuma J J, Stull T L. A universal approach to bacterial molecular epidemiology by polymerase chain reaction ribotyping. J Infect Dis. 1995;171:204–208. doi: 10.1093/infdis/171.1.204. [DOI] [PubMed] [Google Scholar]
- 15.Krasinski K, Holzman R S, Hanna B, Greco M A, Graff M, Bhogal M. Nosocomial fungal infection during hospital renovation. Infect Control. 1985;6:278–282. doi: 10.1017/s0195941700061750. [DOI] [PubMed] [Google Scholar]
- 16.Leenders A, van Belkum A, Janssen S, de Marie S, Kluytmans J, Wielenga J, Lowenberg B, Verbrugh H. Molecular epidemiology of apparent outbreak of invasive aspergillosis in a hematology ward. J Clin Microbiol. 1996;34:345–351. doi: 10.1128/jcm.34.2.345-351.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lentino J R, Rosenkranz M A, Michaels J A, Kurup V P, Rose H D, Rytel M W. Nosocomial aspergillosis. A retrospective review of airborne disease secondary to road construction and contaminated air conditioners. Am J Epidemiol. 1982;116:430–437. doi: 10.1093/oxfordjournals.aje.a113427. [DOI] [PubMed] [Google Scholar]
- 18.Lin D, Lehmann P F, Hamory B H, Padhye A A, Durry E, Pinner R W, Lasker B A. Comparison of three typing methods for clinical and environmental isolates of Aspergillus fumigatus. J Clin Microbiol. 1995;33:1596–1601. doi: 10.1128/jcm.33.6.1596-1601.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loudon K W, Burnie J P, Coke A P, Matthews R C. Application of polymerase chain reaction to fingerprinting Aspergillus fumigatus by random amplification of polymorphic DNA. J Clin Microbiol. 1993;31:1117–1121. doi: 10.1128/jcm.31.5.1117-1121.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loudon K W, Coke A P, Burnie J P, Lucas G S, Liu Yin J A. Invasive aspergillosis: clusters and sources? J Med Vet Mycol. 1994;32:217–224. doi: 10.1080/02681219480000281. [DOI] [PubMed] [Google Scholar]
- 21.Nolard N, Detandt M, Beguin H. Ecology of Aspergillus species in the human environment. In: Vanden Bossche H, Mackenzie D W R, Cauwenbergh G, editors. Aspergillus and aspergillosis. New York, N.Y: Plenum Publishing; 1988. pp. 35–41. [Google Scholar]
- 22.Rinyu E, Varga J, Ferenczy L. Phenotypic and genotypic analysis of variability in Aspergillus fumigatus. J Clin Microbiol. 1995;33:2567–2575. doi: 10.1128/jcm.33.10.2567-2575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robertson M J, Larsen R A. Recurrent fungal pneumonias in patients with acute nonlymphocytic leukemia undergoing multiple courses of intensive chemotherapy. Am J Med. 1988;84:233–239. doi: 10.1016/0002-9343(88)90419-6. [DOI] [PubMed] [Google Scholar]
- 24.Ruutu P, Valtonen V, Tiitanen L, Elonen E, Volin L, Veijalainen P, Ruutu T. An outbreak of invasive aspergillosis in a haematologic unit. Scand J Infect Dis. 1987;19:347–351. doi: 10.3109/00365548709018481. [DOI] [PubMed] [Google Scholar]
- 25.Sherertz R J, Belani A, Kramer B S, Elfenbein G J, Weiner R S, Sullivan M L, Thomas R G, Samsa G P. Impact of air filtration on nosocomial aspergillus infections. Unique risk of bone marrow transplant recipients. Am J Med. 1987;83:709–718. doi: 10.1016/0002-9343(87)90902-8. [DOI] [PubMed] [Google Scholar]
- 26.Spreadbury C L, Bainbridge B W, Cohen J. Restriction fragment length polymorphisms in isolates of Aspergillus fumigatus probed with part of the intergenic spacer region from the ribosomal RNA gene complex of Aspergillus nidulans. J Gen Microbiol. 1990;136:1991–1994. doi: 10.1099/00221287-136-10-1991. [DOI] [PubMed] [Google Scholar]
- 27.Tang C M, Cohen J, Rees A J, Holden D W. Molecular epidemiological study of invasive pulmonary aspergillosis in a renal transplantation unit. Eur J Clin Microbiol Infect Dis. 1994;13:318–321. doi: 10.1007/BF01974610. [DOI] [PubMed] [Google Scholar]
- 28.van Belkum A, Quint W G V, de Pauw B, Melchers W J G, Meis J F. Typing of Aspergillus species and Aspergillus fumigatus isolates by interrepeat polymerase chain reaction. J Clin Microbiol. 1993;31:2502–2505. doi: 10.1128/jcm.31.9.2502-2505.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verweij P E, Meis J F G M, Sarfati J, Hoogkamp-Korstanje J A A, Latge J P, Melchers W J G. Genotypic characterization of sequential Aspergillus fumigatus isolates from patients with cystic fibrosis. J Clin Microbiol. 1996;34:2595–2597. doi: 10.1128/jcm.34.10.2595-2597.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weems J J, Davis B J, Tablan O C, Kaufman L, Martone W J. Construction activity: an independent risk factor for invasive aspergillosis and zygomycosis in patients with haematologic malignancy. Infect Control Hosp Epidemiol. 1987;8:71–75. doi: 10.1017/s0195941700067114. [DOI] [PubMed] [Google Scholar]