Abstract
Introduction
Medical patients, admitted acutely to hospital, are at risk of venous thromboembolism (VTE). Clinical guidelines advise thromboprophylaxis prophylaxis for those at high risk of VTE. VTE is a common sequela of cancer, but guidelines take little consideration of cancer as an independent risk factor and their utility in palliative care patients is unclear. The hospice inpatient deep vein thrombosis (DVT) detection study (HIDDen) reported a 28% prevalence of asymptomatic iliofemoral DVT in hospice patients of poor performance status (PS) and prognosis, calling into question the utility of thromboprophylaxis in the palliative care setting. However, the majority of cancer inpatients receiving palliative care are admitted to hospital through the acute medical setting, yet their risk factors for VTE may differ from those admitted to hospices.
Objective
To better understand the prevalence and behaviours of VTE in patients with cancer receiving palliative care who are admitted as an acute medical emergency.
Design
Multicentre, observational cohort study.
Setting
Secondary care acute hospitals in South Wales, UK.
Patients
We plan to recruit 232 patients≥18 years old with a diagnosis of incurable cancer, and/or receiving palliative or best supportive care who are admitted acutely to hospital. Patients will be followed up for a maximum of 6 months following registration.
Primary outcome
Presence of lower extremity DVT.
Secondary outcomes
Symptom burden attributed to DVT or pulmonary embolism, patient PS, patient demographics and development of new VTE within 90 days of registration.
Analysis
The study statistical analysis plan will document analysis, methodology and procedures.
Ethics and dissemination
Ethical approval was obtained from the Wales Research Ethics Committee, reference 22/WA/0037 (IRAS 306352)—the main trial results will be analysed as soon as practically possible and the publication shared with investigators and on sponsor website; applications to access trial data will be subject to sponsor review process.
Keywords: Thromboembolism, PALLIATIVE CARE, PREVENTIVE MEDICINE
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study explores thromboprophylaxis in a vulnerable adult population, which is often excluded from thromboprophylaxis research.
It is a natural progression of the hospice inpatient deep vein thrombosis detection study, using similar methodology and outcome measures.
Strong patient public involvement has influenced the study design, set up and ongoing trial management.
The results will have a rapid impact on thromboprophylaxis policy within palliative care.
The study does not record pulmonary emboli and so the results may underestimate the true prevalence of venous thromboembolism in this population.
Introduction
The prevention of venous thromboembolism (VTE), comprising of deep vein thrombosis (DVT) and pulmonary embolus (PE), is a priority for NHS England and Wales, which has been demonstrated to reduce avoidable harm and mortality in hospitalised patients.1 It is recommended that all hospitalised patients and, by default, those receiving palliative care, are assessed for their risk of venous thrombosis and if appropriate offered low molecular weight heparin (LMWH) thromboprophylaxis cancer patients are seven times more likely to develop VTE than non-cancer patients, with one in five developing VTE.2 The clinical studies informing thromboprophylaxis guidelines are more than 20 years old and less than 15% of patients recruited to them had cancer.3 There has been considerable debate as to whether these data can be applied to palliative care patients.4 5 Furthermore, these studies excluded palliative care patients,5 6 who are at particular risk of thrombosis since the risk of VTE is greater as cancer becomes more advanced.4 Specific patient exclusion criteria were poor performance status (PS), prognosis of less than 3 months survival, risk of bleeding, renal failure and abnormal liver function. However, this population is one of the most likely to develop VTE and potentially benefit from thromboprophylaxis.7 8 The hospice inpatient DVT detection study (HIDDen) identified a 28% prevalence of DVT in palliative care patients.9 There was minimal-associated symptom burden and no survival difference between those with or without DVT. Patients had high care needs, with a median Australia-modified Karnofsky Performance Scale (AKPS) of 49 and a median survival of 44 days. An accompanying Lancet Haematology editorial concluded that thromboprophylaxis was of limited utility in hospice patients of poor PS and prognosis.10
Rationale
The HIDDen study has been considered practice changing for specialist palliative care units (SPCUs) and hospices, yet its application to the wider palliative care population remains unclear.11 Over 80 000 palliative patients in the UK are admitted acutely to hospital per year, yet thromboprophylaxis may not only be unnecessary but also confer a significant risk of harm.12 LMWH given as a daily injection carries a 2% and 12% risk of major and non-major haemorrhage respectively, and data from 1200 hospice inpatients suggests a 9.8% rate of clinically relevant bleeding.13 14
The HIDDEN2 study represents a natural progression of the original hospice-based HIDDen study as it is to be performed in a ‘healthier’, better prognosis group of patients within the general palliative cancer patient population, which is more representative of the majority of palliative care patients who are admitted to the acute setting. The HIDDen study demonstrated the feasibility of recruiting and performing lower limb imaging in hospice/SPCU-based palliative care cancer patients; it recruited ahead of schedule and gained significant ‘buy-in’ from patients and their respective families.15
There is a clear need to establish and better understand the prevalence, symptom burden and natural history of VTE in patients with advanced cancer admitted to hospital, to better inform clinical practice, avoid unnecessary harm and reduce unwarranted health service costs.
Primary objective
The aims of this study are to better understand the prevalence and behaviours of VTE in patients with cancer receiving palliative care who are admitted acutely to hospital. Specific objectives are to:
Determine the prevalence of radiologically apparent DVT in patients with palliative cancer within 48 hours of hospital admission.
Evaluate the symptom burden attributable to DVT.
Assess the impact of incidental DVT on symptom burden at 3 months.
Determine overall survival at 6 months.
Determine the incidence of new VTE within 90 days of hospital admission
Evaluate the association of DVT incidence with patient demographics including PS.
Methods and analysis
Study design and sample size
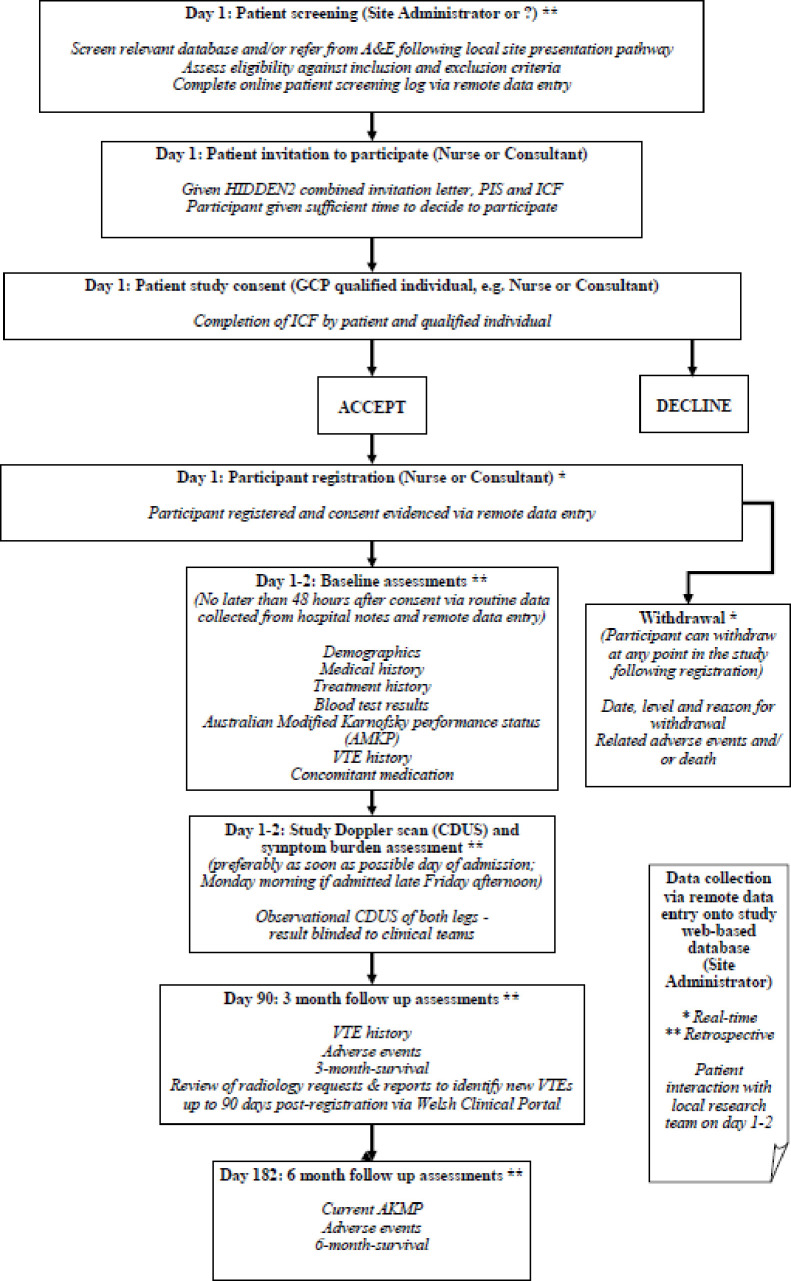
A multicentre, observational cohort study in South Wales, UK (figure 1). The trial opened to recruitment on 04 May 2022 and we plan to close recruitment on 30 September 2023. A target of 232 patients will be recruited and followed up for up to 6 months from study registration. This target will allow us to estimate the prevalence of DVT among patients with advanced cancer admitted to acute hospitals with a 95% CI of no more than ±5 percentage points based on 17% prevalence from the previous HIDDen study and expected dropout of 5%.
Figure 1.
Study schema. CDUS, colour duplex ultrasonography; HIDDen, Hospice inpatient deep vein thrombosis detection; ICF, informed consent form; PIS, participant information sheet; VTE, deep vein thrombosis: A&E, Accident and Emergency; GCP, Good Clinical Practice.
Eligibility criteria
Inclusion criteria
Patients with cancer≥18 years of age who have no physical limitations that would exclude them from taking part in ultrasound assessments are able to give fully informed written consent, and meet at least one of the following criteria: incurable cancer defined as metastatic or locally advance cancer with no curative treatment planned (palliative radiotherapy or systemic anticancer therapy (SACT) is acceptable if being administered for symptom control or palliative intent); under the care of community or hospital palliative care service; or on the General Practioner (GP) community palliative care register.
Exclusion criteria
Patients who meet one or more of the following criteria are excluded: non-melanoma skin cancer; receiving SACT with curative intent; biologically controlled disease, for example, prostate-specific antigen normal prostate cancer; admission for anticipated end-of-life care; or patients who are considered by the clinical team as likely to survive less than 5 days.
Study setting
In total, 232 patients will be recruited from 3 secondary care, acute hospitals in South Wales, UK. The study will be coordinated by the Centre for Trials Research (CTR), Cardiff University and sponsored by Aneurin Bevan University Health Board (ABUHB).
Registration
Patients who consent to take part by authorising the informed consent form (ICF) will be registered on the day of consent by the recruiting site staff using a secure, remote, study-specific web-based database Research Electronic Data Capture (REDCap).16 17 REDCap is a secure, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for data integration and interoperability with external sources.
All patients with cancer 18 years of age or over admitted to participating sites will be screened consecutively prior to consent for eligibility and/or referred to the local study team by the admitting clinician or suitable delegate following the different presentation pathways at each participating site. Screening, eligibility and non-consent will be logged at site on a screening log. Eligible patients will be invited to participate in the study by the admitting clinician and/or suitable delegate as per local patient presentation pathway. Interested patients will be given the HIDDEN2 participant information sheet and ICF and the opportunity to discuss the study with the research team. Participants will be free to withdraw from the study at any time following registration without any impact on their routine hospital treatment or care.
Baseline assessments
Baseline assessment data will be collected on day 1–4 following registration: demographics, medical history and treatment history, routine blood assessments, baseline AKPS status, VTE history and concomitant medication.
Observational colour duplex ultrasonography (CDUS)
Study-specific baseline CDUS assessments will be performed preferably on the day of admission and within no more than 48 hours from study registration (day 1) in order to determine an admission VTE prevalence. However, exceptions will be allowed in the event of a late Friday afternoon admission. In such a case, the patient may be recruited only if their scan can be performed by the following Monday morning, that is, the scan may be delayed until day 4. In this situation, the time-lapse between admission and the scan being conducted will be recorded. For patients who require longer than 4 days to consider participation, the scan can be delayed; however, it would still need to be conducted within 48 hours from study registration. Patients who are happy to proceed with immediate consent will be able to do so since the aim of the study is to find the prevalence of DVT on admission and the study investigation is non-invasive.
CDUS result blinding
In normal practice outside of the study, neither clinician nor patient would be aware of the presence of an asymptomatic DVT on admission to hospital since it is unusual for patients to undergo CDUS on admission and throughout their stay; unless there is a clinical indication that CDUS is required either alone or in combination with compression ultrasonography to diagnose DVT. On the occasion the CDUS is required as part of the patient’s routine care, the whole (upper and lower) leg would be scanned.
The research CDUS imaging differs from this local procedure as it will be limited to the femoral–popliteal segment only (upper thigh) to allow direct comparison to the original HIDDen study and will not include investigation of the calf or superficial veins (lower leg). Thus, the research CDUS will not provide sufficient details for clinical diagnostic purposes. Additionally, the research CDUS would only be valid as an exclusory test for 24 hours post scan, after which time a thrombus could have formed. As such, patients requiring a scan more than 24 hours after the research scan has been performed will require routine CDUS outside of the study following local practice to ensure accuracy.
As HIDDEN2 is an observational study, the study results must not impact on routine clinical management. Access to the research CDUS scan data will be restricted to local site staff delegated on the site staff delegation log. Therefore, the patient, and local clinical team responsible for routine treatment of the patient outside of the study, will be blinded from the results of the research CDUS to avoid influencing clinical management of the patient and usual practice. Unblinding of study CDUS results to these staff will not be permitted.
Due to differences in approaches to routine ultrasonography management, reporting practices and staff individual participating sites will develop local study-specific procedures to mitigate and monitor for results ‘unblinding events’.
Follow-up
The study will comprise of three data collection timepoints: day 1–4 of consent and registration, day 90 post registration for follow-up VTE assessment and day 182 (6 months) post registration for survival. Of these timepoints, only the first visit will require patient interaction as considerable effort has been made to minimise patient visits. From the initial assessment, the team will identify whether DVT is present and collect demographic data in line with usual admission procedures.
On day 1–4, data will be captured from the patient to identify any leg or lung symptoms and to capture any illnesses within the past 90 days. All other required data will be obtained from the patient’s healthcare record (HCR) and recorded within the baseline case report form (CRF).
On day 90, data from radiological investigations undertaken since registration will be reviewed within the patient’s HCR and any new VTE events documented within the day 90 CRF.
On day 182, any deaths that occurred during the study will be recorded on the associated CRF. Any deaths not recorded in real time during the study will be identified on day 182 when the patient’s HCR will be reviewed to determine overall survival.
Primary outcome
The HIDDEN2 study aims to investigate the presence of lower extremity DVT. To ensure consistency throughout the study, all scans will be performed by fully qualified and accredited vascular scientists and/or radiologists with experience of conducting ultrasounds. This will help ensure scan quality and obviate the requirement for the secondary review of scan reporting, which occurred in the original HIDDen study.
Secondary outcomes
Symptom burden attributable to DVT or PE
HIDDEN2 will investigate the presence of pain and/or swelling in each leg, and the presence of breathlessness and/or chest pain evaluated and recorded at baseline.
Patient PS
HIDDEN2 will investigate the following demographics: cancer diagnosis, anticancer treatment within the past 3 months, current medications, history of any potentially reversible risk factor for DVT in the previous 12 weeks and routine blood assessments.
Development of new VTE within 90 days after admission
Any radiological investigations undertaken up to 90 days post registration will be reviewed and any new VTE events documented. This 90-day cut-off is in keeping with the accepted definition of hospital acquired thrombosis and will be of relevance when interpreting the results against current government thromboprophylaxis policy. Any request for a routine CT pulmonary angiogram, ventilation/perfusion scan or CDUS will be triggered by the presence of symptoms suggestive of VTE. The presence of symptoms according to the radiology request will be recorded. Any DVT or PE identified during a scan for any other indication (ie, not primarily looking for VTE) will be recorded as ‘incidental’ DVT or PE. This outcome measure is purely observational and will not affect patient care.
Six-month survival
At 6 months post registration, the Welsh Clinical Portal will be reviewed by the treating site staff to confirm if participants are still alive. Any patient deaths will be recorded along with cause and date of death on the associated CRF. This approach will ensure end-of-life patients and their families are not disturbed or inconvenienced.
Data management
The sponsor will act as a data controller. Cardiff University and individual participating sites will act as data processors. Data management procedures will be documented in a study data management plan in line with the protection impact assessment section of the study risk assessment.
Study data will be collected and managed using REDCap electronic data capture tools hosted at Cardiff University.16 17 Paper CRFs will be used as backup should REDCap be inaccessible. Participating sites will log patient screening on a site-specific electronic screening log and send a redacted version via secure electronic transfer to the CTR for central monitoring purposes.
Patient and public involvement and engagement
The original HIDDen study had strong patient and public involvement (PI) which was evaluated against the National Standards18 and PI has been instrumental in the subsequent development of HIDDEN2.18 Following the publication of HIDDen, a stakeholder meeting was held to discuss the impact of all findings on patient care. This was attended by the study’s PI lead, lay representatives from Hospice UK, Marie Curie, Macmillan, Thrombosis UK, with clinical representation from members of the British Society for Haematology, Multiprofessional Association for Supportive Cancer Care, International Society for Thrombosis and Haemostasis and the Association for Palliative Medicine. In conclusion, as a hospice-based study, the HIDDen study was considered practice changing, but it was not possible to extrapolate the findings to palliative care patients admitted to hospital, who may be at different stages of the cancer journey, particularly with respect to both, better PS and prognosis. Since the majority of palliative care patients are admitted to hospital and not hospices, this was considered a priority area for research. The following patient organisations and charities at the stakeholder meeting helped form the research question for HIDDEN2: Hospice UK, Marie Curie, Macmillan, Thrombosis UK and Anticoagulation UK.
There are two public PI partners on the HIDDEN2 study management group (SMG), one of which supported the original HIDDen study and is also a HIDDEN2 executive committee (EC) member.
Public involvement will be monitored against national standards throughout the HIDDEN2 study and fully documented in the main results publication, or a separate report.
Statistics and data analysis
All participants must have undergone a research CDUS to be included in the primary outcome analysis. We have not planned an interim analysis. The prevalence of DVT at hospital admission will be summarised with a 95% CI. There was no formal sample size calculation for the secondary outcomes. However, further analysis will summarise and compare the characteristics (age, sex, type of cancer diagnosis, treatment, history of DVT, symptoms of DVT or PE, AKPS and baseline blood profiles) of all patients with cancer with and without DVT. Univariable logistic regression models will be performed to create ORs and 95% CIs for the occurrence of the DVTs. The following risk factors will be included in each model: age, sex, baseline DVT and VTE risk factors, use of anticoagulants, AKPS Score, VTE history, bleeding history and bleeding risk. Based on the previous HIDDEN trial, a multivariate logistic regression model will include age, AKPS Score, history of DVT/VTE and the presence of leg oedema. Any additional variables with a p value of less than 0.1 from the univariable analysis will be added to the multivariable model. The final adjusted model will include all the above-named variables, plus those that have a p value<0.05 in the initial multivariable model. Adjusted ORs with 95% CIs will be presented.
The number of patients with new VTE events occurring within 90 days of admission, and the number of patient deaths within 6 months will be reported. Kaplan-Meier curves will be constructed to compare survival according to whether patients had proximal lower limb DVT within 48 hours after the patient’s admission to hospital. A log-rank test will be used to compare survival in by DVT status. Participants who have not died by the end of survival data collection will be censored at the date last known to be alive.
Further exploratory analysis will also be undertaken to evaluate development of symptoms attributable to DVT and pulmonary embolism, bleeding associated with and without thromboprophylaxis and the effect of COVID-19 in our study.
The impact of any missing data on the conclusions drawn from our analyses will be considered. Plausible missing data mechanisms will be considered, allowing us to estimate the strength and direction of relationship between DVT and secondary outcomes. Full analysis details will be documented in a statistical analysis plan.
The study results will be published in a peer-reviewed journal. All study publications will be made publicly available on the study website.
Data sharing
Applications for access to the data, in a pseudonymised format, may be made to the corresponding author and will be reviewed in line with existing CTR SOPs and sponsor processes. It is the intention of the research group to make data available for patient benefit, wherever possible.
Monitoring
The study risk assessment has categorised HIDDEN2 as low risk (comparable to the risk of standard medical care), thus low monitoring levels will be employed following a risk-adapted approach.
There is no formal independent data monitoring committee. A project management group (PMG) will provide oversight on a regular weekly to monthly basis dependent on study stage. The PMG will report to the SMG, including two clinical dependent, one dependent PI and one independent statistician EC members, on a quarterly basis. The CTR cancer trial steering committee will monitor the study once per annum.
Central monitoring will be conducted via routine data queries and quality control checks of ICFs and site participant screening logs, and will focus on accrual, consent, withdrawal, research CDUS results adherence and unblinding, and data integrity and protection.
No site monitoring is planned. However, ad hoc triggered site visits will be conducted if required to address site-related GCP or contractual non-compliance. Non-compliance identified centrally or at site will be reported to research ethics committee (REC), the sponsor and participating sites as applicable, following CTR standard policies and procedures. The study is subject to inspection by REC/institutional review board as the regulatory body, and inspection and audit by ABUHB as sponsor.
Supplementary Material
Acknowledgments
HIDDEN2 is sponsored by Aneurin Bevan University Health Board (ABUHB). ABUHB has delegated day-to-day management of the study to the Centre for Trials Research at Cardiff University.
Footnotes
Twitter: @simonnoble, @Sarah1003Walker
Contributors: Provision of study materials or patients: SN, NP, RA, CB, TG and RW. Collection and assembly of data: TK, EO, LR, JS, LU, IT and AC. Data analysis and interpretation: AC, SN, LR and TK. Patient representation: KS and DS. Manuscript writing: TK, SN, AC, IT and LU. Final approval of manuscript: all authors. Accountable for all aspects of the work: all authors.
Funding: HIDDEN2 is funded by the Welsh Government Health Care Research Wales (HCRW) Research for Public Benefit (RfppB) funding (Ref: RfppB—747(P)). Public involvement resource was sourced via the HCRW support and delivery service. The CTR is a UK Clinical Research Collaboration (UKCRC) registered clinical trials unit. CTR HCRW and CRUK core funds supported RfppB grant submission. SN holds a Marie Curie funded Chair in Supportive and Palliative Medicine at Cardiff University.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods and analysis section for further details.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Hunt BJ. Preventing hospital associated venous thromboembolism. BMJ 2019;365:l4239. 10.1136/bmj.l4239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noble S, Pasi J. Epidemiology and pathophysiology of cancer-associated thrombosis. Br J Cancer 2010;102 Suppl 1:S2–9. 10.1038/sj.bjc.6605599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NICE . Venous Thromboembolism in Over 16s: Reducing the Risk of Hospital‐Acquired Deep Vein Thrombosis or Pulmonary Embolism. NICE guideline [NG89]. London, UK: NICE, 2018. [PubMed] [Google Scholar]
- 4.Johnson MJ, Sproule MW, Paul J. The prevalence and associated variables of deep venous thrombosis in patients with advanced cancer. Clinical Oncology 1999;11:105–10. 10.1053/clon.1999.9023 [DOI] [PubMed] [Google Scholar]
- 5.Noble SIR, Nelson A, Finlay IG. Factors influencing hospice thromboprophylaxis policy: a qualitative study. Palliat Med 2008;22:808–13. 10.1177/0269216308096723 [DOI] [PubMed] [Google Scholar]
- 6.Noble SIR, Finlay IG. Have palliative care teams' attitudes toward venous thromboembolism changed? A survey of thromboprophylaxis practice across British specialist palliative care units in the years 2000 and 2005. J Pain Symptom Manage 2006;32:38–43. 10.1016/j.jpainsymman.2005.11.013 [DOI] [PubMed] [Google Scholar]
- 7.Noble S, Johnson M. Finding the evidence for thromboprophylaxis in palliative care: first let us agree on the question. Palliat Med 2010;24:359–61. 10.1177/0269216310366389 [DOI] [PubMed] [Google Scholar]
- 8.Johnson MJ, McMillan B, Fairhurst C, et al. Primary thromboprophylaxis in hospices: the association between risk of venous thromboembolism and development of symptoms. J Pain Symptom Manage 2014;48:56–64. 10.1016/j.jpainsymman.2013.08.016 [DOI] [PubMed] [Google Scholar]
- 9.White C, Noble SIR, Watson M, et al. Prevalence, symptom burden, and natural history of deep vein thrombosis in people with advanced cancer in specialist palliative care units (hidden): a prospective longitudinal observational study. Lancet Haematol 2019;6:e79–88. 10.1016/S2352-3026(18)30215-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandset PM, Dahm AEA. Is venous thromboembolism a problem in patients with cancer in palliative care? Lancet Haematol 2019;6:e61–2. 10.1016/S2352-3026(18)30218-7 [DOI] [PubMed] [Google Scholar]
- 11.Noble S. Venous thromboembolism in palliative care patients: what do we know? Thromb Res 2020;191 Suppl 1:S128–32. 10.1016/S0049-3848(20)30410-2 [DOI] [PubMed] [Google Scholar]
- 12.NCPC . National Council for palliative care minimum dataset report 2012-13; 2013.
- 13.Samama MM, Cohen AT, Darmon J-Y, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. N Engl J Med 1999;341:793–800. 10.1056/NEJM199909093411103 [DOI] [PubMed] [Google Scholar]
- 14.Tardy B, Picard S, Guirimand F, et al. Bleeding risk of terminally ill patients hospitalized in palliative care units: the RHESO study. J Thromb Haemost 2017;15:420–8. 10.1111/jth.13606 [DOI] [PubMed] [Google Scholar]
- 15.White C, Noble S, Watson M, et al. Optimised clinical study recruitment in palliative care: success strategies and lessons learned. BMJ Support Palliat Care 2020;10:216–20. 10.1136/bmjspcare-2019-001820 [DOI] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seddon K, Elliott J, Johnson M, et al. Using the United Kingdom standards for public involvement to evaluate the impact of public involvement in a multinational clinical study. Res Involv Engagem 2021;7. 10.1186/s40900-021-00264-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.