Abstract
Background
To date, at least 20 different amyloidogenic proteins have been documented. Growing evidence suggests that despite being part of the universal amyloid proteome, apolipoprotein A-IV can be amyloidogenic, accounting for less than 1% of cases.
Case summary
A 75-year-old woman was admitted for paroxysmal nocturnal dyspnoea and intermittent exertional shortness of breath and was found to be in acute heart failure. The patient underwent intravenous diuretic therapy and was discharged after decongestion. She then underwent a battery of outpatient tests to determine aetiology of her heart failure. Cardiac magnetic resonance imaging showed severe concentric left ventricular hypertrophy and diffuse late gadolinium enhancement, concerning for amyloidosis, but serologic evaluation for amyloidogenic light chain (AL) amyloidosis was negative. Tc 99m pyrophosphate (PYP) scan showed Grade 2 uptake at 1 h that was only moderately suggestive of transthyretin (TTR) amyloidosis. She ultimately received a right heart catheterization and endomyocardial biopsy, which showed apolipoprotein A-IV amyloid deposition within Congo red-positive areas of the endomyocardial specimen. The patient continues to report dyspnoea on exertion but has avoided additional heart failure admissions with intensification of her diuretic regimen.
Discussion
In this case, nuclear PYP scan to evaluate for TTR amyloidosis demonstrated focal PYP uptake, but endomyocardial biopsy demonstrated apolipoprotein A-IV deposition without evidence of TTR amyloidosis. Our case increases knowledge of this rare form of amyloidosis, suggests that it may result in false positive nuclear PYP results, and highlights the importance of its evaluation, particularly in circumstances in which investigations do not reveal definitive evidence of AL or TTR amyloidosis.
Keywords: Apolipoprotein A-IV amyloidosis, Cardiac amyloidosis, Restrictive cardiomyopathy, Case report
Graphical Abstract
Graphical Abstract.
Learning points.
Identify apolipoprotein A-IV as a rare but important cause of cardiac amyloidosis as it may produce false positive pyrophosphate results and reinforces the role of endomyocardial biopsy in cases of clinical uncertainty.
Recognize that laser micro-dissection and liquid chromatography-tandem mass spectrometry should be pursued to determine and semi-quantify the primary type of amyloid deposition.
Understand the basic clinical and histologic features of apolipoprotein A-IV amyloidosis.
Primary specialties involved other than cardiology
Radiology, pathology.
Introduction
To date, at least 20 different amyloidogenic proteins have been documented.1 Apolipoprotein A-IV (apo A-IV) is among the rare causes of cardiac amyloidosis. Apolipoprotein A-IV is synthesized in the intestine and secreted into intestinal lymph as a 376 amino acid glycoprotein in association with chylomicron particles.2 The protein has been shown to activate lecithin:cholesterol acyltransferase and modulate both the size and clearance of chylomicrons.3,4 The presence of apo A-IV in amyloid specimens is ubiquitous and deemed part of the universal signature of systemic amyloidosis, regardless of amyloid type.5 However, growing evidence suggests that despite being part of the universal amyloid proteome, apo A-IV can be amyloidogenic, accounting for less than 1% of cases.1,6,7 This report presents a rare case of biopsy-confirmed apo A-IV amyloidosis resulting in clinical heart failure (HF). The data underlying this article are available in the article and in its online Supplementary material.
Case summary
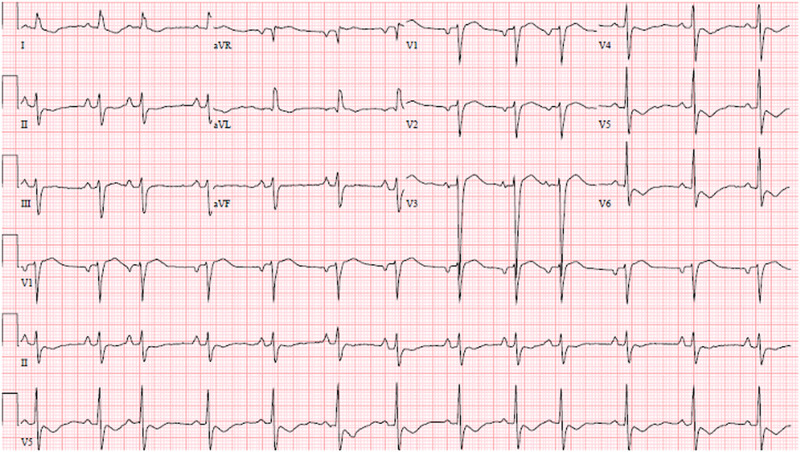
A 75-year-old woman presented to the emergency department with 1 day of paroxysmal nocturnal dyspnoea and intermittent exertional shortness of breath. She had no chest pain or lower extremity swelling. She had no other complaints. The patient’s examination revealed cachexia, jugular venous distention, absence of cardiac murmurs, normal respiratory effort, and clear lungs. An electrocardiogram demonstrated sinus rhythm, left anterior fascicular block, left axis deviation, left ventricular hypertrophy, left atrial enlargement, and T-wave inversions in the lateral leads (Figure 1). Chest radiography revealed small bilateral pleural effusions and mild enlargement of the cardiac silhouette without pulmonary venous congestion or pulmonary oedema. B-type natriuretic peptide (BNP) was elevated at 2603 pg/mL (reference 0–100 pg/mL). High-sensitivity troponin was elevated on admission at 343 pg/mL (reference 0–15 pg/mL). Other admission laboratory studies are noted in Table 1. She was admitted to the hospital for further evaluation.
Figure 1.
Electrocardiogram at presentation. Twelve-lead electrocardiogram demonstrates sinus rhythm with premature atrial contractions at a heart rate of 79, mean QRS axis of −40, left anterior fascicular block with QRS duration of 118 ms, new T-wave inversion affecting leads V4-V6 I aVL, left ventricular hypertrophy by Cornell criteria, and left atrial enlargement.
Table 1.
Serological evaluations
| Value | Reference value | |
|---|---|---|
| CBC and basic chemistry | ||
| WBC | 9.5 × 103 cells/µL | 3.5–10.5 × 103 cells/µL |
| Haemoglobin | 11.5 g/dL | 11.6–15.4 g/dL |
| Sodium | 143 mmol/L | 133–146 mmol/L |
| Potassium | 3.8 mmol/L | 3.5–5.1 mmol/L |
| Bicarbonate | 23 mmol/L | 21–31 mmol/L |
| Creatinine | 2.86 mg/dL | 0.6–1.3 mg/dL |
| eGFR | 16 mL/min/1.73 m2 | ≥60 mL/min/1.73 m2 |
| BNP | 2603 pg/mL | 0–100 pg/mL |
| High-sensitivity troponin | 343 pg/mL | 0–15 pg/mL |
| AL amyloidosis evaluation | ||
| Free kappa light chain | 4.69 mg/dL | 0.33–1.94 mg/dL |
| Free lambda light chain | 3.07 mg/dL | 0.57–2.63 mg/dL |
| Free kappa/lambda light chain ratio | 1.53 | 0.26–1.65 |
| Total protein | 6.9 g/dL | 6.4–8.9 g/dL |
| Albumin | 4.3 g/dL | 3.5–5.7 g/dL |
| Albumin ELP | 4.0 g/dL | 3.2–5.0 g/dL |
| Alpha 1 protein | 0.3 g/dL | 0.1–0.4 g/dL |
| Alpha 2 protein | 1.1 g/dL | 0.6–1.0 g/dL |
| Beta protein | 0.8 g/dL | 0.6–1.3 g/dL |
| Gamma protein | 0.8 g/dL | 0.7–1.5 g/dL |
| IgG | 642 mg/dL | 610–1616 mg/dL |
| IgA | 153 mg/dL | 85–499 mg/dL |
| IgM | 119 mg/dL | 35–242 mg/dL |
AL, amyloidogenic light chain; BNP, B-type natriuretic peptide; CBC, complete blood count; eGFR, estimated glomerular filtration rate; ELP, electrophoresis; WBC, white blood cell count.
The patient’s previous illnesses included advanced stage chronic kidney disease (CKD) not on dialysis (ND) that was attributed to chronic volume depletion from long-standing laxative abuse leading to chronic interstitial fibrosis, secondary hyperparathyroidism and metabolic acidosis, hypothyroidism, chronic pruritus, and vitamin B12 deficiency.
The initial differential diagnosis included HF, acute coronary syndrome (ACS), venous thromboembolism (VTE), myopericarditis, pneumonia, pneumothorax, and anaemia. Given the presence of jugular venous distention, acute HF was of the highest concern.
The patient’s hospital presentation was deemed secondary to acute HF, and she received intravenous diuretic therapy with furosemide 20 mg, with improvement in her symptoms. Troponin elevation was attributable to a Type II myocardial infarction event in the setting of acute HF, and thus further coronary investigations were not performed. She was discharged and then underwent a battery of investigations as an outpatient to determine the aetiology of HF.
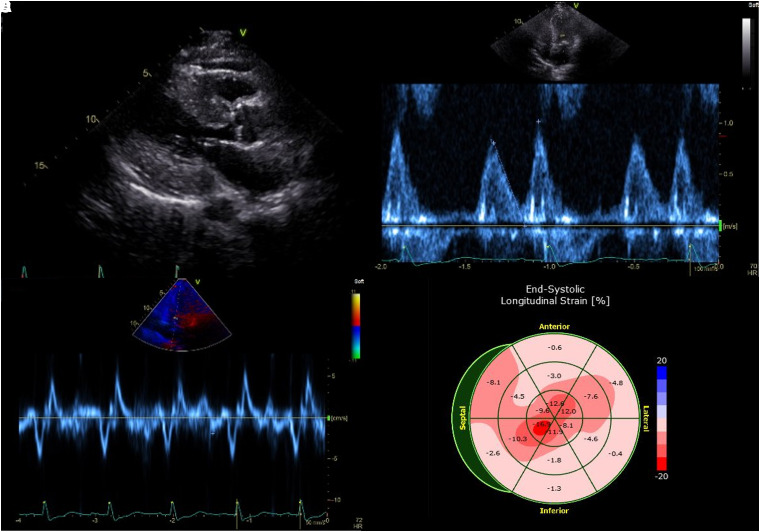
A transthoracic echocardiogram revealed mildly decreased left ventricular systolic function (ejection fraction 47%, calculated by two-dimensional biplane method), global hypokinesis, and severe concentric left ventricular hypertrophy (Figure 2; Supplementary material online, Video S1). Additionally, Grade II diastolic dysfunction was present, with markedly decreased tissue Doppler velocity of the mitral annulus and moderate left atrial enlargement. The left ventricular global longitudinal strain was −6.8%, relative apical sparing ratio was 1.43, and ejection fraction to strain ratio was 6.9.
Figure 2.
Transthoracic echocardiogram at presentation. (A) Transthoracic echocardiogram of the heart in standard parasternal long-axis view demonstrates severe concentric left ventricular hypertrophy, with posterior wall thickness measuring 2.2 cm. (B) Mitral valve inflow evaluation from the same study shows an E/A ratio of 0.79 and E deceleration time of 183 ms. (C) Tissue Doppler imaging exhibits severely reduced tissue velocities with mitral annular septal e′ velocity of 2 cm/s, mitral annular lateral e′ velocity of 2 cm/s, s′ velocity of 6 cm/s, a′ velocity of 5 cm/s, and average mitral E/e′ ratio of 40. (D) Left ventricular global longitudinal strain was −6.8%, with a relative apical sparing ratio of 1.43 and an ejection fraction to strain ratio of 6.9.
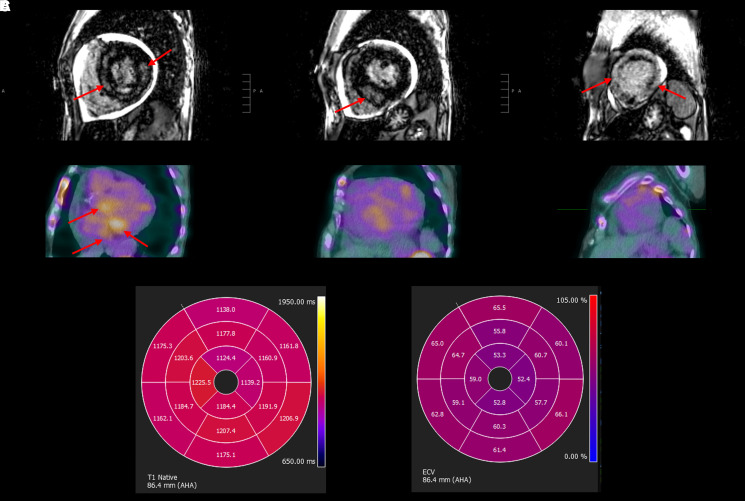
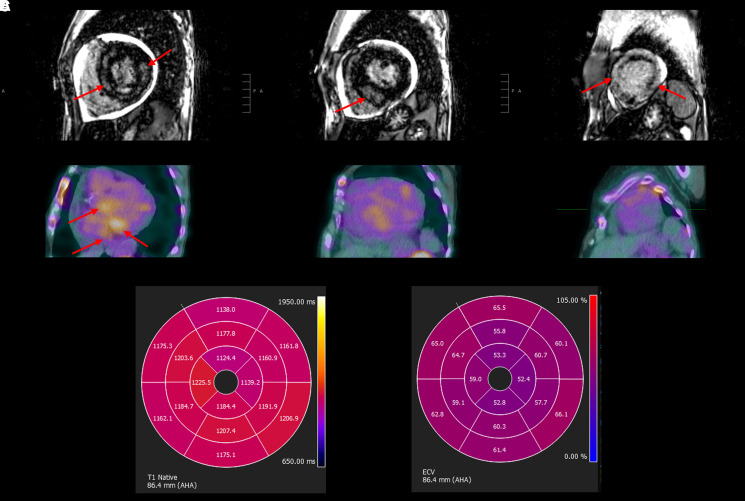
Cardiac magnetic resonance (CMR) imaging revealed severe concentric left ventricular hypertrophy with significant amounts of near transmural late gadolinium enhancement, predominantly involving the mid-wall of the basal and mid-segments, while the apical segments had circumferential, non-transmural sub-endocardial enhancement. The left ventricular ejection fraction was 42%, the extracellular volume (ECV) was markedly elevated at 58%, and there was a small pericardial effusion (Figure 3; Supplementary material online, Video S1).
Figure 3.
Cardiac magnetic resonance imaging and nuclear pyrophosphate scan. (A) Cardiac magnetic resonance of the heart in standard short-axis view demonstrates severe concentric left ventricular hypertrophy and diffuse late gadolinium enhancement within the base, mid-segment, and apex (left to right). (B) Corresponding nuclear technetium Tc 99m pyrophosphate scan demonstrates focal Tc 99m pyrophosphate uptake in the base but no Tc 99m pyrophosphate uptake in the mid-segment or the apex (left to right). (C) Polar maps of native T1 (left) and extracellular volume (right) demonstrating a gradient from base to apex. Native T1 values ranged from 1124 to 1225 ms. Extracellular volume ranged from 52% to 66%.
An evaluation for amyloidogenic light chain (AL) amyloidosis was thus undertaken (Table 1). Serum-free light chains and their respective ratio were within normal limits. There were no restricted bands seen on serum or urine immunofixation.
A nuclear technetium Tc 99m pyrophosphate (99mTc-PYP) scan showed Grade 2 99mTc-PYP uptake at 1 h, Grade 1 uptake at 3 h, and a heart-to-contralateral ratio of 1.4 at both 1 and 3 h. While there was no diffuse 99mTc-PYP uptake in the myocardium, focal 99mTc-PYP uptake was present in the basal segments of the left ventricle on single-photon emission computed tomography imaging (Figure 3). Overall, the nuclear PYP scan did not meet multi-societal expert consensus recommendations as strongly suggestive of TTR cardiac amyloidosis but was moderately suggestive given Grade 2 uptake at 1 h.8
Given the borderline findings on PYP scan, the patient subsequently underwent endomyocardial biopsy. Haemodynamic parameters are outlined in Table 2. Congo red-positive amyloid deposits, exhibiting apple-green birefringence under polarized light, were identified on the biopsy specimen (Figure 4). Liquid chromatography-tandem mass spectrometry (LC-MS/MS) was performed on peptides extracted from Congo red-positive, micro-dissected areas of the paraffin-embedded specimen. Liquid chromatography-tandem mass spectrometry detected apo A-IV amyloid deposition. These findings supported the diagnosis of amyloidosis secondary to apo A-IV–type amyloid deposition.
Table 2.
Haemodynamic measurements from right heart catheterization
| Haemodynamic parameter | Value |
|---|---|
| RA pressure, mm Hg | 7 |
| RV systolic pressure, mm Hg | 42 |
| RV diastolic pressure, mm Hg | 6 |
| PA systolic pressure, mm Hg | 42 |
| PA diastolic pressure, mm Hg | 18 |
| PCWP, mm Hg | 18 |
| Thermodilution cardiac output, L/min | 2.52 |
| Thermodilution cardiac index, L/min/m2 | 1.88 |
| PVR, woods units | 3.63 |
PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RA, right atrial; RV, right ventricular.
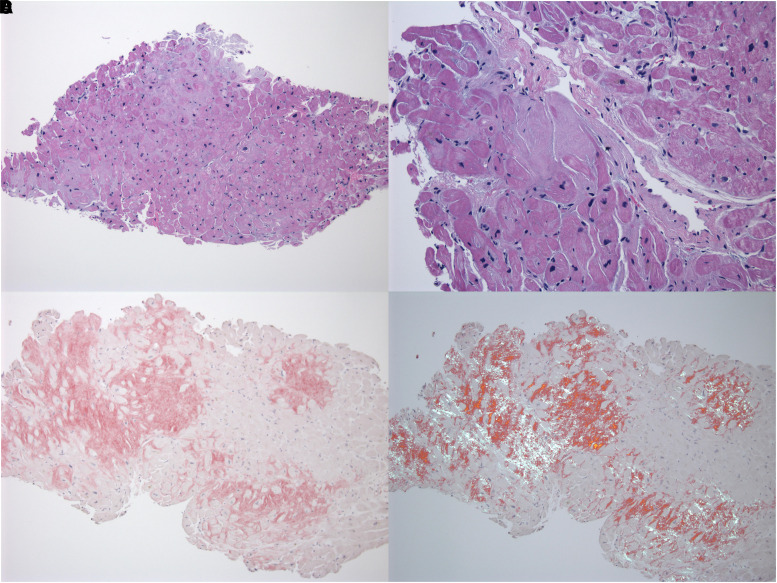
Figure 4.
Endomyocardial biopsy. (A) Haematoxylin–eosin-stained histopathologic sample of a biopsy from the endomyocardium at ×50 magnification. Histology revealed an interstitial infiltrative process. (B) The eosinophilic material has a waxy appearance and causes myocytes to take on angulated shapes. Some interstitial and sub-endocardial nodules are seen. There was micro-vasculopathy, but it was not prominent. (C) Without polarization, amyloid appears red with Congo red staining. (D) The material exhibits apple-green birefringence under polarized light with Congo red staining.
Discussion
To date, at least 20 different amyloidogenic proteins have been documented.1 Apolipoprotein A-IV is among the rare causes of cardiac amyloidosis. Apolipoprotein A-IV is synthesized in the intestine and secreted into intestinal lymph as a 376 amino acid glycoprotein in association with chylomicron particles.2 The protein has been shown to activate lecithin:cholesterol acyltransferase and modulate both the size and clearance of chylomicrons.3,4 The presence of apo A-IV in amyloid specimens is ubiquitous and deemed part of the universal signature of systemic amyloidosis, regardless of amyloid type.5 However, growing evidence suggests that despite being part of the universal amyloid proteome, apo A-IV can be amyloidogenic, accounting for less than 1% of cases.1,6,7
Apolipoprotein A-IV–derived amyloidosis may present with distinctive clinical and histologic features. In the heart, apo A-IV generates interstitial nodular infiltrates. Another notable characteristic is coronary micro-vasculature obstruction, which may cause ischaemic symptoms. In a series of nine cases of apo A-IV–derived cardiac amyloidosis available for clinicopathological review at Mayo Clinic, all patients had obstructive coronary micro-vascular amyloidosis.7 Interestingly, our patient did have involvement of the microvasculature, but it was not prominent.
In our case, there was focal PYP uptake in the left ventricle, but endomyocardial biopsy did not demonstrate evidence of TTR amyloidosis. Although the PYP scan was not strongly suggestive of TTR cardiac amyloidosis, the finding of focal PYP uptake suggests that apo A-IV may result in false positive PYP results and reinforces the role of endomyocardial biopsy in cases of clinical uncertainty.
In the kidney, apo A-IV forms extensive interstitial medullary deposits but spares the cortex.6 Because the glomeruli are spared, kidney decline progresses slowly, and proteinuria is usually either absent or minimal. Our patient has had relatively stable, Stage IV/V CKD ND that was diagnosed over 15 years prior and was attributed to chronic volume depletion leading to acute and chronic tubular damage from long-standing laxative abuse. The finding of apo A-IV cardiac amyloidosis raises the possibility of kidney involvement as a contributor to her CKD. While our patient did not receive a kidney biopsy to confirm deposition of apo A-IV, the steady nature of her kidney function decline, in conjunction with mild proteinuria (28 mg/dL), is consistent with kidney apo A-IV amyloidosis, as well. Notably, kidney biopsy is of relatively low diagnostic yield in cases of apo A-IV amyloidosis due to medullary, as opposed to cortical, protein deposition.
As apo A-IV protein deposits belong to the universal amyloid proteome, immunohistochemistry alone cannot be used to establish a diagnosis of apo A-IV amyloidosis. Mass spectroscopy is required for the diagnosis of apo A-IV amyloidosis as this technique allows for semi-quantitation of apo A-IV protein in the amyloid deposit. Mass spectroscopy becomes diagnostic of apo A-IV amyloidosis when apo A-IV is identified as the primary form of amyloid and has more than two-fold higher abundance.6 Spectra number of apo A-IV protein can be used to differentiate apo A-IV amyloidosis from other forms of systemic amyloidosis. In case series, the average spectra number in apo A-IV amyloidosis ranged from 73 to 85 vs. 20 to 35 in non-apo A-IV cases.6,7 The average spectra number for apo A-IV in our case was 359. Notably, the use of mass spectrometry may be limited given that the technology is inaccessible at many centres, but specimens may be sent to outside centres for analysis.
There are currently no accepted therapies targeted against apo A-IV–derived amyloidosis. However, certain therapies, including cholestyramine, weight loss, and fat-free diets, have demonstrated efficacy at lowering apo A-IV expression or serum levels.9,10 Reducing circulating levels of the amyloidogenic protein (chemotherapy and stem cell transplantation in AL amyloidosis; silencers and liver transplantation in TTR amyloidosis) improves outcomes.11,12 Therefore, in patients with apo A-IV amyloidosis, the use of cholestyramine, weight loss, or fat-free diets to reduce levels of the protein could be a promising therapeutic strategy. Identification of apo A-IV amyloidosis is also important to avoid certain therapies, such as chemotherapy or tafamidis in cases of AL or TTR amyloidosis, respectively.
Since her initial HF presentation, the patient has not had further hospitalizations for HF with intensification of her diuretics to a regimen consisting of torsemide 40 mg daily, with an additional 20 mg taken on an as-needed basis for weight gain of 3 lb or more. She remains alive at the time of the last follow-up (19 months since initial hospitalization). Although clinically appearing euvolemic, she continues to report dyspnoea on exertion and being intolerant of walking beyond one city block. She continues to have stable Stage V CKD ND. In the Mayo Clinic case series, all patients who were alive when the initial diagnosis of apo A-IV–derived cardiac amyloidosis was made were still alive at the time of the last follow-up (median 79.2 months since diagnosis). This suggests that apo A-IV amyloidosis, in comparison with other forms of amyloidosis, may carry a more favourable prognosis.7 Our patient’s course seems to fit with this observation.
Conclusions
Apolipoprotein A-IV is a rare cause of cardiac amyloidosis and may result in false positive, focal PYP uptake on scintigraphy. It should be considered as an aetiology of amyloid when initial evaluation fails to yield evidence of plasma cell dyscrasias or definitive evidence of TTR amyloidosis. Whether therapies that lower apo A-IV levels influence progression of disease in apo A-IV amyloidosis is unclear and requires further investigation.
Lead author biography
 Mohammed Basel Allaw is a third-year internal medicine resident at the McGaw Medical Center of Northwestern University in Chicago, Illinois. He completed his medical training at the Emory University School of Medicine. As a future cardiology fellow, his clinical and research interests include the role of multi-modal cardiac imaging in the diagnosis and treatment of a wide variety of cardiovascular disorders.
Mohammed Basel Allaw is a third-year internal medicine resident at the McGaw Medical Center of Northwestern University in Chicago, Illinois. He completed his medical training at the Emory University School of Medicine. As a future cardiology fellow, his clinical and research interests include the role of multi-modal cardiac imaging in the diagnosis and treatment of a wide variety of cardiovascular disorders.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Slide sets: A fully edited slide set detailing these cases and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for the submission and publication of this case report, including images and associated text has been obtained from the patient in line with COPE guidelines.
Funding: This work was supported by grant KL2TR001424 from the National Institutes of Health/National Center for Advancing Translational Sciences.
Supplementary Material
Contributor Information
Mohammed Basel Allaw, Division of Cardiology, Department of Medicine, Northwestern University Feinberg School of Medicine, 676 N St Clair St, Suite 600, Chicago, IL 60611, USA.
Arjun Sinha, Division of Cardiology, Department of Medicine, Northwestern University Feinberg School of Medicine, 676 N St Clair St, Suite 600, Chicago, IL 60611, USA.
Kambiz Ghafourian, Division of Cardiology, Department of Medicine, Northwestern University Feinberg School of Medicine, 676 N St Clair St, Suite 600, Chicago, IL 60611, USA.
Ryan Avery, Department of Radiology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Richard L Weinberg, Division of Cardiology, Department of Medicine, Northwestern University Feinberg School of Medicine, 676 N St Clair St, Suite 600, Chicago, IL 60611, USA.
Jon W Lomasney, Department of Pathology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Sanjiv J Shah, Division of Cardiology, Department of Medicine, Northwestern University Feinberg School of Medicine, 676 N St Clair St, Suite 600, Chicago, IL 60611, USA.
Ravi B Patel, Division of Cardiology, Department of Medicine, Northwestern University Feinberg School of Medicine, 676 N St Clair St, Suite 600, Chicago, IL 60611, USA.
Data availability
The data underlying this article are available in the article and in its online Supplementary material.
References
- 1.Dasari S, Theis JD, Vrana JA, Rech KL, Dao LN, Howard MT, et al. Amyloid typing by mass spectrometry in clinical practice: a comprehensive review of 16,175 samples. Mayo Clin Proc 2020;95:1852–1864. [DOI] [PubMed] [Google Scholar]
- 2.Elshourbagy NA, Walker DW, Paik YK, Boguski MS, Freeman M, Gordon JI, et al. Structure and expression of the human apolipoprotein A-IV gene. J Biol Chem 1987;262:7973–7981. [PubMed] [Google Scholar]
- 3.Chen CH, Albers JJ. Activation of lecithin: cholesterol acyltransferase by apolipoproteins E-2, E-3, and A-IV isolated from human plasma. Biochim Biophys Acta 1985;836:279–285. [DOI] [PubMed] [Google Scholar]
- 4.Kohan AB, Wang F, Li X, Bradshaw S, Yang Q, Caldwell JL, et al. Apolipoprotein A-IV regulates chylomicron metabolism-mechanism and function. Am J Physiol Gastrointest Liver Physiol 2012;302:G628–G636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vrana JA, Theis JD, Dasari S, Mereuta OM, Dispenzieri A, Zeldenrust SR, et al. Clinical diagnosis and typing of systemic amyloidosis in subcutaneous fat aspirates by mass spectrometry-based proteomics. Haematologica 2014;99:1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dasari S, Amin MS, Kurtin PJ, Vrana JA, Theis JD, Grogg KL, et al. Clinical, biopsy, and mass spectrometry characteristics of renal apolipoprotein A-IV amyloidosis. Kidney Int 2016;90:658–664. [DOI] [PubMed] [Google Scholar]
- 7.Bois MC, Dasari S, Mills JR, Theis J, Highsmith WE, Vrana JA, et al. Apolipoprotein A-IV-associated cardiac amyloidosis. J Am Coll Cardiol 2017;69:2248–2249. [DOI] [PubMed] [Google Scholar]
- 8.Dorbala S, Ando Y, Bokhari S, Dispenzieri A, Falk RH, Ferrari VA, et al. Addendum to ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: part 1 of 2-evidence base and standardized methods of imaging. J Nucl Cardiol 2021;28:1769–1774. [DOI] [PubMed] [Google Scholar]
- 9.Sonoyama K, Nishikawa H, Kiriyama S, Niki R. Cholestyramine and a fat-free diet lower apolipoprotein A-IV mRNA in jejunum and cholestyramine lowers apolipoprotein A-I mRNA in ileum of rats. J Nutr 1994;124:621–627. [DOI] [PubMed] [Google Scholar]
- 10.Lingenhel A, Eder C, Zwiauer K, Stangl H, Kronenberg F, Patsch W, et al. Decrease of plasma apolipoprotein A-IV during weight reduction in obese adolescents on a low fat diet. Int J Obes Relat Metab Disord 2004;28:1509–1513. [DOI] [PubMed] [Google Scholar]
- 11.Palladini G, Dispenzieri A, Gertz MA, Kumar S, Wechalekar A, Hawkins PN, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol 2012;30:4541–4549. [DOI] [PubMed] [Google Scholar]
- 12.Ericzon BG, Wilczek HE, Larsson M, Wijayatunga P, Stangou A, Pena JR, et al. Liver transplantation for hereditary transthyretin amyloidosis: after 20 years still the best therapeutic alternative? Transplantation 2015;99:1847–1854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary material.