Abstract
Background:
Although not routinely assessed, prenatal posttraumatic stress disorder (PTSD) is associated with poor maternal mental health and mother-infant bonding. Prenatal PTSD may also be associated with birth weight and gestational age outcomes, but this remains unclear. This systematic review and meta-analysis investigated the association of prenatal PTSD with risk of low birth weight (LBW) or preterm birth (PTB) (dichotomous medically-defined cut-offs) or with birth weight (BW) or gestational age (GA) (continuous variables).
Methods:
A comprehensive literature search was conducted in Web of Science, MedLine, PubMed, and PsychInfo. Data were collected and processed according to Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines. Study quality was assessed with the Newcastle-Ottowa Quality Assessment Scale. Pooled effect sizes were estimated with random-effects models (correlation for continuous and odds ratios for dichotomous outcomes).
Results:
Sixteen studies with 51,470 participants (prenatal PTSD 8%) were included in 4 meta-analyses. Maternal prenatal PTSD was associated with higher risks of LBW (OR = 1.96; 95% CI, 1.26, 3.03; P = .003), PTB (OR = 1.42 (95% CI, 1.16, 1.73; P = .001), and reduced GA (r = −0.04; 95% CI, −0.06, −0.01; P = .002).
Limitations:
Different designs across studies, variety of PTSD assessment practices, and a small pool of studies were noted.
Conclusions:
Findings suggest prenatal PTSD presents increased risks of LBW, PTB, and reduced GA. Evidence of physical harm to neonates from prenatal PTSD provides a powerful rationale to increase prenatal PTSD screening and identify effective prenatal interventions to improve maternal and child outcomes.
Keywords: posttraumatic stress disorder, PTSD, prenatal, birth weight, preterm, gestational age
1. Introduction
Rates of posttraumatic stress disorder (PTSD) in general samples of pregnant women range widely: from 0.6% in a Nigerian general sample using the Mini International Neuropsychiatric Interview (MINI) (Adewuya et al., 2006; Sheehan et al., 1998) to 16% in a United States general sample using the PTSD Checklist (Morland et al., 2007; Viswasam et al., 2019; Weathers et al., 1993). Among women at high risk of PTSD (defined in the Yildiz et al., 2017 review as those with difficult or traumatic pregnancies or births, severe fears of birth, or histories of sexual/physical violence or child abuse), the range is from 1% in a sample of Turkish women with hyperemesis gravidarum (severe nausea and vomiting during pregnancy) (Annagür et al., 2013) to 40% in a sample of women with hyperemesis gravidarum in the United States (Seng et al., 2013a) with a mean prevalence of 19% across the high-risk samples (Yildiz et al., 2017). Within the prenatal PTSD literature, pregnant women who are considered at particularly high risk for PTSD include African-Americans (Seng et al., 2011a); military veterans (Hugin and Shaw, 2019); inmates (Harner et al., 2015); and those with childhood abuse (Wosu et al., 2015; Yildiz et al., 2017), physical/sexual violence histories (Wosu et al., 2015; Yildiz et al., 2017), substance use disorders (Moylan et al., 2001), severe childbirth fear (Yildiz et al., 2017), or prior pregnancy complications (Yildiz et al., 2017). In 2020, overall rates of PTSD increased concurrent with the novel coronavirus (COVID-19) pandemic (Berthelot et al., 2020).
The American College of Obstetricians and Gynecologists (ACOG) has recommended prenatal screening and referral for depression, domestic violence, and anxiety for several years (American College of Obstetricians and Gynecologists, 2018, 2012a). More recently, researchers called to extend these prenatal mental health screening recommendations to include PTSD (Canfield and Silver, 2020). In April of 2021, ACOG published new recommendations for the care of patients who have experienced trauma (American College of Obstetricians and Gynecologists, 2021a). These recommendations include using a trauma-informed approach, trauma screening, and the provision of educational materials and referrals. Previously, the Association of Women’s Health, Obstetric, and Neonatal Nurses had already recommended universal screening for prenatal PTSD among other perinatal mental health disorders (Association of Women’s Health, Obstetric, and Neonatal Nurses, 2015).
Prenatal PTSD can adversely affect health-risk behaviors and prenatal fetal bonding (Onoye et al., 2009; Radoš et al., 2020; Sanjuan et al., 2020, 2019) and is associated with postpartum PTSD (Onoye et al., 2009), postpartum depression, (Seng et al., 2013b), and disrupted mother-child attachment (Muzik et al., 2012; Seng et al., 2013b; Webb and Ayers, 2015). Multiple studies have suggested that prenatal PTSD may also be associated with more direct risks to the growing fetus, yet, the impact of prenatal PTSD on birth outcomes involving fetal development remains unclear (Cook et al., 2018; Grigoriadis et al., 2018; Murphy et al., 2001). Evidence suggests that PTSD may be associated with lower birth weight and gestational age in a manner similar to prenatal maternal depression (Grigoriadis et al., 2013; Grote et al., 2010). The primary mechanism proposed for this association between prenatal PTSD and birth weight or gestational age is sustained activation of the maternal hypothalamic-pituitary-adrenal (HPA) axis in response to chronic psychological stress. This causes neuroendocrine abnormalities including a suppressed cortisol response in both mother and offspring with changes to the gestational uterine environment (Bowers and Yehuda, 2016; Shapiro et al., 2013). Altered circadian cortisol profiles are associated with general prenatal psychological distress, depression, and anxiety (Van den Heuvel et al., 2018); preterm birth (Gilles et al., 2018); and fetal weight (Diego et al., 2006). Low birth weight (LBW: defined as less than 2500g) and preterm birth (PTB: defined as less than 37 weeks gestation) deliveries are strongly associated with neonatal and long-term morbidity and mortality (Blencowe et al., 2012; Crump et al., 2019; Katz et al., 2013) and also with increased risk for postpartum PTSD and depression (de Paula Eduardo et al., 2019). Thus, the aim of this paper is to determine if PTSD is associated with such adverse birth weight or gestational age outcomes and, if so, to quantify the magnitude of this association to inform maternal psychiatric care.
Two summary studies of related research suggest prenatal PTSD may impact these birth outcomes (sometimes reported as dichotomous measures based on medical definitions of LBW and PTB, and sometimes reported as continuous measures of birth weight (BW) or gestational age (GA)). A qualitative literature review without a meta-analysis (N=11) concluded that prenatal PTSD may be associated with LBW, but the evidence was inconsistent for an association with reduced BW, PTB, or reduced GA (Cook et al., 2018). Also suggesting a link between prenatal PTSD and birth outcomes, a 2018 meta-analysis of anxiety and birth outcomes that included some PTSD studies found associations between prenatal anxiety and LBW, reduced BW, PTB, and reduced GA (Grigoriadis et al., 2018). However, the DSM-5 and ICD-11 Working Groups both concluded that, despite PTSD sharing features of and high comorbidity with both depression and anxiety disorders, the symptom profile and course of PTSD fits poorly with anxiety or depression disorders (American Psychiatric Association, 2013; Barbano et al., 2019; Friedman, 2013; Maercker et al., 2013). Thus, PTSD should be examined in meta-analyses separate from anxiety disorders. Moreover, anxiety alone is associated with an increased risk of LBW and PTB (Ding et al., 2014), so analyzing anxiety data mixed with PTSD data may obscure PTSD effects. Thus, these summary studies, while suggesting a possible relationship, do not fill the critical gap in the literature regarding the consistency and degree of associations specifically between prenatal PTSD and these adverse birth outcomes (Engel et al., 2005; Rogal et al., 2007; Seng et al., 2001; Xiong et al., 2008).
The aim of our meta-analytic study was to focus on PTSD and determine the magnitude of any association of prenatal PTSD with low birth weight (LBW), birth weight (BW), preterm birth (PTB), or gestational age (GA). We hypothesized that prenatal PTSD would be associated with greater risk of LBW, reduced BW, PTB, and reduced GA. We also examined between-study variability to identify potential moderators of the associations.
2. Methods
2.1. Search Strategy
A computerized search was completed by the lead author on July 27, 2020 in the Web of Science, MedLine, PubMed, and PsychInfo databases. MeSH search terms included were: for prenatal period (maternal, prenatal, pregnancy, obstetric), PTSD symptomology (PTSD, posttraumatic stress disorder), and adverse pregnancy outcome (birth weight, preterm birth, gestational age, pregnancy outcome). Searches were limited to English peer-reviewed articles from 1981 to 2020 with a total 1167 articles identified. The Boolean search used in Web of Science was: (((TS=((maternal OR prenatal OR pregnancy OR obstetric) AND (PTSD OR posttraumatic stress disorder) AND (preterm birth OR gestational age OR birth weight OR pregnancy outcome))))). Reference lists of all relevant studies and reviews were examined and forward searches were conducted. To identify any published data novel to the search, lead authors with multiple articles (4 authors considered experts in the field) were contacted, and none were aware of any additional published data sources. Removal of duplicate studies resulted in 803 articles (see Figure 1. PRISMA Flow Diagram (Liberati et al., 2009)). All aspects of the meta-analyses were conducted in accordance with Preferred Reporting Items for Systematic Reviews (PRISMA) (Moher et al., 2009) and Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines (Stroup, 2000).
Figure 1.
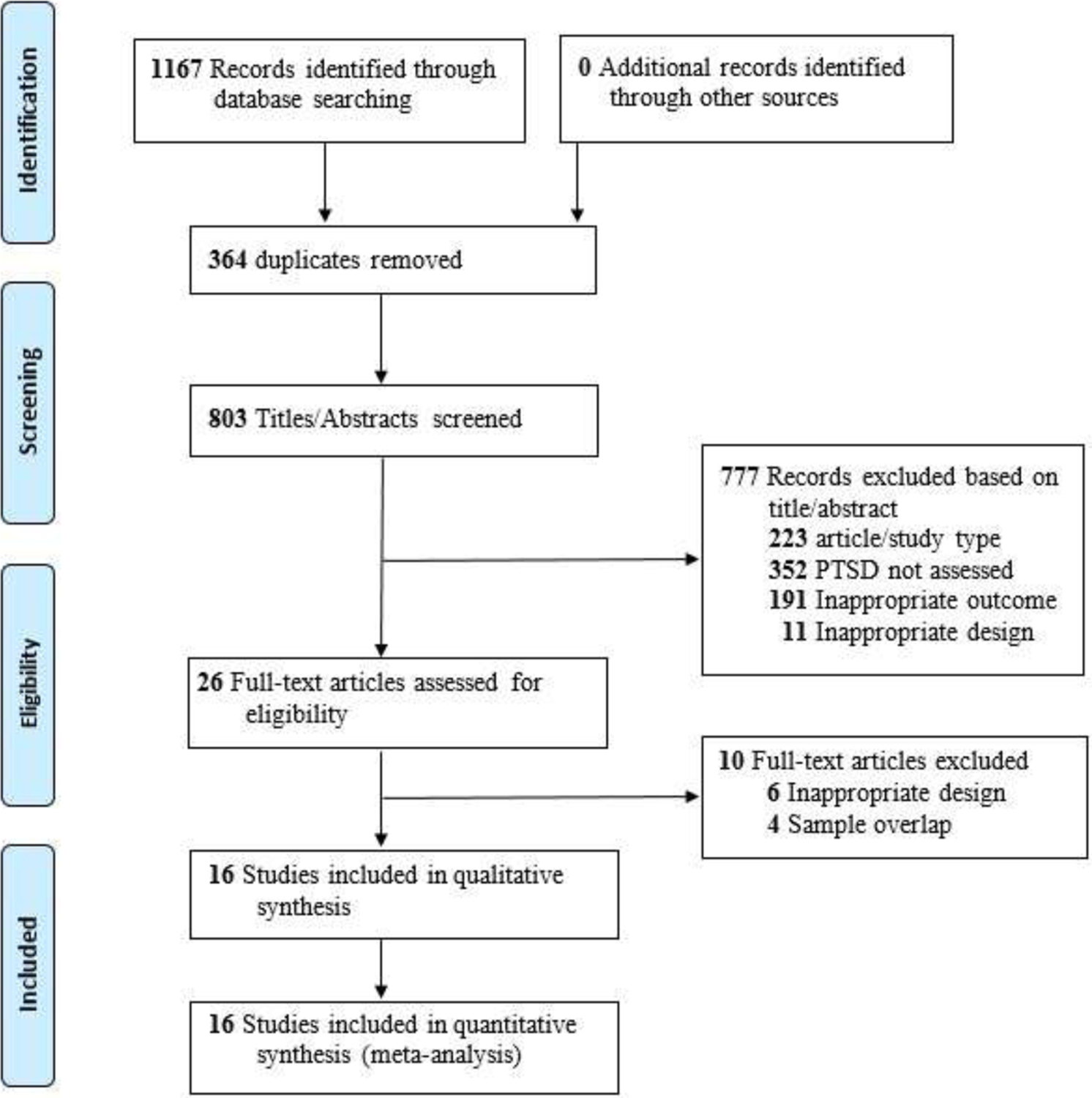
PRISMA Flow Diagram
From: Maher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and .Meta-Analyses: The PRISMA Statement. PLOS Med 6(6): e1000097. doi;10.1371/jornal.pmed1000097 For more information, visit www.prisma-statement.org
2.2. Study Selection and Eligibility Criteria
Four reviewers independently double-screened each title and reviewed abstracts for inclusion criteria. Included were cohort, longitudinal, and cross-sectional (retrospective reports of prenatal PTSD) studies that reported quantitative data, participants with at least probable PTSD prenatally, and at least one measure of adverse birth outcome (i.e., LBW, BW, PTB, GA). The lead author (P.M.S.) resolved discrepancies between reviewers by consensus through group review of abstracts and/or full text. Title/abstract screening excluded 777 articles from the 803 unduplicated identified articles for the following reasons prioritized in this order: 1) N = 223 were not peer-reviewed quantitative journal articles in English, 2) N = 352 articles lacked participants with at least probable prenatal PTSD (per PTSD measure/medical records), 3) N = 191 lacked birthweight or gestational age outcomes, and 4) N = 11 lacked data analyses of prenatal PTSD with a birth outcome. Articles meeting more than one exclusion criteria were excluded based on the first one met in the prioritized list. Twenty-six articles underwent full text analysis: 6 articles were excluded for reason #4 (no analyses of prenatal PTSD with birth outcome) and 4 articles were excluded for sample overlap with included articles. This resulted in 16 total articles for meta-analyses. See Figure 1.
2.3. Data Extraction and Quality Assessment
Data were extracted using a coding form developed by co-author J.S.T. for a prior published meta-analysis (Tonigan et al., 2018) and revised for this topic. Four reviewers independently (J.L., K.F., M.C.H., K.C.; trained by P.M.S.) extracted data from articles (with 2 reviewers per article). Discrepancies were resolved with the fifth reviewer (P.M.S.). PTSD and birth outcome variables were verified a final time (by P.M.S. and A. R.) for each included article prior to conducting each meta-analysis. Extracted participant data included age range, socioeconomic status, race/ethnicity, education, employment, geographic location, special population characteristics, and PTSD classification. Extracted design data included sample size, PTSD measure, PTSD period, study funding, sample origin, exclusion/inclusion criteria, recruitment strategy, source of outcome data, prospective/secondary hypothesis testing, and statistical analyses. Outcomes were low birth weight (LBW), birth weight (BW), preterm birth (PTB), and gestational age (GA). LBW and PTB were defined by studies either by ICD codes or as LBW: <2500 grams and PTB: <37 weeks. PTSD was defined as “at least probable PTSD” and included meeting diagnostic criteria, meeting a severity scale cutoff, or a diagnostic code (ICD or DSM) from medical records. Study quality was rated by reviewers using the Newcastle-Ottowa Quality Assessment Scale for Cohort Studies (NOS) (Wells et al., 2013). See Table 1 for study characteristics.
Table 1.
Characteristics of All Studies Included in the Meta-analyses
| Source | N | N PTSD | Sample Origin | Participant Exclusions | Design | PTSD Meas. | PTSD Var. | PTSD Period | Birth Outcomes | Study Qual. |
|---|---|---|---|---|---|---|---|---|---|---|
| Engel et al. (2005) | 50 | 4 | CT | MP | L, CR | PCL | S | NR | BW, GA | 7 |
| Ferri et al. (2007) | 795 | 62 | OC | MP, MA | CS | CIDI | D | 1-year | LBW | 9 |
| Gelaye et al. (2020) | 4408 | 1519 | OC | MTP, MP, PS,MA, Language | L | PCL | P | 30-day | BW, LBW, GA, PTB | 7 |
| Harville et al. (2015) | 297 | 27 | OC, CO, CT | MP, PS, MA, PC | L, CR | PCL | P | NR | BW, LBW, GA, PTB | 8 |
| Kang-Yi et al. (2017) | 9930 | 269 | DB | Date of delivery | CR | CR | D | 1-year | PTB | 7 |
| Koen et al. (2016) | 366 | 106 | OC | PS, MA | L | MINI | D | Lifetime | LBW, GA, PTB | 4 |
| Lipkind et al. (2010) | 446 | 61 | CO, DB, CT | MP, MC, EGA, MA, EBW, Sm | CS | PCL | P | 30-day | BW, LBW, GA, PTB | 8 |
| MacGinty et al. (2020) | 959 | 126 | OC | PS, MA | L | MPSS | P | NR | BW | 5 |
| Morland et al. (2007) | 101 | 16 | OC, CO | PS, MA | L, CR | PCL | P, S | NR | BW, GA | 3 |
| Rashid et al. (2020) | 450 | 84 | OC | MP, MC, PS, EGA | L | MINI | P | 30-day | LBW | 9 |
| Rogal et al. (2007) | 1100 | 31 | OC | None | L, CR | MINI | D | 30-day | BW, LBW, GA, PTB | 8 |
| Seng et al. (2011b) | 405 | 98 | OC | MTP, MA, MP, Eng, PC | L, CR | NWS | D | 30-day | BW, LBW, GA, PTB | 9 |
| Shaw et al. (2014) | 16334 | 1921 | DB | None | CR | CR | D | 1 year | PTB | 8 |
| Shaw et al. (2018) | 12877 | 361 | DB | None | CR | CR | D | 1-year | PTB | 8 |
| Xiong et al. (2008) | 298 | 13 | OC, CT | MA, Eng | L, CR | PCL | P | NR | LBW, PTB | 7 |
| Yonkers et al. (2014) | 2654 | 129 | OC | MP, PS, MA, MC, Eng | L | MPSS | P | NR | PTB | 8 |
Abbreviations: N = sample size, Meas. = measure, Var. = variable, PTSD = posttraumatic stress disorder, Study Qual. = quality rated using the Newcastle-Ottawa Quality Assessment Scale for Cohort Studies (Wells et al., 2013), NR = Not Reported, CO = community, DB = database, OC = Obstetrics/Prenatal/Maternity Clinic, CT = Common Trauma Sample, MP = multiple pregnancy, MTP = multiparous, MA = Maternal Age, MC = Medical complications, PS = Stage of Pregnancy, PC = prenatal care, EBW = extreme low or high birth weight, EGA = extreme low or high gestational age, Sm = smoking, Eng = non-English speaking, CS = cross sectional, L = longitudinal, CR = Chart review, PCL = PTSD Checklist (-C, −5, or original), MINI = The Mini International Neuropsychiatric Interview, CITI = Composite International Diagnostic Interview, MPSS = Modified PTSD Symptom Scale, NWS = National Women’s Survey, P = Probable PTSD based on a cut-off score on a screening instrument, D = PTSD Diagnosis from clinical interview or medical records, S = PTSD severity (continuous measure), BW = birth weight, LBW = low birth weight, GA = Gestational Age, PTB = preterm birth
2.4. Effect Size Calculation and Statistical Analyses
Separate meta-analyses were conducted for 4 outcomes: 1) LBW (dichotomous), 2) BW (continuous), 3) PTB (dichotomous), and 4) GA (continuous) using Comprehensive Meta-Analysis version 3.3.070 (CMA) (Borenstein et al., 2014). One-tailed P values of .50 and sample sizes were entered for studies reporting non-significant results without statistical values (Rosenthal, 1995) when contacted authors could not provide values (2 studies). Sensitivity analyses were conducted with these 2 studies removed (from each of the 3 meta-analyses that included them). Pearson’s r was computed for effect sizes with continuous birth outcome measures. Odds ratios were computed for effect sizes with dichotomous birth outcomes (OR: odds for PTSD group over odds for non-PTSD group). The actual number of events/non-events (e.g. LBW/no LBW or PTB/no PTB) and sample sizes for conditions (e.g. PTSD/no PTSD) were entered for dichotomous outcome variables, when available (Chang and Hoaglin, 2017). Data for current-PTSD versus trauma-exposed PTSD-resilient comparison groups were entered when more than 2 PTSD groups (e.g., current PTSD, recovered from PTSD, trauma-exposed PTSD-resilient, no trauma) were reported. Forest plots with 95% confidence intervals (CI) for sensitivity and specificity were created. Random effects models were used for all meta-analyses due to heterogeneity across samples pulled from varied sub-populations of pregnant women (Borenstein et al., 2010). Potential publication bias was evaluated by visual inspection of funnel plots (Sterne et al., 2011) and Eggers Tests (Egger et al., 1997).
2.5. Risk of Bias Across Studies
Cochran’s Q and I2 were computed to determine heterogeneity in pooled effect size and true variation between studies, respectively. When statistically significant heterogeneity was observed, we used mixed-effects models to test for moderating effects of 1) type of PTSD measure: self-administered checklist versus interview, 2) type of exclusion criteria: confounding risks for adverse prenatal outcome (e.g., twins, prior PTB) versus study logistics exclusions (e.g., language spoken, clinic enrollment), and 3) PTSD period: prenatal period (e.g., 30-day, 1-year before birth) versus not clearly limited to prenatal period (e.g., lifetime, undefined).
3. Results
3.1. Study Characteristics
Of the 16 articles included, 11 were longitudinal in design, 9 used chart review, and 2 were cross sectional. Among the studies, 10 reported LBW, 7 reported BW, 11 reported PTB, and 7 reported GA outcomes. See Table 1 for study characteristics. Studies had a total of 51,470 participants, 4,334 (8%) with PTSD diagnoses, probable PTSD (based on severity cut-off), or subclinical PTSD (symptoms present but below criteria scoring threshold: combined with PTSD in 1 study). Sample sizes ranged from 50 to 16,334 (mean = 3,216, SD = 5,137). Samples were from the United States (11 articles), Africa (2 articles), Central/South America (2 articles), and Asia (1 article).
3.2. Risk of Bias Within Studies
The average study quality was high at 7.2, SD = 1.76 with a range of 3–9 (the NOS scale measures a range of 0–9; See Table 1). All studies used clinical diagnoses or standardized scales for PTSD with 50% using more conservative interview methods (i.e., interviews require more resources but are generally more reliable than checklists). Half the studies excluded participants with potentially confounding risks for LBW or PTB (e.g., twins, prior PTB), and 56% of studies limited PTSD assessment to a prenatal timeframe (vs. lifetime PTSD or unspecified timeframe). Higher risk of bias within studies was largely related to imprecision in prenatal PTSD determination and, thus, was most likely to result in Type II errors.
3.3. Results for Birth Weight
3.3.1. Low birth weight (LBW: dichotomous)
Prenatal PTSD versus no PTSD was associated with greater odds of LBW. Ten studies (8666 participants) produced a positive combined effect size: OR = 1.96 [95% CI, 1.26 to 3.03]; P = .003; Q = 40.2 (P < .001, I2 = 77.6%); Figure 2. There were no significant subgroup differences for PTSD measure (Q = 0.2, P = .682), exclusion criteria (Q = 2.7, P = .103), or PTSD period (Q = 1.5, P = .223). The funnel plot was symmetrical, suggesting publication bias was unlikely (Figure 3), and the Eggers test was not significant (P = .233).
Figure 2.
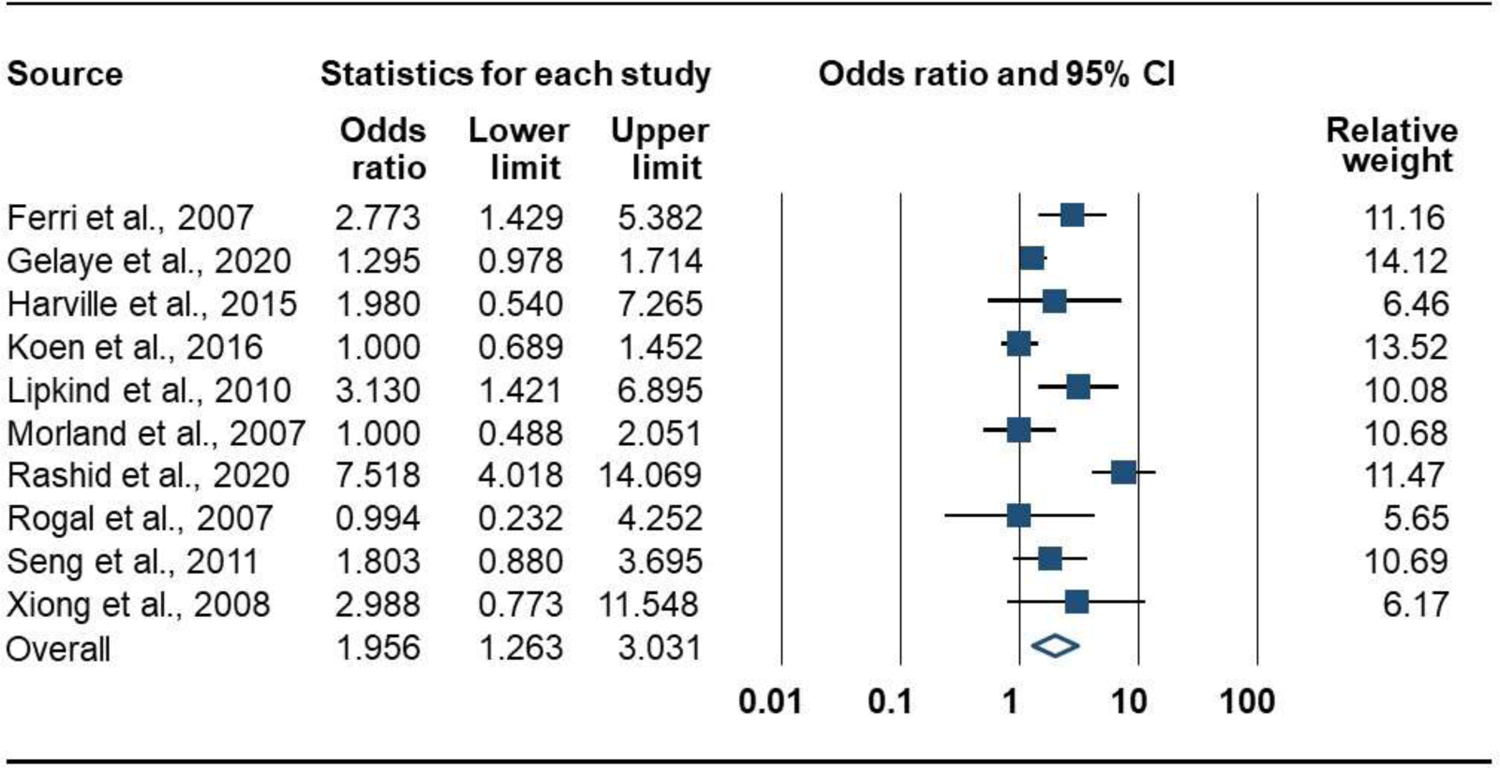
Forrest Plot of the Association of Prenatal PTSD with Risk for Low Birth Weight (dichotomous measure)
Figure 3.
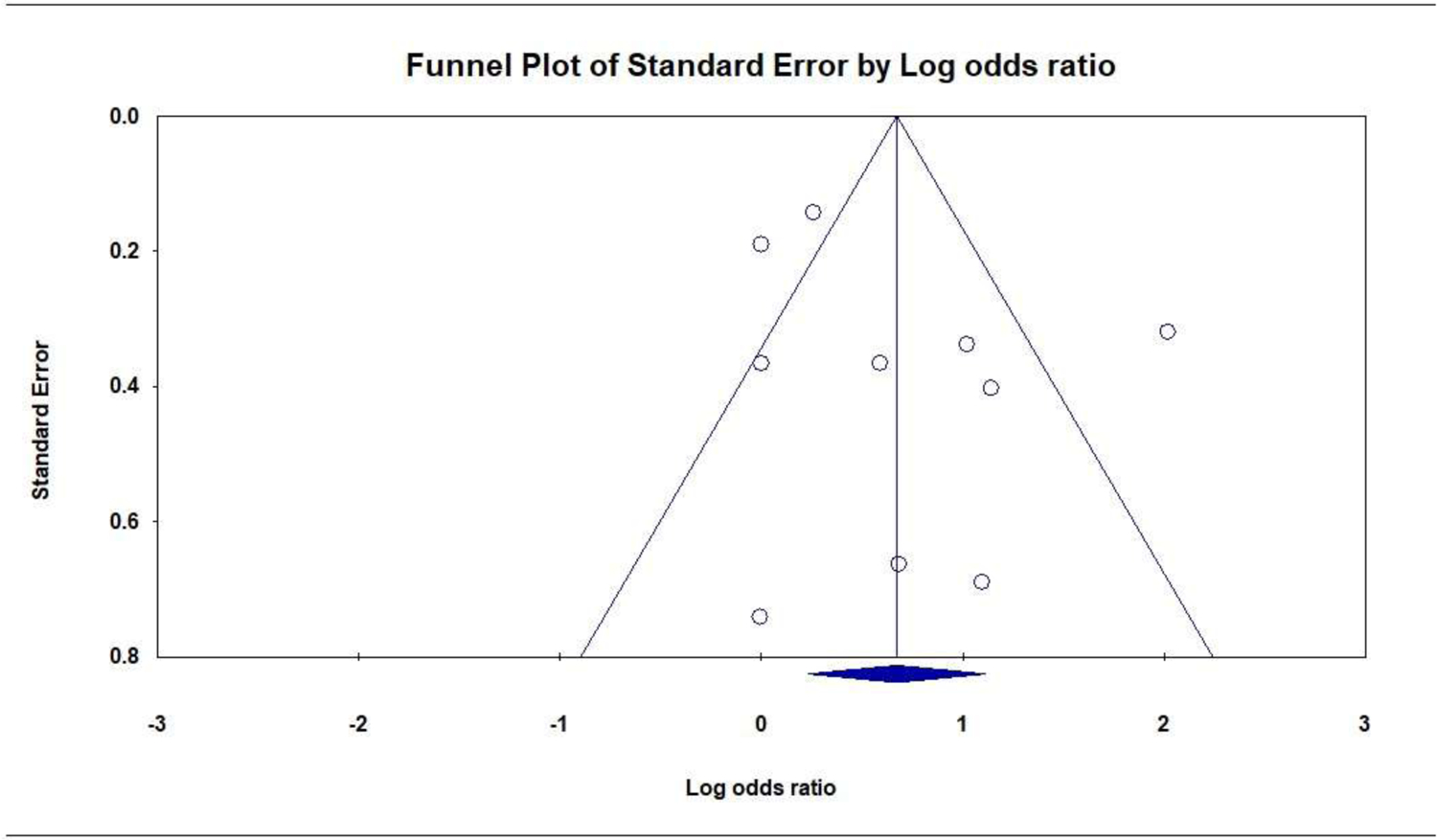
Publication Bias: Funnel Plot of the Association of Prenatal PTSD with Low Birth Weight (dichotomous)
3.3.2. Birth weight (BW: continuous)
BW was reported by 7 studies (7675 participants). The combined effect size, r, was not significant, indicating no association between PTSD and BW: r = −0.04 [95% CI, −0.09 to 0.01]; P = .096; Q = 16.9 (P = .010, I2 = 64.5%); Figure 4. There were no significant subgroup differences for PTSD measure (Q = 1.0, P = .314), exclusion criteria (Q = 2.1, P = .152), or PTSD period (Q = 1.7, P = .188). The symmetrical funnel plot did not suggest publication bias (Figure 5), and the Eggers test was not significant (P = .512).
Figure 4.
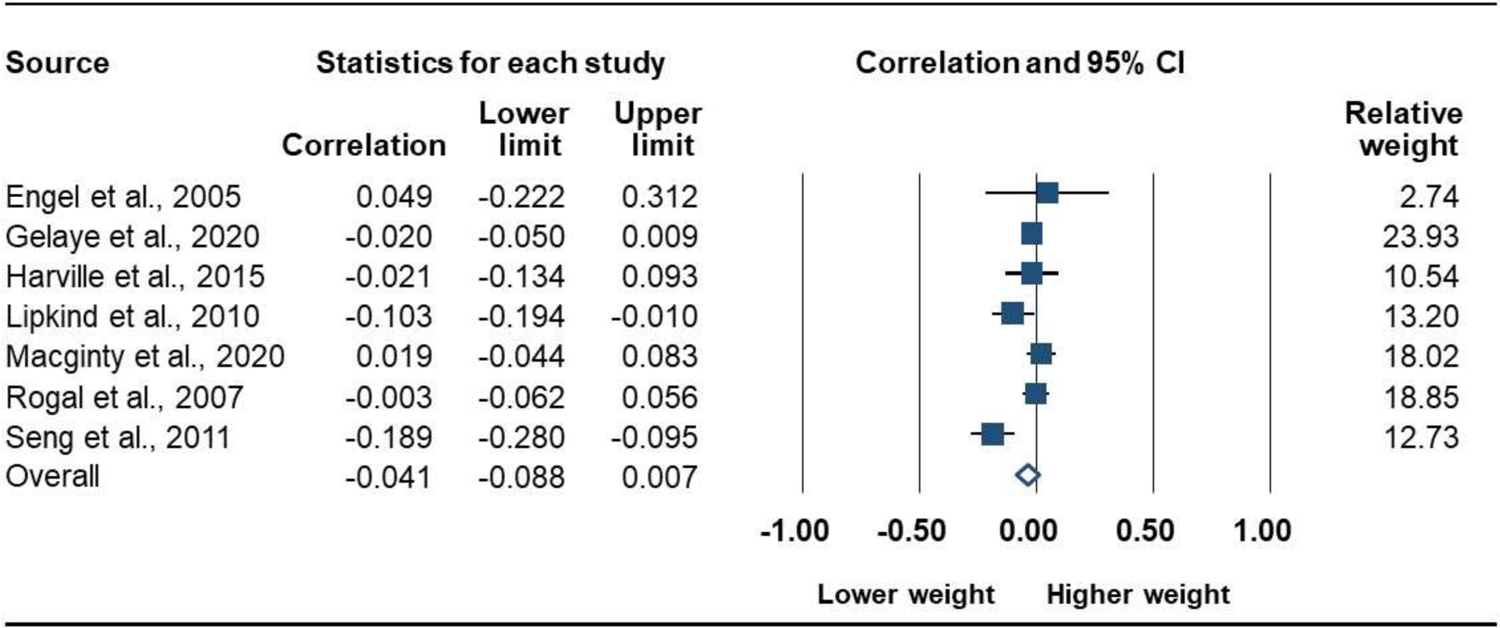
Forrest Plot of the Association of Prenatal PTSD with Birth Weight (continuous measure)
Figure 5.
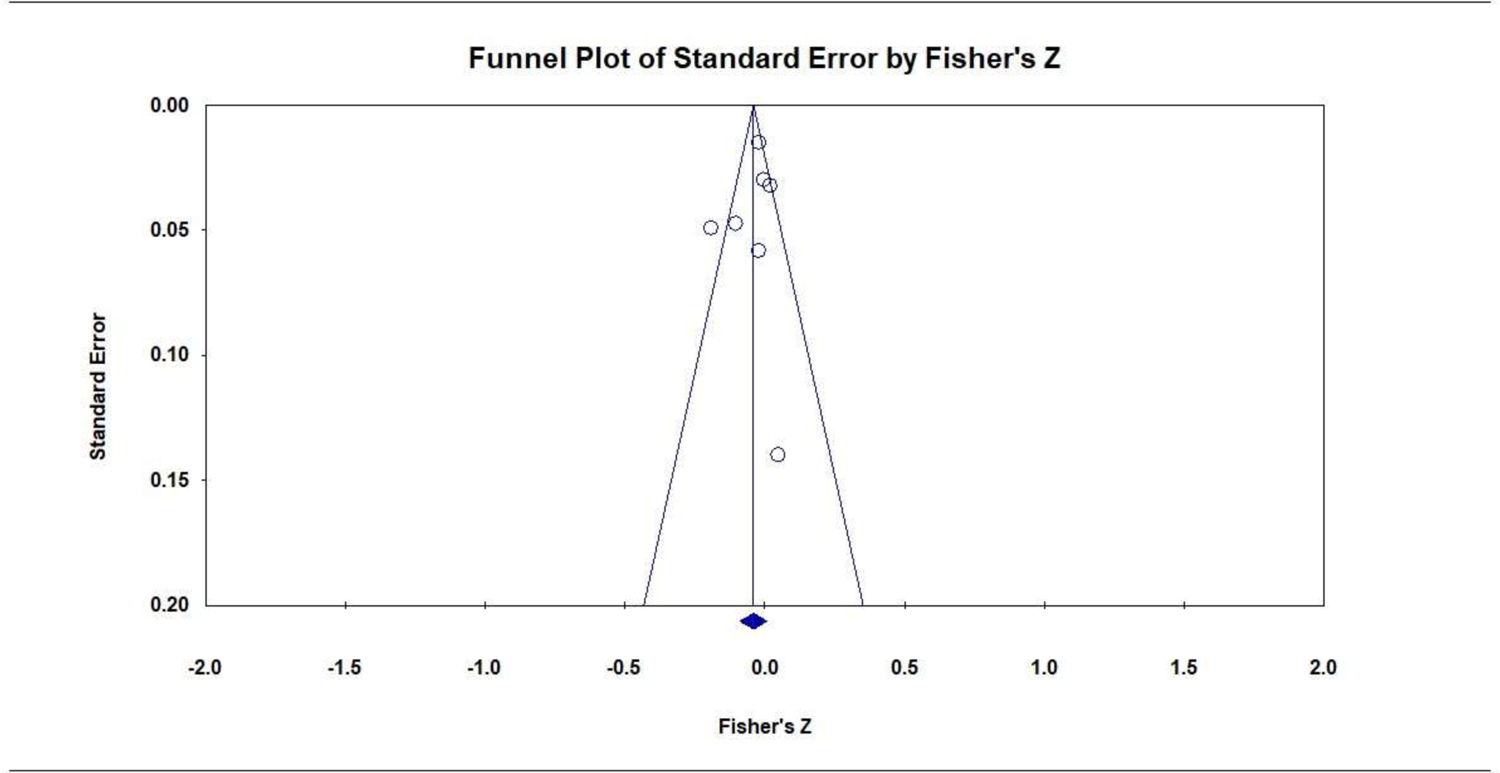
Publication Bias: Funnel Plot of the Association of Prenatal PTSD with Birth Weight (continuous)
3.4. Results for Preterm Birth and Gestational Age
3.4.1. Preterm Birth (PTB: dichotomous)
Prenatal PTSD versus no PTSD was associated with greater odds of PTB. Eleven studies (49,115 participants) produced a positive combined effect size: OR = 1.42 [95% CI, 1.16 to 1.73]; P = .001; Q = 18.3 (P = .032; I2 = 50.7%); Figure 6. There were no significant subgroup differences for PTSD measure (Q = 1.3, P = .247), exclusion criteria (Q = 3.2, P = .075), or PTSD period (Q = 0.3, P = .572). A symmetrical funnel plot did not suggest publication bias (Figure 7), and the Eggers test was not significant (P = .141).
Figure 6.
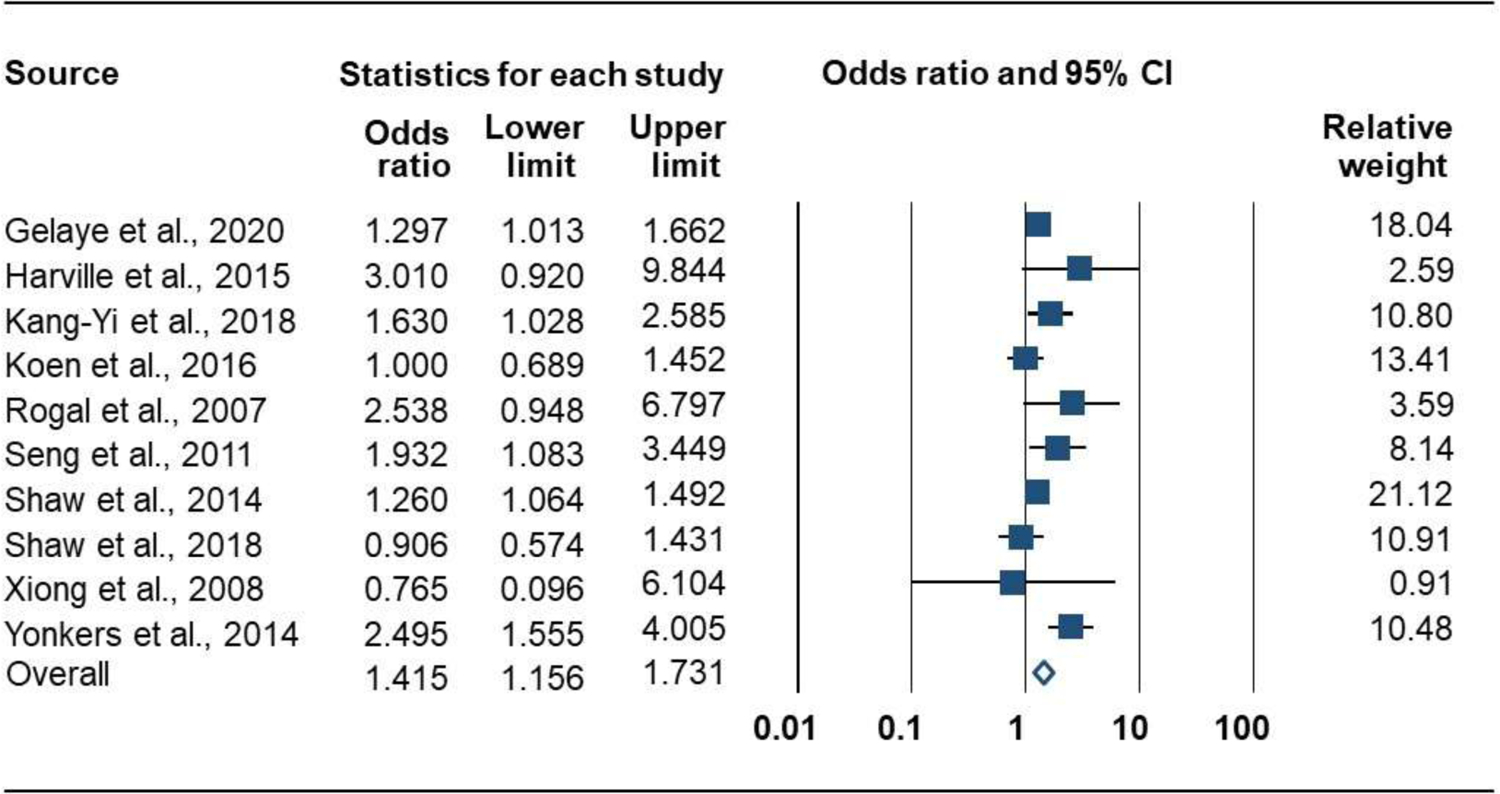
Forrest Plot of the Association of Prenatal PTSD with Risk for Preterm Birth (dichotomous measure)
Figure 7.
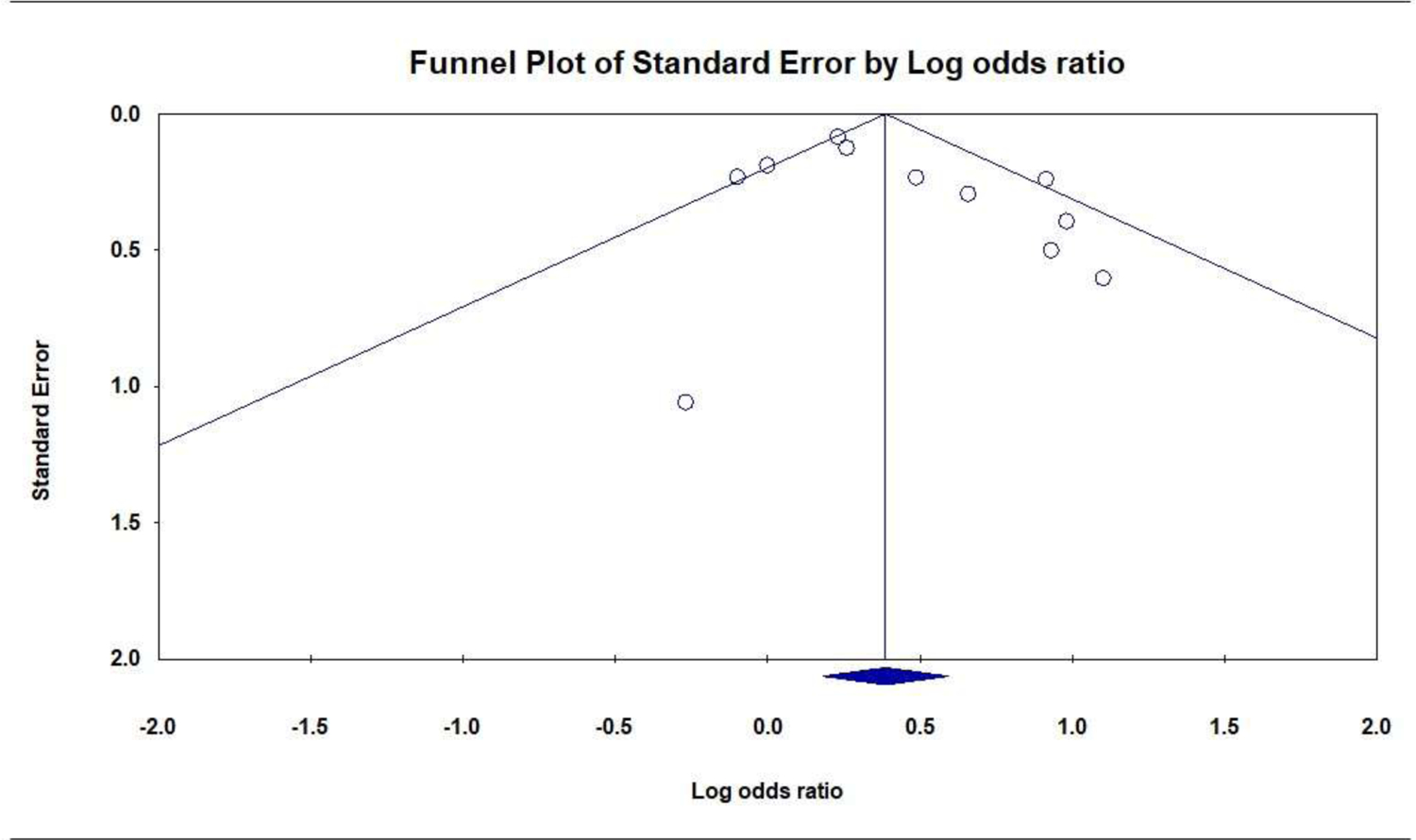
Publication Bias: Funnel Plot of the Association of Prenatal PTSD with Preterm Birth (dichotomous)
3.4.2. Gestational age (GA: continuous)
Prenatal PTSD versus no PTSD was associated with lower GA. Seven studies (7072 participants) produced a negative combined effect size: r = −0.04 [95% CI, −0.06 to −0.01]; P = .002; Q = 5.8 (P = .447; I2 = 0.0%); Figure 8. Subgroup analyses were not conducted because statistically significant heterogeneity was not observed in the analysis. The funnel plot was symmetrical (Figure 9) and the Eggers test was not significant (P = .866), thus there was no suggestion of publication bias.
Figure 8.
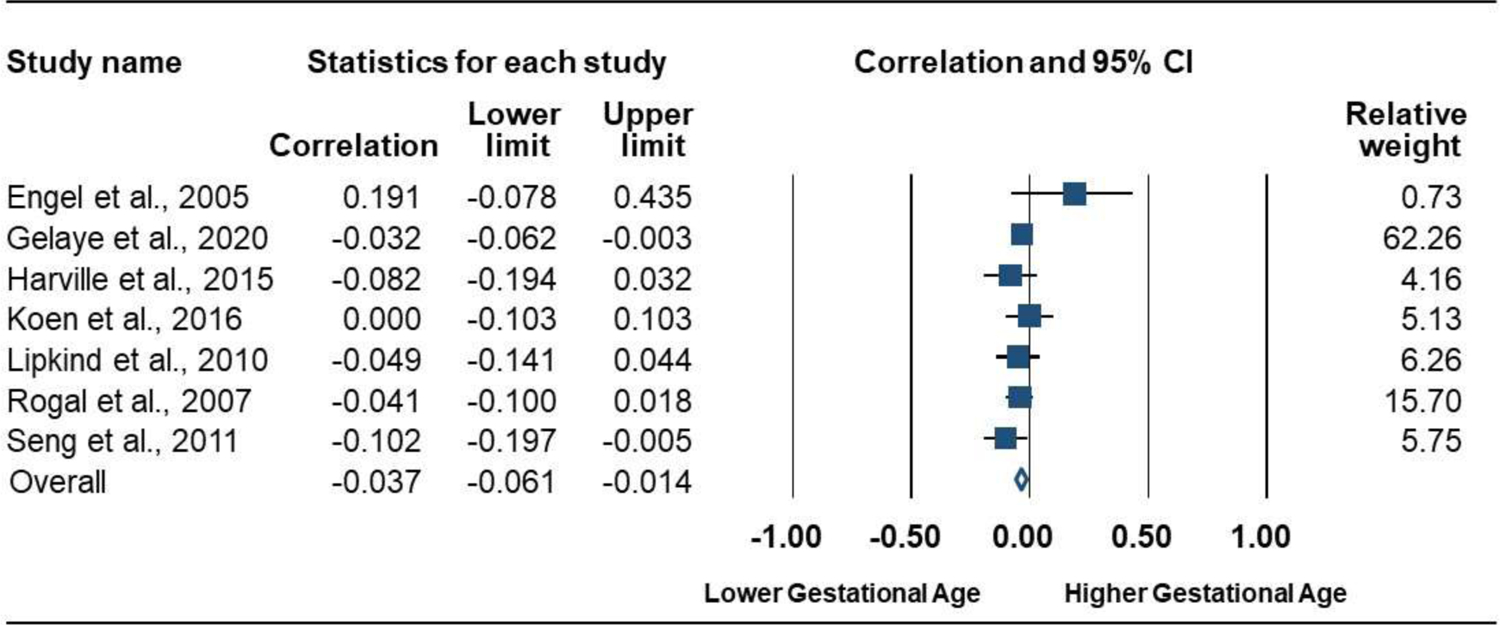
Forrest Plot of the Association of Prenatal PTSD with Gestational Age (continuous measure)
Figure 9.
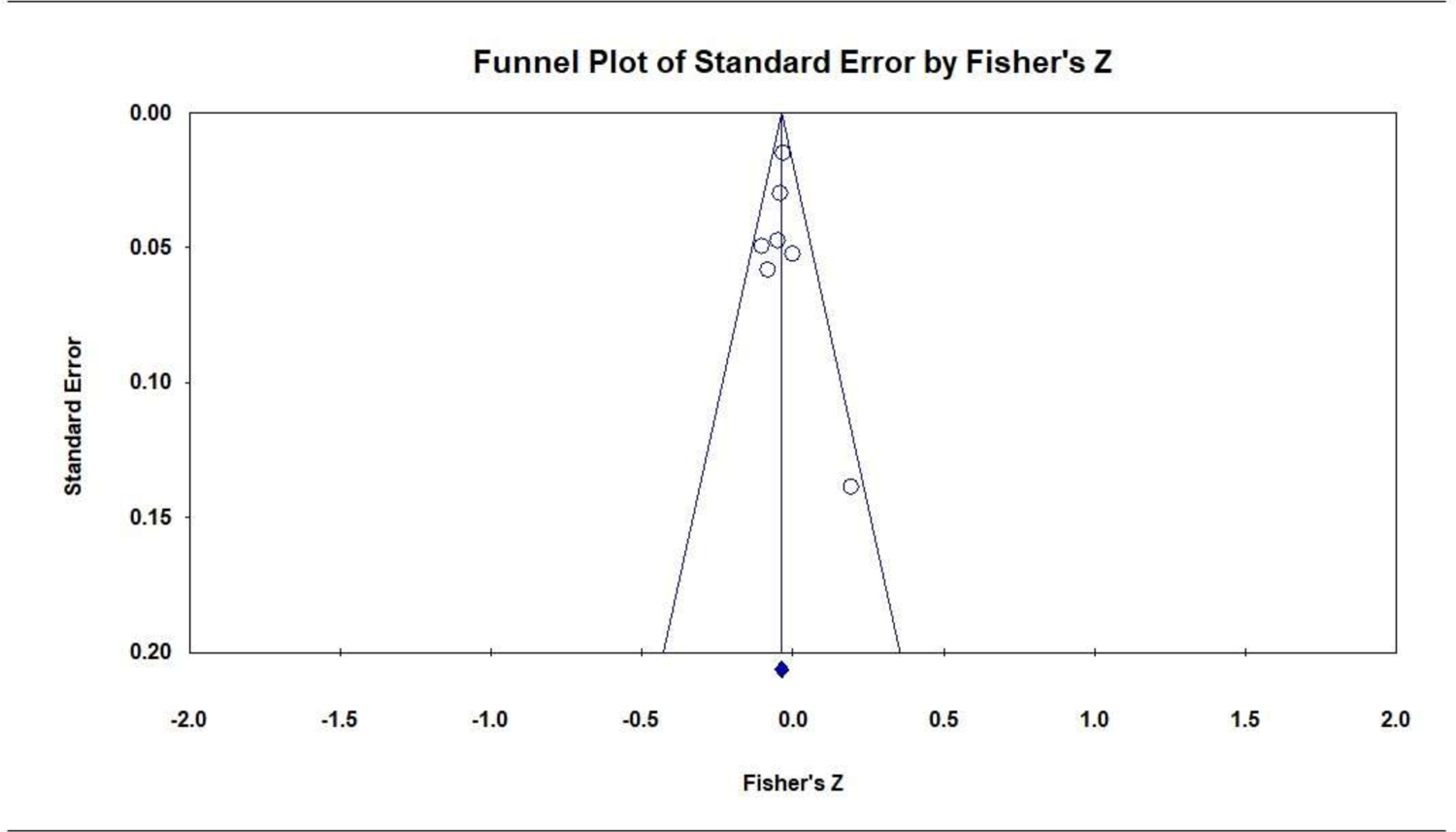
Publication Bias: Funnel Plot of the Association of Prenatal PTSD with Gestational Age (continuous)
3.5. Sensitivity Analyses
Sensitivity analyses removing the 2 studies reporting non-significant results without statistical values (Rosenthal, 1995) did not substantially change the significance of any meta-analyses results: LBW (OR = 2.42 [95% CI, 1.42 to 4.14]; P = .001); PTB (OR = 1.46 [95% CI, 1.25 to 1.94]; P < .000); and GA (r = −0.04 [95% CI, −0.07 to −0.01]; P = .002).
4. Discussion
This meta-analytic review summarizes results from 16 studies examining associations of prenatal PTSD with birth weight and gestational age outcomes. Prenatal PTSD was found to be associated with increased risk of LBW, PTB, and reduced GA. The higher sensitivity of the continuous BW measure, compared to the more clinically-relevant dichotomous LBW variable, may account for not finding a significant association between prenatal PTSD and BW. However, this non-significant association remained directionally consistent with results of the other 3 meta-analyses.
Our results provide the quantitative summary evidence for adverse impacts of prenatal PTSD on LBW, PTB, and reduced GA that was previously lacking in the literature. A qualitative literature review on this topic suggested a prenatal PTSD and LBW association (Cook et al., 2018). Additionally, a very different but related meta-analysis showed prenatal anxiety (that included some PTSD studies) was associated with adverse birth outcomes including LBW, reduced BW, PTB, and reduced GA (Grigoriadis et al., 2018). Our meta-analyses focused specifically on prenatal PTSD, which is common (Viswasam et al., 2019; Yildiz et al., 2017) and is associated with prenatal health-risk behaviors, disrupted prenatal fetal bonding, postpartum depression, and impaired postpartum mother-infant attachment (Onoye et al., 2009; Radoš et al., 2020; Sanjuan et al., 2020, 2019). Our results show prenatal PTSD is significantly associated with LBW, PTB, and reduced GA. These results provide evidence of potential physical harm to the developing fetus posed by prenatal PTSD. This gives urgency to the calls to include prenatal PTSD as an important clinical consideration in prenatal mental health care and for research to determine the best prenatal PTSD treatments (Canfield and Silver, 2020; Geller and Stasko, 2017; Yildiz et al., 2017).
The American College of Obstetricians and Gynecologists (ACOG) recommends prenatal screening for depression, domestic violence, and anxiety (American College of Obstetricians and Gynecologists, 2018, 2012a). Recently, new guidance from ACOG recommends universal screening for current trauma and history of trauma by obstetricians and gynecologists followed by provision of educational materials and appropriate referrals (American College of Obstetricians and Gynecologists, 2021a). Our results support the recommendation that maternity care providers screen and refer patients for trauma and appropriate PTSD treatment. The results of this meta-analysis also reinforce the need for mental health integrated with maternity care (Cox et al., 2017). ACOG previously recommended screening for trauma and PTSD for specific populations including women in the military (American College of Obstetricians and Gynecologists, 2012b) and survivors of sexual assault (American College of Obstetricians and Gynecologists, 2019), and very recently for incarcerated women (American College of Obstetricians and Gynecologists, 2021b). While our results are of particular relevance in clinical populations with higher PTSD risk (e.g., veterans, people from marginalized populations, those with substance use disorders (Harner et al., 2015; Hugin and Shaw, 2019; Moylan et al., 2001; Seng et al., 2011a)), it is of note that the routine universal trauma screening as newly recommended by ACOG is an improvement that will capture cases missed by an overly-narrow focus on only high-risk patients.
It is important to note that the new ACOG recommendation broadly focuses on psychological trauma and not PTSD specifically. As the conditional risk of developing PTSD following a traumatic experience ranges from 2.5% to 17.6%, most people who have experienced trauma do not develop PTSD, and mental health referrals may be inappropriate in many of cases of reported trauma (Atwoli et al., 2015). Screening for PTSD specifically may better capture the potential risk associated with trauma history in pregnant women.
Similar to prenatal anxiety and depression (Rogers et al., 2020), prenatal PTSD is associated with postpartum PTSD (Onoye et al., 2009), postpartum depression, (Seng et al., 2013b), and disrupted mother-child attachment (Muzik et al., 2012; Seng et al., 2013b; Webb and Ayers, 2015). Thus, routine screening and appropriate referral to treatment of pregnant patients for PTSD can have effects transcending birth outcomes. The 5-item Primary Care PTSD Screen for DSM-5 (PC-PTSD-5) developed by researchers at the National Center for PTSD is one option that could be used for such screening (Prins et al., 2016). An earlier 4-item DSM-IV version was validated with a prenatal sample (Wenz-Gross et al., 2016) and further research is warranted using the updated version.
Fortunately, many PTSD treatments are particularly safe during pregnancy (International Society for Traumatic Stress Studies (ISTSS), 2018), but, as with many mental health treatments, PTSD therapies can take weeks to months for full effects. PTSD is associated with altered circadian cortisol profiles (Bowers and Yehuda, 2016; Yehuda et al., 2010), and this may be a primary mechanism by which prenatal PTSD places women at risk of LBW and PTB (Diego et al., 2006). Similarly, depression and anxiety (disorders with PTSD symptom overlap) are also associated with LBW and PTB (Ding et al., 2014; Gelaye et al., 2020; Grote et al., 2010; Lewis et al., 2016). Moreover, altered circadian cortisol profiles are linked to general prenatal psychological distress, depression, and anxiety (Van den Heuvel et al., 2018); mediate the relationship between broad prenatal psychological distress and PTB (Gilles et al., 2018); and predict fetal weight (Diego et al., 2006). This suggests that reduction of PTSD-related distress leading to improved cortisol profiles earlier in pregnancy may be ideal to improve birth outcomes. Additionally, if rapid physiological stress-reduction is a critical treatment goal, this may indicate a role for complementary interventions (often tailored for pregnancy: e.g., prenatal yoga, doulas, tai chi) provided in conjunction with psychotherapy to enhance treatment effects (Beddoe et al., 2009; Dhillon et al., 2017; Field et al., 2013; Hong Gong et al., 2015; International Society for Traumatic Stress Studies (ISTSS), 2018; Lanning and Klaman, 2019). The recent increase in studies examining prenatal PTSD and birth outcomes mirrors the growing interest among clinical providers to consider prenatal psychological disorders overall, however prenatal PTSD treatment research is still at an early stage (Rowe et al., 2014; Weinreb et al., 2018). It remains unknown whether current PTSD interventions can improve birth weight or gestational age outcomes at all, and if so, whether there might exist critical periods for such intervention. Research in these areas is greatly needed to determine whether PTSD treatment can improve these outcomes and, if so, the most effective prenatal PTSD treatments and critical periods for intervention to reduce risk of LBW, PTB, and reduced GA. Additional research might also examine whether different trauma types (e.g., sexual assault, motor vehicle accidents, combat) are differentially associated with these outcomes.
4.1. Limitations
First, as this is an emerging area of research, the pool of studies was modest, with limited PTSD-positive cases in some studies. More studies with higher numbers of positive cases would increase confidence in aggregated findings. The modest sample size also increased the chances of Type II error for the heterogeneity and Eggers tests, which are prone to low power. Second, heterogeneity across studies was detected in 3 meta-analyses. Despite sufficient information for sub-group analyses with some moderators, variation in participant characteristics reported across studies made it impossible to examine other potential moderators (e.g., race, trauma-type, substance use, poverty). For example, one study found trauma history and race mediated PTSD effects on birth outcomes (Seng et al., 2011b). Yet, we could not evaluate this, because trauma history and race (especially as race is not uniformly defined internationally) were not consistently reported across studies. The included studies also differed in PTSD assessment methods (e.g., interview versus checklist, symptom duration). Although we conducted subgroup analyses for some PTSD assessment differences, we could not account for all differences across studies. Third, we also did not include non-English publications because we lacked adequate translation resources.
The potential public health impact of our results is great, despite modest magnitudes of these associations (Chen et al., 2010), as preterm birth is the leading cause of perinatal morbidity and mortality (Goldenberg et al., 2008). Fetal growth restriction is similarly a major risk factor for still-birth as well as neonatal mortality and morbidity (Resnik, 2002). Our findings should be viewed in context with other risks for these outcomes. Many factors beyond mental health are associated with LBW (Lewis et al., 2016) and PTB (Muglia and Katz, 2010), and our results are similar in magnitude to those found for the associations of prenatal depression (Grote et al., 2010) or anxiety disorders (Ding et al., 2014; Grigoriadis et al., 2018) with LBW and PTB. This is consistent with a dimensional framework of psychopathology where biological mechanisms underlie, or are otherwise associated with, psychiatric diagnoses (Kozak and Cuthbert, 2016). Thus, it may be that any of the broader group of affective disorders alter HPA axis functioning, thereby increasing risks of adverse birth weight and gestational age outcomes.
5. Conclusions
Our meta-analyses found prenatal PTSD was associated with increased risk for low birth weight, preterm birth, and reduced gestational age. Our results suggest that prenatal PTSD screening and referral to evidence-based PTSD interventions (similar to recommended prenatal protocols for depression, domestic violence, and anxiety) could provide an opportunity to improve these birth outcomes. More research is critical to determine physiological mechanisms for this relationship and to identify those mental health interventions that best reduce this risk.
Supplementary Material
Highlights.
Prenatal posttraumatic stress disorder is associated with risk of low birth weight.
Prenatal posttraumatic stress disorder is associated with lower gestational age.
Prenatal posttraumatic stress disorder is associated with risk of preterm birth.
Prenatal PTSD may adversely affect maternal and child well-being.
Prenatal PTSD screening and interventions may improve mother and child outcomes.
Acknowledgements
We thank Carly Poremba who assisted substantially with the initial review of abstracts and titles. We also thank the authors we contacted who supplied us with additional information. Finally, we thank Joseph Guydish and Kevin Delucchi at the University of California, San Francisco and Hortensia Amaro at Florida International University for their critiques of drafts and research design advice.
Funding
This study was supported in part by the National Institutes of Health K23AA025094 (Sanjuan), R21DA048058 (Sanjuan), R21MH118765 (Sanjuan), K24AA021157 (Tonigan), and R25DA035163 (Masson, Sorensen).
Role of the Funder/Sponsor
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures
All authors declare they have no conflicts of interest.
References
- Adewuya AO, Ola BA, Aloba OO, Mapayi BM, 2006. Anxiety disorders among Nigerian women in late pregnancy: a controlled study. Arch. Womens Ment. Health 9, 325–328. 10.1007/s00737-006-0157-5 [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists, 2021a. Caring for patients who have experienced trauma. ACOG Committee Opinion No. 825. Obstet. Gynecol 137, e94–9. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists, 2021b. Reproductive health care for incarcerated pregnant, postpartum, and nonpregnant individuals: ACOG Committee Opinion, Number 830. Obstet. Gynecol 138, e24. 10.1097/AOG.0000000000004429 [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists, 2019. ACOG Committee Opinion No. 777 Summary: Sexual Assault. Obstet. Gynecol 133, 850–851. 10.1097/AOG.0000000000003179 [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists, 2018. ACOG Committee Opinion No. 757: screening for perinatal depression. Obstet. Gynecol 132, e208–e212. 10.1097/AOG.0000000000002927 [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists, 2012a. Committee Opinion No. 518: intimate partner violence. Obstet. Gynecol 119, 412–417. 10.1097/AOG.0b013e318249ff74. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists, 2012b. Committee Opinion No. 547: health care for women in the military and women veterans. Obstet. Gynecol 120, 1538–1542. 10.1097/01.AOG.0000423821.70036.5a [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and statistical manual of mental disorders: DSM-5™ (5th ed.). American Psychiatric Publishing, Inc., Arlington, VA US. [Google Scholar]
- Annagür BB, Tazegül A, Gündüz S, 2013. Do psychiatric disorders continue during pregnancy in women with hyperemesis gravidarum: a prospective study. Gen. Hosp. Psychiatry 35, 492–496. 10.1016/j.genhosppsych.2013.05.008 [DOI] [PubMed] [Google Scholar]
- Association of Women’s Health, Obstetric, and Neonatal Nurses, 2015. Mood and Anxiety Disorders in Pregnant and Postpartum Women. J. Obstet. Gynecol. Neonatal Nurs 44, 687–689. 10.1111/1552-6909.12734 [DOI] [Google Scholar]
- Atwoli L, Stein DJ, Koenen KC, McLaughlin KA, 2015. Epidemiology of posttraumatic stress disorder: prevalence, correlates and consequences. Curr. Opin. Psychiatry 28, 307–311. 10.1097/YCO.0000000000000167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbano AC, van der Mei WF, deRoon-Cassini TA, Grauer E, Lowe SR, Matsuoka YJ, O’Donnell M, Olff M, Qi W, Ratanatharathorn A, Schnyder U, Seedat S, Kessler RC, Koenen KC, Shalev AY, 2019. Differentiating PTSD from anxiety and depression: Lessons from the ICD-11 PTSD diagnostic criteria. Depress. Anxiety 36, 490–498. 10.1002/da.22881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddoe AE, Paul Yang C-P, Kennedy HP, Weiss SJ, Lee KA, 2009. The effects of mindfulness-based yoga during pregnancy on maternal psychological and physical distress. J. Obstet. Gynecol. Neonatal Nurs 38, 310–319. 10.1111/j.1552-6909.2009.01023.x [DOI] [PubMed] [Google Scholar]
- Berthelot N, Lemieux R, Garon-Bissonnette J, Drouin-Maziade C, Martel É, Maziade M, 2020. Uptrend in distress and psychiatric symptomatology in pregnant women during the coronavirus disease 2019 pandemic. Acta Obstet. Gynecol. Scand 99, 848–855. 10.1111/aogs.13925 [DOI] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A-B, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, Lawn JE, 2012. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. The Lancet 379, 2162–2172. 10.1016/S0140-6736(12)60820-4 [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR, 2014. Comprehensive Meta Analysis.
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR, 2010. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 1, 97–111. 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- Bowers ME, Yehuda R, 2016. Intergenerational transmission of stress in humans. Neuropsychopharmacology 41, 232–244. 10.1038/npp.2015.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield D, Silver RM, 2020. Detection and prevention of postpartum posttraumatic stress disorder: a call to action. Obstet. Gynecol 136, 1030–1035. 10.1097/AOG.0000000000004093 [DOI] [PubMed] [Google Scholar]
- Chang B-H, Hoaglin DC, 2017. Meta-analysis of odds ratios: current good practices. Med. Care 55, 328–335. 10.1097/MLR.0000000000000696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Cohen P, Chen S, 2010. How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Commun. Stat. - Simul. Comput 39, 860–864. 10.1080/03610911003650383 [DOI] [Google Scholar]
- Cook N, Ayers S, Horsch A, 2018. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: a systematic review. J. Affect. Disord 225, 18–31. 10.1016/j.jad.2017.07.045 [DOI] [PubMed] [Google Scholar]
- Cox EQ, Raines C, Kimmel M, Richardson E, Stuebe A, Meltzer-Brody S, 2017. Comprehensive integrated care model to improve maternal mental health. J. Obstet. Gynecol. Neonatal Nurs 46, 923–930. 10.1016/j.jogn.2017.08.003 [DOI] [PubMed] [Google Scholar]
- Crump C, Sundquist J, Winkleby MA, Sundquist K, 2019. Gestational age at birth and mortality from infancy into midadulthood: a national cohort study. Lancet Child Adolesc. Health 3, 408–417. 10.1016/S2352-4642(19)30108-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paula Eduardo JAF, de Rezende MG, Menezes PR, Del-Ben CM, 2019. Preterm birth as a risk factor for postpartum depression: A systematic review and meta-analysis. J. Affect. Disord 259, 392–403. 10.1016/j.jad.2019.08.069 [DOI] [PubMed] [Google Scholar]
- Dhillon A, Sparkes E, Duarte RV, 2017. Mindfulness-based interventions during pregnancy: A systematic review and meta-analysis. Mindfulness 8, 1421–1437. 10.1007/s12671-017-0726-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diego MA, Jones NA, Field T, Hernandez-Reif M, Schanberg S, Kuhn C, Gonzalez-Garcia A, 2006. Maternal psychological distress, prenatal cortisol, and fetal weight. Psychosom. Med 68, 747–753. 10.1097/01.psy.0000238212.21598.7b [DOI] [PubMed] [Google Scholar]
- Ding X-X, Wu Y-L, Xu S-J, Zhu R-P, Jia X-M, Zhang S-F, Huang K, Zhu P, Hao J-H, Tao F-B, 2014. Maternal anxiety during pregnancy and adverse birth outcomes: A systematic review and meta-analysis of prospective cohort studies. J. Affect. Disord 159, 103–110. 10.1016/j.jad.2014.02.027 [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C, 1997. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Berkowitz GS, Wolff MS, Yehuda R, 2005. Psychological trauma associated with the World Trade Center attacks and its effect on pregnancy outcome. Paediatr. Perinat. Epidemiol 19, 334–341. 10.1111/j.1365-3016.2005.00676.x [DOI] [PubMed] [Google Scholar]
- Ferri CP, Mitsuhiro SS, Barros MC, Chalem E, Guinsburg R, Patel V, Prince M, Laranjeira R, 2007. The impact of maternal experience of violence and common mental disorders on neonatal outcomes: a survey of adolescent mothers in Sao Paulo, Brazil. BMC Public Health 7, 209. 10.1186/1471-2458-7-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Diego M, Delgado J, Medina L, 2013. Tai chi/yoga reduces prenatal depression, anxiety and sleep disturbances. Complement. Ther. Clin. Pract 19, 6–10. 10.1016/j.ctcp.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman MJ, 2013. Finalizing PTSD in DSM-5: getting here from there and where to go next. J. Trauma. Stress 26, 548–556. 10.1002/jts.21840 [DOI] [PubMed] [Google Scholar]
- Gelaye B, Sanchez SE, Andrade A, Gómez O, Coker AL, Dole N, Rondon MB, Williams MA, 2020. Association of antepartum depression, generalized anxiety, and posttraumatic stress disorder with infant birth weight and gestational age at delivery. J. Affect. Disord 262, 310–316. 10.1016/j.jad.2019.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller PA, Stasko EC, 2017. Effect of previous posttraumatic stress in the perinatal period. J. Obstet. Gynecol. Neonatal Nurs 46, 912–922. 10.1016/j.jogn.2017.04.136 [DOI] [PubMed] [Google Scholar]
- Gilles M, Otto H, Wolf IAC, Scharnholz B, Peus V, Schredl M, Sütterlin MW, Witt SH, Rietschel M, Laucht M, Deuschle M, 2018. Maternal hypothalamus-pituitary-adrenal (HPA) system activity and stress during pregnancy: effects on gestational age and infant’s anthropometric measures at birth. Psychoneuroendocrinology 94, 152–161. 10.1016/j.psyneuen.2018.04.022 [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R, 2008. Epidemiology and causes of preterm birth. The Lancet 371, 75–84. 10.1016/S0140-6736(08)60074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis S, Graves L, Peer M, Mamisashvili L, Tomlinson G, Vigod SN, Dennis C-L, Steiner M, Brown C, Cheung A, Dawson H, Rector NA, Guenette M, Richter M, 2018. Maternal anxiety during pregnancy and the association with adverse perinatal outcomes: systematic review and meta-analysis. J. Clin. Psychiatry 79. 10.4088/JCP.17r12011 [DOI] [PubMed] [Google Scholar]
- Grigoriadis S, VonderPorten EH, Mamisashvili L, Tomlinson G, Dennis C-L, Koren G, Steiner M, Mousmanis P, Cheung A, Radford K, 2013. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J. Clin. Psychiatry 74, 321–341. 10.4088/JCP.12r07968 [DOI] [PubMed] [Google Scholar]
- Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ, 2010. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch. Gen. Psychiatry 67, 1012–1024. 10.1001/archgenpsychiatry.2010.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harner HM, Budescu M, Gillihan SJ, Riley S, Foa EB, 2015. Posttraumatic stress disorder in incarcerated women: a call for evidence-based treatment. Psychol. Trauma-Theory Res. Pract. Policy 7, 58–66. 10.1037/a0032508 [DOI] [PubMed] [Google Scholar]
- Harville EW, Giarratano G, Savage J, Barcelona de Mendoza V, Zotkiewicz T, 2015. Birth outcomes in a disaster recovery environment: New Orleans women after Katrina. Matern. Child Health J 19, 2512–2522. 10.1007/s10995-015-1772-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Hong, Ni Chenxu, Shen Xiaoliang, Wu Tengyun, Jiang Chunlei, 2015. Yoga for prenatal depression: a systematic review and meta-analysis. BMC Psychiatry 15, 1–8. 10.1186/s12888-015-0393-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugin M, Shaw JG, 2019. Obstetric outcomes in U.S. veterans: emerging knowledge, considerations, and gaps. Semin. Reprod. Med 37, 17–23. 10.1055/s-0039-1692128 [DOI] [PubMed] [Google Scholar]
- International Society for Traumatic Stress Studies (ISTSS), 2018. ISTSS PTSD prevention and treatment guidelines: Methodology and recommendations. Author. [Google Scholar]
- Kang-Yi CD, Kornfield SL, Epperson CN, Mandell DS, 2017. Relationship between pregnancy complications and psychiatric disorders: a population-based study with a matched control group. Psychiatr. Serv 69, 300–307. 10.1176/appi.ps.201700097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J, Lee AC, Kozuki N, Lawn JE, Cousens S, Blencowe H, Ezzati M, Bhutta ZA, Marchant T, Willey BA, Adair L, Barros F, Baqui AH, Christian P, Fawzi W, Gonzalez R, Humphrey J, Huybregts L, Kolsteren P, Mongkolchati A, Mullany LC, Ndyomugyenyi R, Nien JK, Osrin D, Roberfroid D, Sania A, Schmiegelow C, Silveira MF, Tielsch J, Vaidya A, Velaphi SC, Victora CG, Watson-Jones D, Black RE, CHERG Small-for-Gestational-Age-Preterm Birth Working Group, 2013. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet Lond. Engl 382, 417–425. 10.1016/S0140-6736(13)60993-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koen N, Brittain K, Donald KA, Barnett W, Koopowitz S, Maré K, Zar HJ, Stein DJ, 2016. Psychological trauma and posttraumatic stress disorder: risk factors and associations with birth outcomes in the Drakenstein Child Health Study. Eur. J. Psychotraumatology 7, 28720. 10.3402/ejpt.v7.28720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak MJ, Cuthbert BN, 2016. The NIMH Research Domain Criteria Initiative: background, issues, and pragmatics. Psychophysiology 53, 286–297. 10.1111/psyp.12518 [DOI] [PubMed] [Google Scholar]
- Lanning RK, Klaman SL, 2019. Evaluation of an innovative, hospital-based volunteer doula program. J. Obstet. Gynecol. Neonatal Nurs 48, 654–663. 10.1016/j.jogn.2019.08.004 [DOI] [PubMed] [Google Scholar]
- Lewis AJ, Austin E, Galbally M, 2016. Prenatal maternal mental health and fetal growth restriction: a systematic review. J. Dev. Orig. Health Dis 7, 416–428. 10.1017/S2040174416000076 [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D, 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol 62, e1–e34. 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- Lipkind HS, Curry AE, Huynh M, Thorpe LE, Matte T, 2010. Birth outcomes among offspring of women exposed to the September 11, 2001, terrorist attacks. Obstet. Gynecol 116, 917–925. 10.1097/AOG.0b013e3181f2f6a2 [DOI] [PubMed] [Google Scholar]
- MacGinty R, Kariuki S, Barnett W, Wedderburn C, Hardy A, Hoffman N, Newton C, Zar H, Donald K, Stein D, 2020. Associations of antenatal maternal psychological distress with infant birth and development outcomes: results from a South African birth cohort. Compr. Psychiatry 96, 152128. 10.1016/j.comppsych.2019.152128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maercker A, Brewin CR, Bryant RA, Cloitre M, Reed GM, van Ommeren M, Humayun A, Jones LM, Kagee A, Llosa AE, Rousseau C, Somasundaram DJ, Souza R, Suzuki Y, Weissbecker I, Wessely SC, First MB, Saxena S, 2013. Proposals for mental disorders specifically associated with stress in the International Classification of Diseases-11. The Lancet 381, 1683–1685. 10.1016/S0140-6736(12)62191-6 [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morland L, Goebert D, Onoye J, Frattarelli L, Derauf C, Herbst M, Matsu C, Friedman M, 2007. Posttraumatic stress disorder and pregnancy health: preliminary update and implications. Psychosom. J. Consult. Liaison Psychiatry 48, 304–308. 10.1176/appi.psy.48.4.304 [DOI] [PubMed] [Google Scholar]
- Moylan PL, Jones HE, Haug NA, Kissin WB, Svikis DS, 2001. Clinical and psychosocial characteristics of substance-dependent pregnant women with and without PTSD. Addict. Behav 26, 469–474. 10.1016/S0306-4603(00)00141-6 [DOI] [PubMed] [Google Scholar]
- Muglia LJ, Katz M, 2010. The enigma of spontaneous preterm birth. N. Engl. J. Med 362, 529–535. 10.1056/NEJMra0904308 [DOI] [PubMed] [Google Scholar]
- Murphy CC, Schei B, Myhr TL, Mont JD, 2001. Abuse: a risk factor for low birth weight? a systematic review and meta-analysis. CMAJ 164, 1567–1572. [PMC free article] [PubMed] [Google Scholar]
- Muzik M, Bocknek EL, Broderick A, Richardson P, Rosenblum KL, Thelen K, Seng JS, 2012. Mother–infant bonding impairment across the first 6 months postpartum: the primacy of psychopathology in women with childhood abuse and neglect histories. Arch. Womens Ment. Health 16, 29–38. 10.1007/s00737-012-0312-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoye JM, Goebert D, Morland L, Matsu C, Wright T, 2009. PTSD and postpartum mental health in a sample of Caucasian, Asian, and Pacific Islander women. Arch. Womens Ment. Health 12, 393–400. [DOI] [PubMed] [Google Scholar]
- Prins A, Bovin MJ, Smolenski DJ, Marx BP, Kimerling R, Jenkins-Guarnieri MA, Kaloupek DG, Schnurr PP, Kaiser AP, Leyva YE, Tiet QQ, 2016. The Primary Care PTSD Screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J. Gen. Intern. Med 31, 1206–1211. 10.1007/s11606-016-3703-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radoš SN, Matijaš M, Anđelinović M, Čartolovni A, Ayers S, 2020. The role of posttraumatic stress and depression symptoms in mother-infant bonding. J. Affect. Disord 268, 134–140. 10.1016/j.jad.2020.03.006 [DOI] [PubMed] [Google Scholar]
- Rashid HU, Khan MN, Imtiaz A, Ullah N, Dherani M, Rahman A, 2020. Post-traumatic stress disorder and association with low birth weight in displaced population following conflict in Malakand division, Pakistan: a case control study. BMC Pregnancy Childbirth 20, 166. 10.1186/s12884-020-2841-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnik R, 2002. Intrauterine growth restriction. Obstet. Gynecol 99, 490–496. [DOI] [PubMed] [Google Scholar]
- Rogal SS, Poschman K, Belanger K, Howell HB, Smith MV, Medina J, Yonkers KA, 2007. Effects of posttraumatic stress disorder on pregnancy outcomes. J. Affect. Disord 102, 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A, Obst S, Teague SJ, Rossen L, Spry EA, Macdonald JA, Sunderland M, Olsson CA, Youssef G, Hutchinson D, 2020. Association between maternal perinatal depression and anxiety and child and adolescent development: a meta-analysis. JAMA Pediatr. 174, 1082–1092. 10.1001/jamapediatrics.2020.2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R, 1995. Writing meta-analytic reviews. Psychol. Bull., Writing Articles for Psychological Bulletin 118, 183–192. 10.1037/0033-2909.118.2.183 [DOI] [Google Scholar]
- Rowe H, Sperlich M, Cameron H, Seng J, 2014. A quasi-experimental outcomes analysis of a psychoeducation intervention for pregnant women with abuse-related posttraumatic stress. J. Obstet. Gynecol. Neonatal Nurs 43, 282–293. 10.1111/1552-6909.12312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan PM, Pearson MR, Fokas K, Leeman LM, 2020. A mother’s bond: An ecological momentary assessment study of posttraumatic stress disorder symptoms and substance craving during pregnancy. Psychol. Addict. Behav 34, 269–280. 10.1037/adb0000543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan PM, Pearson MR, Poremba C, Amaro H de LA, Leeman L, 2019. An ecological momentary assessment study examining posttraumatic stress disorder symptoms, prenatal bonding, and substance use among pregnant women. Drug Alcohol Depend. 195, 33–39. 10.1016/j.drugalcdep.2018.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seng JS, Kohn-Wood LP, McPherson MD, Sperlich M, 2011a. Disparity in posttraumatic stress disorder diagnosis among African American pregnant women. Arch. Womens Ment. Health 14, 295–306. 10.1007/s00737-011-0218-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seng JS, Low L, Sperlich M, Ronis D, Liberzon I, 2011b. Post-traumatic stress disorder, child abuse history, birthweight and gestational age: A prospective cohort study. BJOG Int. J. Obstet. Gynaecol 118, 1329–1339. 10.1111/j.1471-0528.2011.03071.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seng JS, Miller J, Sperlich M, van de Ven CJM, Brown S, Carter CS, Liberzon I, 2013a. Exploring dissociation and oxytocin as pathways between trauma exposure and trauma-related hyperemesis gravidarum: A test-of-concept pilot. J. Trauma Dissociation 14, 40–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seng JS, Oakley D, Sampselle CA, Killion C, Graham-Bermann S, Liberzon I, 2001. Posttraumatic stress disorder and pregnancy complications. Obstet. Gynecol 97, 17–22. [DOI] [PubMed] [Google Scholar]
- Seng JS, Sperlich M, Low LK, Ronis DL, Muzik M, Liberzon I, 2013b. Childhood abuse history, posttraumatic stress disorder, postpartum mental health, and bonding: a prospective cohort study. J. Midwifery Womens Health 58, 57–68. 10.1111/j.1542-2011.2012.00237.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro GD, Fraser WD, Frasch MG, Seguin JR, 2013. Psychosocial stress in pregnancy and preterm birth: associations and mechanisms. J. Perinat. Med 41, 631+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JG, Asch SM, Kimerling R, Frayne SM, Shaw KA, Phibbs CS, 2014. Posttraumatic stress disorder and risk of spontaneous preterm birth: Obstet. Gynecol 124, 1111–1119. 10.1097/AOG.0000000000000542 [DOI] [PubMed] [Google Scholar]
- Shaw JG, Nelson DA, Shaw KA, Woolaway-Bickel K, Phibbs CS, Kurina LM, 2018. Deployment and preterm birth among US Army soldiers. Am. J. Epidemiol 187, 687–695. 10.1093/aje/kwy003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC, 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59, 22–33. [PubMed] [Google Scholar]
- Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JPT, 2011. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343. 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- Stroup DF, 2000. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 283, 2008. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- Tonigan JS, Pearson MR, Magill M, Hagler KJ, 2018. AA attendance and abstinence for dually diagnosed patients: a meta-analytic review. Addiction 113, 1970–1981. 10.1111/add.14268 [DOI] [PubMed] [Google Scholar]
- Van den Heuvel MI, Van Assen MALM, Glover V, Claes S, Van den Bergh BRH, 2018. Associations between maternal psychological distress and salivary cortisol during pregnancy: a mixed-models approach. Psychoneuroendocrinology 96, 52–60. 10.1016/j.psyneuen.2018.06.005 [DOI] [PubMed] [Google Scholar]
- Viswasam K, Eslick GD, Starcevic V, 2019. Prevalence, onset and course of anxiety disorders during pregnancy: a systematic review and meta analysis. J. Affect. Disord 255, 27–40. 10.1016/j.jad.2019.05.016 [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz B, Herman D, Huska J, Keane T, 1993. The PTSD Checklist (PCL): Relability, validity, and diagnostic utility, in: Annual Convention of the International Society for Traumatic Stress Studies. San Antonio, TX. [Google Scholar]
- Webb R, Ayers S, 2015. Cognitive biases in processing infant emotion by women with depression, anxiety and post-traumatic stress disorder in pregnancy or after birth: a systematic review. Cogn. Emot 29, 1278–1294. 10.1080/02699931.2014.977849 [DOI] [PubMed] [Google Scholar]
- Weinreb L, Wenz-Gross M, Upshur C, 2018. Postpartum outcomes of a pilot prenatal care-based psychosocial intervention for PTSD during pregnancy. Arch. Womens Ment. Health 21, 299–312. 10.1007/s00737-017-0794-x [DOI] [PubMed] [Google Scholar]
- Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P, 2013. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.
- Wenz-Gross M, Weinreb L, Upshur C, 2016. Screening for post-traumatic stress disorder in prenatal care: prevalence and characteristics in a low-income population. Matern. Child Health J 20, 1995–2002. 10.1007/s10995-016-2073-2 [DOI] [PubMed] [Google Scholar]
- Wosu AC, Gelaye B, Williams MA, 2015. Childhood sexual abuse and posttraumatic stress disorder among pregnant and postpartum women: review of the literature. Arch. Womens Ment. Health 18, 61–72. 10.1007/s00737-014-0482-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Harville EW, Mattison DR, Elkind-Hirsch K, Pridjian G, Buekens P, 2008. Exposure to Hurricane Katrina, post-traumatic stress disorder and birth outcomes. Am. J. Med. Sci 336, 111–115. 10.1097/MAJ.0b013e318180f21c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Bierer LM, Mikhno A, Pratchett LC, Burton CL, Makotkine I, Devanand DP, Pradhaban G, Harvey PD, Mann JJ, 2010. Hydrocortisone responsiveness in Gulf War veterans with PTSD: Effects on ACTH, declarative memory hippocampal [18F]FDG uptake on PET. Psychiatry Res. Neuroimaging 184, 117–127. [DOI] [PubMed] [Google Scholar]
- Yildiz PD, Ayers S, Phillips L, 2017. The prevalence of posttraumatic stress disorder in pregnancy and after birth: A systematic review and meta-analysis. J. Affect. Disord 208, 634–645. 10.1016/j.jad.2016.10.009 [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Smith MV, Forray A, Epperson N, Costello D, Lin H, Belanger K, 2014. Pregnant women with posttraumatic stress disorder and risk of preterm birth. JAMA Psychiatry 71, 897–904. 10.1001/jamapsychiatry.2014.558 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


