Abstract
Background:
Hip displacement is common in children with cerebral palsy (CP). Spasticity in the hip adductor muscles, hip flexors, and medial hamstrings has been identified as a possible cause of progressive hip displacement. Selective dorsal rhizotomy (SDR) aims to reduce lower extremity spasticity in children with CP. Here, we investigate the influence of SDR on hip displacement in children with CP at long-term follow-up, a minimum of 5 years post-SDR.
Methods:
A retrospective review of children undergoing SDR at a Canadian pediatric hospital was completed. Migration percentage (MP) was measured on pelvis radiographs taken in the 6 months before SDR and minimum 5 years post-SDR or before hip surgery. The number of hips with displacement, defined as MP >30%, and the number of children with at least 1 hip displaced were determined. A linear mixed-effects model was used to assess potential risk factors for poor outcome post-SDR, defined as having MP >40% or surgical intervention for hip displacement.
Results:
Ninety children [50 males, 40 females, Gross Motor Function Classification System (GMFCS) levels I to V: 1/13/24/43/9] with a mean follow-up of 8.5 years (SD 5.1) were included. The mean age at SDR was 4.9 years (SD 1.5); more than half of children (52%) had hip displacement at the time of SDR. Post-SDR, MP exceeded 30% in 0 (0%) of children at GMFCS level I, 1 (8%) at II, 11 (46%) at III, 31 (72%) at IV, and 7 (78%) at V. A poor outcome was associated with preoperative MP, age, and GMFCS level.
Conclusions:
The incidence of hip displacement post-SDR was consistent with population-based studies when evaluated by GMFCS. Our findings suggest that SDR has neither a positive nor negative effect on hip displacement when assessed at least 5 years postintervention.
Level of Evidence:
Level IV.
Key Words: cerebral palsy, rhizotomy, displacement, hip
Overall, 1 in 3 children with cerebral palsy (CP) will have hip displacement.1–3 Spasticity and muscle imbalance around the hip have long been felt to be a contributing factor in progressive hip displacement. It has been suggested that increased tone in the hip adductors, hip flexors, and medial hamstrings overpower the relatively weaker antagonist muscles, the hip abductors, and extensors.4,5 This imbalance of muscle forces around the hip is thought to shift the mechanical axis, resulting in the hip dislocating posteriorly and superolaterally.4,6–8 Recent publications have suggested that motor type may have less impact on hip displacement than previously hypothesized.9,10 Rather, it has been suggested that abductor weakness and limitations in weight-bearing lead to changes in the proximal femoral geometry.9–11
Selective dorsal rhizotomy (SDR) is a neurosurgical procedure that aims to reduce spasticity in children with cerebral palsy.12–14 The impact of this decrease in spasticity on hip displacement is unclear. In 2017, a systematic review found 7 articles reporting the impact of rhizotomy on hip displacement.15 These studies reported positive, neutral, and negative effects on hip displacement after SDR. All studies were classified as being a low level of evidence (level IV) and had small sample sizes, ranging from 6 to 82 subjects.
The largest study, with a mean follow-up of 48 ±32.4 months, was from our institution in 2005.16 The authors concluded that SDR was more likely to have a positive effect or no effect on hip subluxation than a negative effect. They also noted that more severe preoperative involvement, as measured using the Gross Motor Function Classification System (GMFCS), may predispose to worsening hip displacement.16–18 This publication was before population-based studies that demonstrated the direct relationship between GMFCS and hip displacement. Unfortunately, as noted by the study authors, a limitation of this review was the use of center-edge angle to measure hip displacement. Migration percentage (MP), defined as the percentage of the femoral head that sits outside the lateral margin of the acetabulum, has been accepted as the most valid and reliable method of monitoring hip displacement from anterior-posterior pelvis radiographs taken with the child in a standardized position.19,20 Center-edge angle relies on the accurate definition of the center of the femoral head and is considered less reliable for measuring hip displacement.4,20
Small sample sizes and lack of long-term follow-up limit the conclusions that can be made from the existing literature on the impact of SDR on hip displacement. The objective of this study was to investigate the influence of SDR on hip displacement in children with CP a minimum of 5 years post-SDR.
METHODS
A retrospective chart review was completed on a consecutive series of children who had an SDR between February 1987 and December 2012 at British Columbia’s Children’s Hospital. Participants were identified from a database of children who underwent an SDR at our institution. The study was reviewed and approved by the local Research Ethics Board. To be included, participants had to have a diagnosis of CP, undergone SDR (L2-S1) before age 18 years, had an anteroposterior (AP) pelvis radiograph in the 6 months before SDR, and an AP pelvis radiograph a minimum of 5 years post-SDR. If hip surgery was performed during this 5-year follow-up period, an AP pelvis radiograph before hip surgery was required.
Age, GMFCS level, hip range of motion, and orthopaedic surgical history were collected pre-SDR, before an orthopaedic hip intervention, and at last follow-up from the child’s medical chart and SDR preoperative physical therapy assessments. When necessary, GMFCS level was retrospectively assigned utilizing detailed functional descriptors in the physical therapy or neurosurgeon notes; it is acceptable to classify GMFCS using reported findings.17 GMFCS levels at the final follow-up were used for analysis. Adductor tone, as measured on the Ashworth Scale or Modified Ashworth scale, was retrieved from pre-SDR, 1-year follow-up and last follow-up assessments.
Radiographs were available digitally in the Picture Archiving and Communication System at our institution starting in 2004; hard copy films of radiographs before this date were retrieved. MP was measured for all preoperative films. The most recent post-SDR radiograph was identified, and MP was measured. For those participants undergoing hip surgery, MP was measured on the last AP pelvis radiograph completed before orthopaedic surgery.
Surgical Procedure
Partial rhizotomies of bilateral dorsal roots were completed from L2 to S1 or S2. The amount of dorsal nerve rootlets cut at these levels varied from 20% to 90%. Initially, the selection of rootlets was based almost exclusively on electrophysiological responses to intraoperative electrical stimulation. Over time, this evolved to include both clinical and intraoperative electrophysiological criteria.21 Between 1997 and 2005, bilateral laminar cuts from L1 to S1 were performed for exposure of rootlets. In 2006, the procedure changed to a laminectomy at the level of the conus medullaris.22
Statistical Analysis
We used descriptive statistics both overall and stratified by post-SDR hip surgery (yes/no) to evaluate MP and hip displacement pre-SDR and post-SDR. The mean MP for both hips was calculated for pre-SDR images and at the final follow-up, which was defined as either most recent pelvis x-ray (minimum 5 y post-SDR) or the last x-ray before hip surgery. The number of hips with displacement, defined as MP >30%, and the number of children with at least 1 hip displaced were determined.
For regression analysis, a poor outcome post-SDR was defined as having MP >40% or surgical intervention for hip displacement. To assess possible risk factors associated with higher postoperative displacement, we used a linear mixed-effects model (to account for pairs of hips from the same individuals), including age at the time of SDR, GMFCS level, preoperative MP, and asymmetry of hip subluxation. Results are summarized as mean differences and 95% CIs. All analyses were conducted using R statistical software version 4.0.3.
RESULTS
Two hundred forty-eight children had an SDR during the study period. Ninety children (50 males, 40 females) were included in the review. The reasons for exclusion are shown in Table 1. Of those included in the present study, 43 (47.8%) were included in the previous study from our institution.16 The mean age at the time of SDR was 4.9 years (SD 1.5 y), ranging from 2.7 to 11.6 years. The mean percentage of dorsal nerve rootlets of L2 to S1 cut was 55.6% (SD 14.9). Before SDR, 1 child had undergone an acetabular osteotomy (pre-SDR MPs 9%, 29%), and 5 had undergone adductor releases. GMFCS levels (Table 2) had to be retrospectively assigned to 25 (27.7%) children at the final follow-up. The GMFCS for 17 (18.9%) participants differed at the final follow-up compared with pre-SDR. No GMFCS level changed by more than 1 level. Six children went to a lower GMFCS level, whereas 9 moved to a higher level; it was most common (6 children) to change from level III to IV. Adductor spasticity decreased on the Ashworth Scale or Modified Ashworth Scale post-SDR in all but 2 participants.
TABLE 1.
Reasons for Exclusion From Study
| Reason for exclusion | No. participants |
|---|---|
| Could not locate radiographs/chart | 68 |
| No pre-SDR hip AP pelvis radiograph | 54 |
| No 5-year post-SDR AP pelvis radiograph | 29 |
| Non-CP diagnosis | 2 |
| Hip surgery post-SDR with no pre-op hip x-ray | 2 |
| SDR completed >18 years | 1 |
| SDR included L5/S1 rootlets only | 2 |
| TOTAL | 158 |
AP indicates anteroposterior; CP, cerebral palsy; SDR, selective dorsal rhizotomy.
TABLE 2.
Participant Data Pre- and Post-Selective Dorsal Rhizotomy
| All | No hip surgery | Hip surgery | |
|---|---|---|---|
| No. children (hips) | 90 (180) | 56 (112) | 34 (68) |
| GMFCS levels (I/II/III/IV/V) | 1/13/24/43/9 | 1/13/16/22/4 | 0/0/8/21/5 |
| Age at SDR (y): mean (SD) | 4.9 (1.5) | 4.8 (1.3) | 5.0 (1.8) |
| Pre-SDR | |||
| MP range (%) | 0–100 | 0–64 | 9 – 100 |
| MP (all hips): mean (SD) | 28.7 (13.9) | 25.1 (12.5) | 34.5 (14.2) |
| Level I | 12.5 (1.5) | 12.5 (1.5) | NA |
| Level II | 17.8 (10.1) | 17.8 (10.1) | NA |
| Level III | 30.1 (14.2) | 26.5 (13.7) | 37.3 (12.9) |
| Level IV | 30.8 (11.5) | 29.7 (11.2) | 31.9 (11.8) |
| Level V | 31.8 (20.4) | 20.1 (9.6) | 41.1 (22.2) |
| No. (%) children: hips MP >30% | 47 (52): 72 (40) | 21 (38): 35 (31) | 26 (77): 37 (54) |
| Level I | 0 (0): 0 (0) | 0 (0): 0 (0) | 0 (0): 0 (0) |
| Level II | 2 (15): 3 (12) | 2 (15): 3 (12) | 0 (0): 0 (0) |
| Level III | 13 (54): 20 (42) | 6 (38): 9 (28) | 7 (88): 11 (69) |
| Level IV | 27 (63): 40 (47) | 12 (55): 21 (48) | 15 (71): 19 (45) |
| Level V | 5 (56): 9 (50) | 1 (25): 2 (25) | 4 (80): 7 (70) |
| Post-SDR | |||
| Length of follow-up/time to hip surgery (y): mean (SD) | 8.5 (5.1) | 11.1 (4.3) | 4.2 (3.2) |
| Range in follow-up time (y) | 0.5–25.0 | 5.0–25.0 | 0.5–13.5 |
| Age at follow-up (y): mean (SD) | 13.4 (5.4) | 16.0 (4.5) | 9.2 (3.9) |
| MP at follow-up or prehip surgery: mean (SD) | 33.7 (24.5) | 27.3 (22.5) | 44.4 (24.2) |
| Level I | 13.5 (4.5) | 13.5 (4.5) | NA |
| Level II | 14.7 (8.1) | 14.7 (8.1) | NA |
| Level III | 33.2 (21.4) | 27.7 (20.0) | 44.3 (20.2) |
| Level IV | 35.5 (23.4) | 28.5 (19.4) | 42.8 (25.5) |
| Level V | 56.7 (32.4) | 63.1 (40.2) | 51.5 (25.6) |
| No. (%)children: hips MP >30% | 50 (56): 70 (39) | 19 (34): 26 (23) | 31 (91): 44 (65) |
| Level I | 0 (0): 0 (0) | 0 (0): 0 (0) | 0 (0): 0 (0) |
| Level II | 1 (8): 1(4) | 1 (8): 1(4) | 0 (0): 0 (0) |
| Level III | 11 (46): 16 (33) | 4 (25): 7 (22) | 7 (88): 9 (56) |
| Level IV | 31 (72): 42 (49) | 11 (50): 13 (30) | 20 (95): 29 (69) |
| Level V | 7 (78): 11 (61) | 3 (75): 5 (63) | 4 (80); 6 (60) |
SDR indicates Selective dorsal rhizotomy; MP, Migration percentage; GMFCS, Gross Motor Function Classification System.
The mean MP of all hips and the number of hips with displacement pre-SDR and post-SDR are shown in Table 2. After SDR, 34 (37.8%) children underwent hip surgery, including 15 adductor releases, 15 varus derotation osteotomies (with or without a pelvic osteotomy), 3 salvage procedures, and 1 hip replacement. MP at the time of hip surgery for all hips was 44.4% (SD 24.2). In considering only the most affected hip in each subject, the mean MP before hip surgery was 58.9% (SD 23.2) at a mean of 4.2 years (SD 3.2) post-SDR. Five subjects had an adductor release, and 2 had reconstructive hip surgery when MPs were <40%. In the group that did not have hip surgery, the mean MP at follow-up was 27.3% (SD 22.5) for all hips or 32.8% (SD 24.5) for only the most affected hip.
Overall, 37 children (42%) met the definition of a poor outcome of either requiring a surgical intervention for hip displacement or having MP ≥40% at the final follow-up. This included 16 hips in 10 children who did not have hip surgery. The 7 subjects who had hip surgery with MP <40% were not included in the regression analysis. Preoperative variables associated with a poor outcome included GMFCS level, preoperative MP, and age at SDR (Fig. 1, Table 3).
FIGURE 1.
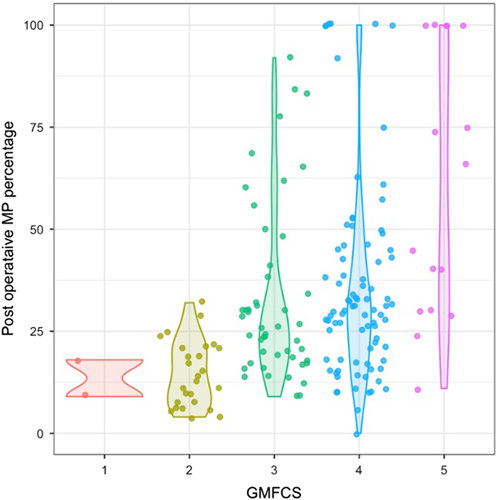
Final migration percentage by gross motor function classification system level. GMFCS indicates Gross Motor Function Classification System; MP, migration percentage.
TABLE 3.
Regression Coefficients for Risk Factors for Greater Migration Percentage Postrhizotomy
| Risk factors | Mean migration percentage (SD) | Adjusted mean difference (95% CI) * |
|---|---|---|
| GMFCS level I | 13.5 (4.5) | Reference |
| GMFCS level II | 14.7 (7.9) | −7.04 (−39.12, 25.05) |
| GMFCS level III | 27.7 (19.7) | 4.14 (−27.52, 35.80) |
| GMFCS level IV | 28.5 (19.1) | 4.50 (−27.09, 36.09) |
| GMFCS level V | 63.1 (37.6) | 27.71 (−5.51, 60.93) |
| Age (per year increase) | — | 2.30 (0.02, 4.58) |
| Asymmetry (per 1 degree increase) | — | −0.03 (−0.41, 0.36) |
| Pre-SDR MP (per 1 degree) | — | 0.68 (0.42, 0.94) |
Estimated using multivariable linear mixed-effects model with above variables and adjustment for length of follow-up time.
GMFCS indicates Gross Motor Function Classification System; MP, migration percentage; SDR, selective dorsal rhizotomy.
DISCUSSION
This study is the largest long-term follow-up study investigating hip displacement after SDR. In this review, hip displacement post-SDR was associated with GMFCS level, and the rate of displacement, when evaluated by GMFCS, was consistent with that reported in published population studies (Table 4). On the basis of our findings, we hypothesize that SDR does not positively or negatively affect hip displacement when reviewed 5 years post-SDR.
TABLE 4.
Comparison of Incidence of Hip Displacement to Literature Values for Patients With Cerebral Palsy
| Current study pre-SDR | Current study at follow-up | Soo et al1 | Hagglund et al2 | |
|---|---|---|---|---|
| Sample size | 90 | 90 | 323 | 212 |
| Age range (y) | 2.5–11.6 | 3.5–31.1 | 6.5–14 | 9–16 |
| Children with MP >30%–33% | ||||
| GMFCS level I | 0 | 0 | 0 | 5 |
| GMFCS level II | 15 | 8 | 15 | 13 |
| GMFCS level III | 54 | 46 | 41 | 50 |
| GMFCS level IV | 63 | 72 | 69 | 62 |
| GMFCS level V | 56 | 78 | 90 | 68 |
GMFCS indicates Gross Motor Function Classification System; MP, migration percentage; SDR, selective dorsal rhizotomy.
Population-based studies have reported between 62% and 90% of children at GMFCS levels IV and V are expected to have displacement defined as an MP >30% to 33%.1–3 We found MP >30% in 72% to 78% of children at GMFCS levels IV and V after SDR. There were more children at GMFCS level IV than any other GMFCS level in our review. Before SDR, 63% of the children at GMFCS level IV already had hip displacement >30% at a mean age of 5.2 years (SD 1.6); this increased to 72% at follow-up. There were a small number of children at GMFCS level V who had an SDR dating back as many as 25 years to a time when SDR was offered to children and families to address goals related to care and comfort. The availability of intrathecal baclofen pumps has changed practice, and SDR is no longer offered to children at GMFCS level V in our institution. Of the 9 children at GMFCS level V who were reviewed, 7 had an MP >30% in 1 or both hips at follow-up. There were 5 dislocated hips in 3 of these 9 children, including 1 child who had a unilateral dislocation before SDR.
Children at GMFCS level III are at moderate risk of hip displacement and are often considered candidates for SDR. In this review, the frequency of MP greater than 30% at final follow-up was again consistent with the literature. We found that 46% of children at GMFCS level III had hip displacement compared with literature values ranging from 41% to 50%.1,2 Although there was a reduction in the percentage of children with MP >30% in this group from pre-SDR to final follow-up, this reflects a change of only 2 children and may be due to the small sample size. Further assessment of the impact of SDR on hip displacement at this GMFCS level is required.
A previous case series suggested that hip displacement may increase post-SDR.23 Our findings do not support any increased risk. The overall incidence of hip displacement in this cohort, defined as MP >30%, was high at 53% compared with the rates between 26% and 35% in population-based studies.1–3,24,25 However, the cohort studied here was not reflective of a population of children with CP; it was heavily weighted to children with more severe CP and, therefore, a higher rate of hip displacement is expected. There was no increased risk when stratified by GMFCS levels. Any increased risk would be particularly concerning in ambulatory children; we found no increased risk of hip displacement in this group. No child at GMFCS level I or II had hip surgery in our study; 1 child at GMFCS level II had 1 hip with MP over 30% at the final follow-up, measuring 32%. Although the incidence (Table 4) of hip displacement in children at GMFCS level II decreased from 15% pre-SDR to 8% at follow-up, this difference again reflects the small sample size, with the difference being due to improvement in MP in 1 child from 32% to 25% between the ages of 4.8 and 10.2 years. Improvement in MP in ambulatory children has previously been described.3,25
Hip displacement can start at a young age, having been reported before 2 years of age, with the greatest risk for displacement being reported as between the ages of 3 to 5 years.2,25–27 The mean age of children at SDR in this review was 4.9 years (SD 1.5), with over half of children already having at least 1 hip with MP >30%. Our finding that outcome was associated with both age at SDR and pre-SDR MP likely reflects this known association between age and MP. In their study of 53 children and youth aged 4 to 15 years, Chan et al28 concluded that SDR had a neutral effect on hip displacement, with preoperative hip radiologic measurement as the most important predictive factor in determining hip status after SDR. In our review, there were 23 children who had an SDR before the age of 4 years. Eight of these children already had at least 1 hip with an MP >30%; all but 1 child either had hip surgery post-SDR or an MP >40% at the final follow-up. Ten of the remaining 15 children were at GMFCS levels III and IV; 1 of 3 children at GMFCS level III and three of 7 children at GMFCS level IV progressed to MP >40%. Although these numbers are too small to draw conclusions, the results suggest progressive hip displacement still occurs even when spasticity is reduced through SDR in a young child who does not yet have hip displacement.
This study has a number of limitations. This was a retrospective review that included data collected from records spanning over 2 decades. As a result, only 35% of those children who underwent an SDR at our institution were included, which may lead to selection bias. The most common reasons for exclusion were the inability to locate records and the lack of pre-SDR hip radiograph. Many children came from other provinces and, therefore, their imaging was done in their home province. There are a number of confounding variables, including additional tone management strategies, such as oral baclofen and botulinum toxin, physiotherapy, and positioning devices, that may influence hip displacement for which we could not control. Four subjects had a procedure related to the hip before their SDR; they were included in the review because imaging was available after their initial procedures, before the SDR. This additional surgery may have had a positive or negative impact on their long-term outcomes. Additional procedures may have been completed between SDR and follow-up without our knowledge. We chose not to evaluate displacement between SDR and the final follow-up, given our institution already published data related to short-term follow-up.16 However, this may have provided additional insights into change in displacement over time. Children in this review were treated before the development of the GMFCS and, therefore, GMFCS level had to be retrospectively assigned for some participants. Although detailed physiotherapy and neurosurgical notes made the assignment of GMFCS levels possible, there remains a risk for error. To improve the overall accuracy of the assignment of the GMFCS, the most recent GMFCS level assigned to the child was utilized. Radiographs were completed before the implementation of the province’s hip surveillance program, and therefore, standardized positioning for MP was not in place. However, when measuring MP, the authors made the decision, based on the degree of hip abduction and adduction, pelvic tilt, and pelvic rotation whether MP could be accurately measured. Finally, although we aimed to have long-term follow-up by only including children with a minimum of 5 years post-SDR follow-up, hip displacement can occur until and beyond skeletal maturity, and continued follow-up is needed to understand final outcomes.
CONCLUSIONS
The incidence of hip displacement post-SDR in our cohort was consistent with literature values when evaluated by GMFCS level. Overall, our findings continue to suggest that the most important factor in determining the risk for hip displacement is a child’s GMFCS level. A multicenter, nonequivalent group design, stratified by GMFCS and appropriately powered to detect a clinical significance, with children followed to skeletal maturity, is required to further understand the impact of SDR on hip displacement.
Footnotes
K.M. reports non-financial support from DePuy Synthes, Johnson & Johnson, Allergan, IPSEN, and Pega Medical; other financial activities with I'm a HIPpy Foundation, Pega Medical, View, Inc. and PrecisionOS; personal fees from IPSEN and OrthoPediatrics; and grants from OrthoPediatrics, Pediatric Orthopaedic Society of North America, AO Foundation, and Canadian Institutes of Health Research, outside the submitted work. The remaining authors declare no conflicts of interest.
Contributor Information
Stacey D. Miller, Email: smiller4@cw.bc.ca.
Maria Juricic, Email: mjuricic@cw.bc.ca.
Jeffrey N. Bone, Email: jeffrey.bone@cw.bc.ca.
Paul Steinbok, Email: psteinbok@cw.bc.ca.
Kishore Mulpuri, Email: kmulpuri@cw.bc.ca.
REFERENCES
- 1.Soo B, Howard JJ, Boyd RN, et al. Hip displacement in cerebral palsy. J Bone Joint Surg [Am]. 2006;88:121–129. [DOI] [PubMed] [Google Scholar]
- 2.Hagglund G, Lauge-Pedersen H, Wagner P. Characteristics of children with hip displacement in cerebral palsy. BMC Musculoskelet Disord. 2007;8:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connelly A, Flett P, Graham HK, et al. Hip surveillance in Tasmanian children with cerebral palsy. J Pediatr Child Health. 2009;45:437–443. [DOI] [PubMed] [Google Scholar]
- 4.Cornell MS. The hip in children with cerebral palsy: predicting the outcome of soft tissue surgery. Clin Orthop. 1995;340:165–171. [DOI] [PubMed] [Google Scholar]
- 5.Valencia FG. Management of hip deformities in cerebral palsy. Orthop Clin North Am. 2010;41:549–559. [DOI] [PubMed] [Google Scholar]
- 6.Miller F, Slomczykowski M, Cope R, et al. Computer, modeling of the pathomechanics of spastic hip dislocation, in children. J Pediatr Orthop. 1999;19:486–492. [DOI] [PubMed] [Google Scholar]
- 7.Kalen V, Bleck EE. Prevention of spastic paralytic dislocation of the hip. Dev Med Child Neurol. 1985;27:17–24. [DOI] [PubMed] [Google Scholar]
- 8.Abel MF, Wenger DR, Mubarak SJ, et al. Quantitative analysis of hip dysplasia in cerebral palsy: a study of radiographs and 3-D reformatted images. J Pediatr Orthop. 1994;14:283–289. [DOI] [PubMed] [Google Scholar]
- 9.Ulusaloglu AC, Asma A, Rogers KJ, et al. The influence of tone on proximal femoral and acetabular geometry in neuromuscular hip displacement: a comparison of cerebral palsy and spinal muscular atrophy. J Child Orthop. 2022;16:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howard JJ, Willoughby K, Thomason P, et al. Hip surveillance and management of hip displacement in children with cerebral palsy: clinical and ethical dilemmas. J Clin Med. 2023;12:1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robin J, Graham HK, Selber P, et al. Proximal femoral geometry in cerebral palsy: a population-based cross-sectional study. J Bone Joint Surg Br. 2008;90:1372–1379. [DOI] [PubMed] [Google Scholar]
- 12.Abbott R, Johann-Murphy M, Shiminski-Maher T, et al. Selective dorsal rhizotomy: outcome and complications in treating spastic cerebral palsy. Neurosurgery. 1993;33:851–857. [DOI] [PubMed] [Google Scholar]
- 13.Peacock WJ, Staudt LA. Functional outcomes following selective posterior rhizotomy in children with cerebral palsy. J Neurosurg. 1991;74:380–385. [DOI] [PubMed] [Google Scholar]
- 14.Peacock WJ, Staudt LA. Spasticity in cerebral palsy and the selective posterior rhizotomy procedure. J Child Neurol. 1990;5:179–185. [DOI] [PubMed] [Google Scholar]
- 15.Miller SD, Juricic M, Hesketh K, et al. Prevention of hip displacement in children with cerebral palsy: a systematic review. Dev Med Child Neurol. 2017;59:1130–1138. [DOI] [PubMed] [Google Scholar]
- 16.Hicdonmez T, Steinbok P, Beauchamp R, et al. Hip joint subluxation after selective dorsal rhizotomy for spastic cerebral palsy. J Neurosurg Pediatr. 2005;103:10–16. [DOI] [PubMed] [Google Scholar]
- 17.Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;3:214–223. [DOI] [PubMed] [Google Scholar]
- 18.Palisano RJ, Rosenbaum P, Bartlett D, et al. Content validity of the expanded and revised Gross Motor Function Classification System. Dev Med Child Neurol. 2008;50:744–750. [DOI] [PubMed] [Google Scholar]
- 19.Pons C, Remy-Neris O, Medee B, et al. Validity and reliability of radiological methods to assess proximal hip geometry in children with cerebral palsy: a systematic review. Dev Med Child Neurol. 2013;5:1089–1102. [DOI] [PubMed] [Google Scholar]
- 20.Reimers J. The stability of the hip in children: a radiological study of results of muscle surgery in cerebral palsy. Acta Orthop Scand. 1980;184:1–100. [DOI] [PubMed] [Google Scholar]
- 21.Steinbok P, Gustavsson B, Kestle JR, et al. Relationship of intraoperative electrophysiological criteria to outcome after selective functional posterior rhizotomy. J Neurosurg. 1995;83:18–26. [DOI] [PubMed] [Google Scholar]
- 22.Park TS, Gaffney PE, Kaufman BA, et al. Selective lumbosacral dorsal rhizotomy immediatelycaudal to the conus medullaris for cerebral palsy spas-ticity. Neurosurgery. 1993;33:929–933. [DOI] [PubMed] [Google Scholar]
- 23.Greene WB, Dietz FR, Goldberg MJ, et al. Rapid progression of hip subluxation in cerebral palsy after selective posterior rhizotomy. J Pediatr Orthop. 1991;11:494–497. [DOI] [PubMed] [Google Scholar]
- 24.Terjesen T. The natural history of hip development in cerebral palsy. Dev Med Child Neurol. 2012;54:951–957. [DOI] [PubMed] [Google Scholar]
- 25.Kentish M, Wynter M, Snape N, et al. Five-year outcome of state-wide hip surveillance of children and adolescents with cerebral palsy. J Pediatr Rehabil Med. 2011;4:205–217. [DOI] [PubMed] [Google Scholar]
- 26.Larnert P, Risto O, Hagglund G, et al. Hip displacement in relation to age and gross motor function in children with cerebral palsy. J Child Orthop. 2014;8:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pruszczynski B, Sees J, Miller F. Risk factors for hip displacement in children with cerebral palsy: systematic review. J Pediatr Orthop. 2016;36:829–833. [DOI] [PubMed] [Google Scholar]
- 28.Chan WM, Choi KYA, Sun KW, et al. Hip development after selective dorsal rhizotomy in patients with cerebral palsy. J Orthop Trauma Rehabil. 2013;17:82–86. [Google Scholar]


