Abstract
Objectives:
Many vaccines require higher/additional doses or adjuvants to provide adequate protection for people with HIV (PWH). Here, we compare coronavirus disease 2019 (COVID-19) vaccine-induced antibody neutralization capacity in PWH vs. HIV-negative individuals following two vaccine doses.
Design:
In Canadian prospective observational cohorts, including a multicentre study of PWH receiving at least two COVID-19 vaccinations (mRNA or ChAdOx1-S), and a parallel study of HIV-negative controls (Stop the Spread Ottawa Cohort), we measured vaccine-induced neutralization capacity 3 months post dose 2 (±1 month).
Methods:
COVID-19 neutralization efficiency was measured by calculating the half maximal inhibitory dilution (ID50) using a high-throughput protein-based neutralization assay for Ancestral (Wuhan), Delta and Omicron (BA.1) spike variants. Univariable and multivariable quantile regression were used to compare COVID-19-specific antibody neutralization capacity by HIV status.
Results:
Neutralization assays were performed on 256 PWH and 256 controls based on specimen availability at the timepoint of interest, having received two vaccines and known date of vaccination. There was a significant interaction between HIV status and previous COVID-19 infection status in median ID50. There were no differences in median ID50 for HIV+ vs. HIV-negative persons without past COVID-19 infection. For participants with past COVID-19 infection, median ICD50 was significantly higher in controls than in PWH for ancestral SARS-CoV-2 and Omicron variants, with a trend for the Delta variant in the same direction.
Conclusion:
Vaccine-induced SARS-CoV-2 neutralization capacity was similar between PWH vs. HIV-negative persons without past COVID-19 infection, demonstrating favourable humoral-mediated immunogenicity. Both HIV+ and HIV-negative persons demonstrated hybrid immunity.
Trial registration:
clinicaltrials.gov NCT04894448.
Keywords: coronavirus disease 2019 vaccination, HIV, humoral immunity, immunogenicity
Introduction
People with HIV (PWH) are vulnerable to acquisition and severe COVID-19 outcomes because of combined risk factors [1,2] and sub-optimal immunogenicity to routine vaccines [3,4]. Studies in PWH early in the pandemic frequently excluded those with risk factors for sub-optimal response to vaccination, including advanced age or lower CD4+ T-cell counts [5,6]. Knowledge gaps fuelled the establishment of a pan-Canadian prospective cohort of PWH receiving COVID-19 vaccines to: assess humoral immunogenicity in diverse PWH; compare immunogenicity responses in PWH vs. HIV-negative controls, and describe safety and tolerability of COVID-19 vaccines in PWH [7,8].
Antibody neutralization capacity is highly predictive of immune protection and vaccine efficacy [9,10]. We previously demonstrated that COVID-19 vaccine-specific immunity, defined as co-positivity for anti-IgG against SARS-CoV-2 Spike (S) and receptor binding domain (RBD) proteins, was achieved in over 90% of PWH and HIV-negative controls at 3 and 6 months post second and 1 month post third doses [8]. Proportions of participants achieving comparable anti-RBD levels were similar by HIV status at each timepoint. We previously observed that anti-S IgG levels were lower in PWH than HIV-negative controls at 3 and 6 months post second vaccine dose [8]. As we had more specimens available at the 3 month rather than 6 month post second-vaccine dose timepoint, we chose to focus on the 3 month post second-dose timepoint for neutralization studies. We chose this timepoint as we had hypothesized that differences in neutralization capacity, if present between PWH vs. HIV-negative controls, may become more evident at this time point.
Methods
The CTN 328 study is a multicentre prospective observational cohort study of PWH recruited from four sites of Canadian cities, as described [8]. Written informed consent was obtained from all participants (Supplementary Information).
Participants
For PWH in the CTN 328 study, inclusion criteria included: age at least 16 years; having received, or planning to receive, at least one dose of COVID-19 vaccine; HIV-seropositive. Exclusion criteria included: receipt of any blood product or immunoglobulin preparation within 1 month of vaccination; signs/symptoms of active COVID-19 at enrolment [8]. Subpopulations of PWH included at least 55 years, HIV immune nonresponders (CD4+ T-cell count remains <350 cells/μl despite suppressed viral load on ART) and PWH with multimorbidity (≥2 comorbidities). We aimed to enrol a diversity of PWH including those who were relatively stable or unstable as evidenced by CD4+ T-cell count less than 350, CD4+/CD8+ less than 0.75 and suppressed viral load [7,8]. For HIV-negative controls, a subset of immunocompetent Stop the Spread Ottawa (SSO) participants were selected and included if they had undergone parallel blood collection in relation to vaccination time [11]. Equal numbers of HIV+ and HIV− participants were randomly selected from individuals who had specimens available at the time point of interest on whose specimens neutralization assays were performed. Matching by participant characteristics was not performed.
Study visits
Participants were to attend up to six visits over 12 months: prevaccination; 1 month following first dose; at 3, 6 and 12 months following the second dose; and 1 month after the third dose. For the presented analysis, we performed neutralization assays on samples collected at 3 months post second dose.
Data collection
Methods related to medical and HIV history, COVID-19 questionnaire administration, sample collection and vaccine safety, in addition to levels of immunoglobulins G (IgG) were previously published [7,8]. Neutralization assays were performed using an automated protein-based surrogate neutralization (sn) ELISA measuring inhibition of ACE2-spike interactions by antibodies. As previously published [12,13], this ELISA-based test functions as a surrogate to live virus neutralization assays for evaluating antibodies that prevent spike and ACE2 receptor interaction. It can measure neutralization efficiency for Ancestral, Delta and Omicron (BA.1) variants. Levels of SARS-CoV-2 Spike trimer (S) protein, receptor-binding domain (RBD) of spike, and nucleocapsid (N) protein were measured using an automated high-throughput chemiluminescent ELISA [12,14]. Vaccine-induced immunity was defined as co-positivity for S and RBD protein and infection-induced immunity was defined as co-positivity of S and N protein (signal to cut-off ratio ≥1.0) [12,14]. This ELISA has been calibrated to the WHO reference standard. As data can be transformed to international units cross-laboratory and cross-assay correlations is facilitated. IgG antibody titters (binding antibody units (BAU)/ml) were generated by a conversion model (four-parameter log-logistic curve based on measurements from the WHO International Standard) (NIBSC 20.136). Assay description was previously published [11,12]. History of previous COVID-19 infection was defined as having endorsed having a positive SARS-CoV-2 PCR test (based on participant or site report) or evidence of co-positivity of S and N protein.
Outcomes
The main outcome of interest was the difference in median COVID-19 neutralization efficiency, as measured by half maximal inhibitory dilution (ID50) for Ancestral (Wuhan), Delta and Omicron (BA.1) spike variants, in PWH vs. HIV-negative controls stratified by prior COVID-19 infection status. An exploratory objective was to determine COVID-19 vaccine-induced neutralization efficiency in PWH 3 months post second dose, stratified by various sub-populations of PWH and based on history of prior COVID-19 infection.
Statistical analyses
All analyses were performed using Statistical Analysis System (SAS) software version 9.4 (SAS Institute, Cary, North Carolina, USA). Quantile regression analysis was used to compare neutralization capacity for Ancestral, Delta and Omicron (BA.1) variant ID50 neutralization titters between PWH and control groups as the data did not conform with normality assumption even after log transformation. The analysis adjusted for vaccine-related variables (vaccine type, time between doses) and participant characteristics (age, sex, race and multimorbidity, defined as at least two comorbidities [yes/no)] [9]. An interaction term between participant groups (HIV+ vs. control) and past COVID infection (yes/no) was included in the regression model to allow for potentially different neutrialization capacity response by these factors. Within PWH, we further performed univariate quantile regression analysis to determine whether there were factors associated with weaker neutralization capacity. After univariate analysis, age, sex, vaccine-related variables and variables with P < 0.1 in univariate analysis were further included in a multivariable model to further explore associations with participant characteristics. Multivariate analysis was only performed within PWH-naive to natural COVID-19 infection, as there was not enough participants with past COVID-19 infection to perform a separate analysis.
Results
A total of 375 PWH and 1002 SSO participants were enrolled. Individuals were excluded if they had received less than two vaccine doses, if the date of the second vaccine was unknown, or if samples were unavailable at the time point of interest (Supplemental Figure 1). We then randomly selected 256 samples out of 311 available for PWH, and 256 samples out of 381 available for controls, at the 3-month post dose 2 (+/− 1 month) time point, to perform neutralization assays.
Baseline characteristics for 256 PWH and 256 HIV-negative controls included in the final analysis are presented in Table 1 and Supplement Table 1. Of 256 in each group, 45 (17.6%) PWH and 56 (21.9%) controls had a history of COVID-19 infection at some point prior to 3 months post dose 2 (+/−1 month). Median ages were 54.2 (IQR 43.4–62.8) and 45.5 years (IQR 35–57) for PWH and controls, respectively. PWH were 72% men vs. 35% of controls, whereas 48% PWH were aged at least 55 years vs. 31% of controls. Median duration of HIV was 17 (IQR 10–25) years (Table 2). Median CD4+ T-cell count was 631 (430.5–838.5) cells/μl and 22% had a nadir CD4+ T-cell count below 100 cells/μl.
Table 1.
Characteristics of participants.
| All participants | Participants without past COVID-19 infection | Participants with past COVID-19 infection | |||||||
| Variable | HIV+ (n = 256) | HIV− (n = 256) | P | HIV+ (n = 211) | HIV− (n = 200) | P | HIV+ (n = 45) | HIV− (n = 56) | P |
| COVID infection prior to 3 months post dose 2 (±1 month) sample [n (%)] | 45 (17.6) | 56 (21.9) | – | 0 (0%) | 0 (0%) | – | 45 (100.0) | 56 (100.0) | – |
| Time since COVID infection, days prior to sample collection | 0.244 | ||||||||
| Median (IQR) | – | – | – | – | 263 (126, 338) | 306 (168, 472) | |||
| Range | – | – | – | – | (25–685) | (17–583) | |||
| Missing | – | – | – | – | 19 | 2 | |||
| Age | <0.001 | <0.001 | 0.133 | ||||||
| Median (IQR) | 54.2 (43.4, 62.8) | 45.5 (35.0, 57.0) | 54.6 (44.0, 63.6) | 45.0 (36.0, 56.0) | 53.3 (42.8, 60.2) | 49.0 (33.0, 58.5) | |||
| Range | (19.7–83.5) | (22.0–79.0) | (19.7–83.5) | (22.0–79.0) | (25.9–73.9) | (23.0–75.0) | |||
| <0.001 | <0.001 | 0.342 | |||||||
| Number missing | 1 | 0 | 1 | 0 | 0 | 0 | |||
| <35 | 30 (11.8) | 59 (23.0) | 24 (11.4) | 42 (21.0) | 6 (13.3) | 17 (30.4) | |||
| 35–44 | 40 (15.7) | 62 (24.2) | 33 (15.7) | 55 (27.5) | 7 (15.6) | 7 (12.5) | |||
| 45–54 | 64 (25.1) | 56 (21.9) | 50 (23.8) | 44 (22.0) | 14 (31.1) | 12 (21.4) | |||
| 55–64 | 73 (28.6) | 52 (20.3) | 60 (28.6) | 39 (19.5) | 13 (28.9) | 13 (23.2) | |||
| 65–74 | 38 (14.9) | 24 (9.4) | 33 (15.7) | 18 (9.0) | 5 (11.1) | 6 (10.7) | |||
| ≥75 | 10 (3.9) | 3 (1.2) | 10 (4.8) | 2 (1.0) | 0 (0.0) | 1 (1.8) | |||
| Sex | <0.001 | <0.001 | 0.04 | ||||||
| Male | 183 (71.5) | 89 (34.8) | 153 (72.5) | 64 (32.0) | 30 (66.7) | 25 (44.6) | |||
| Female | 72 (28.1) | 167 (65.2) | 57 (27.0) | 136 (68.0) | 15 (33.3) | 31 (55.4) | |||
| Prefer to self describe | 1 (0.4) | 0 (0) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Self-declared race or ethnicitya | <0.001 | <0.001 | <0.001 | ||||||
| White | 138 (54.5) | 228 (89.1) | 123 (58.9) | 179 (89.5) | 15 (34.1) | 49 (87.5) | |||
| Black | 59 (23.3) | 0 (0) | 41 (19.6) | 0 (0.0) | 18 (40.9) | 0 (0.0) | |||
| Other | 56 (22.1) | 28 (10.9) | 45 (21.5) | 21 (10.5) | 11 (25.0) | 7 (12.5) | |||
| Unknown | 3 | 0 | 2 | 0 | 1 | 0 | |||
| Subpopulation | |||||||||
| Age >55 years | 121/255 (47.5) | 79/256 (30.9) | <0.001 | 103/210 (49.0) | 59/200 (29.5) | <0.001 | 18/45 (40.0) | 20/56 (35.7) | 0.659 |
| Multimorbidity (≥2 comorbidities) | 90/252 (35.7) | 56/253 (22.1) | <0.001 | 74/208 (35.6) | 44/199 (22.1) | 0.003 | 16/44 (36.4) | 12/54 (22.2) | 0.123 |
Multiple could be selected.
Table 2.
HIV-related characteristics for HIV+ participants.
| All HIV+ participants | HIV + participants without past COVID-19 infection | HIV+ participants without past COVID-19 infection | |
| Variable | N = 256 | N = 211 | N = 45 |
| Duration of HIV infection (years) | |||
| Median (IQR) | 17.0 (10.0–25.0) | 17.0 (10.0–25.5) | 18.0 (9.0–24.0) |
| Range | (0.0–38.0) | (0.0–38.0) | (0.0–34.0) |
| Missing/unknown | 20 | 15 | 5 |
| <10 | 58 (24.6) | 47 (24.0) | 11 (27.5) |
| 10–19 | 84 (35.6) | 73 (37.2) | 11 (27.5) |
| 20+ | 94 (39.8) | 76 (38.8) | 18 (45.0) |
| CD4+ nadir (cells/μl) | |||
| Median (IQR)/range | |||
| Unknown | 86 | 73 | 13 |
| <100 | 38 (22.4) | 26 (18.8) | 12 (37.5) |
| 100–199 | 32 (18.8) | 26 (18.8) | 6 (18.8) |
| 200–299 | 36 (21.2) | 32 (23.2) | 4 (12.5) |
| 300–399 | 21 (12.4) | 19 (13.8) | 2 (6.3) |
| ≥400 | 43 (25.3) | 35 (25.4) | 8 (25.0) |
| CD4+ cell count (cells/μl) | |||
| Median (IQR) | 631.0 (430.5–838.5) | 630.0 (431.0–843.0) | 660.0 (387.0–805.0) |
| Range | (9.0–1800.0) | (9.0–1800.0) | (48.0–1050.0) |
| Missing | 16 | 14 | 2 |
| CD4+ cell count (cells/μl) | |||
| Unknown | 16 | 14 | 2 |
| <250 | 22 (9.2) | 14 (7.1) | 8 (18.6) |
| 250–349 | 15 (6.3) | 14 (7.1) | 1 (2.3) |
| 350–499 | 43 (17.9) | 40 (20.3) | 3 (7.0) |
| 500–999 | 136 (56.7) | 107 (54.3) | 29 (67.4) |
| ≥1000 | 24 (10.0) | 22 (11.2) | 2 (4.7) |
| CD4+/CD8+ ratio | |||
| Median (IQR) | 0.82 (0.56–1.21) | 0.84 (0.55–1.24) | 0.80 (0.59–1.20) |
| Range | (0.00–3.30) | (0.00–2.50) | (0.09–3.30) |
| Missing | 24 | 22 | 2 |
| CD4+/CD8+ ratio ≥0.75 [n (%)] | 129/232 (55.6) | 107/189 (56.6) | 22/43 (51.2) |
| Detectable viral load for at least 6 months [n (%)] | 35/250 (14.0) | 21/207 (10.1) | 14/43 (32.6) |
| If detectable, highest viral load over past 6 months (copies/ml) | |||
| Median (IQR) | 116 (40–1940) | 189 (62–1940) | 42 (25–323) |
| Range | (20–817096) | (20–680000) | (20–817096) |
| ART regimen | |||
| None | 8 (3.1) | 6 (2.8) | 2 (4.4) |
| NRTI-based regimen | 5 (2.0) | 3 (1.4) | 2 (4.4) |
| NNRTI-based regimen | 21 (8.2) | 17 (8.1) | 4 (8.9) |
| PI-based regimen | 8 (3.1) | 7 (3.3) | 1 (2.2) |
| INSTI-based regimen | 175 (68.4) | 149 (70.6) | 26 (57.8) |
| Otherb | 39 (15.2) | 29 (13.7) | 10 (22.2) |
| Subpopulation | |||
| Immune nonrespondera | 24/243 (9.9) | 21/200 (10.5) | 3/43 (7.0) |
| HIV+ stable/reference (CD4+ ≥350, suppressed viral load and ≤1 comorbidity) | 110/241 (45.6) | 94/198 (47.5) | 16/43 (37.2) |
CD4+ <350, CD4+/CD8+ <0.75, suppressed viral load.
regimens containing combinations of above and/or other drug classes (i.e. cell-entry inhibitor).
Fourteen percent of PWH had a detectable HIV viral load within the last 6 months. Nearly all were on ART (96.9%) and 70% were on integrase strand inhibitors. Frequent comorbidities included obesity (24 vs. 16% in PWH and controls, respectively), dyslipidaemia (17 vs. 11%) and hypertension (16 vs. 13%) (Supplement Table 2a).
In the current analysis, performed on March 2023, 88% of PWH and 86% of controls included in the analysis had received at least two mRNA vaccines. BNT162b2 and mRNA-1273 were the most commonly administered vaccines. Time interval between vaccine doses is shown in Table 3.
Table 3.
Types of coronavirus disease 2019 vaccination of participants.
| All participants | HIV + participants without past COVID-19 infection | HIV+ participants without past COVID-19 infection | |||||||
| Variable | HIV+ | HIV− | P | HIV+ | HIV− | P | HIV+ | HIV− | P |
| Types of COVID-19 vaccines received, dose 1 and 2 (%) | 0.004 | 0.002 | 0.341 | ||||||
| mRNA–mRNA | 225 (87.9) | 220 (85.9) | 181 (85.8) | 169 (84.5) | 44 (97.8) | 51 (91.1) | |||
| ChAdOx1–mRNA | 17 (6.6) | 22 (12.9) | 17 (8.1) | 30 (15.0) | 0 (0.0) | 3 (5.4) | |||
| ChAdOx1–ChAdOx1 | 13 (5.1) | 2 (0.8) | 12 (5.7) | 1 (0.5) | 1 (2.2) | 1 (1.8) | |||
| Ad26.COV2.S or Other | 1 (0.4) | 1 (0.4) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 1 (1.8) | |||
| Time between first and second doses in days | |||||||||
| <0.001 | <0.001 | 0.566 | |||||||
| Median (IQR) | 61.0 (51.0–77.0) | 57.0 (34.0–77.0) | 62.0 (51.0–79.0) | 56.0 (31.0–78.0) | 57.0 (36.0–67.0) | 62.0 (35.0–77.0) | |||
| Range | (20.0–238) | (19.0–125.0) | (20.0–135.0) | (19.0–125.0) | (21.0–238.0) | (21.0–112.00 | |||
| Number missing or N/A (Ad26.COV2.S) | 1 | 1 | 1 | 1 | 0 | 0 | |||
| Time between first and second doses in days | |||||||||
| <0.001 | <0.001 | 0.754 | |||||||
| ≤30 | 1 | 1 | 1 | 1 | 0 | 0 | |||
| 31–60 | 24 (9.4) | 56 (22.0) | 18 (8.6) | 49 (24.6) | 6 (13.3) | 7 (12.5) | |||
| >60 | 94 (36.9) | 84 (32.9) | 75 (35.7) | 64 (32.2) | 19 (42.2) | 20 (35.7) | |||
| Number missing or N/A (Ad26.COV2.S) | 137 (53.7) | 115 (45.1) | 117 (55.7) | 86 (43.2) | 20 (44.4) | 29 (51.8) | |||
ID50 after coronavirus disease 2019 vaccination at 3 months post dose 2 (+/−1 month)
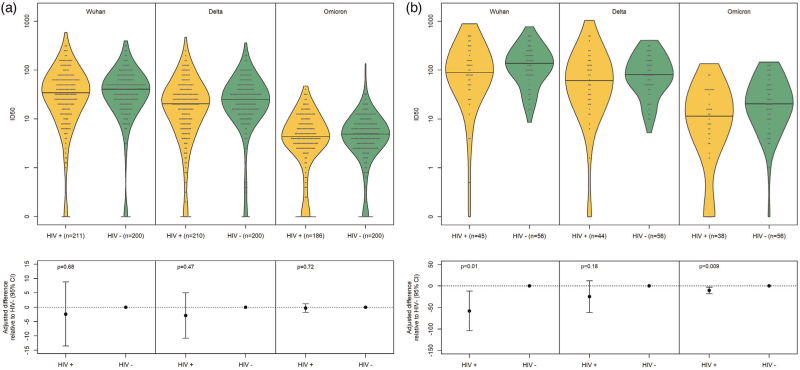
PWH and controls with COVID-19 infection prior to vaccination and during follow-up, up until the time point of interest, were analyzed separately from participants naive to natural COVID-19 infection as the former would be expected to have a more robust response following vaccine administration than the latter [21,22]. In participants naive to natural COVID-19 infection, median ID50 at 3 months post dose 2 (+/−1 month) did not differ between PWH vs. controls for Ancestral, Delta and Omicron variants using adjusted quantile regression (Table 4). In participants with past COVID-19 infection, median (IQR) ID50 was 89.3 (45.1, 185.7), 137.2 (81.3, 222.1) (adjusted difference (95% CI): −58.2 (−104.2, −12.1) (P = 0.013) for the Ancestral variant in PWH vs controls. Median (IQR) ID50 for the Delta variant was 61.0 (22.6, 133.0) in PWH vs controls (81.2 (47.7, 159.8) (adjusted difference (95% CI): −24.9 (−61.6, 11.9) (P = 0.184) and for the Omicron variant 11.5 (4.1, 37.1) vs 20.5 (10.1, 45.8) (adjusted difference (95% CI): −10.4 (−18.2, −2.6)) (P = 0.009). Comparison of ID50 between cohorts is depicted in Fig. 1.
Table 4.
Median (interquartile range) ID50 after COVID-19 vaccination at 3 months post dose 2 (±1 month).
| HIV + participants without past COVID-19 infectiona | HIV+ participants with past COVID-19 infectiona | ||||||||
| Antigen | HIV+ | HIV− | Difference in median for HIV+ vs. HIV− (95% CI)b | P | HIV+ | HIV− | Difference in median for HIV+ vs. HIV− (95% CI)∼ | P | P homo |
| Wuhan | 34.1 (14.4–67.3) | 40.2 (19.9–66.1) | −2.4 (−13.5 to 8.8) | 0.676 | 89.3 (45.1) | 137.2 (81.3–222.1) | −58.2 (−104.2 to −12.1) | 0.013 | 0.026 |
| Delta | 20.4 (7.6–37.8) | 24.7 (13.2–44.3) | −2.9 (−10.8 to 5.0) | 0.465 | 61.0 (22.6–133.0) | 81.2 (47.7–159.8) | −24.9 (−61.6 to 11.9) | 0.184 | 0.269 |
| Omicron | 4.4 (2.7–8.6) | 4.9 (2.7–8.3) | −0.3 (−1.8 to 1.2) | 0.716 | 11.5 (4.1–37.1) | 20.5 (10.1–45.8) | −10.4 (−18.2 to −2.6) | 0.009 | 0.017 |
Phomo is the P value from the test of homogeneity by COVID infection status.
Based on site report, participant questionnaires and IgG assay.
Based on quantile regression with an interaction term between HIV status and COVID infection status and adjusted for age, sex, race (white/black/other), multimorbidity (≥2 comorbidities), vaccine type and time between vaccine doses.
Fig. 1.
Difference in ID50 between cohorts to Ancestral, Delta and Omicron variants in participants without past COVID-19 infection and (a) and with past COVID-19 infection (b) in HIV+ and HIV− participants at 3 months after dose 2 (+/−1 month).
Three months post second vaccine dose (+/−1 month), there was no difference in ID50 within subgroups of PWH in univariate analyses using quantile regression (Table 5). For example, there was no difference in PWH who were immune nonresponders with multimorbidity in univariate analyses (Table 5) or when stratified by sex (Table 6).
Table 5.
Difference in median interquartile range ID50 by participant characteristics within HIV+ participants using quantile regression.
| HIV + participants without past COVID-19 infection (n = 211) | HIV+ participants with past COVID-19 infection (n = 45) | |||||||||||
| Comparison | Wuhan | P | Delta | P | Omicron | P | Wuhan | P | Delta | P | Omicron | P |
| Age >55 years | −10.5 (−23.3, 2.3) | 0.108 | −4.2 (−12.2, 3.8) | 0.301 | −1.1 (−2.2, 0.1) | 0.074 | −37.5 (−121.7, 46.7) | 0.374 | −52.4 (−130.0, 25.1) | 0.180 | 3.3 (−15.8, 22.5), | 0.728 |
| Immune nonresponder (CD4+ <350 cells/μl, CD4+/CD8+ <0.75, suppressed VL) | 4.2 (−34.8, 43.1) | 0.832 | 2.1 (−13.7, 17.9) | 0.795 | 1.0 (−3.6, 5.7) | 0.669 | – | – | – | – | ||
| Multimorbidity (≥2 comorbidities) | −10.2 (−22.7, 2.3) | 0.109 | −1.3 (−9.1, 6.6) | 0.754 | −1.3 (−2.5, 0.0) | 0.047 | 59.2 (−61.1, 179.5) | 0.326 | 29.4 (−59.9, 118.6) | 0.510 | 7.2 (−11.2, 25.6) | 0.431 |
| HIV+ stable/reference (CD4+ ≥350 cells, suppressed VL and ≤1 comorbidity) | 3.0 (−11.4, 17.4) | 0.683 | 0.0 (−5.9, 6.0) | 0.997 | 0.9 (−0.4, 2.1) | 0.167 | −66.0 (−146.7, 14.6) | 0.106 | −33.9 (−114.5, 46.8) | 0.401 | −7.5 (−24.5, 9.4) | 0.372 |
VL, viral load.
Table 6.
Difference in median interquartile range ID50 by participant characteristics within HIV+ participants using quantile regression.
| HIV + participants without past COVID-19 infection (n = 211) | HIV+ participants with past COVID-19 infection (n = 45) | |||||||||||
| Comparison | Wuhan difference (95% CI) | P | Delta difference (95% CI) | P | Omicron difference (95% CI) | P | Wuhan difference (95% CI) | P | Delta difference (95% CI) | P | Omicron difference (95% CI) | P |
| Age (per 10 years increase) | −3.6 (−8.2, 1.0) | 0.122 | −1.7 (−4.7, 1.2) | 0.252 | −0.6 (−1.1, −0.2) | 0.003 | 12.0 (−26.6, 50.6) | 0.533 | 3.3 (−31.3, 37.9) | 0.847 | 2.3 (−2.9, 7.6) | 0.375 |
| <35 | 20.2 (1.7, 38.7) | 0.033 | 16.5 (2.2, 30.7) | 0.024 | 4.5 (2.0, 7.0) | <0.001 | – | – | − | |||
| 35–44 | −0.4 (−18.3, 17.5) | 0.966 | 8.6 (−3.3, 20.6) | 0.157 | 1.5 (−0.7, 3.8) | 0.179 | – | – | – | |||
| 45–54 | 1.6 (−22.9, 26.0) | 0.900 | 10.8 (−0.4, 21.9) | 0.059 | 0.7 (−0.8, 2.3) | 0.358 | – | – | – | |||
| 55–64 | −2.7 (−20.6, 15.2) | 0.768 | 9.3 (−3.0, 21.5) | 0.139 | 0.8 (−0.7, 2.4) | 0.273 | – | – | – | |||
| ≥65 | Referent | Referent | – | – | – | |||||||
| Sex | ||||||||||||
| Male | −9.7 (−30.5, 11.1) | 0.357 | −5.8 (−12.1, 0.5) | 0.073 | −0.6 (−2.5, 1.4) | 0.558 | −46.4 (−130.7, 37.9) | 0.273 | −66.6 (−136.3, 3.0) | 0.060 | −2.5 (−18.8, 13.9) | 0.760 |
| Female | Referent | Referent | Referent | Referent | ||||||||
| Self-declared race or ethnicity | ||||||||||||
| Black | 31.7 (15.6, 47.8) | <0.001 | 16.9 (0.5, 33.3) | 0.043 | 3.6 (1.0, 6.2) | 0.006 | 98.3 (3.9, 192.7) | 0.042 | 76.5 (24.9, 128.1) | 0.005 | 12.6 (−11.1, 36.3) | 0.288 |
| Other | 4.1 (−7.1, 15.3) | 0.469 | 6.4 (−0.6, 13.3) | 0.074 | 0.3 (−1.0, 1.6) | 0.666 | 21.1 (−57.5, 99.7) | 0.591 | 43.7 (−30.2, 117.7) | 0.239 | 9.9 (−9.7, 29.5) | 0.310 |
| White | Referent | Referent | Referent | Referent | ||||||||
| Medical conditions | ||||||||||||
| Hypertension | 4.7 (−27.2, 36.5) | 0.773 | 2.4 (−12.9, 17.7) | 0.759 | 0.0 (−1.8, 1.8) | 0.992 | – | – | – | |||
| Diabetes | -6.0 (−27.9, 15.8) | 0.588 | 1.6 (−11.3, 14.4) | 0.809 | −0.4 (−2.7, 2.0) | 0.767 | – | – | – | |||
| Dyslipidaemia | 4.5 (−17.6, 26.5) | 0.689 | 6.0 (−6.8, 18.8) | 0.355 | −0.2 (−2.1, 1.6) | 0.803 | – | – | – | |||
| Chronic lung disease | −6.0 (−38.0, 25.9) | 0.711 | −4.1 (18.4, 10.2) | 0.576 | 0.0 (−2.3, 2.2) | 0.998 | − | – | – | |||
| Obesity | 1.4 (−22.8, 25.6) | 0.910 | 0.0 (−10.0, 9.9) | 0.994 | −0.7 (−2.2, 0.9) | 0.379 | 74.8 (−100.9, 250.5) | 0.395 | 41.1 (−79.8, 161.9) | 0.496 | 27.3 (2.4, 52.3) | 0.033 |
| BMI per 10-unit increase | −2.7 (−17.9, 12.5) | 0.725 | −0.7 (−6.7, 5.4) | 0.829 | −0.1 (−1.0, 0.7) | 0.786 | 95.2 (−47.1, 237.5) | 0.184 | 88.0 (−46.3, 222.3) | 0.193 | 20.0 (−6.7, 46.7) | 0.138 |
CI, confidence interval.
Association between ID50 and participant characteristics by multivariate quantile regression is shown in Table 7. Within PWH naive to natural COVID-19 infection, when adjusted for age, sex, CD4+ cell count, vaccine type and time between vaccine doses, black African/black Caribbean participants had significantly higher neutralization capacity than white participants for Ancestral but not for Delta or Omicron variants. Correlation between ID50 and antibody level within PWH at month 3 postdose 2 (±1 month) (Supplemental Figure 2), illustrated high concordance between the two levels.
Table 7.
Association between ID50 and patient characteristics by multivariable quantile regression.a
| HIV + participants without past COVID-19 infection (n = 195) | ||||||
| Comparison | Wuhan difference (95% CI) | P | Delta difference (95% CI) | P | Omicron difference (95% CI) | P |
| Age | ||||||
| <35 | 11.9 (−3.9, 27.8) | 0.140 | 6.1 (−10.1, 22.4) | 0.458 | 1.8 (−1.4, 5.0) | 0.258 |
| 35–44 | 0.5 (−15.4, 16.5) | 0.937 | −1.1 (−13.7, 11.5) | 0.866 | 0.9 (−1.3, 3.1) | 0.435 |
| 45–54 | 6.5 (−9.4, 22.3) | 0.476 | 2.6 (−9.4, 14.6) | 0.669 | 0.7 (−1.4, 2.9) | 0.497 |
| 55–64 | −4.1 (−17.5, 9.4) | 0.551 | 3.7 (−7.9, 15.3) | 0.532 | 0.9 (−0.8, 2.6) | 0.286 |
| ≥65 | Referent | Referent | Referent | |||
| Sex | ||||||
| Male | 2.4 (−8.8, 13.5) | 0.676 | 2.8 (−5.7, 11.3) | 0.517 | −0.3 (−1.9, 1.4) | 0.747 |
| Female | Referent | Referent | ||||
| Self-declared race or ethnicity | ||||||
| Black | 20.5 (5.5, 35.6) | 0.008 | 15.6 (−0.7, 31.8) | 0.060 | 1.7 (−1.1, 4.5) | 0.232 |
| Other | −5.1 (−18.6, 8.4) | 0.458 | 7.2 (−2.6, 17.0) | 0.148 | −0.5 (−2.2, 1.1) | 0.530 |
| White | Referent | Referent | Referent | |||
| CD4+ cell count (cells/μl) | ||||||
| CD4+ cell count (per 100 cells/μl increase) | 1.8 (0.1, 3.4) | 0.038 | 0.8 (−0.6, 2.1) | 0.271 | 0.2 (−0.0, 0.4) | 0.127 |
| Types of COVID-19 vaccines received, doses 1 and 2 | ||||||
| mRNA–mRNA | 27.8 (16.1, 39.4) | <0.001 | 13.7 (4.2, 23.2) | 0.005 | 2.6 (0.8, 4.5) | 0.006 |
| ChAdOx1–mRNA | 12.8 (0.1, 25.4) | 0.048 | 10.8 (−0.5, 22.0) | 0.060 | 0.8 (−1.3, 2.9) | 0.463 |
| ChAdOx1–ChAdOx1 | Referent | Referent | Referent | |||
| Time between first and second doses | ||||||
| Time between first and second doses (per 10 days increase) | −0.2 (−2.8, 2.4) | 0.869 | −1.1 (−3.0, 0.8) | 0.252 | 0.2 (−0.2, 0.5) | 0.395 |
CI, confidence interval.
Age, sex, vaccine-related variables and variables with P < 0.1 in the univariate analysis were included in the multivariable model.
Discussion
We previously reported that the vast majority of PWH obtained a detectable antibody titre at 3 and 6 months following second dose, and 1 month following a third or booster dose [8]. We also reported that vaccine-induced antibody titres were overall similar between PWH vs. HIV-negative controls [8]. PWH aged older than 55 years, immune nonresponders and those with multimorbidity achieved similar antibody levels to COVID-19 vaccines compared with HIV-negative controls. In the current study examining neutralization capacity, we focused on the 3 months post second dose timepoint. Reassuringly, we did not find any difference in PWH vs. controls in persons without prior COVID-19 infection. Within PWH without past COVID-19 infection, younger age (<35), higher CD4+ cell count or CD4+/CD8+ ratio, and vaccine type (mRNA) was associated with higher neutralization capacity. Shorter duration between doses was associated with higher neutralization capacity but only in univariate analyses. We had intended to evaluate PWH with past COVID-19 infection, however, our sample size was insufficient to assess all key variables.
In general, hybrid immunity acquired through combined vaccination and natural infection is associated with more robust immune response than either vaccination or natural infection alone [15–17]. Hybrid immunity is thought to specifically enhance Omicron variant neutralization. Verburgh et al. compared severe SARS-CoV-2 vaccine-induced and hybrid B-cell and T-cell responses in middle-aged PWH vs. HIV-negative controls who were highly comparable in terms of demographics and lifestyle [18]. PWH mounted equally robust immunity following vaccination and even more robust stronger immunity was observed in both groups following naturally acquired SARS-CoV-2 infection [18]. Meanwhile, Lu et al. examined humoral longitudinal observational study in PWH following primary inactivated SARS-CoV-2 vaccination. Neutralizing antibodies were detected 1 month following booster vaccine in all PWH and the titre increased six-fold compared with that associated with the primary vaccination, similar to what was observed in HIV-negative controls [19]. Neutralizing antibody titre declined over time after booster vaccine but remained higher at 6 months than following primary vaccine [19]. Neutralizing antibody response was elevated after booster vaccine in PWH with CD4+ cell count less than 200 cells/μl, although this boosting effect was lower compared with higher CD4+ cell count subgroups [19]. It is unclear why PWH with previous COVID-19 infection in our study exhibited higher neutralization capacity than PWH without past infection but weaker neutralization capacity for Ancestral and Omicron variants when compared with HIV-negative controls who also had past infection. As many SSO participants were healthcare workers and individuals working in public-facing environments, these individuals received vaccination, on average, earlier and with shorter intervals between doses than the general population. We speculate that timing of natural infection in relation to vaccination may have contributed to our observation. Therefore, it is possible that uncontrolled confounding may have accounted for this difference between groups. As time of infection was unknown for fewer than half of the HIV+ participants, it was not possible to control for time of infection as a variable.
Within PWH, black African/Caribbeans had higher neutralization capacity than for whites for all variants. In a cross-sectional analysis involving healthcare workers in the United Kingdom, SARS-CoV-2 spike-specific total antibody titre, neutralizing antibody titre and ELISpot count were compared by ethnic group [20]. This study found that humoral and cellular immune responses to SARS-CoV-2 vaccination were stronger in South Asian HCWs than white HCWs, and differences were most pronounced early following vaccination [20]. The reasons for our findings are unclear, although we suspect it relates to uncontrolled confounding.
In the current study, we observed that older age was associated with weaker neutralization capacity for Ancestral, Delta and Omicron variants. Advanced age is associated with diminished vaccine immunogenicity [21], due in part to intrinsic defects in B cells leading to sub-optimal antibody function [22–24]. Furthermore, ‘inflammaging’, which refers to increased low-grade systemic inflammation as persons age, is known to impact PWH [25,26].
We previously did not observe differences in antibody titres in PWH with CD4+ T-cell counts less than 350 cells/μl vs. higher counts. However, other groups have reported diminished anti-S vaccine response and neutralization capacity in PWH vs. HIV-uninfected controls [27,28]. Differences amongst findings in PWH may be because of discrepancies in multimorbidity burden in older adults, type and number of vaccines received and time interval between vaccinations [27–30]. Third dose vaccines elicited better neutralizing antibody responses in PWH with longer time intervals between vaccines [30]. Furthermore, although there is evidence for sex-based differences in humoral immune response with certain types of vaccinations in HIV-negative populations [30], we and others have not observed any sex difference in antibody level or neutralization capacity in the context of COVID-19 vaccination [10–12,19]. Moreover, in both PWH vs. HIV-negative controls, some participants had a high antibody titre but low ID50. However, given the small number of these individuals who had discordance between antibody titre and neutralization capacity, the importance of this finding, if any, is unclear.
We acknowledge several limitations. COVID-19 vaccinations became available in Canada in December 2020 but recruitment commenced in May 2021, resulting in missed opportunity to obtain baseline prevaccination blood in populations prioritized for vaccination (e.g. elderly, indigenous people) [31]. There were differences in vaccine type and dosing interval by province [31]. Numbers of PWH participants not receiving ART therapy and/or low CD4+ cell counts were relatively small. Thus, results may not be generalizable to all PWH [32]. The number of PWH with past COVID-19 infection was also relatively low, precluding robust multivariable assessment.
In summary, adult PWH without past COVID-19 infection with well controlled HIV on ART achieved vaccine-induced SARS-CoV-2 neutralization capacity that is similar to HIV-negative persons. Increasing age is associated with diminished neutralization capacity, and which might be impacted by HIV in those with past COVID-19. This phenomenon requires further exploration. Study of humoral immune response durability, the contribution of cell-mediated immunity and low-grade chronic inflammation will complement our current findings.
Acknowledgements
The authors thank the CTN staff who were involved in the planning and preparation of this study, in addition to the CTN Scientific Review Committee and the Community Advisory Board, for their expedited review and helpful comments. They also thank all of the participants for their time and commitment. In addition, the authors thank study coordinators and study nurses: Florian Bobeuf, Claude Vertzagias, Lina Del Balso, Nathalie Paisible, Guillaume Theriault, Hansi Peiris, Lianne Thai, Rosemarie Clarke, Erin Collins, Nadia Ohene-Adu, Aron Dyks and Jill Jackson; data manager Elisa Lau; laboratory staff: Tara Mabanga, Yulia Alexandrova, Ralph-Sydney Mboumba Bouassa, Erik Pavey, Stephanie Burke-Schinkel, the University of Ottawa Serology and Diagnostics High Throughput Facility: Danielle Dewar-Darch, Justino Hernandez Soto, Abishek Xavier, Nicholas Bradette, Klaudia Baumann, Gwendoline Ward and Yuchu Dou. The authors also thank physicians who assisted with recruitment.
COVID-19 antigens as well as the antihIgG#5-HRP fusion antibody were generously provided by Dr Yves Durocher, National Research Council of Canada (NRC), Montreal.
C.T.C. is supported by a Fonds de recherche du Québec-Santé (FRQS) Junior 2 career award. A.B. holds a Canada Research Chair Tier 2 Canada Research Chair (CRC) in sexually transmitted infection (STI) Prevention. M.A.L. holds a CRC, Tier 2, in human immunovirology. M.A.J. holds a CRC, Tier 2, in human immunovirology. S.W. is supported by a Research Chair in HIV Clinical Care and Aging from the Ontario HIV Treatment Network. D.T. is supported by a Tier 2 CRC in HIV Prevention and STI Research. M.A.B. holds a Canada Research Chair, Tier 2, in viral pathogenesis and immunity. Z.L.B. holds a Scholar Award from the Michael Smith Foundation for Health Research. B.L. is supported by a career award LE 250 from the Quebec's Ministry of Health for researchers in Family Medicine, FRQS Junior 2 career award and holds a Canadian Institutes for Health Research Strategy for Patient Oriented Research (SPOR) Mentorship Chair in Innovative Clinical Trials. J.P.R. holds a Lowenstein Chair in Hematology. Y.G. is supported by a Charles Best and Frederick Bandting (CGS-Doctoral award) from CIHR (476885).
Funding This project was supported by funding from the Public Health Agency of Canada, through the Vaccine Surveillance Reference group and the COVID-19 Immunity Task Force (grant number: 2122-HQ-000075) and the CTN (grant number: N/A). Production of COVID-19 reagents was financially supported by NRC's Pandemic Response Challenge Program.
Authors’ contributions: co-principal investigators of the study are C.T.C., C.L.C. and A.H.A. C.T.C. and C.L.C. conceived the study, led the proposal and protocol development. C.T.C. wrote the first draft of the manuscript. J.S. is the biostatistician who provided methodological expertise and performed sample size calculations. All other authors contributed to protocol development, study design and development of the proposal. C.T.C., M.A.L., Y.G., C.A., M.A.J., M.O., M.B. and Z.B. designed the laboratory evaluations. S.S. oversaw lab specimen processing and lab database development. Y.G., C.A., P.M. and M.A.L. were responsible for studies on humoral immunity. J.S. oversaw data analysis between groups and subgroup analyses. T.L. performed data analyses. All authors critically reviewed and approved the final manuscript.
Conflicts of interest
There are no conflicts of interests.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1.Tesoriero JM, Swain CE, Pierce JL, Zamboni L, Wu M, Holtgrave DR, et al. COVID-19 outcomes among persons living with or without diagnosed HIV infection in New York state. JAMA Netw Open 2021; 4:e2037069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiser JK, Tie Y, Beer L, Neblett Fanfair R, Shouse RL. Racial/ethnic and income disparities in the prevalence of comorbidities that are associated with risk for severe COVID-19 among adults receiving HIV Care, United States, 2014-2019. J Acquir Immune Defic Syndr 2021; 86:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crum-Cianflone NF, Sullivan E. Vaccinations for the HIV-infected adult: a review of the current recommendations, part I. Infect Dis Ther 2017; 6:303–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crum-Cianflone NF, Sullivan E. Vaccinations for the HIV-infected adult: a review of the current recommendations, part II. Infect Dis Ther 2017; 6:333–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergman P, Blennow O, Hansson L, Mielke S, Nowak P, Chen P, et al. COVAXID-collaborator group. Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. EBioMedicine 2021; 74:103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy I, Wieder-Finesod A, Litchevsky V, Biber A, Indenbaum V, Olmer L, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin Microbiol Infect 2021; 27:1851–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costiniuk CT, Singer J, Langlois MA, Kulic I, Needham J, Burchell A, et al. CTN 328: immunogenicity outcomes in people living with HIV in Canada following vaccination for COVID-19 (HIV-COV): protocol for an observational cohort study. BMJ Open 2021; 11:e054208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costiniuk CT, Singer J, Lee T, Langlois MA, Arnold C, Galipeau Y, et al. COVAXHIV Study Group. COVID-19 vaccine immunogenicity in people with HIV. AIDS 2023; 37:F1–F10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert PB, Montefiori DC, McDermott AB, Fong Y, Benkeser D, Deng W, et al. Immune Assays Team§, Moderna, Inc. Team§, Coronavirus Vaccine Prevention Network (CoVPN)/Coronavirus Efficacy (COVE) Team§, United States Government (USG)/CoVPN Biostatistics Team§. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022; 375:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27:1205–1211. [DOI] [PubMed] [Google Scholar]
- 11.Collins E, Galipeau Y, Arnold C, Bosveld C, Heiskanen A, Keeshan A, et al. Cohort profile: Stop the Spread Ottawa (SSO)—a community-based prospective cohort study on antibody responses, antibody neutralisation efficiency and cellular immunity to SARS-CoV-2 infection and vaccination. BMJ Open 2022; 22:e062187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colwill K, Galipeau Y, Stuible M, Gervais C, Arnold C, Rathod B, et al. A scalable serology solution for profiling humoral immune responses to SARS-CoV-2 infection and vaccination. Clin Transl Immunol 2022; 11:e1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abe KT, Li Z, Samson R, Samavarchi-Tehrani P, Valcourt EJ, Wood H, et al. A simple protein-based surrogate neutralization assay for SARS-CoV-2. JCI Insight 2020; 5:142362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cholette F, Fabia R, Harris A, Ellis H, Cachero K, Schroeder L, et al. COVID-19 Immunity Task Force (CITF) working group. Comparative performance data for multiplex SARS-CoV-2 serological assays from a large panel of dried blood spot specimens. Heliyon 2022; 8:e10270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann M, Behrens GMN, Arora P, Kempf A, Nehlmeier I, Cossmann A, et al. Effect of hybrid immunity and bivalent booster vaccination on omicron sublineage neutralisation. Lancet Infect Dis 2023; 23:25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Muecksch F, Schaefer-Babajew D, Finkin S, Viant C, Gaebler C, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021; 595:426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaballa ME, Perez-Saez J, de Mestral C, Pullen N, Lamour J, Turelli P, et al. Specchio-COVID19 study group. Seroprevalence of anti-SARS-CoV-2 antibodies and cross-variant neutralization capacity after the Omicron BA.2 wave in Geneva, Switzerland: a population-based study. Lancet Reg Health Eur 2023; 24:100547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verburgh ML, van Pul L, Grobben M, Boyd A, Wit F, van Nuenen AC, et al. Robust vaccine-induced as well as hybrid B- and T-cell immunity across SARS-CoV-2 vaccine platforms in people with HIV. Microbiol Spectr 2023; 11:e0115523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu T, Chen Z, Cao Y, Ao L, Li Z, Gu X, et al. Dynamic immunogenicity after primary and booster inactivated SARS-CoV-2 vaccination in people living with HIV: a longitudinal observational study. J Med Virol 2023; 95:e28730. [DOI] [PubMed] [Google Scholar]
- 20.Martin CA, Nazareth J, Jarkhi A, Pan D, Das M, Logan N, et al. Ethnic differences in cellular and humoral immune responses to SARS-CoV-2 vaccination in UK healthcare workers: a cross-sectional analysis. EClinicalMedicine 2023; 58:101926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gustafson CE, Kim C, Weyand CM, Goronzy JJ. Influence of immune aging on vaccine responses. J Allergy Clin Immunol 2020; 145:1309–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frasca D, Blomberg BB. Aging induces B cell defects and decreased antibody responses to influenza infection and vaccination. Immun Ageing 2020; 17:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pallikkuth S, De Armas LR, Pahwa R, Rinaldi S, George VK, Sanchez CM, et al. Impact of aging and HIV infection on serologic response to seasonal influenza vaccination. AIDS 2018; 32:1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parmigiani A, Alcaide ML, Freguja R, Pallikkuth S, Frasca D, Fischl MA, et al. Impaired antibody response to influenza vaccine in HIV-infected and uninfected aging women is associated with immune activation and inflammation. PLoS One 2013; 8:e79816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sieg SF, Shive CL, Panigrahi S, Freeman ML. Probing the Interface of HIV and inflammaging. Curr HIV/AIDS Rep 2021; 18:198–210. [DOI] [PubMed] [Google Scholar]
- 26.Pereira B, Xu XN, Akbar AN. Targeting inflammation and immunosenescence to improve vaccine responses in the elderly. Front Immunol 2020; 11:583019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cossu MV, Mileto D, Giacomelli A, Oreni L, Bracchitta F, Pellicciotta M, et al. Does the co-morbidity burden contribute to suboptimal immunological responses to COVID-19 vaccination in people living with HIV?. J Infect Dis 2022; doi: 10.1093/infdis/jiac286. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu S, Zou S, Ming F, Wu M, Guo W, Xing Z, et al. Humoral immune response to inactivated COVID-19 vaccination at the 3rd month among people living with HIV. BMC Infect Dis 2023; 23:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antinori A, Cicalini S, Meschi S, Bordoni V, Lorenzini P, Vergori A, et al. HIV-VAC Study Group. Humoral and cellular immune response elicited by mRNA vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in people living with human immunodeficiency virus receiving antiretroviral therapy based on current CD4 T-lymphocyte count. Clin Infect Dis 2022; 75:e552–e563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Li J, Zhang W, Liu S, Miao L, Li Z, et al. Extending the dosing interval of COVID-19 vaccination leads to higher rates of seroconversion in people living with HIV. Front Immunol 2023; 14:1152695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ministry of Health. COVID-19 vaccine booster recommendation.Version 8.3. July 22. [Google Scholar]
- 32.Noe S, Ochana N, Wiese C, Schabaz F, Von Krosigk A, Heldwein S, et al. Humoral response to SARS-CoV-2 vaccines in people living with HIV. Infection 2022; 50:617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.