ABSTRACT
Ovarian cancer is the most lethal gynecologic malignancy, mostly diagnosed in the advanced stage with multiple sites of metastases. Routes of spread are direct through exfoliation, lymphatic channels, and less commonly hematogenous spread. Skin metastasis in ovarian malignancy is a rare occurence, its incidence range from 1.9% to 5.1% and the most common sites are the abdominal wall and chest wall. The incidence of metastasis to breast and/or axillary lymph nodes is very rare, ranging from 0.03% to 0.6%. We report the case of a 60-year-old female with stage IV B undifferentiated ovarian carcinoma with multiple cutaneous metastases involving the skin over the left breast, scalp, and mediastinal lymph nodes, which are rare sites of metastases. The incidence of cutaneous metastasis in ovarian cancer is 1.9%–5.1% and the overall survival after diagnosis ranges from 2 to 65 months.
KEYWORDS: Cancer antigen 125, cutaneous metastases, gynecologic malignancy, ovarian cancer, scalp nodule
INTRODUCTION
Ovarian cancer is the second most common gynecologic cancer in the United States[1] and the 6th most common cancer in the UK with around 7300 new cases in 2017, almost 6 in 10 cases are diagnosed in late stages, and it accounts for 5% of all cancer deaths in females.[2] In India, ovarian cancer ranks as the 3rd most common cancer after the breast and cervix. This contributes to around 5.9% of total cancers in India, the maximum number of cases (26.3%) being observed between the ages of 45 and 54 years.[3]
Ovarian cancer has the highest mortality among all gynecologic cancers and metastatic spread from the primary tumor has already developed at the time of diagnosis. Ovarian cancer spreads directly to the peritoneal cavity by the exfoliation from the tumor surface, through lymphatic channels to pelvic and para-aortic lymph nodes and less frequently to inguinal and supraclavicular nodes. The most common sites for distant metastases are the pleura, liver, and lung. Skin metastasis rarely occurs, with incidence ranging from 1.9% to 5.1%. The common sites are the abdominal wall, specifically Sister Mary Joseph Nodule, chest wall, and breast.[4] The exact mechanism of metastasis to skin is not fully understood, but according to diffrent theories, spread can occur through direct invasion, lymphatic route or iatrogenic in minimally invasive procedures.[5] Scalp metastasis is rarely reported and may present as soft, balloon-like growth over the occipital region, which might be developed by hematogenous spread.[6]
The appearance of skin metastasis after the ovarian cancer diagnosis might range from 4 to 36 months and the most common histologic types are serous papillary cystadenocarcinoma (78%), endometrioid, and mucinous carcinoma (12%). 78% had poorly differentiated tumors. The overall survival after diagnosis of skin metastasis ranges from 2 to 65 months.[7] Intrathoracic lymph node involvement is very rare and found in 2.3%,[8] and axillary lymph node metastasis without breast cancer is extremely low (0.03%–0.6%),[9] and only 110 cases have been described until 2015.[10]
We report the case of a 60-year-old female with stage IV B of carcinoma ovary with multiple sites of distant metastases to the skin of breast, scalp, and mediastinal lymph nodes.
CASE REPORT
A 60-year-old female presented with a complaint of shortness of breath, progressive over a period of approximately 1 week, with no association of cough or chest pain. This was accompanied with feeling of distension and lump in the abdomen for 1 month. She was taking on and off treatment for recurrent gastrointestinal complaints for the last 4 years.
On physical examination, a large suprapubic fixed lump of approximately 20 cm × 18 cm size was present, which was firm to hard in consistency with irregular margin. A soft to firm, nontender, fixed nodular swelling of size 5 × 5 was present on the scalp in the occipital region [Figure 1]. Multiple skin nodules with erythema were present over the skin of the left breast [Figure 2].
Figure 1.
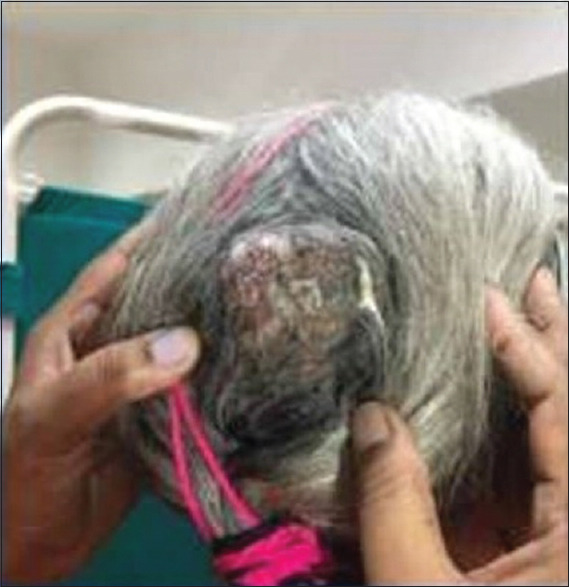
Metastatic scalp nodule
Figure 2.
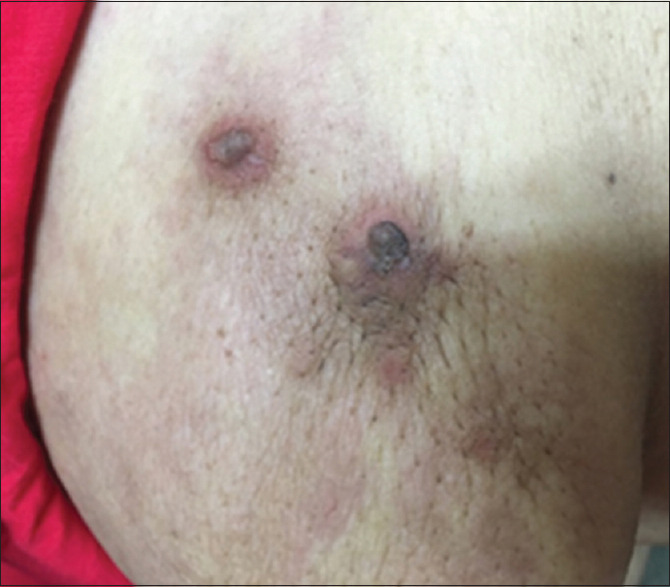
Skin nodule with erythema over breast skin
Chest X-ray showed right-sided massive pleural effusion with malignant cells in exudative fluid. Ultrasound report showed a heterogeneous soft tissue mass lesion of size 166 mm × 133 mm with internal vascularity in the pelvis, suggestive of adnexal origin along with lymphadenopathy in preaortic, para-aortic, and iliac group of lymph nodes, largest measuring 64 mm × 31 mm. Contrast-enhanced computed tomography findings revealed ovarian cancer with malignant soft tissue lesions in the skin of the left breast and multiple retroperitoneal and iliac lymphadenopathy along with involvement of the liver, lung, and mediastinal lymph nodes. Stomach, small and large bowel loops, and urinary bladder showed normal findings. Ascites was minimal and no bone lesion was present [Figure 3]. Cancer antigen 125 was 442.1 IU/ml.
Figure 3.
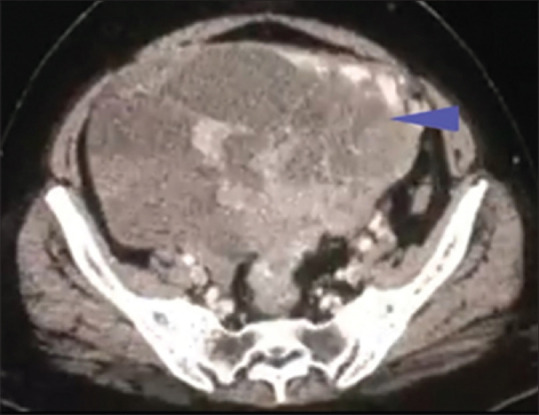
CECT pelvis showing heterogeneous ovarian mass. CECT: Contrast-enhanced computed tomography. The blue arrow head is showing the large, heterogenous mass in the abdomen
Ultrasonography-guided biopsy of abdominopelvic mass revealed undifferentiated carcinoma of the ovary. Skin biopsy of breast lesion [Figure 4] and scalp nodule showed high-grade malignant metastasis from ovarian neoplasm [Figure 5]. The patient was referred to a radiation oncologist for chemotherapy.
Figure 4.
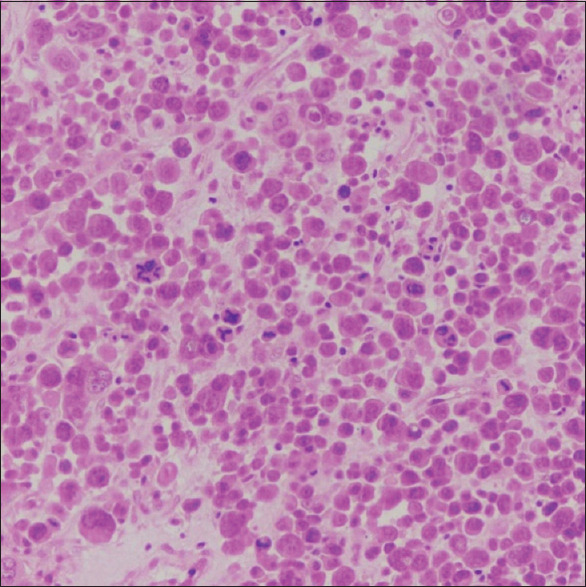
Biopsy from nodular erythematous plaque on the breast showing large, singly scattered and diffusely infiltrating tumor cells and several atypical mitosis
Figure 5.
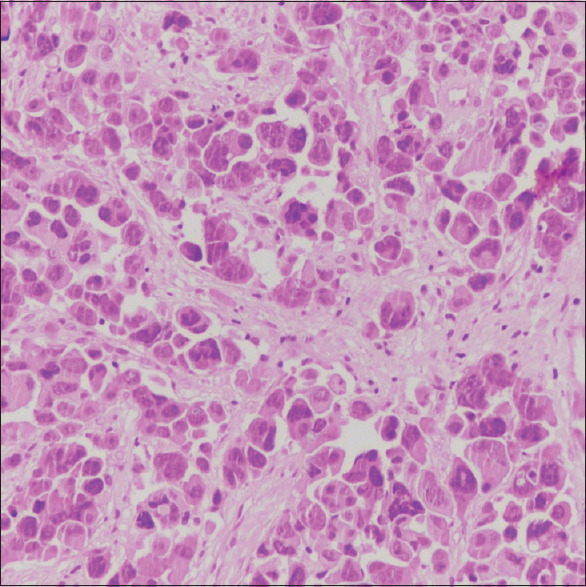
Biopsy from margin of the soft nodular mass, large, singly scattered, and diffusely infiltrating tumor cells
DISCUSSION
Ovarian cancer has high mortality because more than 75% of women present in advanced stages of the disease due to lack or nonspecific symptoms, and only 15% are confined to the primary site at the time of diagnosis.[11]
Distant metastases occur most commonly to pleura, liver, lung, and lymph nodes. It usually spreads directly to the peritoneal cavity, but may also metastasize through the lymphatic and hematogenous routes. Cutaneous metastases are suggestive of widespread intraperitoneal involvement and carry a bad prognosis.[4]
Skin metastasis occurs in 3.3%–4% of cases in primary ovarian malignancy.[4,5,7] The overall survival after diagnosis of skin metastasis was 4 months (range, 2–65 months).[7]
A review of different studies on metastatic ovarian cancer, which included 225 patients showed that 38% of patients were in stage IV disease, 24.7% had malignant pleural effusion with median survival of 6 months and 3.5% had subcutaneous nodules with median survival of 12 months. Metastasis to intrathoracic lymph nodes is extremely rare, few studies reported only 2.3% in advanced stage of the disease.[8]
Scalp metastasis has been rarely reported in very few studies of recurrent ovarian cancer. The pathogenesis was suggested to be through hematogenous spread.[6]
Metastases to the breast and the axillary lymph nodes are very rare in primary ovarian malignancy with an incidence of only 0.03%–0.06%[9] and serious papillary carcinoma is the most common type.[12,13] Route of dissemination may be lymphatic or hematogenous; generally, these lesions are circumscribed masses, while in lymphatic diffuse breast involvement will be there.[14] Secondary breast involvement from ovarian cancer suggests widespread dissemination and is associated with a poor prognosis, survival time ranged from 13 days to 3.5 years, with many patients dying within 1-year.[15]
CONCLUSION
From this case report, we can conclude that all women of age ≥40 years, presenting with vague symptoms and cutaneous lesions, should undergo detailed evaluation with a high index of suspicion to rule out malignancy and awareness of the general population to be done, especially in developing countries.
Consent
Informed consent was obtained from the subject in this case report.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that her name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1. [[Last accessed on January-February 2022]]. Available from: https://www.cdc.gov/cancer/ovarian/statistics/index.htm;Ovarian cancer statistics .
- 2. [[Last accessed on January-February 2022]]. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/ovarian-cancer#heading-Zero .
- 3.Takiar R. Status of Ovarian Cancer in India (2012-14) EC Gynaecology. 2019;85:358–64. [Google Scholar]
- 4.Dauplat J, Hacker NF, Nieberg RK, Berek JS, Rose TP, Sagae S. Distant metastases in epithelial ovarian carcinoma. Cancer. 1987;60:1561–6. doi: 10.1002/1097-0142(19871001)60:7<1561::aid-cncr2820600725>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Cheng B, Lu W, Xiaoyun W, YaXia C, Xie X. Extra-abdominal metastases from epithelial ovarian carcinoma: An analysis of 20 cases. Int J Gynecol Cancer. 2009;19:611–4. doi: 10.1111/IGC.0b013e3181a416d0. [DOI] [PubMed] [Google Scholar]
- 6.Matsui H, Suzuka K, Yamazawa K, Tanaka N, Mitsuhashi A, Seki K, et al. Scalp metastasis of a serous ovarian cancer. Acta Obstet Gynecol Scand. 2002;81:577–8. doi: 10.1034/j.1600-0412.2002.810621.x. [DOI] [PubMed] [Google Scholar]
- 7.Cormio G, Capotorto M, Di Vagno G, Cazzolla A, Carriero C, Selvaggi L. Skin metastases in ovarian carcinoma: A report of nine cases and a review of the literature. Gynecol Oncol. 2003;90:682–5. doi: 10.1016/s0090-8258(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 8.Dhillon SS, Harris K, Pokharel S, Yendamuri S. Calcified mediastinal metastasis of ovarian cancer mimicking broncholithiasis. J Bronchology Interv Pulmonol. 2016;23:229–31. doi: 10.1097/LBR.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 9.El Attrache BF, Highsmith C, Gluck B, Heimann A, Kapenhas E. Ararity in breast disease: Metastatic ovarian carcinoma to the breast mimicking inflammatory breast cancer. J Univer Surg. 2017;5:3. doi: 10.21767/2254-6758.100081. [Google Scholar]
- 10.Thomakos N, Diakosavvas M, Machairiotis N, Fasoulakis Z, Zarogoulidis P, Rodolakis A. Rare distant metastatic disease of ovarian and peritoneal carcinomatosis: A review of the literature. Cancers (Basel) 2019;11:1044. doi: 10.3390/cancers11081044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: A review. Cancer Biol Med. 2017;14:9–32. doi: 10.20892/j.issn.2095-3941.2016.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein RL, Brown AR, Gomez-Castro CM, Chambers SK, Cragun JM, Grasso-Lebeau L, et al. Ovarian cancer metastatic to the breast presenting as inflammatory breast cancer: A case report and literature review. J Cancer. 2010;1:27–31. doi: 10.7150/jca.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore DH, Wilson DK, Hurteau JA, Look KY, Stehman FB, Sutton GP. Gynecologic cancers metastatic to the breast. J Am Coll Surg. 1998;187:178–81. doi: 10.1016/s1072-7515(98)00119-7. [DOI] [PubMed] [Google Scholar]
- 14.Sippo DA, Kulkarni K, Carlo PD, Lee B, Eisner D, Cimino-Mathews A, et al. Metastatic disease to the breast from extramammary malignancies: A multimodality pictorial review. Curr Probl Diagn Radiol. 2016;45:225–32. doi: 10.1067/j.cpradiol.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Ozgüroğlu M, Ersavaşti G, Ilvan S, Hatemi G, Demir G, Demirelli FH. Bilateral inflammatory breast metastases of epithelial ovarian cancer. Am J Clin Oncol. 1999;22:408–10. doi: 10.1097/00000421-199908000-00018. [DOI] [PubMed] [Google Scholar]


