ABSTRACT
Background and Objective:
The prevalence of adenomyosis of the uterus varies from 5% to 70%, and there is no clear consensus on its imaging diagnostic criteria. The objective of this study was to evaluate the role of transvaginal sonography (TVS), combined TVS and color Doppler (TVS-CD), and magnetic resonance imaging (MRI) in the diagnosis of adenomyosis.
Materials and Methods:
This was a tertiary care hospital-based prospective study, in which 365 clinically suspected cases of adenomyosis were enrolled. All three types of imaging (TVS, TVS-CD, and MRI) were done in 233/365 patients, followed by hysterectomy in 50. Imaging features were correlated with the histopathological examination (HPE), which was taken as the gold standard for the diagnosis. The diagnostic performance of each imaging modality was assessed.
Results:
Among patients who underwent hysterectomy, 36/50 (72%) had adenomyosis on HPE, with or without associated benign gynecological abnormalities. Sensitivity, specificity, positive predictive value (PPV), negative PV (NPV), and diagnostic accuracy (DA) of MRI were higher than that of TVS-CD (91.67% vs. 77.78%, 85.71% vs. 78.57%, 94.29% vs. 90.32%, 80% vs. 57.89%, and 90% vs. 78%, respectively). TVS alone had lower diagnostic performance (specificity: 64.29%, PPV 84.85%, NPV 52.94%, and DA74%) than TVS-CD, but equal sensitivity (77.78%). Heterogeneous myometrium was the most sensitive (80.56%), while myometrial cyst was the most specific (92.86%) TVS feature. The maximum junctional zone thickness ≥12 mm was the most sensitive (97.22%), while the hyperintense myometrial focus was the most specific (100%) MRI feature.
Conclusion:
TVS-CD should be used as an initial diagnostic imaging modality in clinically suspected cases of adenomyosis; however, MRI due to better diagnostic efficacy should be the imaging modality of choice before subjecting such patients to hysterectomy.
KEYWORDS: Adenomyosis, junctional zone, magnetic resonance imaging, myometrial cyst, transvaginal sonography
INTRODUCTION
Adenomyosis is defined as the benign invasion of the endometrium into the myometrium, producing a diffusely enlarged uterus, which microscopically exhibits ectopic, nonneoplastic endometrial glands and stroma surrounded by the hypertrophic and hyperplasic myometrium.[1] It most commonly affects perimenopausal and multiparous women.[2] The patients with adenomyosis have nonspecific symptoms, and nearly 50% of them have coexisting leiomyomas, adding to the diagnostic challenge.[3]
Advancement in high-resolution imaging techniques has paved the way to its presurgical diagnosis with high precision. The studies comparing transvaginal sonography (TVS) and magnetic resonance imaging (MRI) offer inconclusive data with some reporting equivalent results, while others indicate the superiority of MRI over TVS.[4] Therefore, the objectives of this study were to evaluate and compare the diagnostic efficacy of TVS, TVS-color Doppler (CD), and MRI for the diagnosis of adenomyosis, and to identify the most sensitive and most specific sonographic and MRI features of adenomyosis and to correlate with histopathological findings.
MATERIALS AND METHODS
All procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 2000. Ethical approval from the Institute’s Ethical Committee was taken (Dean/2017/EC/194 dated October 24, 2017). Informed and written consent was obtained from all patients for being included in the study.
Study design and setting
The study was designed as a hospital-based cross-sectional study and conducted in Sir Sunderlal Hospital (tertiary care hospital), Banaras Hindu University, Varanasi.
Study population
The study was conducted among premenopausal women, aged 40–50 years, who were referred from the outpatient department of obstetrics and gynecology with clinical suspicion of adenomyosis of the uterus and underwent diagnostic imaging by TVS, TVS-CD, and MRI, followed by hysterectomy and histopathological examination (HPE), which was considered the gold standard for the diagnosis of adenomyosis.
Sample size calculation
Sample size (n) was calculated by the following formula:
n = z21-α/2 p.q/e2
Where, “P” = 0.85[5] as sensitivity of imaging modality, “q” = 1–0.85 = 0.15, “e” = 0.10 as absolute error, and “(1-α)” is 95% confidence level; then n = 48.96, i.e., 50.
The participants were chosen as per the following inclusion and exclusion criteria:
Inclusion criteria
The participants who had one or more of the clinical symptoms (menorrhagia, dysmenorrhea, dyspareunia, and chronic pelvic pain) suggested adenomyosis.
Exclusion criteria
Postmenopausal or pregnant women or those with a history of gynecological cancer, minimally invasive treatment of menorrhagia (endometrial ablation/resection), intake of contraceptive pill/gonadotropin-releasing hormone agonists within the past 6 months, and contraindications to surgery or MRI were excluded.
Imaging
TVS and TVS-CD were done in one sitting, while MRI was done within 1 week of the sonography.
2D-Transvaginal sonography and Doppler
TVS and TVS-CD were performed using a 5–9 MHz endocavitary transducer (Diagnostic Ultrasound, iU22-Philips Medical system, California, United States). Diagnosis of adenomyosis was made when ≥3 of the following features were present: heterogeneous myometrial echotexture (presence of an indistinctly myometrial area with decreased or increased echogenicity), globular-appearing uterus, asymmetrical thickness of the anteroposterior wall of the myometrium, subendometrial myometrial cysts (round anechoic areas of 1–7 mm diameter), subendometrial echogenic linear striations (radiate pattern of thin acoustic shadowing not arising from echogenic foci), or poor definition of the endometrial–myometrial junction [Figure 1].[6] The presence of any associated leiomyoma/endometriosis/adnexal lesion was also assessed. On CD, the pattern of vascular distribution was assessed as intralesional or circumferential or both to distinguish focal adenomyosis from leiomyoma.[7] CD also helped in differentiating myometrial cysts from a vascular component.[8]
Figure 1.
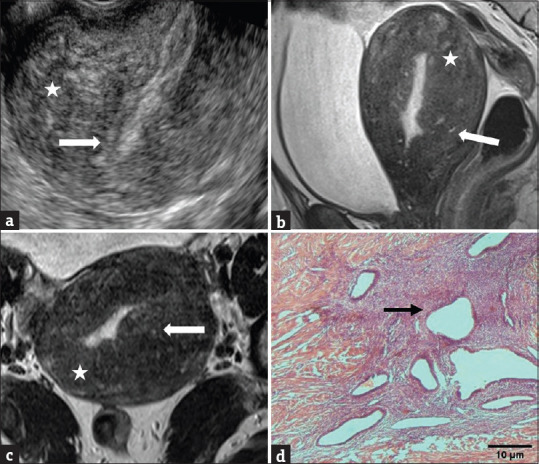
(a) Indistinct endomyometrial junction (arrow) with heterogeneous myometrium (asterisk) on TVS. Sagittal (b) and axial (c) T2-weighted MR images show heterogeneous myometrium, diffuse junctional zone thickening of ≥12 mm (asterisks), and hyperintense myometrial foci (arrows). (d) HPE shows endometrial tissue (arrow) surrounded by hyperplastic smooth muscle. TVS: Transvaginal sonography, MR images: Magnetic resonance images, HPE: Histopathological examination
Magnetic resonance imaging of the pelvis
MRI of the pelvis was done in the late proliferative-secretory phase of the menstrual cycle, using a 1.5 Tesla superconducting magnet (MAGNETOM Avanto, Siemens Medical System, Erlangen, Germany). Axial T1-weighted (with and without fat suppression) and axial, sagittal, and coronal T2-weighted sequences were taken. Diffusion-weighted imaging (DWI) with apparent diffusion coefficient (ADC) map and susceptibility-WI (SWI) were used as problem-solving tools.
Five criteria were evaluated on T2-weighted sequences: (i) borders, size, and uterine symmetry, (ii) maximal junctional zone (JZmax) thickness and/or presence of an ill-defined, relatively homogeneous, low-signal-intensity myometrial area (IDMA), (iii) maximal JZ thickness to myometrial thickness ratio (ratiomax), using the maximal thickness of the JZ and the corresponding thickness of the entire myometrium obtained at the same level, (iv) difference between JZmax and JZmin (JZdif) for the anterior or posterior border (JZ thickness was measured at the thinnest [JZmin] and thickest [JZmax] part at the anterior and posterior walls in the sagittal section and the largest parameter, either anterior or posterior, was used in all calculations), and (v) high-intensity spots within the myometrium.
Adenomyosis on MRI was defined by: (i) a large, regular, asymmetric uterus without leiomyomas, (ii) JZmax of at least 12 mm and/or an ill-defined, IDMA distinguished from well-circumscribed masses related to myoma, (iii) ratiomax >40%, (iv) JZdif >5 mm, and (v) punctate high-intensity myometrial foci.[8,9] The presence of ≥3 features as described above was taken to make the diagnosis of adenomyosis in our study [Figure 1].
Hysterectomy and histopathological examination
Hysterectomy was performed within 3 months of imaging, followed by HPE. The specimens were examined microscopically, in which the presence of ectopic endometrial glands and/or stroma in the myometrium, more than one low-power field away from the endomyometrial junction, was considered diagnostic of adenomyosis.[10]
Statistical analysis
The diagnostic efficiencies of TVS, TVS Doppler, and MRI were assessed in terms of sensitivity, specificity, positive predictive value (PPV), negative PV (NPV), and diagnostic accuracy (DA), and were compared using the Chi-square test and Fisher’s exact test. P < 0.05 was considered statistically significant. Statistical analysis was done using IBM (International Business Machines Corporation, Armonk, New York, United States), SPSS (Statistical Package for Social Sciences) Statistics for Windows, version 20.
RESULTS
All three types of imaging (TVS, TVS-CD, and MRI) were done in 233/365 patients [Figure 2]. For n = 233 patients, the kappa (k) and percentage agreement for the diagnosis of adenomyosis between TVS and TVS-CD, TVS and MRI, and TVS-CD and MRI were 0.896 and 95.71%, 0.246 and 68.67%, and 0.324 and 71.24%, respectively. Fifteen patients were lost on follow-up, while 168 received only conservative treatment. Finally, 50 patients in whom surgical treatment was primarily indicated or who did not respond to 3 months of conservative treatment and underwent hysterectomy were included in the study. On HPE, 36/50 (72.0%) patients were positive, while 14/50 (28.0%) were negative for adenomyosis. The mean age of patients with and without adenomyosis was 44.6 ± 5.52 years and 47.3 ± 5.28 years (P = 0.12), while the mean parity was 2.61 ± 1.05 and 2.64 ± 1.08 (P = 0.93), respectively. Associated leiomyomas were present in 13/36 (36.1%) and endometriomas in 2/36 (5.6%) patients with adenomyosis. Out of 14 adenomyosis-negative patients, leiomyomas were present in 6/14 (42.9%), endometriosis in 4/14 (28.6%), endometrial hyperplasia in 3/14 (21.4%), and endometrial polyp in 1/14 (7.1%).
Figure 2.
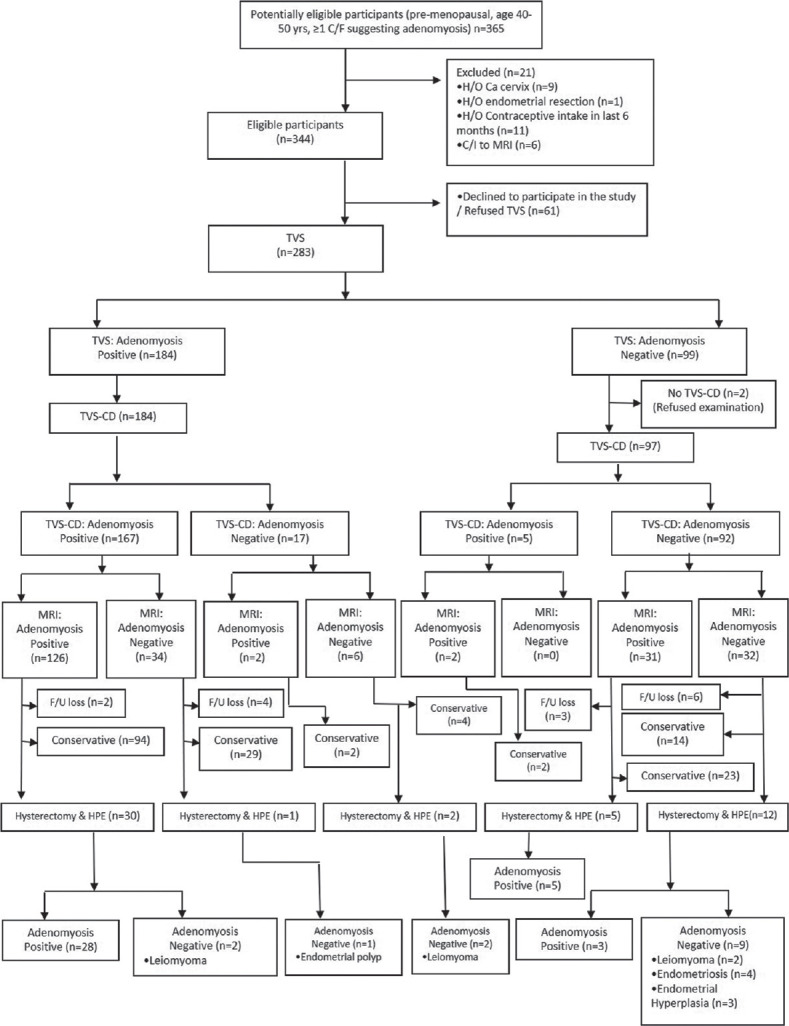
Flow diagram of the study participants
Transvaginal sonography
A correct diagnosis was made in 28/36 (77.8%) cases using TVS with 5/14 (21.4%) false-positive results. “Heterogeneous myometrium” had the highest sensitivity (80.56%), whereas “myometrial cyst” had the highest specificity (92.86%) [Table 1]. In our study, ≥3 features’ criteria on TVS had 77.78% sensitivity, 64.29% specificity, 2.18 positive likelihood ratio (PLR), 0.35 negative LR (NLR), 84.85% PPV, 52.94% NPV, and 74% DA for diagnosis of adenomyosis. The Nagelkerke R2 values for diagnosis of adenomyosis were 64.4% for all the six TVS features and 64.3% for four combined features; i.e., heterogeneous myometrium + indistinct endomyometrial junction + subendometrial echogenic linear striation + wall asymmetry.
Table 1.
Diagnostic performance of transvaginal sonography features of adenomyosis and combination of ≥3 criteria (n=50)
| TVS | 95% CI | ||||
|---|---|---|---|---|---|
|
| |||||
| Sensitivity percentage | Specificity percentage | PPV percentage | NPV percentage | Diagnostic accuracy percentage | |
| Bulky globular uterus | 63.89 (47.5–77.5) | 42.86 (21.3–67.4) | 74.19 (56.75–86.3) | 31.58 (15.36–53.99) | 58 (44.23–70.63) |
| Indistinct endomyometrial junction | 58.33 (42.2–72.86) | 78.57 (52.41–92.43) | 87.5 (69–95.66) | 42.31 (25.54–61.05) | 64 (50.14–75.86) |
| Subendometrial echogenic linear striation | 25 (13.75–41.07) | 85.71 (60.06–95.99) | 81.82 (52.3–94.86) | 30.77 (18.57–46.42) | 42 (29.37–55.77) |
| Wall asymmetry | 58.33 (42.2–72.86) | 64.29 (38.76–83.66) | 80.77 (62.12–91.49) | 37.5 (21.16–57.29) | 60 (46.18–72.39) |
| Heterogeneous myometrium | 80.56 (64.97–90.25) | 57.14 (32.59–78.62) | 82.86 (67.32–91.9) | 53.33 (30.12–75.19) | 74 (60.45–84.13) |
| Myometrial cysts | 19.44 (9.75–35.03) | 92.86 (68.53–98.73) | 87.5 (52.91–97.76) | 30.95 (19.07–46.03) | 40 (27.61–53.82) |
| ≥3 criteria | 77.78 (61.91–88.28) | 64.29 (38.76–83.66) | 84.85 (69.08–93.35) | 52.94 (30.96–73.84) | 74 (60.45–84.13) |
TVS: Transvaginal sonography, PPV: Positive predictive value, NPV: Negative predictive value, CI: Confidence interval
Transvaginal sonography-color Doppler
Using TVS-CD, a correct diagnosis was made in 28/36 (77.8%) cases with 3/14 (21.4%) false-positive results. TVS-CD had 77.78% sensitivity, 78.57% specificity, 3.64 PLR, 0.28 NLR, 90.32% PPV, 57.89% NPV, and 78% DA.
Magnetic resonance imaging of the pelvis
With MRI, a correct diagnosis was made in 33/36 (91.6%) cases with 2/14 (14.3%) false-positive results using a criterion of the presence of ≥3/5 features. This diagnostic criterion had 91.67% sensitivity, 85.71% specificity, 6.41 PLR, 0.10 NLR, 94.29% PPV, 80% NPV, and 90% accuracy. The highest sensitivity of 97.22% was noted for JZ thickness of ≥12 mm and the highest specificity of 100% for hyperintense myometrial foci [Table 2]. The Nagelkerke R2 values for diagnosis of adenomyosis were 88.3% for all the five MRI features and 85.7% for two combined features; i.e., junctional zone thickening ≥12 mm + hyperintense myometrial foci. The receiver operating characteristic curves were plotted for JZmax ≥12 mm, ratiomax >40%, and JZdif >5 mm, and the respective areas under the curves were 0.990, 0.989, and 0.881.
Table 2.
Diagnostic performance of magnetic resonance imaging features of adenomyosis and combination of ≥3 criteria (n=50)
| MRI | 95% CI | ||||
|---|---|---|---|---|---|
|
| |||||
| Sensitivity percentage | Specificity percentage | PPV percentage | NPV percentage | Diagnostic accuracy percentage | |
| Junctional zone thickening ≥12 mm | 97.22 (85.83–99.51) | 78.57 (52.41–92.43) | 92.11 (79.2–97.28) | 91.67 (64.61–98.51) | 92 (81.16–96.85) |
| Maximal JZ thickness to myometrial thickness ratio >40% | 88.89 (74.68–95.59) | 78.57 (52.41–92.43) | 91.43 (77.62–97.04) | 73.33 (48.05–89.1) | 86 (73.81–93.05) |
| Difference between the maximum and minimum thickness of JZ >5 mm | 77.78 (61.91–88.28) | 85.71 (60.06–95.99) | 93.33 (78.68–98.15) | 60 (38.66–78.12) | 80 (66.96–88.76) |
| Myometrial heterogeneous intensity | 91.67 (78.17–97.13) | 50 (26.8–73.2) | 82.5 (68.05–91.25) | 70 (39.68–89.22) | 80 (66.96–88.76) |
| Hyperintense myometrial foci | 72.22 (56.01–84.15) | 100 (78.47–100) | 100 (87.13–100) | 58.33 (38.83–75.53) | 80 (66.96–88.76) |
| ≥3 criteria | 91.67 (78.17–97.13) | 85.71 (60.06–95.99) | 94.29 (81.39–98.42) | 80 (54.81–92.95) | 90 (78.64–95.65) |
MRI: Magnetic resonance imaging, PPV: Positive predictive value, NPV: Negative predictive value, CI: Confidence interval, JZ: Junctional zone
For n = 50 patients, the k and percentage agreement for the diagnosis of adenomyosis between TVS and HPE, TVS-CD and HPE, and MRI and HPE were 0.395 and 74%, 0.508 and 78%, and 0.757 and 90%, respectively.
DISCUSSION
For adenomyosis of the uterus, some researchers have found similar DA of TVS and MRI, while others have shown the superiority of MRI over TVS.[4] In our study, the highest DA for the diagnosis of adenomyosis was reported with MRI (90%) compared to 2D-TVS (74%) and 2D-TVS-CD (78%) [Table 3]. Bazot and Daraï also suggested that MRI was more useful than TVS in the diagnosis of adenomyosis,[4] while they had earlier shown the superiority of the former only in cases associated with leiomyomas.[8] On comparing with the pooled LRs reported by Champaneria et al. in their systematic review with meta-analysis,[11] it was noted that our results were similar for NLR of TVS (0.35 vs. 0.30), PLR (6.41 vs. 6.5), and NLR (0.10 vs. 0.20) of MRI, except for PLR of TVS, which was lower in our study (2.18 vs. 3.7).
Table 3.
Diagnostic performance of transvaginal sonography, transvaginal sonography-color Doppler, and magnetic resonance imaging for adenomyosis (n=50)
| Tests | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Diagnostic accuracy (%) |
|---|---|---|---|---|---|
| TVS | 77.78 | 64.29 | 84.85 | 52.94 | 74 |
| TVS-CD | 77.78 | 78.57 | 90.32 | 57.89 | 78.0 |
| MRI | 91.67 | 85.71 | 94.29 | 80 | 90 |
MRI: Magnetic resonance imaging, PPV: Positive predictive value, NPV: Negative predictive value, TVS: Transvaginal sonography, TVS-CD: TVS-color Doppler
The reason why the results are so conflicting on imaging may be due to several factors. First, there is a lack of clear consensus on diagnostic criteria used on TVS and MRI. Second, there is high variation in the appearance of JZ on MRI, which depends not only on the age and hormonal status of the patient, but also on several other benign uterine conditions.
In our study, the 3 features’ diagnostic criteria used on TVS were the same as that used by Di Donato et al.[6] We also suggested the four main TVS features; the presence of any three of which may yield better results than randomly choosing any three. However, many considered any one feature as sufficient for the diagnosis.[12-14] Therefore, the specificity on TVS observed in our study was higher (64.29%) than that by Puliyathinkal and Surendran (58.6%) and Sun et al. (60.1%).[12,14] However, our study showed almost similar sensitivity (77.78%) to that by the former (80.5%),[12] but less than that by the latter (87.1%).[14] Our sensitivity and specificity both were lower when compared to that by Gupta et al. (89.13% and 90.62%),[13] perhaps due to the subjective nature of the diagnostic criteria of adenomyosis on TVS. A systematic review and meta-analysis carried out by Andres et al. showed pooled sensitivity and specificity of 2D-TVS to be 83.8% and 63.9%, respectively, which is comparable to our findings.[15] Our relatively low sensitivity of 77.7% might be due to associated leiomyomas.
We found that “heterogeneous myometrium” had the highest sensitivity (80.56%) and DA (74%) in contrast to that reported by Sun et al., who noted “subendometrial echogenic linear striations” to have the highest sensitivity (91.8%) and DA (79.3%).[14] “Myometrial cyst,” which had the highest specificity (92.86%) and minimum sensitivity (19.4%) in our study, had maximum sensitivity (60%) and specificity (98.8%) in a study by Bazot et al.[8] “Globular uterine configuration” showed the highest specificity and poor sensitivity in some studies,[12,14] but had the lowest specificity and second-highest sensitivity in our study. Our results were like that by Exacoustos et al., who also noted “heterogeneous myometrium” and “myometrial cyst” as the most sensitive and specific 2D-TVS features.[16] Myometrial heterogeneity correlated with smooth muscle hypertrophy and myometrial cyst with echogenic material representing blood, i.e., active endometrial tissue on HPE.
Adding CD to 2D-TVS reduced the number of false-positive cases in our study from five to three and increased the specificity from 64.29% to 78.57% and PPV from 84.85% to 90.32%. Both cases were those of leiomyomas which were correctly diagnosed by the presence of peripheral vascularity on CD unlike adenomyosis, where the vessels are seen crossing through the areas of adenomyosis. TVS-CD also had better NPV (57.89% vs. 52.94%) and DA (78% vs. 74%), but the same sensitivity (77.78%) when compared to that of TVS alone.
To date, the exact features to be assessed on MRI and the number of features to be fulfilled for the diagnosis of adenomyosis remain controversial. We assessed all five MRI features as suggested by Dueholm et al.,[9] and noted that each imaging feature, although significant, had less contribution in the diagnosis of adenomyosis. We, hence, introduced the criteria of the presence of minimum three features for the preoperative diagnosis of adenomyosis. We also suggested that the two main MRI features, if included in our 3 features’ diagnostic criteria, would yield better results than choosing randomly any three. Our study showed higher sensitivity, specificity, PPV, NPV, PLR, and lower NLR than that reported by Hashad et al.[3] (91.67% vs. 55.2%, 85.71% vs. 81.3%, 94.29% vs. 75.0%, 80% vs. 64.0%, 6.41 vs. 1.81, and 0.10 vs. 0.34, respectively). This could be explained by the fact that we considered all three JZ characteristics to define adenomyosis, while many considered only one or two parameters of JZ. Moreover, we made use of two problem-solving MR imaging techniques: DWI and SWI as suggested by Takeuchi and Matsuzaki.[17] One case in our study showed a focal hyperintense area within an ill-defined low-signal-intensity lesion in the posterior myometrium. Suspicion of primary/secondary malignancy was raised. The focal hyperintense area showed an increase in signal intensity on DWI (T2 shine-through), but did not show any prominent decrease in ADC value, thus ruling out malignancy. We made use of SWI in three cases where we had 2/5 criteria fulfilled, and myometrial hyperintense foci were absent on T2-weighted and absent/indefinite on fat-suppressed T1-weighed sequences. In all three, SWI could delineate signal voids due to hemosiderin deposits indicating old hemorrhagic foci and thus fulfilling the 3/5 criteria for the diagnosis of adenomyosis.
Our 3 features’ diagnostic criteria showed higher sensitivity (91.67% vs. 77.78%), specificity (85.71% vs. 78.57%), PPV (94.29% vs. 90.32%), NPV (80% vs. 57.89%), and DA (90% vs. 78%) compared to that of TVS-CD. The number of false-positive cases reduced further from three to two. Compared to TVS-CD, correctly diagnosed adenomyosis cases on MRI increased from 28 to 33. Out of the five cases missed both on TVS and TVS-CD, three had ill-defined JZ, one had posterior-walled adenomyosis, and one had adenomyosis associated with multiple leiomyomas, highlighting the limitations of sonography.
The knowledge of physiological variations of JZ is very important to avoid misdiagnosis. JZ may change depending on the hormonal status and phase of the menstrual cycle. Therefore, we included only premenopausal nonpregnant females without a history of any hormonal intake in the previous 6 months and performed MRI in the late proliferative-secretory phase. According to Novellas et al., the principal limitation of MRI was that JZ might not be defined in 20% of premenopausal and 50% of postmenopausal women.[18] Transient uterine contractions can lead to either pseudothickening of JZ or T2-weighted hypointense bands perpendicular to JZ. This can be avoided by repeating the acquisition or using cine MR imaging, but neither was done in our study.
Dueholm et al. included JZdif >5 mm in the diagnostic criteria on MRI and showed it to be a better diagnostic marker than JZmax.[9] Our study also included all three features of JZ in diagnostic criteria and showed that JZdif had higher specificity (85.71% vs. 78.57%) but lower DA (80% vs. 92%) than JZmax. The most sensitive (97.22%) MRI feature in our study was “JZ thickness ≥12 mm,” which also had the maximum DA (92%). Bazot et al. also reported maximum DA (85%) with this parameter, but with sensitivity less than ratiomax >40% (62.5% vs. 65.0%).[8] This might be because JZ thickening seen on MRI was due to the proliferation of the inner layer of the myometrium, whereas JZ thickening is actually due to the presence of endometrial glands at a certain distance from the endometrium surrounded by smooth muscle cells oriented in a less coherent pattern. We found hyperintense myometrial foci to have the highest specificity (100%) like that by Bazot et al.(98.8%).[8]
Although MRI is better for diagnosing adenomyosis than TVS/TVS-CD, the latter is still the imaging modality of choice for the disease, as it is relatively accurate in expert hands.[19] Exacoustos and Zupi, in their editorial, mentioned about the strengths of ultrasound in the diagnosis of adenomyosis and suggested to use TVS in all types of patients.[20]
There were many limitations in our study. First, the differentiation of adenomyosis into diffuse or focal type or adenomyoma was not done. Second, for differentiating adenomyosis from leiomyoma, only CD was done, and not spectral Doppler, which could have a potential role as suggested by Sharma et al.[21] Third, MRI was shown to be superior over 2D-TVS; however, comparison with 3D-TVS was not done. Fourth, uterine volume and number of associated leiomyomas were not assessed. Fifth, the sensitivity and specificity of TVS and MRI were not calculated separately for patients with and without leiomyomas. Sixth, repeat MRI acquisition or cine MR imaging was not done to distinguish adenomyosis from transient uterine contraction.
In spite of these limitations, our study used novel MRI diagnostic criteria in the form of the presence of minimum three out of five features. Problem-solving MRI techniques such as DWI and SWI have already been shown to have potential diagnostic roles in adenomyosis; however, to the best of our knowledge, this is the first study to use them in evaluating the MRI diagnostic features. Future studies assessing the role of these and other promising MRI techniques (cine MR imaging, magnetic resonance spectroscopy, and high-resolution 3T MRI) in the diagnosis of adenomyosis are needed. This becomes more important with the newer sonographic classification and reporting system for diagnosing adenomyosis on its way,[19] when the role of MRI in adenomyosis may get limited only to equivocal cases on ultrasound.
CONCLUSION
To conclude, TVS-CD due to its efficacy, safety, wide-spread availability, and low cost remains the primary and initial imaging modality for the diagnosis of adenomyosis. MRI, however, should be recommended as the second-line imaging to confirm the TVS-CD findings in suspected patients before subjecting them to any radical procedure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ferraz Z, Nogueira-Martins N, Nogueira-Martins F. Adenomyosis: Back to the future?Facts Views Vis Obgyn. 2017;9:15–20. [PMC free article] [PubMed] [Google Scholar]
- 2.Senturk LM, Imamoglu M. Adenomyosis: What is new? Womens Health (Lond) 2015;11:717–24. doi: 10.2217/whe.15.60. [DOI] [PubMed] [Google Scholar]
- 3.Hashad AM, Hassan NE, Elbohoty AE, Ibrahim IM, Bakr OB. 3D ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis. Egypt J Hosp Med. 2017;69:3123–33. [Google Scholar]
- 4.Bazot M, Daraï E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil Steril. 2018;109:389–97. doi: 10.1016/j.fertnstert.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Hanafi M. Ultrasound diagnosis of adenomyosis, leiomyoma, or combined with histopathological correlation. J Hum Reprod Sci. 2013;6:189–93. doi: 10.4103/0974-1208.121421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Donato N, Montanari G, Benfenati A, Leonardi D, Bertoldo V, Monti G, et al. Prevalence of adenomyosis in women undergoing surgery for endometriosis. Eur J Obstet Gynecol Reprod Biol. 2014;181:289–93. doi: 10.1016/j.ejogrb.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Van den Bosch T, Dueholm M, Leone FP, Valentin L, Rasmussen CK, Votino A, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: A consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol. 2015;46:284–98. doi: 10.1002/uog.14806. [DOI] [PubMed] [Google Scholar]
- 8.Bazot M, Cortez A, Darai E, Rouger J, Chopier J, Antoine JM, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: Correlation with histopathology. Hum Reprod. 2001;16:2427–33. doi: 10.1093/humrep/16.11.2427. [DOI] [PubMed] [Google Scholar]
- 9.Dueholm M, Lundorf E, Hansen ES, Sørensen JS, Ledertoug S, Olesen F. Magnetic resonance imaging and transvaginal ultrasonography for the diagnosis of adenomyosis. Fertil Steril. 2001;76:588–94. doi: 10.1016/s0015-0282(01)01962-8. [DOI] [PubMed] [Google Scholar]
- 10.Rizvi G, Pandey H, Pant H, Chufal SS, Pant P. Histopathological correlation of adenomyosis and leiomyoma in hysterectomy specimens as the cause of abnormal uterine bleeding in women in different age groups in the Kumaon region: A retroprospective study. J Midlife Health. 2013;4:27–30. doi: 10.4103/0976-7800.109631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: Systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89:1374–84. doi: 10.3109/00016349.2010.512061. [DOI] [PubMed] [Google Scholar]
- 12.Puliyathinkal S, Surendran P. A clinical study on transvaginal ultrasonography and its histopathological correlation in the diagnosis of adenomyosis. Int J Sci Stud. 2017;5:239–42. [Google Scholar]
- 13.Gupta S, Goel G, Agrawal S, Garg P, Khanuja E. Clinical and ultrasonological features of adenomyosis and its histopathological correlation. Int J Reprod Contracept Obstet Gynecol. 2016;5:3283–9. [Google Scholar]
- 14.Sun YL, Wang CB, Lee CY, Wun TH, Lin P, Lin YH, et al. Transvaginal sonographic criteria for the diagnosis of adenomyosis based on histopathologic correlation. Taiwan J Obstet Gynecol. 2010;49:40–4. doi: 10.1016/S1028-4559(10)60007-1. [DOI] [PubMed] [Google Scholar]
- 15.Andres MP, Borrelli GM, Ribeiro J, Baracat EC, Abrão MS, Kho RM. Transvaginal ultrasound for the diagnosis of adenomyosis: Systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:257–64. doi: 10.1016/j.jmig.2017.08.653. [DOI] [PubMed] [Google Scholar]
- 16.Exacoustos C, Brienza L, Di Giovanni A, Szabolcs B, Romanini ME, Zupi E, et al. Adenomyosis: Three-dimensional sonographic findings of the junctional zone and correlation with histology. Ultrasound Obstet Gynecol. 2011;37:471–9. doi: 10.1002/uog.8900. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi M, Matsuzaki K. Adenomyosis: Usual and unusual imaging manifestations, pitfalls, and problem-solving MR imaging techniques. Radiographics. 2011;31:99–115. doi: 10.1148/rg.311105110. [DOI] [PubMed] [Google Scholar]
- 18.Novellas S, Chassang M, Delotte J, Toullalan O, Chevallier A, Bouaziz J, et al. MRI characteristics of the uterine junctional zone: From normal to the diagnosis of adenomyosis. AJR Am J Roentgenol. 2011;196:1206–13. doi: 10.2214/AJR.10.4877. [DOI] [PubMed] [Google Scholar]
- 19.Van den Bosch T, de Bruijn AM, de Leeuw RA, Dueholm M, Exacoustos C, Valentin L, et al. Sonographic classification and reporting system for diagnosing adenomyosis. Ultrasound Obstet Gynecol. 2019;53:576–82. doi: 10.1002/uog.19096. [DOI] [PubMed] [Google Scholar]
- 20.Exacoustos C, Zupi E. A new era in diagnosing adenomyosis is coming. Fertil Steril. 2018;110:858. doi: 10.1016/j.fertnstert.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Sharma K, Bora MK, Venkatesh BP, Barman P, Roy SK, Jayagurunathan U, et al. Role of 3D Ultrasound and Doppler in differentiating clinically suspected cases of leiomyoma and adenomyosis of uterus. J Clin Diagn Res. 2015;9:QC08–12. doi: 10.7860/JCDR/2015/12240.5846. [DOI] [PMC free article] [PubMed] [Google Scholar]


