Abstract
Significance Statement
We hypothesized that triple therapy with inhibitors of the renin–angiotensin system (RAS), sodium–glucose transporter (SGLT)-2, and the mineralocorticoid receptor (MR) would be superior to dual RAS/SGLT2 blockade in attenuating CKD progression in Col4a3-deficient mice, a model of Alport syndrome. Late-onset ramipril monotherapy or dual ramipril/empagliflozin therapy attenuated CKD and prolonged overall survival by 2 weeks. Adding the nonsteroidal MR antagonist finerenone extended survival by 4 weeks. Pathomics and RNA sequencing revealed significant protective effects on the tubulointerstitium when adding finerenone to RAS/SGLT2 inhibition. Thus, triple RAS/SGLT2/MR blockade has synergistic effects and might attenuate CKD progression in patients with Alport syndrome and possibly other progressive chronic kidney disorders.
Background
Dual inhibition of the renin–angiotensin system (RAS) plus sodium–glucose transporter (SGLT)-2 or the mineralocorticoid receptor (MR) demonstrated additive renoprotective effects in large clinical trials. We hypothesized that triple therapy with RAS/SGLT2/MR inhibitors would be superior to dual RAS/SGLT2 blockade in attenuating CKD progression.
Methods
We performed a preclinical randomized controlled trial (PCTE0000266) in Col4a3-deficient mice with established Alport nephropathy. Treatment was initiated late (age 6 weeks) in mice with elevated serum creatinine and albuminuria and with glomerulosclerosis, interstitial fibrosis, and tubular atrophy. We block-randomized 40 male and 40 female mice to either nil (vehicle) or late-onset food admixes of ramipril monotherapy (10 mg/kg), ramipril plus empagliflozin (30 mg/kg), or ramipril plus empagliflozin plus finerenone (10 mg/kg). Primary end point was mean survival.
Results
Mean survival was 63.7±10.0 days (vehicle), 77.3±5.3 days (ramipril), 80.3±11.0 days (dual), and 103.1±20.3 days (triple). Sex did not affect outcome. Histopathology, pathomics, and RNA sequencing revealed that finerenone mainly suppressed the residual interstitial inflammation and fibrosis despite dual RAS/SGLT2 inhibition.
Conclusion
Experiments in mice suggest that triple RAS/SGLT2/MR blockade may substantially improve renal outcomes in Alport syndrome and possibly other progressive CKDs because of synergistic effects on the glomerular and tubulointerstitial compartments.
Keywords: Alport syndrome, CKD, renin–angiotensin system, SGLT2
Introduction
In patients with diabetic and nondiabetic CKD, dual inhibition of the renin–angiotensin system (RAS) and sodium–glucose transporter (SGLT)-2 sustains the decline of GFR more potently than RAS inhibition alone.1 Adding further renoprotective drugs might be even more renoprotective. For example, the nonsteroidal mineralocorticoid receptor antagonist (MRA) finerenone demonstrated an additive reduction of proteinuria in patients with CKD associated with type 2 diabetes beyond RAS inhibition.2 Some positive signals for better efficacy of triple RAS/SGLT2/MR inhibition in humans exist, but these analyses remained underpowered and restricted to proteinuria, similarly to an ongoing phase 2 trial, so a synergistic effect on kidney function decline remains to be tested.3–5 We hypothesized that adding finerenone to dual blockade of RAS/SGLT2 could delay uremic death. To address this, we performed a preclinical randomized controlled trial (pRCT) in a mouse model of spontaneous and progressive CKD, that is, collagen (Col)4a3-deficient mice with Alport nephropathy, to minimize bias and to obtain reproducible results as recommended.6
Methods
Animals and Study Design
Col4a3−/− (Col4a3tm1Dec) mice with a 129/SvJ backgrounds (Jackson Lab, Bar Harbor, ME) are widely used animal model of progressive CKD in Alport syndrome, one of the most standardized animal models for progressive CKD. Col4a3−/− mice were bred under specific pathogen-free housing conditions, cohoused with enrichment in a 12-hour light and dark cycle with free access to chow and autoclaved water, and genotyped as described earlier.7,8 We registered the trial protocol with all prespecified parameters before the start of the study at www.preclinicaltrials.eu (Registration no. PCTE0000266). A detailed trial protocol is provided in Supplemental File 2. All experimental procedures were approved by the local government authorities (approval code Gz. ROB-55.2-2532.Vet_02-20-223) according to the European equivalent of the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (directive 2010/63/EU). Inclusion and exclusion criteria for participant mice are reported in Supplemental File 1. Mice were enrolled between February 2022 and November 2022. Ten male and ten female mice were block-randomized each (10/10:10/10:10/10:10/10) to a food admix of (1) vehicle, (2) 10 mg/kg ramipril, (3) 10 mg/kg ramipril plus 30 mg/kg empagliflozin, or (4) 10 mg/kg ramipril plus 30 mg/kg empagliflozin plus 10 mg/kg finerenone. The different types of food preparations were marked by different food colors starting from age 6 weeks for 8 weeks, and all researchers involved in the study remained blinded to the treatments up to the end of the study. For the cross-sectional analysis, mice in four groups were treated for 2.5 weeks and euthanized for blood sampling from the retro-orbital plexus and kidney collection at age 8.5 weeks. All animals were euthanized by cervical dislocation under isoflurane anesthesia. The prespecified primary end point was mean overall survival.
Randomization; blinding; grouping; primary and secondary end points; measurement of GFR, proteinuria, serum BUN, creatinine, potassium, phosphate, and glucose; histology; immunostaining; immunofluorescence and quantitative analysis; RNA sequencing; transcriptome analysis; animal welfare surveillance; and statistical analysis are reported in detail in the Supplemental Files.
Results
Baseline Characteristics of Col4a3−/− Mice at the Time of Randomization
Up to age 6 weeks, the time of randomization, Col4a3−/− mice developed progressive Alport nephropathy with no significance between group difference in serum creatinine, BUN, or albuminuria (Table 1, Supplemental Figure 1). Tissue analysis at 6 weeks revealed significant glomerulosclerosis, tubular trophy, kidney injury, and alpha smooth muscle actin (αSMA)+/Sirius Red+ interstitial fibrosis (Supplemental Figures 2–4). Thus, we initiated treatments at an advanced stage of CKD.
Table 1.
Baseline characteristics of Col4a3−/− mice at randomization
| Variable | Vehicle | RASi | RASi/SGLT2i | RASi/SGLT2i/MRA |
|---|---|---|---|---|
| N | 20 | 20 | 20 | 20 |
| Male (%) | 50 | 50 | 50 | 50 |
| Weight (g) | 19.8±1.7 | 20.0±2.7 | 20.4±2.5 | 20.4±2.3 |
| GFR (µl/min) | 181±32 | 188±33 | 187±38 | 185±36 |
| UACR (mg/mg) | 51.6±57.7 | 55.7±64.6 | 51.5±44.3 | 49.4±44.4 |
RASi, renin-angiotensin-system inhibitor; MRA, mineralocorticoid receptor antagonist; UACR, urine albumin-creatinine ratio.
Primary End Point: Mean Overall Survival
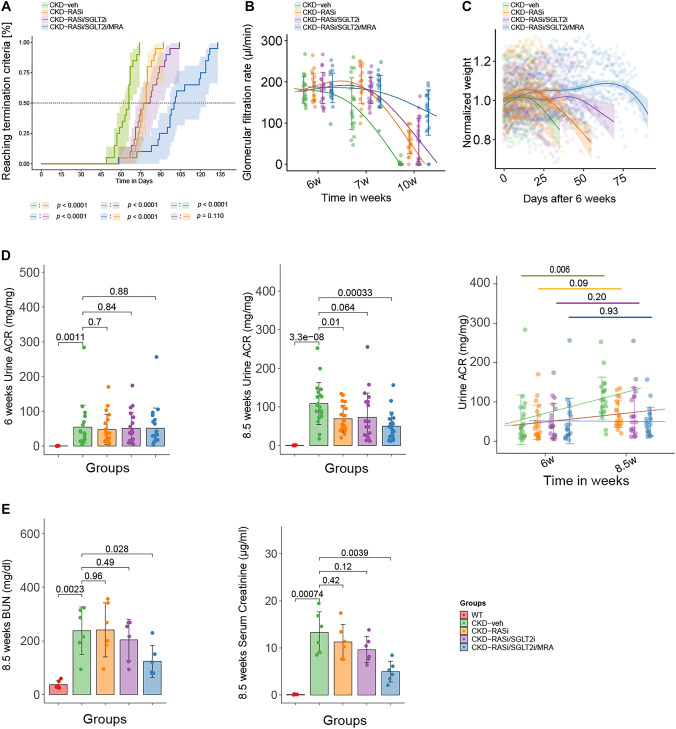
Consistent with previous studies, the mean overall survival of vehicle controls was 63.7±10.0 days.7,9,10 Ramipril monotherapy prolonged mean survival to 77.3±5.3 days. The use of ramipril positively correlated with survival time (R=0.71, P < 0.01). Dual RAS/SGLT2 inhibition prolonged mean survival to 80.3±11.0 days (R=0.67, P < 0.01). Triple therapy including the nonsteroidal MRA finerenone prolonged survival to 103.1±20.3 days (R=0.81, P < 0.01) (Figure 1A). These effects did not differ significantly by sex in stratified Kaplan–Meier analyses (P < 0.05) (Supplemental Figure 5). Thus, triple combination of ramipril, empagliflozin, and finerenone significantly prolonged survival of Col4a3−/− mice with Alport nephropathy, way beyond the ramipril monotherapy (R=0.67, P < 0.01) or the ramipril/empagliflozin combination (R=0.58, P < 0.01).
Figure 1.
Overall survival and other markers of excretory kidney function; albuminuria and other markers of kidney barrier function. (A) Percentage reaching primary end point (overall survival) of WT, CKD-veh, CKD-RASi, CKD-RASi/SGLT2i, and CKD-RASi/SGLT2i/MRA groups. (B) Effects of RASi, RASi/SGLT2i, and RASi/SGLT2i/MRA treatments on GFR. (C) Body weight changes after 6 weeks (shown as a percentage of initial body weight). (D) The progression of proteinuria from 6 to 8.5 weeks. (E) The level of BUN and serum creatinine at 8.5 weeks. WT, wild type; RASi, renin-angiotensin-system inhibitor; SGLT2i, Sodium-glucose-transporter-2 inhibitor; MRA, mineralocorticoid receptor antagonist.
Secondary Analysis: Kidney Function, Histology, and Transcriptome
Longer survival of Col4a3−/− mice should represent a renoprotective effect. To verify this, we measured GFR at weeks 6, 7, and 10. In contrast to vehicle-treated Col4a3−/− mice, which lost their excretory kidney function at week 10, all treatments preserved GFR with the best results for triple therapy (Figure 1B, Supplemental Table 1). Body weight analysis was consistent with the GFR results, suggestive of uremia-related weight loss (Figure 1C). BUN, serum creatinine, and the urine albumin-creatinine ratio were the lowest in 8.5-week-old Col4a3−/− mice on triple therapy (Figure 1, D and E). Serum potassium and inorganic phosphate levels were similar across all five groups (Supplemental Figure 6). In addition, only SGLT2 inhibitor–treated animals showed glucosuria documenting bioactivity (Supplemental Figure 6). Triple therapy maintained kidney weight at the level of wild-type controls (Supplemental Figure 7). Thus, the longer overall survival of treated Col4a3−/− mice was associated with an attenuation of Alport nephropathy.
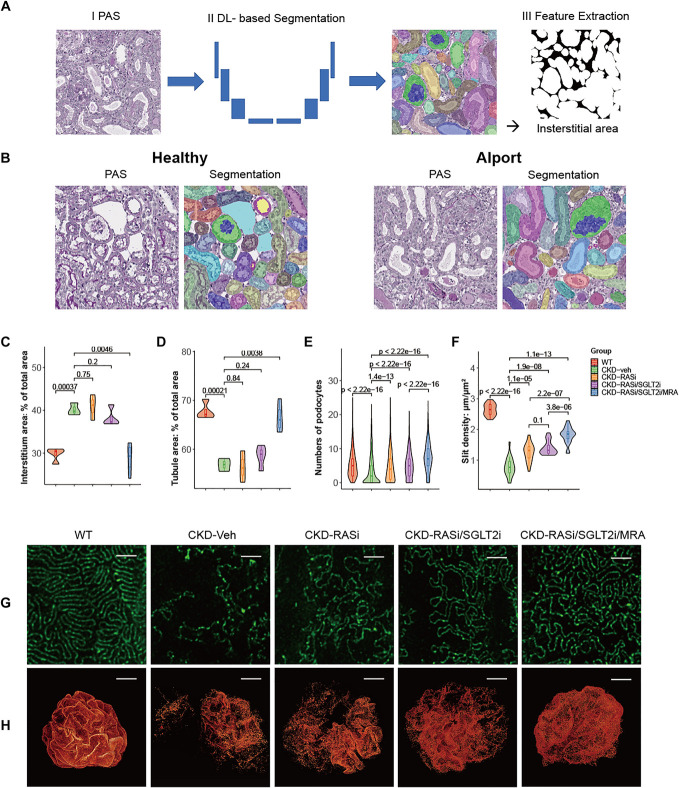
Next, we assessed kidney histology in small subsets of mice after 2.5 weeks of each treatment. A blinded, deep learning–based digital analysis of segmented kidney tissue (pathomics) was used to minimize sources of analytical bias (Figure 2, A and B).11,12 While renin-angiotensin-system inhibitor (RASi) or dual RAS/SGLT2 inhibition had only minor effects on the abnormalities in the tubulointerstitial compartment, triple therapy prevented these changes close to the level of wild-type controls (Figure 2, C and D, Supplemental Figure 8). Picro-Sirius red-stained and αstained kidney sections further demonstrated the significant amelioration of interstitial fibrosis with triple therapy compared with the other groups (Supplemental Figure 9). F4/80 staining, TUNEL staining, and WT-1 staining showed that the triple treatment exhibited a significant reduction in inflammation, cell death, and podocyte loss, respectively (Supplemental Figure 10). In addition, triple therapy significantly sustained podocyte numbers (Figure 2E), consistent with the effects on proteinuria (Figure 1E). Although, adding finerenone still improved the ultrastructure of the filtration slit at the glomerular filtration barrier (Figure 2, F–H).
Figure 2.
Kidney histology in small subsets of mice 2.5 weeks after each treatment. (A) Schematic of the analysis pipeline for segmentation-based extraction of quantitative features of the segmented structures. The interstitial area is presented as an example. (B) Representative examples of segmented images. (C, D) Deep learning–based model for the automated segmentation of major kidney structures in WT, CKD-veh, CKD-RASi, CKD-RASi/SGLT2i, and CKD-RASi/SGLT2i/MRA groups. Overall number of instances—Glomeruli: 4452, Tubules: 364585. (C) Interstitial area: % of total area. (D) Tubule area: % of total area. (E) Deep learning morphometry analysis of WT-1–stained immunohistochemistry slides in WT, CKD-veh, CKD-RASi, CKD-RASi/SGLT2i, and CKD-RASi/SGLT2i/MRA groups. Numbers of podocytes. (F–H) Confocal 3D reconstruction in WT, CKD-veh, CKD-RASi, CKD-RASi/SGLT2i, and CKD-RASi/SGLT2i/MRA groups. (F) Density of slit diaphragms (length/area) was assessed using STED super resolution microscopy. The quantification was performed for five randomly selected areas of each glomerulus in at least five glomeruli per mouse. (G) Representative images of podocyte foot processes using STED super resolution microscopy after tissue clearing of kidney sections from CKD-veh and treated mice. Nephrin stains in green. Scale bars, 2 μm. Magnification, ×100. (H) Three-dimensional reconstruction of glomeruli stained for nephrin on optical tissue clearing. Images show representative glomeruli of mice from each group. Signal represents nephrin protein within the slit diaphragm. Z-series stacks were obtained from 300 μm kidney slices. Images were collected at 1-μm intervals. WT mice show glomeruli with an intact glomerular filtration barrier. CKD-veh mice show large denudated areas (black areas) with podocyte loss and foot process effacement. RASi, RASi/SGLT2i, and RASi/SGLT2i/MRA treatments reduced the area of denudation, and RASi/SGLT2i/MRA especially reduced signs of foot process effacement. Bars, 20 µm. Magnification, ×40. STED, stimulated emission depletion microscopy; MRA, mineralocorticoid receptor antagonist.
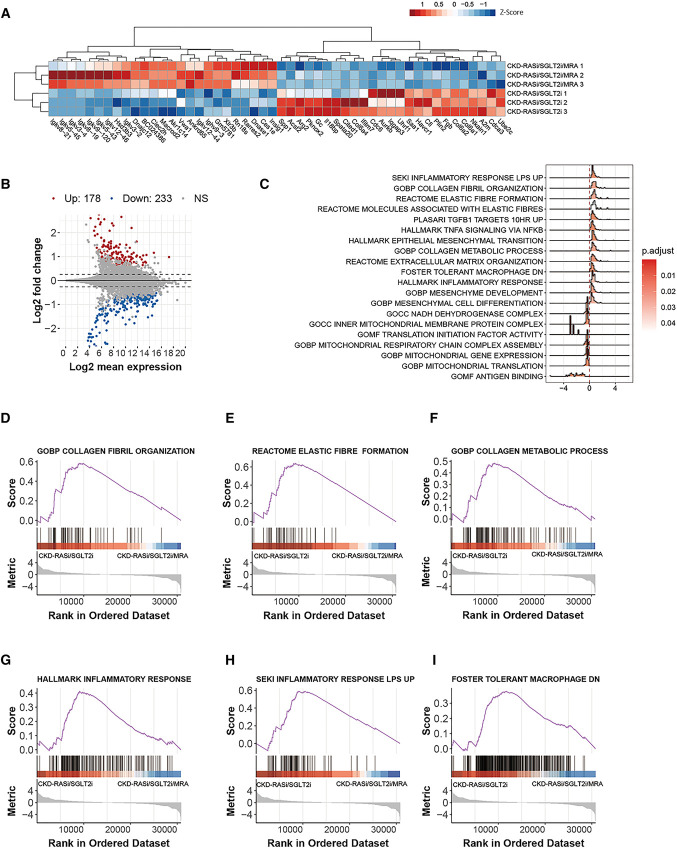
To gain further insights into the renoprotective effect of CKD combination therapy, we performed transcriptome analysis on kidneys obtained after 2.5 weeks of therapy. Cluster analysis clearly separated triple from dual therapy (Figure 3, A and B). RNA sequencing analysis indicated strong effects of the triple therapy on residual inflammation and fibrosis in Col4a3−/− mice on dual RAS/SGLT2 blockade (Figure 3, C–I, Supplemental Figures 11–13). The mRNA expression analysis confirmed the effects of triple therapy on reducing residual tissue fibrosis, inflammation, kidney injury, and podocytes injury in Col4a3−/− mice (Supplemental Figure 14).
Figure 3.
Bulk RNA-seq on kidney of Alport mice on triple versus dual therapy. (A) The heat map presents the biological replicates of the top genes with differing expression levels between the Col4a3−/− mice on triple versus dual therapy. A heat map and dendrogram were used to display the differentially expressed genes' z-scores of normalized counts. (B) An MA plot was also created to display the shrink log2 fold change between kidney of triple versus dual therapy–treated mice. Genes differentially regulated in a significant manner, as indicated by an adjusted P-value, are labeled either in red or blue. (C) The density ridge plot shows the gene expression distribution of core-enriched genes in enriched gene sets, with gradient color indicating adjusted P-values using the Benjamini-Hochberg method. (D–I) The selected enrichment plots from the GSEA analysis on the basis of the gene enrichment profiles of the triple versus dual therapy. The plots highlight the enrichment for transcriptional signatures related to inflammation, fibrosis, and mitochondrial function, GSEA, gene set enrichment analysis.
Together, adding finerenone to dual RAS/SGLT2 inhibition significantly sustains the loss of kidney function and prolonged overall survival in Col4a3−/− mice with Alport nephropathy. The triple combination profoundly suppresses glomerulosclerosis, interstitial inflammation, fibrosis, and hence kidney atrophy.
Discussion
Our results demonstrate that adding finerenone on top of late-onset dual RAS/SGLT2 inhibition potently prolongs overall survival in this animal model of progressive CKD. As the design of our pRCT minimizes common sources of bias, we consider our results a strong rationale for similar trials in human Alport nephropathy and possibly in other forms of nondiabetic CKD. pRCTs are an innovative tool to make more reliable predictions on drug interventions.6 For example, our recent multicenter pRCT correctly predicted poor efficacy of baricitinib in systemic lupus,13 subsequently confirmed by two human trials,14,15 leading to the termination of the trial program.
The additive renoprotective effect of a triple RAS/SGLT2/MR inhibition shown here is consistent with data from a kidney injury model with hypertension16 and a post hoc analysis on a small number of diabetic patients with CKD where such a triple therapy demonstrated an additive effect on proteinuria.4 Another small crossover study reported similar results for eplerenone as a combination partner.3 In contrast to these preliminary studies, we demonstrate a strong effect not only on the surrogate marker proteinuria but on time to kidney failure, the ultimate patient-oriented efficacy end point hardly covered even by large clinical trials.17 In addition, our mechanistic studies demonstrate that add-on blockade of MR signaling strongly suppresses residual tubulointerstitial inflammation and fibrosis beyond RAS/SGLT2 inhibition, known from a wide spectrum of monotherapy or dual therapy experiments.18–21 Indeed, this specific mechanism of action seems to have potent synergistic effects with dual RAS/SGLT2 inhibition, which mostly act on the glomerular compartment. However, also safety issues are important. For example, the sequential addition of spironolactone to ramipril therapy had additive effects on kidney fibrosis in the same Col4a3−/− mouse model.22 However, dual blockade did not prolong overall survival, probably because spironolactone caused fatal hyperkalemia,22 not so in our hands using the nonsteroidal MRA finerenone.
Limitations
We did not perform rigorous pharmacokinetic studies, so effect sizes might be different with other doses. Drug dosing by food admix was based on average food intake and body weight, which may have changed throughout the study. For example, uremia in mice is associated with a decline in food intake and body weight, creating imbalances in drug exposure, when renoprotective therapies delay uremia. Our study assessed effects of late initiation of triple therapy only, and benefits may be even larger when treatment is initiated early.10,23,24 Overall survival cannot be assumed to represent kidney lifespan, but histological assessments were undertaken to support the findings. These results obtained in mice leave obvious uncertainties regarding the possible efficacy and toxicity of triple therapy in humans. Likely, effect size will be different in other forms of nondiabetic kidney disease.
Together, triple RAS/SGLT2/MR blockade in combination substantially increased the mean overall survival of Col4a3−/− mice, beyond dual RAS/SGLT2 inhibition.25 We expect an earlier onset of triple therapy to be even more potent; hence, combination therapy might be a promising and feasible research and treatment perspective to improve kidney outcomes in CKD.
Supplementary Material
Acknowledgments
We thank Jana Mandelbaum, Anna Anifimiadou, and Yvonne Minor for expert technical assistance. Parts of this project were prepared as doctoral theses at the Faculty of Medicine, University of Munich by ZZ and KR. H.-J.A. is a member of the Scientific Network on “Strategies for therapeutic targeting of the Aldosterone-Mineralocorticoid Receptor signaling pathway (ADMIRE network),” funded by the German Research Foundation (DFG – ID 470188766). The companies listed in the Conflict of-Interest statement were unaware of this study or publication.
Disclosures
H.-J. Anders received consultancy fees from Aptarion, AstraZeneca, Bayer, Boehringer-Ingelheim, GSK, Janssen, Kezar, Lilly, Novartis, Sanofi, and Vifor; research funding: Boehringer-Ingelheim; and advisory or leadership role: JASN, NDT. N. Bouteldja reports employer: Ocumeda GmbH. O. Gross received consultancy fees from AstraZeneca, Bayer, Boehringer-Ingelheim, Novartis, and Sanofi. O. Gross also reports consultancy: Codon-X Therapeutics Inc., Galapagos, Genzyme/Regulus Therapeutics, ONO-Pharmaceutical Co. Ltd., Reata Pharmaceuticals, Roche; research funding: Bayer AG; and advisory or leadership role: advisory committees for AstraZeneca, Boehringer-Ingelheim, Reata Pharmaceuticals, Roche, and Sanofi US Services Inc./Genzyme/Regulus Therapeutics. P. Romagnani reports research funding: AstraZeneca. All remaining authors have nothing to disclose.
Funding
H.-J.A. received support from the Deutsche Forschungsgemeinschaft (AN372/27-1 and 30-1). P.B. received supports from the German Research Foundation (DFG, Project IDs 322900939, 454024652, 432698239 & 445703531), European Research Council (ERC Consolidator Grant No 101001791), and the Federal Ministry of Education and Research (BMBF, STOP-FSGS-01GM2202C). O.G. was supported by the Deutsche Forschungsgemeinschaft (GR 1852/6-1), the DFG SINO-GERMAN MOBILITY PROGRAMME (Chinesisch-Deutsches Zentrum für Wissenschaftsförderung, M0166), by Bayer AG (in vivo study in Col4a3−/− mice) and initiated and coordinated the EARLY PRO-TECT Alport trial in children with Alport syndrome, funded by the German Ministry of Education and Research (01KG1104 to O.G.). Z.Z. and C.L. were supported by the Chinese Scholarship Council scholarship program. M.K. was supported by the DFG (CRC TRR332, project A7).
Author Contributions
Conceptualization: Hans-Joachim Anders, Oliver Gross, Zhihui Zhu.
Data curation: Hans-Joachim Anders, Chenyu Li.
Formal analysis: Hans-Joachim Anders, Giulia Antonelli, Peter Boor, Nassim Bouteldja, Roman D. Bülow, Luigi Cirillo, Martin Klaus, Chenyu Li, Alireza Vafaei Sadr, Zhihui Zhu.
Funding acquisition: Hans-Joachim Anders, Peter Boor.
Investigation: Maria-Lucia Angelotti, Yoshihiro Kusunoki, Karoline A.T. Rosenkranz, Zhihui Zhu.
Methodology: Hans-Joachim Anders, Oliver Gross, Yoshihiro Kusunoki, Chenyu Li, Zhihui Zhu.
Project administration: Hans-Joachim Anders.
Resources: Hans-Joachim Anders, Peter Boor, Oliver Gross, Paola Romagnani.
Supervision: Hans-Joachim Anders, Peter Boor, Paola Romagnani.
Validation: Hans-Joachim Anders, Zhihui Zhu.
Visualization: Maria-Lucia Angelotti, Giulia Antonelli, Nassim Bouteldja, Roman D. Bülow, Luigi Cirillo, Paola Romagnani, Alireza Vafaei Sadr.
Writing – original draft: Hans-Joachim Anders.
Writing – review & editing: Hans-Joachim Anders, Maria-Lucia Angelotti, Giulia Antonelli, Peter Boor, Nassim Bouteldja, Roman D. Bülow, Luigi Cirillo, Oliver Gross, Martin Klaus, Yoshihiro Kusunoki, Chenyu Li, Paola Romagnani, Karoline A.T. Rosenkranz, Alireza Vafaei Sadr, Zhihui Zhu.
Data Sharing Statement
The RNA sequencing data have been deposited to the Database of Gene Expression Omnibus https://www.ncbi.nlm.nih.gov/geo/ (Accession number: GSE226353). The preregistered trial protocol is publically available under www.preclinicaltrials.eu (Registry ID: PCTE0000266).
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/JSN/E474.
Supplemental File 1. Material and methods.
Supplemental File 2. Preclinical protocol.
Supplemental File 3. Animal welfare surveillance.
Supplemental File 4. Histology protocols.
Supplemental File 5. Primers used for real-time qRT-PCR.
Supplemental Table 1. Evolution of GFR in Col4a3−/− mice.
Supplemental Figure 1. Assays for kidney function in Col4a3+/+ mice at 6 weeks and Col4a3−/− mice at 6 and 9 weeks.
Supplemental Figure 2. Tissue morphology and periodic-acid schiff staining quantitative assessment of scores from Col4a3+/+ mice at 6 weeks and Col4a3−/− mice at 6 and 9 weeks.
Supplemental Figure 3. Tissue morphology and Sirius red and αSMA staining quantitative assessment of scores from Col4a3+/+ mice at 6 weeks and Col4a3−/− mice at 6 and 9 weeks.
Supplemental Figure 4. TUNEL staining quantitative assessment of scores from Col4a3+/+ mice at 6 weeks and Col4a3−/− mice at 6 and 9 weeks.
Supplemental Figure 5. Subgroup of lifespan in sex.
Supplemental Figure 6. Effects of RASi, RASi/SGLT2i, and RASi/SGLT2i/MRA treatments on potassium and inorganic phosphate serum levels and urinary glucose/creatinine ratio (UGCR) in Col4a3−/− mice.
Supplemental Figure 7. Triple therapy prevented kidney atrophy to the level of wild-type controls.
Supplemental Figure 8. Tissue morphology and periodic-acid schiff staining quantitative assessment of scores from Col4a3−/− mice in WT, Veh, RASi, RASi/SGLT2i, and RASi/SGLT2i/MRA groups.
Supplemental Figure 9. Tissue morphology, αSMA, and Picro-Sirius red staining quantitative assessment of scores from Col4a3−/− mice in WT, Veh, RASi, RASi/SGLT2i, and RASi/SGLT2i/MRA groups.
Supplemental Figure 10. Tissue morphology and F4/80, WT-1, and TUNEL staining quantitative assessment of scores from Col4a3−/− mice in WT, Veh, RASi, RASi/SGLT2i, and RASi/SGLT2i/MRA groups.
Supplemental Figure 11. Bulk RNA-seq on kidney of Veh-treated Alport and wild-type mice.
Supplemental Figure 12. Bulk RNA-seq on kidney of Alport mice on triple therapy versus vehicle.
Supplemental Figure 13. Bulk RNA-seq on kidney of Alport mice on triple versus monotherapy.
Supplemental Figure 14. Relative kidney mRNA expression of kidney fibrosis, inflammation, injury, and podocyte markers.
References
- 1.Baigent C Emberson J Haynes R, et al. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400(10365):1788–1801. doi: 10.1016/s0140-6736(22)02074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakris GL Agarwal R Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–2229. doi: 10.1056/nejmoa2025845 [DOI] [PubMed] [Google Scholar]
- 3.Provenzano M Puchades MJ Garofalo C, et al. Albuminuria-lowering effect of dapagliflozin, eplerenone, and their combination in patients with chronic kidney disease: a randomized crossover clinical trial. J Am Soc Nephrol. 2022;33(8):1569–1580. doi: 10.1681/ASN.2022020207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossing P Filippatos G Agarwal R, et al. Finerenone in predominantly advanced CKD and type 2 diabetes with or without sodium-glucose cotransporter-2 inhibitor therapy. Kidney Int Rep. 2022;7(1):36–45. doi: 10.1016/j.ekir.2021.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green JB Mottl AK Bakris G, et al. Design of the COmbinatioN effect of FInerenone anD EmpaglifloziN in participants with chronic kidney disease and type 2 diabetes using a UACR endpoint study (CONFIDENCE). Nephrol Dial Transplant. 2023;38(4):894–903. doi: 10.1093/ndt/gfac198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nangaku M Kitching AR Boor P, et al. International Society of Nephrology first consensus guidance for preclinical animal studies in translational nephrology. Kidney Int. 2023;104(1):36–45. doi: 10.1016/j.kint.2023.03.007 [DOI] [PubMed] [Google Scholar]
- 7.Ninichuk V Gross O Reichel C, et al. Delayed chemokine receptor 1 blockade prolongs survival in collagen 4A3-deficient mice with Alport disease. J Am Soc Nephrol. 2005;16(4):977–985. doi: 10.1681/ASN.2004100871 [DOI] [PubMed] [Google Scholar]
- 8.Clauss S Gross O Kulkarni O, et al. Ccl2/Mcp-1 blockade reduces glomerular and interstitial macrophages but does not ameliorate renal pathology in collagen4A3-deficient mice with autosomal recessive Alport nephropathy. J Pathol. 2009;218(1):40–47. doi: 10.1002/path.2505 [DOI] [PubMed] [Google Scholar]
- 9.Cosgrove D, Kalluri R, Miner JH, Segal Y, Borza DB. Choosing a mouse model to study the molecular pathobiology of Alport glomerulonephritis. Kidney Int. 2007;71(7):615–618. doi: 10.1038/sj.ki.5002115 [DOI] [PubMed] [Google Scholar]
- 10.Gross O Beirowski B Koepke ML, et al. Preemptive ramipril therapy delays renal failure and reduces renal fibrosis in COL4A3-knockout mice with Alport syndrome. Kidney Int. 2003;63(2):438–446. doi: 10.1046/j.1523-1755.2003.00779.x [DOI] [PubMed] [Google Scholar]
- 11.Bouteldja N Klinkhammer BM Bulow RD, et al. Deep learning-based segmentation and quantification in experimental kidney histopathology. J Am Soc Nephrol. 2021;32(1):52–68. doi: 10.1681/ASN.2020050597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holscher DL Bouteldja N Joodaki M, et al. . Next-generation morphometry for pathomics-data mining in histopathology. Nat Commun. 2023;14(1):470. doi: 10.1038/s41467-023-36173-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lei Y Sehnert B Voll RE, et al. A multicenter blinded preclinical randomized controlled trial on Jak1/2 inhibition in MRL/MpJ-Fas mice with proliferative lupus nephritis predicts low effect size. Kidney Int. 2021;99(6):1331–1341. doi: 10.1016/j.kint.2021.01.024 [DOI] [PubMed] [Google Scholar]
- 14.Morand EF Vital EM Petri M, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 3 trial (SLE-BRAVE-I). Lancet. 2023;401(10381):1001–1010. doi: 10.1016/s0140-6736(22)02607-1 [DOI] [PubMed] [Google Scholar]
- 15.Petri M Bruce IN Dorner T, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 3 trial (SLE-BRAVE-II). Lancet. 2023;401(10381):1011–1019. doi: 10.1016/s0140-6736(22)02546-6 [DOI] [PubMed] [Google Scholar]
- 16.Kolkhof P Hartmann E Freyberger A, et al. Effects of finerenone combined with empagliflozin in a model of hypertension-induced end-organ damage. Am J Nephrol. 2021;52(8):642–652. doi: 10.1159/000516213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weldegiorgis M, de Zeeuw D, Heerspink HJ. Renal end points in clinical trials of kidney disease. Curr Opin Nephrol Hypertens. 2015;24(3):284–289. doi: 10.1097/mnh.0000000000000118 [DOI] [PubMed] [Google Scholar]
- 18.Barrera-Chimal J, Jaisser F, Anders HJ. The mineralocorticoid receptor in chronic kidney disease. Br J Pharmacol. 2022;179(13):3152–3164. doi: 10.1111/bph.15734 [DOI] [PubMed] [Google Scholar]
- 19.Agarwal R Kolkhof P Bakris G, et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42(2):152–161. doi: 10.1093/eurheartj/ehaa736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Droebner K Pavkovic M Grundmann M, et al. Direct blood pressure-independent anti-fibrotic effects by the selective nonsteroidal mineralocorticoid receptor antagonist finerenone in progressive models of kidney fibrosis. Am J Nephrol. 2021;52(7):588–601. doi: 10.1159/000518254 [DOI] [PubMed] [Google Scholar]
- 21.Grune J Beyhoff N Smeir E, et al. Selective mineralocorticoid receptor cofactor modulation as molecular basis for finerenone's antifibrotic activity. Hypertension. 2018;71(4):599–608. doi: 10.1161/hypertensionaha.117.1036 [DOI] [PubMed] [Google Scholar]
- 22.Rubel D, Zhang Y, Sowa N, Girgert R, Gross O. Organoprotective effects of spironolactone on top of ramipril therapy in a mouse model for Alport syndrome. J Clin Med. 2021;10(13):2958. doi: 10.3390/jcm10132958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gross O Licht C Anders HJ, et al. Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int. 2012;81(5):494–501. doi: 10.1038/ki.2011.407 [DOI] [PubMed] [Google Scholar]
- 24.Gross O Tonshoff B Weber LT, et al. A multicenter, randomized, placebo-controlled, double-blind phase 3 trial with open-arm comparison indicates safety and efficacy of nephroprotective therapy with ramipril in children with Alport's syndrome. Kidney Int. 2020;97(6):1275–1286. doi: 10.1016/j.kint.2019.12.015 [DOI] [PubMed] [Google Scholar]
- 25.Boeckhaus J, Gross O. Sodium-glucose cotransporter-2 inhibitors in patients with hereditary podocytopathies, Alport syndrome, and FSGS: a case series to better plan a large-scale study. Cells. 2021;10(7):1815. doi: 10.3390/cells10071815 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The RNA sequencing data have been deposited to the Database of Gene Expression Omnibus https://www.ncbi.nlm.nih.gov/geo/ (Accession number: GSE226353). The preregistered trial protocol is publically available under www.preclinicaltrials.eu (Registry ID: PCTE0000266).