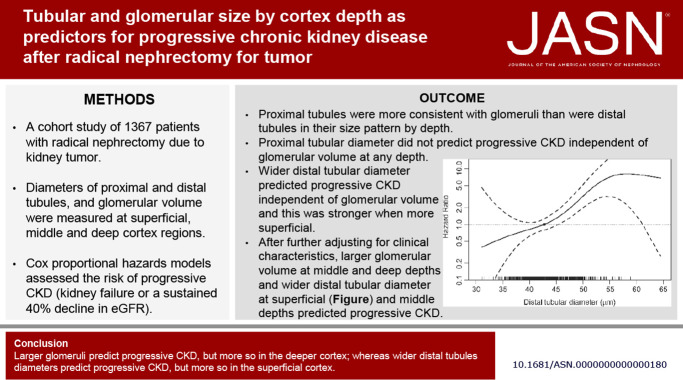
Visual Abstract
Keywords: distal tubule, glomerulus, kidney anatomy, kidney tubule, proximal tubule
Abstract
Significance Statement
Glomerular size differs by cortex depth. Larger nephrons are prognostic of progressive kidney disease, but it is unknown whether this risk differs by cortex depth or by glomeruli versus proximal or distal tubule size. We studied the average minor axis diameter in oval proximal and distal tubules separately and by cortex depth in patients who had radical nephrectomy to remove a tumor from 2019 to 2020. In adjusted analyses, larger glomerular volume in the middle and deep cortex predicted progressive kidney disease. Wider proximal tubular diameter did not predict progressive kidney disease independent of glomerular volume. Wider distal tubular diameter showed a gradient of strength of prediction of progressive kidney disease in the more superficial cortex than in the deep cortex.
Background
Larger nephrons are prognostic of progressive kidney disease, but whether this risk differs by nephron segments or by depth in the cortex is unclear.
Methods
We studied patients who underwent radical nephrectomy for a tumor between 2000 and 2019. Large wedge kidney sections were scanned into digital images. We estimated the diameters of proximal and distal tubules by the minor axis of oval tubular profiles and estimated glomerular volume with the Weibel–Gomez stereological model. Analyses were performed separately in the superficial, middle, and deep cortex. Cox proportional hazard models assessed the risk of progressive CKD (dialysis, kidney transplantation, sustained eGFR <10 ml/min per 1.73 m2, or a sustained 40% decline from the postnephrectomy baseline eGFR) with glomerular volume or tubule diameters. At each cortical depth, models were unadjusted, adjusted for glomerular volume or tubular diameter, and further adjusted for clinical characteristics (age, sex, body mass index, hypertension, diabetes, postnephrectomy baseline eGFR, and proteinuria).
Results
Among 1367 patients were 133 progressive CKD events during a median follow-up of 4.5 years. Glomerular volume predicted CKD outcomes at all depths, but only in the middle and deep cortex after adjusted analyses. Proximal tubular diameter also predicted progressive CKD at any depth but not after adjusted analyses. Distal tubular diameter showed a gradient of more strongly predicting progressive CKD in the superficial than deep cortex, even in adjusted analysis.
Conclusions
Larger glomeruli are independent predictors of progressive CKD in the deeper cortex, whereas in the superficial cortex, wider distal tubular diameters are an independent predictor of progressive CKD.
Introduction
Larger nephrons predict CKD outcomes in living kidney donors and patients with tumor and independently of concurrent clinical evaluation for GFR, proteinuria, and common CKD risk factors.1–5
Hypertrophy of nephrons likely occurs in response to increased metabolic demand (e.g., obesity) or inadequate nephron number because of low nephron endowment or loss of functional nephrons from nephrosclerosis.6–11 Nephron size can be quantified using several different methods, each with limitations.12 Glomerular volume and volume of nonfibrotic cortex per glomerulus can be estimated from nonsclerosed glomerular profiles in the cortex using stereological models.13 However, estimating the size of proximal or distal tubules is more challenging.
It has been well-established in rodent models of early CKD that in addition to glomerular hypertrophy, there is tubular hypertrophy.14 Rodent models have primarily studied proximal tubules,14–18 although a few have also evaluated distal tubules.19,20 A study in human kidneys found that obese patients had a 54% larger cross-sectional area of proximal tubules than nonobese patients.21 We have previously estimated the average cross-sectional area of all tubules in 1 mm2 of cortex (mean tubular profile area).2 This approach does not distinguish proximal from distal tubules, nor can it be used to calculate the true tubular diameter because tubular profiles on biopsy reflect different orientations and curvatures. These issues may explain why the mean cross-sectional tubular area is less prognostic for outcomes than glomerular volume in both living kidney donors1,3,4 and patients with tumor.2 A systematic study of tubular size that distinguishes proximal from distal tubules, considers their depth in the cortex, and evaluates the contribution of tubule size in predicting progressive CKD independent of glomerular size and clinical characteristics would help clarify their clinical and biological importance.
Patients who undergo a radical nephrectomy for a kidney tumor are a unique population for study of tubular size as microstructural outcomes can be assessed across different depths with large wedge sections of the nontumor parenchyma. We have previously reported that glomerular size differed by depth in the cortex and obesity associated more strongly with larger glomeruli in the superficial cortex among patients with kidney tumor.22 There may be differences in tubular size by depth. The prediction of progressive CKD by tubular and glomerular size may also vary by depth. Thus, we measured proximal diameter and distal diameter of tubules and glomerular volume at different depths among patients who underwent radical nephrectomy for a tumor. We then assessed whether these tubular diameters and glomerular volume predicted progressive CKD or kidney failure at different cortical depths independent of each other and independent of kidney function and common CKD risk factors.
Methods
Study Population
The study participants were an expansion of a previously published cohort of Mayo Clinic Nephrectomy Registry patients in the Aging Kidney Anatomy study.2,5,23 From 2000 to 2019, this group had a radical nephrectomy for a kidney tumor at Mayo Clinic Rochester (MN). Only individuals who had a full unilateral nephrectomy for a renal tumor and had no metastatic lesions or positive lymph nodes at the time of surgery were included in the study. The aim was to look at long-term risk of progressive CKD that may be anticipated by kidney microstructural findings on biopsy rather than immediate changes in kidney function caused by the nephrectomy. Thus, postnephrectomy serum creatinine values were used to determine baseline kidney function. A standard follow-up visit with laboratory testing was scheduled at approximately 3 months after nephrectomy to give time for postoperative stabilization of kidney function. Patients who did not have a serum creatinine test between the nephrectomy and 4 months after the nephrectomy or follow-up serum creatinine after 4 months were excluded. Other baseline clinical characteristics were established before or at the time of nephrectomy. Patients who had a cancer recurrence, death, or kidney failure during the first 4 months after their nephrectomy were excluded. Patients with histological evidence of diffuse specific kidney disease (other than mild-moderate diabetic nephropathy), severe and diffuse tubulointerstitial inflammation, severe ischemia with interstitial fibrosis and tubular atrophy (IFTA) (>90%), a large focal scar, or end stage kidney histology (thinned and nearly completely scarred cortex) were also excluded. The Mayo Clinic Institutional Review Board approved the study.
Kidney Function and Risk Factors
Baseline age, sex, body mass index (BMI), serum creatinine (corrected to standardized values if assayed before standardization), 24-hour urine protein, hypertension (as documented in medical records), diabetes mellitus (as documented in medical records) were obtained from the medical records of patients with renal tumors. The eGFR was calculated using the serum creatinine–based 2021 race-free Chronic Kidney Disease Epidemiology Collaboration equation.24 If multiple postnephrectomy serum creatinine levels were available, the one closest to but before 4 months after the nephrectomy was used for baseline. A spot urine protein–osmolality ratio was used to calculate 24-hour urine protein excretion.25 Urine protein testing could have been performed pre-nephrectomy (up to 1 year before) or post-nephrectomy (up to 4 months after) nephrectomy. We preferentially used the post-nephrectomy urine protein test for baseline. If a urine protein level was not obtained within the first 4 months postnephrectomy, a prenephrectomy urine protein level was used for baseline.
Kidney Microstructure
A large wedge segment of the parenchyma that was far from the tumor-involved tissue was cut from the formalin-fixed whole-kidney specimens and then embedded in paraffin. A 3-μm thick section was cut from the paraffin-embedded tissue block, stained with periodic acid–Schiff, and scanned into high-resolution digital images (Aperio AT2 system scanner; Leica Microsystems, Inc., Buffalo Grove, IL; https://www.leicabiosystems.com/us/digital-pathology/). To reduce bias, investigators evaluated biopsy images morphometrically while masked to all clinical factors. The scanned digital images were magnified with Image Scope software (version 12.4.3.7009 Aperio) onto a large touch-screen tablet. The cortex was divided into superficial, middle, and deep depths, as previously reported.22 Briefly, superficial cortex was defined as the subcapsular strip of 3–4 glomerular diameters; deep cortex was defined as the juxtamedullary strip of 3–4 glomerular diameters; and middle cortex as the strip of 3–4 glomerular diameters approximately halfway between superficial and deep cortical strips.
The minor axis of proximal and distal tubular profiles that were circular or oval was measured separately for the superficial, middle, and deep depths of the cortex (Figure 1A). Compared with the proximal tubules, the distal tubules were identified by the lack of apical microvilli on their epithelial cells (the lack of pink brush border) and by having a more prominent lumen.26 Tubular profiles that could not be clearly classified as proximal or distal were not used. Because tubules were often not perfectly oval, we considered the minor axis to the widest portion of the tubule orthogonal to the major axis (on the basis of visual inspection) that connected two opposite points of the tubule basement membrane (Figure 1, inset A1).14,18,27 Then, from all measurements, we calculated the mean diameter of the proximal and distal tubules at each depth. Glomerular volume was calculated from all nonsclerosed glomeruli using Weibel–Gomez stereology models, separately for superficial, middle, and deep cortical depths (Figure 1B).13 The diameter of glomeruli was calculated using the mathematical formula that relates the volume of a sphere to its diameter. Nephrosclerosis measures (% glomerulosclerosis, % IFTA, IFTA foci density and % luminal stenosis due to arteriosclerosis) were obtained as previously described.2,5
Figure 1.
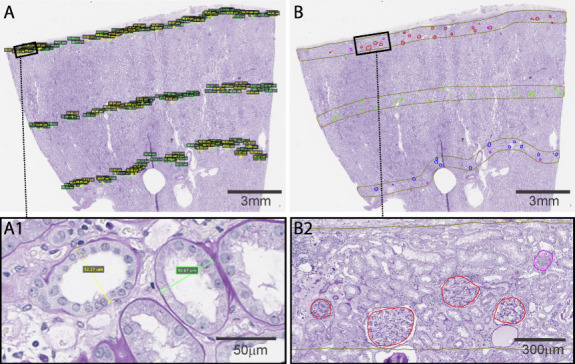
An example of a periodic acid–Schiff-stained wedge section used to obtain measurements for tubular diameters and glomeruli. (A) Proximal and distal tubular diameters and (B) each nonsclerosed and globally sclerosed glomerular profiles were labeled in the superficial, middle, and deep depths. Inset (A1) shows example of a proximal tubule (green line) and a distal tubule (yellow line) with their shortest diameters. Inset (B2) shows four nonsclerosed glomeruli (red trace) and one globally sclerosed glomerulus (pink trace).
Kidney Macrostructure
A semiautomated image processing tool (ITK-SNAP software, version 2.2; University of Pennsylvania, Philadelphia, PA) was used to obtain kidney cortex volume of an unaffected kidney and tumor volume of an affected kidney from the prenephrectomy computerized tomography or magnetic resonance imaging images, as previously reported.28
Outcomes
Patients had a postnephrectomy follow-up visit every 3–6 months for the first year and then every 6–12 months as part of a standardized clinical protocol to screen for cancer recurrence and for CKD. In this study, the final follow-up visit permitted was on February 17, 2023. Per clinical protocol, serum creatinine level was included with these visits. Patients who did not return were contacted by phone and surveyed or provided outside medical records for current serum creatinine levels, dialysis, and kidney transplantation. Progressive CKD has been previously defined as a 40% decline in eGFR29; however, medical record review found that many of the 40% declines in eGFR were very transient and may not even have been recognized clinically as AKI. On medical record review of patients with longer follow-up, we found that requiring a 40% decline in eGFR be sustained for at least 3 months better identified patients who progressed toward kidney failure. Thus, progressive CKD was defined by a 40% or more decline in eGFR from postnephrectomy baseline that was sustained for at least 3 months and an eGFR <10 and at least 5 ml/min per 1.73 m2 below postnephrectomy baseline that was sustained for at least 3 months, dialysis, or kidney transplantation.
Statistical Analyses
The glomerular volume and diameters of proximal and distal tubules were compared between the superficial, middle, and deep depths using the ANOVA test. Nonparametric Spearman correlations were used to compare the diameters of proximal and distal tubules at three cortical depths with each other, glomerular volume at corresponding cortical depths, clinical characteristics, kidney function, biopsy measures of nephrosclerosis, and macroscopic measures of kidney volumes and tumor volume. Similar cross-sectional associations with glomerular volumes at different depths have already been reported in this cohort.22
The risks of progressive CKD with glomerular volume and proximal and distal tubular diameters at three cortical depths were assessed using Cox proportional hazard models with censoring at the last available serum creatinine test, cancer recurrence, the last follow-up visit, or patient's death. To allow meaningful comparisons of effect sizes (hazard ratios), all measures of nephron size were standardized to per SD values in the models. Cox models were unadjusted, adjusted for glomerular volume (for a given cortex depth), and further adjusted for clinical characteristics (age, sex, BMI, hypertension, diabetes, postnephrectomy baseline eGFR and proteinuria). Analyses were repeated using glomerular diameter per SD rather than glomerular volume per SD.
Results
Study Sample Characteristics
We identified 1638 patients at Mayo Clinic who had a radical nephrectomy from 2000 to 2019. There were 30 patients excluded because of kidney transplantation, dialysis, or eGFR <10 ml/min per 1.73 m2, and 119 patients were excluded because of death or cancer recurrence within 4 months after nephrectomy. Another 33 patients were excluded for specific kidney diseases observed on kidney biopsy histology, and 89 patients were excluded because of missing serum creatinine. After exclusions, there were 1367 patients in the final sample to follow for outcomes (Figure 2). Patients with hypertensive nephrosclerosis or mild diabetic nephropathy findings on biopsy were not excluded. Clinical characteristics, biopsy findings, and outcomes are summarized in Table 1. The median time to progressive CKD was 4.5 (25%–75%, 2.1–8.1) years. The median number of follow-up serum creatinine levels available was 14 (25%–75%, 9–26) per patient. Progressive CKD occurred in 62 patients during follow-up.
Figure 2.
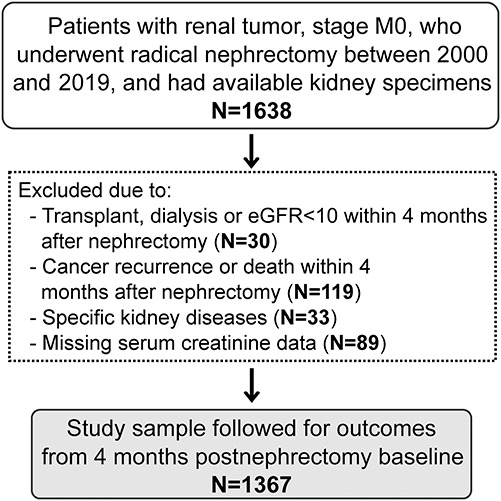
Selection of study population.
Table 1.
Baseline characteristics of 1367 patients with renal tumor
| Characteristic | Mean (SD) or N(%) or median (25%, 75%) |
|---|---|
| Clinical characteristics | |
| Age, yr | 63.9 (11.9) |
| Male | 876 (64.1%) |
| Race | |
| American Indian/Alaskan Native | 14 (1.0%) |
| Asian | 4 (0.3%) |
| Black | 22 (1.6%) |
| Native Hawaiian/Pacific Islander | 1 (0.1%) |
| White | 1269 (92.8%) |
| Unknown/Other | 57 (4.2%) |
| BMI, kg/m2 | 30.8 (6.7) |
| Hypertension | 921 (67.4%) |
| Diabetes mellitus | 186 (13.6%) |
| Kidney function | |
| Prenephrectomy eGFR, ml/min per 1.73 m2 | 72.1 (18.8) |
| Postnephrectomy eGFR, ml/min per 1.73 m2 | 51.2 (13.7) |
| 24-h urine protein, mga | 154 (87, 307) |
| Baseline biopsy measures | |
| Superficial glomerular volume, mm3 | 0.0025 (0.0011) |
| Middle glomerular volume, mm3 | 0.0032 (0.0012) |
| Deep glomerular volume, mm3 | 0.0029 (0.0011) |
| Superficial proximal tubular diameter, μm | 54.2 (6.4) |
| Middle proximal tubular diameter, μm | 58.1 (6.6) |
| Deep proximal tubular diameter, μm | 56.6 (6.5) |
| Superficial distal tubular diameter, μm | 43.1 (4.4) |
| Middle distal tubular diameter, μm | 46.9 (4.7) |
| Deep distal tubular diameter, μm | 47.2 (5.0) |
| % Luminal stenosis | 54.5 (15.5) |
| % Fibrosis | 3.6 (7.5) |
| IFTA foci density, per cm2 | 25.1 (20.8) |
| Macroscopic measuresb | |
| Cortex volume, cm3 | 125.0 (36.9) |
| Medulla volume, cm3 | 48.6 (15.4) |
| Tumor volumec, cm3 | 155 (61, 406) |
| Outcomes during follow-up | |
| Progressive CKD, % | 62 (4.5%) |
BMI, body mass index; IFTA, interstitial fibrosis and tubular atrophy.
Urine protein data missing in 183 patients.
Cortex and medulla volume could not be obtained in 704 patients because of poor cm differentiation on the images or lack of digital images.
Tumor volume missing in 172 patients because of lack of digital images.
Glomerular Volume and Tubular Diameters across the Cortex Depth
The glomeruli and tubules were largest in the middle depth and smallest in the superficial depth (P < 0.0001 for all, Supplemental Table 1, and Figure 3). Distal tubules were smallest in the superficial depth, but there was less evidence of a difference in their size between the middle and deep depths. Proximal tubule diameters were wider than distal tubular diameters at all depths (P < 0.0001 for all). Glomerular volume and diameters of proximal and distal tubules were all correlated with each other across any depth; however, the correlations were generally stronger between the measures at the same cortical depth (Supplemental Table 2).
Figure 3.
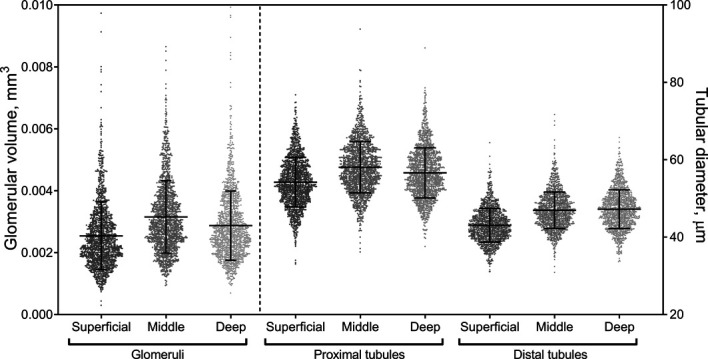
Scatterplot of proximal and distal tubular diameters by cortex depth. At each depth, proximal tubular diameters were significantly larger than the distal tubular diameters.
Clinical and Biopsy Findings Associated with Tubular Diameter
Of the clinical and biopsy characteristics, larger BMI, hypertension, diabetes, proteinuria, % luminal stenosis, % glomerulosclerosis, % IFTA, and cortex and tumor volume had similar associations with wider proximal and distal tubular diameters across the cortical depths (Table 2). There was also evidence of older age, male, and lower eGFR associating with wider distal tubules across the cortical depths. Larger proximal tubule diameter was more correlated with being male in the superficial depth compared with deeper depths (P = 0.005 for test of interaction). None of the other associations with proximal or distal tubule diameter differed by depth (tests for interaction, P > 0.01 for all). The tubular diameters did not associate with IFTA foci density or with medulla volume.
Table 2.
Spearman correlations of clinical characteristics, kidney function, biopsy measures, and macroscopic measures with diameters of proximal and distal tubules by cortex depth
| Characteristics | Mean Proximal Tubular Diameter | Mean Distal Tubular Diameter | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Superficial Cortex | Middle Cortex | Deep Cortex | Superficial Cortex | Middle Cortex | Deep Cortex | |||||||
| Rs | P Value | Rs | P Value | Rs | P Value | Rs | P Value | Rs | P Value | Rs | P Value | |
| Clinical characteristics | ||||||||||||
| Age | 0.03 | 0.34 | 0.01 | 0.68 | 0.03 | 0.24 | 0.06 | 0.04 | 0.07 | 0.009 | 0.06 | 0.04 |
| Male | 0.08 | 0.004 | 0.03 | 0.32 | 0.04 | 0.14 | 0.11 | <0.0001 | 0.12 | <0.0001 | 0.08 | 0.005 |
| BMI | 0.19 | <0.0001 | 0.19 | <0.0001 | 0.16 | <0.0001 | 0.11 | <0.0001 | 0.09 | 0.0008 | 0.10 | 0.0001 |
| Hypertension | 0.14 | <0.0001 | 0.14 | <0.0001 | 0.15 | <0.0001 | 0.08 | 0.003 | 0.13 | 0.0003 | 0.10 | 0.0001 |
| Diabetes mellitus | 0.13 | <0.0001 | 0.11 | <0.0001 | 0.09 | 0.0005 | 0.08 | 0.005 | 0.09 | 0.0009 | 0.11 | <0.0001 |
| Kidney function | ||||||||||||
| Prenephrectomy eGFR | −0.04 | 0.18 | −0.05 | 0.08 | −0.07 | 0.007 | −0.12 | <0.0001 | −0.16 | <0.0001 | −0.11 | <0.0001 |
| 24-h urine proteina | 0.12 | <0.0001 | 0.10 | 0.0007 | 0.11 | 0.0001 | 0.13 | <0.0001 | 0.15 | <0.0001 | 0.12 | <0.0001 |
| Other biopsy measures | ||||||||||||
| % Luminal stenosis | 0.05 | 0.07 | 0.03 | 0.21 | 0.06 | 0.02 | 0.07 | 0.006 | 0.07 | 0.008 | 0.08 | 0.002 |
| % Glomerulosclerosis | 0.17 | <0.0001 | 0.15 | <0.0001 | 0.18 | <0.0001 | 0.17 | <0.0001 | 0.20 | <0.0001 | 0.19 | <0.0001 |
| % IFTA | 0.12 | <0.0001 | 0.09 | 0.0008 | 0.11 | <0.0001 | 0.12 | <0.0001 | 0.17 | <0.0001 | 0.15 | <0.0001 |
| IFTA foci density | 0.04 | 0.16 | 0.03 | 0.26 | 0.03 | 0.24 | −0.01 | 0.74 | 0.03 | 0.30 | 0.04 | 0.13 |
| Contralateral kidney sizeb | ||||||||||||
| Cortex volume | 0.13 | 0.001 | 0.10 | 0.009 | 0.11 | 0.006 | 0.12 | 0.003 | 0.12 | 0.003 | 0.09 | 0.02 |
| Medulla volume | 0.01 | 0.71 | 0.02 | 0.67 | 0.01 | 0.75 | 0.03 | 0.38 | 0.04 | 0.25 | 0.06 | 0.15 |
| Ipsilateral kidney tumor sizec | ||||||||||||
| Tumor volume | 0.07 | 0.01 | 0.09 | 0.002 | 0.08 | 0.007 | 0.06 | 0.04 | 0.06 | 0.047 | 0.08 | 0.004 |
None of correlations between characteristics and proximal and distal tubular diameters clearly differed by depth (tests of interaction >0.01 for all), except the correlation between proximal tubule diameter and male (P = 0.005 for test of interaction). BMI, body mass index; IFTA, interstitial fibrosis and tubular atrophy.
n = 24-h urine protein available in 1184 patients.
Cortex and medulla volumes available in 663 patients.
Tumor volume available in 1195 patients.
Risk of Progressive CKD by Glomerular Volume and Tubular Diameters
In unadjusted analyses, larger glomeruli and wider proximal and distal tubular diameters predicted progressive CKD (Table 3). After adjusting for glomerular volume at the same depth, proximal tubular diameter no longer predicted progressive CKD. After adjustment for glomerular volume at the same depth, wider distal tubular diameter predicted progressive CKD along a gradient by depth. There was a stronger and more statistically significant association in the superficial depth, then middle depth, and no evidence of an association in the deep depth. Larger glomerular volume remained a predictor of progressive CKD at all depths, even after adjusting for distal tubular diameter. In a model further adjusting for clinical characteristics, larger glomerular volume at middle and deep cortical depths and wider distal tubular diameters at superficial and middle depths predicted progressive CKD (Figure 4). Findings were similar using glomerular diameter rather than glomerular volume to predict progressive CKD (Supplemental Table 3).
Table 3.
Measures of nephron size as predictors of progressive CKD from 4 months after a radical nephrectomy
| Predictor (per SD) | Unadjusted | Glomerular Volume and Proximal Tubular Diameter (Model 2) | Glomerular Volume and Distal Tubular Diameter (Model 3) | Model 3 Further Adjusted for Clinical Characteristicsa | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Superficial depth | ||||||||
| Glomerular volume | 1.73 (1.50 to 1.99) | <0.0001 | 1.64 (1.36 to 1.97) | <0.0001 | 1.37 (1.15 to 1.64) | 0.0005 | 1.23 (0.99 to 1.54) | 0.07 |
| Proximal tubular diameter | 1.63 (1.30 to 2.04) | <0.0001 | 1.14 (0.86 to 1.51) | 0.36 | — | — | — | — |
| Distal tubular diameter | 2.25 (1.83 to 2.78) | <0.0001 | — | — | 1.87 (1.46 to 2.38) | <0.0001 | 1.81 (1.40 to 2.35) | <0.0001 |
| Middle depth | ||||||||
| Glomerular volume | 1.75 (1.52 to 2.02) | <0.0001 | 1.94 (1.58 to 2.38) | <0.0001 | 1.53 (1.29 to 1.83) | <0.0001 | 1.51 (1.19 to 1.92) | 0.0007 |
| Proximal tubular diameter | 1.31 (1.04 to 1.66) | 0.02 | 0.80 (0.59 to 1.10) | 0.17 | — | — | — | — |
| Distal tubular diameter | 1.82 (1.48 to 2.24) | <0.0001 | — | — | 1.44 (1.13 to 1.85) | 0.004 | 1.34 (1.03 to 1.74) | 0.03 |
| Deep depth | ||||||||
| Glomerular volume | 1.75 (1.51 to 2.02) | <0.0001 | 1.68 (1.40 to 2.02) | <0.0001 | 1.65 (1.40 to 1.96) | <0.0001 | 1.46 (1.22 to 1.76) | <0.0001 |
| Proximal tubular diameter | 1.54 (1.23 to 1.94) | 0.0002 | 1.11 (0.84 to 1.46) | 0.46 | — | — | — | — |
| Distal tubular diameter | 1.60 (1.25 to 2.04) | 0.0002 | — | — | 1.21 (0.92 to 1.58) | 0.17 | 1.18 (0.89 to 1.56) | 0.26 |
There were 62 events. HR, hazard ratio; CI, confidence interval.
Adjusted for age, sex, BMI, hypertension, diabetes, postnephrectomy baseline eGFR, and proteinuria.
Figure 4.
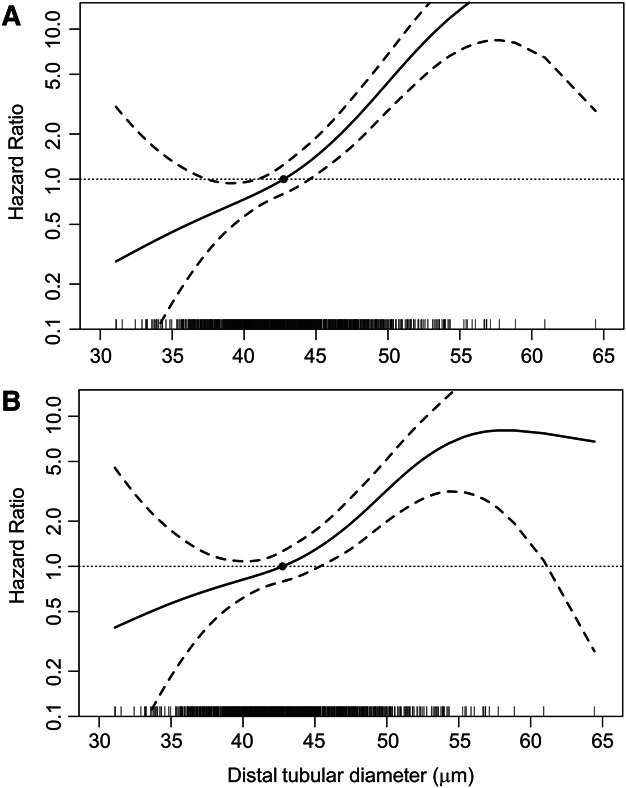
Spline plot of hazard ratio for progressive CKD (solid curve) with 95% confidence interval (dashed curve) by distal tubular diameter. Wider superficial distal tubular diameter is a predictor of progressive CKD in both (A) unadjusted analyses and (B) analyses adjusted for superficial glomerular volume and clinical characteristics (age, sex, body mass index, hypertension, diabetes, postnephrectomy eGFR and proteinuria). Hazard ratios are relative to median distal tubular diameter.
Discussion
Shear stress to the proximal tubular epithelium from glomerular hyperfiltration leads to hypertrophy of the proximal tubule to facilitate increased reabsorption.21,30,31 Hypertrophy of distal tubules can also increase, although by a different unknown mechanism than proximal tubules.31 These enlarged nephrons can eventually collapse and sclerose32,33 and are associated with an increased risk of progressive CKD and kidney failure.1–4,34–38 However, it is unclear whether this CKD risk differs depending on the hypertrophy of different segments or depths of nephrons. In this study, we found that glomerular enlargement was predictive of progressive CKD and kidney failure at middle and deep depths independent of tubular diameters and clinical characteristics. Whereas wider distal tubules that were superficial were predictive of progressive CKD independent of glomerular volume and clinical characteristics, proximal tubule size at any depth was not predictive of progressive CKD independent of glomerular volume.
There were size differences in glomerular volume, proximal tubule diameter, and distal tubule diameter by depth, but the pattern was more consistent between glomerular volume and proximal tubule diameter than with distal tubule diameter. Larger glomerular volume and wider proximal tubule diameter both associated with larger BMI, hypertension, diabetes, and proteinuria.22 Whereas wider distal tubule associates with being male and lower eGFR, associations that were less consistently evident with larger glomeruli or proximal tubules.22 These findings are consistent with glomerular hyperfiltration being the causal mechanism for both glomerular hypertrophy and proximal tubule enlargement. It is well-known that metabolic stressors of obesity and diabetes can cause glomerulomegaly and hyperfiltration.11,39 The glomerular hyperfiltration increases shear stress to the proximal tubular epithelium, leading to hypertrophy to facilitate increased reabsorption with a higher volume of filtrate.21,30,31,40 Thus, it is not surprising that wider proximal tubule diameter does not predict progressive CKD independent of larger glomerular volume given their shared pathway of hyperfiltration. Glomerular hyperfiltration increases intraglomerular pressure, decreases podocyte density, and increases the shear stress on podocytes, and this can lead to global glomerulosclerosis.32,33,41–43 In particular, glomerular enlargement in the deeper cortex seems to predict progressive CKD independent of clinical characteristics, including obesity and diabetes. Age-related ischemic changes in glomerular tufts predominately affect the superficial cortex decreasing estimates of the mean nonsclerosed glomerular volume,22 but these findings were present after age adjustment.
There is likely a different but unknown mechanism for distal tubule enlargement than proximal tubule enlargement.31 Superficial distal tubule enlargement in particular was prognostic for progressive CKD independent of glomerular volume. Notably, microanatomical organization and transepithelial voltage differ between superficial and deeper nephrons, which may be consistent with different physiological functions. For example, deep juxtamedullary nephrons have larger glomeruli and long loops of Henle that descend into the renal medulla and play a greater role in the concentrating mechanism than superficial nephrons with short loops.44 Similarly, increasing glomerular depth is associated with shorter cortical thick ascending limbs. In addition, the connecting tubule of superficial nephrons drains into the collecting duct, whereas in middle and deep nephrons, the connecting tubules form an arcade that in many species drains additional nephrons before emptying into the collecting duct, which may regulate fluid dilution and potassium homeostasis.26
The distal tubule diameter in the superficial cortex may be more representative of the distal convoluted tubule, whereas distal tubule diameter in deeper levels of the cortex may be more representative of the loops of Henle and of collecting ducts. Distal convoluted tubular cells hypertrophy to adapt to increases in dietary intake of sodium chloride.45 Increased activity of the renin-angiotensin-aldosterone system may also contribute to the activation of transporters46 and distal tubular hypertrophy and hyperplasia.47 Continuous furosemide infusion with increased sodium chloride delivery to the distal convoluted tubule also leads to hypertrophy,48,49 and loop diuretics may contribute to progressive CKD by volume depletion while also causing distal tubule hypertrophy. Alternatively, the indication for loop diuretics (such as heart failure) may instead be the mechanism of increased risk of progressive CKD with distal tubule hypertrophy. We did not have data on loop diuretic use in our cohort. It is also possible that a shift in the set point of pressure natriuresis or some other mechanism of increased salt delivery beyond the proximal tubule causes distal tubular hypertrophy.
Older age and male were more strongly associated with wider distal tubules than with wider proximal tubules at all depths. Thus, prior findings in living kidney donors of larger cross-sectional tubular area on needle biopsy being associated with older age28 and male50 may be via larger cross-sectional area of distal rather than proximal tubules. Pertinently, testosterone has been shown to induce distal tubular hypertrophy.51,52 Higher IFTA foci density did not associate with larger tubules. Higher IFTA foci density better reflects the aging component of nephron loss.5 Higher % IFTA did associate with larger tubules and better reflects a disease component of nephron loss that may lead to more compensatory hypertrophy of remaining tubules.5 We found evidence that wider proximal and distal tubular size associated with larger cortical volume, consistent with a prior study in kidney donors.28 More loss of functional nephrons with a larger kidney tumor would also explain a compensatory increase in the size of remaining proximal and distal tubules with larger tumor volume.
This study provides further support to importance of morphometric assessment of kidney parenchyma. Currently, tubular size is rarely ever commented on by pathologists, and glomerular size is often simplified to a binary descriptor of presence or absence of glomerulomegaly. More refined assessment of tubular size using digital pathology with deep learning models to quantify the size of glomeruli and tubules at different depths may be needed to make the morphometric evaluation of kidney biopsies practical.53–58 Assessment of morphometry by depth is a challenge with commonly used needle core biopsies. However, the presence of a capsule can be used to identify superficial cortical depths where distal tubule diameter is more prognostic for progressive CKD. Likewise, the presence of adjacent medulla can identify deep cortex where glomerular size is more prognostic for progressive CKD. While our approach to measuring tubule diameter was time-consuming and tedious, a deep learning image processing algorithm to identify glomeruli and tubules followed by postprocessing calculations may be able to automate the assessment of glomerular volume and tubular diameter.
There are several potential limitations of our study. We only assessed the proximal and distal tubule diameters and not lengths. We were not able to differentiate the length of different tubular segments or separately assess the different segments of distal tubules (loop of Henle, distal convoluted tubule, and collecting duct). We also could not feasibly measure the size of every tubule profile on these large wedge sections. Limiting our data to the minor axis of circular or oval tubular profiles was intended to approximate the true mean diameter of the tubules. Still, there may have been some bias in this approach. We studied the average size of glomeruli and tubules; however, some may have early atrophy while others hypertrophy. Glomerular volume was calculated using the Weibel–Gomez stereology model, which approximates glomeruli to be spheres and assumed 10% variability in their size.13 The cohort consisted of patients with renal tumors who had a radical nephrectomy, and these factors likely played a role in the risk of progressive CKD. However, there are limited clinical settings where a kidney wedge section can be obtained in persons followed for progressive CKD. Proteinuria was estimated from the urine protein–osmolality ratios because 24-hour urine protein or protein-to-creatinine ratios were not routinely measured.
In summary, this study measured glomerular volume and proximal and distal tubule diameters at different depths and assessed their contribution to risk of progressive CKD in a large sample of patients with radical nephrectomy. It is well-known that enlarged glomeruli are prognostic for CKD events,1–4 and we extend this finding by showing that this association is stronger and independent of kidney function and CKD risk factors at deeper depths within the cortex. Furthermore, we demonstrated that wider superficial distal tubules are an important and underappreciated pathological finding that associate with lower eGFR and are predictive of CKD events independent of glomerular volume and clinical characteristics.
Supplementary Material
Acknowledgment
We thank Miloš Denić for assistance with computer algorithms for processing of biopsy annotations data.
Disclosures
L.O. Lerman reports Consultancy: AstraZeneca, Beren Therapeutics, Butterfly Biosciences, and CureSpec; Research Funding: AstraZeneca; Honoraria: AstraZeneca, Butterfly Biosciences, and CureSpec; Patents or Royalties: Cohbar; Stealth Biopharmaceuticals; Advisory or Leadership Role: AstraZeneca, CureSpec; and Other Interests or Relationships: AHA, and NIH. L. Barisoni reports Consultancy: Protalix, Sangamo, Vertex; Honoraria: Protalix, Sangamo, Vertex; Advisory or Leadership Role: Nephcure scientific advisory Board, Nature Review Nephrology, Glomerular Disease Journal; and Other Interests or Relationships: Nephcure. L. Barisoni reports Consultancy: Protalix, Sangamo, Vertex; Honoraria: Protalix, Sangamo, Vertex; Advisory or Leadership Role: Nephcure scientific advisory Board, Nature Review Nephrology, Glomerular Disease Journal; and Other Interests or Relationships: Nephcure. All remaining authors have nothing to disclose.
Funding
This study was supported with funding from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK090358).
Author Contributions
Conceptualization: Andrew D. Rule, Aleksandar Denic.
Data curation: Aleksandar Denic, Mrunanjali Gaddam, Amr Moustafa, Andrew D. Rule.
Formal analysis: Aleksandar Denic, Anthony C. Luehrs, Aidan F. Mullan.
Funding acquisition: Andrew D. Rule.
Methodology: Andrew D. Rule.
Project administration: Andrew D. Rule.
Resources: Vidit Sharma, R. Houston Thompson.
Supervision: Andrew D. Rule.
Writing – original draft: Aleksandar Denic, Andrew D. Rule.
Writing – review & editing: Mariam P. Alexander, Laura Barisoni, Lilach O. Lerman, Anthony C. Luehrs, Aidan F. Mullan, Vidit Sharma, Maxwell L. Smith, R. Houston Thompson.
Data Sharing Statement
Data cannot be shared without a data use agreement and Institutional Review Board approval.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/JSN/E475.
Supplemental Table 1. Comparison of measures of nephron size in three cortical depths.
Supplemental Table 2. Spearman correlations of diameters of proximal and distal tubules and glomerular volume by cortex depth.
Supplemental Table 3. Measures of nephron size (using glomerular diameter rather than volume) as predictors of progressive CKD from 4 months after a radical nephrectomy.
References
- 1.Issa N, Vaughan LE, Denic A, Kremers WK, Chakkera HA, Park WD. Larger nephron size, low nephron number, and nephrosclerosis on biopsy as predictors of kidney function after donating a kidney. Am J Transplant. 2019;19(7):1989–1998. doi: 10.1111/ajt.15259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denic A, Elsherbiny H, Mullan AF, Leibovich BC, Thompson RH, Ricaurte Archila L. Larger nephron size and nephrosclerosis predict progressive CKD and mortality after radical nephrectomy for tumor and independent of kidney function. J Am Soc Nephrol. 2020;31(11):2642–2652. doi: 10.1681/ASN.2020040449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Issa N, Lopez CL, Denic A, Taler SJ, Larson JJ, Kremers WK. Kidney structural features from living donors predict graft failure in the recipient. J Am Soc Nephrol. 2020;31(2):415–423. doi: 10.1681/ASN.2019090964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merzkani MA, Denic A, Narasimhan R, Lopez CL, Larson JJ, Kremers WK. Kidney microstructural features at the time of donation predict long-term risk of chronic kidney disease in living kidney donors. Mayo Clin Proc. 2021;96(1):40–51. doi: 10.1016/j.mayocp.2020.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricaurte L Denic A Mullan A, et al. A higher foci density of interstitial fibrosis and tubular atrophy predicts progressive chronic kidney disease after a radical nephrectomy for tumor. J Am Soc Nephrol. 2021;32(10):2623–2633. doi: 10.1681/ASN.2021020267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimanyi MA, Hoy WE, Douglas-Denton RN, Hughson MD, Holden LM, Bertram JF. Nephron number and individual glomerular volumes in male Caucasian and African American subjects. Nephrol Dial Transplant. 2009;24(8):2428–2433. doi: 10.1093/ndt/gfp116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNamara BJ, Diouf B, Douglas-Denton RN, Hughson MD, Hoy WE, Bertram JF. A comparison of nephron number, glomerular volume and kidney weight in Senegalese Africans and African Americans. Nephrol Dial Transplant. 2010;25(5):1514–1520. doi: 10.1093/ndt/gfq030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luyckx VA, Shukha K, Brenner BM. Low nephron number and its clinical consequences. Rambam Maimonides Med J. 2011;2(4):e0061. doi: 10.5041/RMMJ.10061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denic A, Lieske JC, Chakkera HA, Poggio ED, Alexander MP, Singh P. The substantial loss of nephrons in healthy human kidneys with aging. J Am Soc Nephrol. 2017;28(1):313–320. doi: 10.1681/ASN.2016020154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denic A, Mathew J, Lerman LO, Lieske JC, Larson JJ, Alexander MP. Single-nephron glomerular filtration rate in healthy adults. N Engl J Med. 2017;376(24):2349–2357. doi: 10.1056/NEJMoa1614329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denic A, Glassock RJ. Obesity-related glomerulopathy and single-nephron GFR. Kidney Int Rep. 2020;5(8):1126–1128. doi: 10.1016/j.ekir.2020.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denic A, Rule AD. Authors' reply. J Am Soc Nephrol. 2021;32(2):517–518. doi: 10.1681/ASN.2020111615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weibel ER, Gomez DM. A principle for counting tissue structures on random sections. J Appl Physiol. 1962;17(2):343–348. doi: 10.1152/jappl.1962.17.2.343 [DOI] [PubMed] [Google Scholar]
- 14.Okada K, Takahashi S. Measurement of proximal tubular diameter for evaluation of tubular hypertrophy. Nephron. 1995;70(1):122. doi: 10.1159/000188562 [DOI] [PubMed] [Google Scholar]
- 15.Momeni HR, Eskandari N. Effect of curcumin on kidney histopathological changes, lipid peroxidation and total antioxidant capacity of serum in sodium arsenite-treated mice. Exp Toxicol Pathol. 2017;69(2):93–97. doi: 10.1016/j.etp.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 16.Hemmi S, Matsumoto N, Jike T, Obana Y, Nakanishi Y, Soma M. Proximal tubule morphology in rats with renal congestion: a study involving the in vivo cryotechnique. Med Mol Morphol. 2015;48(2):92–103. doi: 10.1007/s00795-014-0084-x [DOI] [PubMed] [Google Scholar]
- 17.Truter D, Chellan N, Strijdom H, Webster I, Rawstorne J, Kotze SH. Histomorphological changes in the pancreas and kidney and histopathological changes in the liver in male Wistar rats on antiretroviral therapy and melatonin treatment. Acta Histochem. 2018;120(4):347–355. doi: 10.1016/j.acthis.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 18.Leh S, Hultstrom M, Rosenberger C, Iversen BM. Afferent arteriolopathy and glomerular collapse but not segmental sclerosis induce tubular atrophy in old spontaneously hypertensive rats. Virchows Arch. 2011;459(1):99–108. doi: 10.1007/s00428-011-1100-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lebrecht D, Venhoff AC, Kirschner J, Wiech T, Venhoff N, Walker UA. Mitochondrial tubulopathy in tenofovir disoproxil fumarate-treated rats. J Acquir Immune Defic Syndr. 2009;51(3):258–263. doi: 10.1097/qai.0b013e3181a666eb [DOI] [PubMed] [Google Scholar]
- 20.Alkharfy KM, Ahmed M, Yakout SM, Al-Daghri NM. Effects of calcitriol on structural changes of kidney in C57BL/6J mouse model. Int J Clin Exp Med. 2015;8:12390–12396. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4612834 [PMC free article] [PubMed] [Google Scholar]
- 21.Tobar A, Ori Y, Benchetrit S, Milo G, Herman-Edelstein M, Zingerman B. Proximal tubular hypertrophy and enlarged glomerular and proximal tubular urinary space in obese subjects with proteinuria. PLoS One. 2013;8(9):e75547. doi: 10.1371/journal.pone.0075547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denic A, Ricaurte L, Lopez CL, Narasimhan R, Lerman LO, Lieske JC. Glomerular volume and glomerulosclerosis at different depths within the human kidney. J Am Soc Nephrol. 2019;30(8):1471–1480. doi: 10.1681/ASN.2019020183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denic A, Mathew J, Nagineni VV, Thompson RH, Leibovich BC, Lerman LO. Clinical and pathology findings associate consistently with larger glomerular volume. J Am Soc Nephrol. 2018;29(7):1960–1969. doi: 10.1681/ASN.2017121305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y. New creatinine- and Cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson DM, Anderson RL. Protein–osmolality ratio for the quantitative assessment of proteinuria from a random urinalysis sample. Am J Clin Pathol. 1993;100(4):419–424. doi: 10.1093/ajcp/100.4.419 [DOI] [PubMed] [Google Scholar]
- 26.Reilly RF, Ellison DH. Mammalian distal tubule: physiology, pathophysiology, and molecular anatomy. Physiol Rev. 2000;80(1):277–313. doi: 10.1152/physrev.2000.80.1.277 [DOI] [PubMed] [Google Scholar]
- 27.Moskowitz DW, Schneider AN, Lane PH, Schmitz PG, Gillespie KN. Effect of epidermal growth factor in the rat 5/6 renal ablation model. J Am Soc Nephrol. 1992;3(5):1113–1118. doi: 10.1681/ASN.v351113 [DOI] [PubMed] [Google Scholar]
- 28.Denic A, Alexander MP, Kaushik V, Lerman LO, Lieske JC, Stegall MD. Detection and clinical patterns of nephron hypertrophy and nephrosclerosis among apparently healthy adults. Am J Kidney Dis. 2016;68(1):58–67. doi: 10.1053/j.ajkd.2015.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311(24):2518–2531. doi: 10.1001/jama.2014.6634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chagnac A, Zingerman B, Rozen-Zvi B, Herman-Edelstein M. Consequences of glomerular hyperfiltration: the role of physical forces in the pathogenesis of chronic kidney disease in diabetes and obesity. Nephron. 2019;143(1):38–42. doi: 10.1159/000499486 [DOI] [PubMed] [Google Scholar]
- 31.Fine LG, Trizna W, Bourgoignie JJ, Bricker NS. Functional profile of the isolated uremic nephron. Role of compensatory hypertrophy in the control of fluid reabsorption by the proximal straight tubule. J Clin Invest. 1978;61(6):1508–1518. doi: 10.1172/JCI109071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kriz W, Lemley KV. A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J Am Soc Nephrol. 2015;26(2):258–269. doi: 10.1681/ASN.2014030278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodgin JB, Bitzer M, Wickman L, Afshinnia F, Wang SQ, O'Connor C. Glomerular aging and focal global glomerulosclerosis: a podometric perspective. J Am Soc Nephrol. 2015;26(12):3162–3178. doi: 10.1681/ASN.2014080752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denic A, Bogojevic M, Subramani R, Park WD, Smith BH, Alexander MP. Changes in glomerular volume, sclerosis, and ischemia at 5 Years after kidney transplantation: incidence and correlation with late graft failure. J Am Soc Nephrol. 2022;34(2):346–358. doi: 10.1681/ASN.2022040418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haruhara K, Tsuboi N, Sasaki T, Amano H, Tanaka M, Koike K. Volume ratio of glomerular tufts to Bowman capsules and renal outcomes in nephrosclerosis. Am J Hypertens. 2019;32(1):45–53. doi: 10.1093/ajh/hpy147 [DOI] [PubMed] [Google Scholar]
- 36.Hommos MS, Zeng C, Liu Z, Troost JP, Rosenberg AZ, Palmer M. Global glomerulosclerosis with nephrotic syndrome; the clinical importance of age adjustment. Kidney Int. 2018;93(5):1175–1182. doi: 10.1016/j.kint.2017.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okamoto H, Kawamura T, Okonogi H, Tsuboi N, Miyazaki Y, Yokoo T. The role of a low glomerular density and being overweight in the etiology of proteinuria in CKD patients without known glomerular diseases. Clin Exp Nephrol. 2014;18(6):911–917. doi: 10.1007/s10157-014-0940-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuboi N, Kawamura T, Koike K, Okonogi H, Hirano K, Hamaguchi A. Glomerular density in renal biopsy specimens predicts the long-term prognosis of IgA nephropathy. Clin J Am Soc Nephrol. 2010;5(1):39–44. doi: 10.2215/CJN.04680709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okabayashi Y, Tsuboi N, Sasaki T, Haruhara K, Kanzaki G, Koike K. Single-nephron GFR in patients with obesity-related glomerulopathy. Kidney Int Rep. 2020;5(8):1218–1227. doi: 10.1016/j.ekir.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ichikawa I, Hoyer JR, Seiler MW, Brenner BM. Mechanism of glomerulotubular balance in the setting of heterogeneous glomerular injury. Preservation of a close functional linkage between individual nephrons and surrounding microvasculature. J Clin Invest. 1982;69(1):185–198. doi: 10.1172/jci110430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kopp JB, Anders HJ, Susztak K, Podesta MA, Remuzzi G, Hildebrandt F. Podocytopathies. Nat Rev Dis Primers. 2020;6(1):68. doi: 10.1038/s41572-020-0196-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butt L, Unnersjo-Jess D, Hohne M, Edwards A, Binz-Lotter J, Reilly D. A molecular mechanism explaining albuminuria in kidney disease. Nat Metab. 2020;2(5):461–474. doi: 10.1038/s42255-020-0204-y [DOI] [PubMed] [Google Scholar]
- 43.Luyckx VA, Rule AD, Tuttle KR, Delanaye P, Liapis H, Gandjour A. Nephron overload as a therapeutic target to maximize kidney lifespan. Nat Rev Nephrol. 2022;18(3):171–183. doi: 10.1038/s41581-021-00510-7 [DOI] [PubMed] [Google Scholar]
- 44.Jamison RL. Short and long loop nephrons. Kidney Int. 1987;31(2):597–605. doi: 10.1038/ki.1987.40 [DOI] [PubMed] [Google Scholar]
- 45.Ellison DH, Velazquez H, Wright FS. Adaptation of the distal convoluted tubule of the rat. Structural and functional effects of dietary salt intake and chronic diuretic infusion. J Clin Invest. 1989;83(1):113–126. doi: 10.1172/JCI113847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.San-Cristobal P, Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci U S A. 2009;106(11):4384–4389. doi: 10.1073/pnas.0813238106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCormick JA, Ellison DH. Distal convoluted tubule. Compr Physiol. 2015;5(1):45–98. doi: 10.1002/cphy.c140002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaissling B, Bachmann S, Kriz W. Structural adaptation of the distal convoluted tubule to prolonged furosemide treatment. Am J Physiol Renal Physiol. 1985;248(3):F374–F381. doi: 10.1152/ajprenal.1985.248.3.F374 [DOI] [PubMed] [Google Scholar]
- 49.Ellison DH, Velázquez H, Wright FS. Adaptation of the distal convoluted tubule of the rat. Structural and functional effects of dietary salt intake and chronic diuretic infusion. J Clin Invest. 1989;83(1):113–126. doi: 10.1172/JCI113847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elsherbiny HE, Alexander MP, Kremers WK, Park WD, Poggio ED, Prieto M. Nephron hypertrophy and glomerulosclerosis and their association with kidney function and risk factors among living kidney donors. Clin J Am Soc Nephrol. 2014;9(11):1892–1902. doi: 10.2215/CJN.02560314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McAllan BM, Roberts JR, O'Shea T. Effects of testosterone and cortisol on the renal morphology of male Antechinus stuartii (Marsupialia). Gen Comp Endocrinol. 1997;107(3):439–449. doi: 10.1006/gcen.1997.6945 [DOI] [PubMed] [Google Scholar]
- 52.Quinkler M, Bujalska IJ, Kaur K, Onyimba CU, Buhner S, Allolio B. Androgen receptor-mediated regulation of the alpha-subunit of the epithelial sodium channel in human kidney. Hypertension. 2005;46(4):787–798. doi: 10.1161/01.hyp.0000184362.61744.c1 [DOI] [PubMed] [Google Scholar]
- 53.Hermsen M, de Bel T, den Boer M, Steenbergen EJ, Kers J, Florquin S. Deep learning-based histopathologic assessment of kidney tissue. J Am Soc Nephrol. 2019;30(10):1968–1979. doi: 10.1681/ASN.2019020144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lutnick B, Manthey D, Becker JU, Ginley B, Moos K, Zuckerman JE. A user-friendly tool for cloud-based whole slide image segmentation with examples from renal histopathology. Commun Med (Lond). 2022;2(1):105. doi: 10.1038/s43856-022-00138-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang L, Chen W, Dong B, Mei K, Zhu C, Liu J. A deep learning-based approach for glomeruli instance segmentation from multistained renal biopsy pathologic images. Am J Pathol. 2021;191(8):1431–1441. doi: 10.1016/j.ajpath.2021.05.004 [DOI] [PubMed] [Google Scholar]
- 56.Li X, Davis RC, Xu Y, Wang Z, Souma N, Sotolongo G. Deep learning segmentation of glomeruli on kidney donor frozen sections. J Med Imaging (Bellingham). 2021;8(6):067501. doi: 10.1117/1.JMI.8.6.067501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salvi M, Mogetta A, Gambella A, Molinaro L, Barreca A, Papotti M. Automated assessment of glomerulosclerosis and tubular atrophy using deep learning. Comput Med Imaging Graph. 2021;90:101930. doi: 10.1016/j.compmedimag.2021.101930 [DOI] [PubMed] [Google Scholar]
- 58.Hara S, Haneda E, Kawakami M, Morita K, Nishioka R, Zoshima T. Evaluating tubulointerstitial compartments in renal biopsy specimens using a deep learning-based approach for classifying normal and abnormal tubules. PLoS One. 2022;17(7):e0271161. doi: 10.1371/journal.pone.0271161 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data cannot be shared without a data use agreement and Institutional Review Board approval.