Abstract
Vertebrates have some of the most complex and diverse features in animals, from varied craniofacial morphologies to colorful pigmentation patterns and elaborate social behaviors. All of these traits have their developmental origins in a multipotent embryonic lineage of neural crest cells. This “fourth germ layer” is a vertebrate innovation and the source of a wide range of adult cell types. While others have discussed the role of neural crest cells in human disease and animal domestication, less is known about their role in contributing to adaptive changes in wild populations. Here, we review how variation in the development of neural crest cells and their derivatives generates considerable phenotypic diversity in nature. We focus on the broad span of traits under natural and sexual selection whose variation may originate in the neural crest, with emphasis on behavioral factors such as intraspecies communication that are often overlooked. In all, we encourage the integration of evolutionary ecology with developmental biology and molecular genetics to gain a more complete understanding of the role of this single cell type in trait covariation, evolutionary trajectories, and vertebrate diversity.
Keywords: Neural crest cell, Adaptation, Microevolution, Morphological variation
1. Introduction
Neural crest cells (NCCs) are often touted as the “fourth germ layer” [1] for the range of derivative cell types they produce, and many species-defining characteristics like facial shape and coloration are generated from this embryonic lineage. The influence of these cells in human health has been long recognized, with a broad spectrum of conditions classified as neurocristopathies [2] because of their shared developmental origin in NCCs. Within evolutionary variation, NCCs have been linked to animal domestication [3–5]. The domestication syndrome hypothesis proposes that a series of traits observed in domesticated animals—reduced facial structures, loss of pigmentation, and docility, among others—can be unified due to their NCC origins, and should be considered a neurocristopathy [3–5]. However, we still do not have a full view of how changes in NCC development can generate natural phenotypic variation, particularly with regards to behavior. The role of NCCs in tameness has been discussed as it relates to domestication [3–5], but the span of behavioral traits that may be mediated by NCCs and their derivatives has received considerably less attention in the literature than traits such as craniofacial variation and coloration (though see [6,7]). Here, we take a trait-based approach, assessing how phenotypes shaped by natural and sexual selection are diversified through variation in development of NCCs and their derivatives. We extend ideas begun by [3–8] to create a more complete understanding of the types and patterns of phenotypic diversification that trace their origins to NCCs.
We first highlight the types of cells derived from NCCs, developmental sources of phenotypic variation, and how NCC macroevolution may impact microevolutionary diversification. We then focus on a series of traits, discussing how variation in the development of NCCs and their derivatives have or could contribute to the evolution of phenotypic diversity. After addressing co-variation of these traits through examples of domestication and cavefish as neurocristopathies, we suggest future work that will provide fruitful insights into the sources and effects of variation in NCC development on microevolution.
2. Neural crest cell development
The molecular and genetic program that underlies NCC development is well reviewed in the literature. Here, we highlight two major points about these cells: (1) the wide variety of cells differentiating from this single embryonic lineage and (2) developmental changes that produce variation in NCCs and their derivatives. We refer the reader to multiple reviews below for more details on the development of these cells, including primary literature cited therewithin.
NCCs are specified at the boundary between neural and non-neural ectoderm within the neural tube [9,10], and from there migrate extensively throughout the embryo [11,12]. The fate of NCCs is influenced by factors such as anterior-posterior position, signaling pathways, and interactions with their local tissue environment. NCCs differentiate into a multitude of cell types including Schwann cells and neurons of the peripheral nervous system [13,14], neurons of the enteric nervous system [15,16], chromaffin cells of the adrenal gland [17], odontoblasts in the teeth [18], and chondrocytes and osteocytes within the head [19,20] (Table 1). Neural crest cells also produce pigment-producing cells. While mammals and birds only develop black/brown melanocytes, NCCs in fishes and amphibians generate a series of chromatophores including black/brown melanophores, red/yellow xanthophores, and reflective iridophores [21,22].
Table 1.
Some cell types that arise from subpopulations of neural crest cells.
| Neural crest cell subpopulation | Cell type or structure derived |
|---|---|
| Cranial | Osteocytes and chondrocytes within the facial skeleton Odontoblasts of the teeth Sensory ganglia of cranial nerves V, VI, IX, and X Mesenchyme of the parathyroid and thymus Corneal endothelium and stroma Parafollicular cells of the thyroid gland Chromatophores of the skin, including melanocytes, melanophores, xanthophores, and iridophores Carotid body cells |
| Cardiac | Cardiomyocytes of the aortic and pulmonary arteries Portions of the septa of the heart |
| Trunk | Chromatophores of the skin, including melanocytes, melanophores, xanthophores, and iridophores Chromaffin cells of the adrenal gland Neurons and ganglia of the sympathetic nervous system Dorsal root ganglia Schwann cells of peripheral nervous system |
| Vagal and Sacral | Enteric ganglia Neurons and glia of the parasympathetic nervous system Neurons of the enteric ganglia |
NCCs may also influence the development of the sensory organs. While sensory structures are primarily derived from sensory placodes instead of NCCs, their migration and morphogenesis is coordinated with NCCs through a series of reciprocal interactions [23,24]. Through these interactions, NCCs may affect the size, number, or pattern of sensory organs, but not how or what signals are detected by the organ. For example, a tradeoff in cavefish of a reduced size of the optic placode for an expanded olfactory placode [25] may be partially mediated by NCCs through altered co-migration of NCCs and sensory placodes. However, we do not suggest that NCCs are likely to influence evolution of opsin protein types in the retina.
Variation at multiple stages of development of NCCs and their derivatives can produce phenotypic diversification on which natural and sexual selection can act (Fig. 1). We use the development of pigment-producing melanocytes as an example. Altered specification and migration through loss of SOX10 causes a complete loss of these cells, and also impacts other NCC derivatives [26,27]. Mutation of the MITF gene causes multiple subpopulations of migratory NCCs including melanocyte precursors to undergo apoptosis, which produces white patches of skin in dogs and Waardenburg syndrome in humans [28,29]. Additionally, the biosynthesis of the black/brown pigment, eumelanin, in differentiated melanocytes can be affected, resulting in hypopigmented phenotypes such as red hair or albinism (MC1R and TYR mutations, respectively) [30]. These examples highlight how cellular changes throughout NCC development can produce diversity not only within a single cell type, but across NCC derivatives.
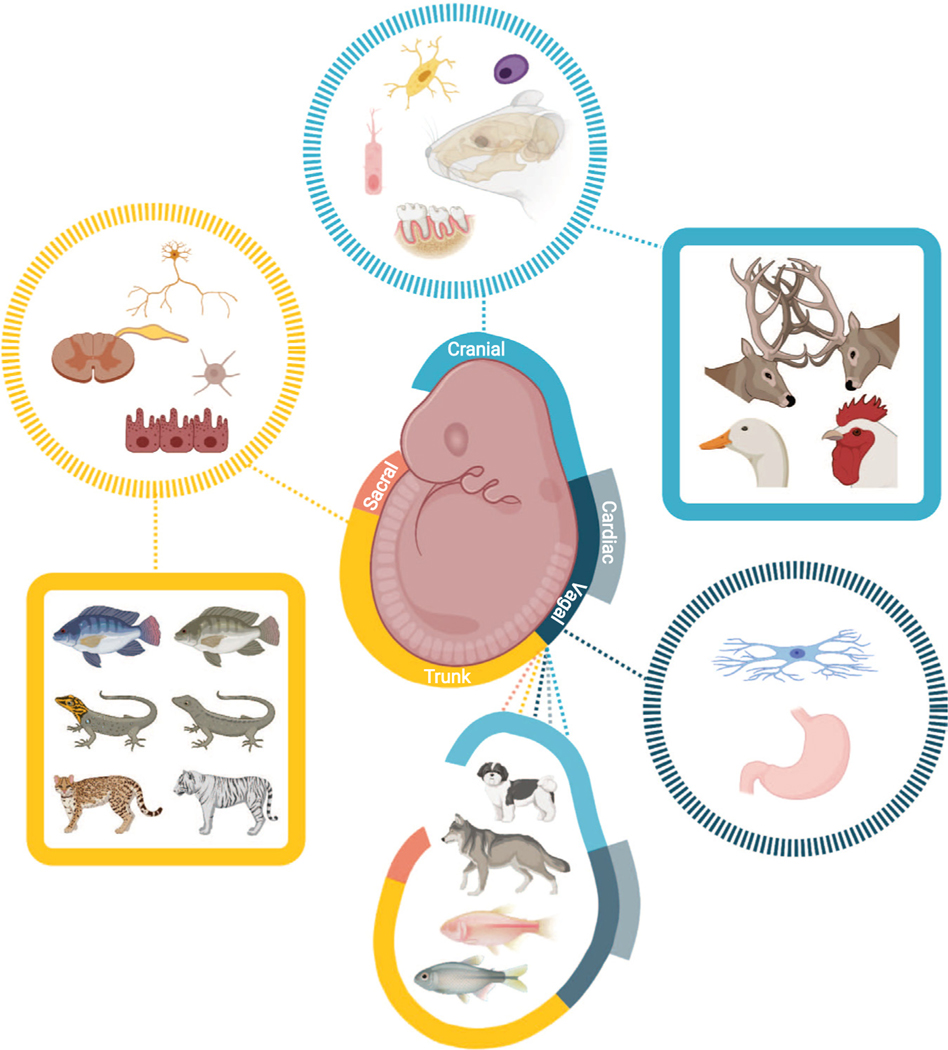
Fig. 1.
Overview of the relationship of neural crest cell embryonic regions, cells, and evolved traits. Embryonic neural crest cell populations are represented in the center. Derivative cells and tissues for regions are illustrated in dotted circles with matching colors. Related variation in evolutionarily selected phenotypes is shown in solid squares, also color-coded. Coordinated changes in NCC derived traits in cavefish adaptation and dog domestication results from multiple populations, as indicated by the solid embryo outline. Created with BioRender.com with permission.
3. Potential legacy of neural crest cell macroevolution
As stated in the seminal “New Head Hypothesis” [31], the innovation of NCCs and a complex head were critical for vertebrate macroevolution, including a shift to active predation. The macroevolutionary origins of NCCs is much discussed in the literature [32–38]. Others propose that it occurred in an incremental manner through a combination of gene duplications, evolution of novel cis-regulatory elements, and co-option of existing sub-networks from other tissues to form the NCC gene regulatory network [32–38].
Discussed less often (but see [39,40]) is how the macroevolutionary history of NCCs facilitates their microevolutionary path and potential. For instance, a critical property co-opted by NCCs in their macroevolution is multipotency [32,33,41]. However, post-embryonic retention of multipotency or an ability to de-differentiate may also expand the microevolutionary plasticity of NCC associated cells and traits. For example, NCC derived Schwann cell precursors in the adult body can also differentiate into enteric and parasympathetic neurons, chromaffin cells, pigment-producing cells, and even odontoblasts [42,43]. This innate ability to produce multiple cell types may allow an animal to have a plastic response or more rapid adaptation in traits influenced by these cells. However, it is unclear the extent that this flexible fate restriction is common across NCCs subpopulations or derivatives, or if this microevolutionary potential is regulated by the same factors that were critical for NCCs to obtain multipotency in the first place. Notably, such questions feed into larger and long-standing debates about the degree to which NCCs are plastic versus pre-programmed [40,41,44–49], and are important considerations for the trajectory and range of phenotypic diversification.
4. Traits influenced by neural crest cell microevolution
We discuss below a series of traits that originate in NCCs or their derivatives and are under natural and sexual selection. Where available, we highlight the genetic or cellular basis for phenotypic diversification.
4.1. Trophic specialization
As NCCs contribute to the majority of the bones and cartilage of the face [19,20], their development is central to producing craniofacial variation. This morphological diversity can confer a biomechanical advantage in foraging or enable an animal to occupy a new feeding niche, such as occurred in textbook examples of adaptive radiations including Darwin’s finches and cichlid fishes.
The multi-faceted and cumulative effect of NCCs in generating species-specific faces has been demonstrated within avian species. While the short-beaked quail and broad-billed duck specify a similar number of NCCs, more cells migrate to the developing jaw in the duck, providing a larger pool of precursors as mesenchyme in the pharyngeal arches [50]. After migration, changes to NCC proliferation can further change the volume and shape of facial prominences. In the bills of ducks and ground finches, increased levels and expanded domains of Bmp4 expression [51,52] and Wnt signaling [53,54], or variation within the length of the cell cycle [50] can all increase proliferation of post-migratory NCCs to produce deeper and wider beak primordia. Additionally, a longer beak can develop from increased expression of Calmodulin within NCC derived mesenchyme [55]. Further variation comes after NCCs differentiate and begin to produce bone. For example, bone development starts earlier in Darwin’s finches with more robust beaks [54]. Also, ossification occurs earlier in the quail at the expense of further proliferation [56], and fine-tuning of bone shape occurs through differential rates of bone resorption [57]. The combinatorial effect of these developmental changes in NCCs and their derivatives generates the distinctive beaks that birds use to exploit different food sources.
The diversification of craniofacial structures within cichlid fishes occurred through similar mechanisms and even some of the same molecules. NCC migration is affected by a single amino acid change in lbh identified in species with shorter mandibles [58], which decreases the post-migratory pool of NCC precursors from which this bone derives. Differential expression of bmp4 and ptch1 are associated with mandible shape variation [59,60]. Additionally, NCC derived osteocytes show altered timing and levels of bone deposition [61] between cichlid species that ultimately have different jaw structures as adults. Finally, facial bones that originated from NCCs can be shaped through bone remodeling due to biomechanical strains during feeding [62]. These combined genetic and epigenetic mechanisms produce jaw morphologies with distinct kinematics that allow species to occupy new trophic niches.
Further foraging specialization occurs through tooth evolution, especially multi-cusped teeth that promote more efficient breakdown of food [63]. NCCs are a major source of the mesenchyme of the developing teeth, which is critical in the formation of tooth cusps [64]. NCCs later differentiate into dentin-secreting odontoblasts in the teeth [65], further implicating this cell type in the microevolution of feeding structures. Work in mammals directly connects NCCs to tooth development, with a loss of BMP signaling within NCCs resulting in molars with more cusps, altered shape, delayed mineralization, and even the complete arrest of tooth development [65–67]. Cichlids again provide an example from nature, with the evolution of unicuspid, bicuspid, and tricuspid teeth [68]. While the molecular mechanisms responsible for dental adaptations in cichlids are not completely understood, changes in BMP, FGF, and Wnt signaling in cichlids can affect both cusp shape and number, mimicking the observed adaptive changes in these fishes [69].
4.2. Intrasexual competition
Craniofacial structures often face multiple selective pressures at the same time. For instance, extreme tooth phenotypes like elephant tusks can be used for foraging, but also intrasexual fights. These male-male or female-female competitions for food, territory, social dominance, and access to mates play a critical role in evolution [70,71]. Transcriptome analysis demonstrates that mineralized headgear like horns and antlers primarily derive from NCCs [72], and may have evolved through a “developmental accident” where cranial NCCs migrated to new embryonic locations [73]. In support of this theory, horns are associated with cis-regulatory changes in genes critical for NCC migration and patterning, including SNAI2, TWIST1, SOX9, and the HOXD gene cluster [72]. Weapons used in intrasexual competition need not be specialized structures, however. Male cichlid fishes use their diet-adapted jaws to fight for social dominance and territories, forgoing the need for elaborate weaponry [74]. Similarly, in several lizard species, craniofacial structures are strongly correlated with fight performance [75,76]. Thus, the series of changes in NCC development detailed above in relation to trophic specialization may also evolve due to strong sexual selection.
Also important to intrasexual competition are NCC derived melanocytes and chromatophores that advertise status to same-sex rivals for mates and resources. Colored feather patches serve as honest signals of health to competitors, and an individual sporting a conspicuously-colored badge usually has higher fitness and larger territory than birds that lack this pigmentation [77]. The aggression-induced coloration exhibited by numerous fish species [78] represents a similar use of pigmentation to signal one’s status to rivals.
4.3. Mate choice
Sexual selection consists of intrasexual selection and mate choice. Structures like antlers are used in both of these interactions [79], but are not the only NCC derived characteristics that attract a mate. Within the craniofacial complex of certain cichlid fishes, an elaboration of the frontonasal region, the nuchal hump, is associated with differential expression of pappa [80] and is predicted to function in mate choice [81]. These changes in expression of the PAPP-A metalloprotease may generate these phenotypes by altering insulin-like growth factor (IGF) signaling [82], and thus NCC migration and differentiation [83,84].
Facial variation associated with trophic specialization (described above) can also impact signaling between potential mates. Beak shape constrains the acoustics and performance of bird songs that are a signal to both mates and competition, such that birds with larger beaks produce slower songs with lower frequencies [85,86]. Thus, natural selection for foraging efficiency that shapes these NCC derived structures also impacts reproductive isolation and sexual selection, and vice versa.
Mate choice is also a strong force in the evolution of pigmentation patterns. Female preference for more brightly colored males is a common theme in animals as divergent as primates [87], birds [88], and fishes [89], and this coloration has its developmental origins in NCCs and NCC derivatives. For instance, cis-regulatory changes in pax3a underlie patches of red and yellow pigmentation in populations of cichlid fishes. This may be due to a fate switch of NCC derived chromatophores, as pax3 promotes the production of red/yellow xanthophores, at the expense of black/brown melanophores [90]. A similar mechanism could underlie the nuptial coloration in stickleback fishes, with a set of genes (pcbd1, slc2a15a, slc24a3, sox10, and csf1) that are all involved in chromatophore fate determination also proposed as candidates that control deposition of red pigmentation in the throat [91].
It is not just hue that is attractive to females, but also pattern. Conspicuous pigmentation on the anal fins of some male cichlids, called egg spots, attract females and are under positive sexual selection. These egg spots are formed from xanthophores, a type of NCC derived chromatophore [92]. Egg spot size and pattern are associated with changes in edn3b and ednrB1a expression [93], a pathway critical for migration of NCCs from the neural tube, and also of migration of mature chromatophores [94]. Egg spot variation also occurs due to modulation of csf1ra expression [92], which is associated with terminal differentiation of xanthophores [95,96], further implicating NCC development in variation of this trait critical for mate choice.
4.4. Aggression and social behavior
The ability of an animal to recognize an individual as a friend or foe and decisions of when to fight are critical for survival. While fighting may result in a fatal injury, flight may result in lost access to food or mates. While neural ectoderm forms much of the nervous system, NCCs are a critical source of neurons in the parasympathetic system. This parasympathetic “rest and digest” response is critical for an animal to self-soothe and respond to an individual as a potential mate rather than a competitor. Through this action, these NCC derived ganglia form a critical part of courtship or social bonding that affect an animal’s fitness [97].
Opposing the action of the parasympathetic system is the “fight or flight” response that mediates risk assessment, exploratory behavior, and defensive actions when faced with a predator or rival. The tendency of an animal to fight is regulated by the sympathetic nervous system and the stress response mediated by the adrenal gland, including NCC derived ganglia and chromaffin cells, respectively. NCC derived chromaffin cells secrete the catecholamine hormones epinephrine and norepinephrine, and thus play a key role in rapid stress response [98]. While variation within the brain and neurotransmitters like serotonin play a key role in aggressive behaviors [99], levels of catecholamine stress hormones are also linked to fighting and boldness across vertebrates [100] and changes in aggression and social status in anoles [101]. Increased production [102] and slower catabolism of epinephrine [103] are both associated with increased aggression in mice. Thus, NCCs are poised to play a crucial role in aggressive behaviors. Others [3–5] have proposed that a decrease in NCC migration and consequent reduction of adrenal gland function was critical for tameness and animal domestication (see Section 5.1). One candidate gene for this evolutionary change in behavior is Sox10, loss of which results in apoptosis of trunk NCCs during migration to the adrenal gland and a loss of chromaffin cells in mice [104]. But we have few empirical examples of how changes in NCC development are linked with evolved changes in adrenal gland morphology, catecholamine secretion, or animal behavior. Such analyses would complement our mechanistic understanding of craniofacial and pigment evolution to give a more complete view of the phenotypic effects of aberrant NCC development.
Sensory structures such as the eyes and lateral line organ, which detects movements and vibration in aquatic animals, are the interface between an animal’s environment and their neural system. These are thus critical structures for an animal to be able to detect predators, rivals, or potential risks and react appropriately. Sensory organs begin as sensory placodes which, as previously described (see Section 2), coordinate with NCCs for their development. Because of this, variation in the amount or location of sensory cells may trace their developmental origins to variation in their co-migration with NCCs. Co-evolution is often observed among changes in the size and distribution of sensory structures, risk-taking behavior, and aggression in vertebrates [105,106]. For instance, variation in the size, number, and distribution of lateral line sensory structures in cichlids are associated with male-male aggression [74]. Further, changes to sensory systems are associated with use of open versus protected habitats, which is often related to an animal’s boldness and overall risk-taking behavior [105]. These examples suggest that assessment of the impact of NCC migration on the evolution of sensory structures may prove a useful avenue of investigation into the developmental origins of variation in an animal’s interaction with competitors and its environment.
4.5. Social communication
An extension of the one-on-one or few-on-one interaction discussed above is a particular type of social behavior that involves the coordination of large groups of conspecific animals. Collective animal behaviors like bird flocking and ungulate herding provide evolutionary benefits such as predator protection, enhanced feeding efficiency, and hydrodynamic, aerodynamic, or thermodynamic advantages [107]. For these social groups to form, the activity of the NCC derived parasympathetic system must override stress responses. But additional NCC derivatives also feed into these social interactions. Sensory systems like vision and lateral lines [108] have each been shown to be important for schooling in cave-dwelling Astyanax [109] and sticklebacks [110]. However, both fishes also demonstrate sensory-independent aspects to this behavior that implicate hormones secreted from NCC derived cells in the adrenal gland. Cave morphs of Astyanax have elevated levels of catecholamine stress hormones [111], a loss of schooling behavior, decreased aggression, and less alarm response compared to surface morphs [112]. Thus, changes in collective behavior in these fishes may originate in NCC development, particularly through chromaffin cells and an ability to withstand the stress of crowding.
Collective animal behaviors may also be influenced by pigmentation patterns produced by NCC derived melanophores. The presence of horizontal stripes in cichlid fishes correlates with shoaling behavior [113], and striped zebrafish show a preference to shoal with fishes who also exhibit horizontal stripes [114,115]. It is thought that these stripes may serve to reduce intraspecific aggression while also concealing the fish from their prey during hunting [113]. This positive correlation between stripes and group interactions extends from fishes to birds and mammals [116], suggesting convergent evolution of this NCC derived trait. Microevolution in this trait can manifest through the presence and number of stripes, and we know much of the genetic basis of this trait from mutant and naturally-occurring variants in zebrafish [117]. In addition to the molecules associated with melanocyte or melanophore development previously discussed (see Section 2), we highlight three additional genes that control distinct aspects of stripe formation through development of NCC derived cells. Asip2b is a master regulator for the presence of stripes versus vertical bars in cichlids and is associated with melanin production in differentiated melanophores [118]. Ednrb1 mutant zebrafish show fewer and disrupted stripes [96]. While endothelin signaling plays a role in migration of NCCs from the neural tube, this pigmentation defect is thought to be due to effects on migration of differentiated melanophores [96]. Finally, the connexin41.8 (leopard) gene in zebrafish controls formation of gap junctions that organize differentiated melanophores into stripes rather than spots [119].
4.6. Predator avoidance
The ability to eat but not be eaten is critical for survival, no matter one’s place in the food chain. One way that animals evade predation is to detect predators before the predators detect them. This is primarily achieved through sensory structures, which as previously discussed (Section 2) may be affected by changes in NCC development.
But this is far from the only way to avoid predation. Another common approach is for prey to camouflage themselves. A series of color patterns made possible by NCC derived pigment-producing cells mediate both cryptic and disruptive coloration, wherein animals try to match their surroundings or obscure their shape, respectively. One widespread mechanism of camouflage in vertebrates is countershading, where the illuminated back of the body is darker than a lighter-colored belly. While countershading may also influence thermoregulation and protection from ultraviolet radiation (see Section 4.7), these dorsal-ventral differences in color help an animal hide from predators [120,121]. Such adaptive color patterns occur based on the development of NCC derived pigment-producing cells, with the examples below specifically relating to eumelanin biosynthesis in melanocytes and melanophores. In mice and zebrafish, countershading is controlled by interactions between Mc1r and Asip that regulate eumelanin deposition [122,123]. Mutations in ASIP may also generate cryptic spotting patterns famously adorned by the leopard [124,125]. A final example is the case of Peromyscus beach mice. Mainland mice exhibit darker coats colors with the pigmentation extending more ventrally, which serves to conceal mice within similarly-colored soil. Likewise, beach mice match their surroundings through a lighter shade and color primarily on the back. This adaptation is mediated by changes in the level and pattern of Agouti expression within NCC derived cells, which alters the distribution and maturation of melanocytes [126], and a single amino acid change in Mc1r that decreases eumelanin biosynthesis [127].
4.7. Thermoregulation and photoprotection
The regulation of body temperature is a critical component of maintaining homeostasis. A distinctive feature of endotherms is the presence of turbinals, which are thin bony structures within the nasal cavity that develop from cranial NCCs [128]. Increases in the surface area of the respiratory turbinals, presumably through the modification of NCC development, allow an animal to regulate moisture exchange or increase evaporative surface to cool the body in a hotter environment. Amphibious mammals [129] and human populations that evolved in hot climates [130] both have increased nasal sinus areas for this purpose, though we do not yet know the molecular basis of this adaptation.
Other NCC derivatives such as pigment-synthesizing chromatophores and melanocytes can also regulate body temperature. For example, some lizards actively change their skin color in response to their thermal environment to regulate the amount of sunlight absorbed, with warm temperatures triggering lighter pigmentation [131]. Even the patterns that may serve roles in predator avoidance might also undergo selection due to thermoregulatory needs. The trademark black and white stripes of a zebra generate differences in air temperature based on their absorption or reflection of sunlight, respectively. These temperature changes create chaotic air motion that accelerates cooling of the animal [132]. The genetic mechanism underlying zebra stripes is unknown, but there may be clues from other mammals. Striping patterns may be regulated by SLC25A2, which generates stripes in tigers [133], or by TBX3, which controls eumelanin deposition in stripes of other equids [134].
Related to thermoregulation, radiation from the sun is another potent abiotic selective pressure, and many vertebrates shield themselves with photoprotective pigments synthesized in NCC derived melanocytes. An example comes from our own species. Humans have evolved skin color variation based on the amount of ultraviolet radiation present in certain geographic areas [135]. High deposition of eumelanin (i.e., darker skin) is beneficial in tropical and subtropical regions to protect from folate degradation. Pigment was subsequently lost as humans migrated to latitudes with lower ultraviolet levels where dark skin was less advantageous [136]. Notably, most of the genes currently associated with human skin color (OCA2, MC1R, TYR, ASIP, and SLC45A2) [137,138] are involved in the biosynthesis of eumelanin within melanocytes that differentiated from NCCs and not due to changes such as NCC migration or fate determination.
5. Coordinated changes
While adaptations like those discussed above can affect a single NCC derived trait, multiple NCC associated traits like craniofacial variation, pigmentation patterns, and neuronal circuitry often co-evolve (Fig. 1). An example from both artificial and natural selection, domestication and cavefish, respectively, are discussed below, but they are far from the only instances of coordinated changes in NCC derived traits. Eumelanin-based coloration is associated with aggression and head shape in a range of species including fishes, reptiles, and mammals [6,7] and see [7] for additional discussion. This trend also occurs in model organisms. Mutagenesis screens for eumelanin-based pigmentation in zebrafish identified mutants like puma (tuba8l3a), which also show altered development of other NCC derivatives including xanthophores, Schwann cells, peripheral nerves, and facial bones [139,140]. Together, these examples demonstrate that multifactorial changes in NCC development, such as changes in the timing, number, and regulation of NCCs, have the potential to produce sweeping changes in a range of NCC derived traits.
5.1. Domestication
A prime example of NCCs causing coordinated phenotypic changes is the domestication of animals, which is reviewed by others [3–5] and we summarize below. Domestication of dogs, sheep, and pigs is associated with a suite of changes, including tameness, loss of pigmentation, reduced tooth size, and shortened snouts [3]. This phenomenon has been coined domestication syndrome, and others [3–5] have proposed that it should be considered a neurocristopathy. This hypothesis posits that selection for docility resulted in reduced migration of all NCCs, in turn causing morphological changes observed in domestication [3–5]. Long-term selection experiments in various canine groups support this idea. For instance, when docility is selected for in foxes, NCC associated traits such as white fur patches and adrenal gland reductions also appear [141]. Further, genomic analyses between feral wolves and domesticated dogs show an enrichment of genetic variation related to NCC development, including members of the Fgf and Wnt signaling pathways. An interesting finding of this genomic study is the possible involvement of the collagen-binding protein SERPINH1 in reducing the number of NCCs migrating to the craniofacial region, leading to reduced cartilages and bones in the head [142]. Importantly, while these findings point to an integral role of NCCs, others have found little evidence of covariance of traits [143,144]. This contradiction highlights the need for more research into the molecular and genetic basis of domestication, and the need to understand the degree to which NCCs underlie this set of phenotypic changes.
5.2. Cavefish
Similar to domestication, we suggest that many adaptations in the cave-dwelling Mexican tetras (Astyanax mexicanus) [145–148] can be traced to changes in NCC development and represent a neurocristopathy. Astyanax fishes exist as two populations (a sighted surface morph and a blind cave morph) with distinct ecomorphological phenotypes. Compared to surface morphs, cavefish have evolved multiple traits associated with NCCs, including larger jaws, craniofacial changes associated with eye loss, increased numbers of maxillary teeth, variation in lateral line neuromast number and pattern, hypopigmentation, and decreased aggressiveness [147,148]. Morphological, genetic, and embryological data all suggest these changes may be coordinated and have a cellular origin in NCCs.
First, several morphological adaptations that co-vary in hybrids between cave and surface morphs have developmental origins in NCCs. Eye size, for instance, shows negative correlations with multiple pigment phenotypes and tooth number, while pigmentation levels co-vary with the length of craniofacial bones and the number of taste buds [149]. Second, multiple traits map to the same genomic intervals. Traits including lens size, taste bud number, and oral jaw size are genetically linked [149], and a separate multi-trait locus influences phenotypic variation in jaw morphology and pigmentation [145]. Whether these traits are controlled through a single gene with pleiotropic effects or through “hotspot” clusters, this genetic architecture generates co-inheritance and correlated evolution [145,150]. Genetic linkage provides a potential mechanism through which NCC derived traits descending from multiple NCC progenitor populations can co-evolve. Finally, and perhaps most convincingly, experimental embryology has demonstrated co-variation of these traits and have directly connected them to NCCs. Cavefish NCCs have intrinsic differences in migration compared to surface fish [150], and NCCs from cavefish are unable to differentiate into some pigment cell types [151]. Transplantation of NCCs from surface to cave morphs demonstrate an autonomous effect on both eye size and pigmentation levels [150], providing direct evidence that NCCs link these distinct traits. In total, these data demonstrate that evolution of multiple traits in cavefish can be traced back to variation in NCCs and their derivatives, and demonstrate the capacity of NCCs to produce suites of phenotypic variation.
6. Conclusions and perspectives
Overall, we highlight NCCs as a central source of microevolutionary diversity in morphology, physiology, and behavior. Viewed through the perspective of traits undergoing natural and sexual selection, we suggest that many if not most vertebrates have phenotypic diversification due to modification of NCC development that directly affects their fitness. This embryonic cell population has a remarkable ability to influence traits as varied as feeding, predation, and mate choice, providing a rich source of evolutionary diversity within vertebrates. While we have extended discussions initiated by others [3–8] to give a more comprehensive analysis of the range of traits with a common developmental origin in NCCs, this is far from exhaustive. Below we suggest several avenues to strengthen our understanding of NCCs and phenotypic diversification.
From the trait perspective, we have a lack of empirical examples that directly link molecular genetics of NCCs to behavioral or neurological traits. This contrasts sharply with the number of details known for trophic adaptation or pigment patterns. We argue that this is not because the connection is rare, but due to challenges quantifying these attributes and a general delay in the study of neurobehavioral work relative to morphological traits in fields such as Evolutionary Developmental Biology [152,153]. To this end, we have included as many theoretical and experimental examples involving phenotypic variation arising from NCCs in the nervous and sensory systems as possible, but acknowledge that there is still much to be learned in these areas. For instance, we still have an incomplete understanding of how variation in NCC migration influences the microevolution of sensory organs or examples of molecular variation that generate adaptation in the adrenal medulla. While the sympathetic nervous system and stress response can directly affect fecundity [154,155], metabolism [156,157], and immune system function [156], it is currently unclear how or if changes in NCC development generate natural variation in these characteristics.
From the cellular perspective, several NCC cell types (Table 1) have been understudied, leaving our view of neurocristopathies in nature incomplete. We highlight Schwann cells and enteric nerves as important examples. First, NCC derived Schwann cells generate myelination of axons, a critical adaptation that accelerated nerve conduction. Variation in the degree and timing of myelination could directly affect fitness through more rapid flight-or-fight responses when faced with life-threatening situations, more complex predatory behaviors such as stalking and chasing prey, or even neural plasticity and learning [158]. Second, among other effects, activity of the enteric nervous system can affect the composition of the gut microbiome [159,160]. Through this, variation in the enteric nervous system could have indirect effects on trophic specification [161], immune function [162], and social behavior [163,164]. While these would be critical for an animal’s fitness, the specific impact of NCCs remains unclear. It is notable, however, that cavefish have altered motility in the gastrointestinal tract, which is hypothesized to be due to changes in the activity or patterning of enteric nerves [165], as well as an altered metabolism [166] that could be indirectly affected. In all, the involvement of NCCs in microevolution of such behavioral and neurological traits remains underexplored and would yield useful insights into the role of NCCs in microevolution.
Finally, we still do not fully understand the evolutionary dynamics that shape NCCs and traits that derive from these cells. As described above, many structures derived from NCCs are selected on by multiple pressures, which may push the phenotype to the same optimum or not. For example, bones of the face impact an animal’s feeding, intrasexual competition, and mate choice, yet we do not have a clear picture of the relative impact of these pressures. Further, the evolutionary order of these selective forces may limit the total phenotypic range possible. For instance, if sexual selection dominated early in evolution, this history may constrain development or change the evolutionary trajectory away from potentially optimal phenotypes for feeding. Additionally, much is to be learned about the plastic and evolutionary potential of NCCs, particularly in comparison to other cell types. Notably, as invertebrates, insects lack NCCs but have evolved many of the same traits that derive from NCCs in vertebrates. Those include sexually-selected pigmentation patterns [167], innervation of the gut [168], social behaviors [167], and even covariation of both pigmentation and sexual behavior due to mutation of a single gene [169]. We suggest it will be beneficial to address whether vertebrates and NCCs may have increased evolutionary potential (i.e. are more evolvable) compared to other cellular lineages. While this would be experimentally challenging, this could include quantifying modularity within NCC regulatory networks or measuring the multipotency of NCCs and NCC derivatives compared to other cell types. NCC derived traits could be assessed in terms of the degree of disparity or plastic responses to environmental changes compared to those derived from non-NCCs. Of particular interest would be to assess if the molecular or genetic basis of this microevolutionary potential of NCCs is regulated by similar factors and mechanisms governing the macroevolutionary origins of this vertebrate innovation. Additional work in these areas will supplement our understanding of how all lineages of NCC derivatives contribute to phenotypic diversification under natural and sexual selection.
Acknowledgements
We thank past and current colleagues for discussions that influenced these ideas. We thank anonymous reviewers for comments and perspectives that significantly improved this work. This work was supported by NSF CAREER #1942178 (KEP), NIH P20GM121342 (KEP), and NIH R15DE029945 (KEP).
Abbreviations:
- NCC
neural crest cell
Footnotes
Competing Interest
The authors do not have any conflicts of interest to declare.
References
- [1].Hall BK, The neural crest as a fourth germ layer and vertebrates as quadroblastic not triploblastic, Evol. Dev. 2 (2000) 3–5, 10.1046/j.1525-142x.2000.00032.x. [DOI] [PubMed] [Google Scholar]
- [2].Bolande R, The neurocristopathies a unifying concept of disease arising in neural crest maldevelopment, Hum. Pathol. 5 (1974) 409–429, 10.1016/S0046-8177(74)80021-3. [DOI] [PubMed] [Google Scholar]
- [3].Wilkins AS, Wrangham RW, Fitch WT, The “domestication syndrome” in mammals: a unified explanation based on neural crest cell behavior and genetics, Genetics 197 (2014) 795–808, 10.1534/genetics.114.165423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sánchez-Villagra MR, Geiger M, Schneider RA, The taming of the neural crest: a developmental perspective on the origins of morphological covariation in domesticated mammals, R. Soc. Open Sci. 3 (2016), 160107, 10.1098/rsos.160107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wilkins AS, A striking example of developmental bias in an evolutionary process: the “domestication syndrome, Evol. Dev. 22 (2020) 143–153, 10.1111/ede.12319. [DOI] [PubMed] [Google Scholar]
- [6].San-Jose LM, Roulin A, Toward understanding the repeated occurrence of associations between melanin-based coloration and multiple phenotypes, Am. Nat. 192 (2018) 111–130, 10.1086/698010. [DOI] [PubMed] [Google Scholar]
- [7].San-Jose LM, Roulin A, On the potential role of the neural crest cells in integrating pigmentation into behavioral and physiological syndromes, Front. Ecol. Evol. 8 (2020), 10.3389/fevo.2020.00278. [DOI] [Google Scholar]
- [8].Donoghue PCJ, Graham A, Kelsh RN, The origin and evolution of the neural crest, BioEssays 30 (2008) 530–541, 10.1002/bies.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rogers CD, Jayasena CS, Nie S, Bronner ME, Neural crest specification: tissues, signals, and transcription factors, Wiley Interdiscip. Rev. Dev. Biol. 1 (2012) 52–68, 10.1002/wdev.8. [DOI] [PubMed] [Google Scholar]
- [10].Bae C-J, Saint-Jeannet J-P, Induction and specification of neural crest cells, in: Neural Crest Cells, Elsevier, 2014, pp. 27–49, 10.1016/B978-0-12-401730-6.00002-8. [DOI] [Google Scholar]
- [11].Szabó A, Mayor R, Mechanisms of neural crest migration, Annu. Rev. Genet. 52 (2018) 43–63, 10.1146/annurev-genet-120417-031559. [DOI] [PubMed] [Google Scholar]
- [12].Theveneau E, Mayor R, Neural crest cell migration. Neural Crest Cells, Elsevier,, 2014, pp. 73–88, 10.1016/B978-0-12-401730-6.00004-1. [DOI] [Google Scholar]
- [13].Newbern JM, Mol. Control Neural crest Peripher. Nerv. Syst. Dev. (2015) 201–231, 10.1016/bs.ctdb.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Prendergast A, Raible DW, Neural crest cells and peripheral nervous system development. Neural Crest Cells, Elsevier,, 2014, pp. 255–286, 10.1016/B978-0-12-401730-6.00014-4. [DOI] [Google Scholar]
- [15].Lake JI, Heuckeroth RO, Enteric nervous system development: migration, differentiation, and disease, Am. J. Physiol. Liver Physiol. 305 (2013) G1–G24, 10.1152/ajpgi.00452.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Barlow AJ, Neural crest cells in enteric nervous system development and disease. Neural Crest Cells, Elsevier,, 2014, pp. 231–253, 10.1016/B978-0-12-401730-6.00013-2. [DOI] [Google Scholar]
- [17].Huber K, Kalcheim C, Unsicker K, The development of the chromaffin cell lineage from the neural crest, Auton. Neurosci. 151 (2009) 10–16, 10.1016/j.autneu.2009.07.020. [DOI] [PubMed] [Google Scholar]
- [18].Mitsiadis TA, Graf D, Cell fate determination during tooth development and regeneration, Birth Defects Res. Part C. Embryo Today Rev. 87 (2009) 199–211, 10.1002/bdrc.20160. [DOI] [PubMed] [Google Scholar]
- [19].Schilling TF, Le Pabic P, Neural crest cells in craniofacial skeletal development. Neural Crest Cells, Elsevier,, 2014, pp. 127–151, 10.1016/B978-0-12-401730-6.00008-9. [DOI] [Google Scholar]
- [20].Santagati F, Rijli FM, Cranial neural crest and the building of the vertebrate head, Nat. Rev. Neurosci. 4 (2003) 806–818, 10.1038/nrn1221. [DOI] [PubMed] [Google Scholar]
- [21].Sommer L, Generation of melanocytes from neural crest cells, Pigment Cell Melanoma Res 24 (2011) 411–421, 10.1111/j.1755-148X.2011.00834.x. [DOI] [PubMed] [Google Scholar]
- [22].Lapedriza A, Petratou K, Kelsh RN, Neural crest cells and pigmentation. Neural Crest Cells, Elsevier,, 2014, pp. 287–311, 10.1016/B978-0-12-401730-6.00015-6. [DOI] [Google Scholar]
- [23].Steventon B, Mayor R, Streit A, Neural crest and placode interaction during the development of the cranial sensory system, Dev. Biol. 389 (2014) 28–38, 10.1016/j.ydbio.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fleenor SJ, Begbie J, Neural crest cell and placode interactions in cranial PNS development. Neural Crest Cells, Elsevier, 2014, pp. 153–165, 10.1016/B978-0-12-401730-6.00009-0. [DOI] [Google Scholar]
- [25].Hinaux H, Devos L, Blin M, Elipot Y, Bibliowicz J, Alié A, Rétaux S, Sensory evolution in blind cavefish is driven by early embryonic events during gastrulation and neurulation, Development 143 (2016) 4521–4532, 10.1242/dev.141291. [DOI] [PubMed] [Google Scholar]
- [26].Aoki Y, Saint-Germain N, Gyda M, Magner-Fink E, Lee Y-H, Credidio C, Saint-Jeannet JP, Sox10 regulates the development of neural crest-derived melanocytes in Xenopus, Dev. Biol. 259 (2003) 19–33, 10.1016/S0012-1606(03)00161-1. [DOI] [PubMed] [Google Scholar]
- [27].Lai X, Liu J, Zou Z, Wang Y, Wang Y, Liu X, Huang W, Ma Y, Chen Q, Li F, Wu G, Li W, Wang W, Yuan Y, Jiang B, SOX10 ablation severely impairs the generation of postmigratory neural crest from human pluripotent stem cells, Cell Death Dis. 12 (2021) 814, 10.1038/s41419-021-04099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Levy C, Khaled M, Fisher DE, MITF: master regulator of melanocyte development and melanoma oncogene, Trends Mol. Med. 12 (2006) 406–414, 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- [29].Baranowska Körberg I, Sundström E, Meadows JRS, Rosengren Pielberg G, Gustafson U, Hedhammar Å, Karlsson EK, Seddon J, Söderberg A, Vilà C, Zhang X, Åkesson M, Lindblad-Toh K, Andersson G, Andersson L, A simple repeat polymorphism in the MITF-M promoter is a key regulator of white spotting in dogs, PLoS One 9 (2014), e104363, 10.1371/journal.pone.0104363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jackson I, Homologous pigmentation mutations in human, mouse and other model organisms, Hum. Mol. Genet 6 (1997) 1613–1624, 10.1093/hmg/6.10.1613. [DOI] [PubMed] [Google Scholar]
- [31].Northcutt RG, Gans C, The genesis of neural crest and epidermal placodes: a reinterpretation of vertebrate origins, Q. Rev. Biol. 58 (1983) 1–28, 10.1086/413055. [DOI] [PubMed] [Google Scholar]
- [32].Munoz WA, Trainor PA, Neural Crest Cell Evol. (2015) 3–26, 10.1016/bs.ctdb.2014.11.001. [DOI] [PubMed] [Google Scholar]
- [33].York JR, McCauley DW, The origin and evolution of vertebrate neural crest cells, Open Biol. 10 (2020), 190285, 10.1098/rsob.190285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Martik ML, Bronner ME, Riding the crest to get a head: neural crest evolution in vertebrates, Nat. Rev. Neurosci. 22 (2021) 616–626, 10.1038/s41583-021-00503-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Green SA, Bronner ME, Gene duplications and the early evolution of neural crest development, Semin. Cell Dev. Biol. 24 (2013) 95–100, 10.1016/j.semcdb.2012.12.006. [DOI] [PubMed] [Google Scholar]
- [36].Hall BK, Gillis JA, Incremental evolution of the neural crest, neural crest cells and neural crest-derived skeletal tissues, J. Anat. 222 (2013) 19–31, 10.1111/j.1469-7580.2012.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Le Douarin NM, Dupin E, The neural crest, a fourth germ layer of the vertebrate embryo. Neural Crest Cells, Elsevier, 2014, pp. 3–26, 10.1016/B978-0-12-401730-6.00001-6. [DOI] [Google Scholar]
- [38].Rebeiz M, Tsiantis M, Enhancer evolution and the origins of morphological novelty, Curr. Opin. Genet. Dev. 45 (2017) 115–123, 10.1016/j.gde.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fish JL, Evolvability of the vertebrate craniofacial skeleton, Semin. Cell Dev. Biol. 91 (2019) 13–22, 10.1016/j.semcdb.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Schneider RA, Neural crest and the origin of species-specific pattern, Genesis 56 (2018), e23219, 10.1002/dvg.23219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dupin E, Calloni GW, Coelho-Aguiar JM, Le Douarin NM, The issue of the multipotency of the neural crest cells, Dev. Biol. 444 (2018) S47–S59, 10.1016/j.ydbio.2018.03.024. [DOI] [PubMed] [Google Scholar]
- [42].Furlan A, Adameyko I, Schwann cell precursor: a neural crest cell in disguise? Dev. Biol. 444 (2018) S25–S35, 10.1016/j.ydbio.2018.02.008. [DOI] [PubMed] [Google Scholar]
- [43].Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A, An Z, Wang L, Hultman I, Ährlund-Richter L, Blom H, Brismar H, Lopes NA, Pachnis V, Suter U, Clevers H, Thesleff I, Sharpe P, Ernfors P, Fried K, Adameyko I, Glial origin of mesenchymal stem cells in a tooth model system, Nature 513 (2014) 551–554, 10.1038/nature13536. [DOI] [PubMed] [Google Scholar]
- [44].Trainor P, Krumlauf R, Plasticity in mouse neural crest cells reveals a new patterning role for cranial mesoderm, Nat. Cell Biol. 2 (2000) 96–102, 10.1038/35000051. [DOI] [PubMed] [Google Scholar]
- [45].Trainor PA, Ariza-McNaughton L, Krumlauf R, Role of the isthmus and FGFs in resolving the paradox of neural crest plasticity and prepatterning, Sci. (80-. ). 295 (2002) 1288–1291, 10.1126/science.1064540. [DOI] [PubMed] [Google Scholar]
- [46].Eames BF, Schneider RA, Quail-duck chimeras reveal spatiotemporal plasticity in molecular and histogenic programs of cranial feather development, Development 132 (2005) 1499–1509, 10.1242/dev.01719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kuehner JN, Bruggeman EC, Wen Z, Yao B, Epigenetic regulations in neuropsychiatric disorders, Front. Genet. 10 (2019), 10.3389/fgene.2019.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sandell LL, Trainor PA, Neural crest cell plasticity, in: Neural Crest Induction Differ., Springer; US, Boston, MA, n.d.: pp. 78–95. 10.1007/978-0-387-46954-6_5. [DOI] [Google Scholar]
- [49].Le Douarin NM, Creuzet S, Couly G, Dupin E, Neural crest cell plasticity and its limits, Development 131 (2004) 4637–4650, 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- [50].Fish JL, Sklar RS, Woronowicz KC, Schneider RA, Multiple developmental mechanisms regulate species-specific jaw size, Development 141 (2014) 674–684, 10.1242/dev.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wu P, Jiang T-X, Suksaweang S, Widelitz RB, Chuong C-M, Molecular shaping of the beak, Sci. (80-. ). 305 (2004) 1465–1466, 10.1126/science.1098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Abzhanov A, Protas M, Grant BR, Grant PR, Tabin CJ, Bmp4 and morphological variation of beaks in Darwin’s finches, Science 305 (2004) 1462–1465, 10.1126/science.1098095. [DOI] [PubMed] [Google Scholar]
- [53].Brugmann SA, Powder KE, Young NM, Goodnough LH, Hahn SM, James AU, Helms JA, Lovett M, Comparative gene expression analysis of avian embryonic facial structures reveals new candidates for human craniofacial disorders, Hum. Mol. Genet. 19 (2010) 920–930, 10.1093/hmg/ddp559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mallarino R, Grant PR, Grant BR, Herrel A, Kuo WP, Abzhanov A, Two developmental modules establish 3D beak-shape variation in Darwin’s finches, Proc. Natl. Acad. Sci. 108 (2011) 4057–4062, 10.1073/pnas.1011480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Abzhanov A, Kuo WP, Hartmann C, Grant BR, Grant PR, Tabin CJ, The calmodulin pathway and evolution of elongated beak morphology in Darwin’s finches, Nature 442 (2006) 563–567, 10.1038/nature04843. [DOI] [PubMed] [Google Scholar]
- [56].Hall J, Jheon AH, Ealba EL, Eames BF, Butcher KD, Mak S-S, Ladher R, Alliston T, Schneider RA, Evolution of a developmental mechanism: species-specific regulation of the cell cycle and the timing of events during craniofacial osteogenesis, Dev. Biol. 385 (2014) 380–395, 10.1016/j.ydbio.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ealba EL, Jheon AH, Hall J, Curantz C, Butcher KD, Schneider RA, Neural crest-mediated bone resorption is a determinant of species-specific jaw length, Dev. Biol. 408 (2015) 151–163, 10.1016/j.ydbio.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Powder KE, Cousin H, McLinden GP, Craig Albertson R, A nonsynonymous mutation in the transcriptional regulator lbh is associated with cichlid craniofacial adaptation and neural crest cell development, Mol. Biol. Evol. 31 (2014) 3113–3124, 10.1093/molbev/msu267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Roberts RB, Hu Y, Albertson RC, Kocher TD, Craniofacial divergence and ongoing adaptation via the hedgehog pathway, Proc. Natl. Acad. Sci. 108 (2011) 13194–13199, 10.1073/pnas.1018456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Albertson RC, Streelman JT, Kocher TD, Yelick PC, From the cover: integration and evolution of the cichlid mandible: the molecular basis of alternate feeding strategies, Proc. Natl. Acad. Sci. 102 (2005) 16287–16292, 10.1073/pnas.0506649102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Parsons KJ, Trent Taylor A, Powder KE, Albertson RC, Wnt signalling underlies the evolution of new phenotypes and craniofacial variability in Lake Malawi cichlids, Nat. Commun. 5 (2014) 3629, 10.1038/ncomms4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Parsons KJ, Concannon M, Navon D, Wang J, Ea I, Groveas K, Campbell C, Albertson RC, Foraging environment determines the genetic architecture and evolutionary potential of trophic morphology in cichlid fishes, Mol. Ecol. 25 (2016) 6012–6023, 10.1111/mec.13801. [DOI] [PubMed] [Google Scholar]
- [63].Constantino PJ, Bush MB, Barani A, Lawn BR, On the evolutionary advantage of multi-cusped teeth, J. R. Soc. Interface 13 (2016), 20160374, 10.1098/rsif.2016.0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Jernvall J, Thesleff I, Tooth shape formation and tooth renewal: evolving with the same signals, Development 139 (2012) 3487–3497, 10.1242/dev.085084. [DOI] [PubMed] [Google Scholar]
- [65].Malik Z, Roth DM, Eaton F, Theodor JM, Graf D, Mesenchymal Bmp7 controls onset of tooth mineralization: a novel way to regulate molar cusp shape, Front. Physiol. 11 (2020), 10.3389/fphys.2020.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zurowski C, Jamniczky H, Graf D, Theodor J, Deletion/loss of bone morphogenetic protein 7 changes tooth morphology and function in Mus musculus: implications for dental evolution in mammals, R. Soc. Open Sci. 5 (2018), 170761, 10.1098/rsos.170761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Li L, Lin M, Wang Y, Cserjesi P, Chen Z, Chen Y, BmprIa is required in mesenchymal tissue and has limited redundant function with BmprIb in tooth and palate development, Dev. Biol. 349 (2011) 451–461, 10.1016/j.ydbio.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Streelman JT, Webb JF, Albertson RC, Kocher TD, The cusp of evolution and development: a model of cichlid tooth shape diversity, Evol. Dev. 5 (2003) 600–608, 10.1046/j.1525-142X.2003.03065.x. [DOI] [PubMed] [Google Scholar]
- [69].Fraser GJ, Bloomquist RF, Streelman JT, Common developmental pathways link tooth shape to regeneration, Dev. Biol. 377 (2013) 399–414, 10.1016/j.ydbio.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Cabrera D, Stankowich T, Stabbing Slinkers: tusk evolution among Artiodactyls, J. Mamm. Evol. 27 (2020) 265–272, 10.1007/s10914-018-9453-x. [DOI] [Google Scholar]
- [71].Emlen DJ, The evolution of animal weapons, Annu. Rev. Ecol. Evol. Syst. 39 (2008) 387–413, 10.1146/annurev.ecolsys.39.110707.173502. [DOI] [Google Scholar]
- [72].Wang Y, Zhang C, Wang N, Li Z, Heller R, Liu R, Zhao Y, Han J, Pan X, Zheng Z, Dai X, Chen C, Dou M, Peng S, Chen X, Liu J, Li M, Wang K, Liu C, Lin Z, Chen L, Hao F, Zhu W, Song C, Zhao C, Zheng C, Wang J, Hu S, Li C, Yang H, Jiang L, Li G, Liu M, Sonstegard TS, Zhang G, Jiang Y, Wang W, Qiu Q, Genetic basis of ruminant headgear and rapid antler regeneration, Science 364 (2019), 10.1126/science.aav6335. [DOI] [PubMed] [Google Scholar]
- [73].Sire J-Y, Teeth outside the mouth in teleost fishes: how to benefit from a developmental accident, Evol. Dev. 3 (2001) 104–108, 10.1046/j.1525-142x.2001.003002104.x. [DOI] [PubMed] [Google Scholar]
- [74].Butler JM, Maruska KP, The mechanosensory lateral line is used to assess opponents and mediate aggressive behaviors during territorial interactions in an African cichlid fish, J. Exp. Biol. 218 (2015) 3284–3294, 10.1242/jeb.125948. [DOI] [PubMed] [Google Scholar]
- [75].Huyghe K, Vanhooydonck B, Scheers H, Molina-Borja M, Van Damme R, Morphology, performance and fighting capacity in male lizards, Gallotia galloti, Funct. Ecol. 19 (2005) 800–807, 10.1111/j.1365-2435.2005.01038.x. [DOI] [Google Scholar]
- [76].Lappin AK, Hamilton PS, Sullivan BK, Bite-force performance and head shape in a sexually dimorphic crevice-dwelling lizard, the common chuckwalla [Sauromalus ater (= obesus)], Biol, J. Linn. Soc. 88 (2006) 215–222, 10.1111/j.1095-8312.2006.00615.x. [DOI] [Google Scholar]
- [77].Veiga JP, Badge size, phenotypic quality, and reproductive success in the house sparrow: a study on honest advertisement, Evolution 47 (1993) 1161–1170, 10.1111/j.1558-5646.1993.tb02143.x. [DOI] [PubMed] [Google Scholar]
- [78].Kim S-Y, Velando A, Stickleback males increase red coloration and courtship behaviours in the presence of a competitive rival, Ethology 120 (2014) 502–510, 10.1111/eth.12224. [DOI] [Google Scholar]
- [79].Morina DL, Demarais S, Strickland BK, Larson JE, While males fight, females choose: male phenotypic quality informs female mate choice in mammals, Anim. Behav. 138 (2018) 69–74, 10.1016/j.anbehav.2018.02.004. [DOI] [Google Scholar]
- [80].Lecaudey LA, Sturmbauer C, Singh P, Ahi EP, Molecular mechanisms underlying nuchal hump formation in dolphin cichlid, Cyrtocara moorii, Sci. Rep. 9 (2019) 20296, 10.1038/s41598-019-56771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Takahashi T, Function of nuchal humps of a cichlid fish from Lake Tanganyika: inferences from morphological data, Ichthyol. Res. 65 (2018) 316–323, 10.1007/s10228-018-0614-y. [DOI] [Google Scholar]
- [82].Oxvig C, The role of PAPP-A in the IGF system: location, location, location, J. Cell Commun. Signal. 9 (2015) 177–187, 10.1007/s12079-015-0259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Li Y, Xiang J, Duan C, Insulin-like growth factor-binding protein-3 plays an important role in regulating pharyngeal skeleton and inner ear formation and differentiation, J. Biol. Chem. 280 (2005) 3613–3620, 10.1074/jbc.M411479200. [DOI] [PubMed] [Google Scholar]
- [84].Onuma TA, Ding Y, Abraham E, Zohar Y, Ando H, Duan C, Regulation of temporal and spatial organization of newborn GnRH neurons by IGF signaling in Zebrafish, J. Neurosci. 31 (2011) 11814–11824, 10.1523/JNEUROSCI.6804-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Derryberry EP, Seddon N, Claramunt S, Tobias JA, Baker A, Aleixo A, Brumfield RT, Correlated evolution of beak morphology and song in the neotropical woodcreeper radiation, Evolution 66 (2012) 2784–2797, 10.1111/j.1558-5646.2012.01642.x. [DOI] [PubMed] [Google Scholar]
- [86].Huber SK, Podos J, Beak morphology and song features covary in a population of Darwin’s finches (Geospiza fortis), Biol. J. Linn. Soc. 88 (2006) 489–498, 10.1111/j.1095-8312.2006.00638.x. [DOI] [Google Scholar]
- [87].Setchell JM, Do female mandrills prefer brightly colored males? Int. J. Primatol. 26 (2005) 715–735, 10.1007/s10764-005-5305-7. [DOI] [Google Scholar]
- [88].Badyaev A, Evolution of sexual dichromatism: contribution of carotenoid-versus melanin-based coloration, Biol. J. Linn. Soc. 69 (2000) 153–172, 10.1006/bijl.1999.0350. [DOI] [Google Scholar]
- [89].Maan ME, Seehausen O, Soderberg L, Johnson L, Ripmeester EAP, Mrosso HD¨J, Taylor MI, van Dooren TJM, van Alphen JJM, Intraspecific sexual selection on a speciation trait, male coloration, in the Lake Victoria cichlid Pundamilia nyererei, Proc. R. Soc. Lond. Ser. B Biol. Sci. 271 (2004) 2445–2452, 10.1098/rspb.2004.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Albertson RC, Powder KE, Hu Y, Coyle KP, Roberts RB, Parsons KJ, Genetic basis of continuous variation in the levels and modular inheritance of pigmentation in cichlid fishes, Mol. Ecol. 23 (2014) 5135–5150, 10.1111/mec.12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Yong L, Peichel CL, McKinnon JS, Genetic architecture of conspicuous red ornaments in female Threespine stickleback, G3 Genes Genomes Genet. 6 (2016) 579–588, 10.1534/g3.115.024505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Salzburger W, Braasch I, Meyer A, Adaptive sequence evolution in a color gene involved in the formation of the characteristic egg-dummies of male haplochromine cichlid fishes, BMC Biol. 5 (2007) 51, 10.1186/1741-7007-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Diepeveen ET, Salzburger W, Molecular characterization of two endothelin pathways in East African cichlid fishes, J. Mol. Evol. 73 (2011) 355–368, 10.1007/s00239-012-9483-6. [DOI] [PubMed] [Google Scholar]
- [94].Lee H-O, Levorse JM, Shin MK, The endothelin receptor-B is required for the migration of neural crest-derived melanocyte and enteric neuron precursors, Dev. Biol. 259 (2003) 162–175, 10.1016/S0012-1606(03)00160-X. [DOI] [PubMed] [Google Scholar]
- [95].Quigley IK, Manuel JL, Roberts RA, Nuckels RJ, Herrington ER, MacDonald EL, Parichy DM, Evolutionary diversification of pigment pattern in Danio fishes:differential fms dependence and stripe loss in D. albolineatus, Development 132 (2005) 89–104, 10.1242/dev.01547. [DOI] [PubMed] [Google Scholar]
- [96].Parichy DM, Mellgren EM, Rawls JF, Lopes SS, Kelsh RN, Johnson SL, Mutational analysis of endothelin receptor b1 (rose) during neural crest and pigment pattern development in the zebrafish Danio rerio, Dev. Biol. 227 (2000) 294–306, 10.1006/dbio.2000.9899. [DOI] [PubMed] [Google Scholar]
- [97].Porges SW, The polyvagal theory: phylogenetic substrates of a social nervous system, Int. J. Psychophysiol. 42 (2001) 123–146, 10.1016/S0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- [98].Carbone E, Borges R, Eiden LE, García AG, Hernández-Cruz A, Chromaffin cells of the adrenal medulla: physiology, pharmacology, and disease. Compr. Physiol, Wiley, 2019, pp. 1443–1502, 10.1002/cphy.c190003. [DOI] [PubMed] [Google Scholar]
- [99].Helmy M, Zhang J, Wang H, Neurobiol. Neural Circuits Aggress. (2020) 9–22, 10.1007/978-981-15-7086-5_2. [DOI] [PubMed] [Google Scholar]
- [100].Haller J, Makara G, Kruk M, Catecholaminergic involvement in the control of aggression: hormones, the peripheral sympathetic, and central noradrenergic systems, Neurosci. Biobehav. Rev. 22 (1997) 85–97, 10.1016/S0149-7634(97)00023-7. [DOI] [PubMed] [Google Scholar]
- [101].Korzan WJ, Summers TR, Ronan PJ, Summers CH, Visible sympathetic activity as a social signal in Anolis carolinensis: changes in aggression and plasma catecholamines, Horm. Behav. 38 (2000) 193–199, 10.1006/hbeh.2000.1619. [DOI] [PubMed] [Google Scholar]
- [102].Sørensen DB, Johnsen PF, Bibby BM, Bottner A, Bornstein SR, Eisenhofer G, Pacak K, Hansen AK, PNMT transgenic mice have an aggressive phenotype, Horm. Metab. Res. 37 (2005) 159–163, 10.1055/s-2005-861301. [DOI] [PubMed] [Google Scholar]
- [103].Volavka J, Bilder R, Nolan K, Catecholamines and aggression: the role of COMT and MAO polymorphisms, Ann. N. Y. Acad. Sci. 1036 (2006) 393–398, 10.1196/annals.1330.023. [DOI] [PubMed] [Google Scholar]
- [104].Reiprich S, Stolt CC, Schreiner S, Parlato R, Wegner M, SoxE proteins are differentially required in mouse adrenal gland development, Mol. Biol. Cell. 19 (2008) 1575–1586, 10.1091/mbc.e07-08-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Webb JF, Bird NC, Carter L, Dickson J, Comparative development and evolution of two lateral line phenotypes in lake malawi cichlids, J. Morphol. 275 (2014) 678–692, 10.1002/jmor.20247. [DOI] [PubMed] [Google Scholar]
- [106].Wark AR, Peichel CL, Lateral line diversity among ecologically divergent threespine stickleback populations, J. Exp. Biol. 213 (2010) 108–117, 10.1242/jeb.031625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Westley PAH, Berdahl AM, Torney CJ, Biro D, Collective movement in ecology: from emerging technologies to conservation and management, Philos. Trans. R. Soc. B Biol. Sci. 373 (2018), 20170004, 10.1098/rstb.2017.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Philip S, Machado JP, Maldonado E, Vasconcelos V, O’Brien SJ, Johnson WE, Antunes A, Fish lateral line innovation: insights into the evolutionary genomic dynamics of a unique mechanosensory organ, Mol. Biol. Evol. 29 (2012) 3887–3898, 10.1093/molbev/mss194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kowalko JE, Rohner N, Rompani SB, Peterson BK, Linden TA, Yoshizawa M, Kay EH, Weber J, Hoekstra HE, Jeffery WR, Borowsky R, Tabin CJ, Loss of schooling behavior in cavefish through sight-dependent and sight-independent mechanisms, Curr. Biol. 23 (2013) 1874–1883, 10.1016/j.cub.2013.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Greenwood AK, Wark AR, Yoshida K, Peichel CL, Genetic and neural modularity underlie the evolution of schooling behavior in threespine sticklebacks, Curr. Biol. 23 (2013) 1884–1888, 10.1016/j.cub.2013.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Bilandžija H, Ma L, Parkhurst A, Jeffery WR, A potential benefit of albinism in Astyanax cavefish: downregulation of the oca2 gene increases tyrosine and catecholamine levels as an alternative to melanin synthesis, PLoS One 8 (2013), e80823, 10.1371/journal.pone.0080823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Hinaux H, Rétaux S, Elipot Y, Social behavior and aggressiveness in Astyanax, in: Biol. Evol. Mex. Cavefish, Elsevier, 2016, pp. 335–359, 10.1016/B978-0-12-802148-4.00017-7. [DOI] [Google Scholar]
- [113].Mayhew Seehausen JJM Van Alphen, Evolution of colour patterns in East African cichlid fish, J. Evol. Biol. 12 (1999) 514–534, 10.1046/j.1420-9101.1999.00055.x. [DOI] [Google Scholar]
- [114].Lewis VM, Saunders LM, Larson TA, Bain EJ, Sturiale SL, Gur D, Chowdhury S, Flynn JD, Allen MC, Deheyn DD, Lee JC, Simon JA, Lippincott-Schwartz J, Raible DW, Parichy DM, Fate plasticity and reprogramming in genetically distinct populations of Danio leucophores, Proc. Natl. Acad. Sci. (2019) 201901021. 10.1073/pnas.1901021116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Rosenthal GG, Ryan MJ, Assortative preferences for stripes in danios, Anim. Behav. 70 (2005) 1063–1066, 10.1016/j.anbehav.2005.02.005. [DOI] [Google Scholar]
- [116].Negro JJ, Doña J, Blázquez MC, Rodríguez A, Herbert-Read JE, de L M. Brooke, Contrasting stripes are a widespread feature of group living in birds, mammals and fishes, 20202021, Proc. R. Soc. B Biol. Sci. 287 (2020), 10.1098/rspb.2020.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Kelsh RN, Harris ML, Colanesi S, Erickson CA, Stripes and belly-spots—a review of pigment cell morphogenesis in vertebrates, Semin. Cell Dev. Biol. 20 (2009) 90–104, 10.1016/j.semcdb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Kratochwil CF, Liang Y, Gerwin J, Woltering JM, Urban S, Henning F, Machado-Schiaffino G, Hulsey CD, Meyer A, Agouti-related peptide 2 facilitates convergent evolution of stripe patterns across cichlid fish radiations, Sci. (80-. ). 362 (2018) 457–460, 10.1126/science.aao6809. [DOI] [PubMed] [Google Scholar]
- [119].Watanabe M, Iwashita M, Ishii M, Kurachi Y, Kawakami A, Kondo S, Okada N, Spot pattern of leopard Danio is caused by mutation in the zebrafish connexin41.8 gene, EMBO Rep. 7 (2006) 893–897, 10.1038/sj.embor.7400757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Steiner CC, Rompler H, Boettger LM, Schoneberg T, Hoekstra HE, The genetic basis of phenotypic convergence in beach mice: similar pigment patterns but different genes, Mol. Biol. Evol. 26 (2008) 35–45, 10.1093/molbev/msn218. [DOI] [PubMed] [Google Scholar]
- [121].Nokelainen O, Brito JC, Scott-Samuel NE, Valkonen JK, Boratyński Z, Camouflage accuracy in Sahara–Sahel desert rodents, J. Anim. Ecol. 89 (2020) 1658–1669, 10.1111/1365-2656.13225. [DOI] [PubMed] [Google Scholar]
- [122].Vrieling H, Duhl DM, Millar SE, Miller KA, Barsh GS, Differences in dorsal and ventral pigmentation result from regional expression of the mouse agouti gene. Proc. Natl. Acad. Sci. 91 (1994) 5667–5671, 10.1073/pnas.91.12.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Cal L, Suarez-Bregua P, Comesaña P, Owen J, Braasch I, Kelsh R, Cerdá-Reverter JM, Rotllant J, Countershading in zebrafish results from an Asip1 controlled dorsoventral gradient of pigment cell differentiation, Sci. Rep. 9 (2019) 3449, 10.1038/s41598-019-40251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Allen WL, Cuthill IC, Scott-Samuel NE, Baddeley R, Why the leopard got its spots: relating pattern development to ecology in felids, Proc. R. Soc. B Biol. Sci. 278 (2011) 1373–1380, 10.1098/rspb.2010.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Schneider A, David VA, Johnson WE, O’Brien SJ, Barsh GS, Menotti-Raymond M, Eizirik E, How the leopard hides its spots: ASIP mutations and melanism in wild cats, PLoS One 7 (2012), e50386, 10.1371/journal.pone.0050386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Manceau M, Domingues VS, Mallarino R, Hoekstra HE, The developmental role of Agouti in color pattern evolution, Sci. (80-. ). 331 (2011) 1062–1065, 10.1126/science.1200684. [DOI] [PubMed] [Google Scholar]
- [127].Hoekstra HE, Hirschmann RJ, Bundey RA, Insel PA, Crossland JP, A single amino acid mutation contributes to adaptive beach mouse color pattern, Sci. (80-. ). 313 (2006) 101–104, 10.1126/science.1126121. [DOI] [PubMed] [Google Scholar]
- [128].Hillenius WJ, The evolution of nasal turbinates and mammalian endothermy, Paleobiology 18 (1992) 17–29. 〈http://www.jstor.org/stable/2400978〉. [Google Scholar]
- [129].Martinez Q, Clavel J, Esselstyn JA, Achmadi AS, Grohé C, Pirot N, Fabre P-H, Convergent evolution of olfactory and thermoregulatory capacities in small amphibious mammals, Proc. Natl. Acad. Sci. 117 (2020) 8958–8965, 10.1073/pnas.1917836117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Irmak MK, Korkmaz A, Erogul O, Selective brain cooling seems to be a mechanism leading to human craniofacial diversity observed in different geographical regions, Med. Hypotheses 63 (2004) 974–979, 10.1016/j.mehy.2004.05.003. [DOI] [PubMed] [Google Scholar]
- [131].Sherbrooke WC, de AM, Castrucci L, Hadley ME, Temperature effects on in vitro skin darkening in the mountain spiny lizard, Sceloporus jarrovi: a thermoregulatory adaptation? Physiol. Zool. 67 (1994) 659–672, 10.1086/physzool.67.3.30163763. [DOI] [Google Scholar]
- [132].Cobb A, Cobb S, Do zebra stripes influence thermoregulation? J. Nat. Hist. 53 (2019) 863–879, 10.1080/00222933.2019.1607600. [DOI] [Google Scholar]
- [133].Xu X, Dong G-X, Hu X-S, Miao L, Zhang X-L, Zhang D-L, Yang H-D, Zhang T-Y, Zou Z-T, Zhang T-T, Zhuang Y, Bhak J, Cho YS, Dai W-T, Jiang T-J, Xie C, Li R, Luo S-J, The genetic basis of white tigers, Curr. Biol. 23 (2013) 1031–1035, 10.1016/j.cub.2013.04.054. [DOI] [PubMed] [Google Scholar]
- [134].Imsland F, McGowan K, Rubin C-J, Henegar C, Sundström E, Berglund J, Schwochow D, Gustafson U, Imsland P, Lindblad-Toh K, Lindgren G, Mikko S, Millon L, Wade C, Schubert M, Orlando L, Penedo MCT, Barsh GS, Andersson L, Regulatory mutations in TBX3 disrupt asymmetric hair pigmentation that underlies Dun camouflage color in horses, Nat. Genet. 48 (2016) 152–158, 10.1038/ng.3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Jablonski NG, Chaplin G, The colours of humanity: the evolution of pigmentation in the human lineage, Philos. Trans. R. Soc. B Biol. Sci. 372 (2017), 20160349, 10.1098/rstb.2016.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Jones P, Lucock M, Veysey M, Beckett E, The vitamin D–folate hypothesis as an evolutionary model for skin pigmentation: an update and integration of current ideas, Nutrients 10 (2018) 554, 10.3390/nu10050554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Deng L, Xu S, Adaptation of human skin color in various populations, Hereditas 155 (2018) 1, 10.1186/s41065-017-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Quillen EE, Norton HL, Parra EJ, Lona-Durazo F, Ang KC, Illiescu FM, Pearson LN, Shriver MD, Lasisi T, Gokcumen O, Starr I, Lin Y, Martin AR, Jablonski NG, Shades of complexity: new perspectives on the evolution and genetic architecture of human skin, Am, J. Phys. Anthropol. 168 (2019) 4–26, 10.1002/ajpa.23737. [DOI] [PubMed] [Google Scholar]
- [139].Parichy DM, Turner JM, Parker NB, Essential role for puma in development of postembryonic neural crest-derived cell lineages in zebrafish, Dev. Biol. 256 (2003) 221–241, 10.1016/S0012-1606(03)00016-2. [DOI] [PubMed] [Google Scholar]
- [140].Larson TA, Gordon TN, Lau HE, Parichy DM, Defective adult oligodendrocyte and Schwann cell development, pigment pattern, and craniofacial morphology in puma mutant zebrafish having an alpha tubulin mutation, Dev. Biol. 346 (2010) 296–309, 10.1016/j.ydbio.2010.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Dugatkin LA, The silver fox domestication experiment, Evol. Educ. Outreach 11 (2018) 16, 10.1186/s12052-018-0090-x. [DOI] [Google Scholar]
- [142].Pendleton AL, Shen F, Taravella AM, Emery S, Veeramah KR, Boyko AR, Kidd JM, Comparison of village dog and wolf genomes highlights the role of the neural crest in dog domestication, BMC Biol. 16 (2018) 64, 10.1186/s12915-018-0535-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Lord KA, Larson G, Coppinger RP, Karlsson EK, The history of farm foxes undermines the animal domestication syndrome, Trends Ecol. Evol. 35 (2020) 125–136, 10.1016/j.tree.2019.10.011. [DOI] [PubMed] [Google Scholar]
- [144].Hansen Wheat C, der Bijl W, Wheat CW, Morphology does not covary with predicted behavioral correlations of the domestication syndrome in dogs, Evol. Lett. 4 (2020) 189–199, 10.1002/evl3.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Protas M, Tabansky I, Conrad M, Gross JB, Vidal O, Tabin CJ, Borowsky R, Multi-trait evolution in a cave fish, Astyanax mexicanus, Evol. Dev. 10 (2008) 196–209, 10.1111/j.1525-142X.2008.00227.x. [DOI] [PubMed] [Google Scholar]
- [146].Jeffery WR, Cavefish as a model system in evolutionary developmental biology, Dev. Biol. 231 (2001) 1–12, 10.1006/dbio.2000.0121. [DOI] [PubMed] [Google Scholar]
- [147].Jeffery WR, Emerging model systems in evo-devo: cavefish and microevolution of development, Evol. Dev. 10 (2008) 265–272, 10.1111/j.1525-142X.2008.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Gross JB, Powers AK, A natural animal model system of craniofacial anomalies: the blind Mexican cavefish, Anat. Rec. 303 (2020) 24–29, 10.1002/ar.23998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Protas M, Conrad M, Gross JB, Tabin C, Borowsky R, Regressive evolution in the Mexican cave tetra, Astyanax mexicanus, Curr. Biol. 17 (2007) 452–454, 10.1016/j.cub.2007.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Yoshizawa M, Hixon E, Jeffery WR, Neural crest transplantation reveals key roles in the evolution of cavefish development, Integr. Comp. Biol. 58 (2018) 411–420, 10.1093/icb/icy006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].McCauley DW, Hixon E, Jeffery WR, Evolution of pigment cell regression in the cavefish Astyanax: a late step in melanogenesis, Evol. Dev. 6 (2004) 209–218, 10.1111/j.1525-142X.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- [152].O’Connell LA, Evolutionary development of neural systems in vertebrates and beyond, J. Neurogenet. 27 (2013) 69–85, 10.3109/01677063.2013.789511. [DOI] [PubMed] [Google Scholar]
- [153].Hoke KL, Adkins-Regan E, Bass AH, McCune AR, Wolfner MF, Co-opting evo-devo concepts for new insights into mechanisms of behavioural diversity, J. Exp. Biol. 222 (2019), 10.1242/jeb.190058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Bonier F, Moore IT, Martin PR, Robertson RJ, The relationship between fitness and baseline glucocorticoids in a passerine bird, Gen. Comp. Endocrinol. 163 (2009) 208–213, 10.1016/j.ygcen.2008.12.013. [DOI] [PubMed] [Google Scholar]
- [155].Breuner CW, Patterson SH, Hahn TP, In search of relationships between the acute adrenocortical response and fitness, Gen. Comp. Endocrinol. 157 (2008) 288–295, 10.1016/j.ygcen.2008.05.017. [DOI] [PubMed] [Google Scholar]
- [156].Chrousos GP, Stress and disorders of the stress system, Nat. Rev. Endocrinol. 5 (2009) 374–381, 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- [157].Patterson ZR, Abizaid A, Stress induced obesity: lessons from rodent models of stress, Front. Neurosci. 7 (2013), 10.3389/fnins.2013.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Miller DJ, Duka T, Stimpson CD, Schapiro SJ, Baze WB, McArthur MJ, Fobbs AJ, Sousa AMM, Sestan N, Wildman DE, Lipovich L, Kuzawa CW, Hof PR, Sherwood CC, Prolonged myelination in human neocortical evolution, Proc. Natl. Acad. Sci. 109 (2012) 16480–16485, 10.1073/pnas.1117943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Furness JB, The enteric nervous system and neurogastroenterology, Nat. Rev. Gastroenterol. Hepatol. 9 (2012) 286–294, 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- [160].Rolig AS, Mittge EK, Ganz J, Troll JV, Melancon E, Wiles TJ, Alligood K, Stephens WZ, Eisen JS, Guillemin K, The enteric nervous system promotes intestinal health by constraining microbiota composition, PLOS Biol. 15 (2017), e2000689, 10.1371/journal.pbio.2000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Härer A, Torres-Dowdall J, Rometsch SJ, Yohannes E, Machado-Schiaffino G, Meyer A, Parallel and non-parallel changes of the gut microbiota during trophic diversification in repeated young adaptive radiations of sympatric cichlid fish, Microbiome 8 (2020) 149, 10.1186/s40168-020-00897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Burr AHP, Bhattacharjee A, Hand TW, Nutritional modulation of the microbiome and immune response, J. Immunol. 205 (2020) 1479–1487, 10.4049/jimmunol.2000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Sherwin E, Bordenstein SR, Quinn JL, Dinan TG, Cryan JF, Microbiota and the social brain, Sci. (80-. ) 366 (2019), 10.1126/science.aar2016. [DOI] [PubMed] [Google Scholar]
- [164].Carabotti M, Scirocco A, Maselli MA, Severi C, The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems, Ann. Gastroenterol. 28 (2015) 203–209. [PMC free article] [PubMed] [Google Scholar]
- [165].Riddle MR, Boesmans W, Caballero O, Kazwiny Y, Tabin CJ, Morphogenesis and motility of the Astyanax mexicanus gastrointestinal tract, Dev. Biol. 441 (2018) 285–296, 10.1016/j.ydbio.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Riddle MR, Aspiras AC, Gaudenz K, Peuβ R, Sung JY, Martineau B, Peavey M, Box AC, Tabin JA, McGaugh S, Borowsky R, Tabin CJ, Rohner N, Insulin resistance in cavefish as an adaptation to a nutrient-limited environment, Nature 555 (2018) 647–651, 10.1038/nature26136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Córdoba-Aguilar A, Wing pigmentation in territorial male damselflies, Calopteryx haemorrhoidalis: a possible relation to sexual selection, Anim. Behav. 63 (2002) 759–766, 10.1006/anbe.2001.1974. [DOI] [Google Scholar]
- [168].Copenhaver PF, How to innervate a simple gut: familiar themes and unique aspects in the formation of the insect enteric nervous system, Dev. Dyn. 236 (2007) 1841–1864, 10.1002/dvdy.21138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Drapeau MD, A novel hypothesis on the biochemical role of the Drosophila Yellow protein, Biochem. Biophys. Res. Commun. 311 (2003) 1–3, 10.1016/j.bbrc.2003.09.106. [DOI] [PubMed] [Google Scholar]