Abstract
A simple screening method for fluconazole susceptibility of Cryptococcus neoformans using 2% dextrose Sabouraud dextrose agar (SabDex) with fluconazole was compared to the National Committee for Clinical Laboratory Standards (NCCLS) broth macrodilution method. By this method, fluconazole-susceptible C. neoformans isolates are significantly smaller on medium with fluconazole than on fluconazole-free medium. Isolates with decreased susceptibility have normal-size colonies on medium containing fluconazole. The 48-h NCCLS broth macrodilution MICs (NCCLS MICs) for isolates with normal-size colonies on 8- or 16-μg/ml fluconazole plates were predicted to be ≥8 or ≥16 μg/ml, respectively. On medium with 16 μg of fluconazole per ml, all strains (84 of 84) for which the NCCLS MICs were <16 μg/ml were correctly predicted, as were all isolates (7 of 7) for which the MICs were ≥16 μg/ml. Agar dilution appears to be an effective screening method for fluconazole resistance in C. neoformans.
Serious fungal infections in immunocompromised patients are increasing in frequency (3–5, 10). Cryptococcal meningitis, caused by Cryptococcus neoformans, remains incurable in the population with AIDS (2, 4, 13). In this setting, the necessary long-term suppressive therapy with antifungal agents may lead to selection of resistant isolates (4, 6). Clinical resistance in Candida spp. is becoming a serious problem (7, 11, 21). While antifungal resistance in Cryptococcus is uncommon, the MICs for some isolates are elevated (4, 6). A rapid, reproducible method for detecting fluconazole resistance would be useful in determining the epidemiology of and optimal treatment for resistant isolates (9, 18, 20).
Recently, a standardized broth macrodilution technique for yeast susceptibility testing has been accepted (9, 12). This technique requires considerable time and expense and is not easily applicable for screening purposes, even with microdilution modifications (1). The National Committee for Clinical Laboratory Standards (NCCLS) method for fluconazole susceptibility testing of invasive yeasts includes both Candida spp. and C. neoformans (19) yet establishes susceptibility breakpoints for only Candida spp. (12). The utility of susceptibility testing of C. neoformans remains controversial (10, 12, 17, 22). We have developed a susceptibility screening method by adding fluconazole to 2% dextrose Sabouraud dextrose agar (2% SabDex) which allows detection of yeasts with decreased fluconazole susceptibility (14, 15). In this study, agar screening was compared with the NCCLS macrodilution method for determining fluconazole susceptibility of C. neoformans.
(This study was presented in part at the 97th General Meeting of the American Society for Microbiology, Miami Beach, Fla., 4 to 8 May 1997 [abstr. F85].)
MATERIALS AND METHODS
Clinical isolates.
Ninety-one clinical C. neoformans isolates that were submitted to the University of Texas Health Science Center Fungus Testing Laboratory (San Antonio, Tex.) and Yale-New Haven Hospital (New Haven, Conn.) for MIC determination by NCCLS methodology (12) were subcultured and evaluated blindly by the agar dilution method.
2 and 4% SabDex.
Two-percent dextrose Sabouraud liquid broth modified antibiotic medium 13 (BBL, Cockeysville, Md.), which contains a final concentration of 20 g of dextrose per liter, was prepared from a powdered medium as suggested by the manufacturer. Bacto Agar (15 g/liter; Difco Laboratories, Detroit, Mich.) was added to a 1.5% final concentration. In addition, 4% SabDex (BBL), which contains a final dextrose concentration of 40 g/liter and agar at 15 g/liter, was prepared from a powdered medium as suggested by the manufacturer. Each medium was brought to a boil in sterile water for 15 to 30 s to dissolve the powdered medium and the agar and was cooled to 45°C in a water bath. Fluconazole intravenous solution (2 mg/ml; Pfizer-Roerig, New York, N.Y.) was added to the media at 45°C, with thorough stirring, to give final concentrations of 8 and 16 μg of fluconazole per ml. These solutions were maintained at 45°C and thoroughly stirred. Approximately 20 ml was poured into sterile 100-mm-diameter petri plates and allowed to cool and harden before use. Hardened plates were stored at 4°C for up to 1 week prior to use.
CHROMagar Candida.
CHROMagar Candida (CHROMagar, Paris, France) was prepared from powdered medium according to the manufacturer’s instructions, with the addition of fluconazole to give 8- and 16-μg/ml concentrations. The prepared medium, which contains chloramphenicol (0.5 g/liter) and agar (15 g/liter), was dispensed (20 ml) into plates and stored as described above.
Plating and interpretation: susceptibility testing.
Fluconazole was added to the media to differentiate resistant yeasts from susceptible yeasts. From each isolate stock (several isolated colonies placed in 3 ml of sterile, deionized H2O), a sterile 10-μl loop was used to inoculate a set of three medium plates containing 0, 8, and 16 μg of fluconazole per ml. Samples were applied to one half of each plate. Plates were incubated at 30°C for 48 and 72 h prior to assessment of growth. Results from the fluconazole-containing medium were recorded as susceptible or mycologically resistant based on growth characteristics. Colonies that demonstrated growth on medium with fluconazole (usually visualized as pinpoint-sized colonies) that was suppressed compared to growth on medium without fluconazole were recorded as susceptible (Fig. 1). Colonies that demonstrated growth that was indistinguishable on medium with or without fluconazole were recorded as mycologically resistant. Results in this study were independently read by two laboratory personnel. Agar dilution susceptibility was tested on groups of 15 to 25 isolates. Control isolates with known susceptibilities were included for comparison with test samples.
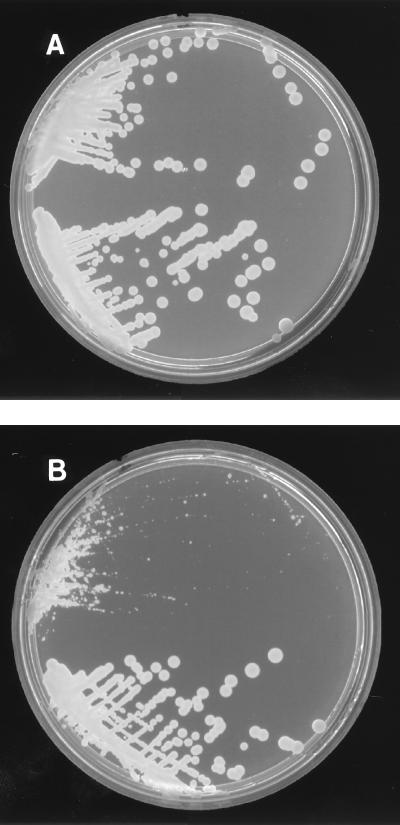
FIG. 1.
Growth of susceptible C. neoformans (tops of plates) and C. neoformans with decreased fluconazole susceptibility (bottoms of plates) on 2% SabDex without fluconazole (A) and on 2% SabDex with 8 μg of fluconazole per ml (B).
RESULTS
Ninety-one clinical isolates of C. neoformans were evaluated by broth macrodilution testing and by agar dilution. A wide range of fluconazole MICs (≤0.125 to >64 μg/ml) were detected, with 13 of 91 isolates (14%) inhibited by ≥8 μg/ml (48-h NCCLS broth macrodilution MICs [referred to hereafter in this work as NCCLS MICs]). Growth of susceptible C. neoformans isolates could be easily differentiated from resistant yeast isolates on fluconazole-containing agar by colony morphology. On 2% SabDex without fluconazole (Fig. 1A), growth characteristics of the susceptible C. neoformans (Fig. 1A, top) and C. neoformans with decreased fluconazole susceptibility (Fig. 1A, bottom) could not be distinguished. Fluconazole-impregnated medium allowed distinction of fluconazole-susceptible C. neoformans isolates (Fig. 1, tops of plates) from C. neoformans with decreased susceptibility (Fig. 1, bottoms of plates). The fluconazole-containing medium suppressed growth of the susceptible strain (Fig. 1B), seen as only pinpoint colonies (Fig. 1B, top), whereas colonies with decreased fluconazole susceptibility were seen to have normal growth characteristics (Fig. 1B, bottom).
Medium containing 8 μg of fluconazole per ml correctly detected 72 of 81 strains for which the NCCLS MICs were <8 μg/ml and 9 of 10 strains for which the NCCLS MICs were ≥8 μg/ml as well (Table 1). One isolate for which the NCCLS MIC was predicted to be <8 μg/ml was found to be inhibited by 8 μg/ml (NCCLS MIC), while nine isolates for which the NCCLS MICs were predicted to be ≥8 μg/ml were inhibited by 1 (n = 1), 2 (n = 3), and 4 (n = 5) μg/ml (NCCLS MICs). Overall agreement on the 8-μg/ml fluconazole plates was within 1 log2 broth macrodilution for 87 of 91 isolates (96%). 2% SabDex agar containing 16 μg of fluconazole per ml correctly detected 84 of 84 strains for which the NCCLS MICs were <16 μg/ml as well as 7 of 7 strains for which the NCCLS MICs were ≥16 μg/ml (Table 2).
TABLE 1.
Correlation between NCCLS MICs and predicted MICs on 2% SabDex agar with 8 μg of fluconazole per ml
| Predicted 2% SabDex agar MIC (μg/ml) | No. (%) of cultures for which NCCLS MIC (μg/ml) was:
|
|
|---|---|---|
| <8 (n = 81) | ≥8 (n = 10) | |
| <8 | 72 (89) | 1a |
| ≥8 | 9b | 9 (90) |
The NCCLS MIC for this C. neoformans isolate was 8 μg/ml.
The NCCLS MICs for one, three, and five C. neoformans isolates were 1, 2, and 4 μg/ml, respectively. The NCCLS MICs for 78% of the C. neoformans isolates predicted to be mycologically resistant were ≥4 μg/ml.
TABLE 2.
Correlation between NCCLS MICs and predicted MICs on 2% SabDex agar with 16 μg of fluconazole per ml
| Predicted 2% SabDex agar MIC (μg/ml) | No. (%) of cultures for which NCCLS MIC (μg/ml) was:
|
|
|---|---|---|
| <16 (n = 84) | ≥16 (n = 7) | |
| <16 | 84 (100) | 0 |
| ≥16 | 0 | 7 (100) |
Studies using either 4% SabDex or CHROMagar medium were less successful in determining susceptibility. 4% SabDex or CHROMagar containing 8 μg of fluconazole per ml correctly detected 7 of 17 strains (41%) or 9 of 17 strains (53%), respectively, for which the NCCLS MICs were <8 μg/ml, and 6 of 6 strains (both media) for which the NCCLS MICs were ≥8 μg/ml. Examination of these isolates on either 4% SabDex or CHROMagar medium containing 16 μg of fluconazole per ml correctly detected 13 of 20 strains (65%) or 9 of 20 strains (45%), respectively, for which the NCCLS MICs were <16 μg/ml and 3 of 3 (both media) strains for which the NCCLS MICs were ≥16 μg/ml.
The sensitivities of correctly predicting yeasts with increased resistances by normal colony growth on 2% SabDex medium containing 8 or 16 μg of fluconazole per ml were 90 and 100%, respectively. The specificities of correctly predicting isolates to be fluconazole susceptible based on suppressed growth on 2% SabDex media containing fluconazole at either 8 or 16 μg/ml were 89 and 100%, respectively.
DISCUSSION
Screening C. neoformans for susceptibility to antifungals has been difficult. In the present studies, 2% SabDex containing 8 μg of fluconazole per ml correctly detected 89% of the clinical C. neoformans isolates for which the NCCLS MICs were <8 μg/ml and 90% of the strains for which the NCCLS MICs were ≥8 μg/ml. Medium containing 16 μg of fluconazole per ml correctly detected all isolates for which the NCCLS MICs were <16 μg/ml as well as all isolates for which the NCCLS MICs were ≥16 μg/ml. Overall, agreement was within 1 log2 broth tube macrodilution for 87 of 91 isolates (96%) on the 8-μg/ml fluconazole medium and 91 of 91 isolates (100%) on the 16-μg/ml fluconazole medium. While this screening method correlates well with the NCCLS M27-A method, the clinical significance of C. neoformans susceptibility testing remains unclear (15).
The sensitivity of correctly predicting mycologically resistant yeasts by normal colony growth on medium containing 8- or 16-μg/ml fluconazole was 100% with either 4% SabDex or CHROMagar. However, the specificities of correctly predicting isolates to be fluconazole susceptible based on suppressed growth on medium containing fluconazole at either 8 or 16 μg/ml were 41 and 65%, respectively, on 4% SabDex medium and 53 and 45%, respectively, on CHROMagar medium containing fluconazole. Thus, 2% SabDex appeared superior to these other media for susceptibility screening. The use of Yeast Nitrogen Base agar could possibly have improved the growth of C. neoformans but was not tested in this study (10, 12, 22). Data obtained in these experiments supports previous observations that medium-specific differences pertaining to MIC determination exist (5, 8, 12, 16, 22).
The use of 2% SabDex medium with fluconazole appears to be a rapid, simple, and sensitive method for detection of fluconazole-resistant yeasts. Additional studies should be conducted to determine the utility of this method in screening clinical samples and in establishing the optimal management of resistant cryptococcal infections.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health/National Institute for Dental Research grant 5 R01-DE11381, by grant M01-RR-01346 to the Frederic C. Bartter General Clinical Research Center of the South Texas Veterans Medical Center, San Antonio, and by Pfizer, Inc. Chromogenic medium was kindly provided by CHROMagar Candida.
REFERENCES
- 1.Barchesi F, Colombo A L, McGough D A, Rinaldi M G. Comparative study of broth macrodilution and microdilution techniques for in vitro antifungal susceptibility testing of yeasts by using the National Committee for Clinical Laboratory Standards’ proposed standard. J Clin Microbiol. 1994;32:2494–2500. doi: 10.1128/jcm.32.10.2494-2500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barchesi F, Hollis R J, Messer S A, Scalise G, Rinaldi M G, Pfaller M A. Electrophoretic karyotype and in vitro susceptibility of Cryptococcus neoformans isolates from AIDS patients. Diagn Microbiol Infect Dis. 1995;23:99–103. doi: 10.1016/0732-8893(95)00169-7. [DOI] [PubMed] [Google Scholar]
- 3.Brandt M E, Pfaller M A, Hajjeh R A, Gravis E A, Rees J, Spitzer E D, Pinner R W, Mayer L W the Cryptococcal Disease Active Surveillance Group. Molecular subtypes and antifungal susceptibilities of serial Cryptococcus neoformans isolates in human immunodeficiency virus-associated cryptococcosis. J Infect Dis. 1996;174:812–820. doi: 10.1093/infdis/174.4.812. [DOI] [PubMed] [Google Scholar]
- 4.Casadevall A, Spitzer E D, Webb D, Rinaldi M G. Susceptibilities of serial Cryptococcus neoformans isolates from patients with recurrent cryptococcal meningitis to amphotericin B and fluconazole. Antimicrob Agents Chemother. 1993;37:1383–1386. doi: 10.1128/aac.37.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen S C A, O’Donnell M L, Gordon S, Gilbert G L. Antifungal susceptibility testing using the E-test: comparison with the broth macrodilution technique. J Antimicrob Chemother. 1996;37:265–273. doi: 10.1093/jac/37.2.265. [DOI] [PubMed] [Google Scholar]
- 6.Colombo A L, Barchiesi F, McGough D A, Rinaldi M G. Comparison of E-test and National Committee for Clinical Laboratory Standards broth macrodilution method for azole antifungal susceptibility testing. J Clin Microbiol. 1995;33:535–540. doi: 10.1128/jcm.33.3.535-540.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dermoumi H. In vitro susceptibility of yeast isolates from the blood to fluconazole and amphotericin B. Chemotherapy. 1992;38:112–117. doi: 10.1159/000238950. [DOI] [PubMed] [Google Scholar]
- 8.Espinel-Ingroff A, Steele-Moore L, Galgiani J N. Evaluation of 80% inhibition standards for the determination of fluconazole minimum inhibitory concentrations in three laboratories. Diagn Microbiol Infect Dis. 1994;20:81–86. doi: 10.1016/0732-8893(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 9.Fromtling R A, Galgiani J N, Pfaller M, Espinel-Ingroff A, Bartizal K F, Bartlett M S, Body B A, Frey C, Hall G, Roberts G D, Nolte F B, Odds F C, Rinaldi M G, Sugar A M, Villareal K. Multicenter evaluation of a broth macrodilution antifungal susceptibility test for yeasts. Antimicrob Agents Chemother. 1993;37:39–45. doi: 10.1128/aac.37.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghannoum M A, Fu Y, Ibrahim A S, Mortara L A, Shafiq M C, Edwards J E, Jr, Criddle R S. In vitro determination of optimal antifungal combinations against Cryptococcus neoformans and Candida albicans. Antimicrob Agents Chemother. 1995;39:2459–2465. doi: 10.1128/aac.39.11.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Millon L, Manteaux A, Reboux G, Drobacheff C, Monod M, Barale T, Michel-Briand Y. Fluconazole-resistant recurrent oral candidiasis in human immunodeficiency virus-positive patients: persistence of Candida albicans strains with the same genotype. J Clin Microbiol. 1994;32:1115–1118. doi: 10.1128/jcm.32.4.1115-1118.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. NCCLS document M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 13.Patterson, T. F. 1997. Cryptococcosis in HIV-infected and non-HIV-infected hosts. Int. J. Infect. Dis. 1(Suppl. 1):S64–S69.
- 14.Patterson T F, Kirkpatrick W R, Revankar S G, McAtee R K, Fothergill A W, McCarthy D I, Rinaldi M G. Comparative evaluation of macrodilution and chromogenic agar screening for fluconazole susceptibility of Candida albicans. J Clin Microbiol. 1996;34:3237–3239. doi: 10.1128/jcm.34.12.3237-3239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patterson T F, Revankar S G, Kirkpatrick W R, Dib O P, Fothergill A W, Redding S W, Sutton D A, Rinaldi M G. Simple method for detecting fluconazole-resistant yeasts with chromogenic agar. J Clin Microbiol. 1996;34:1794–1797. doi: 10.1128/jcm.34.7.1794-1797.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaller M A, Berry A L. Evaluation of a novel colorimetric broth microdilution method for antifungal susceptibility testing of yeast isolates. J Clin Microbiol. 1994;32:1992–1996. doi: 10.1128/jcm.32.8.1992-1996.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powderley W G. Editorial response: management of cryptococcal meningitis—have we answered all the questions? Clin Infect Dis. 1996;22:329–330. doi: 10.1093/clinids/22.2.329. [DOI] [PubMed] [Google Scholar]
- 18.Rex J H, Pfaller M A, Barry A L, Nelson P W, Webb C W. Antifungal susceptibility testing of isolates from a randomized, multicenter trial of fluconazole versus amphotericin B as treatment of nonneutropenic patients with candidemia. Antimicrob Agents Chemother. 1995;39:40–44. doi: 10.1128/aac.39.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, Berry A L. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and candida infections. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 20.Rex J H, Pfaller M A, Rinaldi M G, Polak A, Galgiani J N. Antifungal susceptibility testing. Clin Microbiol Rev. 1993;6:367–381. doi: 10.1128/cmr.6.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rex J H, Rinaldi M G, Pfaller M A. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witt M D, Lewis R J, Larsen R A, Milefchik E N, Leal M A E, Haubrich R H, Richie J A, Edwards J E, Jr, Ghannoum M A. Identification of patients with acute AIDS-associated cryptococcal meningitis who can be effectively treated with fluconazole: the role of antifungal susceptibility testing. Clin Infect Dis. 1996;22:322–328. doi: 10.1093/clinids/22.2.322. [DOI] [PubMed] [Google Scholar]