Abstract
The protocols described in this book chapter are meant to be used as an outline and guideline to explore the use of a cationic, polymeric, and synthetic carrier—poly (amidoamine) (PAMAM) dendrimers. The amineterminated, hyperbranched structures have been identified as a vehicle for the delivery of nucleic acids. As such, clear protocols for the optimization of dendrimer usage should be set in place. This chapter details the experiments used to determine the ratio that dendrimers and nucleic acids should be complexed at through the use of binding assays, nuclease protection assays, and competitive binding assays.
Keywords: PAMAM dendrimers, NANPs, Delivery, Carrier, Binding assay, Gel electrophoresis, Nuclease protection
1. Introduction
Dendrimers are polymers with extensively branching structures and easily adaptable surfaces. Their structures exhibit a restricted molecular weight range, molecular homogeneity, a versatile terminal surface, and specified size and shape properties. These features are essential in functionalization, where the surface chemistry is modified to add new capabilities and properties. Surface chemistry can be manipulated through synthesis processes, resulting in changes in charge, solubility, charge, and size [1]. It can additionally function as a bioconjugate in diseased cells to target pharmacological properties and deliver therapeutic oligonucleotides by attaching to the functional groups on the surface or enclosing in the intramolecular cavity of the dendrimer [2].
The structures of dendrimers begin with the central core and then bloom into expanding branching units and their terminal groups. The generation of a dendrimer is determined by the increase in the number of recurrent branching units, which creates the globular structure of a dendrimer. Hydrophobic and hydrophilic interactions can create binding to the surface of these dendrimers, strongly encouraging their usage in systems for drug and gene delivery [2]. The recurrent branching units in dendrimerbased carrier platforms provide many attaching sites while protecting them from enzymatic nucleic acid degradation [3]. The tertiary amines located inside the dendrimer’s interior serve as a buffer, allowing the dendrimer and its associated content to escape from the endosome following uptake by the cell by an osmosis-driven process known as the “proton sponge” effect [4]. Dendrimer surfaces are amenable to functional group alteration and targeted moiety modification. Dendrimers have been described as superior to traditional biological and chemical linear polymers because of the potential in the dendrimer’s ability to deliver nucleic acids to target locations [5].
Nucleic acids are large biomolecules that play essential roles in all cells and viruses. A major function of nucleic acids involves storing and expressing genomic information. Nucleic acid nanoparticles (NANPs) provide a highly adaptable molecular foundation for the precise distribution of a multitude of therapeutics and the prevention of disease-causing protein expansion [6].
Combinations of various nucleic acid nanoparticles (NANPs) to carriers allow for their delivery into cells and can prevent undesired immunological and therapeutic effects, depending on the carrier being used [7]. Numerous studies have presented extensive evidence that, in conjunction with the physicochemical qualities and therapeutic benefits of NANPs, their interactions with immune system cells may be modulated by numerous independently programmable architectural features. The findings also show that NANPs’ specified immunomodulation can either promote their immunoquiescent administration or be exploited to stimulate favorable immune responses [8].
Since NANPs require a delivery vehicle to enter cells while protecting them from naturally occurring nucleases they may encounter on their way into cells, detailed physicochemical characterization is needed to understand the NANP-carrier interactions. To address these challenges, this chapter will characterize the complexing of nucleic acid constructs to dendrimers through experimental methods.
2. Materials and Equipment
PAMAM dendrimers—from Dendritech
2.1. Nucleic Acid Nanoparticle (NANP) Preparation
Nanodrop or spectrophotometer.
5X Assembly buffer (AB): 89 mM Tris–borate (pH 8.2), 2 mM MgCl2, 50 mM KCl.
Heat blocks that can be held at constant temperatures of 95 °C and 30 °C.
Bio-Rad ChemiDoc MP System and ethidium bromide for band visualization in gels for analysis.
2.2. Determination of N/P Ratio Using Binding Assays
Agarose.
1X TBE buffer.
0.2 mL PCR tubes.
- Agarose loading buffer.
- To make 15 mL of agarose loading buffer:
- Pipette 10.2 mL ddiH2O into a 15 mL Falcon tube.
- Add 4.6 mL glycerol (Pour this instead of pipetting—it’s very viscous!).
- Add 100 μL 1% bromophenol blue.
- Add 100 μL 1% xylene cyanol.
- Vortex and centrifuge thoroughly.
Bio-Rad ChemiDoc MP System.
1X Assembly buffer: 1-part endotoxin-free water: 4 parts 5X AB.
2.3. Nuclease Protection Assay
A DNA duplex is used for this step of the characterization protocols. The duplex comprises one fluorescently labeled strand, and the other strand will be functionalized with a quencher and assembled as described in Subheading 3.1.
DNA duplex.
RQ1 DNase.
0.2 mL PCR tubes with lids.
1X Assembly buffer.
Bio-Rad CFX96 RT-thermocycler.
Bio-Rad RT-PCR software.
Excel.
2.4. Competitive Binding Assay
Nucleic acid constructs of interest.
Dendrimers.
Aqueous heparin.
1.5 mL tubes.
- 8% native-PAGE.
- Acrylamide, bis-acrylamide, and TEMED.
Ethidium bromide.
- Native loading buffer.
- To make 15 mL of native loading buffer:
- Pipette 4.3 mL ddiH2O into a 15 mL Falcon tube.
- Add 3 mL 5X AB.
- Add 7.5 mL glycerol.
- Add 100 μL 1% bromophenol blue.
- Add 100 μL 1% xylene cyanol.
- Vortex and centrifuge thoroughly.
Bio-Rad ChemiDoc MP System and ethidium bromide for band visualization in gels for analysis.
3. Methods
3.1. Nanoparticle Preparation
Calculate concentrations and required volumes of duplex strands 1 and 2 for equimolar addition of each strand for assembly using a NanoDrop 2000.
Label Duplex 1.5 mL tube.
Add the calculated volume of endotoxin-free water to tube.
Mix strands 1 and 2 in an equimolar ratio.
Place the duplex solution in the 1.5 mL tube(s) on a 95 °C heat block for 2 min.
Add 5X assembly buffer at 20% volume.
Keep at room temperature for 20 min.
Place on ice or store in the −20 °C freezer.
Confirm assembly using an 8% native-PAGE.
3.2. Binding Assays to Experimentally Determine and Confirm N/P Ratios
Use a fluorescently labeled nucleic acid construct of which you know the sequence to account for the number of phosphates per molecule.
Calculate the volume of dendrimer and duplex needed to reach the desired ratio of interest (see Note 1).
To form the dendriplexes, combine the volumes of each molecule—dendrimer and duplex—into a 0.2 mL PCR tube.
Tris–borate EDTA (45 mM Tris–borate, 1 mM EDTA, pH 8.3 buffer).
Allow each sample to incubate at room temperature for 30 min.
After the 30-min incubation period, add 1X AB to each sample so that all final volumes of each sample are equal (see Note 2).
Assemble a 2.0% 50 mL agarose gel.
Load each sample into the agarose gel using a 1:1 ratio of your dendriplex to the loading buffer (see Note 3).
Run the gel at 200 V for 20 min.
Once the gel has been run for the allotted time, position the gel on the ChemiDoc, gel imaging system, and image the gel (see Note 4). After the allotted time, the gel should resemble Fig. 1a.
Fig. 1.
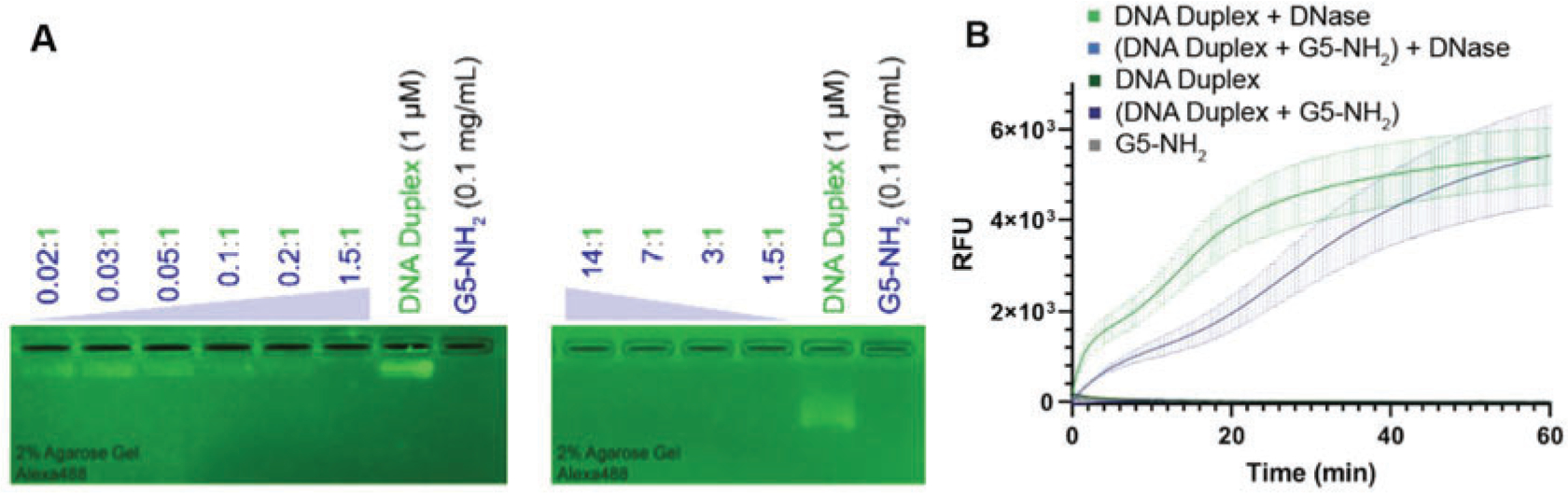
(a) Binding assays using generation 5 amine-terminated PAMAM dendrimers with a fluorescently labeled DNA duplex. The assays are being tested on a 2% agarose gel and visualized using the Chemi-Doc. (b) Resulting fluorescence profiles from nuclease resistance assays
3.3. Nuclease Protection Assay
This experimental protocol uses a FRET-paired DNA duplex, wherein one strand is labeled with a fluorophore (Alexa 488), and another strand is functionalized with a quencher (Iowa Black). Upon nuclease digestion, the FRET pair will be released, and fluorescence can be measured over a period of time to determine the ability and time frame at which dendrimers can provide and maintain protection of the DNA duplex or the nucleic acid construct of interest.
Combine your FRET pair nucleic acid construct at the N/P ratio that was previously determined in a 0.2 mL PCR tube, and incubate at room temperature for 30 min (see Note 5).
After the 30-min incubation period, place your samples in the Bio-Rad RT PCR samples holder.
Rapidly add 3 μL of RQ1 DNase to all the samples, and set the CFX96 RT-thermocycler to hold a constant temperature of 37 °C and to read the relative fluorescence every 30 s for up to 4 h for a time-lapse experiment (see Note 6). Once all fluorescence measurements have been taken, plot the data in relative fluorescence units (RFU) vs time, as seen in Fig. 1b.
3.4. Competitive Binding Assay
To investigate the integrity that dendrimers can provide nucleic acid constructs, a competitive binding assay using heparin can be used. Heparin carries a highly negative charge which can outcompete the dendrimer-NANP complex interaction therein, resulting in the release of the nucleic acid construct. A native-PAGE can then be run to compare the constructs’ integrity before and after exposure to heparin.
Using the same N/P ratios, which were determined through the binding assays, complex the dendrimer generation of interest with the nucleic acid construct of interest within that same ratio in a 1.5 mL tube, and allow them to incubate at room temperature for 30 min.
In another tube, combine the nucleic acid constructs and dendrimer again, also letting this sample incubate at room temperature for 30 min.
Once the incubation period has ceased, add aqueous heparin sulfate to the dendriplex solution at a nucleic acid/heparin ratio of 1:6 w/w to one of the sample tubes, and incubate again at 37 °C for 30 min.
Both samples can then be analyzed via an 8% native-PAGE (see Note 7).
4. Notes
Depending on the generation and the nucleic acid construct, the N/P ratios will vary.
Dilution of all samples to the same volume will ensure that a decrease in fluorescence is attributed to binding and not because the sample is too dilute.
To determine the true binding ratio, a range of ratios should be used to show the stepwise progression of binding and when complete and optimal binding occurs.
The lack of a free Alexa 488 band in the gel indicates complete binding between the dendrimer and nucleic acid construct.
Because there is a minimum volume of 30 μL for the CFX96 RT-thermocycler, 1X AB can be used on the samples to make all the solutions be the same dilution and volume.
The negative control sample should not be treated with RQ1 DNase, as it will be used as your initial threshold for lack of fluorescence. There should also be a positive control where the DNA duplex without dendrimers is degraded with the RQ1 DNase.
The native-PAGE gel should show any changes in morphology if the band shifts differently after the heparin release are completed when compared to the non-exposed sample.
Acknowledgments
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R35GM139587 (to K.A.A.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Abedi-Gaballu F, Dehghan G, Ghaffari M, Yekta R, Abbaspour-Ravasjani S, Baradaran B et al. (2018) PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl Mater Today 12:177–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmerston Mendes L, Pan J, Torchilin VP (2017) Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules 22(9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Li M, Wang M, Xu H, Wang Z, Li Y et al. (2021) Effects of the surface charge of polyamidoamine dendrimers on cellular exocytosis and the exocytosis mechanism in multidrug-resistant breast cancer cells. J Nano-biotechnol 19(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wojnilowicz M, Glab A, Bertucci A, Caruso F, Cavalieri F (2019) Super-resolution imaging of proton sponge-triggered rupture of endosomes and cytosolic release of small interfering RNA. ACS Nano 13(1):187–202 [DOI] [PubMed] [Google Scholar]
- 5.Zeng Y, Kurokawa Y, Win-Shwe TT, Zeng Q, Hirano S, Zhang Z et al. (2016) Effects of PAMAM dendrimers with various surface functional groups and multiple generations on cytotoxicity and neuronal differentiation using human neural progenitor cells. J Toxicol Sci 41(3):351–370 [DOI] [PubMed] [Google Scholar]
- 6.Johnson MB, Chandler M, Afonin KA (2021) Nucleic acid nanoparticles (NANPs) as molecular tools to direct desirable and avoid undesirable immunological effects. Adv Drug Deliv Rev 173:427–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avila YI, Chandler M, Cedrone E, Newton HS, Richardson M, Xu J et al. (2021) Induction of cytokines by nucleic acid nanoparticles (NANPs) depends on the type of delivery carrier. Molecules 26(3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandler M, Johnson MB, Panigaj M, Afonin KA (2020) Innate immune responses triggered by nucleic acids inspire the design of immunomodulatory nucleic acid nanoparticles (NANPs). Curr Opin Biotechnol 63:8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]


