Abstract
Introduction
Lactococcus garvieae, a zoonotic pathogen, may rarely infect humans through the consumption of fish. Documented manifestations of L. garvieae infection in humans include infective endocarditis, prosthetic joint infections, liver abscesses, peritoneal dialysis-associated peritonitis, osteomyelitis, meningitis, infective spondylodiscitis, acalculous cholecystitis, and urinary tract infection.
Case report
An 87-year-old female was hospitalized for coffee-ground emesis secondary to acute gastritis after eating cooked fish. One week after her discharge, she developed new-onset confusion and was returned to the hospital. Chest computed tomography revealed total consolidation of the left lung and a multiloculated left pleural effusion. The patient required intubation and direct admission to the intensive care unit. Pleural fluid and blood cultures grew L. garvieae, which was susceptible to ceftriaxone, penicillin, and vancomycin. Despite intensive antibiotic therapy and supportive care for thirteen days, the patient remained in irreversible shock, and the family opted for comfort care.
Conclusions
Heretofore unreported, this case demonstrates that L. garvieae can cause bronchopneumonia and empyema.
Keywords: Lactococcus garvieae, empyema, septic shock, critical care
Introduction
Lactococcus garvieae (L. garvieae, previously known as Enterococcus seriolicida) is a Gram-positive, catalase-negative, and facultative anaerobic pathogen. This organism was initially identified in the 1930s as the cause of hemorrhagic septicemia in several fish species, and later found to infect ruminants (i.e., cattle) and other aquatic animals. L. garvieae was originally classified within the Streptococcus genus due to its phenotypic resemblance to Enterococcus. Genetic analyses in the 1980s enabled the accurate re-classification of this organism under its own genus, Lactococcus.1
L. garvieae is felt to be an emerging human pathogen. The increased number of reported cases largely reflects the advancement of diagnostic bacteriology. The most common L. garvieae infection in humans is infective endocarditis.2 Other documented manifestations include prosthetic joint infections,3 liver abscesses,4 peritoneal dialysis-associated peritonitis,5 osteomyelitis,6 meningitis,7 infective spondylodiscitis,8 acalculous cholecystitis,9 and urinary tract infection.10 To our knowledge, this is the first case that documents an empyema caused by L. garvieae.
Case report
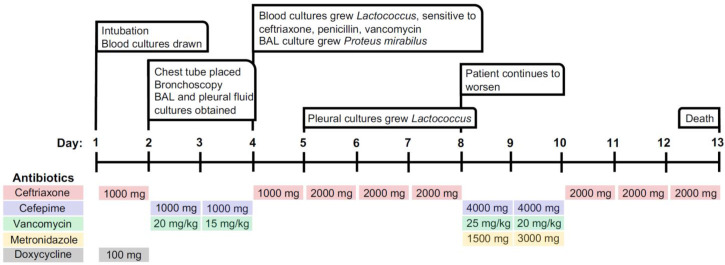
An 87-year-old female with a history of heart failure and hemodialysis-dependent end-stage renal disease had recently been hospitalized for coffee-ground emesis. She related the occurrence of her gastrointestinal upset to recent consumption of cooked fish. This hospitalization was also notable for a positive COVID-19 test. Her symptoms resolved with supportive care, and she was discharged to her nursing home. She was re-admitted to the hospital one week later due to new-onset altered mental status. A timeline of the patient’s hospital course and antibiotic therapy is detailed in Figure 1.
Figure 1.
Timeline detailing the patient’s hospital course and antibiotic therapy
BAL – bronchoalveolar lavage.
Antibiotic dosing is represented as total mg daily.
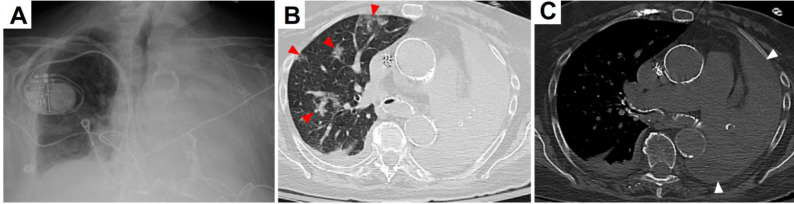
In the emergency department (ED), the patient was afebrile, pale, and in respiratory distress. Her blood pressure was 185/79 mmHg, heart rate 98 beats/minute, respiratory rate 24 breaths/minute, and oxygen saturation 82% on ambient air. Coarse scattered rhonchi were present over the right lung, and breath sounds were markedly decreased on the left. Her Glasgow coma score was 14 (eyes 4, verbal 4, motor 6). Neurological exam revealed neither motor nor sensory deficits. Complete blood count showed a white blood cell count (WBC) of 8.29 thousand/µL (ref. range: 4.31-10.16) with 87% neutrophilia (ref. range: 43-75), hemoglobin 8.8 g/dL (ref. range: 11.5-15.4), and MCV 96% (ref. range: 82-98). The serum creatinine was 3.95 mg/dL (ref. range: 0.6-1.3; patient’s baseline: 3-4) and BUN was 26 mg/dL (ref. range: 5-25). Although numerous leukocytes were present in the urine, urine cultures were negative. Severe inflammation was strongly evidenced by an elevated serum procalcitonin of 295 ng/mL (ref. range: <0.25) and C-reactive protein 128 mg/L (ref. range: <3). Venous blood gas analysis showed a pH of 7.3 (ref. range: 7.3-7.4), pCO2 63.3 mmHg (ref. range: 42-50), and bicarbonate 30.9 mmol/L (ref. range: 24-30). Complete opacification of the left hemithorax and prominent interstitial markings on the right were seen on a chest X-ray performed in the ED (Figure 2A). By chest computed tomography (CT), a massive multiloculated pleural effusion and total lung collapse were evident on the left side. In addition, the right lung had basilar consolidation, scattered ground glass opacities, and subtotal endobronchial obstruction of the mainstem bronchus (Figure 2B,C). While in the ED, two sets of blood cultures were obtained. The patient was given a dose of intravenous (IV) ceftriaxone 1000 mg and IV doxycycline 100 mg. Due to her recent diagnosis of COVID-19, IV tocilizumab 400 mg, IV remdesivir 200 mg, and IV methylprednisolone 35 mg every 12 hours were administered. Upon hospitalization, additional COVID-19 medications included oral (PO) ascorbic acid 1000 mg every 12 hours, PO zinc sulfate 220 mg daily, and PO atorvastatin 40 mg daily.
Figure 2.
(A) Chest X-ray showing complete opacification of the left hemithorax. (B) Lung window of chest CT without contrast demonstrating right and left mainstem bronchi with endobronchial debris, scattered right-sided ground glass opacities (red arrowheads) and complete left lung atelectasis. (C) Abdominal window of chest CT without contrast revealing multiloculated pleural effusion surrounding the left lung (white arrowheads).
On the first day of hospitalization, rapidly worsening hypoxemia required transfer to the intensive care unit for intubation. On day two, broad spectrum IV antibiotic therapy included cefepime 1000 mg and vancomycin 20 mg/kg. At this time, left thoracentesis yielded 360 cc of serosanguinous fluid. Pleural fluid analysis revealed an LDH of 1135 U/L, protein <2 g/dL, glucose <1 mg/dL, and WBC 348/µL with a differential of 90% neutrophils. The pleural LDH/serum LDH ratio was 4.67 U/L (Light’s criteria: exudative if >0.6), meeting criteria for an exudative pathology. Thick purulent mucus plugs in both main stem bronchi were removed by bronchoscopy, and the bronchoalveolar lavage (BAL) was sent for culture. On day four, the results of both blood cultures performed in the ED proved positive for Lactococcus garvieae, as determined by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) rapid organism identification. The organism was sensitive to ceftriaxone, penicillin, and vancomycin. Additionally, pan-sensitive Proteus mirabilis was cultured from the BAL specimen. Cultures for fungal and acid-fast organisms were negative. Based upon the combined bacteriological results, IV ceftriaxone 1000 mg was chosen as the optimal antibiotic therapy, and methylprednisolone was immediately stopped. The patient progressively declined due to ensuing hypotension despite intensive vasopressor support. The absence of endocarditic vegetations on echocardiography supported that our patient’s sepsis likely arose from the overwhelming empyema. On day 5, one of two sets of pleural fluid cultures grew Lactococcus garvieae. The dose of ceftriaxone was increased from 1000 mg to 2000 mg. Video assisted thoracoscopic surgery with decortication was recommended by thoracic surgery, however the family declined surgical intervention. Several tPA/DNase instillations through the pleural drain led to only minor improvement of the effusion.
The family requested the patient be transitioned to comfort care in the light of inexorable cardiovascular collapse.
Discussion
The literature is replete with cases of human L. garvieae infections associated with the consumption and exposure to various foods. These include beef, poultry, raw cow’s milk, goat cheese, pork, and most notably, raw or cooked fish. In a review of 25 cases, the potential risk factors in the development of L. garvieae infections included previous valvular heart disease, prosthetic heart valves and devices, colonic polyps, and gastrointestinal disease.1 Risk of infection and disease severity may also be influenced by an immunocompromised state.4
Our patient’s consumption of cooked fish appears to have led to her L. garvieae infection. The portals of entry may have been via invasion of the gastrointestinal mucosa or by aspiration. Evidence supporting the latter was the bronchoscopic findings of bilateral bronchial aspiration and confluent bronchopneumonia that eventuated in a massive empyema. Further, the presence of enteric Proteus mirabilis in the BAL specimen substantiates the aspiration basis of this patient’s portal of infection. In the light of her extensive L. garvieae infection, the degree to which Proteus mirabilis and COVID-19 contributed to our patient’s morbidity and mortality unfortunately must remain moot. However, the absence of Proteus mirabilis in the cultures of the blood and pleural effusion also mitigates against its significant role in the severity of her disease.
Similar to Enterococcus, L. garvieae forms diplococci or short chains, is pyrrolidonylarylamidase (PYR) positive, and demonstrates growth on 6.5% sodium chloride and bile esculin agar. The fact that these morphologic and growth characteristics are shared by L. garvieae and Enterococcus has led to misclassification and underdiagnosis in the past. The organisms in our case were definitively identified by MALDI-TOF mass spectrometry, a diagnostic technology not routinely available in all laboratories. Alternative methods of identification include 16S RNA PCR, API 32 Strep Kit (bioMérieux, France), Vitek 2 with GP identification card (bioMérieux), and BD automated Phoenix System (Becton, Dickinson and Company, USA).1
Antibiotic selection for the treatment of L. garvieae is based on the data gathered from case reports and treatment of viridans-group streptococci and enterococcal infective endocarditis. Depending on organism susceptibilities, the treatment recommendations include ampicillin (2 g q4h), amoxicillin (200 mg/kg/day), or ceftriaxone (2 g q12h). Alternatively, vancomycin (30 mg/kg/day) alone or in combination with an aminoglycoside, such as gentamicin (3 mg/kg/day), may be chosen. Notably, penicillin and clindamycin resistance has been documented.1 The duration of treatment may last from one to six weeks. In our case, recommended therapeutic regimens were administered. However, the rapid demise of the patient precluded what likely would have been a long course of treatment.
Conclusions
Our case of L. garvieae pneumonia rapidly progressed to a massive empyema, which has heretofore been unreported. Awareness of the risk factors associated with L. garvieae can aid in the early recognition, rapid diagnosis, and effective treatment of this rare human pathogen.
Footnotes
Authors’ contributions: Conception and design: ST, FI, IE. Acquisition of data: ST, FI, IE. Analysis and interpretation of data: ST, FI, IE, NY, SL. Drafting of the manuscript: ST, FI, IE. Revision of the manuscript: ST, FI, SL. Final approval of manuscript: FI, IE, ST, NY, SL. All authors read and approved the final version of the manuscript.
Conflicts of interest: All authors – none to declare.
Funding: None to declare.
Consent: Written informed consent was obtained from the next-of-kin for the publication of this case report and the accompanying images.
References
- 1.Malek A, De la Hoz A, Gomez-Villegas SI, Nowbakht C, Arias CA. Lactococcus garvieae, an unusual pathogen in infective endocarditis: case report and review of the literature. BMC Infect Dis. 2019;19:301. doi: 10.1186/s12879-019-3912-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rösch RM, Buschmann K, Brendel L, Schwanz T, Vahl CF. Lactococcus garvieae endocarditis in a prosthetic aortic valve: a case report and literature review. J Investig Med High Impact Case Rep. 2019;7:2324709619832052. doi: 10.1177/2324709619832052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubin GG, Bémer P, Guillouzouic A, et al. First report of a hip prosthetic and joint infection caused by Lactococcus garvieae in a woman fishmonger. J Clin Microbiol. 2011;49:2074–6. doi: 10.1128/JCM.00065-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mofredj A, Baraka D, Cadranel JF, LeMaitre P, Kloeti G, Dumont JL. Lactococcus garvieae septicemia with liver abscess in an immunosuppressed patient. Am J Med. 2000;109:513–4. doi: 10.1016/S0002-9343(00)00534-9. [DOI] [PubMed] [Google Scholar]
- 5.Chao CT, Lai CF, Huang JW. Lactococcus garvieae peritoneal dialysis peritonitis. Perit Dial Int. 2013;33:100–1. doi: 10.3747/pdi.2012.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James PR, Hardman SM, Patterson DL. Osteomyelitis and possible endocarditis secondary to Lactococcus garvieae: a first case report. Postgrad Med J. 2000;76:301–3. doi: 10.1136/pmj.76.895.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tandel K, Bhatt P, Ranjan P, Rathi KR. Meningitis caused by Lactococcus garvieae. Med J Armed Forces India. 2017;73:94–6. doi: 10.1016/j.mjafi.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan JF, Woo PC, Teng JL, et al. Primary infective spondylodiscitis caused by Lactococcus garvieae and a review of human L. garvieae infections. Infection. 2011;39:259–64. doi: 10.1007/s15010-011-0094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JH, Go J, Cho CR, et al. First report of human acute acalculous cholecystitis caused by the fish pathogen Lactococcus garvieae. J Clin Microbiol. 2013;51:712–4. doi: 10.1128/JCM.02369-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tariq EF, Irshad Y, Khalil HB, Khakwani AS, Khan UA. Urinary tract infection caused by the novel pathogen, Lactococcus garvieae: a case report. Cureus. 2020;12:e9462. doi: 10.7759/cureus.9462. [DOI] [PMC free article] [PubMed] [Google Scholar]