Abstract
Background:
Attachment to a regular primary care provider is associated with better health outcomes, but 15% of people in Canada lack a consistent source of ongoing primary care. We sought to evaluate trends in attachment to a primary care provider in Ontario in 2008–2018, through an equity lens and in relation to policy changes in implementation of payment reforms and team-based care.
Methods:
Using linked, population-level administrative data, we conducted a retrospective observational study to calculate rates of patients attached to a regular primary care provider from Apr. 1, 2008, to Mar. 31, 2019. We evaluated the association of patient characteristics and attachment in 2018 using sex-stratified, adjusted, multivariable logistic regression models and used segmented piecewise regression to evaluate changing trends before and after implementation of a policy that restricted physician entry to alternate models.
Results:
Attachment increased from 80.5% (n = 10 352 385) in 2008 to 88.9% of the population (n = 12 537 172) in 2018, but was lower among people with low comorbidity, high residential instability, material deprivation, rural residence and recent immigrants. Inequities narrowed for recent immigrants, males and people with lower incomes over the study period, but disparities persisted for these groups. Attachment grew by 1.47% annually until 2014 (p < 0.0001), but was stagnant thereafter (annual percent change of 0.13, p = 0.16).
Interpretation:
Lack of sustained progress in attachment followed reduced levels of physician entry to alternate funding models. Although disparities narrowed for many groups over the study period, persistent gaps remained for immigrants and people with lower incomes; targeted interventions and policy changes are needed to address these persistent gaps.
Strong primary care is fundamental to effective, efficient and equitable health care systems.1,2 Attachment to a regular primary care provider, defined as formal or informal patient access to the same individual primary care provider or group of providers,3 is associated with delivery of more preventive care, better chronic disease management and lower rates of hospital admission.4–7 Lack of attachment to a primary care provider is associated with higher mortality; higher rates of emergency department visits, hospital admissions and readmissions; presentation to care with more advanced disease; and poor patient experiences.8–10 Some groups are less likely to be attached (e.g., people who are new immigrants, have low income, were previously incarcerated, were prescribed opioid agonist treatment or have serious mental illness).11–17
Despite the importance of consistent primary care access, 14.5% of Canadians aged 12 years and older (about 4.6 million people) reported not having a regular primary care provider in 2019.18 High numbers of unattached patients have important health systems impacts, such as high use emergency department and walk-in clinic use, poor follow-up after hospital discharge and high morbidity.8,9
Understanding trends in primary care attachment is a key policy priority19 and is critical for ensuring effective health system planning that reduces inequities for structurally marginalized groups. Some drivers of attachment include recruitment and retention of family physicians. Professional organizations have called for alternate payment models and expansion of team-based care as factors that can incentivize physicians to practise family medicine.20 Policy changes between 2012 and 2015 to restrict access to alternate payment models may have negatively affected patient attachment, and trends may have differed for some groups. Thus, we sought to evaluate trends in attachment to a primary care provider in Ontario in 2008–2018, through an equity lens and in relation to policy changes in implementation of payment reforms and team-based care.
Methods
Study setting
The study was set in Ontario (population of more than 15 million21), in which family physician and nurse practitioner visits are insured and free at the point of care. In 2002, Ontario increased investment and implemented voluntary reforms in the delivery and payment of primary care aimed at improving access, quality of care and physician retention.22 Under the reforms, most physicians shifted from exclusive fee-for-service remuneration to one of several models that incorporated blended capitation payments, patient enrolment and, in some cases, access to interdisciplinary teams. Several models require patient enrolment (collectively described as patient enrolment models), including those in which physicians are paid by blended capitation (monthly age-and sex-adjusted payments and a small proportion of fee-for-service payments), and those paid by fee for service. Beginning in 2012, the Ontario government began to limit new physicians entering capitation-based models, culminating in 2015, when the government restricted new positions in some patient-enrolment models to 20 per month in areas of high physician need, or to replacement of physicians in existing teams.23
Study design
We conducted a repeated cross-sectional study using population-level administrative data. Study participants included all Ontario residents with a health card number in each year from Apr. 1, 2008, to Mar. 31, 2019.
Data sources and linkages
We used linked administrative data sets to evaluate trends in attachment at the patient level. Using a confidential and secure proprietary algorithm, health card numbers are converted to unique encoded identifiers, and are linked and analyzed at ICES.24 ICES is an independent, nonprofit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, without consent, for health system evaluation and improvement.
We used the Primary Care Population data set (PCPOP), an ICES-derived, population-level data set that includes all eligible people in Ontario. An eligible person would be an Ontario resident who is alive at the index, has had contact with the health care system within 9 years of index and has Ontario Health Insurance Plan (OHIP) eligibility. We linked PCPOP with the Registered Persons Database (a health insurance registry), the Corporate Provider Database (a registry of providers and groups eligible to bill OHIP for their services), the Client Agency Program Enrolment database (which identifies patients enrolled in different primary care models over time) and the Community Health Centre (CHC) database (which lists patients receiving health services at CHCs, nonprofit health centres that provide primary care and health promotion to priority populations in which primary care providers are salaried). We assessed emergency department visits using the National Ambulatory Care Reporting System and hospital admissions using the Discharge Abstracts Database.
Outcome
The dependent variable was the percentage of eligible Ontario residents attached to a primary care provider, identified in administrative data using an algorithm developed and validated by our group against survey responses, with excellent sensitivity (90.5%) and modest specificity (46.1%).25 The algorithm involved hierarchical assignment of attachment. First, patients enrolled to a patient enrolment model were considered attached. Next, patients receiving clinical care at a community health centre were included as attached. Next, patients were included as attached if they were virtually rostered to a primary care provider with the highest billings for that patient, with higher physician-level continuity of care. We sought to limit categorizing virtually rostered patients who received a substantial proportion of their care from physicians with low continuity of care for their patients, such as those practising in walk-in clinics. Therefore, virtually rostered patients were considered attached only if they received most of their primary care over the preceding 2-year period from a primary care provider with greater than 10% physician-level continuity of care. Physician-level continuity of care is a visit-based measure of the proportion of patients receiving ongoing care with the same provider and was determined with a numerator of patients virtually rostered to a primary care provider divided by the denominator of all unique patients the same primary care provider has seen over 2 years. Finally, and consistent with a previously validated algorithm used to evaluate access to pediatric health services,26 children who were virtually attached to a primary care pediatrician were also considered attached.25 All others were considered uncertainly attached (described in additional detail in Appendix 1, available at www.cmajopen.ca/content/11/5/E809/suppl/DC1).
Covariates
We derived age, sex, rurality and immigration status from the Registered Persons Database. We measured rurality using the postal code and the Rurality Index for Ontario, categorized as urban (score 0–9), suburban (score 10–39) and rural (score ≥ 40).27 We used postal codes and the Ontario Marginalization Index to derive participants’ Material Deprivation and Residential Instability quintiles. The Ontario Marginalization Index is an area-based index derived using variables from the Census that seeks to understand differences in health between population groups or between geographical areas.28 Material deprivation includes indicators such as the proportion of the adult population who are lone-parent families, are receiving government transfer payments, are low income, are unemployed or have no high school diploma. Residential instability is a measure of area-level concentration of people who experience high rates of family or housing instability and includes indicators of the proportion of people living alone, the proportion of dwellings that are apartment buildings and the proportion of the population who have moved in the previous 5 years. We identified people with first-time health care coverage in Ontario within the previous 10 years, most of whom are recent immigrants to Canada.29 We used the Johns Hopkins Adjusted Clinical Groups System Version 10 to capture comorbidity according to Aggregated Diagnostic Groups (ADGs), in which the diagnostic codes describing each person’s health conditions are assigned to 1 or more of 32 diagnostic groups based on clinical and expected health services use.30 We used hospital admissions and OHIP claims from the preceding 2 years to determine the ADGs and Resource Utilization Bands, which are robust and validated measures of comorbidity and expected resource use. We categorized ADGs as low (0–4 ADGs), moderate (5–9 ADGs) or high comorbidity (≥ 10 ADGs). We categorized Resource Utilization Bands as nonuser or healthy user (0–1), low (2), moderate (3) or high expected resource use (≥ 4).
Statistical analysis
We identified attached and uncertainly attached populations for each year between 2008/09 and 2018/19, their characteristics and annual rates of emergency department visits and hospital admissions. We evaluated changes in attachment over time, stratified by demographic group. Next, we used logistic regression models using complete case analysis to evaluate the association between patient characteristics and attachment in 2018/19, adjusting for sex, age, rurality, comorbidity, resource utilization, recent immigration (≤ 10 yr v. those who had immigrated > 10 yr previously or those who were born in Canada), material deprivation and residential instability. We tested for and identified an interaction between age and sex, and developed stratified multivariable models for males and females of factors associated with attachment in 2018, using prespecified variables selected a priori from published literature. We did not use a model section process. Tolerance and variance inflation factors were consistent with lack of multi-collinearity in the multivariable models.
To assess the association with restricted entry to alternate funding models in 2015, we used segmented piecewise linear regression models with correlated residuals, including year, policy change in 2015 and time after policy change as predictors. We tested for and found no evidence of autocorrelation (β for AR(1) = 0.57, p = 0.39, AR(2) = 0.59, p = 0.19). Therefore, we dropped the autoregressive terms from the regression model and included only time before and time after the policy change in the model.
We completed all analyses with SAS Enterprise Edition.
Ethics approval
The use of the data in this project is authorized under section 45 of Ontario’s Personal Health Information Protection Act and does not require review by a Research Ethics Board.
Results
In 2008, 10 352 385 (80.5%) of 12 863 036 eligible Ontario residents were attached to a primary care provider (Appendix 2, available at www.cmajopen.ca/content/11/5/E809/suppl/DC1). Attachment increased over the study period to 12 537 172 (88.9%) of the 14 096 100 population in 2018. The characteristics of the attached and general population are summarized in 2008, 2014 and 2018 (Table 1). Proportionately fewer males were attached at baseline (77.4% v. 83.5% females) and in 2018 (86.9% v. 90.9% females). Young adults (aged 19–34 yr) had lower rates of attachment compared with all other age groups at baseline (71.5%) and study end (83.6%). Children and youth had the highest rates of attachment, followed by older adults. Attachment was lower among those who lived in rural areas, those with low comorbidity, those with the highest residential instability, those with the highest material deprivation and recent immigrants throughout the study period. About 25% of uncertainly attached people visited the emergency department, which remained stable throughout the study period. Rates of hospital admission for uncertainly attached patients decreased from 12.1% in 2008 to 9.8% in 2018. Health system use was higher for attached patients, of whom about 37% visited the emergency department and 20%–22% were admitted to hospital in a given year.
Table 1:
Patient demographic characteristics
| Variable | 2008 | 2014 | 2018 | Difference 2018–2008, % | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| No. (%) of attached patients | Total population | No. (%) of attached patients | Total population | No. (%) of attached patients | Total population | Absolute difference | Relative difference | |
| Overall | 10 352 385 (80.5) | 12 863 036 | 11 972 070 (88.1) | 13 371 946 | 12 537 172 (88.9) | 14 096 100 | 8.4 | 10.4 |
| Sex | ||||||||
|
| ||||||||
| Male | 4 902 611 (77.4) | 6 336 768 | 5 731 257 (86.3) | 6 641 622 | 6 021 636 (86.9) | 6 928 191 | 9.5 | 12.3 |
|
| ||||||||
| Female | 5 449 774 (83.5) | 6 526 268 | 6 240 813 (90.6) | 6 886 323 | 6 515 536 (90.9) | 7 167 909 | 7.4 | 8.9 |
|
| ||||||||
| Age category, yr | ||||||||
|
| ||||||||
| < 19 | 2 731 580 (91.6) | 2 983 281 | 2 707 855 (93.7) | 2 889 839 | 2 688 182 (93.6) | 2 872 967 | 2 | 2.2 |
|
| ||||||||
| 19–34 | 1 941 613 (71.5) | 2 713 735 | 2 387 721 (82.8) | 2 883 509 | 2 491 779 (83.6) | 2 979 286 | 12.1 | 16.9 |
|
| ||||||||
| 35–49 | 2 345 430 (76.3) | 3 073 175 | 2 468 965 (86.4) | 2 856 163 | 2 471 632 (86.7) | 2 850 490 | 10.4 | 13.6 |
|
| ||||||||
| 50–64 | 1 947 237 (80.2) | 2 429 426 | 2 536 267 (89.0) | 2 849 501 | 2 708 959 (89.6) | 3 024 685 | 9.4 | 11.7 |
|
| ||||||||
| 65–79 | 1 038 837 (83.3) | 1 246 586 | 1 402 343 (91.3) | 1 536 482 | 1 646 130 (91.9) | 1 791 552 | 8.6 | 10.3 |
|
| ||||||||
| ≥ 80 | 347 688 (83.4) | 416 833 | 468 919 (91.5) | 512 451 | 530 490 (91.9) | 577 120 | 8.5 | 10.2 |
|
| ||||||||
| Rurality Index for Ontario | ||||||||
|
| ||||||||
| Urban (0–9) | 7 397 897 (79.8) | 9 275 239 | 8 692 101 (88.2) | 9 855 613 | 9 144 956 (88.8) | 10 302 737 | 9 | 11.3 |
|
| ||||||||
| Small town (10–39) | 2 116 215 (84.8) | 2 496 232 | 2 345 182 (90.9) | 2 579 570 | 2 434 140 (90.9) | 2 676 741 | 6.1 | 7.2 |
|
| ||||||||
| Rural (≥ 40) | 765 279 (78.2) | 978 283 | 857 518 (87.4) | 980 713 | 874 527 (87.9) | 994 441 | 9.7 | 12.4 |
|
| ||||||||
| Missing | 72 994 (64.4) | 113 282 | 77 269 (69.0) | 112 049 | 83 549 (68.4) | 122 181 | 4 | 6.2 |
|
| ||||||||
| Comorbidity (ADG) | ||||||||
|
| ||||||||
| No or low comorbidity (0–4) | 4 977 558 (73.3) | 6 791 348 | 6 068 182 (83.8) | 7 245 411 | 6 237 180 (84.0) | 7 427 923 | 10.7 | 14.6 |
|
| ||||||||
| Moderate comorbidity (5–9) | 4 272 094 (88.7) | 4 816 930 | 4 625 684 (94.0) | 4 920 446 | 4 859 500 (94.5) | 5 142 000 | 5.8 | 6.5 |
|
| ||||||||
| High comorbidity (≥ 10) | 1 102 733 (87.9) | 1 254 758 | 1 278 204 (93.8) | 1 362 088 | 1 440 492 (94.4) | 1 526 177 | 6.5 | 7.4 |
|
| ||||||||
| Resource Utilization Band | ||||||||
|
| ||||||||
| Nonuser or healthy user (0–1) | 1 026 238 (48.4) | 2 118 830 | 1 472 205 (67.5) | 2 182 561 | 1 539 471 (67.3) | 2 286 918 | 18.9 | 39.1 |
|
| ||||||||
| Low morbidity (2) | 2 218 280 (84.8) | 2 616 422 | 2 457 443 (90.6) | 2 711 249 | 2 454 723 (91.0) | 2 696 051 | 6.2 | 7.3 |
|
| ||||||||
| Moderate morbidity (3) | 5 248 159 (87.5) | 5 999 986 | 5 818 534 (93.0) | 6 254 661 | 6 042 110 (93.6) | 6 452 615 | 6.1 | 7.0 |
|
| ||||||||
| High morbidity (≥ 4) | 1 859 708 (87.4) | 2 127 798 | 2 223 888 (93.5) | 2 379 474 | 2 500 868 (94.0) | 2 660 516 | 6.6 | 7.6 |
|
| ||||||||
| Recent immigrant | ||||||||
|
| ||||||||
| No | 7 920 620 (80.1) | 9 882 644 | 9 466 538 (88.6) | 10 682 618 | 10 045 967 (89.0) | 11 287 661 | 8.9 | 11.1 |
|
| ||||||||
| Yes | 924 122 (67.8) | 1 363 337 | 970 576 (79.8) | 1 216 706 | 975 069 (81.4) | 1 198 483 | 13.6 | 20.1 |
|
| ||||||||
| Residential instability quintile | ||||||||
|
| ||||||||
| 1 (lowest instability) | 2 245 592 (83.8) | 2 678 771 | 2 746 156 (90.9) | 3 019 913 | 2 858 167 (91.3) | 3 130 363 | 7.5 | 9.0 |
|
| ||||||||
| 2 | 2 091 120 (83.3) | 2 511 738 | 2 311 451 (90.4) | 2 556 842 | 2 412 349 (90.6) | 2 661 479 | 7.3 | 8.8 |
|
| ||||||||
| 3 | 1 917 243 (82.1) | 2 335 277 | 2 142 267 (89.5) | 2 393 882 | 2 280 527 (89.9) | 2 535 978 | 7.8 | 9.5 |
|
| ||||||||
| 4 | 1 893 272 (79.6) | 2 377 687 | 2 136 073 (88.1) | 2 425 107 | 2 213 598 (88.5) | 2 500 126 | 8.9 | 11.2 |
|
| ||||||||
| 5 (highest instability) | 2 087 599 (75.1) | 2 780 816 | 2 524 839 (84.9) | 2 972 369 | 2 671 039 (85.5) | 3 123 843 | 10.4 | 13.9 |
|
| ||||||||
| Material deprivation quintile | ||||||||
|
| ||||||||
| 1 (lowest deprivation) | 2 381 696 (83.4) | 2 857 306 | 2 623 982 (90.2) | 2 910 272 | 2 893 438 (90.4) | 3 201 555 | 7.0 | 8.4 |
|
| ||||||||
| 2 | 2 099 290 (82.5) | 2 545 256 | 2 518 205 (90.2) | 2 791 259 | 2 663 134 (90.5) | 2 942 539 | 8 | 9.7 |
|
| ||||||||
| 3 | 1 982 173 (81.0) | 2 447 798 | 2 297 416 (89.1) | 2 577 049 | 2 382 518 (89.6) | 2 659 189 | 8.6 | 10.6 |
|
| ||||||||
| 4 | 1 863 131 (79.4) | 2 346 986 | 2 199 123 (87.9) | 2 503 068 | 2 244 028 (88.3) | 2 540 744 | 8.9 | 11.2 |
|
| ||||||||
| 5 (highest deprivation) | 1 908 536 (76.7) | 2 486 943 | 2 222 060 (85.9) | 2 586 465 | 2 252 562 (86.4) | 2 607 762 | 9.7 | 12.7 |
|
| ||||||||
| ED visit in previous 2 years | ||||||||
|
| ||||||||
| Yes | 3 760 038 (85.4) | 4 403 177 | 4 397 211 (91.5) | 4 805 605 | 4 708 543 (92.1) | 5 113 652 | 6.7 | 7.5 |
|
| ||||||||
| No | 6 592 347 (77.9) | 8 459 859 | ||||||
|
| ||||||||
| Hospital admission in previous 2 years | ||||||||
|
| ||||||||
| Yes | 2 338 830 (88.5) | 2 642 562 | 2 551 439 (93.8) | 2 719 265 | 4 708 543 (92.1) | 2 761 144 | 3.6 | 4.1 |
|
| ||||||||
| No | 8 013 555 (78.4) | 10 220 474 | ||||||
Note: ADG = Aggregated Diagnostic Group, ED = emergency department.
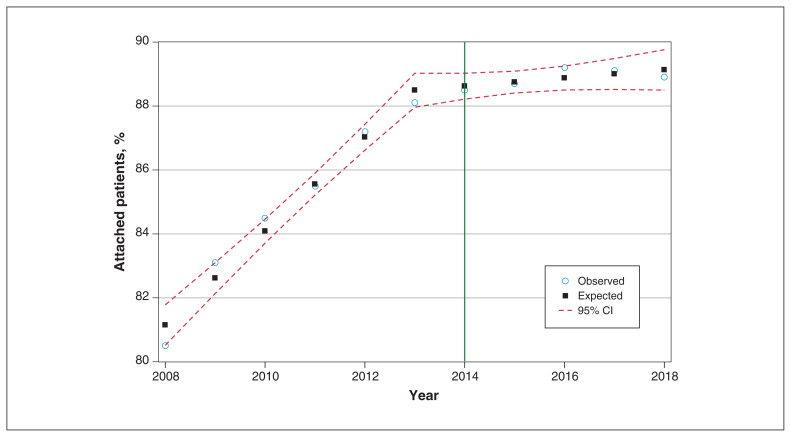
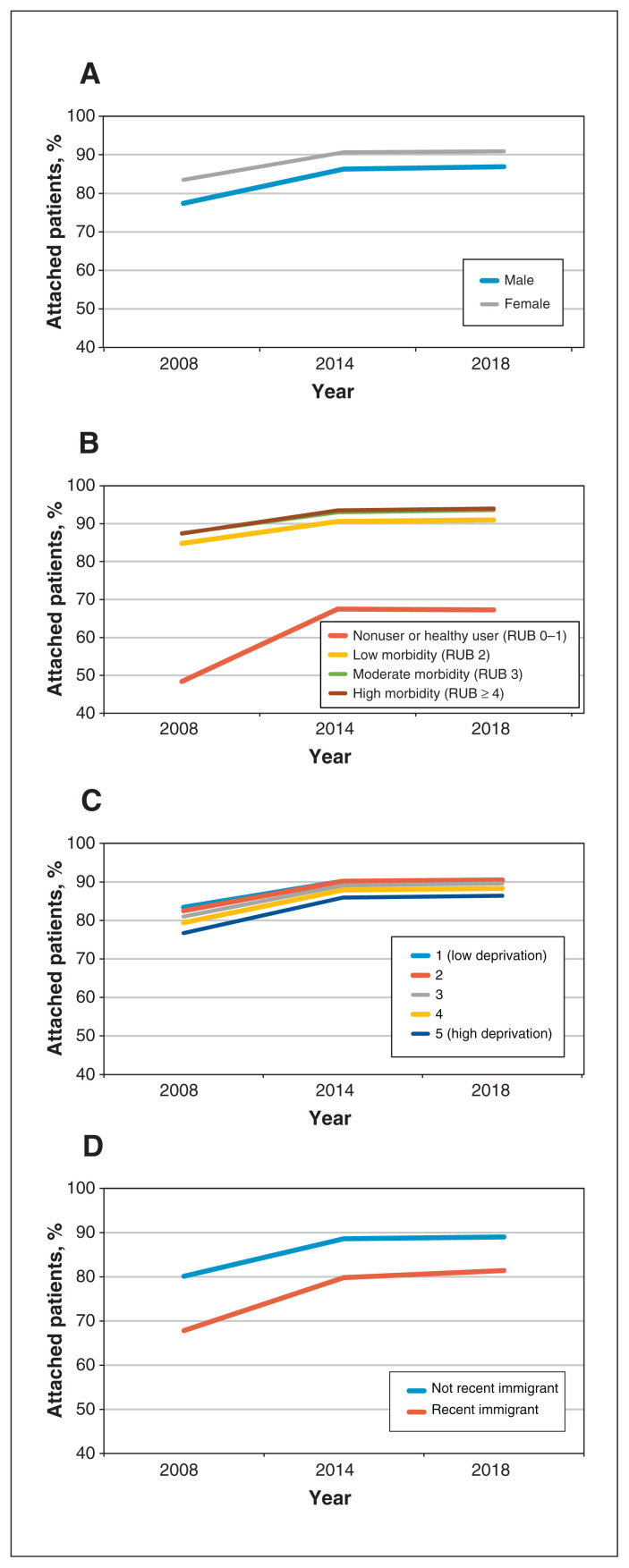
Attachment increased over the study period overall and for all demographic groups, with the largest relative gains seen among new immigrants, patients aged 19–34 years and patients with low comorbidity. Overall, we observed gains between 2008 and 2014, after which attachment plateaued (Figure 1). Gaps between some groups narrowed from 2008 to 2014, after which the rate of change slowed overall (Figure 2). The disparity for recent immigrants continued to close after 2014, though more slowly than before 2014. We observed rapid gains in the proportion of attached patients among those with low comorbidity until 2014, after which the rate was essentially unchanged. We observed limited reduction in disparities by material deprivation between 2008 and 2014, but the gap continued to close throughout the study period.
Figure 1:
Proportion of patients attached to a primary care provider, 2008–2018. Note: CI = confidence interval.
Figure 2:
Proportion of patients attached to a primary care provider, 2008–2018 by (A) sex, (B) Resource Utilization Band (RUB), (C) material deprivation quintile and (D) recent immigrant status.
We used sex-stratified, unadjusted, single variable (Table 2) and multivariable models of 2018 data to further evaluate predictors of attachment (Table 3). Compared with adults aged 50–64 years, children and youth were most likely to be attached (males: adjusted odds ratio [OR] 2.70, 95% confidence interval [CI] 2.67–2.73; females: adjusted OR 2.40, 95% CI 2.37–2.43). Adults aged 19–34 years were least likely to be attached (males: adjusted OR 0.86, 95% CI 0.86–0.87; females: 0.83, 95% CI 0.83–0.84). Older adult males were more likely to be attached to a provider, but not older females. Males and females with moderate-to-high comorbidity had higher odds of attachment, as did those with moderate-to-high health care use. Urban and small-town residents had higher odds of attachment than those living in rural areas.
Table 2:
Unadjusted, single-predictor logistic regression models for association between patient characteristics and patient attachment in 2018, stratified by sex
| Variable | OR (95% CI) | |
|---|---|---|
| Male n = 6 009 381 |
Female n = 6 297 372 |
|
| Age category, yr | ||
| < 19 | 1.99 (1.97–2.01) | 1.41 (1.40–1.42) |
| 19–34 | 0.57 (0.57–0.57) | 0.64 (0.63–0.64) |
| 35–49 | 0.71 (0.70–0.71) | 0.83 (0.82–0.84) |
| 50–64 | Ref. | Ref. |
| 65–79 | 1.45 (1.44–1.47) | 1.16 (1.15–1.17) |
| ≥ 80 | 1.54 (1.52–1.57) | 1.10 (1.08–1.11) |
| Rurality Index for Ontario | ||
| Urban (0–9) | 1.08 (1.07–1.09) | 1.07 (1.06–1.08) |
| Small town (10–39) | 1.33 (1.32–1.34) | 1.44 (1.42–1.45) |
| Rural (≥ 40) | Ref. | Ref. |
| Comorbidity (ADG) | ||
| No or low comorbidity (0–4) (Ref.) | Ref. | Ref. |
| Moderate comorbidity (5–9) | 3.33 (3.31–3.35) | 3.03 (3.01–3.05) |
| High comorbidity (≥ 10) | 3.28 (3.24–3.32) | 2.89 (2.86–2.92) |
| Morbidity (Resource Utilization Band) | ||
| Nonuser or healthy user (0–1) | Ref. | Ref. |
| Low comorbidity (2) | 4.38 (4.36–4.41) | 5.85 (5.80–5.89) |
| Moderate morbidity (3) | 6.51 (6.47–6.54) | 7.67 (7.62–7.71) |
| High morbidity (≥ 4) | 7.13 (7.07–7.19) | 7.64 (7.58–7.71) |
| Recent immigrant | ||
| No | Ref. | Ref. |
| Yes | 0.56 (0.56–0.56) | 0.50 (0.50–0.51) |
| Residential instability quintile | ||
| 1 (lowest instability) | Ref. | Ref. |
| 2 | 0.90 (0.89–0.91) | 0.95 (0.94–0.96) |
| 3 | 0.82 (0.82–0.83) | 0.89 (0.88–0.89) |
| 4 | 0.70 (0.70–0.71) | 0.77 (0.77–0.78) |
| 5 (highest instability) | 0.53 (0.53–0.54) | 0.59 (0.58–0.59) |
| Material deprivation quintile | ||
| 1 (lowest deprivation) | Ref. | Ref. |
| 2 | 1.01 (1.00–1.01) | 1.03 (1.02–1.03) |
| 3 | 0.90 (0.90–0.91) | 0.94 (0.93–0.94) |
| 4 | 0.78 (0.78–0.79) | 0.83 (0.82–0.84) |
| 5 (highest deprivation) | 0.65 (0.64–0.65) | 0.71 (0.71–0.72) |
Note: ADG = Aggregated Diagnostic Group, CI = confidence interval, OR = odds ratio, Ref. = reference category.
Table 3:
Multivariable logistic regression for association between patient characteristics and patient attachment in 2018, stratified by sex
| Variable | OR (95% CI) | |
|---|---|---|
| Male n = 6 009 381 |
Female n = 6 297 372 |
|
| Intercept | 2.23 (2.20–2.26) | 2.39 (2.35–2.43) |
| Age category, yr | ||
| < 19 v. 50–64 | 2.70 (2.67–2.73) | 2.40 (2.37–2.43) |
| 19–34 v. 50–64 | 0.86 (0.86–0.87) | 0.83 (0.83–0.84) |
| 35–49 v. 50–64 | 0.92 (0.91–0.92) | 1.01 (1.00–1.02) |
| 65–79 v. 50–64 | 1.13 (1.12–1.14) | 1.00 (0.99–1.01) |
| ≥ 80 v. 50–64 | 1.14 (1.13–1.16) | 0.91 (0.90–0.92) |
| Rurality Index for Ontario | ||
| Urban v. rural | 1.11 (1.10–1.12) | 1.11 (1.09–1.12) |
| Small town v. rural | 1.28 (1.27–1.30) | 1.35 (1.33–1.37) |
| Comorbidity (ADG) | ||
| Moderate v. low comorbidity | 1.41 (1.40–1.42) | 1.33 (1.31–1.34) |
| High v. low comorbidity | 1.58 (1.56–1.61) | 1.36 (1.34–1.38) |
| Resource Utilization Band | ||
| Low user v. nonuser | 3.90 (3.87–3.93) | 5.43 (5.38–5.48) |
| Moderate user v. nonuser | 5.32 (5.28–5.36) | 6.95 (6.89–7.01) |
| High user v. nonuser | 4.82 (4.76–4.89) | 7.07 (6.98–7.16) |
| Recent immigrant | ||
| Immigrant v. nonimmigrant | 0.63 (0.63–0.64) | 0.60 (0.59–0.60) |
| Residential instability quintile | ||
| Q2 v. Q1 (lowest instability) | 0.93 (0.92–0.94) | 0.94 (0.93–0.95) |
| Q3 v. Q1 (lowest instability) | 0.88 (0.88–0.89) | 0.91 (0.90–0.92) |
| Q4 v. Q1 (lowest instability) | 0.81 (0.81–0.82) | 0.84 (0.83–0.85) |
| Q5 (highest) v. Q1 (lowest instability) | 0.67 (0.67–0.68) | 0.72 (0.71–0.73) |
| Material deprivation quintile | ||
| Q1 v. Q1 (lowest deprivation) | 0.98 (0.97–0.99) | 0.98 (0.98–0.99) |
| Q3 v. Q1 (lowest deprivation) | 0.92 (0.91–0.93) | 0.93 (0.92–0.94) |
| Q4 v. Q1 (lowest deprivation) | 0.85 (0.84–0.86) | 0.87 (0.86–0.88) |
| Q5 (highest) v. Q1 (lowest deprivation) | 0.75 (0.75–0.76) | 0.80 (0.79–0.80) |
Note: ADG = Aggregated Diagnostic Group, CI = confidence interval, OR = odds ratio.
However, we also identified lower odds of attachment for people who had recently immigrated to Ontario (males: adjusted OR 0.63, 95% CI 0.63–0.64; females: adjusted OR 0.60, 95% CI 0.59–0.60). In addition, we observed lower odds of attachment for those with higher residential instability (highest instability males: adjusted OR 0.67, 95% CI 0.67–0.68; highest instability females: adjusted OR 0.72, 95% CI 0.71–0.73) and higher material deprivation (adjusted OR highest deprivation males 0.75 [0.75–0.76], females 0.80 [0.79–0.80]). Both marginalization measures followed a gradient by quintile, with lower odds of attachment for more vulnerable males than females.
We modelled change in the percentage of attached patients using segmented regression models, including initial slope, intercept and a paravermis at 2014 as variables, with correlated residuals. Given the lack of evidence of either first-or second-order autocorrelation, we assumed the residuals to be independent and thus dropped the autoregressive terms. We observed a significant trend before 2014 (slope = 1.47% increase in attachment rate per year, p < 0.0001), which flattened after 2014 (slope = 0.13%, p = 0.16).
Interpretation
The crude number of attached patients increased by 21.1% over the study period, a rate in excess of population growth (9.6%), but plateaued after 2014, when it matched but no longer exceeded population growth.
Rapid growth in attachment occurred during the period of growth policy reforms, including new models of primary care based on patient enrolment and blended capitation payment. Attachment plateaued around the time that the Ontario government restricted entry to blended capitation models, many of which were also interprofessional teams.31 From 2012 to 2015, primary care was affected by a series of policy changes aimed at containing costs, including restricted access to new family health teams, government-imposed fee cuts and discontinuation of a new patient fee code. Finally, expansion of alternate models was limited to physicians practising in underserved areas or addressing attrition within existing teams. In 2015, 122 physicans entered these new models, compared with 489 in 2014. Our results support a strong rationale for investment in funding reform and expansion of interdisciplinary teams in primary care. Expansion of patient enrolment models was included in the recently approved Ontario Physician Services Agreement, although specific implementation details remain unclear.32
A substantial proportion of uncertainly attached people had frequent contact with the health system, including about 25% with an emergency department visit and 10%–12% who were admitted to hospital in a given year. Although these proportions were lower than those seen for attached people (38% with an emergency department visit and 21%–23% admitted to hospital), each of these encounters represents an opportunity for attachment, which will require appropriate policy innovations.
Overall equity in attachment improved. In contrast to other jurisdictions, we found higher attachment among people with higher comorbidity, likely because those with lower comorbidity were less likely to seek care, and therefore had fewer enrolment opportunities. However, important gaps in attachment remained for specific groups, particularly new immigrants and people living with economic and residential insecurity. Targeted interventions are needed to reach these communities, who have not benefited as much from policy reforms.33
In other jurisdictions, attachment has either decreased or remained fixed over time. In the United States, attachment among adults decreased from 77% (95% CI 76%–78%) in 2002 to 75% (95% CI 74%–76%) in 2015 (adjusted OR 0.90, 95% CI 0.82–0.98).34 Another study reported reduced attachment of older adults from 94.2% in 2010 to 91.0% in 2016 (p < 0.0001).35 Both studies found lower attachment among males, people with lower incomes or those whose race or ethnicity was Black or Latino, even after controlling for insurance status. In New Zealand, 93%–95% of the population was enrolled in primary care from 2015 to 2019, with lower attachment among Maori people and those living with higher deprivation.13
Nationally, Ontario has the lowest proportion of residents who are unattached to a primary care provider.18 Data from the Canadian Community Health Survey show that Quebec and the Western provinces fare considerably worse and, nationwide, more than 4.5 million people in Canada do not have access to a regular primary care provider.18 Some provinces have established centralized wait lists to improve attachment.19 Cross-sectional studies have shown increased attachment with this strategy; however, people with fewer comorbid conditions were preferentially enrolled and demand exceeded primary care capacity.36,37 Longitudinal analyses of centralized waitlists are underway. Additional measures taken in Canada include payment reforms, implementation of interdisciplinary teams, specific fee codes for attachment of complex patients, expansion of nurse practitioner roles and geographic attachment.38 Our work underscores the importance of payment reform and interdisciplinary team models for supporting attachment.39
Overall gains in attachment may be threatened by upcoming trends in health human resources. About 14.4% of Ontario family physicians are aged 65 years and older,40 and the mean age of retirement is 70.5 years.41 Increased pressures during the COVID-19 pandemic have accelerated retirement plans of older physicians,42 and almost 20% of Toronto primary care providers report considering closing their practice in the next 5 years.43 In addition, the comprehensiveness of practice has been decreasing.44 Overall patient panel sizes are reduced in all career phases41 and practice patterns are shifting away from comprehensive primary care practices to more focused practices and roles in hospital and emergency departments. 45 The combined impact of fewer medical students ranking family medicine as their first choice for residency training46 and an aging family physician workforce47 suggest upcoming problems in health human resources, which could substantially erode the gains observed in our study.
Limitations
Administrative data cannot be used to track services provided by nurse practitioners, except in CHCs. In Ontario, 25 nurse practitioner–led clinics serve around 100 000 patients, largely located in rural and remote settings.48 Although they play an important role in these communities, the volume of service is unlikely to change the overall trends. In addition, although the attachment algorithm showed high sensitivity, specificity was more modest, meaning that some uncertainly attached individuals may have been misclassified. In addition, measures of income and residential instability were all determined at a neighbourhood level using Census data. Area-level measures are economical and widely used to examine population-level differences, but are limited by their inability to capture variation within neighbourhoods.49 Some young adults without clear primary care providers may have been temporarily living outside Ontario, which we could not identify in our data. We also could not assess the quality of attachment or whether unattached patients were seeking attachment. Finally, the associations found do not imply causation and additional unmeasured reasons may contribute to lack of attachment to a primary care provider.
Conclusion
Attachment to a primary care provider in Ontario increased between 2008 and 2014, but was unchanged after 2014, following reduced physician entry to alternate funding and interdisciplinary team models. Targeted interventions are needed to address persistent gaps for immigrants and people with low incomes. Upcoming trends in health human resources may erode the gains seen. Future research should use robust longitudinal designs to evaluate trends during the COVID-19 pandemic and health outcomes associated with attachment for different patient populations.
Supplementary Material
Acknowledgement
The authors thank Dr. Rahim Moineddin for assistance with the statistical analysis.
Footnotes
Competing interests: Kamila Premji reports research funding from the University of Ottawa Faculty of Medicine and Institut du Savoir Montfort; consulting fees from the Ontario College of Family Physicians and the Ontario Medical Association; honoraria from the Centre for Effective Practice and the Ontario College of Family Physicians; travel support from INSPIRE-PHC-2 and the University of Ottawa Department of Family Medicine; and roles as chair of the board of directors with the Canadian Women in Medicine and as member of the Ontario Provincial Primary Care Advisory Table. Michael Green reports research funding from the Canadian Institutes of Health Research, Ontario Health, the Ontario College of Family Physicians and the College of Family Physicians of Canada; honoraria from the University of Toronto and The Ottawa Hospital; and travel support from the Frederick National Laboratory. He is president-elect of the College of Family Physicians of Canada, a board member with AMS Healthcare and a medical advisor with the Lawson Foundation. No other competing interests were declared.
This article has been peer reviewed.
Contributors: All of the authors contributed to the conception and design of the work, and to data acquisition, analysis and interpretation. All of the authors drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was supported by the Innovations Strengthening Primary Health Care through Research (INSPIRE PHC) program, which was funded through the Ontario Ministry of Health. This study was also supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health. Michael Green is supported by the Queen’s University Brian Hennen Chair in Family Medicine.
Disclaimer: The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication. This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and Ministry of Long-Term Care (MLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOH or MLTC is intended or should be inferred.
Data sharing: The data set from this study is held securely in coded form at ICES. Although legal data sharing agreements between ICES and data providers (e.g., health care organizations and government) prohibit ICES from making the data set publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at https://www.ices.on.ca/DAS (email: das@ices.on.ca). The full data set creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/11/5/E809/suppl/DC1.
References
- 1.Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457–502. doi: 10.1111/j.1468-0009.2005.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Realising the potential of primary health care. Paris (FR): Organisation for Economic Co-operation and Development; 2020. [Google Scholar]
- 3.Marshall EG, Breton M, Green M, et al. CUP study: protocol for a comparative analysis of centralised waitlist effectiveness, policies and innovations for connecting unattached patients to primary care providers. BMJ Open. 2022;12:e049686. doi: 10.1136/bmjopen-2021-049686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosano A, Loha CA, Falvo R, et al. The relationship between avoidable hospitalization and accessibility to primary care: a systematic review. Eur J Public Health. 2013;23:356–60. doi: 10.1093/eurpub/cks053. [DOI] [PubMed] [Google Scholar]
- 5.Wolters RJ, Braspenning JCC, Wensing M. Impact of primary care on hospital admission rates for diabetes patients: a systematic review. Diabetes Res Clin Pract. 2017;129:182–96. doi: 10.1016/j.diabres.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 6.DeVoe JE, Fryer GE, Phillips R, et al. Receipt of preventive care among adults: insurance status and usual source of care. Am J Public Health. 2003;93:786–91. doi: 10.2105/ajph.93.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McIsaac WJ, Fuller-Thomson E, Talbot Y. Does having regular care by a family physician improve preventive care? Can Fam Physician. 2001;47:70–6. [PMC free article] [PubMed] [Google Scholar]
- 8.Peel A, Gutmanis I, Bon T. Disparities in health outcomes among seniors without a family physician in the North West Local Health Integration Network: a retrospective cohort study. CMAJ Open. 2019;7:E94–100. doi: 10.9778/cmajo.20180004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duero Posada JG, Moayedi Y, Zhou L, et al. Clustered emergency room visits following an acute heart failure admission: a population-based study. J Am Heart Assoc. 2018;7:e007569. doi: 10.1161/JAHA.117.007569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crooks VA, Agarwal G, Harrison A. Chronically ill Canadians’ experiences of being unattached to a family doctor: a qualitative study of marginalized patients in British Columbia. BMC Fam Pract. 2012;13:69. doi: 10.1186/1471-2296-13-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hay C, Pacey M, Bains N, et al. Understanding the unattached population in Ontario: evidence from the Primary Care Access Survey (PCAS) Healthc Policy. 2010;6:33–47. [PMC free article] [PubMed] [Google Scholar]
- 12.Batista R, Pottie KC, Dahrouge S, et al. Impact of health care reform on enrolment of immigrants in primary care in Ontario, Canada. Fam Pract. 2019;36:445–51. doi: 10.1093/fampra/cmy082. [DOI] [PubMed] [Google Scholar]
- 13.Irurzun-Lopez M, Jeffreys M, Cumming J. The enrolment gap: who is not enrolling with primary health organizations in Aotearoa New Zealand and what are the implications? An exploration of 2015–2019 administrative data. Int J Equity Health. 2021;20:93. doi: 10.1186/s12939-021-01423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talbot Y, Fuller-Thomson E, Tudiver F, et al. Canadians without regular medical doctors. Who are they? Can Fam Physician. 2001;47:58–64. [PMC free article] [PubMed] [Google Scholar]
- 15.Bradford DW, Kim MM, Braxton LE, et al. Access to medical care among persons with psychotic and major affective disorders. Psychiatr Serv. 2008;59:847–52. doi: 10.1176/ps.2008.59.8.847. [DOI] [PubMed] [Google Scholar]
- 16.Kouyoumdjian F, Kim M, Kiran T, et al. Attachment to primary care and team-based primary care: retrospective cohort study of people who experienced imprisonment in Ontario. Can Fam Physician. 2019;65:e433–42. [PMC free article] [PubMed] [Google Scholar]
- 17.Spithoff S, Kiran T, Khuu W, et al. Quality of primary care among individuals receiving treatment for opioid use disorder. Can Fam Physician. 2019;65:343–51. [PMC free article] [PubMed] [Google Scholar]
- 18.Primary health care providers, 2019. Ottawa: Statistics Canada; 2020. [accessed 2022 Jan. 24]. Available https://www150.statcan.gc.ca/n1/en/pub/82-625-x/2020001/article/00004-eng.pdf?st=TYoGj5oC. [Google Scholar]
- 19.McKay M, Lavergne MR, Lea AP, et al. Government policies targeting primary care physician practice from 1998–2018 in three Canadian provinces: a jurisdictional scan. Health Policy. 2022;126:565–75. doi: 10.1016/j.healthpol.2022.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Strengthening health care: access done right [position statement] Mississauga (ON): The College of Family Physicians of Canada; 2021. Aug 30, [accessed 2022 Nov. 16]. Available: https://www.cfpc.ca/CFPC/media/Resources/Health-Care-Delivery/Access-Done-Right_ENG_Final.pdf. [Google Scholar]
- 21.Ontario Demographic Quarterly: highlights of first quarter. Oshawa (ON): Minister of Finance Tax Office; 2020. [accessed 2022 Nov. 16]. updated 2022 Dec. 15. Available: https://www.ontario.ca/page/ontario-demographic-quarterly-highlights-first-quarter. [Google Scholar]
- 22.Hutchison B, Glazier RH. Ontario’s primary care reforms have transformed the local care landscape, but a plan is needed for ongoing improvement. Health Aff (Millwood) 2013;32:695–703. doi: 10.1377/hlthaff.2012.1087. [DOI] [PubMed] [Google Scholar]
- 23.Areas of high physician need. Toronto: ServiceOntario — INFOline; [accessed 2022 Apr. 30]. modified 2020 Mar. 26. Available: https://www.health.gov.on.ca/en/pro/programs/highneed/income_stabilization.aspx. [Google Scholar]
- 24.Schull MJ, Azimaee M, Marra M, et al. ICES: data, discovery, better health. Int J Popul Data Sci. 2020;4:1135. doi: 10.23889/ijpds.v4i2.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaakkimainen L, Bayoumi I, Glazier R, et al. Development and validation of an algorithm using health administrative data to define patient attachment to primary care providers. J Health Organ Manag. 2021;35:733–43. doi: 10.1108/JHOM-05-2020-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guttmann A, Shipman SA, Lam K, et al. Primary care physician supply and children’s health care use, access, and outcomes: findings from Canada. Pediatrics. 2010;125:1119–26. doi: 10.1542/peds.2009-2821. [DOI] [PubMed] [Google Scholar]
- 27.Kralji B. Measuring rurality: RIO2008 BASIC — methodology and results. Toronto: Ontario Medical Association; 2009. [accessed 2022 Feb. 26]. Available: https://docplayer.net/91599736-Measuring-rurality-rio2008_basic-methodology-and-results.html. [Google Scholar]
- 28.Matheson FI, Moloney G, van Ingen T. Ontario marginalization index: user guide. 1st revision. Toronto: St. Michael’s Hospital (Unity Health Toronto); 2022. [accessed 2022 Jan. 12]. Ontario Agency for Health Protection and Promotion (Public Health Ontario) 2016. Available: https://www.publichealthontario.ca/-/media/documents/O/2017/on-marg-userguide.pdf. [Google Scholar]
- 29.Data tables, 2016 Census. Ottawa: Statistics Canada; [accessed 2022 Oct. 16]. modified 2019 June 17. Available: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/dt-td/Rp-eng.cfm?LANG=E&APATH=3&DETAIL=0&DIM=0&FL=A&FREE=0&GC=0&GID=0&GK=0&GRP=1&PID=111869&PRID=10&PTYPE=109445&S=0&SHOWALL=0&SUB=0&Temporal=2017&THEME=127&VID=0&VNAMEE=&VNAMEF= [Google Scholar]
- 30.Johns Hopkins ACG® System. Baltimore: The Johns Hopkins Hospital; [accessed 2022 July 2]. Available https://www.hopkinsacg.org/ [Google Scholar]
- 31.Grant K. Ontario’s curious shift away from family health teams. The Globe and Mail. 2015. Feb 15, [accessed 2022 Feb. 3]. Available: https://www.theglobeandmail.com/life/health-and-fitness/health/ontarios-curious-shift-away-from-family-health-teams/article22989363/#:~:text=The%20province%20decided%20to%20limit,represents%20the%20province's%2028%2C000%20doctors.
- 32.Ontario’s doctors ratify new three-year agreement with province [news release] Toronto: Ontario Medical Association; 2022. Mar 28, [accessed 2022 Nov. 16]. Available: https://www.oma.org/newsroom/news/2022/march/ontarios-doctors-ratify-new-three-year-agreement-with-province/ [Google Scholar]
- 33.Kiran T, Kopp A, Glazier RH. Those left behind from voluntary medical home reforms in Ontario, Canada. Ann Fam Med. 2016;14:517–25. doi: 10.1370/afm.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine DM, Linder JA, Landon BE. Characteristics of Americans with primary care and changes over time, 2002–2015. JAMA Intern Med. 2020;180:463–6. doi: 10.1001/jamainternmed.2019.6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganguli I, McGlave C, Rosenthal MB. National trends and outcomes associated with presence and type of usual clinician among older adults with multi-morbidity. JAMA Netw Open. 2021;4:e2134798. doi: 10.1001/jamanetworkopen.2021.34798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breton M, Smithman MA, Brousselle A, et al. Assessing the performance of centralized waiting lists for patients without a regular family physician using clinical-administrative data. BMC Fam Pract. 2017;18:1. doi: 10.1186/s12875-016-0573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breton M, Brousselle A, Boivin A, et al. Who gets a family physician through centralized waiting lists? BMC Fam Pract. 2015;16:10. doi: 10.1186/s12875-014-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peckham S, Hutchison B. Developing primary care: the contribution of primary care research networks. Healthc Policy. 2012;8:56–70. [PMC free article] [PubMed] [Google Scholar]
- 39.Mitra G, Grudniewicz A, Lavergne MR, et al. Alternative payment models: a path forward. Can Fam Physician. 2021;67:805–7. doi: 10.46747/cfp.6711805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Health Workforce in Canada, 2020 quick Stats. Ottawa: Canadian Institute for Health Information; [accessed 2022 Feb. 3]. Available: https://www.cihi.ca/en/health-workforce-in-canada-2020-quick-stats. [Google Scholar]
- 41.Simkin S, Dahrouge S, Bourgeault IL. End-of-career practice patterns of primary care physicians in Ontario. Can Fam Physician. 2019;65:e221–30. [PMC free article] [PubMed] [Google Scholar]
- 42.Kiran T, Green ME, Wu FC, et al. Family physicians stopping practice during the COVID-19 pandemic in Ontario, Canada. Ann Fam Med. 2022;20:460–3. doi: 10.1370/afm.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiran T, Wang R, Handford C, et al. Keeping doors open: a cross-sectional survey of family physician practice patterns during COVID-19, needs, and intentions. medRxiv. 2021 Dec 21; doi: 10.1101/2021.12.20.21267918.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lavergne MR, Rudoler D, Peterson S, et al. Declining comprehensiveness of services delivered by Canadian family physicians is not driven by early-career physicians. Ann Fam Med. 2023;21:151–6. doi: 10.1370/afm.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hedden L, Banihosseini S, Strydom N, et al. Modern work patterns of “classic” versus millennial family doctors and their effect on workforce planning for community-based primary care: a cross-sectional survey. Hum Resour Health. 2020;18:67. doi: 10.1186/s12960-020-00508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National data on CMF applicants and quota in the R-1 match by disciplines (first iteration only) Ottawa: Canadian Resident Matching Service; [accessed 2022 Oct. 26]. Available: https://www.carms.ca/data-reports/r1-data-reports/r-1-match-interactive-data/ [Google Scholar]
- 47.Premji K, Ryan B. Green. HHR impacts of an aging family physician workforce. Innovations Strengthening Primary Health Care Through Research (INSPIRE-PHC) Stakeholders Meeting; 2022. [Google Scholar]
- 48.Nurse practitioner led clinics in Ontario: an overview of the nurse practitioner led clinic model and recommendations for further development. Toronto: Nurse Practitioner Association of Ontario; 2019. [accessed 2022 Feb. 26]. pp. 1–24. Available: https://npao.org/wp-content/uploads/2019/11/NPLC-Overview-Document-1.pdf. [Google Scholar]
- 49.Buajitti E, Chiodo S, Rosella LC. Agreement between area- and individual-level income measures in a population-based cohort: implications for population health research. SSM Popul Health. 2020;10:100553. doi: 10.1016/j.ssmph.2020.100553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.