Abstract
Background
Clostridioides difficile infection (CDI) is a significant healthcare-associated infection with implications for patient morbidity, mortality, and healthcare costs. However, the connection between CDI and coronavirus disease 2019 (COVID-19) infection and its influence on patient outcomes remain uncertain. This study aimed to examine the association between CDI and COVID-19, specifically investigating whether CDI worsens outcomes in patients with COVID-19. By utilizing the extensive National Inpatient Sample (NIS) database and analyzing pertinent factors, this research endeavored to enhance our understanding of CDI within the context of COVID-19.
Methods
The NIS database was searched for adult patients hospitalized with a primary diagnosis of COVID-19 infection in 2020. Patients with a secondary diagnosis of CDI were identified and separated into two groups based on CDI status. Baseline characteristics, Charlson Comorbidity Index (CCI), and outcomes were compared between the two groups using Chi-square and t-tests. Multivariate logistic and linear regressions were performed for the identification of independent predictors of CDI and mortality.
Results
A total of 1,045,125 COVID-19 hospitalizations were included, of which 4,920 had a secondary diagnosis of CDI. Patients with CDI and COVID-19 were older (mean age 69.9 vs. 64.2 years; P < 0.001), more likely to be female (54.1% vs. 47.1%; P < 0.001) and white (60% vs. 52.4%; P < 0.001). The CDI and COVID-19 group had a longer length of stay (14.1 vs. 7.42 days; P < 0.001), higher total hospital costs ($42,336 vs. $18,974; P < 0.001), and higher inpatient mortality (21.6% vs. 11%; P < 0.001) compared to the COVID-19 group without CDI. Patients in the CDI and COVID-19 group had a higher CCI score (51.7% with a score of 3 or more vs. 27.7%; P < 0.001), indicating a higher comorbidity burden. Multivariate logistic regression analysis revealed CDI was independently associated with increased mortality (odds ratio (OR) 1.37; P = 0.001) and showed that the female gender and several pre-existing comorbidities were associated with a higher likelihood of CDI.
Conclusion
CDI is independently associated with increased mortality in patients admitted with COVID-19 infection. Female gender and several pre-existing comorbidities are independent predictors of CDI in COVID-19 patients.
Keywords: Clostridioides difficile infection, Colitis, COVID-19, National Inpatient Sample
Introduction
Clostridioides difficile (C. difficile) is an anaerobic, gram-positive bacterium that is typically found as part of the normal gut flora [1]. However, when antibiotics disrupt the normal colonic flora, C. difficile can cause a range of diseases, including C. difficile colitis (CDC), which has a spectrum of severity ranging from mild to severe, fulminant disease, pseudomembranous colitis, and toxic megacolon [2-4]. C. difficile infection (CDI) has become the leading cause of healthcare-associated infections (HAIs), particularly infective diarrhea, in hospitals [5]. In the United States, CDI is estimated to cause 500,000 infections and 29,000 deaths each year [6]. While coronavirus disease 2019 (COVID-19) primarily affects the respiratory system, it can cause systemic inflammation that affects many organ systems, including the gastrointestinal (GI) tract [7]. GI symptoms have been frequently reported in COVID-19 patients, with the incidence ranging widely from 2% to 79.1% [8-10]. Studies have suggested that the dysregulation of the immune response in the GI tract caused by COVID-19 infection may increase the risk of coinfection with other GI pathogens, such as C. difficile [7]. While studies have indicated potential associations between COVID-19 and GI symptoms, the specific relationship between COVID-19 and CDI, as well as their combined impact on patient outcomes, remains poorly understood. To address this knowledge gap, we conducted a study utilizing data from the National Inpatient Sample (NIS) database, the largest hospital database available. We specifically focused on identifying the risk factors associated with the development of CDI in patients primarily admitted to the hospital with COVID-19 infection. Additionally, we assessed the effect of CDI on mortality and hospital length of stay (LOS) in patients with COVID-19 infection. Our study will provide important insights into the potential relationship between these two infections and inform clinical practice in managing patients with COVID-19 who may be at increased risk of developing CDI.
Materials and Methods
Data source
Data were searched and collected from the NIS database (produced by the Agency for Healthcare Research and Quality) in 2020. This database is the largest all-payer inpatient database which has 7 million hospitalizations every year. From the last quarter of 2015, the International Classification of Diseases (ICD) diagnostic and procedure codes were changed from the ninth revision to the tenth revision (ICD-10). The study was exempt from the Institutional Review Board (IRB) review as NIS database is a limited dataset that does not require IRB approval.
Study population
The NIS 2020 was queried for all hospitalizations of patients ≥ 18 years who had a discharge diagnosis of COVID-19 infection. ICD-10 codes used to identify COVID-19 infection were U00, U07.1, and U49. CDI was identified using A04.7, A04.71, and A04.72 ICD-10 diagnostic codes. Patients with a secondary diagnosis of CDI were also identified. The study population was divided into two groups based on CDI status. The first group included COVID-19 hospitalizations with no CDI and the second group included COVID-19 hospitalizations with CDI.
Patient characteristics and outcome variables
Baseline characteristics were compared between the two groups. Baseline patient characteristics included age, sex, race, insurance status, and comorbidities. Elixhauser comorbidity software was utilized to obtain 31 comorbidities. Baseline hospital characteristics including hospital region, size, and teaching status were also compared between the two study groups.
The primary outcomes were mortality and LOS with secondary outcomes being the cost of hospitalization, septic shock, acute liver failure, acute renal failure, and the need for mechanical ventilation.
Statistical analysis
STATA version 15 was used for data analysis. Categorical variables were analyzed using the Chi-squared test and linear variables were compared using Student’s t-test. The statistical significance was determined based on a P-value of ≤ 0.05. Univariate analysis was performed to calculate the unadjusted odds ratio (OR) for the outcomes. The variables that had a P-value of < 0.2 in the univariate analysis were included in the multivariate analysis. Logistic regression analysis was used for binary outcomes and linear regression analysis was used for the continuous outcomes. Multivariate logistic regression analysis assessed the independent impact of CDI on mortality. Moreover, it was used to identify factors associated with the development of CDI in patients with COVID-19 infection. Multivariate linear regression analysis was performed to measure the independent predictors of increased LOS.
Results
A total of 1,045,125 hospitalizations for COVID-19 patients were found, of which 4,920 had a secondary diagnosis of CDI, representing 0.47% of all COVID-19 hospitalizations. The patients with COVID-19 and CDI had a higher mean age of 69.9 years compared to those with COVID-19 but without CDI who had a mean age of 64.2 years (P < 0.001). In addition, the CDI and COVID-19 group had a higher percentage of females at 54.1% compared to 47.1% in the COVID-19 group without CDI, representing a difference of 14.9% (P < 0.001), and whites at 60% compared to 52.4% in the COVID-19 group without CDI, representing a difference of 14.5% (P < 0.001).
The study also found that patients with COVID-19 and CDI had a longer mean length of hospital stay of 14.1 days compared to 7.42 days in those without CDI, representing a difference of 90% (P < 0.001), and higher total hospital costs at $42,336 compared to $18,974 in those without CDI (P < 0.001). Furthermore, the CDI and COVID-19 group had higher comorbidity burden, with 51.7% having a Charlson Comorbidity Index (CCI) score of 3 or more compared to 27.7% in the COVID-19 group without CDI (P < 0.001) (Table 1).
Table 1. Baseline Demographics and Hospital Outcomes in Individuals With COVID-19 and No CDI Versus Those With Both CDI and COVID-19.
| Variables | COVID-19 and no CDI (N = 1,045,125) | Both CDI and COVID-19 (N = 4,920) | P-value |
|---|---|---|---|
| Baseline demographic characteristics | |||
| Mean age (years) | 64.2 | 69.3 | < 0.001 |
| Gender | < 0.001 | ||
| Male | 52.8% | 45.8% | |
| Female | 47.1% | 54.1% | |
| Race | < 0.001 | ||
| White | 52.4% | 60.0% | |
| Black | 18.4% | 19.6% | |
| Hispanic | 20.7% | 13.6% | |
| Asian or Pacific Islander | 3.2% | 1.7% | |
| Native American | 1.04% | 1.46% | |
| Other | 4.11% | 3.44% | |
| Charlson Comorbidity Index | < 0.001 | ||
| 0 | 28.1% | 13.2% | |
| 1 | 27.8% | 17.2% | |
| 2 | 16.2% | 17.6% | |
| 3 | 27.2% | 51.8% | |
| Hospital outcomes | |||
| Died during hospitalization | 11.0% | 21.6% | < 0.001 |
| Length of stay (days) | 7.42 | 14.1 | < 0.001 |
| Total cost of hospitalization (USD) | 18,974.51 | 42,336.23 | < 0.001 |
| Septic shock | 4.0% | 13.4% | < 0.001 |
| Acute liver | 0.6% | 1.5% | < 0.001 |
| Acute kidney failure | 25.2% | 41.8% | < 0.001 |
| Acute respiratory failure | 51.9% | 45.9% | < 0.001 |
| Mechanical ventilation | 9.7% | 22.4% | < 0.001 |
| Fluid and electrolytes imbalance | 48.0% | 71.2% | < 0.001 |
| Blood loss | 0.3% | 1.0% | < 0.001 |
Patients in the CDI group were older with higher comorbidity burdens. A comparison of clinical outcomes showed worse outcomes in the CDI group, including higher inpatient mortality and longer length of stay. CDI: Clostridioides difficile infection; COVID-19: coronavirus disease 2019.
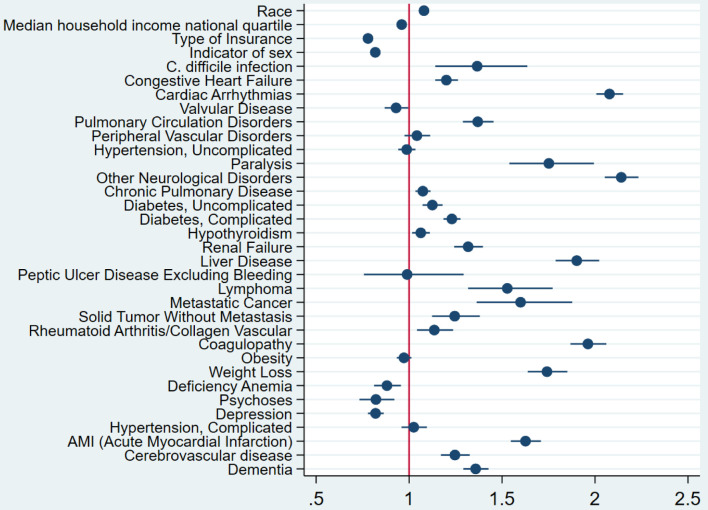
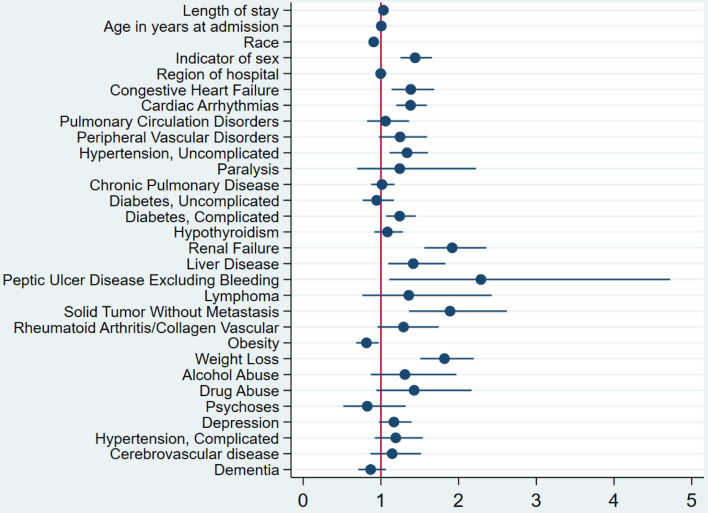
The study also revealed that CDI was independently associated with a higher risk of mortality, with an OR of 1.37 (P = 0.001) (Fig. 1). The multivariate logistic regression analysis conducted to identify predictors of CDI in COVID-19 patients found that several factors were associated with a higher likelihood of CDI. These included female gender, peptic ulcer disease, renal failure, weight loss, diabetes mellitus with complications, congestive heart failure, cardiac arrhythmias, liver disease, and non-metastatic solid organ tumors (Fig. 2). The multivariate analysis showed that peptic ulcer disease and renal failure were the risk factors with the highest odds of association with CDI in COVID-19 patients. The OR for peptic ulcer disease was 2.3 (95% confidence interval (CI): 1.1 - 4.7), while the OR for renal failure was 1.9 (95% CI: 1.6 - 2.4).
Figure 1.
Forest plot for the multivariate logistic regression analysis. The analysis has shown that CDI is associated with increased odds of mortality in patients with COVID-19 infection after adjustment for potential confounders. CDI: Clostridioides difficile infection; COVID-19: coronavirus disease 2019.
Figure 2.
Forest plot of the multivariate logistic regression analysis for predictors of CDI in patients with COVID-19 infection. It showed that among COVID-19-infected patients, female patients, patients with congestive heart failure, peptic ulcer disease, renal failure, weight loss, diabetes mellitus with complications, cardiac arrhythmias, liver disease, and non-metastatic solid organ tumors have higher odds of development CDI. CDI: Clostridioides difficile infection; COVID-19: coronavirus disease 2019.
Discussion
CDI is a common HAI, that predominantly affects older hospitalized patients, causing diarrhea [11]. COVID-19 infection, on the other hand, primarily affects the lungs, but it may also impair multiple organ systems, including the GI tract, leading to GI-related symptoms, such as diarrhea [12]. Previous studies have shown a potential association between CDI and COVID-19 infection, but the magnitude of this association is not well established. The aim of our study was to investigate the association between CDI and COVID-19, as limited literature exists on this topic. We sought to assess the incidence, severity, and outcomes of CDI in COVID-19 patients, identify risk factors for CDI development, and contribute to clinical practice in managing coinfected patients. By filling this knowledge gap, our study aimed to provide important insights into the relationship between these two infections and guide future research and interventions.
This retrospective cohort study included 1,045,125 patients with COVID-19, of which 4,920 (0.47%) had a secondary diagnosis of CDI. The study found that patients with CDI and COVID-19 coinfection were older and more likely to be female and white, with a higher comorbidity burden, longer hospital stays, and higher total hospital costs than COVID-19 alone. The inpatient mortality rate was significantly higher in patients with CDI and COVID-19 coinfection than in those with COVID-19 alone. Furthermore, in the multivariate analysis, the study identified peptic ulcer disease and renal failure as the comorbidities with the highest odds of association with CDI.
These findings are consistent with previous studies that have shown a higher incidence of CDI in COVID-19 patients compared to non-COVID-19 patients [13, 14]. In a study by Boeriu et al, CDI incidence was significantly higher in COVID-19 patients, and the study identified several risk factors for CDI in COVID-19 patients, including advanced age, antibiotic use, and proton pump inhibitor (PPI) use [13]. Similarly, a systematic review and meta-analysis by Granata et al found that COVID-19 patients had a significantly higher risk of CDI compared to non-COVID-19 patients and identified several risk factors for CDI in COVID-19 patients, including advanced age, antibiotic use, and severe COVID-19 disease [15]. These findings emphasize the importance of considering CDI as a potential complication in COVID-19 patients, particularly in the presence of identified risk factors.
In our study, we observed that patients in the CDI and COVID-19 group were more likely to be female and white when compared to the COVID-19 group without CDI. These findings align with the CDC’s 2020 annual report on CDI, which also reported higher incidence rates of CDI among women compared to men and higher rates among white individuals compared to those of other races [16]. This consistency in demographics reinforces the understanding of CDI epidemiology and provides further support for the association between CDI and COVID-19 in specific population groups.
Patients in the CDI and COVID-19 group exhibited prolonged hospital stays and higher total hospital costs, consistent with previous research indicating that extended hospitalizations are a significant risk factor for CDI in COVID-19 patients. Lewandowski et al have shown that each additional day of hospitalization increases the risk of CDI by 3% [17]. The economic burden of CDI is substantial, estimated to contribute to 3 - 20 additional hospital days per patient and incur costs exceeding one billion dollars annually in the US [18]. These findings underscore the importance of implementing effective measures to prevent and manage CDI in COVID-19 patients, aiming to alleviate the strain on healthcare resources.
Furthermore, patients in the CDI and COVID-19 group had a higher comorbidity burden, as indicated by a higher percentage having a CCI score of 3 or more. This finding suggests that CDI is associated with increased comorbidities, particularly in elderly patients. The coinfection of CDI and COVID-19 was also associated with a higher mortality rate, with an inpatient mortality rate of 21.6% in the CDI and COVID-19 group compared to 11% in the COVID-19 group without CDI. Mortality due to CDI and COVID-19 coinfection increases with age. A meta-analysis of COVID-19 patients found a significant increase in mortality with age, particularly in patients aged 60 and above. The mortality rate was six times higher in patients aged 80 and above compared to younger patients [19].
Our study revealed that peptic ulcer disease and renal failure were the most significant risk factors for CDI in COVID-19 patients. PPI use in peptic ulcer disease patients likely contributed to the increased risk. Additionally, we found that renal disease, diabetes mellitus, solid-organ malignancies, liver disease, congestive heart failure, and weight loss were associated with CDI in our cohort. These findings enhance our understanding of the comorbidities that may predispose COVID-19 patients to CDI, informing clinical management and preventive strategies.
The strength of this study lies in its large and diverse sample size, including over 1 million patients with COVID-19. This enables robust statistical analysis and enhances the generalizability of the findings. Additionally, the study employed a multivariate analysis to identify specific comorbidities strongly associated with CDI, providing valuable insights into risk factors for coinfection in COVID-19 patients. The study’s retrospective design limits the ability to establish causal relationships between CDI and COVID-19. The reliance on secondary diagnosis codes for CDI identification may lead to underestimation or misclassification of cases. The study did not assess specific factors such as antibiotic use or hospital-acquired infections, which could further inform the understanding of CDI in COVID-19 patients.
In conclusion, our study provides valuable insights into the incidence, outcomes, and risk factors of CDI in COVID-19 patients. We found a higher incidence of CDI in this population, along with increased comorbidity burden, longer hospital stays, higher costs, and elevated mortality rates. Our study raises important research questions for future investigations, including understanding the underlying mechanisms of increased CDI incidence in COVID-19 patients, exploring the role of medications and gut microbiota, and developing targeted interventions. Additionally, further research is needed to examine the impact of CDI on the clinical outcomes of COVID-19 patients, identify high-risk subgroups, and determine associated risk factors. These inquiries will contribute to a better understanding of CDI in the context of COVID-19 and inform strategies for prevention and management in clinical practice.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Conception and design: Deda, Elfert, Nayudu. Acquisition of data: Deda, Elfert. Analysis and interpretation of data: Elfert, Elromisy, Guevara. Drafting of manuscript: Deda, Elfert, Ghandi, Malik. Critical revision of manuscript: Bechtold, Matthew. Statistical expertise: Elfert, Bechtold. Final approval: Bechtold.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
References
- 1. Centers for Disease Control and Prevention. Clostridioides difficile Infection. Available at: https://www.cdc.gov/cdiff/index.html. Accessed on April 11, 2023.
- 2.Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, McFarland LV. et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478–498. doi: 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]
- 3.Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372(16):1539–1548. doi: 10.1056/NEJMra1403772. [DOI] [PubMed] [Google Scholar]
- 4.Khanna S, Pardi DS. Clostridium difficile infection: new insights into management. Mayo Clin Proc. 2012;87(11):1106–1117. doi: 10.1016/j.mayocp.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R. et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Available at: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Accessed on April 11, 2023.
- 7.Gu J, Han B, Wang J. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020;158(6):1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J. et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(7):667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY. et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159(1):81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redd WD, Zhou JC, Hathorn KE, McCarty TR, Bazarbashi AN, Thompson CC, Shen L. et al. Prevalence and characteristics of gastrointestinal symptoms in patients with severe acute respiratory syndrome coronavirus 2 infection in the United States: a multicenter cohort study. Gastroenterology. 2020;159(2):765–767.e762. doi: 10.1053/j.gastro.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magill SS, O'Leary E, Janelle SJ, Thompson DL, Dumyati G, Nadle J, Wilson LE. et al. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med. 2018;379(18):1732–1744. doi: 10.1056/NEJMoa1801550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boeriu A, Roman A, Dobru D, Stoian M, Voidazan S, Fofiu C. The impact of clostridioides difficile infection in hospitalized patients: what changed during the pandemic? Diagnostics (Basel) 2022;12(12):3196. doi: 10.3390/diagnostics12123196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuya-Kanamori L, Stone JC, Clark J, McKenzie SJ, Yakob L, Paterson DL, Riley TV. et al. Comorbidities, exposure to medications, and the risk of community-acquired clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2015;36(2):132–141. doi: 10.1017/ice.2014.39. [DOI] [PubMed] [Google Scholar]
- 15.Granata G, Petrosillo N, Al Moghazi S, Caraffa E, Puro V, Tillotson G, Cataldo MA. The burden of clostridioides difficile infection in COVID-19 patients: a systematic review and meta-analysis. Anaerobe. 2022;74:102484. doi: 10.1016/j.anaerobe.2021.102484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. https://www.cdc.gov/hai/eip/Annual-CDI-Report-2019.html .
- 17.Lewandowski K, Rosolowski M, Kaniewska M, Kucha P, Meler A, Wierzba W, Rydzewska G. Clostridioides difficile infection in coronavirus disease 2019 (COVID-19): an underestimated problem? Pol Arch Intern Med. 2021;131(2):121–127. doi: 10.20452/pamw.15715. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A, Ananthakrishnan AN. Economic burden and cost-effectiveness of therapies for Clostridiodes difficile infection: a narrative review. Therap Adv Gastroenterol. 2021;14:17562848211018654. doi: 10.1177/17562848211018654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonanad C, Garcia-Blas S, Tarazona-Santabalbina F, Sanchis J, Bertomeu-Gonzalez V, Facila L, Ariza A. et al. The effect of age on mortality in patients with COVID-19: a meta-analysis with 611,583 subjects. J Am Med Dir Assoc. 2020;21(7):915–918. doi: 10.1016/j.jamda.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article.