Abstract
Background
The role for radiotherapy or surgery in the upfront management of brain metastases (BrM) in epidermal growth factor receptor mutant (EGFRm) or anaplastic lymphoma kinase translocation positive (ALK+) non-small cell lung cancer (NSCLC) is uncertain because of a lack of prospective evidence supporting tyrosine kinase inhibitor (TKI) monotherapy. Further understanding of practice heterogeneity is necessary to guide collaborative efforts in establishing guideline recommendations.
Methods
We conducted an international survey among medical (MO), clinical (CO), and radiation oncologists (RO), as well as neurosurgeons (NS), of treatment recommendations for asymptomatic BrM (in non-eloquent regions) EGFRm or ALK+ NSCLC patients according to specific clinical scenarios. We grouped and compared treatment recommendations according to specialty. Responses were summarized using counts and percentages and analyzed using the Fisher exact test.
Results
A total of 449 surveys were included in the final analysis: 48 CO, 85 MO, 60 NS, and 256 RO. MO and CO were significantly more likely than RO and NS to recommend first-line TKI monotherapy, regardless of the number and/or size of asymptomatic BrM (in non-eloquent regions). Radiotherapy in addition to TKI as first-line management was preferred by all specialties for patients with ≥4 BrM. NS recommended surgical resection more often than other specialties for BrM measuring >2 cm.
Conclusions
Recommendations for the management of BrM from EGFRm or ALK+ NSCLC vary significantly according to oncology sub-specialties. Development of multidisciplinary guidelines and further research on establishing optimal treatment strategies is warranted.
Keywords: Brain metastases (BrM), non-small cell lung cancer (NSCLC), epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK)
Highlight box.
Key findings
• Medical and clinical oncologists are more likely than radiation oncologists and neurosurgeons to recommend upfront systemic therapy for asymptomatic brain metastases in patients with metastatic non-small cell lung cancer (NSCLC).
• Size and number of brain metastases influence upfront treatment recommendations.
What is known and what is new?
• Tyrosine kinase inhibitors (TKIs) against ALK and EGFR alterations can penetrate the central nervous system and have intra-cranial activity.
• The optimal frontline strategy is not well established for patients with asymptomatic brain metastases.
• This international survey provides relevant insight into the practice patterns among various oncological specialties in their upfront treatment strategies for this patient population.
What is the implication and what should change now?
• Multi-disciplinary approach to the management of asymptomatic brain metastases is essential given the recent and evolving treatment landscape.
• In patients with a limited number of small brain metastases from ALK or EGFR positive NSCLC, upfront systemic therapy with TKIs is a common approach among oncologists.
Introduction
More than 25% of non-small cell lung cancer (NSCLC) patients develop brain metastases (BrM) during the course of their disease, a feature that is associated with poorer outcomes (1). BrM are especially prevalent among patients with NSCLC with epidermal growth factor receptor mutations (EGFRm) or anaplastic lymphoma kinase rearrangements (ALK+), affecting 20% to 35% of patients at presentation (2,3). EGFRm NSCLC comprises approximately 15% of new NSCLC cases in predominantly western populations (4). ALK+ NSCLC comprises 5% of all NSCLC cases and also carries a high risk of BrM (5). Both ALK+ and EGFRm NSCLC patients often present at an advanced stage in young, never-smokers. Among all NSCLC patients, including those with BrM, EGFRm and ALK+ patients survive significantly longer than patients without oncogenic driver mutations (6).
EGFR tyrosine kinase inhibitors (TKIs) (e.g., erlotinib, gefitinib, afatinib, and osimertinib) are effective first-line therapies for EGFRm NSCLC with response rates ranging from 70% to 80% and median progression-free survival (PFS) rates of approximately 19 months, resulting in significant and clinically relevant improvements in symptom control and quality of life, compared to chemotherapy (7). For the first generation TKI erlotinib, data from one retrospective study indicated lower rates of central nervous system (CNS) progression in patients who received first-line TKI compared to patients who received chemotherapy, with a 12-month risk of CNS progression of 6% in the erlotinib group compared with 19% in the chemotherapy group (8). Similarly, in a combined analysis of LUX-LUNG 3 and LUX-LUNG 6, afatinib significantly improved objective response rates among patients with either asymptomatic or controlled (including previous whole brain radiotherapy) BrM (9). For the third generation TKI osimertinib, pooled analyses of AURA extension (NCT01802632) and AURA2 (NCT02094261) showed that BrM patients with the T790m mutation who received second-line osimertinib had a CNS overall response rate (ORR) and disease control rate (DCR) of 54% and 92%, respectively (10). The randomized phase 3 trial (AURA3) demonstrated a 70% CNS ORR in patients treated with osimertinib compared to 31% in patients with asymptomatic, stable BrM treated with chemotherapy. Median duration of response was 8.9 months for osimertinib and 5.7 months for chemotherapy and median CNS PFS was 11.7 and 5.6 months, respectively (11). Finally, the randomized, double blinded, phase 3 FLAURA trial, where patients with CNS involvement were required to be “neurologically stable”, demonstrated decreased CNS progression among patients treated with osimertinib compared to first generation TKIs (6% vs. 15%) (12). A meta-analysis of osimertinib by Erickson et al. that included 15 studies with 324 patients reported a CNS ORR of 64% and CNS DCR of 90% (13). No studies that evaluated osimertinib reported detailed data on prior CNS focused treatments with radiation and/or surgery.
TKIs used to treat ALK+ NSCLC have similar CNS activity. A pooled analysis of a single-arm phase 2 trial (PROFILE 1005) and second-line randomized trial (PROFILE 1007), both of which tested the efficacy of crizotinib, demonstrated a 12-week intracranial disease control rate (iDCR) of 56% among 109 patients with untreated asymptomatic BrM. In the subset of patients not treated with RT, iDCR was 18% (14). Alectinib, a second generation ALK-targeting TKI, was superior to crizotinib in the phase 3 ALEX study, including among patients who had measurable CNS disease (prior radiation therapy to BrM was permitted) at baseline (CNS ORR of 79% vs. 40%) (15). In the ALEX study (Alectinib study arm), 4% of patients had prior brain surgery, 19% of patients had radiosurgery, and 63% had whole brain radiotherapy. However, in the subgroup analysis, prior brain radiation treatments [hazard ratio (HR) 0.33, 95% CI: 0.14–0.74] vs. no prior treatment (HR 0.52, 95% CI: 0.36–0.73) had no significant difference, with both favoring alectinib, supporting the ability of alectinib to penetrate the blood brain barrier and prevent disease progression. In a randomized phase 3 trial, the intracranial response rate of brigatinib compared to crizotinib among patients with measurable CNS disease was 78% vs. 29% (16). In multiple phase 2 trials of lorlatinib, the objective intracranial response rates among patients with measurable CNS disease at baseline ranged from 53–66% (17). Finally, the CROWN Study, which enrolled patients with asymptomatic treated or untreated BrM, reported improved iORR (82% vs. 23%) for the third generation TKI lorlatinib compared to crizotinib, including an impressive 71% iCR for lorlatinib (18). The current standard of care for fit patients with 1–4 BrM, none of which require surgical resection, is stereotactic radiosurgery (SRS), whereas whole brain radiation remains the standard for patients with extensive BrM (19). Surgical resection is typically reserved for large or symptomatic BrMs or for obtaining diagnostic tissue. However, there is limited guidelines on the standard of care management for BrM from EGFRm or ALK+ NSCLC, i.e., whether or not treatment should include TKI alone (monotherapy) versus radiotherapy or surgery (20,21). To ascertain treatment recommendations within specific clinical practices, we conducted an international survey among medical oncologists (MO), clinical oncologists (CO), radiation oncologists (RO), and neurosurgeons (NS) that are involved in the management of BrM in patients with EGFRm or ALK+ NSCLC. We present this article in accordance with the SURGE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-697/rc).
Methods
Participants and study design
This was an international study conducted from January 2019 to February 2020. Participating clinicians were grouped into four cohorts: MO, who prescribe systemic anti-cancer therapies; CO, who prescribe both systemic anti-cancer therapies and also radiation treatments (depending on country and jurisdiction of practice); RO, clinicians who prescribe only radiation treatments; and NS who identified themselves as being involved in the surgical and multidisciplinary management of patients with EGFRm or ALK+ NSCLC BrM (in non-eloquent regions). The survey was distributed in-person during the February 2019 Canadian Lung Cancer Conference; 300 surveys were distributed resulting in 29 responses. Otherwise the survey was distributed electronically from March 2019 through September 2019. The electronic survey opened in March 2019 and was closed in January 2020. The following professional societies distributed the survey through either email directories or their spring 2019 newsletters: Society for Neuro-Oncology (North America), Medical Oncology Group of Australia, Canadian Association of Radiation Oncology, Taiwan Lung Cancer Society, Taiwan Oncology Society, Indian Society of Neuro-oncology, National Cancer Center of Japan, Royal Australian and New Zealand College of Radiologists, Society for Neuro-Oncology of Latin America, Brazilian Society of medical oncology, and the Brazilian Society of radiation oncology. Reminder emails were sent out at the discretion of those societies. In addition, the authors emailed approximately 3,500 members of the American Society of Radiation Oncology and the European Society of Medical Oncology who had expertise in the treatment of BrM or lung cancer according to their online email directories. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study and its protocol were approved by University Health Network Ethics Review Board (No. 18-6258). Informed consent was not required since completing the survey implied consent for the results to be used.
Survey development and distribution
Two surveys (A and B) were developed through an iterative process based on feedback from the manuscript’s authors. International members also provided face validity for their specific countries or regions. Our goal was to elicit treatment recommendations based on well-defined clinical scenarios based on the unique expertise of participating clinicians. Of note, the survey also contained a separate set of scenarios that focused on melanoma BrM; the results of that effort will be reported in a separate manuscript. Survey A was designed for MO and CO (because they are involved in systemic treatment decisions and management) and survey B was designed for RO and NS. The surveys consisted of four parts: demographics (part I); approach to BrM (part II); scenarios of primary lung cancers with targetable oncogenic driver mutations (EGFRm and ALK+) (part III); and a comments section (part IV). The difference between survey A and B was limited to part II, which for MO and CO addressed questions about the proportion of EGFRm and ALK+ patients in their practice and access to specific TKIs. Both surveys comprised 12 unique clinical scenarios followed by multiple choice questions. The first set of 6 scenarios were based on a patient with NSCLC EGFRm BrM and the second set of 6 scenarios were based on a patient with NSCLC ALK+ BrM. In all scenarios, the patient was asymptomatic from their BrM, and no BrMs were located within the brainstem, nor did the patient have symptomatic edema, leptomeningeal disease or impending herniation. In scenarios where 1 BrM was >2 cm in maximum diameter, it was located in a non-eloquent brain region. All BrMs described were potentially operable. Participants were then asked whether first-line treatment should include an appropriate (but unspecified) TKI alone, TKI in combination with radiotherapy (WBRT or SRS), or surgical resection followed by TKI and radiotherapy. Response rates and participation rates were not prospectively collected as part of this study.
Data analysis and statistical analysis
Treatment options for EGFRm and ALK+ lung cancer were summarized using counts and percentages. Responses were compared among the four cohorts of clinician respondents. Descriptive statistics were used to summarize the cohorts. Responses were analyzed and compared among the cohorts within each scenario using the Fisher exact test, using R software version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria—www.r-project.org). P values less than 0.05 were considered statistically significant.
Results
A total of 571 surveys (A and B combined) were completed: 198 by MO or CO and 373 completed by either a RO or NS. After review, a total of 6 surveys were excluded due to the specific type of specialist being missing; 5 were excluded due to listing their specialty as neuro-oncology because the size of that cohort (total number of individuals who completed the survey) was considered too small to provide an accurate comparison to other specialties. Of the 12 lung scenarios (6 EGFR and 6 ALK), 111 physicians answered none of the questions and were excluded. Therefore, a total of 449 surveys from over 20 countries were used in the analyses. Demographic information from all respondents in all four cohorts is summarized in Table 1. This analysis revealed heterogeneity in the demographic and practice settings among the cohorts, including location of practice, academic affiliation. Minor differences were also seen among CO and MO with respect to experience using EGFR and ALK TKIs (Table 2).
Table 1. Demographic data from all four cohorts of all respondents for medical, clinical, radiation, and neurosurgery oncologists.
| Covariate | Clinical oncology (n=48), n [%] |
Medical oncology (n=85), n [%] |
Neurosurgery (n=60), n [%] |
Radiation oncology (n=256), n [%] |
P value |
|---|---|---|---|---|---|
| How many years have you practiced as a medical clinical oncologist following completion of your training? | <0.001* | ||||
| <5 years | 21 [44] | 29 [34] | 7 [12] | 73 [29] | |
| 5–15 years | 22 [46] | 29 [34] | 22 [37] | 75 [29] | |
| >15 years | 5 [10] | 27 [32] | 31 [52] | 108 [42] | |
| Are you currently an attending staff or a fellow? | <0.001* | ||||
| Attending staff | 36 [86] | 34 [76] | 57 [97] | 241 [96] | |
| Fellow | 6 [14] | 11 [24] | 2 [3] | 9 [4] | |
| Missing | 6 | 40 | 1 | 6 | |
| Location of clinical practice | <0.001* | ||||
| Asia | 37 [77] | 11 [14] | 28 [49] | 61 [24] | |
| Europe | 1 [2] | 3 [4] | 4 [7] | 13 [5] | |
| North America | 9 [19] | 47 [59] | 15 [26] | 150 [59] | |
| Other | 1 [2] | 19 [24] | 10 [18] | 30 [12] | |
| Missing | 0 | 5 | 3 | 2 | |
| Do you regularly consult ASCO or guidelines for management of brain metastases as a reference to guide decision making? | <0.001* | ||||
| Yes | 15 [31] | 36 [42] | 23 [38] | 46 [18] | |
| N/A | 33 [69] | 49 [58] | 37 [62] | 210 [82] | |
| Do you regularly consult ASTRO guidelines for management of brain metastases as a reference to guide decision making | <0.001* | ||||
| Yes | 21 [44] | 6 [7] | 7 [12] | 170 [66] | |
| N/A | 27 [56] | 79 [93] | 53 [88] | 86 [34] | |
| Are you affiliated with an academic center? | <0.001* | ||||
| Yes | 24 [50] | 71 [84] | 53 [88] | 176 [69] | |
| No | 24 [50] | 14 [16] | 7 [12] | 80 [31] | |
| Are you part of a multi-disciplinary oncology team? | 0.32 | ||||
| Yes | 39 [81] | 78 [92] | 52 [87] | 219 [86] | |
| No | 9 [19] | 7 [8] | 8 [13] | 37 [14] | |
| Based on your best estimate how many patients with a new diagnosis of brain metastases from primary lung cancer synchronous or metachronous do you see in a month? | <0.001* | ||||
| <3 cases | 28 [62] | 48 [59] | 22 [38] | 75 [29] | |
| 3–10 cases | 17 [38] | 32 [40] | 28 [48] | 149 [58] | |
| >10 cases | 0 [0] | 1 [1] | 8 [14] | 32 [12] | |
| Missing | 3 | 4 | 2 | 0 | |
*, P<0.05. N/A, data not available (i.e., no response in questionnaire); ASCO, American Society of Clinical Oncology, ASTRO, American Society for Radiation Oncology.
Table 2. Demographic data from all two cohorts of respondents for medical and clinical oncologists.
| Covariate | Clinical oncology cohort (n=48), n [%] | Medical oncology cohort (n=85), n [%] | P value |
|---|---|---|---|
| Based on your best estimate how many new patients with primary lung cancers do you see in a month? | 0.42 | ||
| <5 cases | 18 [40] | 28 [35] | |
| 5–15 cases | 24 [53] | 41 [51] | |
| >15 cases | 3 [7] | 12 [15] | |
| Missing | 3 | 4 | |
| Based on your best estimate what proportion of the lung cancer patients in your practice have drug targetable EGFR mutations? | 0.11 | ||
| <10% | 10 [22] | 26 [32] | |
| 10–30% | 22 [49] | 44 [54] | |
| >30% | 13 [29] | 11 [14] | |
| Missing | 3 | 4 | |
| Based on your best estimate what proportion of the lung cancer patients in your practice have drug targetable ALK mutations? | 0.5 | ||
| <5% | 32 [71] | 65 [80] | |
| 5–10% | 11 [24] | 14 [17] | |
| >10% | 2 [4] | 2 [2] | |
| Missing | 3 | 4 | |
| Based on your best estimate what proportion of the lung cancer patients in your practice have drug targetable BRAF MET RET or ROS1 mutations? | 0.45 | ||
| <5% | 40 [91] | 77 [95] | |
| 5–10% | 4 [9] | 4 [5] | |
| >10% | 0 [0] | 0 [0] | |
| Missing | 4 | 4 | |
| Access to tyrosine kinase inhibitors EGFR specific in your practice environment? | N/A | ||
| Yes | 36 [100] | 28 [100] | |
| Missing | 12 | 57 | |
| Access to tyrosine kinase inhibitors ALK specific in your practice environment? | 0.0019* | ||
| Yes | 21 [58] | 26 [93] | |
| No | 15 [42] | 2 [7] | |
| Missing | 12 | 57 | |
*, P<0.05. Missing, data not available (i.e., no response in questionnaire); EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; N/A, P value not calculated.
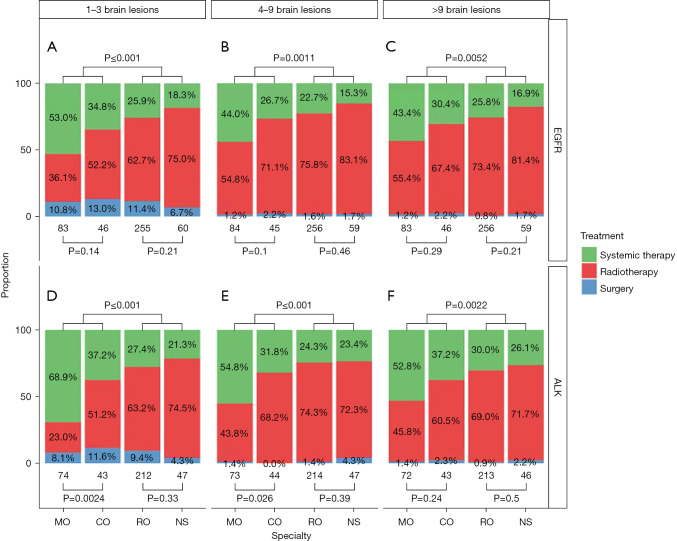
Clinical scenarios with BrM <2 cm
In EGFRm clinical scenarios where all BrM measured <2 cm and the patient had 1–3 BrM, 53% (MO), 34.8% (CO), 25.9% (RO), 18,3%(NS) recommended upfront systemic therapy with TKI alone, respectively (Figure 1A). When the number of BrM increased to ≥4 (Figure 1B,1C), the proportion recommending upfront surgery decreased, while those recommending radiation increased. There was significant practice variability among the cohorts for all four clinical scenarios, shown in Figure 1. In identical clinical scenarios in which BrM measured <2 cm for a patient with ALK fusion positive (instead of EGFRm) NSCLC, 68.9% (MO), 37.2% (CO), 27.4% (RO), 21.3% (NS) recommended upfront TKI alone (Figure 1D). Similar to the trend observed with the EGFRm scenarios, more respondents recommended radiation and fewer recommended upfront surgery as the number of lesions increased (Figure 1E,1F). Again, management recommendations differed significantly between the four specialty cohorts.
Figure 1.
Clinical scenarios with all lesions measured at <2 cm—comparison among the four cohorts. Both EGFRm (A-C) and ALK+ (D-F) specific scenarios are represented. EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; MO, medical oncologist; CO, clinical oncologist; RO, radiation oncologist; NS, neurosurgery.
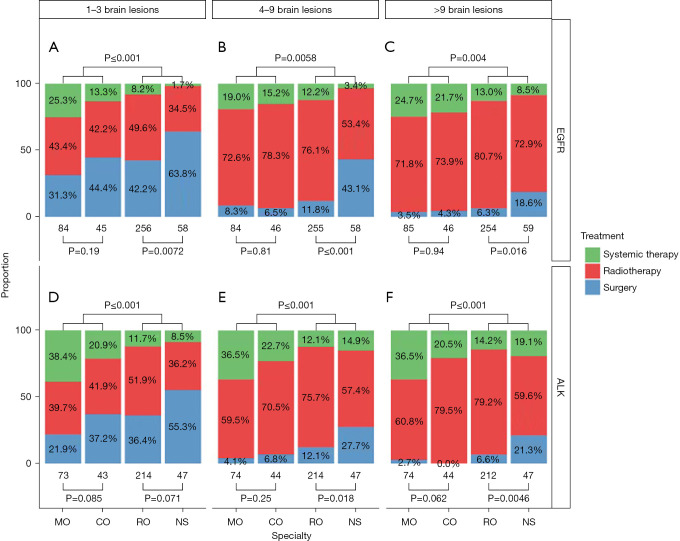
Clinical scenarios with BrM >2 cm
In EGFRm clinical scenarios where at least one BrM measured >2 cm but the total number of BrM remained 1–3, a higher proportion of respondents from all specialties recommended upfront surgery: 31.3% (MO), 44.4% (CO), 42.2% (RO), and 63.8% (NS) (Figure 2A). NS respondents were significantly more likely to recommend surgical resection than RO for EGFRm patients (P=0.0072; Figure 2A). When the number of BrM was increased to 4–9 or >9, surgical intervention was still more commonly recommended by NS respondents than by other specialist cohorts, shown in Figure 2B and Figure 2C. Similar specialty-specific recommendation patterns were identified in the ALK+ clinical scenarios: 21.9% (MO), 37.2% (CO), 36.4% (RO), and 55.3% (NS) recommended upfront surgery for patients with 1–3 BrM with at least one lesion >2 cm (Figure 2D). Among all specialties, recommendations trended away from surgery in favor of radiotherapy as the number of lesions increased (Figure 2E,2F).
Figure 2.
Clinical scenarios with one or more lesions measured at >2 cm—comparison among the four cohorts. Both EGFRm (A-C) and ALK+ (D-F) specific scenarios are represented. EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; MO, medical oncologist; CO, clinical oncologist; RO, radiation oncologist; NS, neurosurgery.
Discussion
Radiotherapy, including whole-brain radiotherapy (WBRT), SRS, and fractionated stereotactic radiotherapy (fSRT), remains the most common treatment modality for BrM (7) from lung and other solid tumors. While targeted small molecule inhibitors like EGFR and ALK TKIs used to treat EGFRm and ALK+ NSCLC have significant CNS activity, the specific criteria for recommending initial systemic therapy for BrM remain ill-defined. In this study, we identified that most MO and CO recommend upfront TKI alone as initial therapy for EGFRm and ALK+ NSCLC patients who present with 1–3 small, asymptomatic BrM. In our analysis of clinical scenarios where patients had 4 or more BrM, the addition of RT was preferred across specialties, regardless of size of the BrM. The only scenario in which most respondents recommended surgery was among NS when there was a BrM ≥2 cm and no more than 2 additional smaller BrM.
It is perhaps not surprising that our data demonstrate MO and CO are more likely to recommend initial TKI monotherapy for patients with EGFRm or ALK+ BrM compared to RO or NS. This may reflect the fact that MO and CO are more familiar with these drugs as prescribers and have more experience with TKI use and toxicity management, and greater awareness of CNS efficacy outcomes although this was not addressed in this survey. On the other hand, given that RO and NS have more experience with their treatment modalities, it is also not surprising that these specialists offer what they are more familiar with, i.e., radiation or surgical interventions. This is in line with previous reports that RO are likely to use their treatment modalities even in the presence of driver mutation positive BrM (22). In addition, given the financial incentives associated with different treatment options, it is conceivable that each specialty would have an incentive and bias to recommend their specific treatments, previously reported in the multi-disciplinary care oncology of oncology patients (23). It is also important to note that the optimal follow-up and monitoring schedule for patients with BrM has not been established and that our study did not seek to obtain a consensus on this matter. Although a common approach includes monitoring via brain imaging every 3 months or sooner if symptoms arise, formal prospective studies evaluating this approach depending on initial treatment approach chosen (TKI vs. TKI in combination with either RT and/or surgery) are required.
It is important to note that in both of our surveys, the TKI available in each scenario was not specified. This decision was based on the variable availability of certain TKIs during the course of our study among the different healthcare regions surveyed. We also did not specify the form of radiotherapy available in any scenario. Although SRS is considered the standard of care for well-performing patients with up to 4 BrM, some centers regularly treat patients with >4 BrM. Furthermore, the resources required to deliver SRS are not necessarily available to all oncologists in all regions or for all clinical scenarios. Thus, the expectation was that survey respondents would assume that the patient could receive SRS or WBRT based upon practices they used within the clinical environment in which they worked.
The additional benefit of RT added to TKI for EGFRm or ALK+ lung cancer BrM has not been tested in prospective trials. One retrospective multi-institutional analysis of 351 EGFRm NSCLC patients with BrM compared the outcomes of patients treated with upfront SRS and TKI, WBRT and TKI, or TKI alone. This analysis suggested that OS among patients treated with SRS was superior compared to those receiving upfront WBRT (46 vs. 30 months) or TKI alone (25 months) (24). In addition, a 2019 meta-analysis reported that the combination of TKI and RT significantly improved OS and PFS compared to TKI alone (25). However, other retrospective studies (with the caveat that older generation EGFR TKIs such as erlotinib were overwhelmingly used) have shown PFS without OS benefit, highlighting the uncertainty about the best approach in these clinical situations (26,27). A recently published retrospective study of both EGFRm and ALK+ lung cancer patients showed no significant difference between patients treated with TKI alone vs. TKI in combination with radiation therapy, which is likely explained by the fact that this analysis evaluated new-generation agents with known CNS activity (28). Currently, the Trans-Tasman Oncology Group is conducting a randomized phase 2 trial of Osimertinib with or without SRS for EGFRm NSCLC with BrM (NCT03497767) and a similar study is being conducted within Canada (NCT03769103). In addition to these trials, the HALT trial (NCT03256981) is an ongoing randomized phase 2 trial evaluating the benefit of radiation in mutation positive NSCLC patients with initial response to TKI therapy. Such trials will provide valuable data to help optimize the management of advanced mutation positive NSCLC patients.
Our study does have limitations. Our survey was conducted in 2019 and early 2020, during which time the third generation TKI osimertinib was not widely available in some surveyed regions. Osimertinib has better CNS activity than prior generation EGFR TKIs (29). Similarly, newer generation ALK TKIs have superior CNS activity compared to earlier generation drugs (e.g., lorlatinib). It is possible that our survey results may have been impacted if we had specified the TKI available. A similar issue applies to RT techniques in that if we had specified SRS vs. WBRT as being available in each scenario that may have the affected survey responses of some participants. In addition, the clinical scenarios developed are in contrast to real world cases, which are often more nuanced (e.g., region of brain affected, magnitude and severity of symptoms, patient preference, details of type of mutations/variants, etc.). In addition, in practice, clinical decision making can be influenced by various factors including multi-disciplinary case conference discussions and patient preference. Furthermore, in patients with mild to moderate symptoms or involved eloquent brain, a preference for brain-directed treatments (i.e., not only TKI therapy) may be considered the optimal approach and was not evaluated in this study. Another limitation is that scenarios for different number and size of lesions were completed by the same respondent and therefore further analysis, including univariate and multivariate, were not done. As such, assessing the impact of various characteristics (e.g., years of practice, location of practice, academic vs. non-academic practice) on decision making for each clinical scenario, including the ability to capture nuances from case to case, was not done to avoid the impact of multiple testing of the same respondent. Finally, our analysis, interpretation, and generalizability is limited given the small number of respondents of the total population approached, in addition to the small number of participants from European centres. Of note, European practice trends have been previously reported in another survey-based study of European lung cancer specialists; however, this was also prior to the introduction of next generation TKIs (30).
Conclusions
In this international survey, significant variation in treatment recommendations was seen across experts from different specialties with regard to the upfront management of asymptomatic BrM (in non-eloquent regions) in newly diagnosed EGFRm or ALK+ NSCLC. Our results demonstrate that, in the absence of prospective data, upfront management recommendations vary significantly according to specialty. Overall, RT in addition to TKI was the most recommended option, especially with increasing number of BrMs. TKI monotherapy was preferred among MO in cases with limited numbers of small sized BrMs and surgery was commonly recommended for a patient with one larger sized BrM (>2 cm) among up to 3 smaller BrM. Our results highlight the importance of routinely presenting and discussing cases at multi-disciplinary tumor boards where available, and the need for both expert consensus guidelines and prospective randomized controlled trials to provide definitive evidence about the optimal management for these patient cohorts.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study and its protocol were approved by University Health Network Ethics Review Board (No. 18-6258). Informed consent was not required since completing the survey implied consent for the results to be used.
Footnotes
Reporting Checklist: The authors have completed the SURGE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-697/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-697/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-697/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-697/coif). NM serves on the advisory boards of Pfizer, Novartis, and Seagen. NM reports honoraria from Takeda Oncology and Astra Zeneca. FYM reports consulting fees from Elekta, and honoraria from Astra Zeneca and IASLC; grants and contracts from CTAQ Queen’s University. JCHY reports participation in advisory board with Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly, Merck, Novartis, Ono Pharmaceuticals, Pfizer, Roche/Genentech, Takeda, Yuhan Pharmaceuticals, JNJ, Puma Technology, Gilead, and GSK; grant support with Astra Zeneca. JHL reports participation in advisory boards with Novartis, Takeda, Eli Lilly, and Astra Zeneca; consulting fees from Pfizer, and payments or honoraria from Bayer, Pfizer, Daiichi-Sankyo, Novartis and Boehringer Ingelheim. NP reports research support from Pfizer, Bayer, and Roche; Honoraria from Boehringer Ingelheim, Pfizer, Roche, Takeda, Pieere-Faber; Advisory board participation from Boehringer Ingelheim, MSD, Astra Zeneca, Merck, Bristol Meyers Squibb, Pfizer, Roche, Takeda, AllVascular, Beigene, Novartis. PK reports financial compensation from Medexus Pharmaceuticals Canada. MD reports consulting fees from Roche, Astra Zeneca, Takeda, Eisai, and Merck; honoraria from Roche and Astra Zeneca. NBL reports research support from Amgen, Array, Astra Zeneca, Bayer, Eli Lilly, EMD Serono, Pfizer, Roche, Guardant Health, Takeda; Honoraria from Amgen, Astra Zeneca, BMS, Janssen, MSD, Novartis, Pfizer, Roche, Sanofi, and Takeda. The other authors have no conflicts of interest to declare.
References
- 1.Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol 2005;23:6207-19. 10.1200/JCO.2005.03.145 [DOI] [PubMed] [Google Scholar]
- 2.Shin DY, Lee DH, Kim CH, et al. Epidermal growth factor receptor mutations and brain metastasis in patients with nonadenocarcinoma of the lung. J Cancer Res Ther 2016;12:318-22. 10.4103/0973-1482.154024 [DOI] [PubMed] [Google Scholar]
- 3.Shi W, Dicker AP. CNS Metastases in Patients With Non-Small-Cell Lung Cancer and ALK Gene Rearrangement. J Clin Oncol 2016;34:107-9. 10.1200/JCO.2015.63.9682 [DOI] [PubMed] [Google Scholar]
- 4.Bethune G, Bethune D, Ridgway N, et al. Epidermal growth factor receptor (EGFR) in lung cancer: an overview and update. J Thorac Dis 2010;2:48-51. [PMC free article] [PubMed] [Google Scholar]
- 5.Pikor LA, Ramnarine VR, Lam S, et al. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer 2013;82:179-89. 10.1016/j.lungcan.2013.07.025 [DOI] [PubMed] [Google Scholar]
- 6.Sperduto PW, Yang TJ, Beal K, et al. Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). JAMA Oncol 2017;3:827-31. 10.1001/jamaoncol.2016.3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baik CS, Chamberlain MC, Chow LQ. Targeted Therapy for Brain Metastases in EGFR-Mutated and ALK-Rearranged Non-Small-Cell Lung Cancer. J Thorac Oncol 2015;10:1268-78. 10.1097/JTO.0000000000000615 [DOI] [PubMed] [Google Scholar]
- 8.Heon S, Yeap BY, Lindeman NI, et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res 2012;18:4406-14. 10.1158/1078-0432.CCR-12-0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuler M, Wu YL, Hirsh V, et al. First-line afatinib versus chemotherapy in patients with non-small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol 2016;11:380-90. 10.1016/j.jtho.2015.11.014 [DOI] [PubMed] [Google Scholar]
- 10.Goss G, Tsai CM, Shepherd FA, et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: pooled data from two phase II trials. Ann Oncol 2018;29:687-93. 10.1093/annonc/mdx820 [DOI] [PubMed] [Google Scholar]
- 11.Wu YL, Ahn MJ, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol 2018;36:2702-9. 10.1200/JCO.2018.77.9363 [DOI] [PubMed] [Google Scholar]
- 12.Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 2020;382:41-50. 10.1056/NEJMoa1913662 [DOI] [PubMed] [Google Scholar]
- 13.Erickson AW, Brastianos PK, Das S. Assessment of effectiveness and safety of osimertinib for patients with intracranial metastatic disease: a systematic review and meta-analysis. JAMA Netw Open 2020;3:e201617. 10.1001/jamanetworkopen.2020.1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol 2015;33:1881-8. 10.1200/JCO.2014.59.0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gadgeel S, Peters S, Mok T, et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol 2018;29:2214-22. 10.1093/annonc/mdy405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2027-39. 10.1056/NEJMoa1810171 [DOI] [PubMed] [Google Scholar]
- 17.Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018;19:1654-67. 10.1016/S1470-2045(18)30649-1 [DOI] [PubMed] [Google Scholar]
- 18.Shaw AT, Bauer TM, de Marinis F, et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N Engl J Med 2020;383:2018-29. 10.1056/NEJMoa2027187 [DOI] [PubMed] [Google Scholar]
- 19.Suh JH, Kotecha R, Chao ST, et al. Current approaches to the management of brain metastases. Nat Rev Clin Oncol 2020;17:279-99. 10.1038/s41571-019-0320-3 [DOI] [PubMed] [Google Scholar]
- 20.Ernani V, Stinchcombe TE. Management of Brain Metastases in Non-Small-Cell Lung Cancer. J Oncol Pract 2019;15:563-70. 10.1200/JOP.19.00357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fung AS, Leighl NB. Improving the management of brain metastases in oncogene-addicted non-small-cell lung cancer. J Oncol Pract 2019;15:571-2. 10.1200/JOP.19.00575 [DOI] [PubMed] [Google Scholar]
- 22.Kraft J, Mayinger M, Willmann J, et al. Management of multiple brain metastases: a patterns of care survey within the German Society for Radiation Oncology. J Neurooncol 2021;152:395-404. 10.1007/s11060-021-03714-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell AP, Rotter JS, Patel E, et al. Association Between Reimbursement Incentives and Physician Practice in Oncology: A Systematic Review. JAMA Oncol 2019;5:893-9. 10.1001/jamaoncol.2018.6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of brain metastases in tyrosine kinase inhibitor-naïve epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol 2017;35:1070-7. 10.1200/JCO.2016.69.7144 [DOI] [PubMed] [Google Scholar]
- 25.Dong K, Liang W, Zhao S, et al. EGFR-TKI plus brain radiotherapy versus EGFR-TKI alone in the management of EGFR-mutated NSCLC patients with brain metastases. Transl Lung Cancer Res 2019;8:268-79. 10.21037/tlcr.2019.06.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El Shafie RA, Seidensaal K, Bozorgmehr F, et al. Effect of timing, technique and molecular features on brain control with local therapies in oncogene-driven lung cancer. ESMO Open 2021;6:100161. 10.1016/j.esmoop.2021.100161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He ZY, Li MF, Lin JH, et al. Comparing the efficacy of concurrent EGFR-TKI and whole-brain radiotherapy vs EGFR-TKI alone as a first-line therapy for advanced EGFR-mutated non-small-cell lung cancer with brain metastases: a retrospective cohort study. Cancer Manag Res 2019;11:2129-38. 10.2147/CMAR.S184922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas NJ, Myall NJ, Sun F, et al. Brain metastases in EGFR- and ALK-positive NSCLC: outcomes of central nervous system-penetrant tyrosine kinase inhibitors alone versus in combination with radiation. J Thorac Oncol 2022;17:116-29. 10.1016/j.jtho.2021.08.009 [DOI] [PubMed] [Google Scholar]
- 29.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018;378:113-25. 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 30.Levy A, Faivre-Finn C, Hasan B, et al. Diversity of brain metastases screening and management in non-small cell lung cancer in Europe: Results of the European Organisation for Research and Treatment of Cancer Lung Cancer Group survey. Eur J Cancer 2018;93:37-46. 10.1016/j.ejca.2018.01.067 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as