Abstract
Background
The benefits of selective coronary vein bypass grafting (SCVBG) for patients with severe diffuse lesions of the right coronary artery (RCA) who are unsuitable for coronary endarterectomy (CE) are unclear.
Methods
We recruited patients with diffuse lesions of the RCA undergoing coronary artery bypass surgery between January 2015 and December 2018 and matched SCVBG and CE patients on propensity score (PS). We evaluated the degree of single-stenosis in the RCA, incidence of perioperative myocardial infarction (MI) and major adverse cardiovascular and cerebrovascular events (MACCE), influencing factors of perioperative MACCE, long-term survival rate, and long-term MACCE incidence.
Results
Overall, 430 patients were enrolled: 344 (80%) underwent CE and 86 (20%) underwent SCVBG (n=78 and n=64, respectively, after PS matching). The incidence of perioperative MI and MACCE were significantly lower in the SCVBG group (5.1% vs. 1.5%, P<0.05; and 10.2% vs. 4.7%, P<0.05). When the vascular flow rate of the graft anastomosed to RCA in the SCVBG group was above 100 mL/min, the incidence of perioperative MACCE significantly increased [odds ratio (OR): 1.94, 95% confidence interval (CI): 1.42–2.03]. Choosing the bilateral internal mammary artery for SCVBG reduced the incidence of perioperative MACCE (OR: 0.82, 95% CI: 0.68–0.92). There was no significant difference in the rates of long-term survival or MACCE between the two groups before or after PS matching (P>0.05).
Conclusions
SCVBG is an acceptable surgical intervention for patients with severe diffuse RCA lesions.
Keywords: Diffuse coronary artery disease (DCAD), right coronary artery (RCA), coronary endarterectomy (CE), selective coronary vein bypass grafting (SCVBG)
Highlight box.
Key findings
• Selective coronary vein bypass grafting (SCVBG) is an acceptable surgical intervention for patients with severe diffuse right coronary artery (RCA) lesions.
What is known and what is new?
• Diffuse coronary artery disease (DCAD) require coronary artery bypass grafting (CABG). If DCAD occurs in the RCA, the rate of occlusion of the RCA anastomosis increases further; therefore, increasing the long-term patency rate of the RCA after CABG is essential to ensure good prognoses of patients with DCAD.
• The study aimed to compare the incidence of MI, short- and long-term incidence of major adverse cardiovascular and cerebrovascular events (MACCE), and survival rate among patients undergoing SCVBG vs. coronary endarterectomy (CE) of the RCA as a more effective surgical approach for DCAD of the RCA.
What is the implication, and what should change now?
• SCVBG with bilateral internal mammary artery (BIMA) is a more appropriate surgical strategy for patients with severe DCAD in the RCA than for those with conventional diffuse lesions in the RCA and should be the first-line treatment for the former.
Introduction
Diffuse coronary artery disease (DCAD) is defined as a coronary artery lesion with a degree of stenosis of >50% in any segment of the coronary artery with the stenotic segment being >75% of the length of the segment. It currently accounts for 12–13% for patients with coronary heart disease and the incidence of DCAD is increasing. The 1-year mortality rate of patients with DCAD who do not undergo revascularization is 30% or higher, and the 5-year survival rate is less than 50% (1). The location of DCAD is most commonly the left anterior descending (LAD) or right coronary artery (RCA) (2). According to the coronary revascularization guidelines (3), approximately 75% of patients with DCAD require coronary artery bypass grafting (CABG) (4). The rate of occlusion of the RCA anastomosis after CABG is significantly higher than that for other coronary arteries. Once occluded, the overall patency of sequential graft vessels will be affected (5). If DCAD occurs in the RCA, the rate of occlusion of the RCA anastomosis increases further (6); therefore, increasing the long-term patency rate of the RCA after CABG is essential to ensure good prognoses of patients with DCAD. Complete revascularization of the RCA can be achieved by CABG after coronary endarterectomy (CE); however, although the long-term efficacy has been confirmed, the incidence of perioperative myocardial infarction (MI) is 3–5 times higher than that of patients undergoing conventional CABG (7). For severe cases of diffuse RCA lesions, CE often fails to create a suitable anastomosis target for subsequent RCA revascularization (8). Selective coronary vein bypass grafting (SCVBG) can provide retrograde perfusion of the myocardium from the middle cardiac vein (MCV), effectively improving myocardial perfusion of the ischemic area of the RCA (9,10). However, there are few reports on the clinical efficacy of SCVBG. The present retrospective study aimed to compare the incidence of MI, short- and long-term incidence of major adverse cardiovascular and cerebrovascular events (MACCE), and survival rate among patients undergoing SCVBG vs. CE of the RCA as a more effective surgical approach for DCAD of the RCA. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-350/rc).
Methods
Study design
We retrospectively analyzed patients undergoing coronary artery bypass surgery between January 2015 and December 2018 in the Department of Cardiac Surgery, Beijing AnZhen Hospital, Capital Medical University. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Beijing AnZhen Hospital, Capital Medical University (approval No. AEEI-2014-042) and informed consent was taken from all the patients.
Patients
We recruited all patients undergoing coronary artery bypass surgery for whom coronary angiography showed three lesions of the coronary artery and DCAD of the RCA. The graft vessels were internal mammary arteries (IMA) or great saphenous vein (GSV). Exclusion criteria were as follows: undergoing CABG under cardiopulmonary bypass, undergoing secondary CABG, and other cardiac procedures. Baseline data including sex, age, angina pectoris grade, Euro Score, SYNTAX Score, comorbidities at admission (diabetes, hypertension), smoking history, dyslipidemia, previous MI, previous percutaneous coronary intervention (PCI), peripheral vascular disease (PVD), preoperative low cardiac output, and emergency surgery were collected by reviewing the medical records. The SYNTAX scores of individual RCA lesions were evaluated based on coronary angiography. For right dominant coronary artery patients, we evaluated segments 1, 2, 3, 4, 16, 16a, 16b, and 16c of the coronary artery. For left dominant coronary artery patients, we evaluated segments 1, 2, and 3 of the coronary artery. The scores were assessed by three physicians and the average value was defined as the final score.
CE and SCVBG
For CE, all patients underwent conventional CABG without cardiopulmonary bypass. In the case of RCA diffuse lesions unsuitable for conventional CABG, CABG combined with CE was chosen as the surgical strategy. The area of the RCA in which the grafting anastomosis was to be performed was stabilized using a cardiac stabilizer. The RCA was opened 8–15 mm longitudinally, and the intima of the coronary artery removed using fine tweezers and forward scissors. The calcified endometrium was thickened by the clip. End and proximal lesions were completely stripped and removed, CABG was completed, and the grafting flow was measured using a Veri Q flow meter (Medi-Stim Inc., Oslo area, Norway).
SCVBG was performed for patients who were unable to undergo CE, or where results of CE were not satisfactory (i.e., pulsatility index of >5 and blood flow of <10 mL/min after CABG). First, 6–0 Prolene was used to block the proximal end of the cardiac vein associated with the posterior descending artery, and the end of the sequential GSV was end-to-side anastomosed with the MCV using 7–0 Prolene. After anastomosis, the Veri Q flowmeter was used to test the blood flow of grafts.
Evaluation of complications
Postoperative complications that were assessed included: perioperative all-cause mortality, MI or MACCE, cerebral infarction rate, atrial fibrillation or supraventricular tachycardia, ventricular fibrillation or ventricular arrhythmia, secondary thoracotomy rate, wound infection rate, gastrointestinal bleeding, requirement for intra-aortic balloon pump (IABP) implantation and ExtraCorporeal Membrane Oxygenation (ECMO) use. Among these, MI was defined as postoperative troponin I (TnI) 10 times higher than normal accompanied by new Q wave or ST segment elevation of >2 mm in electrocardiogram (ECG) or other imaging findings showing new MI; MACCE was defined as cardiac death, nonfatal MI, unplanned revascularization, or stroke; cerebral infarction was defined as postoperative findings of cerebral infarction at >48 h and computed tomography (CT) or evidence of cerebral infarction by magnetic resonance imaging (MRI); atrial fibrillation or supraventricular tachycardia was defined as postoperative ECG showing atrial fibrillation greater than 1 min or supraventricular arrhythmia of >10 beats/min or supraventricular tachycardia; ventricular fibrillation or ventricular arrhythmia was defined as postoperative findings of ventricular arrhythmia >10 beats/min or ventricular fibrillation by heart electrical monitor; gastrointestinal bleeding was defined as the appearance of symptoms such as fecal occult blood positive, melena, hematemesis, or hemoglobin decline.
Prognosis
Postoperative follow-up was carried out using outpatient appoints or telephone. The follow-up data that were collected included postoperative survival, recurrent cardiovascular and cerebrovascular adverse events, and long-term MACCE. All data (including preoperative basic conditions, perioperative complications, and follow-up data) were strictly recorded, supervised, and proofread by two examiners.
Statistical analysis
All data were processed using SPSS 21.0 statistical software. Data are presented as mean ± standard deviation. And add number of patients at risk and individual patient information on censoring. Continuous variables were compared using the t-test, classification data were compared using the χ2 chi-square test. Survival was estimated by the Kaplan-Meier method, and any differences in survival were evaluated with a stratified log-rank test. Multivariable analyses with the Cox proportional-hazards model were used to estimate the simultaneous effects of prognostic factors on MACCE. Propensity score (PS) matching was used to adjust baseline data of the SCVBG and CE groups. In the PS matching process, age, dyslipidemia, MI history, previous PCI history, and SYNTAX score (which showed statistical differences between the two groups at baseline) were used as matching variables. Survival curves were compared using log-rank analysis and Cox proportional hazards analysis. All P values were 2-sided and P<0.05 was considered statistically significant.
Results
The degree of RCA diffuse lesions was more severe after SCVBG than CE
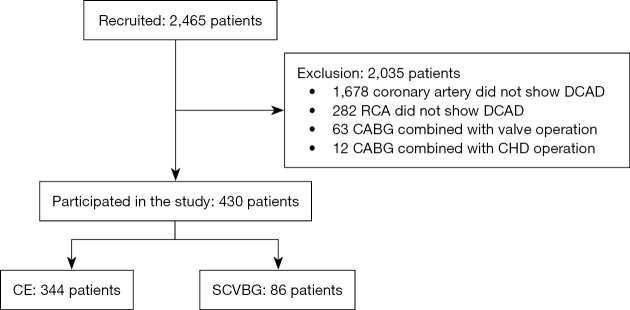
The study flow chart is presented in Figure 1. We recruited 2,465 patients who underwent CABG at the Beijing AnZhen Hospital, Capital Medical University between January 2015 and December 2018. Coronary angiography revealed DCAD in 787 patients; among them, 505 patients exhibited DCAD in the RCA, including 63 who underwent CABG combined with valvuloplasty/replacement surgery and 12 who underwent CABG combined with surgery for congenital heart disease. Finally, 430 patients met the inclusion criteria and were enrolled. Of these, 344 (80%) underwent CE and 86 (20%) underwent SCVBG.
Figure 1.
Flow chart showing the inclusion of patients. DCAD, diffuse coronary artery disease; RCA, right coronary artery; CABG, coronary artery bypass grafting; CHD, coronary heart disease; CE, coronary endarterectomy; SCVBG, selective coronary vein bypass grafting.
Before PS, the mean age, prevalence of dyslipidemia, history of MI, previous PCI, average SYNTAX score, and SYNTAX score of the RCA single coronary artery were significantly higher in the SCVBG group than the CE group. Other preoperative baseline data were not statistically different between the two groups (Table 1). After PS, the CE and SCVBG groups included 78 and 64 patients, respectively. A single coronary SYNTAX score was obtained for the RCA in both groups. The SYNTAX score of the RCA single coronary artery was significantly higher in SCVBG than the CE group (Table 2).
Table 1. Baseline clinical characteristics of the study population.
| Variables | Overall patients | Propensity-matched patients | |||||
|---|---|---|---|---|---|---|---|
| CE (n=344) | CVBG (n=86) | P value | CE (n=78) | CVBG (n=64) | P value | ||
| Age, years | 61.3±4.3 | 67.9±9.1 | <0.05 | 64.0±10.1 | 64.4±10.5 | NS | |
| Female sex | 95 (27.6) | 23 (26.7) | NS | 22 (28.2) | 17 (26.6) | NS | |
| Diabetes | 127 (36.9) | 32 (37.2) | NS | 28 (35.9) | 18 (28.1) | NS | |
| Hypertension | 200 (58.1) | 48 (55.8) | NS | 45 (57.7) | 37 (57.8) | NS | |
| Smoking | 86 (25.0) | 22 (25.6) | NS | 19 (24.4) | 16 (25.0) | NS | |
| Dyslipidemia | 189 (54.9) | 50 (58.1) | <0.05 | 44 (56.4) | 36 (56.3) | NS | |
| Previous MI | 122 (35.5) | 32 (37.2) | <0.05 | 28 (35.8) | 23 (35.9) | NS | |
| Previous PCI | 39 (11.3) | 16 (18.6) | <0.05 | 13 (16.7) | 11 (17.2) | NS | |
| PVD | 9 (2.6) | 2 (2.3) | NS | 3 (3.8) | 1 (1.6) | NS | |
| Preoperative low cardiac output, LVEF <40% | 42 (12.2) | 10 (11.6) | NS | 8 (10.3) | 5 (7.8) | NS | |
| Emergency surgery | 8 (2.3) | 2 (2.3) | NS | 2 (2.6) | 0 (0) | NS | |
| Bilateral internal mammary artery grafting | 33 (9.6) | 9 (10.4) | NS | 8 (10.3) | 7 (10.9) | NS | |
| CCSC IV | 27 (7.8) | 7 (8.1) | NS | 6 (7.7) | 5 (7.8) | NS | |
| Euro score | 5.8±2.1 | 5.3±3.5 | NS | 5.4±1.8 | 5.7±5.1 | NS | |
| SYNTAX score | 68.2±21.1 | 79.4±29.4 | <0.05 | 73.6±11.4 | 75.2±15.1 | NS | |
| SYNTAX for RCA | 21.3±2.5 | 26.4±6.7 | <0.05 | 23.4±2.1 | 25.7±3.2 | <0.05 | |
Continuous data are presented as mean ± standard deviation, categorical data as number (%). Abbreviations: CE, coronary endarterectomy; CVBG, coronary vein bypass grafting; NS, not significant; MI, myocardial infarction; PCI, percutaneous transluminal coronary intervention; PVD, peripheral vascular disease; LVEF, left ventricular ejection fraction; CCSC IV, Canadian Cardiovascular Society Classification IV; RCA, right coronary artery.
Table 2. In-hospital postoperative complications (before propensity score matching).
| Outcome | CE (n=344) | CVBG (n=86) | P value |
|---|---|---|---|
| In-hospital mortality | 4 (1.2) | 1 (1.1) | NS |
| Myocardial infarction | 13 (4.3) | 2 (2.3) | NS |
| MACCE | 28 (8.1) | 6 (7.0) | NS |
| Stroke | 5 (1.5) | 1 (1.2) | NS |
| Atrial fibrillation/supraventricular tachycardia | 31 (9.1) | 9 (10.4) | NS |
| Ventricular tachycardia/ventricular fibrillation | 4 (1.1) | 1 (1.2) | NS |
| Mean postoperative stay, days | 16.7±3.5 | 15.2±4.2 | NS |
| Reoperation for bleeding | 11 (3.2) | 2 (2.3) | NS |
| Wound Infection | 24 (7.0) | 3 (3.5) | NS |
| Postoperative gastrointestinal bleeding | 5 (1.5) | 1 (1.2) | NS |
| IABP use | 29 (8.4) | 7 (8.1) | NS |
| ECMO use | 4 (1.1) | 1 (1.2) | NS |
Continuous data are presented as mean ± standard deviation, categorical data as number (%). CE, coronary endarterectomy; CVBG, coronary vein bypass grafting; NS, not significant; MACCE, major adverse cardiovascular and cerebrovascular events; IABP, intra-aortic balloon pump; ECMO, extracorporeal membrane oxygenation.
SCVBG significantly reduces the incidence of perioperative MI and major cardiovascular and cerebrovascular adverse events in patients with severe DCAD in the RCA
There were no significant differences in perioperative complications between the two groups before PS (Table 2). After PS, the incidence of perioperative MI and MACCE were significantly higher in the CE group than in the SCVBG group. The incidence of other perioperative complications was not statistically different (Table 3).
Table 3. In-hospital postoperative complications (after propensity score matching).
| Outcome | CE (n=78) | CVBG (n=64) | P value |
|---|---|---|---|
| Hospital mortality | 1 (1.2) | 0 | NS |
| Myocardial infarction | 4 (5.1) | 1 (1.5) | <0.05 |
| MACCE | 8 (10.2) | 3 (4.7) | <0.05 |
| Stroke | 2 (2.6) | 0 | NS |
| Atrial fibrillation/supraventricular tachycardia | 7 (9.0) | 5 (7.8) | NS |
| Ventricular tachycardia/ventricular fibrillation | 2 (2.6) | 1 (1.6) | NS |
| Mean postoperative stay, days | 15.2±3.7 | 14.1±4.2 | NS |
| Reoperation for bleeding | 3 (3.8) | 1 (1.6) | NS |
| Wound infection | 2 (2.6) | 2 (3.1) | NS |
| Postoperative gastrointestinal bleeding | 2 (2.6) | 0 (0) | NS |
| IABP use | 6 (7.7) | 4 (6.2) | NS |
| ECMO use | 1 (1.2) | 0 | NS |
Continuous data are presented as mean ± standard deviation, categorical data as number (%). CE, coronary endarterectomy; CVBG, coronary vein bypass grafting; NS, not significant; MACCE, major adverse cardiovascular and cerebrovascular events; IABP, intra-aortic balloon pump; ECMO, extracorporeal membrane oxygenation.
The results of multivariate logistic regression analysis with regards to influencing factors for perioperative MACCE in the SCVBG group are shown in Table 4. The rate of perioperative MACCE significantly increased when the vascular flow rate of the GSV anastomosed with the RCA was greater than 100 mL/min. The use of bilateral internal mammary artery (BIMA) for SCVBG significantly reduced the incidence of perioperative MACCE.
Table 4. Results of multivariate logistic regression analysis of risk factors for in-hospital occurrence of major adverse cardiovascular and cerebrovascular events after selected coronary vein bypass grafting.
| Variables | Odds ratio | 95% CI | P value |
|---|---|---|---|
| RCA flow rate of >100 mL/min | 1.94 | 1.42–2.03 | <0.05 |
| Bilateral internal mammary artery grafting | 0.82 | 0.68–0.92 | <0.05 |
| Age (per a decade incremental) | 1.83 | 0.96–2.01 | NS |
| Female | 1.02 | 0.97–1.22 | NS |
| Diabetes | 1.05 | 0.82–1.11 | NS |
| LVEF <40% | 1.12 | 0.95–1.23 | NS |
| Previous MI | 1.07 | 0.95–1.16 | NS |
| PVD | 1.03 | 0.81–1.21 | NS |
CI, confidence interval; RCA, right coronary artery; NS, not significant; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PVD, peripheral vascular disease.
Long-term survival was not significantly different following SCVBG or CE
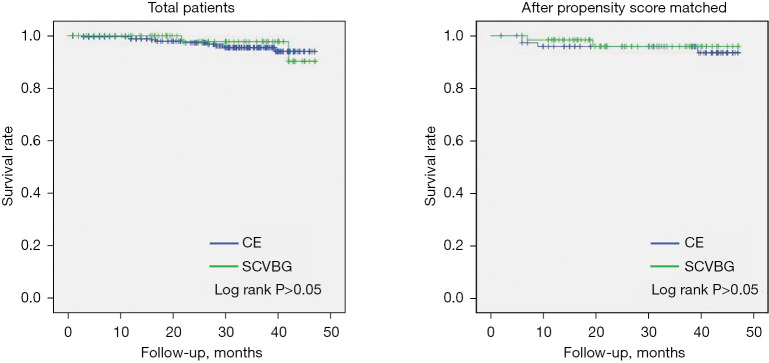
Follow-up of 29.3±13.9 months was completed for 430 patients; 16 patients were lost to follow-up, 15 from in the CE group and one from the SCVBG group (total loss to follow-up =3.7%). There were three deaths in the SCVBG group and 10 in the CE group. There was no significant difference in long-term survival rate between the groups (P>0.05). Twenty-three and 7 patients experienced MACCE in the CE and SCVBG groups, respectively; this difference was not significant (P>0.05). After PS, three patients from the matched CE group and two from the matched SCVBG group died; there was no significant difference in long-term survival (P>0.05) between two groups. There were five and four patients with MACCE in the PS-matched CE and SCVBG groups, respectively; this difference was not significant (P>0.05) (Figures 2,3).
Figure 2.
Survival of patients undergoing selective coronary vein bypass grafting versus coronary endarterectomy. CE, coronary endarterectomy; SCVBG, selective coronary vein bypass grafting.
Figure 3.
Freedom from postoperative major adverse cardiovascular and cerebrovascular events for patients undergoing selective coronary vein bypass grafting versus coronary endarterectomy. CE, coronary endarterectomy; SCVBG, selective coronary vein bypass grafting.
Discussion
Coronary heart disease is a serious hazard to human health. Approximately 12–30% of patients with CAD suffer from DCAD (11), which develops diffuse lesions as a pathogenic factor acting on existing coronary lesions, leading to aggravation of the lesion and eventual formation of DCAD (12). Comorbidities such as advanced age, diabetes, hypertension, dyslipidemia, and previous MI or PCI all contribute to the progression of coronary lesions (13,14), but the progression of coronary artery disease in relation to various comorbidities is unknown, especially in the case of RCA lesions. The present study compared baseline data of the two groups before PS, which revealed that higher average age, dyslipidemia, and previous MI or PCI were significantly higher among patients undergoing CE compared with those undergoing SCVBG. Abnormal lipid metabolism causes lipoproteins to be phagocytosed by macrophages to form foam cells which are deposited on the vascular wall and cause aggravation of lesions. The presence of oxidized low-density lipoprotein can activate the complement cascade and aggravate immune damage (15). Thus, previous MI and PCI creates chronic aggravation of RCA lesions. Multiple acute-phase proteins have been shown to promote intimal hyperplasia and thrombosis, affect glucose metabolism and lipid metabolism, aggravate coronary artery lesions, and further increase the incidence of DCAD (16). This study is, to the best of our knowledge, the first to quantify RCA lesions using the SYNTAX score, and it demonstrated that SYNTAX score of the RCA was significantly higher among patients undergoing SCVBG compared with those undergoing CE. This suggests that SCVBG is performed in more severe cases of DCAD in the RCA. It can be speculated that dyslipidemia, previous MI, and previous PCI contribute to the progression of diffuse lesions in the RCA.
CE (17) is currently the primary surgical approach for treatment of DCAD in the RCA. Wang et al. conducted a meta-analysis of observational study of CABG combined with CE (18), which revealed that the all-cause mortality rate at 30 days after combined surgery was 1.87 times that of CABG alone. Other meta-analyses have reported a 10% increase in perioperative MI and 7% increase in mortality compared with CABG alone (19), especially in patients undergoing CE in the RCA (4). These results may be related to the activation of internal coagulation mechanisms after CE (20). Because grafting of the vessel that is anastomosed to the RCA is usually the end of the sequential grafting, and the blood supply region of the RCA supplies the important cardiac conduction system, postoperative mortality increases significantly if the anastomosis is blocked (4,5). Therefore, CE should be carried out for the treatment of DCAD in the RCA with caution. Given that SCVBG does not damage the target vascular endothelium, we propose that complete RCA revascularization can be achieved by SCVBG when the RCA exhibits severe DCAD or when revascularization cannot be completed by CE.
The surgical procedure of SCVBG has been proposed by scholars as early as the 1970s (10,21), and the effectiveness of this procedure has been verified in animal experiments (21). Improvement of myocardial ischemia, especially in the case of acute MI (AMI), has been reported following CVBG (22,23), while SCVBG has been shown to effectively infuse the infarcted myocardium, maintain normal myocardial cell metabolism in the infarcted area, limit infarct size, and maintain left ventricular function (22,23). The results of the present study indicate that the complexity of patients undergoing SCVBG is greater than those undergoing CE, but the perioperative efficacy of SCVBG may be similar to CE. The reduced incidence of MI and MACCE in the SCVBG group after PS and agreement of the former with previous studies reporting perioperative MI after CABG suggest that SCVBG is an effective surgical procedure for severe DCAD in the RCA, or for patients who are unsuitable for CE.
Several studies (24,25) have confirmed that venous grafting in conditions of high pressure, high-speed blood flow, and high shear stress in arteries can cause acute damage to vascular endothelial cells after conventional CABG, thus increasing the risk of perioperative MI. This situation can also occur in the MCV system after SCVBG. It has been calculated that the blood flow in the MCV cannot exceed 50 mL/min according to the pressure and average diameter of the MCV (26). When the GSV is used as the grafting vessel anastomosed to the MCV, the flow rate is often too large because of the high intravascular pressure created at the end of the GSV anastomosed to the aorta. We discovered that a flow rate of greater than 100 mL/min at the anastomosis could increase the risk of short-term MACCE. Thus, we speculate that when the grafting flow rate of the MCV is more than 100 mL/min, this will cause excessive expansion of MCV and consequent endothelial injury, thereby increasing the risk of perioperative MI. In addition, excessive blood flow in a short period of time will increase postoperative myocardial edema and increase the risk of MACCE. However, we demonstrate that BIMA for SCVBG can effectively prevent postoperative in-hospital MACCE. The reasons for this effect may be as follows: (I) the graft directly connects the ascending aortic root and blood pressure and flow rate are higher, while the IMA arises from the subclavian artery and the blood pressure is lower than in the GSV; (II) as the middle layer of the GSV is thin and its ability to regulate blood flow is poor, the coronary vein cannot transfer excessive flow which may lead to myocardial hemorrhage and edema, while the middle layer of the IMA is mainly composed of elastic fiber and the smooth muscle components can automatically regulate blood flow (27), (III) compared with the GSV, the IMA can release a large number of vasodilator factors including endothelial nitric oxide synthase (eNOS), induced oxidative nitrogen synthase (iNOS), and prostaglandin I2 (PGI2), all of which have strong antithrombotic properties (24,28). In addition, the efficacy of complete arterial myocardial revascularization has been confirmed to be higher for BIMA than SCVBG (3).
The efficacy of CE for DCAD has been confirmed (6). A study carried out by Byrne and colleagues confirmed that the rates of long-term survival and major complications were not significantly higher after CE than after simple CABG (19,20). In the present study, SCVBG was associated with a higher number of RCA lesions. Patients who did not experience complete revascularization through CE were put onto the waiting list for heart transplant. However, we demonstrate that there is no significant difference in the long-term survival and incidence of MACCE between patients undergoing SCVBG and CE, suggesting that the long-term efficacy of SCVBG for patients with severe DCAD in the RCA is acceptable. Studies have shown that SCVBG can promote formation of the collateral circulation of the myocardium (29), which can improve the blood supply to myocardial cells and improve the long-term survival rate following SCVBG (30,31). Therefore, the present study highlights SCVBG as a new and effective surgical strategy for the treatment of patients who had diffuse lesions in the RCA but were not suitable candidates for CE. In addition, SCVBG is suitable for patients with severe chronic total obstruction in the RCA or left dominant coronary artery. Such patients do not have sufficient hedging blood between the RCA and MCV after SCVBG. Although this study confirms the short- and long-term effects of SCVBG, this procedure is not suitable as a routine surgical procedure for the treatment of DCAD in RCA because SCVBG is feasible only for a small percentage of patients with diffuse RCA lesions.
Limitations
First, the number of patients in the SCVBG group was small and significantly different from that in the CE group. Second, the mechanism of SCVBG was not elucidated. At present, there is a lack of intuitive and effective clinical imaging evidence confirming the surgical mechanism of SCVBG. Third, this study did not investigate long-term complications; thus, we cannot draw conclusions regarding the long-term efficacy of SCVBG.
Conclusions
Our study shows that SCVBG can effectively reduce the perioperative incidence of MI and MACCE in patients with severe DCAD in the RCA and that the efficacy of SCVBG is higher when the IMA is used as the grafting vessel. We demonstrate that, if SCVBG is performed with the GSV, then the grafting flow should be maintained below 100 mL/min. Therefore, SCVBG with BIMA is a more appropriate surgical strategy for patients with severe DCAD in the RCA than for those with conventional diffuse lesions in the RCA and should be the first-line treatment for the former.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors of this study would like to thank all the study participants.
Funding: This study was supported by the Beijing Municipal Science & Technology Commission (No. Z151100004015177), which provided financial support for the research. The sponsor had no role in the design or conduct of this research.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Beijing AnZhen Hospital, Capital Medical University (approval No. AEEI-2014-042) and informed consent was taken from all the patients.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-350/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-350/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-350/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-350/coif). The authors have no conflicts of interest to declare.
References
- 1.Khalifa AA, Cornily JC, David CH, et al. Medium-term survival of diffuse coronary artery disease patients following coronary artery reconstruction with the internal thoracic artery. Cardiology 2011;120:192-9. 10.1159/000335068 [DOI] [PubMed] [Google Scholar]
- 2.Nishigawa K, Fukui T, Yamazaki M, et al. Ten-Year Experience of Coronary Endarterectomy for the Diffusely Diseased Left Anterior Descending Artery. Ann Thorac Surg 2017;103:710-6. 10.1016/j.athoracsur.2016.11.028 [DOI] [PubMed] [Google Scholar]
- 3.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. Correction appears in Eur Heart J 2019;40:3096.
- 4.Konstanty-Kalandyk J, Bartuś K, Piątek J, et al. Is right coronary artery chronic total vessel occlusion impacting the surgical revascularization results of patients with multivessel disease? A retrospective study. PeerJ 2018;6:e4909. 10.7717/peerj.4909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taniguchi Y, Sakakura K, Adachi Y, et al. In-hospital outcomes of acute myocardial infarction with cardiogenic shock caused by right coronary artery occlusion vs. left coronary artery occlusion. Cardiovasc Interv Ther 2018;33:338-44. 10.1007/s12928-017-0490-9 [DOI] [PubMed] [Google Scholar]
- 6.Soylu E, Harling L, Ashrafian H, et al. Should we consider off-pump coronary artery bypass grafting in patients undergoing coronary endarterectomy? Interact Cardiovasc Thorac Surg 2014;19:295-301. 10.1093/icvts/ivu116 [DOI] [PubMed] [Google Scholar]
- 7.Stavrou A, Gkiousias V, Kyprianou K, et al. Coronary endarterectomy: The current state of knowledge. Atherosclerosis 2016;249:88-98. 10.1016/j.atherosclerosis.2016.03.036 [DOI] [PubMed] [Google Scholar]
- 8.Ariyaratnam P, Javangula K, Papaspyros S, et al. Long-term survival from 801 adjunctive coronary endarterectomies in diffuse coronary artery disease. Eur J Cardiothorac Surg 2012;42:e140-5. 10.1093/ejcts/ezs510 [DOI] [PubMed] [Google Scholar]
- 9.Chowdhry MF, Davies J, McCance A, et al. Lack of durability of surgical arterialization of coronary veins for the treatment of ischemic heart disease. J Card Surg 2005;20:326-8. 10.1111/j.1540-8191.2005.200489.x [DOI] [PubMed] [Google Scholar]
- 10.Arealis EG, Volder JG, Kolff WJ. Arterialization of the coronary vein coming from an ischemic area. Chest 1973;63:462-3. 10.1378/chest.63.3.462 [DOI] [PubMed] [Google Scholar]
- 11.Murray CSG, Siddiqui T, Keller N, et al. Physiology-Guided Management of Serial/Diffuse Coronary Artery Disease. Curr Cardiol Rep 2019;21:25. 10.1007/s11886-019-1105-0 [DOI] [PubMed] [Google Scholar]
- 12.Isner JM. Myocardial gene therapy. Nature 2002;415:234-9. 10.1038/415234a [DOI] [PubMed] [Google Scholar]
- 13.Jin C, Lu L, Zhang RY, et al. Association of serum glycated albumin, C-reactive protein and ICAM-1 levels with diffuse coronary artery disease in patients with type 2 diabetes mellitus. Clin Chim Acta 2009;408:45-9. 10.1016/j.cca.2009.07.003 [DOI] [PubMed] [Google Scholar]
- 14.Gould KL, Johnson NP. Physiologic severity of diffuse coronary artery disease: hidden high risk. Circulation 2015;131:4-6. 10.1161/CIRCULATIONAHA.114.013815 [DOI] [PubMed] [Google Scholar]
- 15.Calvo C, Olmos A, Ulloa N, et al. Lipoprotein particles LpA-I, LpA-I: A-II and LpB in coronary artery disease. Rev Med Chil 2000;128:9-16. [PubMed] [Google Scholar]
- 16.Firdous S. Correlation of CRP, fasting serum triglycerides and obesity as cardiovascular risk factors. J Coll Physicians Surg Pak 2014;24:308-13. [PubMed] [Google Scholar]
- 17.Ellouze M, Bouchard D, Pham M, et al. Coronary endarterectomy in patients with diffuse coronary artery disease: assessment of graft patency with computed tomography angiography. Can J Surg 2022;65:E635-41. 10.1503/cjs.011121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Gu C, Yu W, et al. Short- and Long-Term Patient Outcomes From Combined Coronary Endarterectomy and Coronary Artery Bypass Grafting: A Meta-Analysis of 63,730 Patients (PRISMA). Medicine (Baltimore) 2015;94:e1781. 10.1097/MD.0000000000001781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soylu E, Harling L, Ashrafian H, et al. Adjunct coronary endarterectomy increases myocardial infarction and early mortality after coronary artery bypass grafting: a meta-analysis. Interact Cardiovasc Thorac Surg 2014;19:462-73. 10.1093/icvts/ivu157 [DOI] [PubMed] [Google Scholar]
- 20.Ivert T, Welti R, Forssell G, Landou C. Coronary endarterectomy--angiographic and clinical results. Scand J Thorac Cardiovasc Surg 1989;23:95-102. 10.3109/14017438909105976 [DOI] [PubMed] [Google Scholar]
- 21.Bhayana JN, Olsen DB, Byrne JP, et al. Reversal of myocardial ischemia by arterialization of the coronary vein. J Thorac Cardiovasc Surg 1974;67:125-32. [PubMed] [Google Scholar]
- 22.Munz M, Amorim MJ, Faria M, et al. Cardiac venous arterialization in acute myocardial infarction: how great is the benefit? Interact Cardiovasc Thorac Surg 2013;16:307-13. 10.1093/icvts/ivs471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Y, Yan XL, Wei H, et al. Off-pump sequential bilateral internal mammary artery grafting combined with selective arterialization of the coronary venous system. Chin Med J (Engl) 2011;124:3017-21. [PubMed] [Google Scholar]
- 24.Tu QM, Wang ZW. Study on mechanism of c-Myc in restenosis after coronary artery bypass grafting. Eur Rev Med Pharmacol Sci 2016;20:2363-7. [PubMed] [Google Scholar]
- 25.Eda T, Teshima Y, Suga K, et al. Coronary artery bypass grafting for in-stent restenosis probably caused by allergic response; Report of a case. Kyobu Geka 2016;69:545-7. [PubMed] [Google Scholar]
- 26.Hochberg MS, Roberts WC, Morrow AG, et al. Selective arterialization of the coronary venous system. Encouraging long-term flow evaluation utilizing radioactive microspheres. J Thorac Cardiovasc Surg 1979;77:1-12. [PubMed] [Google Scholar]
- 27.Raja SG, Haider Z, Ahmad M, et al. Saphenous vein grafts: to use or not to use?. Heart Lung Circ 2004;13:150-6. Correction appears in Heart Lung Circ 2007;16:329. [DOI] [PubMed]
- 28.Zhu Y, Wang HS, Li XM, et al. Establishment of a rabbit model of coronary artery bypass graft and endothelial nitric oxide synthase gene transfection. Genet Mol Res 2015;14:1479-86. 10.4238/2015.February.20.3 [DOI] [PubMed] [Google Scholar]
- 29.Yu Y, Li HT, Gao MX, et al. Outcomes of middle cardiac vein arterialization via internal mammary/thoracic artery anastomosis. PLoS One 2013;8:e80963. 10.1371/journal.pone.0080963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Resetar ME, Ullmann C, Broeske P, et al. Selective arterialization of a cardiac vein in a model of cardiac microangiopathy and macroangiopathy in sheep. J Thorac Cardiovasc Surg 2007;133:1252-6. 10.1016/j.jtcvs.2006.12.037 [DOI] [PubMed] [Google Scholar]
- 31.Gardner RS, Magovern GJ, Park SB, et al. Arterialization of coronary veins in the treatment of myocardial ischemia. J Thorac Cardiovasc Surg 1974;68:273-82. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as