Abstract
Prolonged COVID-19 following B-cell depleting immunotherapy for malignant lymphoma is characterized by repeated cycles of remission followed by symptom recurrence, persistent detection of SARS-CoV-2, and profound humoral immunodeficiency. To the best of our knowledge, the present report is the first to describe dual antiviral therapy with remdesivir and ensitrelvir for prolonged COVID-19 following B-cell depleting immunotherapy for malignant lymphoma. A 59-year-old, female patient with a history of follicular lymphoma treated with obinutuzumab and bendamustine contracted COVID-19 despite receiving a single course of standard remdesivir therapy. She received dual antiviral therapy with remdesivir following a five-day course of oral ensitrelvir, which improved her clinical symptoms and chest radiology findings and cleared SARS-CoV-2 from respiratory samples. Dual antiviral therapy with remdesivir and ensitrelvir may be sufficient to stop viral replication and promote clinical resolution in prolonged COVID-19 following B-cell depleting immunotherapy for malignant lymphoma.
Keywords: Prolonged COVID-19, B-cell depleting immunotherapy, Remdesivir, Ensitrelvir, Dual antiviral therapy
Introduction
Immunocompromised patients, such as those with a hematological malignancy, are associated with a high risk of severe coronavirus disease 2019 (COVID-19) and poor outcomes of acute infection [1]. Recently, several studies of lymphoid malignancy treated with B-cell depleting therapy, such as rituximab and obinutuzumab, addressed prolonged COVID-19 symptoms with or without viremia and persistent severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) detection in respiratory samples [1], [2]. There is no clear definition of prolonged COVID-19 with persistent viral detection in the context of hematological malignancies, including malignant lymphoma treated with B-cell depleting therapy and characterized by 1) persistent or recurrent COVID-19 symptoms with or without pulmonary lesions (>30 days); 2) recent anti-CD20 therapy for a hematological malignancy, including malignant lymphoma (<1 year); 3) persistent SARS-CoV-2 detection in respiratory samples with or without concurrent viremia; 4) undetectable anti-SARS-CoV-2 antibody titer in the blood after infection or vaccination; 5) impairment of humoral and cellular immunity, such as lymphopenia (low CD19+B and CD4+T cell count) and hypogammaglobulinemia [1], [2], [3]. Prolonged COVID-19 results in poor outcomes, such as a longer hospital stay [2], impaired functional activity status, and even death. There are currently no consensus guidelines or a definite standard of care for patients with this condition [2].
We herein report the first case of successful management of prolonged COVID-19 using dual antiviral therapy with remdesivir and ensitrelvir following B-cell-depleting immunotherapy for malignant lymphoma.
Case report
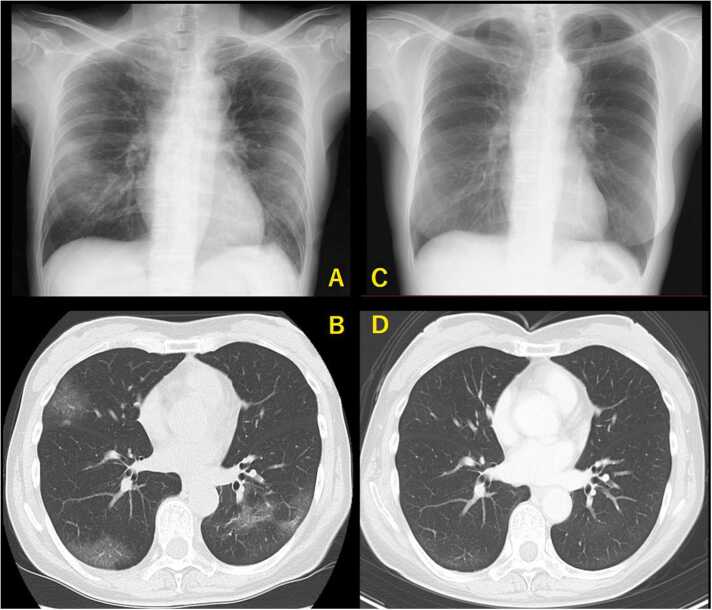
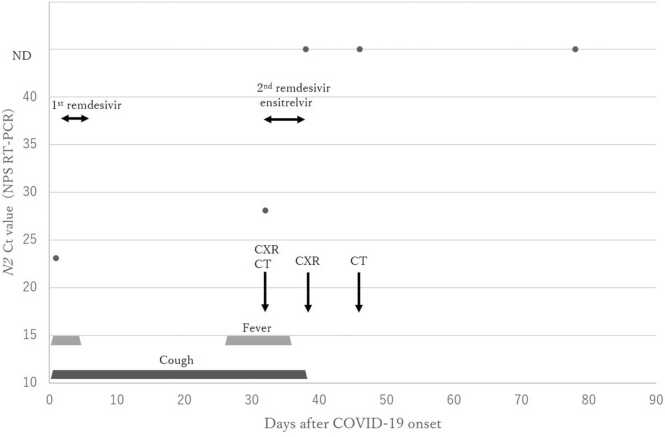
A 59-year-old, female patient with a medical history of follicular lymphoma received four cycles of obinutuzumab and bendamustine (GB) between August 2022 and November 2022 (eight to five months before COVID-19 onset) without maintenance therapy because of persistent, grade 3 neutropenia. She was also receiving antimicrobial prophylaxis with levofloxacin, acyclovir, and atovaquone. The patient demonstrated a complete metabolic response from December 2022 and had received three doses of the BNT162b2 vaccine before the initiation of GB therapy and an intramuscular tixagevimab / cilgavimab injection as prophylaxis in January 2023 (three months before COVID-19 onset). In April 2023 (on day 1 of COVID-19), a fever and cough developed, and COVID-19 was diagnosed after qualitative real-time polymerase chain reaction (RT-PCR) (Xpert®, Xpress SARS-CoV-2, BECKMAN COULTER) testing of nasopharyngeal swabs (NPS) returned positive for SARS-CoV-2, with a cycle threshold (Ct) value of 25.6 and 23.1 for the E and N2 gene, respectively. The patient did not require supplemental oxygen and demonstrated no lung lesions on chest X-ray. However, persistent, grade 3 neutropenia and severe lymphopenia were observed, and treatment with molnupiravir was begun but was later discontinued because of severe anorexia development. On day 2 after COVID-19 onset, the patient was hospitalized and received a five-day course of remdesivir (200 mg on day 1 and 100 mg on days 2–5). The patient achieved partial, clinical improvement with defervescence but still had a protracted cough but was discharged on day 11 after COVID-19 onset. On day 26 after COVID-19 onset, the fever recurred, and the cough had deteriorated. The patient was hospitalized for the second time on day 32 after COVID-19 onset. On admission, RT-PCR of NPS repeatedly returned positive for the virus, with a Ct value of 31.6 and 28.2 for the E and N2 gene, respectively. Chest X-ray and chest computed tomography (CT) demonstrated diffuse, bilateral ground-glass opacities consistent with COVID-19 pneumonia (Fig. 2). Lymphopenia with a depleted CD19+B and CD4+T cell count of 0/µL and 220/µL, respectively and low gammaglobulinemia (IgG 848 mg/dL, IgA 64 mg/dL, IgM 21 mg/dL) were also observed. Based on these findings, prolonged COVID-19 following B-cell depleting immunotherapy for malignant lymphoma was newly diagnosed. To exploit a possible, additive effect of dual antiviral therapy [19], administration of a three-day course of standard-dose remdesivir (200 mg on day 1 and 100 mg on days 2–3) was begun following a five-day course of oral ensitrelvir 375 mg on day 1, which was tapered to 125 mg on days 2–5 avoid a drug interaction or adverse events resulting from the combined administration of the medications. On day 36 after COVID-19 onset (or day 5 after commencement of dual antiviral therapy), the fever abated, and clinical improvement was achieved. On day 38 after COVID-19 onset, RT-PCR of NPS returned negative, and the patient was discharged with complete resolution of her lung lesions (Fig. 2) without any adverse events. Follow-up RT-PCR using NPS repeatedly returned negative on day 46 after COVID-19 onset and thereafter. An antibody test for SARS-CoV-2 nucleocapsid protein (Elecsys®, Anti-SARS-CoV-2 RUO, Roche Diagnostics, K.K.) returned negative. Follow-up lung CT also demonstrated the resolution of the lung lesions (Fig. 2). Fig. 1 shows a summary of the patient’s clinical course and management during COVID-19.
Fig. 2.
Chest X-ray and lung computed tomography (CT). A, B) On day 32, diffuse, bilateral ground-glass opacities consistent with COVID-19 pneumonia were observed. C, D) On day 38 and day 46, the opacities had resolved, and no further lung lesions were observed.
Fig. 1.
Summary of patient’s clinical course and treatment based on the N2 cycle threshold (Ct) values of SARS-CoV-2 in nasopharyngeal swabs (NPS). On day 1, the patient had a fever, cough, and anorexia. The N2 Ct value of a nasopharyngeal swab (NPS) on real-time polymerase chain reaction (RT-PCR) was 23.1, demonstrating a medium viral load. A five-day, single, standard dose of remdesivir therapy achieved defervescence, but the cough remained. The patient was discharged on day 11. On day 26, the fever recurred, and the cough worsened. The patient was readmitted on day 32 with diffuse, bilateral ground-glass opacites and a N2 Ct value on NPS RT-PCR of 28.2, demonstrating a persistent, medium, viral load. Dual antiviral therapy with a three-day standard dose of remdesivir following a five-day course of oral ensitrelvir was begun. On day 36, the fever abated. The patient fully recovered and was finally discharged on day 38 after RT-PCR of NPS returned negative, and the lung lesions had resolved. Thereafter, the patient has been in stable condition, and RT-PCR tests of NPS consistently returned negative. Abbreviations: ND, not detected; CXR, chest X-ray; CT, lung computed tomography.
Discussion
Patients with a lymphoid malignancy receiving B-cell depleting therapy have insufficient humoral immunity and dysregulated cellular immunity [1]. A decrease in the CD4+T cell count and compensatory innate immune and CD8+T cell activation combined with loss of B-cell function can enable the control of an acute infection and/or viremia caused by SARS-CoV-2 but are insufficient to achieve adequate viral clearance without the requisite level of neutralizing antibody [1]. The incomplete viral clearance correlates with symptomatic relapses and results in prolonged COVID-19. Thus, effective treatment of prolonged COVID-19 involves focusing on clearing residual viruses especially from the blood and respiratory tract.
There are several management options for prolonged COVID-19, such as antiviral therapy, immunotherapy, and even a multimodal strategy combining the two [4], [5], [6], [7], [8], [9], [10], [11], [12], [19], [20]. Remdesivir, a RNA-dependent RNA polymerase (RdRP) inhibitor, is the first antiviral agent to be approved for use against SARS-CoV-2 infection [9]. Several courses of remdesivir lasting five to ten days each can achieve temporary suppression of the virus but cannot achieve viral clearance in respiratory samples [7], [8]. Further, a mutation of RdRP following treatment with remdesivir in prolonged COVID-19 has been reported [9]. Single-dose remdesivir therapy may have a role in controlling the disease course but is insufficient for achieving resolution [4], [5], [6]. With regard to the humoral immunodeficiency seen in prolonged COVID-19, immunotherapy with convalescent plasma (CP) [13], [14], intravenous immunoglobulin (IVIG)[15] or anti-SARS-CoV-2 monoclonal antibody (mAB) [16] may be considered. While there are some reports of successful treatment with CP and a multimodal approach including mAb for prolonged COVID-19, the use of CP in patients with severe COVID-19 pneumonia as well as those with COVID-19 pneumonia requiring hospitalization failed to demonstrate a significant, clinical benefit [14], and emerging and circulating variants of SARS-CoV-2 with spike protein mutations restrict its clinical value [17]. Moreover, non-neutralizing antibodies in IVIG can worsen a SARS-CoV-2 infection through antibody-dependent enhancement [15].
Ensitrelvir, a novel, oral, SARS-CoV-2 3C-like protease (3CLpro)-inhibitor approved in Japan for mild to moderate COVID-19, demonstrated a rapid and significant reduction in the titer [18] of currently circulating SAR-CoV-2 variants, including omicron (BA1.1, BA2, BA2.75, BA.4, BA.5, BQ1.1, XBB.1, and XE), mu, lambda, and theta. Further, it demonstrated an inhibitory effect on variants with the 3CLpro mutation [17]. Another 3CLpro -inhibitor, nirmatrelvir/ritonavir, induced rapid remission of symptoms and viral clearance in several cases of prolonged COVID-19 [10], [11], [12]. These treatments are currently the only effective antiviral therapy for patients with COVID-19 with an inadequate host immune response [11].
Previous studies have reported the success of dual antiviral therapy with remdesivir and nirmatrelvir/ritonavir in treating prolonged COVID-19 [19], [20]. This combination therapy has the potential, additive effect of reducing the likelihood the virus’ evading treatment via mutations conferring resistance to either of the medications. Nirmatrelvir/ritonavir and ensitrelvir may also be good options for improving outcomes in prolonged COVID-19 if administered concurrently with remdesivir because they also inhibit 3CLpro.
Our patient received anti-CD20 immunochemotherapy for follicular lymphoma six months before COVID-19 onset. Her level of anti-SARS-CoV-2 antibody was undetectable. She had lymphopenia and hypogammaglobulinemia. Despite vaccination against SARS-CoV-2, prophylactic use of intramuscular tixagevimab/cilgavimab injections three months before onset, and a single remdesivir infusion during the first hospitalization, she was able to achieve only a partial response; her disease became prolonged, and RT-PCR testing of NPS were persistently positive for the virus for 30 days, thus resulting in a second hospitalization. Our case is a typical example of prolonged COVID-19 following B-cell-depleting immunotherapy for malignant lymphoma. Dual antiviral therapy with remdesivir and ensitrelvir during the patient’s second hospital admission resulted in favorable clinical and virological outcomes without any notable adverse effect. Although further research with larger cohorts and clinical trials are needed to verify the efficacy of remdesivir combined with ensitrelvir against prolonged COVID-19, in the current absence of other treatment strategies, this combination therapy may be a viable option for treating patients with COVID-19.
In conclusion, patients with prolonged COVID-19 following B-cell depleting immunotherapy for malignant lymphoma have features that are distinct from those of other, immunocompromised populations as well as immunocompetent patients with COVID-19. Effective antiviral therapy is a requirement for achieving SARS-CoV-2 clearance and favorable treatment outcomes. Even though the current study had a small sample pool, its findings, together with those of other reports [19], [20], suggest that dual antiviral therapy with remdesivir and ensitrelvir may be sufficient to suppress viral replication and promote clinical resolution of prolonged COVID-19 following B-cell depleting immunotherapy for malignant lymphoma.
Ethics approval
Written informed consent was obtained from the patient for the publication of the details of this case and the accompanying images.
Funding
This study did not receive any specific grant from any funding agencies in the public, commercial or not-for-profit sector.
CRediT authorship contribution statement
Seowoong Jung: Conceptualization, Writing – original draft preparation. Yu Yagi, Yukari Nishikawa, Taiichiro Kobayashi, Keishiro Yajima: Writing – review & editing. Kazuaki Fukushima, Masaru Tanaka, Atsushi Ajusawa, Akifumi Imamura: Writing – review & editing, Supervision.
Declaration of Competing Interest
None.
Acknowledgements
The authors thank the patient for her consent to publish this report.
References
- 1.Lee C.Y., Shah M.K., Hoyos D., Solovyov A., Douglas M., Taur Y., et al. Prolonged SARS-CoV-2 infection in patients with lymphoid malignancies. Cancer Discov. 2022;12:62–73. doi: 10.1158/2159-8290.CD-21-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duléry R., Lamure S., Delord M., Di Blasi R., Chauchet A., Hueso T., et al. Prolonged in-hospital stay and higher mortality after Covid-19 among patients with non-Hodgkin lymphoma treated with B-cell depleting immunotherapy. Am J Hematol. 2021;96:934–944. doi: 10.1002/ajh.26209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yasuda H., Mori Y., Chiba A., Bai J., Murayama G., Matsushita Y., et al. Resolution of one-year persisting COVID-19 pneumonia and development of immune thrombocytopenia in a follicular lymphoma patient with preceding rituximab maintenance therapy: a follow-up report and literature review of cases with prolonged infections. Clin Lymphoma Myeloma Leuk. 2021;21:e810–e816. doi: 10.1016/j.clml.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueda Y., Asakura S., Wada S., Saito T., Yano T. Prolonged COVID-19 in an immunocompromised patient treated with obinutuzumab and bendamustine for follicular lymphoma. Intern Med. 2022;61:2523–2526. doi: 10.2169/internalmedicine.9136-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camprubí D., Gaya A., Marcos M.A., Martí-Soler H., Soriano A., Mosquera M., del M., et al. Persistent replication of SARS-CoV-2 in a severely immunocompromised patient treated with several courses of remdesivir. Int J Infect Dis. 2021;104:379–381. doi: 10.1016/j.ijid.2020.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kajova M., Kekäläinen E., Anttila V.J., Paajanen J. Successful treatment with a short course of remdesivir in a case of prolonged COVID-19 in a lymphoma patient. Infect Dis. 2022;54:455–459. doi: 10.1080/23744235.2022.2028896. [DOI] [PubMed] [Google Scholar]
- 7.Sepulcri C., Dentone C., Mikulska M., Bruzzone B., Lai A., Fenoglio D., et al. The longest persistence of viable SARS-CoV-2 with recurrence of viremia and relapsing symptomatic COVID- 19 in an Immunocompromised patient-a case study. Open Forum Infect Dis. 2022:8. doi: 10.1093/ofid/ofab217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helleberg M., Niemann C.U., Moestrup K.S., Kirk O., Lebech A.M., Lane C., et al. Persistent COVID-19 in an immunocompromised patient temporarily responsive to two courses of remdesivir therapy. J Infect Dis. 2020;222:1103–1107. doi: 10.1093/infdis/jiaa446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinot M., Jary A., Fafi-Kremer S., Leducq V., Delagreverie H., Garnier M., et al. Emerging RNA-dependent RNA polymerase mutation in a remdesivir-treated B-cell immunodeficient patient with protracted coronavirus disease 2019. Clin Infect Dis. 2021;73:E1762–E1765. doi: 10.1093/cid/ciaa1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franceschini E., Pellegrino M., Todisco V., Dolci G., Bettelli F., Meschiari M., et al. Persistent SARS-CoV-2 infection with multiple clinical relapses in two patients with follicular lymphoma treated with bendamustine and obinutuzumab or rituximab. Infection. 2023:1–5. doi: 10.1007/s15010-023-02039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu C., Yoke L.H., Bhattacharyya P., Cassaday R.D., Cheng G.-S., Escobar Z.K., et al. Successful treatment of persistent symptomatic coronavirus disease 19 infection with extended-duration nirmatrelvir-ritonavir among outpatients with hematologic cancer. Open Forum Infect Dis. 2023:10. doi: 10.1093/ofid/ofad306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graziani L., Gori L., Manciulli T., Basile G., Campolmi I., Borchi B., et al. Successful use of nirmatrelvir/ritonavir in immunocompromised patients with persistent and/or relapsing COVID-19. J Antimicrob Chemother. 2023;78:555–558. doi: 10.1093/jac/dkac433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karataş A., İnkaya A.Ç., Demiroğlu H., Aksu S., Haziyev T., Çınar O.E., et al. Prolonged viral shedding in a lymphoma patient with COVID-19 infection receiving convalescent plasma. Transfus Apher Sci. 2020:59. doi: 10.1016/j.transci.2020.102871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martínez-Barranco P., García-Roa M., Trelles-Martínez R., Arribalzaga K., Velasco M., Guijarro C., et al. Management of persistent SARS-CoV-2 infection in patients with follicular lymphoma. Acta Haematol. 2022;145:384–393. doi: 10.1159/000521121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kos I., Balensiefer B., Roth S., Ahlgrimm M., Sester M., Schmidt T., et al. Prolonged course of COVID-19-associated pneumonia in a B-cell depleted patient after rituximab. Front Oncol. 2020:10. doi: 10.3389/fonc.2020.01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thornton C.S., Huntley K., Berenger B.M., Bristow M., Evans D.H., Fonseca K., et al. Prolonged SARS-CoV-2 infection following rituximab treatment: clinical course and response to therapeutic interventions correlated with quantitative viral cultures and cycle threshold values. Antimicrob Resist Infect Control. 2022:11. doi: 10.1186/s13756-022-01067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawashima S., Matsui Y., Adachi T., Morikawa Y., Inoue K., Takebayashi S., et al. Ensitrelvir is effective against SARS-CoV-2 3CL protease mutants circulating globally. Biochem Biophys Res Commun. 2023;645:132–136. doi: 10.1016/j.bbrc.2023.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukae H., Yotsuyanagi H., Ohmagari N., Doi Y., Sakaguchi H., Sonoyama T., et al. Efficacy and safety of ensitrelvir in patients with mild-to-moderate coronavirus disease 2019 (COVID-19): the phase 2b part of a randomized, placebo-controlled, phase 2/3 study. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trottier C.A., Wong B., Kohli R., Boomsma C., Magro F., Kher S., et al. Dual antiviral therapy for persistent coronavirus disease 2019 and associated organizing pneumonia in an immunocompromised host. Clin Infect Dis. 2023;76:923–925. doi: 10.1093/cid/ciac847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peracchi F., Merli M., Rogati C., Ravano E., Puoti M., Cairoli R., et al. Dual antiviral therapy in haematological patients with protracted SARS-CoV-2 infection. Br J Haematol. 2023 doi: 10.1111/bjh.18827. [DOI] [PubMed] [Google Scholar]